Abstract
Background
In heart failure with reduced left ventricular ejection fraction (HFrEF) patients the effects of exercise-based cardiac rehabilitation on top of state-of-the-art pharmacological and device therapy on mortality, hospitalization, exercise capacity and quality-of-life are not well established.
Design
The design of this study involved a structured review and meta-analysis.
Methods
Evaluation of randomised controlled trials of exercise-based cardiac rehabilitation in HFrEF-patients with left ventricular ejection fraction ≤40% of any aetiology with a follow-up of ≥6 months published in 1999 or later.
Results
Out of 12,229 abstracts, 25 randomised controlled trials including 4481 HFrEF-patients were included in the final evaluation. Heterogeneity in study population, study design and exercise-based cardiac rehabilitation-intervention was evident. No significant difference in the effect of exercise-based cardiac rehabilitation on mortality compared to control-group was found (hazard ratio 0.75, 95% confidence interval 0.39–1.41, four studies; 12-months follow-up: relative risk 1.29, 95% confidence interval 0.66–2.49, eight studies; six-months follow-up: relative risk 0.91, 95% confidence interval 0.26–3.16, seven studies). In addition there was no significant difference between the groups with respect to ‘hospitalization-for-any-reason’ (12-months follow-up: relative risk 0.79, 95% confidence interval 0.41–1.53, four studies), or ‘hospitalization-due-to-heart-failure’ (12-months follow-up: relative risk 0.59, 95% confidence interval 0.12–2.91, four studies; six-months follow-up: relative risk 0.84, 95% confidence interval 0.07–9.71, three studies). All studies show improvement of exercise capacity. Participation in exercise-based cardiac rehabilitation significantly improved quality-of-life as evaluated with the Kansas City Cardiomyopathy Questionnaire: (six-months follow-up: mean difference 1.94, 95% confidence interval 0.35–3.56, two studies), but no significant results emerged for quality-of-life measured by the Minnesota Living with Heart Failure Questionnaire (nine-months or more follow-up: mean difference –4.19, 95% confidence interval –10.51–2.12, seven studies; six-months follow-up: mean difference –5.97, 95% confidence interval –16.17–4.23, four studies).
Conclusion
No association between exercise-based cardiac rehabilitation and mortality or hospitalisation could be observed in HFrEF patients but exercise-based cardiac rehabilitation is likely to improve exercise capacity and quality of life.
Keywords: Meta-analysis, systematic review, heart failure, exercise training, cardiac rehabilitation
Introduction
Chronic heart failure with reduced left ventricular ejection fraction (HFrEF) remains a major health problem with increasing prevalence.1,2 Within the next 25 years, an increase in heart-failure-related hospital admissions by 50% is expected, mainly due to the aging population.3 Today, a broad spectrum of therapeutic options is available for HFrEF patients including state-of-the-art medications, device therapy (e.g. implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT)), interventional or surgical repair of mitral valve regurgitation and, finally, implantation of ventricular assist devices or heart transplantation.1,2 In spite of the therapeutic progress, the prognosis of HFrEF patients remains poor.4 During an average follow-up of 47 months, a mortality of 32% has been reported in patients with left ventricular ejection fraction (LV-EF) of 35–50%, increasing to 41% in patients with LV-EF below 35%.5 In addition, HFrEF is characterised by significant exercise intolerance caused primarily by skeletal muscle atrophy and dysfunction, which limits daily living activities and reduces health-related quality of life (QoL).1,2,6 Moreover, the prevalence of frailty in HFrEF patients is high (15–74%) and known to be associated with disability, poor QoL, and outcome and/or higher hospital admittance rates.7 Following proof-of-concept studies by Coats et al.8 and Hambrecht et al.9 which documented the efficacy and safety, respectively, of exercised-based cardiac rehabilitation (ebCR), multiple studies documented its potential to improve functional capacity, QoL and prognosis in patients with heart failure.10–17 In these studies, however, the populations under investigation were heterogeneous with respect to LV-EF (<35% up to >50%), New York Heart Association (NYHA) classification, and follow-up period. However, the effect of ebCR on morbidity and mortality on top of evidence-based state-of-the-art pharmaco- and device-therapy in high-risk patients with moderately to severely reduced LV-EF is not yet fully established.10–17 Although current guidelines recommend ebCR as an effective and safe therapeutic intervention,1,2,6 HFrEF patients are still clearly underrepresented in CR settings worldwide.
Hence, the aim of this systematic review and meta-analysis was to evaluate whether or not ebCR is effective in reducing all-cause-mortality and hospitalization and improving exercise capacity (peak oxygen uptake (VO2peak)) and QoL in HFrEF-patients with reduced LV-EF ≤40% on guideline-recommended pharmaco- and device-therapy.
Methods
This systematic review was conducted and reported according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA).18 The study protocol has been published previously in PROSPERO (CRD42017054833).
Study eligibility criteria
The study selection criteria (population, intervention, control, outcomes, and study designs) are outlined in detail in Table 1.
Table 1.
The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): inclusion criteria.
|
Population:
| |
| Age: ≥18 years. Origin and presentation heart failure: patients with chronic heart failure of any aetiology with either reduced left ventricular ejection fraction (HFrEF; left ventricular ejection fraction (LVEF)≤40% or preserved left ventricular ejection fraction (HFpEF). Baseline therapeutic requirements: included studies must have been performed during the era of chronic heart failure treatment including at least beta-blockers in combination with either angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs) and/or aldosterone antagonist. Patients must not have previously received exercise-based cardiac rehabilitation. | |
|
Intervention:
| |
| (a) Any form of structured and supervised exercise intervention (i.e. aerobic exercise training or resistance training or endurance-resistance training) alone or in combination with a comprehensive rehabilitation intervention (e.g. including information, education, motivation, psychological interventions etc.). (b) The exercise programme should meet the following minimal requirements: – Exercise duration per week: ≥90 min – Duration of the exercise programme: ≥3 weeks – Exercise frequency: at least two training sessions per week (c) These requirements must clearly be documented in the manuscript or by a written confirmation of the authors. (d) Patients may be supervised by general practitioners and/or resident cardiologists following the local medical conventions. | |
|
Control:
| |
| (a) Patients in the control group should not participate in any exercise-based rehabilitation programme or any form of structured exercise intervention. Neither in a cardiac rehabilitation centre nor at home. (b) Patients may be supervised by general practitioners and/or resident cardiologists following the local medical conventions. (c) Control group – patients may participate in unstructured and unspecified prevention programmes. | |
|
Reported follow up:
| |
| The follow-up period must be six months or longer after randomization. | |
|
Accepted study designs:
| |
| (a) Randomised controlled trials (RCTs). (b) Controlled non-randomised studies, if adjusted for baseline characteristics, and addressing risk of bias by adequate statistical methods. | |
|
Outcomes: clinical course after the cardiac
rehabilitation
| |
| Primary outcome Secondary outcomes | All-cause mortality. (a) Cardiovascular mortality. (b) Hospitalization of any course. (c) Hospitalization due to worsening heart failure. (d) Combined endpoint of mortality and hospitalization of any cause. (e) Cardiopulmonary exercise capacity (peak oxygen uptake (VO2peak)). (f) Quality of life (Short-Form-36-Questionnaire [SF36], Minnesota Living With Heart Failure Questionnaire or other validated questionnaires). |
| Publication year: | |
| Studies published in 1999 or later. | |
To ascertain guideline-recommended heart failure treatment (e.g. medical treatment including beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, aldosterone receptor blockers, and devices (ICD, CRT, etc.)) only studies published in 1999 or later were included in this systematic review.
All-cause-mortality was defined as the primary endpoint. Predefined secondary endpoints were cardiovascular mortality, hospitalizations, cardiopulmonary exercise capacity and QoL, as outlined in Table 1.
Data sources, search strategies and identification of studies
A graduated information scientist Master of Library and Information Science [MIM] (MG) developed highly sensitive search strategies.
Two systematic literature searches were performed, the first finished on 10 September 2015, the second on 12 February 2019. The following databases have been used: PubMed (via National Center for Biotechnology Information [NCBI]-Platform); Cochrane Database of Systematic Reviews (CDSR); Database of Abstracts of Reviews of Effects (DARE); Cochrane Central Register of Controlled Trials (CENTRAL); Cochrane Methodology Register (CMR); Health Technology Assessment Database (HTA); Economic Evaluation Database (EED); all via Cochrane library; Web of Science Core Collection; CINAHL and PsychInfo (via EBSCO-HOST); Current Contents Medicine CCMED (German database via LIVIVO); ClinicalTrials.gov. For the second structured search, the following databases were used: PubMed, Web of Science Core Collection, CINAHL, PsychInfo. The structured search was supplemented by manual searches using reference lists of other reviews and key literature.10–13 Details of all search strategies are documented in the supplementary material (Supplementary Material Table SM1).
Study selection
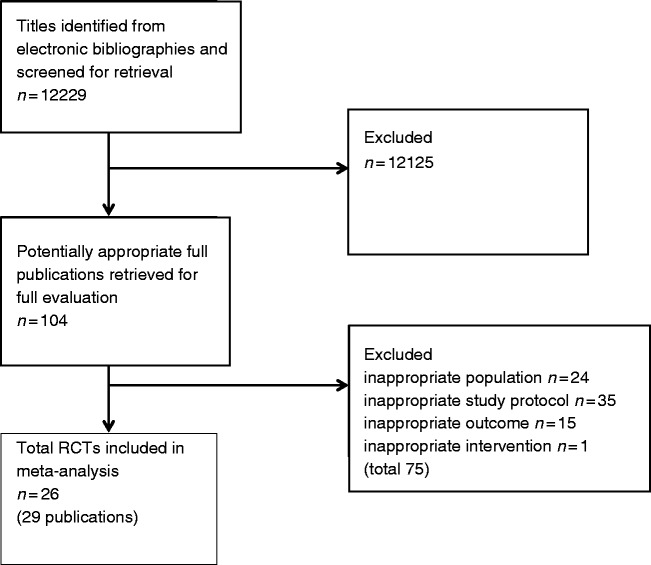
The study selection process is outlined in Figure 1. The EndNote X7 system was used by all study participants for literature management. Two independent expert reviewers (RN, BBW) selected the studies according to the predefined Population-Intervention-Control-Outcome (PICO) criteria (Table 1.) Each single reference was evaluated independently by the two reviewers according to a predefined scheme. The result of this primary selection was forwarded to the Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF) steering committee (BR, SG, MH, KJ) for internal control. Full text articles were assessed for final decision of eligibility. Studies were finally selected based on a consensus between the two expert reviewers and the steering committee (RN, BBW, BR, SG, MH, KJ). In case of disagreement, the decision of the majority was approved.
Figure 1.
A summary of study inclusion/exclusion process.
Study evaluation and risk of bias assessment
The literature search yielded no controlled cohort study that fulfilled the study selection criteria. Hence, this systematic review and meta-analysis includes randomised controlled trials (RCTs) only (Figure 1). The risk of bias was measured using the Cochrane Collaboration’s tool for assessing risk of bias.19 Additionally, imbalances regarding the baseline characteristics of the study groups were evaluated as a further criterion for assessing the risk of bias.20
Data extraction
A standardised data extraction form was used to individually extract data from full-text copies of included trials by two biometricians (MH, KJ). The following data were extracted: name of first author, year of publication, study location (country), study design; population: data sources, sample size, index events, other inclusion criteria and characteristics, inclusion period, mean age and gender proportion; intervention: number of participants; type of intervention (exercise only CR (CR-ex) or multikomponent CR (CR-mult) and aerobic intervention or aerobic plus resistance intervention); duration of single exercise and/or total number or CR sessions, number of CR exercise sessions per week, exercise mode and CR setting. Control: number of participants, treatment; outcome: follow-up-period, outcomes according to the CROS-HF protocol, instruments of measurement for QoL; overall results with respect to CROS-HF-protocol endpoints (Table 2).9,21–47 The primary reasons for study exclusion at the PICO selection level are given in Supplementary Material Table SM2.
Table 2.
Detailed characteristic of all integrated trials.
| Study, year, country | Study design | Population: (a) Data sources (b) Number of included participants (n) (c) Index events (d) Inclusion period (e) other inclusion criteria and characteristics (f) Age (yrs, mean±sd or as stated) (g) Gender (male %) | Intervention: (a) Number (n) (b) CR including exercise only (CR-ex) or multicomponent CR including exercise (CR-mult) (c) Duration (time period and /or total number or CR sessions) (d) Frequency (CR exercise sessions per week) (e) Exercise mode (f) CR setting | Control: (a) Number (n) (b) Treatment | Outcome: (a) Follow-up period (b) Outcome according to CROS_HF -protocol (number according to Table 1) (c) Other outcome | Overall results with respect to CROS-HF-protocol EPs: EP 1: All-cause-mortality EP 2: CV-mortality EP 3: Hospitalization EP 4: Hospitalization due to HF EP 5: Composite endpoint of mortality and hospitalization EP 6: Peak VO2 EP 7: Quality of life | Remarks |
| Belardinelli et al., 1999, Italy21 | RCT | (a) Institutional (b) n=99 (c) HFrEF, LV-EF≤40% (d) NSp (e) Clinically stable ≥3 months (f) 59 ± 14 yrs (g) 88% male | (a) n = 50 (b) CR-ex(c) 14 months (d) 8 wks, 3 sessions/wk, 60 min, one year 2 sessions/wk 60 min (e) Aerobic exercise; 60% peak VO2 (f) Out-patient | (a) n = 49 (b) UC | (a) 14 months (b) 1, 2, 4, 6, 7 (c) Thallium activity score, echo-cardio-graphic results | EP 1: HR 0.37, 95% CI (0.16–0.84) EP 2: Events/patient at risk after 54 months: CR-ex: 9/50 vs UC: 20/49 All deaths were cardiac in nature (see EP 1) EP 4: HR 0.29, 95% CI (0.11–0.84) EP 6: After 12–14 months MD 3.90, 95% CI (3.24–4.56) EP 7: MLWHF 9+ months MD –10, 95% CI (–18.98– –1.02) | |
| Keteyian et al., 1999, USA22 | RCT | (a) Institutional (b) n = 51 (c) HFrEF, LV-EF≤35%, (d) NSp (e) NYHA class II–III, optimal medical therapy≥30 day (f) CR-ex: 57 ± 12 vs UC: 55 ± 12 yrs (g) 100% male | (a) n = 26 (b) CR-ex (c) 6 months (d) 3 sessions/wk, 33 min (e) Aerobic exercise; 50–80% HRR (f) Out-patient | (a) n = 25 (b) UC | (a) 6 months (b) 1, 6, 7 (c) Plasma norepinephrine | EP 1: Events/patient at risk after 6 months: CR-ex: 0/21 vs UC: 1/23 EP 6: After 6 months MD 3.10, 95% CI (0.62–5.58) EP 7: MLWHF up to 6 months MD 5.00, 95% CI (–8.45–18.45) | |
| Myers et al., 2000, Switzerland23 | RCT | (a) Institutional (b) n = 25 (c) HFrEF, LV-EF<40% (d) NSp (e) Clinically stable (f) CR-mult:_ 56 ± 5 vs UC: 55 ± 7 yrs (g) 100% male | (a) n = 12; (b) CR-mult (c) 2 months (d) 2 sessions/day, 60 min walking + 4 sessions/wk 45 min cycle ergometer (e) Aerobic exercise; 60–70% peak VO2 (f) In-patient | (a) n = 13 (b) UC | (a) 12 months (b) 1, 2, 6 (c) Echocardiographic results, physical activity | EP 1: Events/patient at risk after 12 months: CR-mult: 1/12 vs UC: 1/13 EP 2: Events/patient at risk after 12 months: CR-mult: 1/12 vs UC: 0/13 EP 4: Events/patient at risk after 12 months: CR-mult: 0/12 vs UC: 2/13 EP 6: After 12–14 months MD 4.70, 95% CI (0.46–8.94) | |
| Hambrecht et al., 2000, Germany9 | RCT | (a) Institutional (b) n = 73 (c) HFrEF, LV-EF<40% (d) 1994–1999 (e) Aged 70 yrs, PWC>25 watt, clinically stable ≥ 3 months (f) CR-ex: 54 ± 9 vs UC: 55 ± 8 yrs (g) 100% male | (a) n = 36 (b) CR-ex (c) 6 months (d) 2 wks, 4–6 sessions/day, 10 min; 6 months, one session/day 20 min. (e) Aerobic exercise; 70% peak VO2 (f) 2 wks in-hospital, 6 months out-patient/home-based | (a) n = 37 (b) UC | (a) 6 months (b) 1, 4, 6 (c) Echocardiographic results, endothelial function | EP 1: Events/patient at risk after 6 months: CR-ex: 3/34 vs UC: 2/35 EP 2: Events/patient at risk after 6 months: CR-ex: 3/34 vs UC: 2/35 EP 4: Events/patient at risk after 6 months: CR-ex: 2/34 vs UC: 2/35 EP 6: After 6 months MD 4.90, 95% CI (2.73–7.07) | |
| McKelvie et al., 2002, Canada24 | RCT | (a) EXERT (b) n = 181 (c) HFrEF, LV-EF<40% (d) NSp (e) NYHA Class II–III, 6-MWD<500 m, (f) CR-ex: 64.8 ± 1.1 vs UC: 66.1 ± 1 yrs (g) 89.5% male | (a) n = 90 (b) CR-ex (c) 3 months supervised and (d) 9 months home-based (e) 2 sessions/wk, 45–60 min (f) Aerobic exercise; 60–70% HRmax, resistance training 60% 1RM (g) 3 months out-patient, 9 months home-based | (a) n = 91 (b) UC | (a) 12 months (b) 1, 5, 6, 7 (c) adherence to exercise, 6-MWD, dynamic muscle strength, rest radionuclide ventriculography | EP 1: Events/patient at risk after 12 months: CR-ex: 9/73 vs UC: 8/83 EP 7: MLWHF 9+ months MD –0.10, 95% CI (–5.86–5.66) | |
| Giannuzzi et al., 2003, Italy25 | RCT | (a) ELVD-CHF-Trial (b) n = 90 (c) HFrEF, LV-EF<35% (d) NSp (e) NYHA class II or III, peak VO2<20 ml/kg/min, clinical stability at ≥ 3 months, optimised therapy (f) CR-ex: 60 ± 7 vs UC: 61 ± 7 yrs (g) NSp | (a) n = 45 (b) CR-ex (c) 6 months (d) Supervised 3 sessions/wk, 30 min, daily 60 min walking and calisthenics home-based (e) Aerobic exercise; 60% peak VO2 (f) Out-patient and home-based | (a) n = 45 (b) UC | (a) 6 months (b) 1, 4, 6, 7 (c) Echocardiographic results, 6-MWD | EP 1: Events/patient at risk after 6 months: CR-ex: 0/45 vs UC: 1/45 EP 2: Events/patient at risk after 6 months: CR-ex: 0/45 vs UC: 1/45 EP 4: Events/patient at risk after 6 months: CR-ex: 2/45 vs UC: 1/45 EP 6: After 6 months MD 2.50, 95% CI (1.26–3.74) | |
| Koukouvou et al., 2004, Greece26 | RCT | (a) Institutional (b) n = 29 (c) HFrEF, LV-EF<40% (d) NSp (e) NYHA class II or III (f) 52.5 ± 9.8 yrs (g) 100% male | (a) n = 16; (b) CR-ex (c) 6 months (d) 3–4 sessions/wk, 60 min (e) Aerobic exercise; 50–70% peak VO2, calisthenics, resistance training (f) Out-patient | (a) n = 10, (b) UC | (a) 6 months (b) 6, 7 (c) EPQ, BDI, HADS | EP 6: After 6 months MD 7.50, 95% CI (3.70–11.30) EP 7: MLWHF up to 6 months MD –11.10, 95% CI (–19.57– –2.63) | |
| Sabelis et al., 2004, the Netherlands27 | RCT | (a) Institutional (b) n = 29 (c) HFrEF, LV-EF<35% (d) NSp (e) NYHA class II or III, history of CHF >6 months (f) 60 ± 8 yrs (g) 100% male | (a) n = 16 (b) CR-ex (c) 6 months (d) 2 sessions/wk a 60 min supervised and 2 sessions/wk home-based (e) Aerobic IT (30/60 s); 50% of max. short-term exercise capacity, resistance training (f) Out-patient and home-based | (a) n = 13 (b) UC | (a) 6 months (b) 6 (c) Endothelial marker | EP 1: Events/patient at risk after 6 months: CR-ex: 0/16 vs UC: 0/13 EP 2: Events/patient at risk after 6 months: CR-ex: 0/16 vs UC: 0/13 EP 6: After 6 months MD 2.80, 95% CI (–0.70–6.30) | |
| Austin et al., 2005, UK28 | RCT | (a) Institutional (b) n = 200 (c) HFrEF, LV-EF < 40% (d) NSp (e) Age > 60 yrs, NYHA class II or III (f) CR-mult: 71.9 ± 6.3 vs UC: 71.8 ± 6.8 yrs (g) 66% male | (a) n = 100 (b) CR-mult (c) 8 wks CR, 16 wks community based (d) CR: 2 sessions/wk aerobic exercise, resistance training; (e) Community based 1 session/wk, 60 min; according to HF-guidelines (f) Out-patient; community based | (a) n = 100 (b) UC | (a) 6 months (b) 1, 3, 7 (c) 6-MWD, MLSQ | EP 1: Events/patient at risk after 6 months: CR-ex: 3/88 vs UC: 4/98 EP 4: Events/patient at risk after 6 months: CR-ex: 9/85 vs UC: 19/94 EP 7: MLWHF 9 + months MD –1.60, 95% CI (–10.26–7.06) | |
| Austin et al., 2008, UK29 | (a) 60 months (b) 1, 3, 7 | Long-term results of Austin et al., 200528 | |||||
| Klocek et al., 2005, Poland30 | RCT | (a) Institutional (b) n = 42 (c) HFrEF, LV-EF<40% (d) 1999–2002 (e) Age<65 yrs (f) 55.9 ± 8.1 yrs (g) 100% male | (a) n = 28; (A n = 14; B n = 14) (b) CR-ex (c) 6 months (d) 3 sessions/wk, 45 min (e) Group A: IT, 4 min, 60% HRmax and 1 min rest; Group B: progressive constant level aerobic exercise, 75% HRmax (f) Out-patient | (a) n = 14 (b) UC | (a) 6 months (b) 6 (c) Echocardiographic results PGWB; SSA-p | EP 6: After 6 months MD 3.70, 95% CI (–0.79–8.19) | |
| de Mello Franco et al., 2006, USA31 | RCT | (a) Institutional (b) n = 29 (c) HFrEF, LV-EF<40% (d) NSp (e) Aged 35–60, NYHA class II–III (f) 54.9 ± 1.9 yrs (g) 75.9% male | (a) n = 17 (b) CR-ex (c) 4 months supervised and 4 months home-based (d) 3 sessions/wk 60 min (e) 25–40 min aerobic exercise, resistance training, NSp (f) Out-patient and home-based | (a) n = 12 (b) UC | (a) 8 months (b) 6, 7 (c) FBF, muscle sympathetic nerve activity | EP 6: After 6 months MD 0.90, 95% CI (–2.44–4.24) EP 7: MLWHF up to 6 months MD –3.00, 95% CI (–18.31–12.31) | Group comparison only available for 4 months results. |
| Passino et al., 2006, Italy32 | RCT | (a) Institutional (b) n = 95 (c) HFrEF, LV-EF<40% (d) 2002–2004 (e) VO2 peak<25 ml/min/kg, clinically stable >1 month, optimal therapy (f) 60 ± 1 yrs (g) 84.2% male | (a) n = 47 (b) CR-ex (c) 9 months (d) 3 sessions/wk, 30 min (e) Aerobic exercise, 65% VO2 peak (f) Out-patient | (a) n = 12 (b) UC | (a) 9 months (b) 6, 7 (c) Echocardiographic results, BNP/NT-proBNP, neuro-hormones | EP 6: After 12–14 months MD 4.00, 95% CI (1.23–6.77) EP 7: MLWHF 9+ months MD –21.00, 95% CI (–33.55– –8.45) | |
| Mueller et al., 2007, Switzerland33 | RCT | (a) Institutional (b) n = 50 (c) HFrEF, LV-EF<40% (d) NSp (e) clinically stable >1 month (f) 55.0 ± 10 yrs (g) 100% male | (a) n = 25 (b) CR-mult (c) 1 month (d) 5 sessions/wk, 30 min; 2 sessions/day 45 min (e) Aerobic exercise, 60–80% HRR (f) In-patient | (a) n = 25 (b) UC | (a) 74 months (b) 1, 4, 6 (c) physical activity | EP 1: HR 0.8, 95% CI (0.34–1.92) EP 4: Events/patient at risk after 30+ months: CR-mult: 8/23 vs UC: 5/25 EP 6: After 6 months MD 4.20, 95% CI (1.75–6.65) | HR extracted with Tierney-tool (method 7) |
| Klecha et al., 2007, Poland34 | RCT | (a) Institutional (b) n = 50 (c) HFrEF, LV-EF < 35% (d) NSp (e) NYHA class II, III, clinically stable >6 weeks on unchanged treatment (f) 60.1 ± 9.2 yrs(g) 76% male | (a) n = 25 (b) CR-ex (c) 6 months (d) 3 sessions/wk 60 min (e) 25 min aerobic exercise, 80% HRmax, relaxation (f) Out-patient | (a) n = 25 (b) UC | (a) 6 months (b) 6 (c) MRI left ventricular evolution | EP 1: Events/patient at risk after 6 months: CR-ex: 0/25 vs UC: 0/25 EP 2: Events/patient at risk after 6 months: CR-ex: 0/25 vs UC: 0/25 EP 6: After 6 months MD 5.10, 95% CI (3.32–6.88) | |
| Dracup et al., 2007, USA35 | RCT | (a) Institutional (b) n = 173 (c) HFrEF, LV-EF < 40% (d) NSp (e) Aged 18–80 yrs NYHA class II–IV (f) 54 ± 12.5 yrs (g) 71.7% male | (a) n = 86 (b) CR-ex (c) 6 months (d) 4 sessions/wk 45 min (e) Aerobic exercise, 60% HRmax, resistance training 80% 1RM (f) Home-based | (a) n = 87 (b) UC | (a) 6 and 12 months (b) 6 months 6, 7; 12 months 3, 5 (c) 6-MWD, MAACL | EP 1: Events/patient at risk after 12 months: CR-ex: 9/87 vs UC: 8/86 EP 3: Events/patient at risk after 12 months: CR-ex: 35/87 vs UC: 37/86 EP 5: Events/patient at risk after 12 months: CR-ex: 37/87 vs UC: 38/86 EP 6: After 6 months MD 0.40, 95% CI (–0.81–1.61) EP 7: MLWHF up to 6 months MD –7.50, 95% CI (–15.12–0.12) | Combined endpoint for cardiovascular events |
| Nilson et al., 2008, Norway36 | RCT | (a) Institutional (b) n = 80 (c) HFrEF, LV-EF<40%, or ≥40 with clinical symptoms of HFpEF (d) NSp (e) Aged 18–80 yrs, NYHA class II–IIIB (f) 70.1 ± 7.9 yrs(g) 71.7% male | (a) n = 40 (b) CR-ex (c) 4 months (d) 2 sessions/wk, 50 min (e) Aerobic exercise, 60% HRmax, resistance training (f) Out-patient | (a) n = 40 (b) UC | (a) 6 and 12 months; (b) 1, 7 (c) 6-MWD | EP 1: Events/patient at risk after 6 months: CR-ex: 2/37 vs UC: 1/38 EP 7: MLWHF up to 6 months MD –5.00, 95% CI (12.57–2.57) | |
| O'Connor et al., 2009, USA37 | RCT | (a) HF-ACTION trial (b) n = 2331 (c) HFrEF, LV-EF≤35% (d) 2003–2007 (e) NYHA class II to IV, >6 wks optimal HF-treatment (f) 59 yrs (g) 72% male | (a) n = 1159 (b) CR-ex (c) Supervised 3 months; home-based 9–10.5 months (d) 3 sessions/wk 15–35 min; 5 sessions/wk, 40 min (e) Aerobic exercise, 60–70% HRR (f) Out-patient, home-based | (a) n = 1172 (b) UC | (a) Median 30 months (b) 1, 2, 5, 6 | EP 1: HR 0.96, 95% CI (0.79–1.17) EP 2: HR 0.92, 95% CI (0.74–1.15) EP 3: Events/patient at risk after 30 months: CR-ex: 729/1159 vs UC: 760/1171 EP 5: HR 0.93, 95% CI (0.85–1.02) EP 6: After 12–14 months MD 0.60, 95% CI (0.34–0.86) EP 7: KCCQ up to 6 months MD 1.93, 95% CI (0.85–3.01) | Combined endpoint for cardiovascular events Sample sizes for peak oxygen consumption was derived by overall sample size divided by 2. |
| Flynn et al., 2009, USA38 | RCT | (a) HF-ACTION trial (see O'Connor et al., 2009)37 | (a) 3, 6, 9, 12, months then annually for 4 yrs, median 2.5 yrs (b) 7 | HF-ACTION trial (see O'Connor et al., 2009)37 | |||
| Jolly et al., 2009, UK39 | RCT | (a) BRUM-CHF-study (b) n = 169 (c) HFrEF, LV-EF≤40% (d) 2004–2005 (e) ≥NYHA class II, >4 wks clinically stable with optimal therapy (f) CR-ex: 65.9 ± 12.5 vs UC: 70 ± 12.5 yrs (g) 74.6% male | (a) n = 84; (b) CR-ex (c) 3 supervised sessions (d) 6 months home-based with telephone support (e) 5 sessions/wk, 20–30 min (f) Aerobic exercise, 70% VO2 peak, resistance training (g) Home-based | (a) n = 85 (b) UC | (a) 12 months (b) 1, 3, 4, 5, 7 (c) EQ-5D-5L, HADS, physical activity score, ISWT | EP 1: Events/patient at risk after 12 months: CR-ex: 7/84 vs UC: 5/85 EP 3: Events/patient at risk after 12 months: CR-ex: 16/84 vs UC: 20/85 EP 4: Events/patient at risk after 12 months: CR-ex: 4/84 vs UC: 2/85 EP 5: HR 1.45, 95% CI (0.43–4.86) EP 7: MLWHF 9+ months MD 2.76, 95% CI (–4.47–9.87) | Combined endpoint for cardiovascular events |
| Belardinelli et al., 2012, Italy40 | RCT | (a) Institutional (b) n = 123 (c) HFrEF, LV-EF≤40% (d) NSp (e) ≥3 months clinically stable (f) 59 ± 14 yrs (g) 78% male | (a) n = 63 (b) CR-ex (c) 120 months (d) 3 months 3 session/wk; 117 months 2 sessions/wk, 60 min (e) Aerobic exercise, 70% VO2 peak (f) Out-patient | (a) n = 60 (b) UC | (a) 120 months, evaluation every 12 months (b) 1, 2, 3, 6, 7 (c) Echocardiographic results | EP 1: HR 0.68, 95% CI (0.30–1.54) EP 2: Events/patient at risk after 120 months: CR-ex: 4/63 vs UC: 10/60 All death were cardiac nature (see EP 1) EP 3: HR 0.64, 95% CI (0.34–0.81) EP 4: Events/patient at risk after 30+ months: CR-ex: 8/63 vs UC: 10/60 EP 6: after 12–14 months MD 3.40, 95% CI (1.15–5.65) | |
| Norman et al., 2012, Netherlands41 | RCT | (a) Institutional (b) n = 42 (c) HFrEF, LV-EF≤40% (d) NSp (e) Aged≥21 yrs, NYHA class II–IV, >30 days clinically stable with optimal therapy (f) CR-ex: 56.0 ± 2.7 vs UC: 63.0 ± 3.4 yrs (g) 54.8% male | (a) n = 22; (b) CR-ex (c) 3 wk (d) 3 wk, 3 sessions/wk, 60 min, (e) Aerobic exercise, 40–60% HRR, resistance training (f) Out-patient | (a) n = 20 (b) UC (Attention-UC) | (a) 6 months (b) 7 (c) BNP, 6-MWD | EP 1: Events/patient at risk after 6 months: CR-ex: 1/21 vs UC: 0/20 EP 2: Events/patient at risk after 6 months: CR-ex: 0/21 vs UC: 0/20 EP 7: KCCQ up to 6 months MD 3.10.95% CI (–6.68–12.88) | |
| de Meirelles et al., 2014, Brasil42 | RCT | (a) Institutional (b) n = 30 (c) HFrEF, LV-EF≤40% (d) NSp (e) ≥3 months clinically stable, exercise capacity ≥3 METs (f) CR ex 54 ± 3 vs UC 55 ± 2 yrs (g) 46.7% male | (a) n = 15, (b) CR-ex (c) 6 months (d) 3 sessions/wk, >30 min (e) 30 min aerobic exercise, 15% >VAT, resistance training 3 sets 10–15 reps (f) Out-patient | (a) n = 15 (b) UC | (a) 6 months (b) 6 (c) Inflammatory markers, biomarkers of oxidative stress platelet aggregation | EP 6: After 6 months MD 5.50, 95% CI (4.93–6.07) | |
| Dehkordi et al., 2015, Iran43 | RCT | (a) Institutional (b) n = 66 (c) HFrEF, LV-EF ≤ 40% (d) NSp (e) Optimal therapy (f) 65 ± 15 yrs (g) 62% male | (a) n = 33 (b) CR-ex (c) 6 months (d) 3 sessions/wk, 40 min (e) Aerobic, walking 70% HRR (f) Out-patient | (a) n = 33 (b) UC with educational support | (a) 6 months (b) 7 (c) Echocardiographic results | EP 7: After 6 months CR-ex: 61.01 ± 14.9 to 63.34 ± 12.69 vs UC: 62.34 ± 11.25 to 58.43 ± 8.67 | MacNew Heart Disease Health-related Quality of Life questionnaire |
| Höllriegel R et al., 2016, Germany44 | RCT | (a) Institutional (b) n = 37 (c) HFrEF, LV-EF<30% (d) NSp (e) NYHA class IIIb, clinically stable >2 months, optimal therapy (f) 60 ± 3 yrs (g) 100% male | (a) n = 18 (b) CR-ex (c) 12 months (d) 3 wks: 3–6 sessions daily, 5–20 min; 12 month: daily 20–30 min home-based and one supervised session at 60 min weekly (e) Aerobic exercise training (cycle ergometer) 50–60% maximal exercise capacity (f) In-patient and home-based | (c) n = 19 (a) UC | (d) 12 months (e) 1, 2, 3, 4, 6 (f) Echocardiographic results | EP 1: Events/patient at risk after 12 months: CR-ex 0/15 vs UC: 1/9 EP 2: Events/patient at risk after 12 months: CR-ex 0/15 vs UC: 1/9 EP 3: Total number of observed events: CR-ex: 7 vs UC: 7 EP 4: Total number of observed events: CR-ex: 3 vs UC: 5 EP 6: after 12 months MD 4.60, 95% CI (2.22–6.98) | |
| Ellingsen et al., 2017, Europe45 | RCT | (a) SMARTEX heart failure study (b) n = 261 (c) HFrEF, LV-EF ≤ 35% (d) 2009–2014 (e) NYHA class II–III, optimal therapy (f) Median 60 yrs; CR-ex: 78, 54 ± 3 vs UC: 55 ± 2 yrs (g) 81% male | (a) n = 78 (b) CR-ex (c) 3 months (d) 3 sessions/wk, 47 min (e) Moderate continues aerobic exercise*, 60–70% HRmax (f) Out-patient | (a) n = 81, (b) UC advised to physical activity (c) HIIT n = 88* | (a) 12 months (b) 1, 2, 3, 4, 6, 7 (c) Echocardiographic results, IPAQ 7 short form, HADS | EP 1: Events/patient at risk after 12 months: CR-ex: 3/73 vs UC: 1/76 EP 2: Events/patient at risk after 12 months: CR-ex: 1/73 vs UC: 0/76 EP 3: Events/patient at risk after 12 months: CR-ex: 16/73 vs UC: 38/76 EP 4: Events/patient at risk after 12 months: CR-ex: 6/73 vs UC: 15/76 EP 6: After 12–14 months MD –1.80, 95% CI (–4.56–0.96) EP 7: KCCQ 9+ months MD –2.00, 95% CI (–11.93–7.93) | Two* intervention groups (only the moderate continuous training group used) |
| Dalal et al., 2018, UK46 | RCT | (a) REACH-HF (b) n = 216 (c) HFrEF, LV-EF≤40% (d) NSp (e) Confirmed diagnosis of HFrEF, aged≥18 yrs (f) CR-ex: 69.7 ± 10.9 vs UC: 69.9 ± 11 (g) 78% male | (a) n = 107 (b) CR-mult (c) 12 weeks (d) 3 sessions/wk (e) Structured exercise programme with chair-based exercise and a progressive walking programme (f) Home-based comprehensive self- management CR with regular telephone contacts | (a) n = 109 (b) UC | (a) 12 months (b) 1, 2, 3, 4, 7 (c) EQ-5D, Heart QoL, HADS, SCHFI, NT-proBNP, costs | EP 1: Events/patient at risk after 12 months: CR-ex: 4/96 vs UC: 4/97 EP 2: Events/patient at risk after 12 months: CR-ex: 1/96 vs UC: 4/97 EP 3: Events/patient at risk after 12 months: CR-ex: 19/107 vs UC: 24/109; OR 0.80 95% CI (0.47–1.36) EP 4; CR-ex: 3/107 vs UC: 6/109; OR 0.51 95% CI (0.13–196) EP 7: MLWHF after 12 months MD –3.40, 95% CI (–10.51–2.12) | |
| Lang et al., 2018, UK47 | (a) REACH-HF (b) n = 50 (c) HFpEF, LV-EF≥45% (d) NSp (e) Confirmed HFpEF-diagnosis within the last 6 months (f) 73.9 yrs (g) 56% male | (a) n = 25 (b) CR-mult (c) 12 weeks (d) 3 sessions/wk (e) Structured exercise programme with chair-based exercise and a progressive walking programme (f) Home-based comprehensive self- management CR with regular telephone contacts | (a) n = 25 (b) UC | (a) 6 months (b) 3, 4, 7 (c) Heart QoL, ISWT, HADS, SCHFI, EQ-5D-5L | EP 3: Events/patient at risk after 6 months: CR-mult: 4/25 vs UC: 7/25 EP 4: Events/patient at risk after 6 months: CR-mult: 0/25 vs UC: 4/25 EP 7: MLWHF after 6 months final values: MD –9.5 95% CI (–25.86–6.86), change score: MD –11.5, 95% CI (–22.8–0.3) | HFpEF –patients only |
1RM: one repetition maximum; 6-MWD: 6-minute-walking distance; BDI: Beck Depression Inventory Questionnaire; BNP: B-type naturetic peptide; BRUM-CHF-study: Birmingham Rehabilitation Uptake Maximization study for patients with Congestive Heart Failure; CHF: chronic heart failure; CI: confidence interval; CR: cardiac rehabilitation; CR-ex: cardiac rehabilitation including exercise only; CR-mult: multicomponent cardiac rehabilitation including exercise; CROS-HF: Cardiac Rehabilitation Outcome Study in Heart Failure; CV: cardiovascular; ELVD-CHF-Trial: Exercise in Left Ventricular Dysfunction and Chronic Heart Failure Trial; EP: endpoint; EPQ: Eysenck Personality Questionnaire; EQ-5D-5L: Five-Level-EuroQol-Five-Dimentional-Questionnaire; EXERT: the Exercise Rehabilitation Trial; FBF: forearm blood flow; HADS: Hospital Anxiety and Depression Scale; HF: heart failure; HF-ACTION: a controlled trial investigating outcomes of exercise training; HFprEF: heart failure with preserved left ventricular ejection fraction; HFrEF: heart failure with reduced left ventricular ejection fraction; HR: hazard ratio; HRmax: maximal heart rate; HIIT: high intensity interval training; HRR: heart rate reserve; IPAQ: International Physical Activity Questionnaire; ISWT: Shuttle Walk Test; IT: interval training; KCCQ: Kansas City Cardiomyopathy Questionnaire; LVEF: left ventricular ejection fraction; MAACL: Multiple Affect Adjective Checklist; max: maximum; MD: mean difference; MET: metabolic equivalent; min: minutes; MLWHF: Minnesota Living with Heart Failure Questionnaire; MLSQ: Modified Linkert Symptom Questionnaire; MRI: magnetic resonance imaging; n: number; NSp: not specified; NYHA: New York Heart Association; PGWB: Psychological Well-being Index; proBNP: pro-brain natriuretic peptide; PWC: physical work capacity; QoL: Quality of Life; RCT: randomised controlled trial; REACH-HF: Rehabilitation EnAblement in CHronicHeart Failure; reps: repetitions; sd: standard deviation; SCHFI: Self-Care of Heart Failure Index; SSA-p: Subjective Symptoms Assessment profile; UC: usual care; VAT: ventilatory threshold; VO2: oxygen capacity; wk: week; yrs: years.
Statistical methods
The primary meta-analysis included all studies comparing ebCR with control groups. Where possible a subgroup analysis was performed comparing different types of intervention during CR (e.g. CR-ex vs CR-mult, aerobic exercise alone vs aerobic plus resistance exercise). The hazard ratio (HR) and its 95% confidence interval (CI) was used as an effect measure for time-to-event data. If HRs and associated variances could not be extracted directly from the trial publications, the data were obtained indirectly using methods as described by Parmar et al.48 and Tierney et al.49 For event data, the relative risk (RR) with its 95% CI was chosen as an effect measure. Ratios were defined as the effect in the ebCR group divided by the effect in the control group. For continuous outcomes, the mean difference (MD) of the final values with corresponding 95% CI was used. All MDs were calculated by subtracting the mean values of the control from the mean values of the ebCR group. Some publications only provided medians and interquartile ranges (IQRs) instead of means and standard deviations (SDs). In these cases mean and SD were estimated by methods proposed by Wan et al.50
Random-effects meta-analyses were used to calculate overall effect estimates and CIs, as heterogeneity between the ‘true’ effects of the various ebCR programmes being evaluated was assumed. As a random-effects model, the ‘Knapp-Hartung approach’ was applied.51 Due to the high number of studies with no predefined event occurring during follow-up, the beta-binomial model52 was applied to estimate an overall effect for the event data. Only to visualise the study specific RR in the forest plots and to evaluate the statistical heterogeneity, a continuity correction of 0.5 was applied to studies without events.
Statistical heterogeneity of the results was checked by I2 statistics19 with 50–75% representing substantial and 75–100% considerable heterogeneity. A potential publication bias was evaluated by visual examination of funnel plot asymmetry for the primary outcome. As sensitivity analysis, a fixed-effect model for the primary endpoint was applied. To evaluate the influence of the HF-ACTION trial, a further sensitivity analysis was conducted by excluding the results of the HF-ACTION trial. Due to insufficient data, no further subgroup analyses were conducted. R version 3.4.4 (R Foundation for Statistical Computing, 2018) and the R meta package version 4.9-1 (developed by Guido Schwarzer) were used for statistical analyses.
Results
Study characteristics
Literature search yielded a total of 12,229 titles. Following the review of titles and abstracts, 104 publications remained for full text evaluation. Finally, 26 RCTs (29 publications) fulfilled all inclusion criteria of the CROS-HF-study and, therefore, were selected for the final review (Figure 1).
Within these finally selected RCTs, one pilot study including heart failure patients with preserved ejection fraction (HFpEF) was excluded47 (Table 2). Therefore, this review focusses on studies evaluating HF-patients with reduced ejection fraction (25 studies; 4481 patients, LV-EF ≤40%). Out of these 25 studies, seven studies (2759 patients)22,25,27,34,37,44,45 included patients with LV-EF<35% only. The majority of the included studies represent single-centre studies (21 studies). The number of participants in most studies was small (25–216 participants) being dominated by one large randomised controlled study (HF-ACTION 2009). HF-ACTION contributed to 52% of all included patients.37,38 The follow-up periods varied widely from six months up to 10 years.
Four studies provided CR-mult and 21 studies applied CR-ex. All studies evaluated continuous aerobic exercise training. In 10 studies, continuous training was combined with resistance exercise.24,26–28,31,35,36,39,41,42 Two studies evaluated two intervention groups with different exercise modalities.30,45 In the CROS-HF evaluation, the two intervention-groups of Klocek et al.40 were analysed together. In Ellingsen et al.,45 the results from the high intensity interval training (HIIT) group were not included in the analysis because this training modality was significantly different from that used in all other studies.
The dose of exercise training ranged widely across the studies under investigation with single session durations of 30–105 min, session frequencies of 2–7 sessions per week, and total programme durations of 4–54 weeks. Therefore, the total exercise volume varied widely between 960–7800 min (Table 2).
Risk of bias
In 10 of the studies included, the randomization sequence generation has clearly been described.9,28,36,37,39,43–47 Four of these studies9,36,43,44 failed to report the allocation concealment. Blinding of the participants and staff was not possible due to the type of intervention, resulting in a high risk of performance bias.
Most outcomes (all-cause-mortality, cardiovascular (CV) mortality, hospital admission due to heart failure) are unlikely to be influenced by a lack of blinding of the outcome assessment. In contrary, the assessment of the remaining endpoints (QoL and VO2peak) might be influenced.
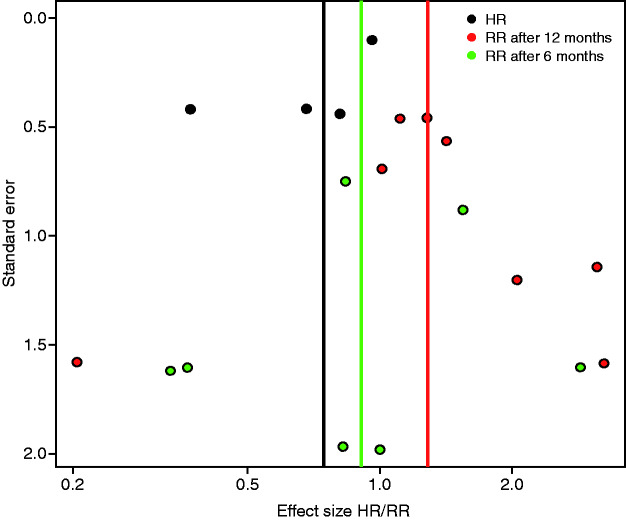
Nineteen trials were rated as low risk of bias concerning incomplete data. In four studies,21,24,28,44 dropout rates differed between ebCR and control group. Therefore, the risk of an attrition bias is regarded as high in these trials. The HF-ACTION37 trial had a dropout rate of 33% after 12 months for the outcome VO2peak, whereas all randomised patients were analysed for the other outcomes. Only in seven publications, has the existence of a study protocol been reported.37,39,43–46 Six studies9,23,26,30,33,44 only included men, and therefore cannot be regarded as representative. In some studies, the quality of reporting was poor (e.g. contradiction between text and tables, confidence intervals not covering the point estimator etc.). In nine trials22,25,30,31,34,39,45–47 the baseline characteristics of the intervention group were unbalanced at baseline. A summary of the risk of bias is provided in Figure 2. The visual examination of the funnel plot revealed no visible publication bias (Figure 3).
Figure 2.
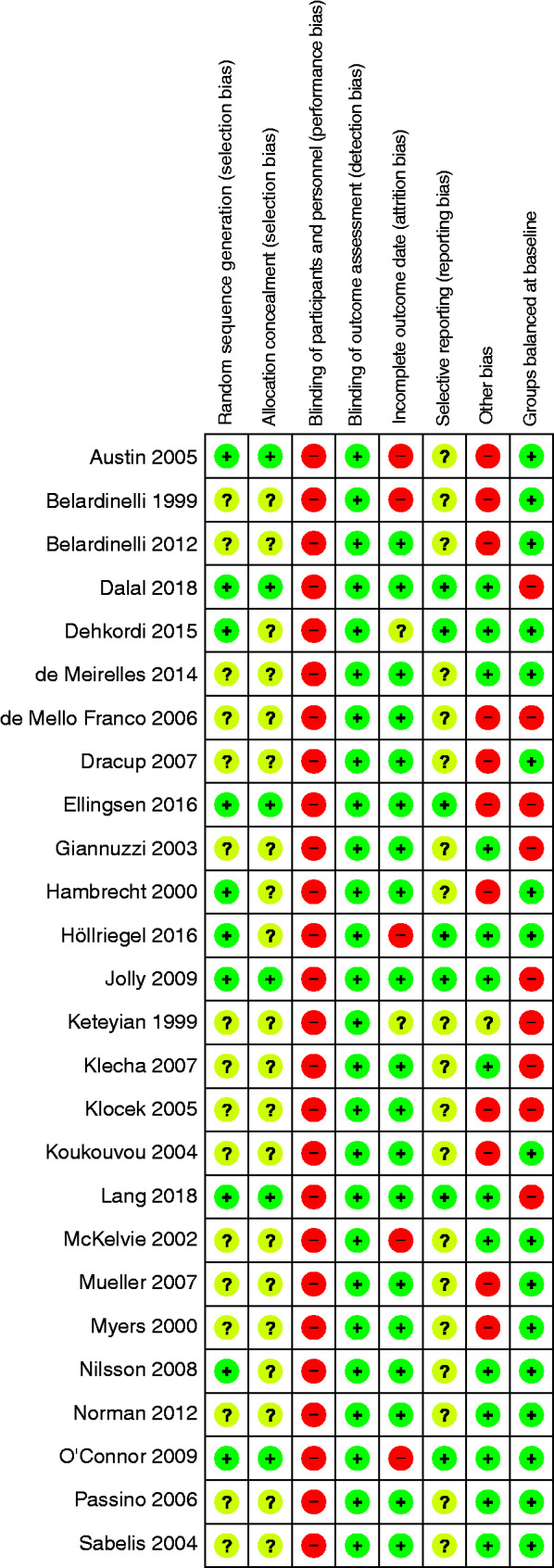
Risk of bias for individual studies. HR: hazard ratio; RR: relative risk.
Figure 3.
Risk of bias: the visual examination of the funnel plot.
Outcomes
Primary endpoint: all-cause-mortality
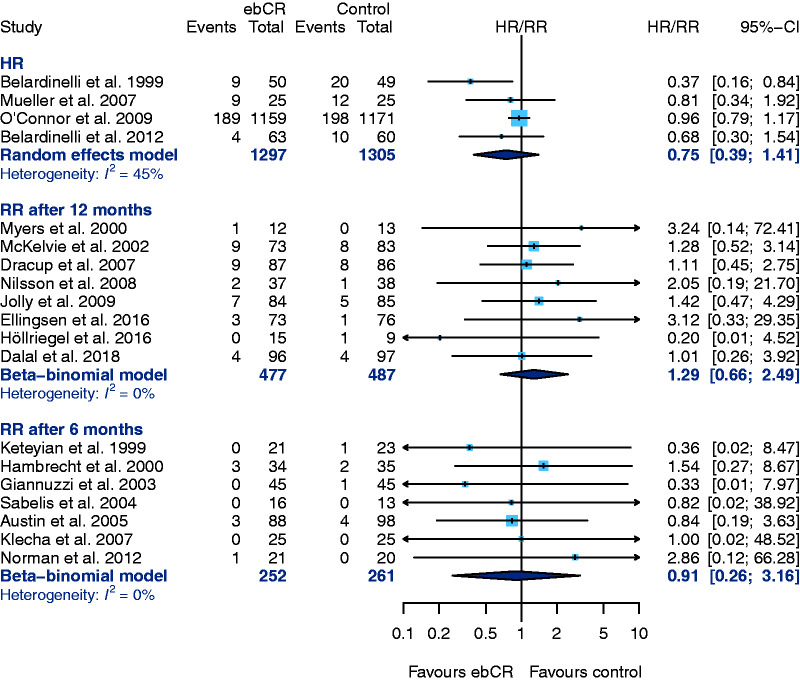
Data for the primary endpoint – all-cause-mortality – was provided in 19 studies.
Four studies (follow-up-periods ranging from 30 months up to 10 years) used HR for evaluating all-cause-mortality (Figure 3; HR 0.75, 95% CI 0.39–1.41, I2=45%). The only study with a statistically significant mortality reduction in the ebCR group was published in 1999.21
The application of a fixed-effect model for the reported HR as a sensitivity analysis confirms these result of the primary analysis (HR 0.89, 95% CI 0.74–1.07), although the effect measure is shifted towards the null effect. The studies evaluated are dominated by the HF-ACTION trial.37 If the HF-ACTION trial was excluded from the analysis, the result still did not reach statistical significance in favour of ebCR (HR 0.58, 95% CI 0.21–1.62, I2=0%).
Eight studies reported count data for mortality after a follow-up of 12 months and seven studies after six-months follow-up. A significant difference was neither found after 12 months (RR 1.29, 95% CI 0.66–2.49, I2 = 0%) nor after six-months follow-up (RR 0.91, 95% CI 0.26–3.16, I2 = 0%) (Figure 4).
Figure 4.
Primary endpoint: all-cause-mortality, results of the primary analysis. CI: confidence interval; ebCR: exercise-based cardiac rehabilitation; HR: hazard ratio; RR: relative risk.
The subgroup analysis (e.g. CR-mult vs CR-ex and aerobic exercise only vs aerobic plus resistance exercise) did not show any differences between the subgroups, respectively (Table 3).
Table 3.
Results of the subgroup analysis (multicomponent cardiac rehabilitation (CR) vs CR exercise only and aerobic exercise only vs aerobic and resistance exercise) for the primary endpoint mortality.
| Outcome | Population/ number of studies (follow-up) | Number of patients (ebCR) | Number of patients (control) | Pooled HR, (95% CI) | Pooled RR, (95% CI) |
|---|---|---|---|---|---|
| All-cause- mortality subgroup analysis | CR-ex: n = 3 studies CR-mult: n = 1 study | 1272 25 | 1280 25 | 0.69 (0.21–2.27) 0.81 (0.34–1.92) | |
| CR-ex: n = 5 studies (12 months) CR-mult: n = 3 studies (12 months) | 285 192 | 292 195 | 1.24 (0.21–8.59) 1.35 (0.43–3.62) | ||
| CR-ex: n = 2 studies (6 months) CR-mult: n = 5 studies (6 months) | 109 141 | 118 141 | NA 0.49 (0.024–9.99) | ||
| aerobic exercise only: n = 5 studies (12 months) combined exercise: n = 3 studies (12 months) | 283 194 | 281 206 | 1.22 (0.37–4.01) 1.37 (0.31–5.95) |
CI: confidence interval; ebCR: exercise-based cardiac rehabilitation; HR: hazard ratio; RR: relative risk.
CR-ex refers to cardiac rehabilitation including exercise only; CR-mult refers to multicomponent cardiac rehabilitation including exercise, combined exercise means combined aerobic and resistance exercises.
Secondary endpoints
CV mortality
Twelve studies provided data for CV mortality. Three of them reported the HR for CV mortality. In two studies,21,40 all deaths were of CV origin, and therefore were already included into the evaluation of all-cause-mortality. The third study reporting CV mortality was the HF-ACTION trial (HR 0.92, 95% CI 0.74–1.15).37
Four studies23,44–46 provide count data for CV mortality after 12 months and five studies9,25,27,34,41 after six-months follow-up, respectively. Only a few events occurred within these studies. Results for CV mortality are summarised in Table 4.
Table 4.
A summary of cardiovascular mortality.
| Study | Follow-up(in months) | ebCR |
Control |
||
|---|---|---|---|---|---|
| n | CV death | n | CV death | ||
| More than 12 months | |||||
| Belardinelli et al., 201240 | 120 | 63 | 4 | 60 | 10 |
| Belardinelli et al., 199921 | 54 | 50 | 9 | 49 | 20 |
| O'Connor et al., 200937 | 30 | 1159 | 131 | 1171 | 143 |
| 12 months | |||||
| Myers et al., 200023 | 12 | 12 | 1 | 13 | 0 |
| Ellingsen et al., 201745 | 12 | 73 | 1 | 76 | 0 |
| Höllriegel et al., 201644 | 12 | 15 | 0 | 9 | 1 |
| Dalal et al., 201846 | 12 | 96 | 1 | 97 | 3 |
| 6 months | |||||
| Hambrecht et al., 20009 | 6 | 34 | 3 | 35 | 2 |
| Giannuzzi et al., 200325 | 6 | 45 | 0 | 45 | 1 |
| Sabelis et al., 200427 | 6 | 16 | 0 | 13 | 0 |
| Klecha et al., 200734 | 6 | 25 | 0 | 25 | 0 |
| Norman et al., 201241 | 6 | 21 | 0 | 20 | 0 |
CV: cardiovascular; ebCR: exercise-based cardiac rehabilitation.
Composite endpoint of mortality and hospitalization
Three studies reported an HR for the composite endpoint of mortality and hospitalization, Dracup et al.36 (HR 1.23, 95% CI 0.78–1.93), O’Connor et al.37 (HR 0.93, 95% CI 0.85–1.02) and Jolly et al.39 (HR 1.45, 95% CI 1.45–4.86).
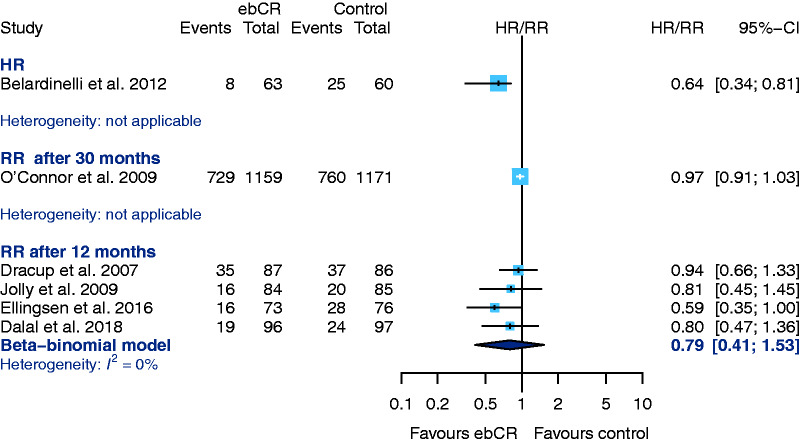
Hospital admission
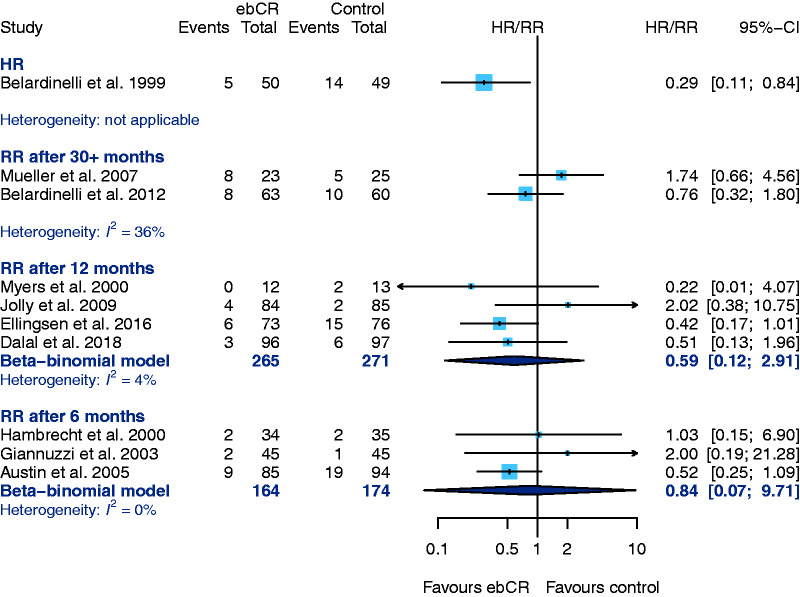
From an overall six studies that provided data for the outcome ‘hospital admission due to any reason’, Belardinelli et al.40 is the only one that reported an HR. The observed HR is statistically significant in favour of ebCR. The CIs in this study, however, are asymmetrical around the HR. Five studies evaluating ‘hospital admission after 12-months follow-up’ failed to show any differences between the treatment groups35,39,44–46 (RR 0.79, 95% CI 0.41–1.53; I2 = 0%) (Figure 5). Höllriegel et al.44 only provided the total numbers of events and was therefore not included into the analysis.
Figure 5.
Secondary endpoint: hospital admission for any reason. CI: confidence interval; ebCR: exercise-based cardiac rehabilitation; HR: hazard ratio; RR: relative risk.
Ten studies provided results concerning ‘hospitalization due to worsening of heart failure’ (Figure 6). The HR has been calculated by only one study21 (HR 0.29, 95% CI 0.11–0.84). Excluding Höllriegel et al. (see above-mentioned reasons) four studies reported results after 12-months follow-up23,39,45,46 (RR 0.59, 95% CI 0.12–2.91, I2 = 4%). Three studies provided data for six-months follow-up (RR 0.84, 95% CI 0.07–9.71, I2 = 0%) (Figure 6).
Figure 6.
Secondary endpoint: hospital admission due to worsening heat failure. CI: confidence interval; ebCR: exercise-based cardiac rehabilitation; HR: hazard ratio; RR: relative risk.
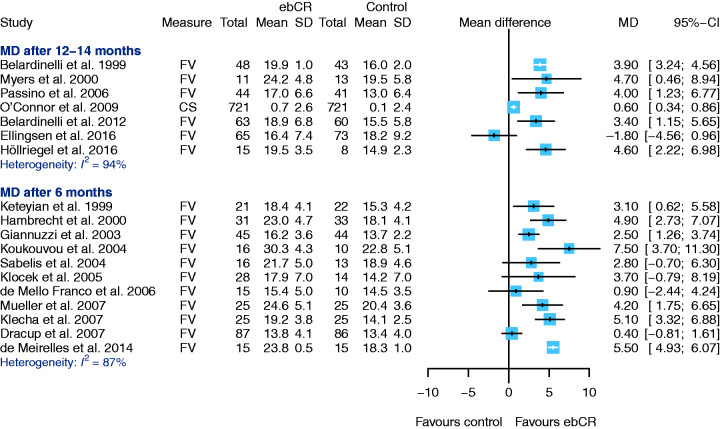
Cardiopulmonary exercise capacity
Eighteen studies reported data on cardiopulmonary exercise capacity (VO2peak ml/min/kg).
The VO2peak was measured using cardiopulmonary exercise testing (n = 13 cycle ergometer or n = 5 treadmill, using different protocols) performed at baseline, after the intervention and follow-up, to document increments in exercise capacity. In one study only,37 respiratory exchange ratio (RER)>1.0 was required as a load criterion. Ten studies9,21–23,25,26,33,37,42,45 reported mean RER values in all tests performed (n = 9 mean RER > 1.0) (see overview in Supplementary Material Table SM3). Follow-up periods were 12–14 months (seven studies) or six months (11 studies) (Figure 7). Due to a considerable statistical heterogeneity effect, sizes have not been pooled (heterogeneity after 12–14 months I2 = 94.38%; heterogeneity after six months I2 = 86.66%). All but one study45 show an increase of exercise capacity in the ebCR group. In the single study not showing this favourable effect,45 a significant imbalance at baseline in favour of the control group was observed (MD at baseline –2.2, 95% CI –4.4–0.0).
Figure 7.
Secondary endpoint: cardiopulmonary exercise capacity (peak oxygen uptake (VO2peak)). No pooled effect sizes estimated due to high heterogeneity (mean difference (MD) after 12–14 months: 94.92%, MD after six months: (86.66%). CI: confidence interval; ebCR: exercise-based cardiac rehabilitation; FV: final value (raw value obtained after end of intervention); SD: standard deviation.
QoL
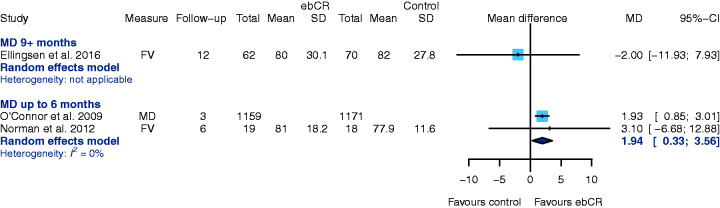
QoL has been evaluated by a variety of instruments, but the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Minnesota Living With Heart Failure Questionnaire (MLWHF) have predominantly been used in the studies selected in CROS-HF. Therefore, data based on either KCCQ or MLWHF were used for further analysis.
Using KCCQ after six-months follow-up (two studies),37,41 ebCR is associated with an increased QoL as compared to the control group (MD 1.94, 95% CI 0.35–3.56, I2 = 0%); higher KCCQ scores indicate a better QoL (Figure 8). This improvement could not be observed after 12 months in one study.45
Figure 8.
Secondary endpoint: health-related quality of life: Kansas City Cardiomyopathy Questionnaire (KCCQ). CI: confidence interval; ebCR: exercise-based cardiac rehabilitation; FV: final value (raw value obtained after end of intervention); MD: mean difference (publication reported mean difference); SD: standard deviation.
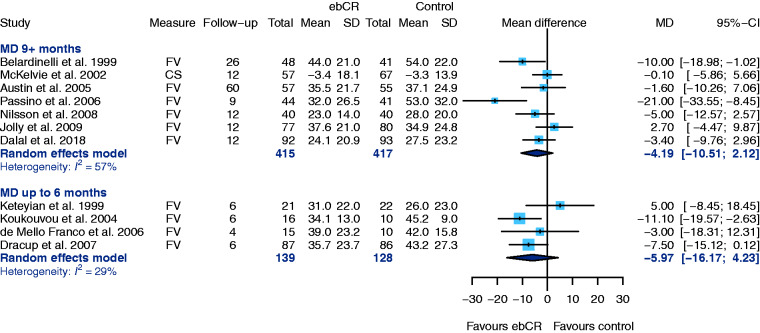
MLWHF questionnaires have been used in 12 studies, whereby lower scores indicate a better QoL. Due to inconsistently reported results, the study of Bellardinelli et al.40 was excluded.
Seven trials reported QoL after a follow-up of ≥9 months and heterogeneity was substantial. Pooled MD was – although not statistically significant – in favour of ebCR (MD –4.19, 95% CI –10.51–2.12, I2 = 57%) (Figure 9). Four trials provided data after six-months follow-up, and the results again were in favour of ebCR (MD –5.97, 95% CI –16.17–4.23, I2 = 29%) (Figure 9).37,45
Figure 9.
Secondary endpoint: health-related quality of life: Minnesota Living With Heart Failure Questionnaire (MLWHF). CI: confidence interval; CS: change score (difference between final value and baseline value; ebCR: exercise-based cardiac rehabilitation; FV: final value (raw value obtained after end of intervention); SD: standard deviation.
Discussion
In this systematic review and meta-analysis including HFrEF patients with LV-EF ≤40% and guideline-recommended medical therapy, ebCR (a) did not have a significant effect on all-cause-mortality, CV mortality or hospital readmission, and (b) was associated with improved QoL and an increased exercise capacity.
The lack of any prognostic effect of ebCR in HFrEF comes as a disappointment to many exercise physiologists and rehabilitation experts, who believed that ebCR could indeed influence the underlying disease process by reducing left ventricular (LV)-dilation, improving LV-EF, and partially reversing skeletal muscle dysfunction. Neurohormonal changes like reduction of circulating catecholamines and reduced sympathetic tone, improved nitric oxide (NO) availability and endothelial function provided a pathophysiological framework to expect prognostic benefit.9,17,45,53
So why could no association between ebCR and a reduction of all-cause-mortality or cardiovascular mortality be observed?
Underpowered studies with small sample size: one may hypothesise that the major improvements in survival related to the introduction of standardised Renin-Angiotensin-Aldosteron-System (RAAS) blockade in combination with beta-blockers and mineralocorticoid antagonists reduced cardiac mortality to a degree, where additional effects of ebCR could only have been detected with much larger patient numbers. As exercise intervention studies do not promise the return on investment expected from drug trials, large well-powered ebCR RCTs are very rare. In fact, the Food and Drug Administration (FDA)-sponsored HF-ACTION trial is the only adequately sized mortality trial.
Lack of adequate patient compliance with the prescribed training programme: in HF-ACTION,37 however, a different problem may have prevented a positive study result (i.e. a significant mortality reduction): lack of patient compliance (approximately 60%). After adjustment for highly prognostic predictors of the primary endpoint in this study, exercise training was, however, associated with modest significant reductions for both all-cause-mortality or hospitalization and CV mortality or heart failure hospitalization.37 Subsequent sub-analysis confirmed a dose-response relation between training intensity (as measured by MET-h per week) and outcome improvement indicating significant reductions in adjusted HRs for all-cause/CV mortality or all-cause/cardiac hospitalization between 3–7 MET-h per week.54 Due to the large patient number in the HF-ACTION trial (2331 patients) the lack of any prognostic benefit in the primary non-adjusted analysis dominated all subsequent meta-analyses. However, if we accept that non-pharmacological interventions should be tested according to the same rules as pharmacological therapies we must also accept the intention-to-treat principle in randomised studies. On-treatment effects are therefore merely hypothesis-generating.
Inherent therapeutic limitations due to an irreversibly damaged heart muscle: due to a consistently improved drug and device therapy, the well-known deleterious circulatory consequences of chronic heart failure largely could be prevented and the risk of sudden cardiac death in these patients significantly has been reduced. Therefore, the current therapeutic approaches in severe chronic heart failure may – at least in part – be exhausted.
On the upside, the current meta-analysis confirmed the positive effects of ebCR on exercise capacity and QoL. Individually adapted exercise training is able to effectively prevent muscular deconditioning with all its negative implications on activities of daily living. In this way, regular exercise training does help to stabilise physical fitness and to increase QoL in these severely ill patients, as shown in this meta-analysis.
To the best of our knowledge, CROS-HF is the first meta-analysis focusing on a clearly defined cohort of HFrEF-patients with a LV-EF ≤40% and a minimum follow-up of six months. In addition, studies were included only if they were published in the era of modern heart failure therapy (1999 or later), and if ebCR guaranteed a minimum of supervised exercise therapy as outlined in Table 1 (CROS-HF inclusion criteria). These baseline requirements of CROS-HF resulted in a study selection different from the majority of meta-analyses published so far (see Table 2 and Supplementary Material Table SM2). The CROS-HF-results are in line with two recently published meta-analyses12,13 that also failed to show a clear effect of ebCR on mortality. In contrast to CROS-HF, these meta-analyses included a broad group of ‘low’ up to ‘high risk’ heart failure patients with an LV-EF spectrum from HFrEF over heart failure with mid-range ejection fraction (HFmrEF)12,13 to HFpEF.12
With respect to the effect of ebCR on hospitalization, rates recently published reviews show variable results. Taylor et al. did not show significant effects (hospitalization due to any reason: HR 0.90, 95% CI 0.76–1.06; hospitalization due to worsening of heart failure: HR 0.98, 95% CI 0.72–1.35)13 while the analysis of Long et al.12 showed statistical significance in favour of ebCR (hospitalization due to any reason up to 12 months: RR 0.70, 95% CI 0.60–0.83; hospitalization due to worsening of heart failure: RR 0.59, 95% CI 0.42–0.84). The finding that CROS-HF did not show a significant reduction in hospital readmission might be explained as well by the fact that all patients had a markedly reduced LV-EF and were on a state-of-the-art pharmaco- and device therapy. Hence, more studies evaluating larger cohorts of patients with different levels of reduced LV-EF are needed to strengthen the tendency of our results. Reduced physical performance and exercise-induced dyspnoea are key symptoms of heart failure. In HFrEF-patients the VO2peak (ml/min/kg) is a significant prognostic predictor of all-cause mortality and a 1 ml/min/kg lower VO2peak is associated with a 16% higher all-cause-mortality.55 A recently published study evaluated the impact of the baseline VO2peak as well as of the increment of the exercise capacity during ebCR on the primary endpoints of death or hospitalization (n = 421 HF-patients; 85 cases during 2.5 year follow-up). The results revealed an independent association between high exercise capacity at ebCR baseline and primary endpoints, during follow-up (HR 0.54, 95% CI 0.34–0.85).56 Moreover, high exercise capacity at baseline combined with major improvement in VO2peak during ebCR was associated with 81% risk reduction for primary endpoints (HR 0.19, 95% CI 0.08–0.43). Importantly, a significant risk reduction was also evident in patients with low baseline values but significant increment of exercise capacity during ebCR (HR 0.41, 95% CI 0.23–0.74).56 These results emphasise the importance of improvement in exercise capacity through ebCR. All effects for cardiopulmonary exercise capacity beside those of one study45 are in favour of the ebCR group in the current systematic review. A previously published meta-analysis57 also revealed a significant improvement by MD + 2.82 ml/min/kg (95% CI 1.97–3.67) achieved through ebCR in patients with HFrEF. Higher exercise intensity was associated with greater improvements in exercise capacity.57,58 In addition, the effectiveness was similarly influenced by exercise frequency and volume.58 The potential impact of the quality of exercise-based intervention being under evaluation, the adherence to exercise within and/or across trials, as well as variations in the characteristics of ‘usual care’ as control, has to be discussed in this context.13 Nevertheless, an univariate meta-regression analysis of Long et al.12 did not show any significant impact of type of exercise, exercise dose, type of rehabilitation, or exercise setting on all-cause-mortality, all hospitalizations or QoL.
Although all but one study showed improvements in exercise capacity this effect was not translated into mortality results in the CROS-HF review. This might in part be explained by the study populations included in CROS-HF-analysis, that despite our strict selection criteria showed a substantial heterogeneity, including patients with severely damaged myocardium, regarding cardiorespiratory fitness, disease history and aetiology as well as age. These factors might have influenced the individual response to exercise training. One study evaluating the impact of the response to exercise training classified those patients as non-responders who improved their VO2peak by less than 6% during ebCR. The results showed non-responders to have a significantly higher risk for all-cause-mortality and hospitalization, as compared to responders during a follow-up of five years. Factors associated with no response to ebCR were age, baseline VO2peak and adherence to ebCR. The inability to improve VO2peak by ebCR was associated with a doubling of the risk for death or hospitalization.59
The CROS-HF study revealed significant improvement of KCCQ scores after six-months follow-up. After six months and after ≥9 months the pooled MLWHF results were in favour of ebCR as well, but the difference was not statistically significant. The results for MLWHF in this study are comparable to those of Long et al.,12 showing significant reduction in MLWHF-scores during short-term (<12 months) and long-term (>12 months) follow-up.
Usually, all aspects of QoL are significantly impaired in patients with heart failure.54 Improvements in health related QoL caused by interventions like ebCR or multidisciplinary transition-to-care clinic programmes were associated with a reduced hospitalization rate60,61 and all-cause-mortality of heart failure patients.60 Hence, improvement of QoL is one of the most important goals in heart failure therapy, even though the mechanisms and the interrelationship between health-related QoL and prognostic outcome, are not yet fully understood.60
Limitations
In real life, individuals with HFpEF contribute to at least half of the patients with chronic heart failure.60 The initial aim of CROS-HF, to evaluate the effect of ebCR on HFpEF patients as well, could not be realised, as only one pilot study fulfilled the CROS-HF selection criteria.47 In 2017, a multicentre RCT started in a large cohort of HFpEF-patients focusing on the efficacy of 12 months supervised exercise. This trial, among others, will give more insight into this very important patient cohort.62
The CROS-HF inclusion criteria were met by only a few studies. In addition, most of the studies included were small. Hence, the results were dominated by the large HF-ACTION trial. Moreover, integrated trials showed a considerable heterogeneity in study design, ebCR intervention and patient populations varied substantially with regard to age, disease history and aetiology as well as comorbidity and cardiorespiratory fitness. All of these characteristics do influence the individual response to exercise training making it difficult to compare study results.
Conclusion
In patients with chronic heart failure and LV-EF≤40% being on current guideline-recommended pharmacological and device therapy, ebCR was associated with an increase in QoL and exercise capacity. A significant advantage regarding mortality and hospitalization, however, could not be found.
Supplemental Material
Supplemental Material for Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis by Birna Bjarnason-Wehrens, R Nebel, K Jensen, M Hackbusch, M Grilli, S Gielen, B Schwaab, B Rauch and for the German Society of Cardiovascular Prevention and Rehabilitation (DGPR) in European Journal of Preventive Cardiology
Acknowledgments
All authors have read and approved the submission of the manuscript; the manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language.
Author contribution
BBW, SG, KJ, MH, RN and BR designed this systematic review. MG performed the systematic literature search. BBW and RN selected and evaluated retrieved studies. KJ and MH extracted the data, assessed the risk of bias and analysed the extracted data. BBW, BS BR, SG and RN were responsible for drafting the report. All authors reviewed the manuscript, gave their final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: During the last three years BBW received lecture honoraria from Ergoline outside the submitted work. During the last three years RN received lecture honoraria from Ergoline and Boehringer Ingelheim outside the submitted work. The Institute of Medical Biometry and Informatics of the University Heidelberg (MH, KJ) and the University Library, Medical Faculty of the University of Mannheim-Heidelberg (MG) received grants from the German Society of Cardiovascular Prevention and Rehabilitation. During the last three years BR received honoraria as a consultant of Abbott Medical Devices and lecture honoraria from the Deutsche Hochdruckliga e.V. SG and BS report no conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: the authors thank the German Society of Cardiovascular Prevention and Rehabilitation (DGPR e.V.) for supporting the study by grants to the Institute of Medical Biometry and Informatics of the University Heidelberg and the University Library, Medical Faculty of the University of Mannheim-Heidelberg.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence: Clinical Guidelines. NG106. Chronic Heart Failure in Adults: Diagnosis and Management. National Guideline Centre (UK). London: National Institute for Health and Care Excellence (UK); September 2018. [PubMed]
- 4.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: A report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somaratne JB, Berry C, McMurray JJ, et al. The prognostic significance of heart failure with preserved left ventricular ejection fraction: A literature-based meta-analysis. Eur J Heart Fail 2009; 11: 855–862. [DOI] [PubMed] [Google Scholar]
- 6.Piepoli MF, Conraads V, Corra U, et al. Exercise training in heart failure: From theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011; 13: 347–357. [DOI] [PubMed] [Google Scholar]
- 7.Vigorito C, Abreu A, Ambrosetti M, et al. Frailty and cardiac rehabilitation: A call to action from the EAPC Cardiac Rehabilitation Section. Eur J Prev Cardiol 2017; 24: 577–590. [DOI] [PubMed] [Google Scholar]
- 8.Coats AJ, Adamopoulos S, Meyer TE, et al. Effects of physical training in chronic heart failure. Lancet 1990; 335: 63–66. [DOI] [PubMed] [Google Scholar]
- 9.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA 2000; 283: 3095–3101. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014; 4: CD003331–CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagar VA, Davies EJ, Briscoe S, et al. Exercise based rehabilitation for heart failure: Systematic review and meta-analysis. Open Heart 2015; 2: e000163–e000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019; 1: CD003331. [DOI] [PMC free article] [PubMed]
- 13.Taylor RS, Walker S, Smart NA, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: An individual patient data meta-analysis of randomised trials. Eur J Heart Fail 2018; 20: 1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doletsky A, Andreev D, Giverts I, et al. Interval training early after heart failure decompensation is safe and improves exercise tolerance and quality of life in selected patients. Eur J Prev Cardiol 2018; 25: 9–18. [DOI] [PubMed] [Google Scholar]
- 15.Iliou MC, Vergès-Patois B, Pavy B, et al. Effects of combined exercise training and electromyostimulation treatments in chronic heart failure: A prospective multicentre study. Eur J Prev Cardiol 2017; 24: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 16.Panagopoulou N, Karatzanos E, Dimopoulos S, et al. Exercise training improves characteristics of exercise oscillatory ventilation in chronic heart failure. Eur J Prev Cardiol 2017; 24: 825–832. [DOI] [PubMed] [Google Scholar]
- 17.Billebeau G, Vodovar N, Sadoune M, et al. Effects of a cardiac rehabilitation programme on plasma cardiac biomarkers in patients with chronic heart failure. Eur J Prev Cardiol 2017; 24: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339: b2535–b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT and Green S (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011),www.handbook.cochrane.org (accessed 15 January 2019).
- 20.Anderson L, Thompson DR, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016; 1: CD001800–CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999; 99: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 22.Keteyian SJ, Brawner CA, Schairer JR, et al. Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J 1999; 138: 233–240. [DOI] [PubMed] [Google Scholar]
- 23.Myers J, Goebbels U, Dzeikan G, et al. Exercise training and myocardial remodeling in patients with reduced ventricular function: One-year follow-up with magnetic resonance imaging. Am Heart J 2000; 139: 252–261. [DOI] [PubMed] [Google Scholar]
- 24.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: The Exercise Rehabilitation Trial (EXERT). Am Heart J 2002; 144: 23–30. [DOI] [PubMed] [Google Scholar]
- 25.Giannuzzi P, Temporelli PL, Corra U, et al. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: Results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation 2003; 108: 554–559. [DOI] [PubMed] [Google Scholar]
- 26.Koukouvou G, Kouidi E, Lacovides A, et al. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J Rehabil Med 2004; 36: 36–41. [DOI] [PubMed] [Google Scholar]
- 27.Sabelis LW, Senden PJ, Fijnheer R, et al. Endothelial markers in chronic heart failure: Training normalizes exercise-induced vWF release. Eur J Clin Invest 2004; 34: 583–589. [DOI] [PubMed] [Google Scholar]
- 28.Austin J, Williams R, Ross L, et al. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail 2005; 7: 411–417. [DOI] [PubMed] [Google Scholar]
- 29.Austin J, Williams WR, Ross L, et al. Five-year follow-up findings from a randomized controlled trial of cardiac rehabilitation for heart failure. Eur J Cardiovasc Prev Rehabil 2008; 15: 162–167. [DOI] [PubMed] [Google Scholar]
- 30.Klocek M, Kubinyi A, Bacior B, et al. Effect of physical training on quality of life and oxygen consumption in patients with congestive heart failure. Int J Cardiol 2005; 103: 323–329. [DOI] [PubMed] [Google Scholar]
- 31.de Mello Franco FG, Santos AC, Rondon MU, et al. Effects of home-based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail 2006; 8: 851–855. [DOI] [PubMed] [Google Scholar]
- 32.Passino C, Severino S, Poletti R, et al. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol 2006; 47: 1835–1839. [DOI] [PubMed] [Google Scholar]
- 33.Mueller L, Myers J, Kottman W, et al. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil 2007; 21: 923–931. [DOI] [PubMed] [Google Scholar]
- 34.Klecha A, Kawecka-Jaszcz K, Bacior B, et al. Physical training in patients with chronic heart failure of ischemic origin: Effect on exercise capacity and left ventricular remodeling. Eur J Cardiovasc Prev Rehabil 2007; 14: 85–91. [DOI] [PubMed] [Google Scholar]
- 35.Dracup K, Evangelista LS, Hamilton MA, et al. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J 2007; 154: 877–883. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson BB, Westheim A, Risberg MA. Long-term effects of a group-based high-intensity aerobic interval-training program in patients with chronic heart failure. Am J Cardiol 2008; 102: 1220–1224. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 153: 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jolly K, Taylor RS, Lip GY, et al. A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: The Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure (BRUM-CHF) study. Eur J Heart Fail 2009; 11: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belardinelli R, Georgiou D, Cianci G, et al. 10-Year exercise training in chronic heart failure: A randomized controlled trial. J Am Coll Cardiol 2012; 60: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 41.Norman JF, Pozehl BJ, Duncan KA, et al. Effects of exercise training versus attention on plasma B-type natriuretic peptide, 6-minute-walk test and quality of life in individuals with heart failure. Cardiopulm Phys Ther J 2012; 23: 19–25. [PMC free article] [PubMed] [Google Scholar]
- 42.de Meirelles LR, Matsuura C, Resende Ade C, et al. Chronic exercise leads to antiaggregant, antioxidant and anti-inflammatory effects in heart failure patients. Eur J Prev Cardiol 2014; 21: 1225–1232. [DOI] [PubMed] [Google Scholar]
- 43.Dehkordi AH, Far AK. Effect of exercise training on the quality of life and echocardiography parameter of systolic function in patients with chronic heart failure: A randomized trial. Asian J Sports Med 2015; 6: e22643–e22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollriegel R, Winzer EB, Linke A, et al. Long-term exercise training in patients with advanced chronic heart failure: Sustained benefits on left ventricular performance and exercise capacity. J Cardiopulm Rehabil Prev 2016; 36: 117–124. [DOI] [PubMed] [Google Scholar]
- 45.Ellingsen Ø, Halle M, Conraads V, et al. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 2017; 135: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalal HM, Taylor RS, Jolly K, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol 2019; 26: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang CC, Smith K, Wingham J, et al. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: The REACH-HFpEF pilot study. BMJ Open 2018; 8: e019649–e019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analysis of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 49.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan X, Wang W, Liu J, et al. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed]
- 51.Veroniki AA, Jackson D, Viechtbauer W, et al. Recommendations for quantifying the uncertainty in the summary intervention effect and estimating the between-study heterogeneity variance in random-effects meta-analysis. Cochrane Database Syst Rev 2015; 1: 25–27. [Google Scholar]
- 52.Kuss O. Statistical methods for meta-analyses including information from studies without any events—add nothing to nothing and succeed nevertheless. Stat Med 2015; 34: 1097–1116. [DOI] [PubMed] [Google Scholar]
- 53.Adams V, Reich B, Uhlemann M, Niebauer J. Molecular effects of exercise training in patients with cardiovascular disease: Focus on skeletal muscle, endothelium, and myocardium. Am J Physiol Heart Circ Physiol 2017; 313: H72–H88. [DOI] [PubMed] [Google Scholar]
- 54.Keteyian SJ, Leifer ES, Houston-Miller N, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol 2012; 60: 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keteyian SJ, Patel M, Kraus WE, et al. HF-ACTION Investigators. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol 2016; 67: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabbag A, Mazin I, Rott D, et al. The prognostic significance of improvement in exercise capacity in heart failure patients who participate in cardiac rehabilitation programme. Eur J Prev Cardiol 2018; 25: 354–361. [DOI] [PubMed] [Google Scholar]
- 57.Uddin J, Zwisler AD, Lewinter C, et al. Predictors of exercise capacity following exercise-based rehabilitation in patients with coronary heart disease and heart failure: A meta-regression analysis. Eur J Prev Cardiol 2016; 23: 683–693. [DOI] [PubMed] [Google Scholar]
- 58.Vromen T, Kraal JJ, Kuiper J, et al. The influence of training characteristics on the effect of aerobic exercise training in patients with chronic heart failure: A meta-regression analysis. Int J Cardiol 2016; 208: 120–127. [DOI] [PubMed] [Google Scholar]
- 59.Bakker EA, Snoek JA, Meindersma EP, et al. Absence of fitness improvement is associated with outcomes in heart failure patients. Med Sci Sports Exerc 2018; 50: 196–203. [DOI] [PubMed] [Google Scholar]
- 60.Luo N, O'Connor CM, Cooper LB, et al. Relationship between changing patient-reported outcomes and subsequent clinical events in patients with chronic heart failure: Insights from HF-ACTION. Eur J Heart Fail 2019; 21: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitaker-Brown CD, Woods SJ, Cornelius JB, et al. Improving quality of life and decreasing readmissions in heart failure patients in a multidisciplinary transition-to-care clinic. Heart Lung 2017; 46: 79–84. [DOI] [PubMed] [Google Scholar]
- 62.Edelmann F, Bobenko A, Gelbrich G, et al. Exercise training in diastolic heart failure (Ex-DHF): Rationale and design of a multicentre, prospective, randomized, controlled, parallel group trial. Eur J Heart Fail 2017; 19: 1067–1074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis by Birna Bjarnason-Wehrens, R Nebel, K Jensen, M Hackbusch, M Grilli, S Gielen, B Schwaab, B Rauch and for the German Society of Cardiovascular Prevention and Rehabilitation (DGPR) in European Journal of Preventive Cardiology