Abstract
Heparin and heparan sulfate (HS) are attractive components for constructing biomaterials due to their ability to recruit and regulate the activity of growth factors. The structural and functional heterogeneity of naturally derived heparin and HS is, however, an impediment for the preparation of biomaterials for regenerative medicine. To address this problem, we have prepared hydrogels modified by well-defined synthetic HS-derived disaccharides. Human induced pluripotent cell-derived neural stem cells (HIP-NSCs) encapsulated in a polyethylene glycol-based hydrogel modified by a pendent HS disaccharide that is a known ligand for fibroblast growth factor-2 (FGF-2) exhibited a significant increase in proliferation and self-renewal. This observation is important because evidence is emerging that undifferentiated stems cells can yield significant therapeutic benefits via their paracrine signaling mechanisms. Our data indicate that the HS disaccharide protects FGF-2, which has a very short biological half-live, from degradation. It is anticipated that, by careful selection of a synthetic HS oligosaccharide, it will be possible to control retention and release of specific growth factor, which in turn will provide control over cell fate.
Graphical Abstract
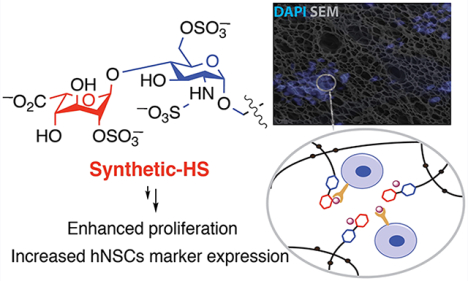
Hydrogels, which are formed by chemical or physical cross-linking of water-soluble polymers, provide attractive scaffolds for tissue regrowth and regeneration.1–3 The bulk properties of hydrogels can influence stem cell fate, and in particular, mechanical properties and chemical entities such as cell adhesion peptides have been explored to influence proliferation and differentiation.4 Glycosaminoglycans, such as heparin and heparan sulfate (HS), are also attracting considerable interest for the development of hydrogels for regenerative medicine.5
HS are highly sulfated linear polysaccharides that are ubiquitously expressed within the stem cell microenvironment or niche where they interact with numerous signaling proteins, growth factors, and extracellular matrix components.6,7 The biosynthesis of HS occurs in the Golgi by initially forming a linear polymer composed of alternating D-glucuronic acid (GlcA) and N-acetyl-D-glucosamine (GlcNAc) moieties, which are modified by a series of enzymatic transformations involving N-deacetylation/N-sulfation, epimerization of GlcA to l-iduronic acid (IdoA), and sulfation of the C-2 hydroxyl of IdoA and the C-6 hydroxyl of glucosamine. These transformations proceed only partially, resulting in as many as 20 different disaccharide moieties that can be arranged in different ways to create specific HS epitopes.8
Evidence is emerging that cells can create unique HS epitopes by regulating the expression of isoforms of HS biosynthetic enzymes.9,10 The so-called “HS sulfate code hypothesis” is based on the notion that such epitopes can recruit or retain specific HS-binding proteins. Indeed, the structure of HS changes during stem cell differentiation,11 which in turn is important for the stage dependent recruitment of morphogens such as the fibroblast growth factors (FGFs), bone morphogenetic protein (BMP), and wingless-type MMTV integration site family proteins (Wnts).12
Polymeric matrixes modified by heparin or heparan sulfate offer interesting vehicles for the controlled delivery of growth factors.13 For example, the use of collagen modified by heparin in combination with recombinant fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) provided sustained release, induced vascularization, and reduced hypoxic conditions.14 In other studies, it has been found that functionalization of hyaluronic acid-based hydrogels with heparin diminished the burst release of BMP-2 and prolonged osteogenic activity.15 Despite these promising results, many challenges remain for the use of heparin or HS for biomaterial construction. In particular, the structural and functional complexity of these biopolymers is an impediment because it can cause adverse effects due to binding of many different proteins.16 In an interesting approach, this problem was addressed by using partial desulfated heparin, which could modify growth factor presentation and influence cell fate in a PEG-based hydrogel.17 In another study, affinity purification was employed to obtain an HS fraction that bound potently to BMP-2, greatly enhances BMP-2-induced bone formation in vitro and in vivo, and was much more efficacious than mucosal HS or heparin.18 These approaches are promising but still offer limited capabilities to control the binding properties of HS.
Recent progress in the chemical19 and chemoenzymatic20 synthesis of HS oligosaccharides is making it possible to probe ligand requirements of HS binding proteins. These studies reveal that relatively small structures, such as di- and tetra-saccharides, can bind with high affinity and selectivity to HS-binding proteins.21–23 Furthermore, the attachment of such compounds to polymeric backbones can provide neoglycopolymers that recapitulate properties of HS.24–27 Therefore, we were compelled to investigate whether small synthetic HS oligosaccharides can be employed for hydrogel formation to influence cell fate.
In a proof-of-principle study, we have synthesized a HS disaccharide that is a known ligand of FGF-228 and used it to fabricate a neural tissue mimic that can encapsulate, recruit, and maintain the undifferentiated state of neural stem cells (NSCs). These findings are important because NSCs have tremendous therapeutic potential to treat neurological deficits that manifest after injury or disease of the central nervous system (CNS). Although the focus on promoting the neuronal differentiation of NSCs to facilitate functional repair of neurological deficits has its merits, emerging evidence suggests that NSCs in their undifferentiated state promote more favorable functional outcomes via their paracrine signaling mechanisms rather than through differentiation and cell replacement per se.29 It is therefore plausible that strategies to promote “bystander” signaling by undifferentiated NSCs could carry a significant therapeutic benefit.
RESULTS AND DISCUSSION
Chemical Synthesis
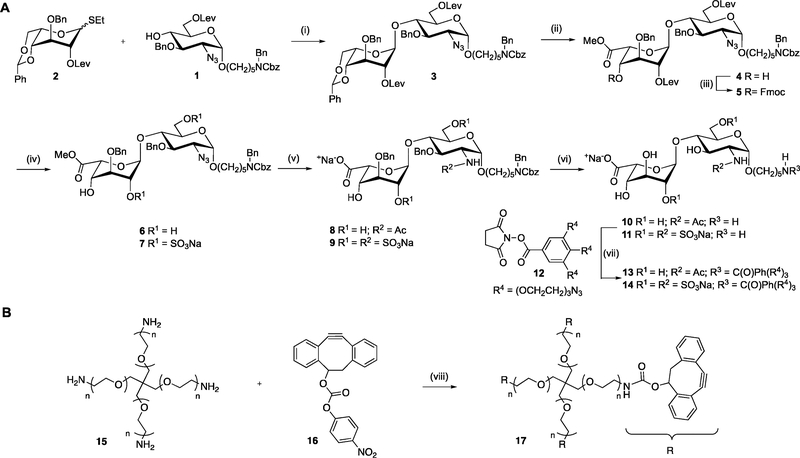
Conventional approaches to incorporate heparin into biomaterial scaffolds rely on modification of the saccharide backbone with a reactive functional group.30,31 Such an approach is unsuitable for presenting small synthetic oligosaccharides because vital functional groups would be destroyed. Therefore, we developed a so-called pendant approach in which synthetic HS oligosaccharides (e.g., 10 and 11, Scheme 1) are modified with an artificial anomeric aminopentyl linker, which can react with an NHS activated triazide PEG linker such as 12 to yield HS oligosaccharides that possess three azide moieties (e.g., 13 and 14). It was anticipated that these azide-modified compounds could be cross-linked with a 4-armed PEG derivative modified with dibenzylcyclooctynol (17, DIBO) to yield stable hydrogels.32 This approach would appropriately place the HS disaccharides in the hydrogel lattice to facilitate functional interactions with HS-binding proteins. For the purpose of this study, compound 14 was selected as a high affinity ligand of FGF-228 whereas compound 13, which is devoid of sulfate groups, was used as a negative control. We anticipated that a hydrogel modified with a FGF-2 binding HS oligosaccharide will retain and stabilize this growth factor, which in turn would promote NSCs self-renewal and maintenance.
Scheme 1. Chemical Synthesis of HS Hydrogel Precursorsa.
a(A) Unsulfated-HS-triazide (13) and sulfated-HS-triazide (14); (B) DIBO-4-armed PEG polymer (17). Reagents and conditions: (i) NIS, TMSOTf, DCM, −20 °C, 0.5 h; (ii) (a) DCM/TFA/H2O, 0 °C to rt, 1 h; (b) TEMPO, BAIB, DCM/H2O, 1 h; (c) CH2N2, THF, 0.5 h; (iii) FmocCl, pyridine, DMAP (cat.), 2 h; (iv) (a) hydrazine acetate, EtOH/toluene (2/1, v/v), 2 h; (b) SO3·pyridine complex, DMF, 8 h; (c) Et3N/MeOH (1/1, v/v), 0.5 h; for 6, only step a on 4; for 7, steps a, b, and c on 5; (v) (a) 1.0 M aq. LiOH, aq. H2O2 (50%), 8 h then pH ~ 14 with 4.0 M NaOH, 16 h; (b) 1.0 M PMe3 in THF, NaOH, 1 h; (c) Ac2O, NEt3, MeOH; (d) SO3-pyridine complex, MeOH, Et3N, 0.1 M NaOH, 16 h; for 8, steps a, b, and c on 6; for 9, stepd a, b, and d on 7; (vi) (a) Pd/C, H2 (atm.), tBuOH/H2O (1/1, v/v), 2 h; (b) Pd(OH)2/C, H2 (atm.), tBuOH/H2O (1/1, v/v), 16 h, 10 from 8 and 11 from 9; (vii) DIPEA, DMSO, 0 °C to rt, 16 h, 13 from 10 and 14 from 11; (viii) Et3N, DCM, 0 °C to 16 h.
Functionalized HS disaccharides 13 and 14 were assembled from glycosyl acceptor 1 and idosyl donor 2, which were coupled using NIS/TMSOTf as the promoter system to give disacharide 3 in good yield as only the α-anomer (Scheme 1A).33 The benzylidene acetal of 3 was removed by a mixture of trifluoroacetic acid, dichloromethane, and water to give a diol, which was subjected to TEMPO/BAIB to selectively oxidize the primary alcohol to a carboxylic acid, which was esterified by treatment with diazomethane to yield methyl ester 4. The C-4′ hydroxyl of 4 was protected as Fmoc by treatment with FmocCl in pyridine in the presence of a catalytic amount of DMAP to give disaccharide 5. Next, the levulinoyl (Lev) esters of 5 were removed by treatment with hydrazine acetate, and the resulting hydroxyls were sulfated using sulfur trioxide/pyridine complex in DMF, followed by Fmoc removal to provide 7. The methyl ester of 7 was saponified with H2O2 and LiOH in THF. The azide of the resulting compounds was reduced under Staudinger conditions using PMe3, and the resulting amine was N-sulfated, employing a sulfur trioxide/pyridine complex in MeOH in the presence of Et3N and NaOH to provide 9.
In parallel, disaccharide 4 was subjected to a similar sequence of reactions without O-sulfation and N-acetylation instead of N-sulfation to provide 8. The target disaccharides 10 and 11 were obtained by hydrogenation of 8 and 9, respectively, over Pd/C in a mixture of tBuOH/H2O (1/1) to cleave the protecting group of the linker followed by further hydrogenation over Pd(OH)2 /C to remove the benzyl ethers, and finally purification by size exclusion chromatography over a P-2 column. Treatment of the latter compounds with the NHS activated triazide PEG linker 12 (Supplementary Scheme 1)34 resulted in the formation of the desired derivatives 13 and 14, respectively. The DIBO-functionalized 4-armed PEG 17 was synthesized (Scheme 1B) by reaction of p-nitrophenyl carbonate of DIBO (16) with commercially available PEG tetra-amine (15, 10 kDa).
Preparation and Characterization of HS-Containing Hydrogels
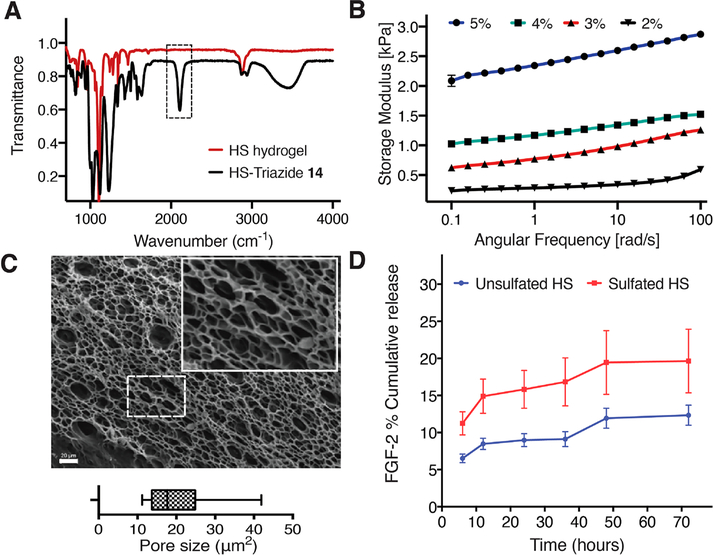
Stoichiometric quantities of DIBO-functionalized 4-armed PEG 17 and triazide-functionalized sulfated-HS disaccharide 14 (1:1 molar ratio of azide and DIBO) were mixed, resulting in the formation of stable hydrogels within 20 min. The absence of the N3 stretch vibration at 2095 cm−1 in the IR spectra of the lyophilized sulfated-HS hydrogels indicated that the cycloaddition had proceeded to completion (Figure 1A). By varying the concentration of the components, the storage modulus of the sulfated-HS hydrogels could be tuned to match the material properties of the physiological host tissue (Figure 1B). The gels displayed minimal strain hardening at high frequency (100 rad s−1) and high strain (5%). Sulfated-HS hydrogels with 3 wt % of compound 17 had a storage modulus (0.5–1 kPa) appropriate for NSCs cultures and was employed for further studies.
Figure 1.
Physio-mechanical characterization of HS hydrogels. (A) An overlay of FTIR spectra of sulfated-HS-triazide (14) and lyophilized sulfated-HS hydrogel showing the consumption of azide (2095 cm−1). (B) Storage moduli of 2, 3, 4, and 5 wt % sulfated-HS hydrogels over a frequency range of 0.1 to 100 rad s−1 at a constant strain of 5% at 25 °C. (C) Scanning electron micrograph (SEM) of a lyophilized 3 wt % sulfated-HS hydrogel at magnification of 500× (inset represents a magnified image of the dashed box); pore size analysis of the SEM images displays an average pore size of 20.16 μm2. (D) Cumulative % release of FGF-2 encapsulated in sulfated-HS and unsulfated-HS hydrogels determined by ELISAs.
Swelling studies of this hydrogel showed an acceptable swelling ratio of 2.2 over a period of 10 days (Supplementary Figure 1). The microarchitecture observed using SEM and pore size analysis of the SEM images revealed an average pore size of 20.16 μm2 indicating that the material is sufficiently porous to facilitate the migration and proliferation of encapsulated NSCs (Figure 1C).
FGF-2 is a potent mitogen for neural progenitor cells; however, it is highly unstable, and its concentration rapidly declines when incubated at 37 °C, probably due to thermal denaturation, proteolytic digestion and cellular uptake. It has been shown that heparin can prevent degradation and its presence leads to faster cell growth.35,36 To evaluate FGF-2 stabilization and retention capacity of the hydrogels, we quantified the cumulative % release of FGF-2 of hydrogels modified by the sulfated (14) and control oligosaccharide (13) over a period of 72 h. For this experiment, human FGF-2, HS-triazide (13 or 14), and DIBO-4-armed PEG polymer (17, 3 wt % final concentration, 1:1 molar ratio of azide group and DIBO group) were mixed to form a hydrogel, which was overlaid with PBS. At regular time intervals, the PBS was replaced and the concentration of FGF-2 was determined by an enzyme-linked immunosorbent assay (ELISA, Figure 1D). The total amount of FGF-2 released from the sulfated-HS hydrogels was higher than that from the unsulfated-HS hydrogel. The enhanced cumulative release of FGF-2 detected in the sulfated-HS construct is likely due to significantly enhanced protection and lower heat induced degradation of FGF-2 activity in sulfated-HS matrixes when compared to unsulfated-HS matrixes.37–40
NSCs Cell Fate in HS-Containing Hydrogel
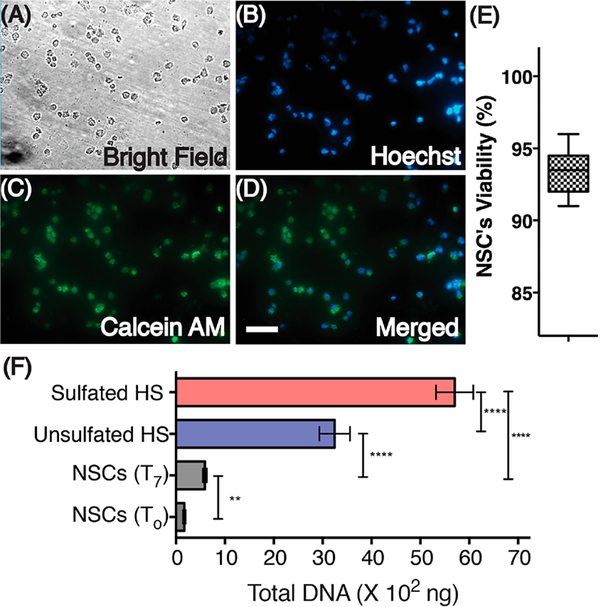
We performed a hydrogel contact viability assay in order to evaluate the effects of HS hydrogels on NSC viability. For these assays, NSCs were cultured for 2 h on a poly-D-lysine coated surface. Subsequently, HS hydrogel components reconstituted in neurobasal media were overlaid on top of the NSC monolayer and allowed to cross-link. After formation of stable hydrogels, hydrogels were overlaid with expansion media and incubated for 24 h before staining with calcein AM and Hoechst stain. NSC viability was assessed by comparing live cells (green fluorescence) to live or fluorescence) and to bright field images of the NSCs. We observed that the hydrogels formed from 14 and 17 did not induce cytotoxicity and that the majority of the encapsulated NSCs were alive 24 h post encapsulation (94%) (Figure 2A–E).
Figure 2.
Cytocompatibility and proliferation of NSCs encapsulated in HS hydrogels. NSCs were stained for nuclei (Hoechst) and live cell (Calcein AM) detection after culturing for 24 h. A bright field image and an overlay of these images are also presented (A–D). Scale, 50 μm. (E) Viability of NSCs. (F) Total isolated DNA from NSCs grown on Geltrex coated culture plates (T0, day 0 and T7, day 7) and from NSCs encapsulated in the sulfated (14)-HS and unsulfated (13, control)-HS hydrogel. Statistical differences observed between groups are represented by **** indicating P < 0.001 and ** indicating P < 0.01.
In order to investigate the influence of HS oligosaccharides on proliferation, NSCs were encapsulated in the hydrogels modified by the sulfated (14) and control (13) oligosaccharide. Unlike the contact viability assay, where NSCs were cultured in a monolayer, NSCs in these assays were encapsulated by the hydrogel matrix after resuspending the NSC pellet with the HS hydrogel mix. This results in NSCs being distributed throughout the volume of the hydrogel. Hydrogel encapsulated NSCs were cultured in FGF-2 containing media (conditioned media) for 24 h, after which they were cultured in media without FGF-2 (unconditioned media) for an additional 6 days. Next, the rate of proliferation was determined by quantifying total DNA. Both sulfated- and unsulfated-HS hydrogel encapsulated NSCs yielded significantly greater quantities of total DNA, indicating higher proliferation when compared to NSCs cultured under standard 2D culture conditions. Among the hydrogel encapsulated NSCs, those cultured in sulfated-HS matrixes demonstrated significantly greater proliferation when compared to unsulfated-HS controls (Figure 2F), as inferred from the total DNA recovered. Our results also demonstrate that FGF-2 deprived NSCs grown under standard 2D culture conditions began differentiating into neural lineage cells within 48 h postseeding (Supplementary Figure 3) when compared to hydrogel encapsulated NSC, which suggests that hydrogels can efficiently engage endogenously released growth factors and promote NSCs proliferation. Although the cytocompatible cross-linking chemistry and optimum mechanical properties of our synthetic heparin constructs ensure cell viability (Figure 2E), the absence of cell-adhesion peptides in the 3D matrix results in the NSCs exhibiting a rounded morphology,41 which is typical of the proliferative state of hydrogel encapsulated NSCs.
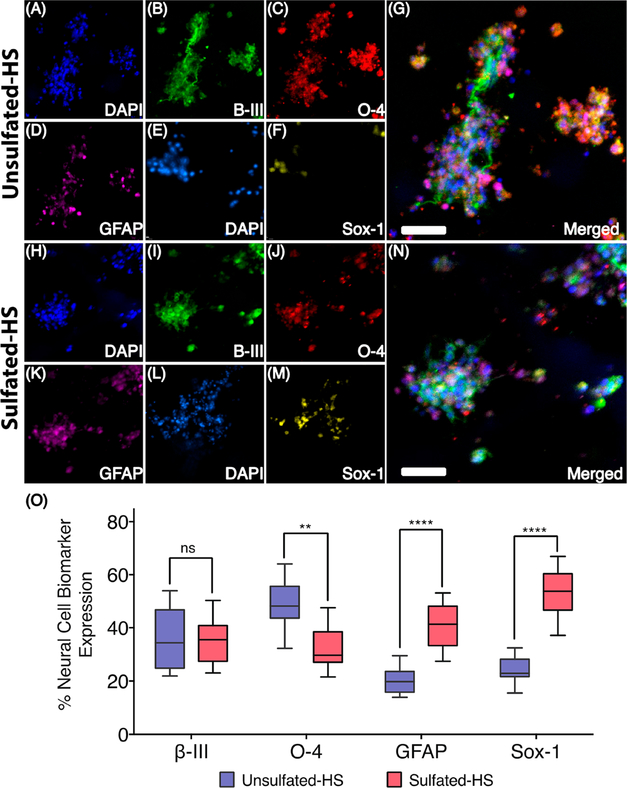
Next, we investigated whether the sulfate moieties of the HS disaccharide influences cell differentiation. For this purpose, the relative expression of relevant phenotypic markers of stem cell differentiation was performed by immunocytochemical staining using appropriate antibodies. These studies demonstrated that sulfated-HS oligosaccharide 14, which can stabilize FGF-2, greatly impacted cell differentiation (Figure 3). Thus, NSCs cultured in this hydrogel displayed significantly higher expression of the self-renewal biomarker Sox-1 (P < 0.001) and the astrocytic marker GFAP (P < 0.001). Furthermore, we detected a significantly (P = 0.02) lower expression of the oligodendrocyte marker O-4. Although no significant difference in neuronal differentiation as determined by β-III tubulin was observed, the higher expression of the Sox-1 is indicative of NSCs that are maintaining their undifferentiated state. In addition, we believe higher GFAP expression is indicative of glial lineage NSCs that can differentiate into neurons and glial cells.42 Furthermore, the lower expression of the oligodendrocyte marker O-4 indicates NSCs self-renewal and maintenance. The maintenance of self-renewal and proliferative capacity of NSCs is likely due to recruitment and/or stabilization of FGF-2 in the hydrogel containing a HS ligand for this growth factor.
Figure 3.
Immunocytochemistry results of NSCs encapsulated in HS hydrogels. (A–G) A representative image (Z-stack) of NSCs encapsulated in a 3 wt % unsulfated-HS hydrogel conditioned for 24 h with FGF-2 and cultured for 6 days without FGF-2; Group-1 unsulfated-HS hydrogels were stained for nuclei, neuronal, oligodendrocyte, and astrocyte markers, represented by DAPI (A), β-III tubulin (B), O-4 (C), and GFAP (D), respectively. An overlay of these images (A–D) is also presented (G); Group-2 unsulfated-HS hydrogels were stained for nuclei and stem cell marker, represented by DAPI (E) and Sox-1 (F), respectively. An overlay of these images (E, F) is presented in Supplementary Figure 4; (H–N) NSCs encapsulated in a 3 wt % sulfated-HS hydrogel conditioned for 24 h with FGF-2 and cultured for 6 days without additional FGF-2. Group-1 sulfated-HS hydrogels were stained for nuclei, neuronal, oligodendrocyte, and astrocyte markers and are represented by DAPI (H), β-III tubulin (I), O-4 (J), and GFAP (K), respectively. An overlay of these images (H–K) is also presented (N); Group-2 sulfated-HS hydrogels were stained for nuclei and stem cell marker, represented by DAPI (L) and Sox-1 (M), respectively. An overlay of these images (L, M) is presented in Supplementary Figure 4; scale, 20 μm. (O) Quantification of markers β-III tubulin, O-4, GFAP, and Sox-1 expression in NSCs encapsulated in unsulfated-HS and sulfated-HS hydrogel. Statistical differences observed between groups are represented by ns indicating not significant, **** indicating P < 0.001, and ** indicating P < 0.01.
Conclusions
An important goal in regenerative medicine is to create three dimensional matrixes that resemble properties of the extracellular matrix to guide cell fate.43 Integrins are transmembrane receptors that facilitate adhesion of extracellular matrix components such as laminin, collagen, and fibronectin. Peptides derived from these extracellular matrix proteins, such as RGDS, IKVAV, and YIGSR have found wide use in the construction of functional materials for cell adhesion, and their presence can also influence cell fate.44,45 HS is also an important component of the extracellular matrix that is involved in growth factor retention and signaling, and efforts have been made to incorporate heparin into biomaterials.5,16,30,31 The pleiotropic nature and binding promiscuity of heparin can, however, result in undesired effects. For example, growth differentiation factor 5 (GDF5), which is involved in cartilage/skeletal formation and homeostasis, is inhibited by heparin.46 In addition, heparin can cause hemorrhagic side effects and induce thrombocytopenia. It is derived from animal products exhibiting batch to batch differences and has been contaminated with other sulfated molecules.47,48 To address these liabilities of natural derived heparin, we demonstrate here that incorporation of a small and well-defined synthetic HS oligosaccharide into a hydrogel can influence cell fate. In particular, the use of a tailor-made saccharide ligand for FGF-2 greatly increased self-renewal and proliferative capacity of NSCs.
Chemical synthesis makes it now possible to identify HS oligosaccharides that interact with specific HS-binding proteins. Furthermore, a synthetic approach allows the incorporation of an artificial linker to facilitate controlled attachment of cross-linking functionalities for hydrogel formation. Here, we modified an HS oligosaccharide with a linker having three azido moieties, and a stable hydrogel could be formed by addition of a PEG-derivative modified by four DIBO moieties. We developed DIBO for strain promoted azide–alkyne cycloaddition (SPAAC),49 and this reagent reacts spontaneously with azides in the absence of a catalyst and, thus, is attractive for biomedical application. It was found that the presence of a sulfated oligosaccharide did not impede hydrogel formation, and by tuning the concentration of the reaction components, a hydrogel could be formed that has a storage modulus resembling that of neuronal tissue. It is likely that the placement of a HS disaccharide in a pendant fashion within hydrogel scaffolds creates a multivalent display, which augments binding avidity for growth factors owing to the cluster effect,50 contributes to thermal stability, and protects against proteolytic cleavage.39,40 It is anticipated that, by careful selection of a HS oligosaccharide, it will be possible to control the retention and release of a specific growth factor, which in turn will give greater control over cell fate.
In this study, a HS disaccharide was employed that can bind FGF2 but not induce cell signaling through the formation of a ternary complex with FGFR1.28 Future studies will focus on the incorporation of an HS oligosaccharide that can induce the formation of such a ternary complex, and possible cellular responses will be investigated. Furthermore, PEG was selected as the polymeric material because it is widely used in tissue engineering and approved by FDA for human use. The synthetic approach gives, however, limited control over HS oligosaccharide spacing and valency. Future studies will focus on the use of alternative polymeric backbones, such as dextran or hyaluronic acid, that make it possible to attach HS oligosaccharides at different densities. Such an approach can be combined with the use of other cell-responsive and cell-instructive components such as cell adhesion peptides and protease-cleavable linkers that can promote degradation of a hydrogel.
Traumatic brain injuries and stroke result in significant brain tissue loss and atrophy, which leads to lifelong disabilities and a cost-of-care burden. There is a dire need for therapies for these conditions in order to prevent rampant brain tissue atrophy and promote functional repair. Our future studies will focus on the design of tailored synthetically derived glycomaterials that can better regulate cellular repair cascades in order to facilitate functional recovery following severe brain trauma.
MATERIALS AND METHODS
Chemical Synthesis
Preparation of HS-Triazide: General Procedure for Amide Coupling (Scheme 1)
To the stirring solution of amino compound (10 or 11) in DMSO (0.5 mL for 0.05 mmol) and DIPEA (4 equiv) at 0 °C was added compound 12 (2 equiv., in 0.5 mL of DMSO). The progress of the reaction was monitored by TLC (EtOAc/pyridine/H2O/AcOH, 8/5/3/1, v/v/v/v, or EtOAc/MeOH/H2O, 7/2/1, v/v/v). After stirring for 16 h, the reaction mixture was diluted with water (1.0 mL) and lyophilized. The obtained solid residue was purified by reverse phase chromatography (RP-18 silica gel column, H2O/MeOH, 9/1 → 1/1, v/v). The appropriate fractions were concentrated in vacuo, and the residue was passed through a column Dowex 50 × 8 Na+ resin (0.6 × 5 cm) using MeOH/H2O, 9/1, as eluent to afford the desired product (13, 0.038 g, 67.9%; 14, 0.026 g, 71.8%).
DIBO-Functionalized 4-Armed PEG Polymer (17)
To the stirring mixture of 4-armed PEG-amine polymer (15, MW 10K, 0.30 g, 0.03 mmol, CreativePEGworks) in anhyd. CH2Cl2 (3.0 mL) at 0 °C was added p-nitrophenyl carbonate of DIBO (16, 0.11 g, 0.3 mmol) and Et3N (0.15 mL). The resulting reaction mixture was left stirring in a melting ice bath for 16 h. After completion of the reaction, confirmed by TLC (Rf of 0.65, EtOAc/IPA/H2O/NH4OH, 3/3/2/1, v/v/v/v), the reaction mixture was concentrated under vacuum to give a yellow oil, which was further purified over a LH-20 column (CH2Cl2/CH3OH, 1/1, v/v) to give the desired compound 17 (0.27 g, 83.4%) as a white solid. The detailed synthetic procedures and spectral data of intermediate compounds are provided in the Supporting Information.
Preparation of HS Hydrogels
Stochiometric equivalents of DIBO-4-armed PEG polymer (150 μL, 10 wt % in PBS, 17) and unsulfated- or sulfated-HS-triazide (150 μL, 13 or 14 in PBS, 1:1 molar ratio of azide group and DIBO group) were mixed by vortexing in a glass vial and left to cross-link for 20 min at 37 °C. The formation of stable hydrogels was confirmed by the vial-tilting method (Supplementary Figure 1).
Fourier Transform Infrared (FTIR) Spectroscopy
To determine the degree of azide functional group consumption, FTIR measurements of lyophilized sulfated-HS hydrogel (3 wt %, final concentration of DIBO-4-armed PEG polymer, 17) samples were taken with a Nicolet model 6700 with a grazing angle attenuated total reflectance (GATR) accessory at 64 scans with a 4 cm−1 resolution (Figure 1A).
Rheological Evaluation of HS Hydrogels
The pendant approach circumvents interference of HS disaccharide in strain promoted cycloaddition between triazide and DIBO polymer. Although the strategic placement of HS has little influence on rheological properties, we nevertheless conducted mechanical characterization on hydrogels that were functionalized using sulfated-HS-triazide. Hydrogels (300 μL each of 2, 3, 4, and 5 wt % final concentration of DIBO-4-armed PEG polymer, 17) were prepared by mixing an equal volume of 17 (150 μL) and sulfated-HS-triazide (14, 150 μL, 1:1 molar ratio of azide group and DIBO group) in a PDMS mold (16 mm diameter and 3 mm thickness) swelled in PBS for 24 h at 37 °C. The hydrogels were characterized by isothermal frequency sweep experiments in triplicate from 0.1 to 100 rad’s−1 and 5% strain using a parallel plate rheometer (Anton Paar) (Figure 1B).
Swelling Study of HS Hydrogels
The swelling ratio of HS hydrogels was measured over a period of 10 days. Sulfated-HS hydrogels (3 wt %, final concentration of DIBO-functionalized 4-armed PEG polymer, 17, 100 μL discs, in triplicate) were prepared on the surface of a 14 mm2 coverslip affixed to glass bottom Petri dishes (In Vitro scientific). The initial weight (Wto) of each hydrogel was obtained by subtracting the weight of glass bottom Petri dishes before and after casting the hydrogels. Each hydrogel disc was subsequently overlaid with 100 μL of PBS and placed in a tissue culture incubator set at 37 °C, 5% CO2, and 95% humidity. Swollen hydrogels were weighed (Wt) immediately after the removal of excess media every 24 h for 7 days. The hydrogels were overlaid with 100 μL of PBS post-weighing. The swelling ratios were calculated by Wt/Wto and plotted (Supplementary Figure 2).
Scanning Electron Microscopy (SEM)
The microarchitecture of HS hydrogels was observed using a Zeiss 1450EP scanning electron microscope (Carl Zeiss). Sulfated-HS hydrogels (3 wt %, final concentration of DIBO-functionalized 4-armed PEG polymer, 17) were prepared in a polydimethysiloxane (PDMS) mold (16 mm diameter and 3 mm thickness), swelled in PBS for 24 h at 37 °C, flash frozen in liquid nitrogen, and then lyophilized for 48 h. The lyophilized gels were mounted on 10 mm SEM stubs and sputter coated (Structure Probe Inc.) with gold for 60 s before being imaged under an accelerated voltage of 20 kV. Images were acquired at 1000× and 1500× magnifications to observe the porosity and structure of the hydrogels. For pore size analysis, 1000× magnified images were processed using ImageJ software (NIH) (Figure 1C).
FGF-2 Retention in HS Hydrogels
HS hydrogels were cross-linked with human FGF-2, and its cumulateve % release profile was established using ELISA. DIBO-functionalized 4-armed PEG polymer (25 μL of 6 wt %, 17) was dispensed into each well of a 96-well plate (in triplicate) followed by the addition of either unsulfated-HS-triazide (13, 25 μL) or sulfated-HS-triazide (14, 25 μL) (1:1 molar ratio of azide group and DIBO group) containing FGF-2 (10 ng, R&D Systems, Inc.), mixed by pipetting, and left to cross-link at 37 °C for 30 min. Upon hydrogel formation, 50 μL of hydrogel was overlaid with 50 μL of PBS; the plate was then incubated at 37 °C, 5% CO2, and 95% humidity. At 6, 12, 24, 36, 48, and 72h, the overlaid PBS was collected and stored at −80 °C and replaced with 50 μL of fresh PBS. Due to the nondegradable nature of the HS-PEG hydrogels, the hydrogel bound FGF-2 cannot be retrieved. As a result, the extent of FGF-2 retention was determined as the difference between the initial FGF-2 loaded and the cumulative FGF-2 released. The FGF-2 released by unsulfated-HS and sulfated-HS hydrogels was determined by a human basic FGF Quantikine ELISA kit (R&D Systems, Inc.) according to the manufacturer’s instructions, and the cumulative % recovery of FGF-2 was plotted (Figure 1D).
NSC Expansion
Human induced pluripotent cell-derived neural stem cells (HIP-NSCs, MTI-GlobalStem) were expanded on a Geltrex coated plate in expansion media consisting of Neurobasal medium (Life Technologies), B-27 supplement (Life Technologies), MEM nonessential amino acids (Life Technologies), GlutaMAX supplement (Life Technologies), and basic fibroblast growth factor (20 ng mL−1, R&D Systems, Inc.) and incubated at 37 °C, 5% CO2, and 95% humidity. Cells were fed with expansion media every other day unless passaged or extracted for use in assays.
Viability Assessment of Hydrogel Overlaid NSCs
Coverslips affixed to glass bottom Petri dishes (In Vitro scientific) were coated with poly-D-lysine hydrobromide (100 μg mL−1, Sigma-Aldrich) overnight at 37 °C and washed with PBS before platting 20 × 103 HIP-NSCs. After 1 h, expansion media was aspirated, and sulfated-HS hydrogels (3 wt %, final concentration of DIBO-4-armed PEG polymer, 17, 100 μL discs) were prepared on top of the adhered NSCs. Alternatively, NSCs were encapsulated in HS hydrogels by mixing 25 μL of 6 wt % DIBO-functionalized-4-armed PEG polymer (17, containing 20 × 103 NSCs) and sulfated-HS-triazide (14, 25 μL) (1:1 molar ratio of azide group and DIBO group) and left to cross-link at 37 °C for 20 min. The obtained NSC encapsulated hydrogels were overlaid with expansion media and incubated at 37 °C, 5% CO2, and 95% humidity for 24 h before being stained with Calcein AM (Thermo Fisher) and Hoechst stain (Thermo Fisher) according to the manufacturer’s instructions. Live cells (green fluorescence) were compared to live or dead cells (blue fluorescence) and to bright field images of the cells. Images were analyzed using a Leica DM IRB series microscope (Leica Microsystems, Inc.). Cell viability was assessed using colocalization of Calcein green fluorescence to Hoechst blue fluorescence in 40× images for at least four images per hydrogel sample, using cell colocalization tools associated with Volocity software (PerkinElmer) (Figure 2A-E).
Total DNA Isolation from Hydrogel Encapsulated NSCs
In order to evaluate the proliferation capacity of the hydrogels and the effect of sulfation on proliferation, total DNA isolated from NSCs grown on a Geltrex coated culture plate was compared with those grown in unsulfated-HS and sulfated-HS hydrogels. 25 μL of 6 wt % DIBO-functionalized-4-armed PEG polymer (17, with 20 × 103 NSCs) was mixed with either unsulfated-HS-triazide (13, 25 μL) or sulfated-HS-triazide (14, 25 μL) (1:1 molar ratio of azide group and DIBO group) by pipetting and left to cross-link at 37 °C for 30 min. In addition, a culture plate was Geltrex coated and seeded with NSCs (20 × 103). Culture plates and plates containing HS hydrogels were overlaid with 2.0 mL of expansion media and incubated at 37 °C, 5% CO2, and 95% humidity. After 24 h, expansion media was replaced with basal media (expansion media without FGF-2), and NSC incubation was continued in a FGF-2 deprived condition. After 7 days, the total amount of DNA present in NSCs grown on Geltrex coated culture plates (at time zero, T0 and time day 7, T7) and unsulfated-HS and sulfated-HS hydrogels was determined by a Zymoclean gel DNA recovery kit (Zymo research corp.) according to the manufacturer’s instructions, and the total DNA isolated was plotted (Figure 2F).
Immunocytochemistry
NSCs encapsulated in unsulfated-HS (two groups, in triplicate) and sulfated-HS (two groups, in triplicate) hydrogels were fixed with 4% paraformaldehyde containing 0.4 M sucrose in PBS. After 20 min, the hydrogels were rinsed thrice with PBS and permeabilized in blocking buffer (PBS containing 4% goat serum and 0.5% Triton-X100) for 1 h. Subsequently, the group-1 hydrogels (both unsulfated- and sulfated-HS hydrogels) were incubated at 4 °C with blocking buffer containing primary antibodies (at dilutions of 1:500) against β-III tubulin (Millipore), a neuronal cytoskeletal marker; O-4 (R&D Systems, Inc.), an oligodendrocyte marker; glial fibrillary acidic protein (GFAP, R&D Systems, Inc.), an astrocyte marker. The group-2 hydrogels (both unsulfated- and sulfated-HS hydrogels) were incubated with a neural stem cell marker: Sox-1 (R&D Systems, Inc.). After 16 h, the hydrogels were rinsed thrice with PBS and incubated with blocking buffer again for 1 h followed by the addition of appropriate AlexaFluor 488 (Life Technologies), AlexaFluor 555 (Life Technologies), and Cy5 (Life Technologies) labeled secondary antibodies and incubation for 1 h. After rinsing hydrogels thrice with PBS, a nuclear stain (NucBlue, Life Technologies, CA) was added to the hydrogels; they were then incubated for 5 min and rinsed thrice with PBS. The hydrogels were subsequently mounted with fluoromount-G (SouthernBiotech) and a coverslip was placed on top the next day. NSCs were imaged using a Leica DM IRB series microscope (Leica Microsystems, Inc.). Representative high-magnification images (15 in total, from three replicates) of unsulfated-HS and sulfated-HS hydrogels were analyzed using cell population tools in Volocity software (PerkinElmer) (Figure 3). The Manders overlap coefficient was used to measure the degree of colocalization between fluorescent images, as described previously.51 Each separate signal intensity was quantified against the DAPI signal to provide the percentages of cells positive for markers found in the FITC, TRITC, or Cy5 channels.
Statistical Analysis
All statistical analyses were performed using a one-way analysis of variance (ANOVA) for significance with appropriate posthoc tests using GraphPad Prism or SigmaPlot software. Direct mean comparisons were evaluated using a Student’s t-test. All studies were performed in triplicate at the minimum. Statistical differences observed between groups are represented by ns indicating not significant, **** indicating P < 0.001, and ** indicating P < 0.01.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institute of General Medicine (NIGMS; Research Resource for Integrated Glycotechnology P41GM103390 to G.-J.B.) and the National Institute of Neurological Disorders and Stroke (NINDS; R01NS099596 to L.K.) of the National Institutes of Health (NIH).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.9b00401.
Full experimental details, characterization and spectral data of the compounds, hydrogel swelling data, 2D culturing of NSCs, and immunocytochemical results for Sox-1 (PDF)
REFERENCES
- (1).Peppas NA, Hilt JZ, Khademhosseini A, and Langer R (2006) Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater 18, 1345–1360. [Google Scholar]
- (2).Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, and Peppas NA (2009) Hydrogels in Regenerative Medicine. Adv. Mater 21, 3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Seliktar D (2012) Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 336, 1124–1128. [DOI] [PubMed] [Google Scholar]
- (4).Ventre M, and Netti PA (2016) Engineering Cell Instructive Materials to Control Cell Fate and Functions through Material Cues and Surface Patterning. ACS Appl Mater. Interfaces 8, 14896–14908. [DOI] [PubMed] [Google Scholar]
- (5).Freudenberg U, Liang Y, Kiick KL, and Werner C (2016) Glycosaminoglycan-Based Biohybrid Hydrogels: A Sweet and Smart Choice for Multifunctional Biomaterials. Adv. Mater 28, 8861–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bishop JR, Schuksz M, and Esko JD (2007) Heparan Sulphate Proteoglycans Fine-tune Mammalian Physiology. Nature 446, 1030–1037. [DOI] [PubMed] [Google Scholar]
- (7).Sarrazin S, Lamanna WC, and Esko JD (2011) Heparan Sulfate Proteoglycans. Cold Spring Harbor Perspect. Biol 3, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Turnbull J, Powell A, and Guimond S (2001) Heparan sulfate: Decoding a Dynamic Multifunctional Cell Regulator. Trends Cell Biol. 11, 75–82. [DOI] [PubMed] [Google Scholar]
- (9).Kamhi E, Joo EJ, Dordick JS, and Linhardt RJ (2013) Glycosaminoglycans in Infectious Disease. Biol. Rev 88, 928–943. [DOI] [PubMed] [Google Scholar]
- (10).García B, Merayo-Lloves J, Martin C, Alcalde I, Quirós LM, and Vazquez F (2016) Surface Proteoglycans as Mediators in Bacterial Pathogens Infections. Front. Microbiol 7, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kraushaar DC, Dalton S, and Wang L (2013) Heparan sulfate: a Key Regulator of Embryonic Stem Cell Fate. Biol. Chem 394, 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Yu C, Griffiths LR, and Haupt LM (2017) Exploiting Heparan Sulfate Proteoglycans in Human Neurogenesis-Controlling Lineage Specification and Fate. Front. Integr. Neurosci 11, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Joung YK, Bae JW, and Park KD (2008) Controlled Release of Heparin-binding Growth Factors using Heparin-containing Particulate Systems for Tissue Regeneration. Expert Opin. Drug Delivery 5, 1173–1184. [DOI] [PubMed] [Google Scholar]
- (14).Nillesen STM, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, and van Kuppevelt TH (2007) Increased Angiogenesis and Blood Vessel Maturation in Acellular Collagen–Heparin Scaffolds containing both FGF2 and VEGF. Biomaterials 28, 1123–1131. [DOI] [PubMed] [Google Scholar]
- (15).Bhakta G, Rai B, Lim ZXH, Hui JH, Stein GS, van Wijnen AJ, Nurcombe V, Prestwich GD, and Cool SM (2012) Hyaluronic Acid-based Hydrogels Functionalized with Heparin that Support Controlled Release of Bioactive BMP-2. Biomaterials 33, 6113–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ayerst BI, Merry CLR, and Day AJ (2017) The Good the Bad and the Ugly of Glycosaminoglycans in Tissue Engineering Applications. Pharmaceuticals 10, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Freudenberg U, Zieris A, Chwalek K, Tsurkan MV, Maitz MF, Atallah P, Levental KR, Eming SA, and Werner C (2015) Heparin Desulfation Modulates VEGF Release and Angiogenesis in Diabetic Wounds. J. Controlled Release 220, 79–88. [DOI] [PubMed] [Google Scholar]
- (18).Murali S, Rai B, Dombrowski C, Lee JLJ, Lim ZXH, Bramono DS, Ling L, Bell T, Hinkley S, Nathan SS, Hui JH, Wong HK, Nurcombe V, and Cool SM (2013) Affinity-Selected Heparan Sulfate for Bone Repair. Biomaterials 34, 5594–5605. [DOI] [PubMed] [Google Scholar]
- (19).Mende M, Bednarek C, Wawryszyn M, Sauter P, Biskup MB, Schepers U, and Bräse S (2016) Chemical Synthesis of Glycosaminoglycans. Chem. Rev 116, 8193–8255. [DOI] [PubMed] [Google Scholar]
- (20).Peterson S, Frick A, and Liu J (2009) Design of Biologically Active Heparan Sulfate and Heparin using an Enzyme-based Approach. Nat. Prod. Rep 26, 610–627. [DOI] [PubMed] [Google Scholar]
- (21).Hu Y-P, Zhong Y-Q, Chen Z-G, Chen C-Y, Shi Z, Zulueta MML, Ku C-C, Lee P-Y, Wang C-C, and Hung S-C (2012) Divergent Synthesis of 48 Heparan Sulfate-Based Disaccharides and Probing the Specific Sugar-Fibroblast Growth Factor-1 Interaction. J. Am. Chem. Soc 134, 20722–20727. [DOI] [PubMed] [Google Scholar]
- (22).Zong C, Venot A, Li X, Lu W, Xiao W, Wilkes J-SL, Salanga CL, Handel TM, Wang L, Wolfert MA, and Boons G-J (2017) Heparan Sulfate Microarray Reveals that Heparan Sulfate-Protein Binding Exhibits Different Ligand Requirements. J. Am. Chem. Soc 139, 9534–9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Yang J, Hsieh P-H, Liu X, Zhou W, Zhang X, Zhao J, Xu Y, Zhang F, Linhardt RJ, and Liu J (2017) Construction and Characterisation of a Heparan Sulphate Heptasaccharide Microarray.Chem. Commun 53, 1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).de Paz JL, Noti C, Böhm F, Werner S, and Seeberger PH (2007) Potentiation of Fibroblast Growth Factor Activity by Synthetic Heparin Oligosaccharide Glycodendrimers. Chem. Biol 14, 879–887. [DOI] [PubMed] [Google Scholar]
- (25).Sheng GJ, Oh YI, Chang S-K, and Hsieh-Wilson LC (2013) Tunable Heparan Sulfate Mimetics for Modulating Chemokine Activity. J. Am. Chem. Soc 135, 10898–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Huang ML, Smith RAA, Trieger GW, and Godula K (2014) Glycocalyx Remodeling with Proteoglycan Mimetics Promotes Neural Specification in Embryonic Stem Cells. J. Am. Chem. Soc 136, 10565–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Loka RS, Yu F, Sletten ET, and Nguyen HM (2017) Design, Synthesis, and Evaluation of Heparan Sulfate Mimicking Glycopolymers for Inhibiting Heparanase Activity. Chem. Commun 53, 9163–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Li Y-C, Ho IH, Ku C-C, Zhong Y-Q, Hu Y-P, Chen Z-G, Chen C-Y, Lin W-C, Zulueta MML, Hung S-C, Lin M-G, Wang C-C, and Hsiao C-D (2014) Interactions that Influence the Binding of Synthetic Heparan Sulfate based Disaccharides to Fibroblast Growth Factor-2. ACS Chem. Biol 9, 1712–1717. [DOI] [PubMed] [Google Scholar]
- (29).Martino G, and Pluchino S (2006) The Therapeutic Potential of Neural Stem Cells. Nat. Rev. Neurosci. 7, 395–406. [DOI] [PubMed] [Google Scholar]
- (30).Sakiyama-Elbert SE (2014) Incorporation of Heparin into Biomaterials. Acta Biomater. 10, 1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Liang Y, and Kiick KL (2014) Heparin-Functionalized Polymeric Biomaterials in Tissue Engineering and Drug Delivery Applications. Acta Biomater. 10, 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zheng J, Smith Callahan LA, Hao J, Guo K, Wesdemiotis C, Weiss RA, and Becker ML (2012) Strain-Promoted Cross-Linking of PEG-Based Hydrogels via Copper-Free Cycloaddition. ACS Macro Lett. 1, 1071–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Arungundram S, Al-Mafraji K, Asong J, Leach FE, Amster IJ, Venot A, Turnbull JE, and Boons G-J (2009) Modular Synthesis of Heparan Sulfate Oligosaccharides for Structure-Activity Relationship Studies. J. Am. Chem. Soc 131, 17394–17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Song H-O, Lee B, Bhusal RP, Park B, Yu K, Chong C-K, Cho P, Kim SY, Kim HS, and Park H (2012) Development of a Novel Fluorophore for Real-Time Biomonitoring System. PLoS One 7, No. e48459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Caldwell MA, Garcion E, Ter Borg MG, He X, and Svendsen CN (2004) Heparin Stabilizes FGF-2 and Modulates Striatal Precursor Cell Behavior in Response to EGF. Exp. Neurol 188, 408–420. [DOI] [PubMed] [Google Scholar]
- (36).Chen G, Gulbranson DR, Yu P, Hou Z, and Thomson JA (2012) Thermal Stability of Fibroblast Growth Factor Protein Is a Determinant Factor in Regulating Self-Renewal, Differentiation, and Reprogramming in Human Pluripotent Stem Cells. Stem Cells 30, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Gospodarowicz D, and Cheng J (1986) Heparin Protects Basic and Acidic FGF from Inactivation. J. Cell. Physiol 128, 475–484. [DOI] [PubMed] [Google Scholar]
- (38).Habuchi H, Suzuki S, Saito T, Tamura T, Harada T, Yoshida K, and Kimata K (1992) Structure of a Heparan Sulphate Oligosaccharide that Binds to Basic Fibroblast Growth Factor. Biochem. J 285, 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Damon DH, Lobb RR, D’Amore PA, and Wagner JA (1989) Heparin Potentiates the Action of Acidic Fibroblast Growth Factor by Prolonging its Biological Half-life. J. Cell. Physiol 138, 221–226. [DOI] [PubMed] [Google Scholar]
- (40).Damon DH, Halegoua S, D’Amore P, and Wagner JA (1992) Rapid Fibroblast Growth Factor-induced Increases in Protein Phosphorylation and Ornithine Decarboxylase Activity: Regulation by Heparin and Comparison to Nerve Growth Factor-induced Increases. Exp. Cell Res 201, 154–159. [DOI] [PubMed] [Google Scholar]
- (41).Karumbaiah L, Enam SF, Brown AC, Saxena T, Betancur MI, Barker TH, and Bellamkonda RV (2015) Chondroitin Sulfate Glycosaminoglycan Hydrogels Create Endogenous Niches for Neural Stem Cells. Bioconjugate Chem. 26, 2336–2349. [DOI] [PubMed] [Google Scholar]
- (42).Kriegstein A, and Alvarez-Buylla A (2009) The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu. Rev. Neurosci 32, 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Liu X, Holzwarth JM, and Ma PX (2012) Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci 12, 911–919. [DOI] [PubMed] [Google Scholar]
- (44).Streuli CH (2009) Integrins and Cell-fate Determination. J. Cell Sci 122, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Huettner N, Dargaville TR, and Forget A (2018) Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 36, 372–383. [DOI] [PubMed] [Google Scholar]
- (46).Ayerst BI, Smith RAA, Nurcombe V, Day AJ, Merry CLR, and Cool SM (2017) Growth Differentiation Factor 5-Mediated Enhancement of Chondrocyte Phenotype Is Inhibited by Heparin: Implications for the Use of Heparin in the Clinic and in Tissue Engineering Applications. Tissue Eng. Part A 23, 275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Blossom DB, Kallen AJ, Patel PR, Elward A, Robinson L, Gao G, Langer R, Perkins KM, Jaeger JL, Kurkjian KM, Jones M, Schillie SF, Shehab N, Ketterer D, Venkataraman G, Kishimoto TK, Shriver Z, McMahon AW, Austen KF, Kozlowski S, Srinivasan A, Turabelidze G, Gould CV, Arduino MJ, and Sasisekharan R (2008) Outbreak of Adverse Reactions Associated with Contaminated Heparin. N. Engl. J. Med 359, 2674–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al- Hakim A, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, and Sasisekharan R (2008) Oversulfated Chondroitin Sulfate is a Contaminant inHeparin Associated with Adverse Clinical Events. Nat. Biotechnol 26, 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ning X, Guo J, Wolfert MA, and Boons G-J (2008) Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Free and Fast Huisgen Cycloadditions. Angew. Chem., Int. Ed 47, 2253–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Mammen M, Choi S-K, and Whitesides GM (1998) Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem., Int. Ed 37, 2754–2794. [DOI] [PubMed] [Google Scholar]
- (51).Betancur MI, Mason HD, Alvarado-Velez M, Holmes PV, Bellamkonda RV, and Karumbaiah L (2017) Chondroitin Sulfate Glycosaminoglycan Matrices Promote Neural Stem Cell Maintenance and Neuroprotection Post-Traumatic Brain Injury. ACS Biomater. Sci. Eng 3, 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.