Abstract
Background
Electronic nicotine delivery systems (ENDS; e-cigarettes), consisting of a battery, heating element and e-liquid, have evolved significantly with wide variation in design, components, operating powers, and chemical constituents. Generated aerosols have been reported to contain potentially toxic substances. We conducted a systematic review to assess what is known about the presence of toxicants in ENDS aerosols in order to inform how system design could mitigate risk.
Methods
Articles reporting on or evaluating design characteristics of ENDS and aerosol constituents were included and summarized.
Results
The search identified 2,305 articles, of which 92 were included after full-text review. Findings were grouped into 6 major categories of potentially harmful chemicals: carbonyls, volatile organic chemicals, trace elements, reactive oxygen species and free radicals, polycyclic aromatic hydrocarbons, and tobacco-specific nitrosamines. In general, higher concentrations of aerosol toxicants are associated with increased power or voltage. Aerosol toxicants are also associated with e-liquid flavoring agents existing as primary ingredients or as products of thermal degradation.
Conclusions
Improved ENDS design can reduce toxicant levels. Additional research is needed to develop a framework for optimizing system characteristics to minimize exposure, especially with respect to heating power and e-liquids. Both manufacturers and regulatory agencies have roles in reducing toxicants and potential health risks from ENDS.
Introduction
Electronic nicotine delivery systems (ENDS) consist of a battery, a heating element (usually consisting of a coil and wick), and liquid (“e-liquid”). The heated coil aerosolizes the e-liquid which consists of propylene glycol (PG), vegetable glycerin (VG), nicotine, and flavoring agents. ENDS were introduced commercially in China in 2003 and to the United States and Europe between 2006 and 2007 [1]. The ENDS market was a $19.3 billion global industry in 2018 [2] which is expected to increase to $58 billion by 2026 [3]. ENDS have been proposed as a smoking cessation strategy [4], or for smoking reduction [5], but concerns also exist that ENDS may serve as a “gateway” to conventional tobacco products among adolescents [6].
The aerosols produced by ENDS have been reported to contain potentially toxic substances, which the user inhales [7]. Previous literature reviews evaluated aerosol carbonyls [8], trace metals [9], and methodological approaches to analysis [10]. A broader review [11] evaluated carbonyls, volatile organic compounds (VOCs), metals, polycyclic aromatic hydrocarbons (PAHs), and tobacco-specific nitrosamines (TSNAs) in aerosols, as well as flavors and solvents in refill solutions, and cartridges. No reviews have summarized the literature relating to the impact of ENDS design on all identified aerosol toxicants. Potential toxicants originate from e-liquid components, degradation of ENDS materials, and reactions between the device and e-liquid delivering chemicals such as VOCs, trace elements [12], and carbonyls such as formaldehyde, acrolein [13], and acetaldehyde [14]. Investigators have suggested that differences in the engineering process, individual modifications [15], and design features affect aerosol composition [16].
We conducted a systematic review of the literature in order to collate and integrate the literature assessing the design characteristics of ENDS associated with the production or release of potentially harmful substances in inhaled aerosols. We approached our review from the perspective of advancing understanding of how ENDS design could inform scientific and policy discussion around how to modify potential risks associated with their use.
Methods
A registered protocol does not exist for this systematic review. A PRISMA checklist is available in supporting information (S1 Checklist).
Inclusion criteria
The review included articles reporting on (i.e., listing ENDS characteristics) or evaluating (i.e., assessing how design differences impact output) ENDS design characteristics and aerosol constituents, with a specific focus on the measurement of chemical species in aerosols produced by heated-coil ENDS.
Exclusion criteria
We excluded articles first by duplicates (n = 287), then a title and abstract screening, and finally by a full-text screening. Irrelevant studies included (n = 1,835), but were not limited to, articles not assessing ENDS aerosol, letters, commentaries, or other reviews, second-hand or third-hand aerosol/residue assessments from ENDS, and environmental exposure studies. Additionally, studies retrieved that were not available in English or were published anonymously were also eliminated at this stage. At the full-text screening, studies were eliminated upon the basis of 4 criteria: wrong study design (n = 32; e.g., indirect measures of toxicity or cytotoxicity studies, exhaled breath or saliva studies, or only analyzed e-liquid), no primary data (n = 28; e.g., perspective, comments, or responses to previous literature), no full text (n = 17), and assessed major aerosol constituents only (n = 14; studies exclusively analyzing the known major and expected aerosol constituents nicotine, PG, and VG) were also excluded as these are expected aerosol constituents when liquids containing them are aerosolized.
We defined risk of bias a priori as the utilization of analytical techniques employed by research groups externally (Group A), internally (Group B), or non-replicated (Group C) for a specific major chemical category. Because we did not quantitatively combine study results, no articles were excluded based on risk of bias.
Search strategy
We searched Ovid MEDLINE, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions electronic databases, selecting articles published from 1946 until May 07, 2020. We employed the following keywords: "electronic nicotine device," “vape,” “vaping,” "electronic nicotine delivery system," "electronic cigarette," "e-cigarette," “ecig,” and "e-cigs." These terms were combined with the following terms: “acetaldehyde,” “acrolein,” “aerosol,” “air pollutant,” “aldehyde,” "aromatic amine," “atomizer,” “carbonyl,” “coil wire,” “e-liquid,” “exp flavoring agents,” “exp metals, heavy,” “exp temperature,” “formaldehyde,” "free radical," “glycerol,” "heavy metal," “nitrosamine,” “phenol,” "polycyclic aromatic hydrocarbon," “propylene glycol,” “thermal degradation,” “toxic,” “toxicity,” “vocs,” “volatile,” and "volatile organic compounds".
Results
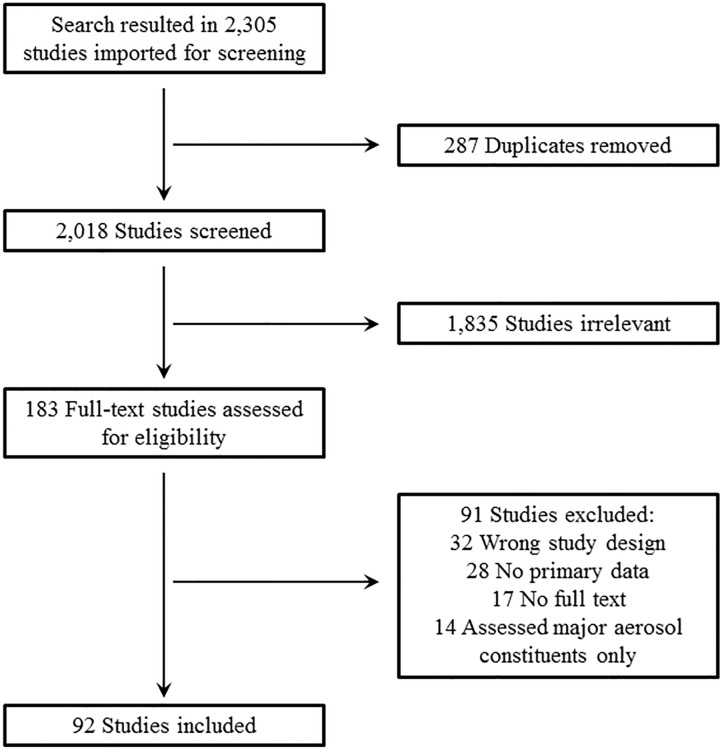
Our search strategy identified 2,305 articles, and full-text review resulted in inclusion of 92 studies (Fig 1). We grouped studies into 6 major chemical groups. Five were included on the basis of their designation as “harmful and potentially harmful constituents” in tobacco products by the US Food & Drug Administration (FDA) [17]: carbonyls, VOCs, trace elements, PAHs, and TSNAs. In addition, we added free radicals and reactive oxygen species (ROS) because of their potential to cause tissue injury and disrupt physiological function [18]. If a particular chemical did not fit into one of these categories, it was categorized as “other” and not included in the presented tables.
Fig 1. Methodological approach for systematic review and article selection.
Because there is overlap in the physical properties of categories, chemicals were sorted on the basis of general consensus in the literature as well as physical characteristics. The carbonyl category consisted of chemicals containing a carbonyl moiety; examples include formaldehyde and acetaldehyde. VOCs were defined as organic compounds with a boiling point between 50 and 250 °C [19], such as benzene and styrene. Elemental analysis was required for inclusion in the trace elements category; common examples include copper (Cu), lead (Pb), and zinc (Zn). ROS and free radicals included studies analyzing ROS, free radicals, or flux in ROS. PAHs included compounds exclusively containing carbon and hydrogen atoms and at least 2 benzene rings bonded in particular arrangements such as clustered, angular, and linear [20]. Common examples seen in the ENDS literature are benzo[a]pyrene and chrysene. TSNAs are N-nitroso derivatives of pyridine alkaloids such as nicotine and nornicotine that are naturally occurring in tobacco and increase throughout the curing process [21]; N’-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) are notable examples. Articles were not combined quantitatively due to heterogeneity in collection methods, analytical methods, device parameters, and reported units.
Our search identified publications reporting ENDS characteristics and major toxicant chemical groups in aerosols (Table 1; S1 Table). We identified carbonyl compounds as the most commonly analyzed toxicants and further characterized articles by the specific carbonyl chemical analyzed (Table 2). The majority of these studies reported power, device type (i.e., manufacturer or other device identifying characteristics regarding atomizers, cartomizers, or clearomizers), and e-liquid flavor (i.e., commercially available or laboratory-prepared) and evaluated aerosol constituents of formaldehyde, acetaldehyde, and acrolein.
Table 1. Number of studies reporting ENDSa characteristics and measured major chemical groups in aerosol.
| Characteristic | Carbonyls | VOCs | Trace Elements | ROS and Free Radicals | PAHs | TSNAs |
|---|---|---|---|---|---|---|
| Power (W) | 28 [22–49] | 7 [39, 44, 47–51] | 5 [49, 51–54] | 4 [55–58] | 2 [49, 59] | 1 [49] |
| Voltage (V) | 23 [23–26, 28–30, 33, 36, 38, 42, 43, 60–70] | 5 [51, 64, 66, 69, 71] | 7 [51–53, 60, 69, 71, 72] | 1 [73] | 2 [60, 69] | 1 [69] |
| Resistance (Ω) | 34 [22, 23, 25–29, 31–36, 38, 39, 41–48, 60, 62, 64, 65, 67, 69, 70, 74–77] | 10 [39, 44, 47, 48, 50, 51, 64, 69, 76, 77] | 5 [51–53, 60, 69] | 4 [56–58, 73] | 3 [60, 69, 76] | 2 [69, 76] |
| Temperature (°C) | 1 [41] | 2 [56, 58] | ||||
| Coil Material | 6 [22, 25, 27, 45, 48, 69] | 3 [48, 51, 69] | 5 [51, 52, 54, 69, 78] | 3 [56–58] | 1 [69] | 1 [69] |
| Device | 45 [14, 22–32, 34–47, 49, 60, 62, 64, 65, 67–70, 74–77, 79–84] | 12 [39, 44, 47, 49–51, 69, 76, 77, 79, 83, 85] | 13 [49, 51–54, 60, 69, 72, 79, 83, 86–88] | 10 [14, 55–58, 73, 84, 88–90] | 7 [49, 59, 60, 69, 76, 83, 85] | 6 [49, 69, 76, 79, 83, 91] |
| E-Liquid | 52 [14, 22–49, 60, 62, 64–70, 74–77, 79, 80, 83, 84, 92–97] | 19 [39, 44, 47–51, 64, 66, 69, 71, 76, 77, 79, 83, 85, 93–95] | 14 [49, 51–54, 60, 69, 71, 79, 83, 86–88, 98] | 10 [14, 55–58, 73, 84, 88–90] | 7 [49, 59, 60, 69, 76, 83, 85] | 6 [49, 69, 76, 79, 83, 91] |
a Abbreviations: ENDS, electronic nicotine delivery device; VOC, volatile organic compound; ROS, reactive oxygen species; PAH, polycyclic aromatic hydrocarbon; TSNA, tobacco-specific nitrosamines
Table 2. Number of studies reporting ENDS characteristics and measured carbonyl compounds.
| Characteristic | Formaldehyde | Acetaldehyde | Acrolein | Crotonaldehyde | Acetone | Propionaldehyde | Methylglyoxal | Glyoxal | Dihydroxyacetone | Butyraldehyde (butanal) | Benzaldehyde | Isovaleraldehyde (3-methylbutanal) | valeraldehyde (pentanal) | Diacetyl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Power (W) | 23 [22–26, 28–33, 36–42, 45–49] | 22 [22, 23, 25–29, 32, 33, 35–42, 44–49] | 22 [22, 23, 25–29, 32, 33, 35–42, 44, 46–49] | 6 [36, 38, 41, 42, 47, 49] | 14 [22, 28, 29, 32, 33, 37, 40–42, 44, 45, 47–49] | 14 [22, 27, 29, 32, 33, 36, 38–41, 44, 45, 47, 49] | 6 [22, 32, 33, 41, 48, 49] | 7 [32, 33, 38, 41, 47–49] | 2 [34, 44] | 5 [29, 38, 41, 47, 49] | 4 [32, 38, 43, 47] | 5 [32, 38, 41, 45, 47] | 1 [49] | |
| Voltage (V) | 19 [23–26, 28–30, 33, 36, 38, 42, 60–65, 69, 70] | 18 [23, 25, 26, 28, 29, 33, 36, 38, 42, 60–66, 69, 70] | 18 [23, 25, 26, 28, 29, 33, 36, 38, 42, 60–66, 69, 70] | 8 [36, 38, 42, 61, 62, 65, 69, 70] | 10 [28, 29, 33, 42, 61, 62, 65, 66, 69, 70] | 10 [29, 33, 37, 38, 61–63, 65, 69, 70] | 2 [33, 69] | 3 [33, 38, 69] | 7 [38, 61–63, 65, 69, 70] | 7 [38, 43, 61, 62, 67, 68, 70] | 4 [61–63, 70] | 6 [38, 61–63, 65, 70] | 2 [64, 69] | |
| Resistance (Ω) | 28 [22, 23, 25, 26, 28, 29, 31–33, 36, 38, 39, 41, 42, 45–48, 60, 62, 64, 65, 69, 70, 74–77] | 30 [22, 23, 25–29, 32, 33, 35, 36, 38, 39, 41, 42, 44–48, 60, 62, 64, 65, 69, 70, 74–77] | 29 [22, 23, 25–29, 32, 33, 35, 36, 38, 39, 41, 42, 44, 46–48, 60, 62, 64, 65, 69, 70, 74–77] | 11 [36, 38, 41, 42, 47, 62, 65, 69, 70, 74, 76] | 19 [22, 28, 29, 32, 33, 41, 42, 44, 45, 47, 48, 62, 65, 69, 70, 74–77] | 19 [22, 27, 29, 32, 33, 36, 38, 39, 41, 44, 45, 47, 62, 65, 69, 70, 74, 76, 77] | 8 [22, 32, 33, 41, 48, 49, 69, 77] | 9 [32, 33, 38, 41, 47–49, 69, 77] | 3 [34, 44, 77] | 11 [29, 38, 41, 47, 49, 62, 65, 69, 70, 74, 76] | 8 [32, 38, 43, 47, 62, 67, 70, 74] | 3 [62, 70, 74] | 9 [32, 38, 41, 45, 47, 62, 65, 70, 74] | 3 [64, 69, 77] |
| Temperature (°C) | 1 [41] | 1 [41] | 1 [41] | 1 [41] | 1 [41] | 1 [41] | 1 [41] | 1 [41] | 1 [41] | 1 [41] | ||||
| Coil Material | 5 [22, 25, 45, 48, 69] | 6 [22, 25, 27, 45, 48, 69] | 5 [22, 25, 27, 48, 69] | 1 [69] | 4 [22, 45, 48, 69] | 4 [22, 27, 45, 69] | 3 [22, 48, 69] | 2 [48, 69] | 1 [69] | 1 [45] | 1 [69] | |||
| Device | 38 [14, 22–26, 28–32, 36–42, 45–47, 49, 60, 62, 64, 65, 69, 70, 74–77, 79, 80, 83, 84] | 38 [14, 22, 23, 25–29, 32, 35–42, 44–47, 49, 60, 62, 64, 65, 69, 70, 74–77, 79, 80, 83, 84] | 36 [14, 22, 23, 25–29, 32, 35–42, 44, 46, 47, 49, 60, 62, 64, 65, 69, 70, 74–77, 79, 80, 83] | 16 [14, 36, 38, 41, 42, 47, 49, 62, 65, 69, 70, 74, 76, 79, 83] | 24 [14, 22, 28, 29, 32, 37, 40–42, 44, 45, 47, 49, 62, 65, 69, 70, 74–77, 79, 84] | 24 [14, 22, 27, 29, 32, 36, 38–41, 44, 45, 47, 49, 62, 65, 69, 70, 74, 76, 77, 79, 84] | 6 [22, 32, 41, 49, 69, 77] | 7 [32, 38, 41, 47, 49, 69, 77] | 3 [34, 44, 77] | 11 [29, 38, 41, 47, 49, 62, 65, 69, 70, 74, 76] | 10 [32, 38, 43, 47, 62, 67, 68, 70, 74, 79] | 4 [62, 70, 74, 79] | 10 [32, 38, 41, 45, 47, 62, 65, 70, 74, 79] | 6 [38, 49, 64, 69, 77, 82] |
| E-Liquid | 43 [14, 22–26, 28–33, 36–42, 45–49, 60, 62, 64, 65, 69, 70, 74–77, 79, 80, 83, 84, 94, 96, 97] | 44 [14, 22, 23, 25–29, 32, 33, 35–42, 44–49, 60, 62, 64–66, 69, 70, 74–77, 79, 80, 83, 84, 94, 96, 97] | 42 [14, 22, 23, 25–29, 32, 33, 35–42, 44, 46–49, 60, 62, 64–66, 69, 70, 74–77, 79, 80, 83, 93, 94, 96] | 16 [14, 36, 38, 41, 42, 47, 49, 62, 65, 69, 70, 74, 76, 79, 83, 97] | 28 [14, 22, 28, 29, 32, 33, 37, 40–42, 44, 45, 47–49, 62, 65, 66, 69, 70, 74–77, 79, 84, 94] | 27 [14, 22, 27, 29, 32, 33, 36, 38–41, 44, 45, 47, 49, 62, 65, 69, 70, 74, 76, 77, 79, 84, 94, 97] | 8 [22, 32, 33, 41, 48, 49, 69, 77] | 9 [32, 33, 38, 41, 47–49, 69, 77] | 3 [34, 44, 77] | 11 [38, 41, 47, 49, 62, 65, 69, 70, 74, 76, 95] | 13 [32, 38, 43, 47, 62, 67, 68, 70, 74, 79, 94, 95, 97] | 5 [62, 70, 74, 79, 95] | 12 [32, 38, 41, 45, 47, 62, 65, 70, 74, 79, 95, 97] | 6 [49, 64, 69, 77, 92, 97] |
Associations between ENDS characteristics and aerosol toxicants
We further categorized articles based on their reporting of ENDS attributes and how differences in devices and operating conditions influence the presence of aerosol toxicants (Table 3); the absence of a correlation did not prevent a particular study from being included in Table 3. Below, the studies are grouped by the toxicants examined, and their main findings are summarized.
Table 3. Number of studies reporting associations between varying ENDSa characteristics and aerosol toxicants.
| Characteristic | Carbonyls | VOCs | Trace Elements | ROS and Free Radicals | PAHs | TSNAs |
|---|---|---|---|---|---|---|
| Power (W) | 21 [22–27, 29, 31–38, 40, 43–45, 47, 48] | 4 [44, 47, 48, 50] | 1 [52] | 3 [55–57] | ||
| Voltage (V) | 11 [23–26, 29, 33, 38, 43, 62, 64, 70] | 2 [64, 71] | ||||
| Resistance (Ω) | 14 [22, 26, 29, 32–36, 38, 44, 45, 47, 48, 64] | 5 [44, 47, 48, 50, 64] | 1 [52] | |||
| Temperature (°C) | 1 [56] | |||||
| Coil Material | 2 [22, 48] | 1 [48] | 1 [52] | |||
| Device | 19 [14, 22, 23, 26, 29, 32, 34–38, 40, 44, 45, 47, 64, 79, 80, 83] | 6 [44, 47, 50, 64, 79, 83] | 6 [52, 54, 72, 79, 83, 87] | 4 [14, 55, 89, 90] | 1 [83] | 2 [79, 83] |
| E-Liquid | 31 [14, 23, 25, 28, 29, 33, 35, 36, 38–40, 42, 43, 46, 47, 60, 62, 64, 67, 68, 70, 74, 75, 79, 80, 83, 84, 92–94, 97] | 11 [39, 47, 50, 51, 64, 71, 79, 83, 85, 93, 94] | 8 [52, 54, 60, 79, 83, 86, 87, 98] | 9 [14, 55–58, 73, 84, 89, 90] | 4 [59, 60, 83, 85] | 3 [79, 83, 91] |
a Abbreviations: ENDS, electronic nicotine delivery device; VOC, volatile organic compound; ROS, reactive oxygen species; PAH, polycyclic aromatic hydrocarbon; TSNA, tobacco-specific nitrosamines
Carbonyls/VOCs
Numerous studies have evaluated the relationship between power supplied to the atomizer heating element and aerosol carbonyl concentrations. Salamanca et al. [31] reported carbonyl levels of 1.20 μg/mg e-liquid consumed when an ENDS was operated at a power of 10 watts (W) and 4.43 μg/mg e-liquid with the power at 15 W. Beauval et al. [22] observed that carbonyl concentrations in aerosol were higher when the ENDS was operated at 30 W than at 18 W. Korzun et al. [27] marked propionaldehyde, acetaldehyde, acrolein, glycoaldehyde, hydroxyacetone below the limits of detection and quantification at 11, 13, and 17 W, but all carbonyls were above the limit of quantification at 24 W. El-Hellani et al. [40] concluded that power significantly correlated with total aldehydes in ENDS aerosol. In a particular ENDS recommended for use at 15–60 W, Uchiyama et al. [48] detected carbonyls at high levels at 50 W (4400 μg/15 puffs) and even higher at 60 W (6200 μg/15 puffs). Jensen et al. [44] identified carbonyls and VOCs in ENDS aerosol in single-puff experiments and observed increases in the measured compounds when power increased from 4 W to 6 W; the aerosol produced had a unique fingerprint that distinguished it from the unaerosolized e-liquid. Ogunwale et al. [29] observed that increasing ENDS power from 11.7 W to 16.6 W dramatically increased the aerosol concentrations of acetaldehyde, acrolein, formaldehyde, propionaldehyde, and butyraldehyde. Geiss et al. [25] observed that carbonyl production significantly increased with increasing power, with a sharp rise at 15 W and 20 W. Talih et al. [32] concluded that carbonyl concentrations in ENDS aerosols are correlated with both power applied per surface area of coil and liquid consumed. Vreeke et al. [35] observed that acrolein and acetaldehyde remained below the limit of detection when power increased from 55 W to 65 W on a sub-ohm (<1 ohm resistance) heating coil, but these toxicants increased when power increased from 9 W to 11 W on a supra-ohm (>1 ohm resistance) heating coil. Vreeke et al. [34] found that device temperature increases with increasing power and corresponds directly to dihydroxyacetone (DHA) production. Pankow et al. [50] observed that benzene concentrations in aerosol increased with increasing wattage from 6 W to 13 W on one device, but remained undetectable when wattage increased from 6 W to 20 W on a different device. Gillman et al. [26] observed that an increase in the efficiency of aerosol production, defined as (milligrams aerosol per puff) per watt of power applied to the coil, was associated with lower levels of aldehydes in the aerosol. Farsalinos et al. [24] observed that aerosol formaldehyde concentrations increased with increasing power applied to the heating coil, but also concluded that daily exposure to formaldehyde from consuming 3 grams of liquid would be 32% lower than from smoking cigarettes. Overall, the findings suggest that increased power is associated with increased carbonyl production.
The available literature suggests a similar relationship between voltage and the amount of carbonyls in ENDS aerosol. Uchiyama et al. [33] observed that carbonyls increased when applied voltage increased from 3.2 V to 4.4 V. Kośmider et al. [62] observed that PG-containing fluids were associated with significantly higher levels of formaldehyde and acetone when an ENDS was operated at 4.8 V than at 3.2 V. Sleiman et al. [64] observed that increasing the voltage applied to a single-coil device from 3.3 V to 4.8 V tripled total aldehyde emission rates from 53 to 165 μg/puff, and reusing the device several times increased aldehydes by more than 60%. Behar et al. [43] observed hydroxyacetone in ENDS aerosols at concentrations ranging from ~100 to ~10,000 μg/mL at 5 V but not at 3 V. Zhao et al. [71] observed that as voltage increased from 2.2 to 5.7 V, benzene increased 11-fold and toluene doubled. Similarly to increasing power, an increase in voltage has been observed to be associated with increased carbonyls in aerosol at a fixed resistance.
Suboptimal ENDS operating conditions result in what is commonly referred to as a “dry puff”, during which coil heating occurs in the absence of sufficient e-liquid to produce aerosol. Suboptimal ENDS operation can result in higher concentrations of carbonyls in aerosol. Farsalinos et al. [37] observed aldehydes under non–dry puff conditions but at substantially lower levels than with conventional cigarettes; however, dry puff conditions raised ENDS aerosol concentrations of formaldehyde, acetaldehyde, and acrolein by 30 to 250 times, potentially higher levels than a conventional cigarette. Available evidence seems to suggest that maintenance of optimal operation conditions (e.g., adequate wick saturation and avoiding excessive coil heating) is associated with lower levels of carbonyls.
Coil location, orientation, and resistance may also affect carbonyl production. Farsalinos et al. [23] observed that top-coil atomizers produce more carbonyls at higher voltages than at lower voltages, and bottom-coil atomizers produce extremely low levels of carbonyls at both high and low voltages. Gillman et al. [26] also observed that top-coil atomizers produce more carbonyls than bottom-coil atomizers at the same wattage. Pankow et al. [50] reported that benzene concentrations were largely undetectable in an ENDS with a single vertical coil with a cotton wick, but were more readily detected with a single horizontal coil with a silica wick. Vreeke et al. [34] observed that aerosol concentrations of DHA were directly proportional to the mass of aerosolized VG:PG, but a vertical coil produced less DHA than a horizontal coil. Stephens et al. [45] similarly observed more carbonyls with a top coil than a bottom coil; insufficient wick saturation was associated with coil build-up and greater carbonyl output compared to a stainless steel organic cotton coil which provided ample wick saturation with minimal coil deposits and fewer carbonyls. Son et al. [47] identified a top coil device generated increased concentrations of formaldehyde (4.80 μg/puff) compared to a mod device with a Clapton-style coil (0.47 μg/puff) Top-coil horizontal atomizers have the potential to generate more carbonyls compared to bottom-coil horizontal atomizers, while vertical coils produced fewer carbonyls than horizontal coils.
Several studies, but not all, have observed that carbonyl and VOC production varies by device type. Uchiyama et al. [33] reported significant differences in carbonyl production between different brands, whereas Flora et al. [83] observed low formaldehyde concentrations with low variability (0.09 to 0.33 μg/puff) across 4 of the same ENDS products. Gillman et al. [46] examined the formaldehyde, acetaldehyde, and acrolein output of 10 identical ENDS products noting the inherent variability observed from the devices. Goniewicz et al. [79] observed 4 carbonyls (among 15 analyzed) and 2 VOCs (among 11 analyzed) that were present in aerosols generated from almost all 12 ENDS brands. Bitzer et al. [14] observed some significant differences between several non-refillable closed-system devices and 4 carbonyls. Wagner et al. [85] did not detect 1,3 butadiene; isoprene; acrylonitrile; benzene; or toluene in the aerosol of 6 top-selling, commercially available ENDS. Laugesen et al. [80] observed across 9 ENDS brands an average formaldehyde concentration of 1.07 μg/L of vapor, 0.81 μg/L of acetaldehyde, and 1.06 μg/L of acrolein; however, the concentrations of aldehydes on average have decreased over time. El-Hellani et al. [40] concluded that ENDS brand significantly correlated with total aldehydes in ENDS aerosol. Vreeke et al. [35] reported fewer carbonyl compounds produced from a sub-ohm tank than from a supra-ohm clearomizer (atomizer and cartridge). Gillman et al. [26] reported that sub-ohm devices produced lower levels of carbonyls than supra-ohm devices at similar wattages. Device type has been shown to potentially impact carbonyl and VOC output.
Numerous articles have reported that e-liquid constituents impact carbonyl aerosol concentrations. Kim et al. [51] observed that carbonyls were present in aerosol associated with major flavoring groups but not in un-aerosolized liquid. Herrington et al. [94] observed that acetaldehyde and acrolein exist in ENDS aerosol but not in e-liquid solutions. Conklin et al. [42] demonstrated that VG generates higher levels of formaldehyde and acrolein and PG generates higher levels of acetaldehyde. Klager et al. [97] observed median formaldehyde concentrations of 626 μg/m3 in aerosol produced with 24 e-liquid flavors. Beauval et al. [60] observed formaldehyde and acetaldehyde close to 1 ng/mL of emission with no substantial differences between flavorings, with the exception of acrolein concentrations from aerosol produced with “blond tobacco” flavored e-liquid. Papoušek et al. [93] observed that acrolein was present in higher concentrations in tobacco-flavored than in fruit-flavored e-liquid. Zhao et al. [71] observed that menthol flavor generated 330% more benzene and 120% more toluene than in tobacco flavor. Qu et al. [74] observed that the carbonyl compounds emission factors increased linearly 1.0- to 92-fold when flavor content of the e-liquid increased from 5% to 50% and concluded that most carbonyls in e-liquids were from flavorings. Allen et al. [92] detected diacetyl in aerosols in 39 of 51 e-liquids. Kośmider et al. [67] detected benzaldehyde in 108 of 145 flavored e-liquids and observed the highest levels (5.1–141.2 μg/30 puffs) in cherry-flavored products. Pankow et al. [50] observed that benzene concentrations in ENDS aerosol increased with the addition of benzoic acid and benzaldehyde to the e-liquid solution. Vreeke et al. [35] observed that e-liquid containing 10% flavoring compound was associated with increased carbonyls in aerosol compared with e-liquids containing only VG:PG. Duell et al. [39] observed that the concentrations of aldehydes (propionaldehyde, acetaldehyde, glycolaldehyde, and acrolein) increased as sucralose concentration in the e-liquid increased. The relative concentrations of carbonyls in the aerosol have varied from study to study [36, 38]. Reilly et al. [84] observed no differences in formaldehyde and acetone across the aerosols of 4 different JUUL flavors. Additionally, Mallock et al. [75] observed that European and American versions of JUUL pods produced similar levels of carbonyls. Available evidence suggests that the chemical constituency of aerosol is dependent upon the matrix of the e-liquid as carbonyl compounds are present in the aerosol as both flavoring agents (i.e., diacetyl) and products of thermal degradation (i.e., aldehydes).
E-liquid composition also impacts the transfer of chemicals to the aerosol. Erythropel et al. [68] observed that benzaldehyde carryover (i.e., percentage of chemical transferred from e-liquid to aerosol) significantly increased when PG content increased from 0% PG to 100% PG. This suggests that the concentration of toxicants in aerosol can be influenced by the bulk e-liquid solution.
A common technique for carbonyl analysis is to bubble aerosol through 2,4-dinitrophenylhydrazine (DNPH). This allows carbonyls to form carbonyl-DNPH adducts that can be detected with high performance liquid chromatography. Hemiacetals are formed through the reaction of a carbonyl with a hydroxyl, such as one on PG or VG, and become unreactive to DNPH. The stability of hemiacetal adducts formed when reactive carbonyls, such as formaldehyde, interact with VG or PG is controversial, as are the implications of the results. Though not directly observed, some investigators suggest that the stability of the carbonyl-DNPH adduct drives equilibria of hemiacetals, acetals, and free carbonyls towards that of the carbonyl-DNPH adduct and the formation of hemiacetals is reversed in DNPH solution [26]; others maintain that the formation of hemiacetals leads to a severe underestimation of the absolute amount of carbonyls [31, 99]. Since an ENDS user would inhale the hemiacetal or acetal [68], both are excluded as carbonyls in the tables.
Various ENDS operating parameters increase the concentrations of hemiacetals in aerosol. Jensen et al. [99] reported a significant change in the presence of formaldehyde hemiacetals as voltage increased from 3.3 V (not detected) to 5.0 V (380 ± 90 μg/10 puffs). Salamanca et al. [31] observed approximately 4 times the amount of formaldehyde and formaldehyde hemiacetals when a device was powered at 15 W than at 10 W. However, both power settings had the same ratio of formaldehyde to formaldehyde hemiacetals. Vreeke et al. [35] increased power from 55 W to 65 W for a newer generation device and identified increased formaldehyde hemiacetals. Similarly, power increases from 9 W to 11 W significantly increased formaldehyde hemiacetals. Additionally, Vreeke et al. [35] reported that the addition of triacetin, a common flavor additive, increased levels of formaldehyde hemiacetals. Duell et al. [39] observed that formaldehyde hemiacetals increased with increasing concentrations of sucralose added to the e-liquid. Erythropel et al. [68] reported carryover rates for both aldehydes and corresponding acetals to be 50–80% of the original concentration in e-liquid. Power and voltage have been demonstrated to increase formaldehyde hemiacetals in aerosol which can be further influenced by e-liquid composition.
Trace elements
Important relationships have been observed between trace elements in ENDS aerosols and variations in power, device, air-flow, coil material, and e-liquid. Zhao et al. [52] observed that when power was increased on an open-system device from 20 W to 40 W, median arsenic (As), chromium (Cr), Cu, iron (Fe), manganese (Mn), nickel (Ni), Pb, antimony (Sb), tin (Sn), and Zn concentrations increased 14, 54, 17, 30, 41, 96, 14, 81, 631, and 7-fold, respectively. Zhao et al. [52] observed that open-system ENDS with kanthal (Fe, Cr, and aluminum [Al] alloy) and stainless steel coils had consistently higher Fe and Ni levels. Goniewicz et al. [79] identified Cd, Ni, and Pb in all vapors generated from 12 different first-generation ENDS models. Prokopowicz et al. [72] detected Pb in the aerosol of 2-tank-system ENDS. Ting et al. [54] identified 5% of ENDS and e-liquid combinations tested emitted Cr at levels that exceeded permissible daily exposure limits. Williams et al. [87] analyzed disposable ENDS and observed that aerosols generated at low and high air-flow rates produced the same pattern of elements, although the total element concentration decreased at the higher air-flow rate. In this study, at least 35 trace elements were identified in ENDS aerosol; Si was the dominant element, but other elements such as calcium (Ca), sodium (Na), Cu, magnesium (Mg), Sn, Pb, Zn, boron (B), selenium (Se), Al, Fe, germanium (Ge), Sb, Ni, and strontium (Sr) were present in most ENDS aerosols, with relative concentrations varying by ENDS model. Beauval et al. [60] evaluated 6 e-liquid refills and observed that only Cd, Cr, and Sb were present in aerosol, with concentrations up to 0.14, 3.4, and 0.47 pg/mL puff, respectively. The concentrations of metals varied minimally with the presence or absence of flavoring and nicotine. In contrast, Mikheev et al. [86] observed that that As, Cr, Ni, Cu, Sb, Sn, and Zn levels in ENDS aerosol varied widely across nicotine- and non-nicotine-containing flavors. Liu et al. [98] observed certain As species significantly increased in concentration from e-liquids to aerosol. Not all published articles have detected trace elements. Flora et al. [83] did not detect As and cadmium (Cd) in ENDS aerosol across 4 commercial e-cigarette non-refillable closed-system products. The ten most examined elements in descending order were Cu, Ni, Zn, Pb, Cd, As, Fe, Cr, Sn, and Al.
ROS and free radicals
The concentration of free radicals in aerosol has been reported to vary by power and associated coil temperature. Bitzer et al. [56] observed increases in free radicals with increases in coil temperature from 100 to 300 °C and power from 10 W to 50 W. Haddad et al. [55] observed that ROS flux increased 3-fold when the power increased from 5 W to 11 W on a single coil device, and increased approximately 7-fold when the power increased from 100 W to 200 W on an octuple coil device. Son et al. [57] observed that a device formed more hydroxyl radicals at 31.3 W than at 6.4 W. Free radicals have been demonstrated to increase with an increase in power.
The effects of device type on free radicals have been examined. Bitzer et al. [14] observed that free radicals are produced by non-refillable closed-system ENDS. Shein et al. [89] observed similar amounts of free radicals in aerosols using standard wicking and heating, a mesh coil, and an air control. Hasan et al. [90] found free radical concentration from two closed-system ENDS were significantly lower than conventional tobacco cigarettes, but the oxidative potency from the free radicals originating from the ENDS was much higher. No clear relationship between free radicals and device design has been observed.
The composition of e-liquid affects the concentrations of free radicals in aerosol. Bitzer et al. [56] observed increases in free radicals with changing VG:PG ratios from 100:0 to 0:100. Son et al. [57] observed that VG- and VG:PG-based e-liquids formed twice as many radicals than exclusively PG-based e-liquids; specifically, 90- and 170-mL puffs (3.8 s each) generated 4.1- and 10.3-fold more hydroxyl radicals than a 35-mL puff (also 3.8 s). Reilly et al. [84] analyzed aerosol from JUUL and observed that free radicals were 2.95 ± 0.81 nmol/g for tobacco flavor at a 70:30 VG:PG ratio; the concentration was higher (3.01 ± 0.28 nmol/g) when the e-liquid composition was 70:30 VG:PG without flavoring. Additionally significance was reached when the e-liquid was 40:60 VG:PG without flavoring (4.68 ± 0.58 nmol/g), and increased further to 4.78 ± 0.73 nmol/g when citral was added to the 40:60 VG:PG base. Goel et al. [73] generated aerosols from 3 different e-liquids generated by a tank system and estimated free-radical production to be 10.3, 4.0 and 2.5 × 1013 radicals per puff of menthol, citrus, and tobacco flavors, respectively. Bitzer et al. [58] analyzed the free radicals generated from 49 commercially available e-liquids and observed that nearly 43% of the flavors resulted in significant increases in radical production compared with the base VG:PG (40:60) mixture, but the amount varied greatly among the flavors; the flavorings dipentene, ethyl maltol, citral, linalool, and piperonal promoted radical formation in a concentration-dependent manner, whereas ethyl vanillin inhibited radical formation. Overall, ROS and free radicals in ENDS aerosol vary by e-liquid composition, but the precise relationship has yet to be determined.
PAHs
PAH concentrations in aerosol vary with device and e-liquid but are generally low if detected. Flora et al. [83] analyzed aerosol from 4 commercially available e-cigarette products and observed that benzo[a]pyrene was below the limit of detection of 10 ng/device. Similarly, Wagner et al. [85] did not detect benzo[a]pyrene in the aerosol of 6 top-selling commercially available ENDS. Beauval et al. [60] systematically detected naphthalene and acenaphthylene in ENDS aerosol at low concentrations (up to 4.10 and 0.37 pg/mL puff, respectively). Eddingsaas et al. [59] analyzed aerosols created from vanilla, cinnamon, and mango e-liquids and observed cadalene in the mango flavor. Available evidence suggests that PAH concentrations are low which is consistent with the knowledge that PAHs are primarily products of combustion [100] and ENDS operate at lower temperatures than combusted tobacco products.
TSNAs
Studies of different ENDS products have observed low or undetectable levels of TSNAs. Flora et al. [83] did not detect TSNAs (NNN and NNK) in aerosols from 4 commercially available ENDS products. Goniewicz et al. [79] analyzed 12 ENDS products and observed NNN concentrations ranging from not detected to 4.3 ± 2.4 ng and NNK from not detected to 28.3 ± 13.2 per 150 puffs. Farsalinos et al. [91] observed that no TSNAs were above the level of detection for aerosols generated from 3 tobacco-flavored e-liquids aerosolized by a second-generation ENDS. Though TSNAs are carcinogenic and their concentrations eclipse many other carcinogenic compounds in conventional cigarettes [101], they are less prevalent in ENDS.
Additional articles
Garcia-Gomez et al. [102] analyzed the aerosol of an ENDS through secondary electrospray ionization mass spectrometry, which identified more than 250 chemical species, including alkaloids and various flavoring compounds. The authors noted that some substances increased with increasing power of the device. Behar et al. [103] found cinnamaldehyde in 20 (51%) of 39 refill fluids, and aerosols contained several different constituents at 5 V than at 3 V. Soussy et al. [104] reported that emissions of 5-hydroxymethylfurfural and furfural were correlated with device power and sweetener concentration in the e-liquid. These observations support the notion that power and voltage influence aerosol toxicant concentration.
Lee et al. [105] detected more carbonyls in ENDS aerosol generated from tobacco flavors than from menthol. Kośmider et al. [28, 70] observed that aerosol carbonyls varied by puffing protocol when different e-liquid nicotine concentrations were assessed. Uchiyama et al. [106] observed large variations in carbonyl concentrations between and within unnamed devices. El-Hage et al. [107] reported the transfer of pyrazines, a class of flavoring compounds, from e-liquid to aerosol. Rawlinson et al. [108] identified the chemical profiles of aerosols generated from 4 e-liquids contained between 30–90 compounds mostly characterized as “unknown” exceeding the 5 ng/puff threshold which may include PAHs.
El-Hellani et al. [109] focused on how device modifications such as power, coil material, and coil geometry may affect the emission of small gasses, notably carbon monoxide; they reported that power and coil material significantly affect analyzed chemical species, with nickel wire being the most reactive. Wang et al. [110] reported a device-independent study wherein various ratios of VG and PG were aerosolized at a range of temperatures, yielding carbonyl compounds. VG generated much higher concentrations of formaldehyde than PG; acrolein was detected only when VG was present in the solution and the temperature was ≥270 °C. Saliba et al. [111] explored the device-independent effect of the metal coil on subsequent carbonyl production. The wires had a catalytic impact on carbonyls, producing species at 250 °C, as opposed to 460 °C without wire. Moreover, the investigators found the material and age of the wire had a strong correlation with carbonyl production, with new nichrome (Ni and Cr alloy) being the least reactive and old nichrome being the most reactive. Williams et al. [112, 113] reported a comprehensive metal analysis of ENDS aerosol the sources of the metals to be the metal components of the heating elements such as coils, wires, or solder joints.
Belushkin et al. [114] completed a comprehensive toxicant panel of 34 ENDS and 57 e-liquids. Benzene was identified in some high power mod systems, but was not as prevalent in non-refillable closed systems. Carbonyl emissions were lower in closed systems than open systems. Particularly noted, formaldehyde increased with the depletion of e-liquid in the reservoir, starting at approximately 50% capacity. Trace elements, TSNAs, and PAHs were seldom above the limit of quantification.
Risk of bias assessment
Table 4 demonstrates that the analytical techniques used to assess major chemical groups of aerosol toxicants have been largely replicated.
Table 4. Risk of bias determined by analytical technique utilized by studies and number of studies of measured major chemical groups in aerosol.
| Technique | Carbonyls | VOCsa | Trace Elements | ROS and Free Radicals | PAHs | TSNAs |
|---|---|---|---|---|---|---|
| Group A | ||||||
| HPLC | 41 [14, 22–26, 28, 30–33, 36–38, 40, 41, 45–48, 60–65, 67, 69, 70, 74–76, 79, 80, 82–84, 97, 106, 110, 111, 114] | 3 [47, 50, 69] | ||||
| GC-FID | 3 [39, 68, 92] | 2 [39, 71] | ||||
| TD-GC-MS | 2 [94, 95] | 4 [50, 64, 94, 95] | ||||
| GC-MS | 11 [29, 33, 34, 39, 41–43, 69, 81, 93, 105] | 13 [39, 48, 49, 51, 59, 69, 76, 79, 83, 85, 93, 105, 114] | 7 [49, 59, 69, 76, 83, 85, 114] | |||
| EPR | 8 [14, 56, 58, 73, 84, 88–90] | |||||
| ICP-MS | 11 [49, 52–54, 60, 69, 78, 79, 83, 86, 114] | |||||
| ICP-OES | 4 [51, 87, 112, 113] | |||||
| SF-ICP-MS | 1 [71] | |||||
| UPLC-MS/MS | 1 [91] | |||||
| UPLC-MS | 1 [79] | |||||
| LC-MS/MS | 5 [49, 69, 76, 83, 114] | |||||
| SPME GC-MS | 1 [96] | |||||
| Group B | ||||||
| NMR | 5 [27, 34, 35, 39, 44] | 2 [39, 44] | ||||
| GC-MS/MS | 1 [60] | |||||
| Group C | ||||||
| PTR-MS | 1 [66] | 1 [66] | ||||
| GC-FID/TOFMS | 1 [108] | 1 [108] | 1 [108] | |||
| HPLC-HR-MS | 1 [77] | 1 [77] | ||||
| SESI-HR-MS | 1 [102] | 1 [102] | ||||
| Fluorescenceb | 1 [55] | |||||
| Fluorescencec | 1[57] | |||||
| electrothermal AA | 1 [72] | |||||
| AA | 1 [88] | |||||
| HPLC-ICP-MS | 1 [98] | |||||
| EDXRF | 1 [105] | |||||
| FT-ICR-MS | 1 [42] | |||||
| Total Individual Studies | ||||||
| 60 | 24 | 20 | 10 | 9 | 7 | |
aAbbreviations: VOCs, volatile organic compound; ROS, reactive oxygen species; PAH, polycyclic aromatic hydrocarbon; TSNA, tobacco-specific nitrosamines; HPLC, high performance liquid chromatography; GC-FID, gas chromatography–coupled flame ionization detector; TD-GC-MS, thermal desorption gas chromatography mass spectrometry (MS); GC-MS, gas chromatography MS; EPR, electronic paramagnetic resonance spectroscopy; ICP-MS, inductively coupled plasma MS; ICP-OES, inductively coupled plasma optical emission spectrometry; SF-ICP-MS, sector field inductively coupled plasma MS; UPLC-MS/MS, ultra performance liquid chromatography tandem MS; UPLC-MS, ultra performance liquid chromatography MS; LC-MS/MS, liquid chromatography tandem MS; SPME GC-MS, solid phase microextraction gas chromatography MS; NMR, nuclear magnetic resonance spectroscopy; GC-MS/MS, gas chromatography tandem MS; PTR-MS, proton transfer reaction MS; GC-FID/TOFMS, gas chromatography–coupled flame ionization detector coupled time of flight MS; HPLC-HR-MS, HPLC high resolution-MS; SESI-HR-MS, secondary electrospray ionization HR-MS; electrothermal AA, electrothermal atomic absorption spectroscopy; AA, atomic absorbance spectrometry; EDXRF, energy dispersive X-ray fluorescence spectroscopy; FT-ICR-MS, Fourier transform ion cyclotron resonance MS
b flurorescence SpectraMax M5 microplate
c fluorescence Synergy 4 multidetection microplate
Discussion
We identified 92 articles reporting ENDS characteristics and aerosol toxicants, the majority of which used analytic techniques replicated by other researchers and many of which attempted to define relationships between changes in ENDS characteristics and aerosol constituents. Device characteristics relevant to the current review of ENDS are power, voltage, resistance, temperature, coil material, device, and e-liquid. The major chemical constituents were differentiated into carbonyls, VOCs, trace elements, ROS and free radicals, PAHs, and TSNAs.
The ENDS industry evolves rapidly, and contemporary scientific observations may not maintain relevancy over time. Williams et al. [115] characterized ENDS manufactured from 2011 to 2017, noting significant variation in atomizer design over time within and between brands and indicating the importance of acknowledging and monitoring device design changes. Despite these rapid changes, the extant literature provides insight into ways that the technology could be improved and aerosol toxicants minimized either by the actions of ENDS manufacturers or through regulation, thereby reducing potential adverse health impacts.
First, improving the safety profile of e-liquids would be a critical first step in manufacturing and regulation to reduce the likelihood of adverse health consequences from ENDS use. The risk associated with inhaling ENDS aerosol is associated with “unintentional” contaminants (i.e., impurities), “intentional” constituents (i.e., PG, VG, and flavorings), and the conversion of unintentional and intentional constituents into new chemical species during aerosolization. The literature suggests that numerous new chemicals are created during aerosolization that are not present in the native e-liquid [51, 59, 94]. Part of the complexity of aerosol and e-liquid composition analysis arises from the addition of flavoring compounds. Flavorings are an integral aspect of the customization of ENDS. Almost all ENDS users report using a flavored e-liquid; only 1% of users prefer the chemically simpler unflavored e-liquid [116]. E-liquid manufacturers are not mandated to list all flavoring additives as a part of the ingredients list. Moreover, many flavorings have not been tested for toxicity or potential harm caused by chemical transformations through heated aerosolization and inhalation. A handful of chemical agents used in the flavoring of e-liquid have been specifically investigated. Diacetyl and acetyl propionyl, which produce buttery or creamy flavors, have received attention for associations with bronchiolitis obliterans (i.e., “popcorn lung”) among microwave popcorn industry workers [117, 118] and have been investigated in e-liquid flavoring [82, 92]. Cinnamaldehyde has been observed to be cytotoxic [103]. Additionally, ethyl maltol and maltol have both been observed to interact with Cu or Fe to generate hydroxypyranone complexes that can promote the generation of radicals [58]. Although not within the scope of this review, cell toxicity and animal studies have been and can be leveraged to determine toxicity associated with e-liquids. Identification and characterization of toxicants both in the native e-liquid and those produced through aerosolization should be required of manufacturers. Regulatory agencies should provide guidance on safe levels of exposure in order to facilitate industry adherence.
Second, the relationship between power, resistance, and voltage (power = voltage2/resistance) affects the potential risk associated with the use of ENDS. Aerosol toxicants generally increase with increased power and voltage applied to the metal coil for heating. Power per surface area of coil may be a stronger predictor of toxicant production in aerosol than power alone [32]. Sub-ohm coils were introduced into the market to improve aerosol production through rapid coil heating; however, this phenomenon occurs at the expense of battery life. Sub-ohm devices have enabled ENDS to be operated at high wattages. Manufacturer-recommended operating ranges reach into the triple digits but produce less carbonyl than supra-ohm coils at the same power setting [26]. Researchers have suggested for manufacturers to limit power flow to coils to reduce exposure to formaldehyde, acetaldehyde, and acrolein [114]. More research needs to be conducted to understand the impact of newer-generation coils (e.g., mesh, Clapton, alien, staple) on toxicant production. Researchers can advance the science through consistent reporting of power, resistance, and voltage as well as use of standardized units of measure.
Third, atomizer design, specifically as it relates to the adequate supply of e-liquid to a heating coil, is critical to avoid the observed phenomenon of higher toxicant production from coil overheating caused by a deficiency of e-liquid for aerosolization (i.e., dry puff). This appears to be more common among top-coil designs due to inadequate wicking and could be mitigated with improved wicking to the top coil or the use of bottom, vertically oriented coils. In addition to coil location, wicking material has also evolved. In earlier generations of ENDS, the wick was primarily silica-based. With newer generations, the ENDS industry has begun to explore other wicking materials such as cotton, stainless steel, bamboo, and ceramic. Various wicking materials will deliver e-liquid to the coil at different rates; to avoid the dry puff phenomenon, these rates should be taken into consideration to ensure that the coil is properly saturated with e-liquid at all operating temperatures. The viscosity and density of the e-liquid will also determine its mobility, capillary action, and delivery to the coil, influencing the likelihood of a dry puff. Some ENDS manufacturers have included microprocessors that will deactivate heating once a certain temperature threshold is reached (e.g. >350 °C), which may minimize toxicant production from a dry puff without compromising user experience by suppressing aerosol production.
Fourth, atomizer heating elements are an identified source of aerosol metals, and metals used for construction of coils and other components (i.e., clamps, wicks, and soldering) should be selected to minimize metal breakdown and leaching. Commonly identified metals were Cu, Pb, Ni, Sn, and Zn. Coils are traditionally Ni, Ti, kanthal (Fe, Cr, and Al), stainless steel, and nichrome (Ni and Cr), whereas solder joints are Sn or Pb and wires are Cu [87]. The resistance of the alloys kanthal and nichrome remain relatively constant with changing temperature, making them ideal for use in wattage-control mode (i.e., fixed power supply with variable coil temperature); stainless steel and titanium are better suited for temperature-controlled devices (fixed coil temperature and variable power supply). High-quality metals with few defects might be less likely to leach under repeated heating-cooling cycles; coupled with timely replacement of coils, this could lead to reduced metal concentrations in aerosol. The literature suggests that the age of the coil influences carbonyl production [111], with higher carbonyl emissions associated with older devices or components [64]. To minimize potential toxicant exposure, ENDS manufacturers should select high-quality metals, minimize soldering parts, and recommended timing of coil replacement as a function of coil use.
Our review has several limitations. First, due to the variability in reporting we were unable to quantitatively estimate and provide confidence intervals around the relationships between device operative parameters (e.g., temperature, wattage) and resulting toxicant levels in the aerosol. Second, we did not specifically assess the influence of human puffing topography variability on toxicity. The ability to quantitate “estimates of effect” would become increasingly possible if the market were to contract, products were regulated, devices were standardized for clinical therapeutic interventions, and researchers were to rigorously report operating parameters to allow comparison across studies.
Conclusion
Toxicants with potential risk to health exist in aerosol produced by ENDS. Available literature suggests that ENDS can be designed to minimize exposure to potentially harmful aerosol toxicants. Minimizing toxicants in aerosol through consideration and optimization of design could serve to reduce health risks associated with the use of ENDS as part of a comprehensive approach to reducing tobacco harm on the population level.
Supporting information
(DOC)
(DOCX)
Data Availability
All relevant data is within the paper and Supporting Information files. The supporting information and bibliography includes all of the articles used in this study.
Funding Statement
This work is made possible by the generosity of HH Sheikh Hamed Bin Zayed Al Nahyan. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Andrikopoulos GI, Zagoriti Z, Topouzis S, Poulas K. Oxidative stress induced by electronic nicotine delivery systems (ENDS): Focus on respiratory system. Current Opinion in Toxicology. 2019;13:81–9. 10.1016/j.cotox.2018.09.002. . [DOI] [Google Scholar]
- 2.Jones L. Vaping: How popular are e-cigarettes?: BBC News; 2019 [updated September 15]. Available from: https://www.bbc.com/news/business-44295336.
- 3.Global Electronic Cigarette Market Outlook Report 2017–2019 & 2026 PR Newswire: Research and Markets; 2019 [updated September 02]. https://www.prnewswire.com/news-releases/global-electronic-cigarette-market-outlook-report-2017-2019--2026-300910267.html.
- 4.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med. 2019;380(7):629–37. Epub 2019/01/31. 10.1056/NEJMoa1808779 . [DOI] [PubMed] [Google Scholar]
- 5.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Syst. Rev. 2014; 12 Art. No.: CD010216. 10.1002/14651858.CD010216.pub2 [DOI] [PubMed] [Google Scholar]
- 6.Primack BA, Shensa A, Sidani JE, Hoffman BL, Soneji S, Sargent JD, et al. Initiation of Traditional Cigarette Smoking after Electronic Cigarette Use Among Tobacco-Naive US Young Adults. Am J Med. 2018;131(4):443.e1–e9. Epub 2017/12/16. 10.1016/j.amjmed.2017.11.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Electronic Nicotine Delivery Systems and Electronic Non-Nicotine Delivery Systems (ENDS/ENNDS). WHO Framework Convention on Tobacco Control; 2016; Delhi, India: WHO. https://www.who.int/fctc/cop/cop7/FCTC_COP_7_11_EN.pdf
- 8.Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–200. 10.3390/ijerph111111192 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaur S, Agnihotri R. Health Effects of Trace Metals in Electronic Cigarette Aerosols-a Systematic Review. Biol Trace Elem Res. 2019;188(2):295–315. 10.1007/s12011-018-1423-x . [DOI] [PubMed] [Google Scholar]
- 10.Farsalinos KE, Gillman G. Carbonyl Emissions in E-cigarette Aerosol: A Systematic Review and Methodological Considerations. Front Physiol. 2017;8:1119 10.3389/fphys.2017.01119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23 Suppl 2:ii11–7. 10.1136/tobaccocontrol-2013-051482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balbo S, Stepanov I. The Wild West of E-Cigarettes. Chem Res Toxicol. 2018;31(9):823–4. 10.1021/acs.chemrestox.8b00214 . [DOI] [PubMed] [Google Scholar]
- 13.Bansal V, Kim KH. Review on quantitation methods for hazardous pollutants released by e-cigarette (EC) smoking. Trends Analyt Chem. 2016;78:120–33. 10.1016/j.trac.2016.02.015. . [DOI] [Google Scholar]
- 14.Bitzer ZT, Goel R, Reilly SM, Bhangu G, Trushin N, Foulds J, et al. Emissions of Free Radicals, Carbonyls, and Nicotine from the NIDA Standardized Research Electronic Cigarette and Comparison to Similar Commercial Devices. Chem Res Toxicol. 2019;32(1):130–8. 10.1021/acs.chemrestox.8b00235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23 Suppl 2:ii4–10. 10.1136/tobaccocontrol-2013-051476 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao T, Shu S, Guo Q, Zhu Y. Effects of design parameters and puff topography on heating coil temperature and mainstream aerosols in electronic cigarettes. Atmos Environ. 2016;134:61–9. 10.1016/j.atmosenv.2016.03.027 [DOI] [Google Scholar]
- 17.Center for Tobacco Products. Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke Under Section 904(a)(3) of the Federal Food, Drug, and Cosmetic Act. Food and Drug Administration, 2012. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reporting-harmful-and-potentially-harmful-constituents-tobacco-products-and-tobacco-smoke-under.
- 18.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. ISBN-13: 978-0-16-084078-4. https://www.ncbi.nlm.nih.gov/books/NBK53017/. [PubMed]
- 19.Nathanson T. Indoor air quality in office buildings: A technical guide. Federal-Provincial Advisory Committee on Environmental and Occupational Health: Health Canada, 1995. https://irp-cdn.multiscreensite.com/562d25c6/files/uploaded/Canada_Indoor%20Air%20Quality%20in%20Office%20Buildings_A%20Technical%20Guide_1995.pdf.
- 20.Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt J Pet. 2016;25(1):107–23. 10.1016/j.ejpe.2015.03.011 [DOI] [Google Scholar]
- 21.Edwards SH, Rossiter LM, Taylor KM, Holman MR, Zhang L, Ding YS, et al. Tobacco-Specific Nitrosamines in the Tobacco and Mainstream Smoke of U.S. Commercial Cigarettes. Chem Res Toxicol. 2016;30(2):540–51. 10.1021/acs.chemrestox.6b00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beauval N, Verriele M, Garat A, Fronval I, Dusautoir R, Antherieu S, et al. Influence of puffing conditions on the carbonyl composition of e-cigarette aerosols. Int J Hyg Environ Health. 2019;222(1):136–46. 10.1016/j.ijheh.2018.08.015 . [DOI] [PubMed] [Google Scholar]
- 23.Farsalinos KE, Kistler KA, Pennington A, Spyrou A, Kouretas D, Gillman G. Aldehyde levels in e-cigarette aerosol: Findings from a replication study and from use of a new-generation device. Food Chem Toxicol. 2018;111:64–70. 10.1016/j.fct.2017.11.002 . [DOI] [PubMed] [Google Scholar]
- 24.Farsalinos KE, Voudris V, Spyrou A, Poulas K. E-cigarettes emit very high formaldehyde levels only in conditions that are aversive to users: A replication study under verified realistic use conditions. Food Chem Toxicol. 2017;109(Pt 1):90–4. 10.1016/j.fct.2017.08.044 . [DOI] [PubMed] [Google Scholar]
- 25.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health. 2016;219(3):268–77. 10.1016/j.ijheh.2016.01.004 . [DOI] [PubMed] [Google Scholar]
- 26.Gillman IG, Kistler KA, Stewart EW, Paolantonio AR. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol. 2016;75:58–65. 10.1016/j.yrtph.2015.12.019 . [DOI] [PubMed] [Google Scholar]
- 27.Korzun T, Lazurko M, Munhenzva I, Barsanti KC, Huang Y, Jensen RP, et al. E-Cigarette Airflow Rate Modulates Toxicant Profiles and Can Lead to Concerning Levels of Solvent Consumption. ACS Omega. 2018;3(1):30–6. 10.1021/acsomega.7b01521 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kośmider L, Kimber CF, Kurek J, Corcoran O, Dawkins LE. Compensatory Puffing With Lower Nicotine Concentration E-liquids Increases Carbonyl Exposure in E-cigarette Aerosols. Nicotine Tob Res. 2018;20(8):998–1003. 10.1093/ntr/ntx162 . [DOI] [PubMed] [Google Scholar]
- 29.Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, et al. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega. 2017;2(3):1207–14. 10.1021/acsomega.6b00489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salamanca JC, Meehan-Atrash J, Vreeke S, Escobedo JO, Peyton DH, Strongin RM. E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci. 2018;8(1):7559 10.1038/s41598-018-25907-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salamanca JC, Munhenzva I, Escobedo JO, Jensen RP, Shaw A, Campbell R, et al. Formaldehyde Hemiacetal Sampling, Recovery, and Quantification from Electronic Cigarette Aerosols. Sci. 2017;7(1):11044 10.1038/s41598-017-11499-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talih S, Salman R, Karaoghlanian N, El-Hellani A, Saliba N, Eissenberg T, et al. "Juice Monsters": Sub-Ohm Vaping and Toxic Volatile Aldehyde Emissions. Chem Res Toxicol. 2017;30(10):1791–3. 10.1021/acs.chemrestox.7b00212 . [DOI] [PubMed] [Google Scholar]
- 33.Uchiyama S, Senoo Y, Hayashida H, Inaba Y, Nakagome H, Kunugita N. Determination of Chemical Compounds Generated from Second-generation E-cigarettes Using a Sorbent Cartridge Followed by a Two-step Elution Method. Anal Sci. 2016;32(5):549–55. 10.2116/analsci.32.549 . [DOI] [PubMed] [Google Scholar]
- 34.Vreeke S, Korzun T, Luo W, Jensen RP, Peyton DH, Strongin RM. Dihydroxyacetone levels in electronic cigarettes: Wick temperature and toxin formation. Aerosol Sci Technol. 2018;52(4):370–6. 10.1080/02786826.2018.1424316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vreeke S, Peyton DH, Strongin RM. Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols. ACS Omega. 2018;3(7):7165–70. 10.1021/acsomega.8b00842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farsalinos KE, Voudris V. Do flavouring compounds contribute to aldehyde emissions in e-cigarettes? Food Chem Toxicol. 2018;115:212–7. 10.1016/j.fct.2018.02.059 . [DOI] [PubMed] [Google Scholar]
- 37.Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in 'dry puff' conditions. Addiction. 2015;110(8):1352–6. 10.1111/add.12942 . [DOI] [PubMed] [Google Scholar]
- 38.Khlystov A, Samburova V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ Sci Technol. 2016;50(23):13080–5. 10.1021/acs.est.6b05145 . [DOI] [PubMed] [Google Scholar]
- 39.Duell AK, McWhirter KJ, Korzun T, Strongin RM, Peyton DH. Sucralose-Enhanced Degradation of Electronic Cigarette Liquids during Vaping. Chem Res Toxicol. 2019;32(6):1241–9. 10.1021/acs.chemrestox.9b00047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Hellani A, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, et al. Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine Tob Res. 2018;20(2):215–23. 10.1093/ntr/ntw280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talih S, Salman R, El-Hage R, Karam E, Karaoghlanian N, El-Hellani A, et al. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob Control. 2019. 10.1136/tobaccocontrol-2018-054616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu XA, et al. Electronic cigarette-generated aldehydes: The contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci Tech. 2018;52(11):1219–32. 10.1080/02786826.2018.1500013. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behar RZ, Luo W, McWhirter KJ, Pankow JF, Talbot P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. 2018;8(1):8288 10.1038/s41598-018-25575-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen RP, Strongin RM, Peyton DH. Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci. 2017;7:42549. 10.1038/srep42549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephens WE, de Falco B, Fiore A. A Strategy for Efficiently Collecting Aerosol Condensate Using Silica Fibers: Application to Carbonyl Emissions from E-Cigarettes. Chem Res Toxicol. 2019;32(10):2053–62. 10.1021/acs.chemrestox.9b00214 [DOI] [PubMed] [Google Scholar]
- 46.Gillman IG, Pennington ASC, Humphries KE, Oldham MJ. Determining the impact of flavored e-liquids on aldehyde production during Vaping. Regul Toxicol Pharmacol. 2020;112 10.1016/j.yrtph.2020.104588 [DOI] [PubMed] [Google Scholar]
- 47.Son Y, Bhattarai C, Samburova V, Khlystov A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. Int J Environ Res Public Health. 2020;17(8). 10.3390/ijerph17082767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uchiyama S, Noguchi M, Sato A, Ishitsuka M, Inaba Y, Kunugita N. Determination of Thermal Decomposition Products Generated from E-Cigarettes. Chem Res Toxicol. 2020;33(2):576–83. Epub 2020/01/18. 10.1021/acs.chemrestox.9b00410 . [DOI] [PubMed] [Google Scholar]
- 49.Nicol J, Fraser R, Walker L, Liu C, Murphy J, Proctor CJ. Comprehensive Chemical Characterization of the Aerosol Emissions of a Vaping Product Based on a New Technology. Chem Res Toxicol. 2020;33(3):789–99. Epub 2020/03/04. 10.1021/acs.chemrestox.9b00442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, et al. Benzene formation in electronic cigarettes. PLoS ONE. 2017;12(3):e0173055 10.1371/journal.pone.0173055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SA, Smith S, Beauchamp C, Song Y, Chiang M, Giuseppetti A, et al. Cariogenic potential of sweet flavors in electronic-cigarette liquids. PLoS ONE. 2018;13(9):e0203717 10.1371/journal.pone.0203717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao D, Navas-Acien A, Ilievski V, Slavkovich V, Olmedo P, Adria-Mora B, et al. Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ Res. 2019;174:125–34. 10.1016/j.envres.2019.04.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palazzolo DL, Crow AP, Nelson JM, Johnson RA. Trace metals derived from electronic cigarette (ECIG) generated aerosol: Potential problem of ECIG devices that contain nickel. Front Physiol. 2016;7:663 Epub 2017/01/26. 10.3389/fphys.2016.00663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ting CY, Ahmad Sabri NA, Tiong LL, Zailani H, Wong LP, Agha Mohammadi N, et al. Heavy metals (Cr, Pb, Cd, Ni) in aerosols emitted from electronic cigarettes sold in Malaysia. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2020;55(1):55–62. Epub 2019/09/19. 10.1080/10934529.2019.1665950 . [DOI] [PubMed] [Google Scholar]
- 55.Haddad C, Salman R, El-Hellani A, Talih S, Shihadeh A, Saliba NA. Reactive Oxygen Species Emissions from Supra- and Sub-Ohm Electronic Cigarettes. J Anal Toxicol. 2019;43(1):45–50. 10.1093/jat/bky065 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bitzer ZT, Goel R, Reilly SM, Foulds J, Muscat J, Elias RJ, et al. Effects of Solvent and Temperature on Free Radical Formation in Electronic Cigarette Aerosols. Chem Res Toxicol. 2018;31(1):4–12. 10.1021/acs.chemrestox.7b00116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Son Y, Mishin V, Laskin JD, Mainelis G, Wackowski OA, Delnevo C, et al. Hydroxyl Radicals in E-Cigarette Vapor and E-Vapor Oxidative Potentials under Different Vaping Patterns. Chem Res Toxicol. 2019;32(6):1087–95. 10.1021/acs.chemrestox.8b00400 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, et al. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic Biol Med. 2018;120:72–9. 10.1016/j.freeradbiomed.2018.03.020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eddingsaas N, Pagano T, Cummings C, Rahman I, Robinson R, Hensel E. Qualitative Analysis of E-Liquid Emissions as a Function of Flavor Additives Using Two Aerosol Capture Methods. Int J Environ Res Public Health. 2018;15(2):323 10.3390/ijerph15020323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beauval N, Antherieu S, Soyez M, Gengler N, Grova N, Howsam M, et al. Chemical Evaluation of Electronic Cigarettes: Multicomponent Analysis of Liquid Refills and their Corresponding Aerosols. J Anal Toxicol. 2017;41(8):670–8. 10.1093/jat/bkx054 . [DOI] [PubMed] [Google Scholar]
- 61.Jo SH, Kim KH. Development of a sampling method for carbonyl compounds released due to the use of electronic cigarettes and quantitation of their conversion from liquid to aerosol. J Chromatogr. 2016;1429:369–73. 10.1016/j.chroma.2015.12.061 . [DOI] [PubMed] [Google Scholar]
- 62.Kośmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–26. 10.1093/ntr/ntu078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee MH, Szulejko JE, Kim KH. Determination of carbonyl compounds in electronic cigarette refill solutions and aerosols through liquid-phase dinitrophenyl hydrazine derivatization. Environ Monit Assess. 2018;190(4):200 10.1007/s10661-018-6553-2 . [DOI] [PubMed] [Google Scholar]
- 64.Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, et al. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ Sci Technol. 2016;50(17):9644–51. 10.1021/acs.est.6b01741 . [DOI] [PubMed] [Google Scholar]
- 65.Talih S, Balhas Z, Salman R, Karaoghlanian N, Shihadeh A . "Direct Dripping": A High-Temperature, High-Formaldehyde Emission Electronic Cigarette Use Method. Nicotine Tob Res. 2016;18(4):453–9. 10.1093/ntr/ntv080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blair SL, Epstein SA, Nizkorodov SA, Staimer N. A Real-Time Fast-Flow Tube Study of VOC and Particulate Emissions from Electronic, Potentially Reduced-Harm, Conventional, and Reference Cigarettes. Aerosol Sci Technol. 2015;49(9):816–27. 10.1080/02786826.2015.1076156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kośmider L, Sobczak A, Prokopowicz A, Kurek J, Zaciera M, Knysak J, et al. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71(4):376–7. 10.1136/thoraxjnl-2015-207895 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erythropel HC, Jabba SV, DeWinter TM, Mendizabal M, Anastas PT, Jordt SE, et al. Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob Res. 2019;21(9):1248–58. 10.1093/ntr/nty192 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, et al. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem Res Toxicol. 2016;29(10):1662–78. 10.1021/acs.chemrestox.6b00188 . [DOI] [PubMed] [Google Scholar]
- 70.Kośmider L, Cox S, Zaciera M, Kurek J, Goniewicz ML, McRobbie H, et al. Daily exposure to formaldehyde and acetaldehyde and potential health risk associated with use of high and low nicotine e-liquid concentrations. Sci. 2020;10(1). 10.1038/s41598-020-63292-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J, Nelson J, Dada O, Pyrgiotakis G, Kavouras IG, Demokritou P. Assessing electronic cigarette emissions: linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhal Toxicol. 2018;30(2):78–88. 10.1080/08958378.2018.1450462 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prokopowicz A, Sobczak A, Szula-Chraplewska M, Ochota P, Kosmider L. Exposure to Cadmium and Lead in Cigarette Smokers Who Switched to Electronic Cigarettes. Nicotine Tob Res. 2019;21(9):1198–205. 10.1093/ntr/nty161 . [DOI] [PubMed] [Google Scholar]
- 73.Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, et al. Highly reactive free radicals in electronic cigarette aerosols. Chem Res Toxicol. 2015;28(9):1675–7. 10.1021/acs.chemrestox.5b00220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qu Y, Kim KH, Szulejko JE. The effect of flavor content in e-liquids on e-cigarette emissions of carbonyl compounds. Environ Res. 2018;166:324–33. 10.1016/j.envres.2018.06.013 . [DOI] [PubMed] [Google Scholar]
- 75.Mallock N, Trieu HL, Macziol M, Malke S, Katz A, Laux P, et al. Trendy e-cigarettes enter Europe: chemical characterization of JUUL pods and its aerosols. Arch Toxicol. 2020. 10.1007/s00204-020-02716-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rudd K, Stevenson M, Wieczorek R, Pani J, Trelles-Sticken E, Dethloff O, et al. Chemical Composition and In Vitro Toxicity Profile of a Pod-Based E-Cigarette Aerosol Compared to Cigarette Smoke. Appl In Vitro Toxicol. 2020;6(1):11–41. 10.1089/aivt.2019.0015 [DOI] [Google Scholar]
- 77.Li Y, Burns AE, Burke GJP, Poindexter ME, Madl AK, Pinkerton KE, et al. Application of High-Resolution Mass Spectrometry and a Theoretical Model to the Quantification of Multifunctional Carbonyls and Organic Acids in e-Cigarette Aerosol. Environ Sci Technol. 2020;54(9):5640–50. 10.1021/acs.est.9b07387 [DOI] [PubMed] [Google Scholar]
- 78.Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, Aherrera A, et al. Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ Health Perspect. 2018;126(2):027010 10.1289/EHP2175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goniewicz ML, Knysak J, Gawron M, Kośmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9. 10.1136/tobaccocontrol-2012-050859 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laugesen M. Nicotine and toxicant yield ratings of electronic cigarette brands in New Zealand. N Z Med J. 2015;128(1411):77–82. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25820506. [PubMed] [Google Scholar]
- 81.Pellegrino RM, Tinghino B, Mangiaracina G, Marani A, Vitali M, Protano C, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig. 2012;24(4):279–88. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22913171. [PubMed] [Google Scholar]
- 82.Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17(2):168–74. 10.1093/ntr/ntu176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flora JW, Meruva N, Huang CB, Wilkinson CT, Ballentine R, Smith DC, et al. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul Toxicol Pharmacol. 2016;74:1–11. 10.1016/j.yrtph.2015.11.009 . [DOI] [PubMed] [Google Scholar]
- 84.Reilly SM, Bitzer ZT, Goel R, Trushin N, Richie JP Jr. Free Radical, Carbonyl, and Nicotine Levels Produced by Juul Electronic Cigarettes. Nicotine Tob Res. 2018. 10.1093/ntr/nty221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagner KA, Flora JW, Melvin MS, Avery KC, Ballentine RM, Brown AP, et al. An evaluation of electronic cigarette formulations and aerosols for harmful and potentially harmful constituents (HPHCs) typically derived from combustion. Regul Toxicol Pharmacol. 2018;95:153–60. 10.1016/j.yrtph.2018.03.012 . [DOI] [PubMed] [Google Scholar]
- 86.Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob Res. 2016;18(9):1895–902. 10.1093/ntr/ntw128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS ONE. 2017;12(4):e0175430 10.1371/journal.pone.0175430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ Pollut. 2015;198:100–7. Epub 2015/01/13. 10.1016/j.envpol.2014.12.033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shein M, Jeschke G. Comparison of Free Radical Levels in the Aerosol from Conventional Cigarettes, Electronic Cigarettes, and Heat-Not-Burn Tobacco Products. Chem Res Toxicol. 2019;32(6):1289–98. 10.1021/acs.chemrestox.9b00085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hasan F, Khachatryan L, Lomnicki S. Comparative Studies of Environmentally Persistent Free Radicals on Total Particulate Matter Collected from Electronic and Tobacco Cigarettes. Environ Sci Technol. 2020;54(9):5710–8. 10.1021/acs.est.0c00351 [DOI] [PubMed] [Google Scholar]
- 91.Farsalinos KE, Gillman G, Poulas K, Voudris V. Tobacco-Specific Nitrosamines in Electronic Cigarettes: Comparison between Liquid and Aerosol Levels. Int J Environ Res Public Health. 2015;12(8):9046–53. 10.3390/ijerph120809046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2016;124(6):733–9. 10.1289/ehp.1510185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papoušek R, Pataj Z, Novakova P, Lemr K, Bartak P. Determination of acrylamide and acrolein in smoke from tobacco and E-cigarettes. Chromatographia. 2014;77(17–18):1145–51. 10.1007/s10337-014-2729-2. [DOI] [Google Scholar]
- 94.Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr. 2015;1418:192–9. 10.1016/j.chroma.2015.09.034 . [DOI] [PubMed] [Google Scholar]
- 95.Kim YH, Kim KH. A novel method to quantify the emission and conversion of VOCs in the smoking of electronic cigarettes. Sci. 2015;5:16383 10.1038/srep16383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sala C, Medana C, Pellegrino R, Aigotti R, Bello FD, Bianchi G, et al. Dynamic measurement of newly formed carbonyl compounds in vapors from electronic cigarettes. Eur J Mass Spectrom. 2017;23(2):64–9. 10.1177/1469066717699078 . [DOI] [PubMed] [Google Scholar]
- 97.Klager S, Vallarino J, MacNaughton P, Christiani DC, Lu Q, Allen JG. Flavoring Chemicals and Aldehydes in E-Cigarette Emissions. Environ Sci Technol. 2017;51(18):10806–13. 10.1021/acs.est.7b02205 . [DOI] [PubMed] [Google Scholar]
- 98.Liu Q, Huang C, Chris Le X. Arsenic species in electronic cigarettes: Determination and potential health risk. J Environ Sci. 2020;91:168–76. 10.1016/j.jes.2020.01.023 [DOI] [PubMed] [Google Scholar]
- 99.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–4. 10.1056/NEJMc1413069 . [DOI] [PubMed] [Google Scholar]
- 100.Polycyclic Aromatic Hydrocarbons (PAHs): Agency for Toxic Substances & Disease Registry; [updated March 3, 2011]. https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=25. [PubMed]
- 101.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9(6):875–84. 10.1093/carcin/9.6.875 [DOI] [PubMed] [Google Scholar]
- 102.Garcia-Gomez D, Gaisl T, Barrios-Collado C, Vidal-de-Miguel G, Kohler M, Zenobi R. Real-Time Chemical Analysis of E-Cigarette Aerosols By Means Of Secondary Electrospray Ionization Mass Spectrometry. Chem Eur J. 2016;22(7):2452–7. 10.1002/chem.201504450 . [DOI] [PubMed] [Google Scholar]
- 103.Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, et al. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control. 2016;25(Suppl 2):ii94–ii102. 10.1136/tobaccocontrol-2016-053224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soussy S, El-Hellani A, Baalbaki R, Salman R, Shihadeh A, Saliba NA. Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tob Control. 2016;25(Suppl 2):ii88–ii93. 10.1136/tobaccocontrol-2016-053220 . [DOI] [PubMed] [Google Scholar]
- 105.Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health. 2017;16(1):42 10.1186/s12940-017-0249-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci. 2013;29(12):1219–22. 10.2116/analsci.29.1219 . [DOI] [PubMed] [Google Scholar]
- 107.El-Hage R, El-Hellani A, Salman R, Talih S, Shihadeh A, Saliba NA. Fate of pyrazines in the flavored liquids of e-cigarettes. Aerosol Sci Technol. 2018;52(4):377–84. 10.1080/02786826.2018.1433293 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr. 2017;1497:144–54. 10.1016/j.chroma.2017.02.050 . [DOI] [PubMed] [Google Scholar]
- 109.El-Hellani A, Al-Moussawi S, El-Hage R, Talih S, Salman R, Shihadeh A, et al. Carbon Monoxide and Small Hydrocarbon Emissions from Sub-ohm Electronic Cigarettes. Chem Res Toxicol. 2019;32(2):312–7. 10.1021/acs.chemrestox.8b00324 . [DOI] [PubMed] [Google Scholar]
- 110.Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, et al. A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS ONE. 2017;12(1):e0169811 10.1371/journal.pone.0169811 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saliba NA, El Hellani A, Honein E, Salman R, Talih S, Zeaiter J, et al. Surface Chemistry of Electronic Cigarette Electrical Heating Coils: Effects of Metal Type on Propylene Glycol Thermal Decomposition. J Anal Appl Pyrol. 2018;134:520–5. 10.1016/j.jaap.2018.07.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE. 2013;8(3):e57987 10.1371/journal.pone.0057987 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Williams M, To A, Bozhilov K, Talbot P. Strategies to Reduce Tin and Other Metals in Electronic Cigarette Aerosol. PLoS ONE. 2015;10(9):e0138933 10.1371/journal.pone.0138933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Belushkin M, Tafin Djoko D, Esposito M, Korneliou A, Jeannet C, Lazzerini M, et al. Selected Harmful and Potentially Harmful Constituents Levels in Commercial e-Cigarettes. Chem Res Toxicol. 2019;33(2):657–68. 10.1021/acs.chemrestox.9b00470 [DOI] [PubMed] [Google Scholar]
- 115.Williams M, Bozhilov KN, Talbot P. Analysis of the elements and metals in multiple generations of electronic cigarette atomizers. Environ Res. 2019;175:156–66. 10.1016/j.envres.2019.05.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dawkins L, Turner J, Roberts A, Soar K.‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013;108(6):1115–25. Epub 2013/04/05. 10.1111/add.12150 . [DOI] [PubMed] [Google Scholar]
- 117.Park RM, Gilbert SJ. Pulmonary Impairment and Risk Assessment in a Diacetyl-Exposed Population: Microwave Popcorn Workers. J Occup Environ Med. 2018;60(6):496–506. Epub 2018/02/15. 10.1097/JOM.0000000000001303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Rooy FG, Smit LA, Houba R, Zaat VA, Rooyackers JM, Heederik DJ. A cross-sectional study of lung function and respiratory symptoms among chemical workers producing diacetyl for food flavourings. Occup Environ Med. 2009;66(2):105–10. Epub 2008/09/23. 10.1136/oem.2008.039560 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data is within the paper and Supporting Information files. The supporting information and bibliography includes all of the articles used in this study.