Abstract
With the exponential surge in patients with coronavirus disease 2019 (COVID-19) worldwide, the resources needed to provide continuous kidney replacement therapy (CKRT) for patients with acute kidney injury or kidney failure may be threatened. This article summarizes subsisting strategies that can be implemented immediately. Pre-emptive weekly multicenter projections of CKRT demand based on evolving COVID-19 epidemiology and routine workload should be made. Corresponding consumables should be quantified and acquired, with diversification of sources from multiple vendors. Supply procurement should be stepped up accordingly so that a several-week stock is amassed, with administrative oversight to prevent disproportionate hoarding by institutions. Consumption of CKRT resources can be made more efficient by optimizing circuit anticoagulation to preserve filters, extending use of each vascular access, lowering blood flows to reduce citrate consumption, moderating the CKRT intensity to conserve fluids, or running accelerated KRT at higher clearance to treat more patients per machine. If logistically feasible, earlier transition to intermittent hemodialysis with online-generated dialysate, or urgent peritoneal dialysis in selected patients, may help reduce CKRT dependency. These measures, coupled to multicenter collaboration and a corresponding increase in trained medical and nursing staffing levels, may avoid downstream rationing of care and save lives during the peak of the pandemic.
Index Words: Acute kidney injury (AKI), citrates, continuous renal replacement therapy (CRRT), coronavirus 19 disease (COVID-19), dialysis solutions, kidney failure, nursing staff, pandemics, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), workload, resource management
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to a tragic loss of lives, and the exponential increase in infections has overwhelmed health care systems with a surge in demand for critical care, along with continuous kidney replacement therapy (CKRT) for patients with acute kidney injury (AKI) or kidney failure. The reported incidence of KRT in intensive care unit (ICU) or mechanically ventilated patients with COVID-19 ranges from 16% to 23%.1, 2, 3, 4 The pandemic has placed unprecedented demands on the supply of CKRT machines and consumables, which is worsened by the global lockdown and disrupted supply chains. The provision of CKRT in an austere environment poses profound challenges in that traditional evidence-based management needs to be balanced with resource limitations, and pre-emptive planning has to be flexible based on the rapidly changing disease epidemiology and logistical considerations.
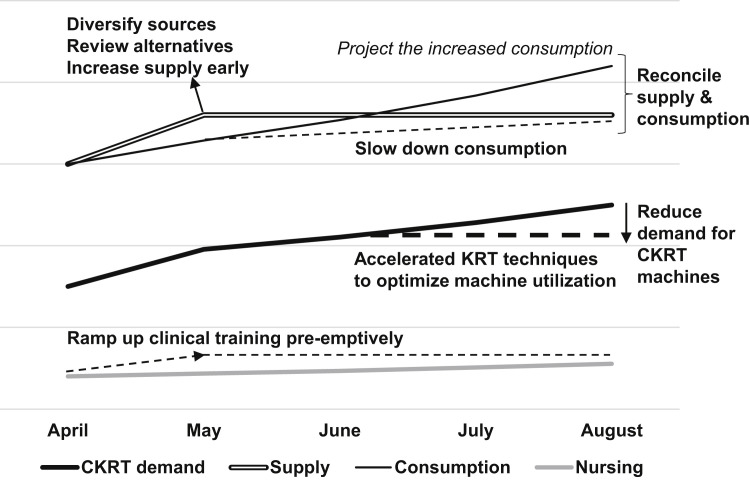
Our work group of nephrologists and intensivists from the 3 public health care clusters in Singapore has outlined strategies to ensure the medium-term sustainability of CKRT in the ICU during the peak of the COVID-19 pandemic. These strategies could be implemented immediately and would remain relevant for future contingency planning during similar health care crises. Specifically, we aimed to: (1) develop projections regarding the demand for CKRT that are revised weekly in accordance with prevailing rates, (2) improve the efficiency of existing care practices to avoid future rationing, (3) optimize stocks of consumables by trimming current consumption and diversifying sources while preventing hoarding, (4) ramp up clinical and nursing training but maintain realistic workforce awareness, and most importantly, (5) collaborate widely at a national and regional level and stay in close communication despite the social disconnect forced by COVID-19 (Fig 1 ).
Figure 1.
Overview of continuous kidney replacement therapy (CKRT) sustainability plan.
Approach
Projecting Demand
We first examined the annual consumption of CKRT fluids and estimated the current use in the 8 publicly funded acute-care hospitals in Singapore, based on routine rates and a total capacity of 9,404 hospital beds.5 We factored in another 586 acute-care beds in the National Centre for Infectious Diseases, which attends to the majority of hospitalized COVID-19 cases in Singapore. We projected incident COVID-19 cases per week based on current epidemiology6 and inferred a corresponding CKRT incidence of 1%,1 with an average 6 days of CKRT.7 This allowed us to estimate the assets and consumables for standard CKRT support and predict the critical threshold of incident COVID-19 cases beyond which the CKRT capacity would be exceeded (Table 1; Box 1 ).
Table 1.
Multicenter Weekly Projection of CKRT Assets and Consumables Required, Based on Theoretical Surge in New COVID-19 Cases
| Predicted New COVID-19 Cases/wk |
|||||
|---|---|---|---|---|---|
| 3,000 | 5,000 | 10,000 | 15,000 |
||
| −Special Measuresa | +Special Measuresa | ||||
| Routine CKRT volume | |||||
| CKRT sessions per week | 223 | 223 | 223 | 223 | 112 |
| No. of corresponding HD sessions per week | 112 | 112 | 112 | 112 | 56 |
| Incident COVID-CKRT cases per weekb | 30 | 50 | 100 | 150 | 150 |
| Estimated CKRT days per case | 6 | 6 | 6 | 6 | 5 |
| No. of corresponding intermittent HD sessions per week | 90 | 150 | 300 | 450 | 600 |
| Total KRT (CKRT + intermittent HD) patients per week | 67 | 87 | 137 | 187 | 169 |
| Total CKRT session-days per week | 403 | 523 | 823 | 1,123 | 862 |
| Total HD sessions per week | 202 | 262 | 412 | 562 | 656 |
| No. of 5-L bags of isonatremic low-bicarbonate calcium-free fluid | 736 | 955 | 1,502 | 2,049 | 1,468 |
| No. of 5-L bags of conventional fluids | 1,936 | 2,512 | 3,952 | 5,392 | 3,102 |
| No. of 5-L bags of low-sodium low-bicarbonate calcium-free dialysate | 194 | 251 | 395 | 539 | 310 |
| No. of 0.5-L bags of ACDA | 232 | 301 | 474 | 647 | 331 |
| No. of 1.5-L bags of 4% TSC | 65 | 84 | 132 | 180 | 90 |
| No. of 5-L bags of 0.5% citrate fluid | 1,162 | 1,507 | 2,371 | 3,235 | 1,654 |
| No. of 5,000-IU vials of unfractionated heparin | 755 | 980 | 1,541 | 2,103 | 1,124 |
| No. of CKRT filters | 302 | 392 | 617 | 842 | 648 |
| No. of CKRT machines per day | 58 | 75 | 118 | 160 | 107 |
| No. of intermittent HD machines per day | 34 | 44 | 69 | 94 | 109 |
| No. of RO machines per dayc | 34 | 44 | 69 | 94 | 109 |
| No. of vascular cathetersd | 134 | 174 | 274 | 374 | 241 |
| No. of nurses for CKRT care per day | 173 | 224 | 353 | 481 | 321 |
| No. of nurses for HD care per day | 50 | 65 | 103 | 140 | 109 |
Note: Assumptions used for calculating consumption levels in the absence of conservation strategies are shown in the top half of Box 1.
Abbreviations: ACDA, anticoagulant citrate dextrose A solution; CKRT, continuous kidney replacement therapy; COVID-19, coronavirus disease 2019; HD, hemodialysis; Qb, blood flow rate; RO, reverse osmosis; TSC, trisodium citrate.
Special measures for conservation of resources are depicted in the bottom half of Box 1.
Based on a 1% incidence rate.
A 1:1 ratio of HD machines to RO machines.
Based on 20 KRT days per patient.
Box 1. Assumptions and Conservation Strategy Practices for Projections of CKRT Needs.
Assumptions for Calculating Consumption Under Standard Conditions
-
•
50% RCA circuits; 50% circuits using conventional/non-RCA fluids
-
•
Protocols used for RCA circuits: ACDA (10%), 4% citrate (10%), and 0.5% citrate (80%)
-
•
Qb = 150 mL/min; citrate dose = 3.0 mmol/L
-
•
Prevalent target CKRT dose of 30 mL/kg/h (to compensate for estimated downtime)
-
•
Average filter life: 24 h for non-RCA circuits, 48 h for RCA circuits
-
•
Each vascular catheter lasts 10 days on average
-
•
Each patient receives 3 HD sessions following transition from CKRT
-
•
For non-RCA CKRT and HD circuits, 90% use unfractionated heparin
-
•
2 trained nurses per CKRT session-day on 1:1 nursing (×1.5 replacement factor)
-
•
2 trained nurses per HD machine-day on 1:2 nursing (×1.5 replacement factor)
Strategy for Conservation of Resources
-
•
↓ Qb to 100 mL/min
-
•
↓ CKRT dose to 20-25 mL/kg/h
-
•
Extend filter life by 30%
-
•
Extend average catheter life to 14 days
-
•
Earlier transition to HD; cut CKRT days
-
•
Cut routine CKRT volume by half by deferring elective workload
-
•
Consider 12-h accelerated KRT to conserve machines
-
•
Reduce nurse to HD session ratio to 1:3
Note: See Table 1 for projections based on these parameters.
Abbreviations: ACDA, anticoagulant citrate dextrose A solution; CKRT, continuous kidney replacement therapy; HD, (intermittent) hemodialysis; Qb, blood flow rate; RCA, regional citrate anticoagulation.
Diversifying Supply
CKRT Machines
We have diversified our sources of CKRT machines from multiple vendors to ensure a continuity of supply. In Singapore, these include Prismaflex (Baxter), Prismax (Baxter), multiFiltrate (Fresenius Medical Care), Omni (B. Braun), Aquarius (Nikkiso), and HF440 (Infomed). Oversight of national demand is maintained to avoid disproportionate hoarding with respect to service load among institutions.
Regional Citrate Anticoagulation Protocols
Different types of CKRT machines use different protocols and fluids for regional citrate anticoagulation (RCA). These include the 0.5% citrate solution (diluted citrate concentration of 18 mmol/L) paired with an isonatremic calcium-free low bicarbonate solution,8 and 4% trisodium citrate (concentrated citrate of 136 mmol/L) paired with a low-sodium, low-bicarbonate, and calcium-free solution.9 In the event that the fluids for either RCA regimen become depleted, an alternative in the form of anticoagulant citrate dextrose A solution (containing 75 mmol/L of citrate and 38 mmol/L of citric acid) is available.10 In our experience, anticoagulant citrate dextrose A at low volume is circuit compatible with conventional isonatremic solution; the latter runs as a postfilter replacement if it contains calcium. Prescribers need to draft new protocols on alternative RCA regimens and initiate immediate clinical training in preparation for the use of new machines.
Conventional CKRT Fluids
Most machines allow common connector compatibility and cross-use of fluids from various vendors without special adaptors, but there are exceptions in that certain machine types require specific fluids, limiting flexibility. Conventional CKRT fluids are mostly used in non-RCA circuits and contain physiologic concentrations of electrolytes, including sodium, 140 mEq/L; chloride, 109 mEq/L; calcium, 3 mEq/L; magnesium, 1 mEq/L; and bicarbonate, 35 mEq/L.11 Some fluids include low quantities of organic anions such as lactate. CKRT fluids contain potassium at either 0, 2, or 4 mEq/L. Interchangeable conventional fluids should be actively sourced to maintain supply and connector compatibility to available machines. It is best to keep a consistent practice with regard to fluid potassium content to avoid its misadministration. Attention should be given to the glucose content because it affects caloric calculation and nutritional management during CKRT.12 Pharmacy-compounded CKRT fluids reduce the dependency on commercially available options, but intensive safety checks are essential.13 , 14
Filters
CKRT filters and lines may be specific for certain machine types based on unique connectors; this restricts their diversification. Most current filter membranes are synthetic and high flux. Prismaflex uses a filter made from a copolymer membrane of acrylonitrile and sodium methallyl sulfonate (AN69) that is conjoined with lines. Fresenius Medical Care and Nikkiso machines incorporate filter membranes made from polysulfone; the filters come separate from the lines, making the connection less restrictive, but the priming time may be longer. Filter membrane surface areas range from 0.9 to 1.9 m2 for adult CKRT and institutions must work with the vendors to ensure a supply of filter sizes that correspond to the most common range of body weight encountered in their practice. Certain filter membranes putatively aid immunoadsorption of cytokines.15 , 16 It is unknown whether these would be beneficial in patients with COVID-19, and their use should be reserved for specific indications or clinical trials.
Vascular Catheters
Administrative oversight is vital to ensure adequate diversified stocks of both tunneled and nontunneled vascular catheters for KRT, as well as catheter sizes appropriate for various venous sites. Different catheter guidewire rigidity or dilatation techniques may affect the end-user competency in catheterization. Experienced proceduralists should be tasked with handling new catheter types so as to allow trainee operators to use catheters that they are familiar with. A common procurement process for ICUs and dialysis centers within the same institution would avoid duplication of efforts and excessive demand.
Reducing Unnecessary Consumption
To ensure service sustainability, all domains of CKRT practice must be reviewed to ensure that adequate but rational care is delivered to current patients, with minimal compromise in service (Box 2 ).
Box 2. Suggested Strategies for More Efficient Consumption of CKRT Resources.
CKRT Machines
-
•
Work with vendors regarding planning for adequate numbers of machines and filters
-
•
Consider modifying CKRT prescription to allow sharing of CKRT machine between 2 or more patients within a 24-h period
-
•
Consider prolonged intermittent/hybrid KRT
-
•
Consider urgent-start peritoneal dialysis to transfer patients from CKRT
Vascular Access
-
•
Work with vendors regarding planning for adequate vascular catheters
-
•
Aim to optimize each vascular access, preferably at least 7 d
-
•
Use thrombolytic therapy for blocked nontunneled vascular access
-
•
Use tunneled vascular access catheters when appropriate
-
•
In patients receiving ECMO, run CKRT concurrent with ECMO circuit
-
•
In patients with kidney failure, consider using their arteriovenous fistula/graft for accelerated KRT
CKRT Filters
-
•
Adopt measures to prolong filter life, eg, good vascular access, filtration fraction < 25%, higher blood flows
-
•
Optimize circuit anticoagulation
-
•
Use a combination of regional citrate anticoagulation and heparinization in appropriate patients
CKRT Fluids
-
•
Work with available vendors regarding planning for adequate CKRT fluids
-
•
Use dynamic dosing of CKRT targeted to metabolic status
-
•
Incorporate net ultrafiltration or patient fluid removal rate into effluent dose
-
•
Explore compatibility of different CKRT fluids with different models of CKRT machines
-
•
Consider pharmacy-compounded CKRT fluids
-
•
Consider “re-using” effluent fluid disposal bags
-
•
Consider using extension tubing for direct drainage of effluent
Abbreviations: CKRT, continuous kidney replacement therapy; ECMO, extracorporeal membrane oxygenation.
Vascular Access
Vascular access is an important determinant for delivery of efficient CKRT. A good access minimizes complications during insertion, limits the risk for catheter-related infections and thrombosis, and provides adequate uninterrupted blood flow for CKRT. Clinicians should optimize each vascular access to conserve resources, but remain mindful of unexplained leukocytosis and elevated inflammatory marker levels that suggest catheter-related infection, given that CKRT may mask fever.17 It is prudent to ensure at least 7 days of use per nontunneled vascular catheter, within which period the catheter colonization or infection rate is low.18 , 19 Systematic vascular catheter change in the absence of complications is best avoided because there is no clear threshold duration beyond which the risk for catheter-related bloodstream infection increases significantly in the ICU.18 Choosing the most appropriate catheter site and size upfront minimizes downstream catheter dysfunction and interrupted CKRT, especially in patients with COVID-19 receiving prone ventilation for extended hours daily. Femoral venous access does not necessarily worsen the risk for malfunction during prone ventilation as compared with internal jugular venous access,20 but the latter would be easier to monitor for bleeding or dislodgement.
Evidence of fibrinolytic therapy with alteplase or urokinase for catheter malfunction is extrapolated from maintenance hemodialysis and tunneled catheter studies.21 Although safety and efficacy are unclear in the context of CKRT, fibrinolytic therapy may provide rapid salvage of dysfunctional catheters, is noninvasive, and arguably leads to fewer vascular complications and less use of consumables than new catheterization. Extended femoral venous catheterization for CKRT and guidewire exchange of dysfunctional vascular catheters over an optimal sterile field can be performed with very low colonization or infection risk.19 This helps conserve central venous sites, which is important because KRT may continue for weeks in AKI survivors.22 Additionally, CKRT circuits may be combined in parallel with extracorporeal membrane oxygenation in patients.23 For patients without COVID-19 who could be readily transferred for fluoroscopy, an initial tunneled vascular catheter over the traditional nontunneled-first approach is feasible and more efficacious for KRT.24 This helps conserve nontunneled catheter stocks for patients with COVID-19. Existing arteriovenous access may be used for CKRT in patients with kidney failure; vigilance is advised for needle dislodgement, extravasation, and access hematoma.25
CKRT Fluids
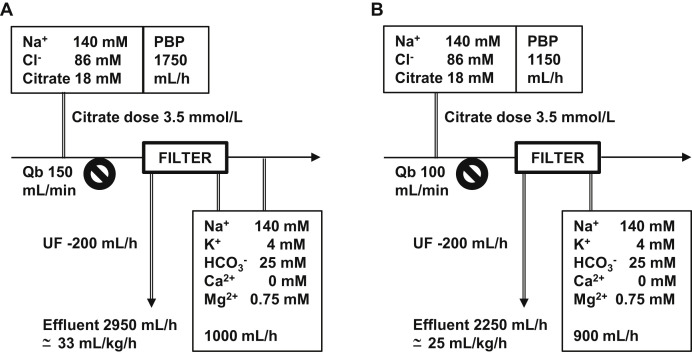
Measures to improve the efficiency of CKRT fluid use could potentially save 30% of cumulative consumption and help counterbalance the increase in demand (Table 1; Box 1; Fig 2 ). The accepted intensity of CKRT is 25 mL/kg/h for most patients with AKI in the ICU26; this is subject to individual variations due to illness severity, catabolism, and acid-base and electrolyte derangements. There is a general consensus to prescribe ~30 mL/kg/h to achieve the desired CKRT dose in view of circuit downtime.27 A higher initial CKRT intensity may be associated with greater improvements in patients’ hemodynamics and vasopressor requirements.28 However, concerns about the CKRT fluid supply should prompt clinicians (if not required by severe hyperkalemia or acidosis) to order relatively lower dialysate and/or replacement fluid rates initially and uptitrate to achieve the desired intensity rather than the reverse. Furthermore, the net ultrafiltration augments the effluent rate, which determines clearance. Replacement fluid rate could also be reduced in tandem with increased ultrafiltration rate to maintain a constant effluent dose. As CKRT progresses over days and the patient’s kidney function improves, adequate clearance could be maintained at a CKRT intensity < 20 mL/kg/h.29 Antibiotic and antiviral drug dosing need to be correspondingly adjusted with the changes in intrinsic kidney function and KRT dose to optimize the antimicrobial efficacy with adequate drug clearance. Individualized treatment goals and frequent reviews of the prescription could further reduce unnecessary fluid consumption.
Figure 2.
Citrate and continuous kidney replacement therapy (CKRT) fluid consumption under (A) standard conditions, with higher blood flow rate (Qb) and pre–blood pump (PBP) rate, versus (B) conditions designed to reduce consumption. Variations in PBP (citrate) flow rate based on Qb; a higher Qb may be more ideal for hemofiltration but results in greater citrate delivery. The paired bicarbonate-buffered solution that contains key electrolytes, for example, potassium and magnesium, is often maintained to achieve metabolic equilibrium, resulting in greater overall CKRT fluid consumption. Lower blood flow reduces citrate consumption by 30%; paired bicarbonate-based solution is reduced proportionally, and delivered as a dialysate to reduce the filtration fraction. Ultrafiltration (UF) rate augments the overall CKRT dose. Delivered dose calculated based on 75 kg of body weight with correction factor applied (due to PBP dilution).
Anticoagulation and Filter Life
Anticoagulation-free CKRT allows 12 to 16 hours of filter life on average.30 , 31 In conditions of shortage, this represents dangerously excessive use of filters, particularly given that dedicated filters are often needed for each machine type. The currency of contraindications to circuit anticoagulation must be actively reviewed and the longevity of filters targeted to a minimum of 2 days.
In Singapore, most ICUs adopted the RCA protocol that uses 0.5% citrate solution, which also serves as a replacement fluid contributing to at least half the CKRT intensity and buffering capacity. With conventional blood flow settings and a citrate dose of 3.0 to 3.5 mmol/L of blood, citrate consumption exceeds 1.5 L/h. The paired bicarbonate-based solution that contains potassium and magnesium is often maintained in proportion to the former for biochemical equilibrium. The resulting CKRT intensity usually exceeds 30 mL/kg/h to avert citrate accumulation. Reducing the blood flow toward 100 mL/min attains a more favorable citrate dose over total citrate delivery and reduces the metabolic burden, but a diffusive-based therapy is preferred to avoid a high filtration fraction (Fig 2). In patients without COVID-19, citrate dose may even be lowered to 2.5 mmol/L, which is noninferior to higher citrate dosing for an optimal filter life in general.32
In addition, we suggest a balance between RCA and heparinization to allow proportionate consumption of both. Importantly, premature filter clotting is commonly observed in patients with COVID-19.33 This is postulated to be in part due to a hypercoagulable state, and systemic anticoagulation is frequently necessary to prolong filter life and manage thromboembolic complications.34 , 35 Monitoring anti-Xa activity is suggested during treatment with unfractionated heparin due to the numerous interactions with inflammatory proteins that interfere with activated partial thromboplastin time prolongation.36 A combined anticoagulation strategy with RCA-CKRT deployed concurrently to heparinized extracorporeal membrane oxygenation has been described in critically ill patients, with good filter life and no increased bleeding events.37 Nonanticoagulation measures such as a predominant diffusive-based CKRT versus convective clearance would reduce circuit failures.38 The optimal blood flow for circuit patency is unclear39 but needs to be adjusted for an appropriate filtration fraction in hemofiltration.
Ensuring Sufficient Machines
Optimizing Machine Use and Conservative Treatment Goals
Purchasing CKRT machines is limited by supplier inventory and the substantial cost. The continuous nature of CKRT implies a ratio of 1 patient to 1 machine, which becomes unsustainable as demand surges. It may be possible to treat 2 patients per machine within a 24-hour period by compressing the run of KRT to 10 to 12 hours at correspondingly higher clearance to compensate for efficacy.40 , 41 This accelerated KRT could be run as hemodiafiltration to optimize both dialysate and replacement fluid pumps and accommodate the doubled fluid consumption rate. Admittedly such therapy would consume at least 1 filter per patient treatment; moreover, the higher ultrafiltration rate could induce hemodynamic instability. Although the strategy saves on the nursing hours and machines, avoids prolonged circuit anticoagulation, and reduces cumulative heparin consumption, it does not save on effort, filters, and fluids. Antimicrobial administration would need to be staggered to be delivered posttreatment, and the dose moderated with transition from CKRT.
Therapy may even be staggered according to predetermined treatment goals because not all patients would require an augmented solute clearance even if the duration is shortened, and KRT could be terminated when targets are reached (such as the case for targeted correction of hyperkalemia and acidosis in patients with kidney failure). Ultrafiltration could be geared toward a projected negative balance required for the day and achieved in the initial hours of therapy. This approach can be adopted in patients with relatively improved stability, lower vasoactive drug requirements, and less complex metabolic derangements.
Alternatives to CKRT
Clinicians could allow an earlier transition to intermittent hemodialysis using online-generated dialysate, but the latter will also be subject to resource constraints, including water supply, drainage, and reverse osmosis machines. The nursing requirement with intermittent hemodialysis is less compared with that of CKRT (Table 1).
In select patients with AKI, peritoneal dialysis (PD) may be advantageous over extracorporeal KRT.42 Similar mortality has been reported in both.43 Technical simplicity, minimal infrastructural requirements, lack of requirement for anticoagulation, and less nursing time make PD especially attractive in pandemics. Flexible PD catheters are preferred over rigid catheters due to a higher risk for complications and peritonitis associated with the latter and the high dialysate flow rates achieved with the former.44 High primary success rate and efficient use of resources are reported in tunneled Tenckhoff catheter insertions by trained nephrologists using the Seldinger technique. Bowel injury and early pericatheter leak are recognized complications, but uncommon.45 Fluoroscopy, mini-laparotomy, or laparoscopic catheter insertion occurs in routine practice but would be challenging in COVID-19.
Manual PD exchange remains an option, but PD is best delivered using an automated cycler to optimize staff time and use of personal protective equipment. Icodextrin dwell during prone ventilation alternating with cycler use when supine may meet fluid removal and clearance targets while overcoming drainage issues and alarms.
Although recognizing the difficulties in clearance measurement in the context of a pandemic, titration to best possible solute and volume balance is essential. Weekly Kt/Vurea of 3.5, which matches the dose of daily hemodialysis, is recommended.46 With a dialysate to plasma urea ratio of 0.6 in 1 hour,47 , 48 30 to 40 L of daily PD exchange volume may be required in a 70-kg patient. In some patients, weekly Kt/Vurea of 2.1 may be acceptable.46 , 49 Non–PD-trained aides or volunteers could also be trained specially to deliver manual PD exchanges in service exigencies.50 , 51
Workforce Training
A CKRT program cannot be implemented without the integral contributions by nurses. Projections of staffing needs in tandem with increasing CKRT demand are detailed in Table 1, and the training needs to start pre-emptively. Nursing staff have to monitor circuit dynamics, titrate volume management, ensure metabolic stability during RCA, and perform infection control.52 The complexity increases with diversification of machines, platforms, filters, fluids, and RCA protocols. A close collaboration between critical care and nephrology nursing is highly desirable. It may be more efficient to have a core group of nursing supervisors to oversee machine assignments, perform the initial setup, and train a larger pool of bedside nurses to monitor ongoing CKRT. Involvement of the lead nurses, along with physicians, in drafting new protocols would provide concurrent education to rapidly increase everyone’s domain knowledge. If physicians and nurses from departments other than the ICU and nephrology will be needed, particular care should be given to developing easily accessible and simplified protocols, as well as audiovisual learning aides that are accessible in the ICU or hospital intranet.
Centralized Coordination
Many large hospitals run several ICUs and CKRT machines are often forward-allocated to individual ICUs for routine deployments. In the context of a pandemic, demand may become unpredictable and there is a need for responsive allocation (and redistribution) of machines to areas experiencing a surge in patient load. It is vital to share information across institutions regarding patient-level CKRT needs, as well to understand the supply chain that vendors use so as to pre-empt threats to resource availability at the national and regional level. A centralized machine and patient census would help administrators coordinate, track, and optimize use. To minimize therapy downtime due to technical issues, pre-emptive machine maintenance is necessary despite the movement restrictions in force. The use of consumables and resupply should be tracked weekly to allow a greater lead time to respond to any supply chain disruptions.
Conclusion
It is imperative that critical care and nephrology teams work together to address shortfalls in life-saving medical treatments exposed by the pandemic. CKRT provision is only a part of the end-to-end management of patients with critical illness and kidney diseases. Upstream management to optimize volume balance and lung-protective ventilation may help moderate AKI severity in patients with acute respiratory distress syndrome and prevent deterioration to the point that KRT is required. Many survivors of KRT-requiring AKI develop chronic kidney disease,53 , 54 and long-term management needs to be planned for them. A careful review of current practice patterns with good clinician oversight of resource limitations could potentially save lives by preventing an undesired rationing of care during the peak of a pandemic.
Article Information
Authors’ Full Names and Academic Degrees
Horng-Ruey Chua, MMed, Graeme MacLaren, MBBS, MSc, Lina Hui-Lin Choong, MMed, Chang-Yin Chionh, MMed, Benjamin Zhi En Khoo, MMed, See-Cheng Yeo, PhD, Duu-Wen Sewa, MBBS, Shin-Yi Ng, MMed, Jason Chon-Jun Choo, MMed, Boon-Wee Teo, MD, Han-Khim Tan, PhD, Wen-Ting Siow, MMed, Rohit Vijay Agrawal, MD, Chieh-Suai Tan, MMed, Anantharaman Vathsala, MD, Rajat Tagore, MD, Terina Ying-Ying Seow, MD, Priyanka Khatri, MD, Wei-Zhen Hong, MMed, and Manish Kaushik, MD.
Support
None.
Financial Disclosure
Dr Chua reports prior research funding from Baxter in an investigator-initiated trial on CKRT. Dr Kaushik reports speaker fees from Baxter and Fresenius Medical Care. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received April 25, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from an Associate Editor and a Deputy Editor. Accepted in revised form May 27, 2020.
Footnotes
Complete author and article information provided before references.
Contributor Information
Horng-Ruey Chua, Email: horng_ruey_chua@nuhs.edu.sg.
Manish Kaushik, Email: manish.kaushik@singhealth.com.sg.
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health Singapore Beds in inpatient facilities and places in non-residential long-term care facilities. Resources & Statistics. 2019 https://www.moh.gov.sg/resources-statistics/singapore-health-facts/beds-in-inpatient-facilities-and-places-in-non-residential-long-term-care-facilities [Google Scholar]
- 6.Ministry of Health Singapore COVID-19 situation report. https://www.moh.gov.sg/docs/librariesprovider5/2019-ncov/situation-report---24-apr-2020.pdf
- 7.Intensive Care National Audit & Research Centre (ICNARC) Case Mix Programme Database ICNARC report on COVID-19 in critical care. https://www.icnarc.org/DataServices/Attachments/Download/7fabeb0c-db96-ea11-9125-00505601089b [DOI] [PMC free article] [PubMed]
- 8.Tolwani A.J., Prendergast M.B., Speer R.R., Stofan B.S., Wille K.M. A practical citrate anticoagulation continuous venovenous hemodiafiltration protocol for metabolic control and high solute clearance. Clin J Am Soc Nephrol. 2006;1(1):79–87. doi: 10.2215/CJN.00040505. [DOI] [PubMed] [Google Scholar]
- 9.Morgera S., Schneider M., Slowinski T. A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Crit Care Med. 2009;37(6):2018–2024. doi: 10.1097/CCM.0b013e3181a00a92. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell A., Daul A.E., Beiderlinden M. A new system for regional citrate anticoagulation in continuous venovenous hemodialysis (CVVHD) Clin Nephrol. 2003;59(2):106–114. doi: 10.5414/cnp59106. [DOI] [PubMed] [Google Scholar]
- 11.Oudemans-van Straaten H., Chua H.R., Oliver J.B., Bellomo R. Metabolic aspects of CRRT. In: Oudemans-van Straaten H., Forni L., Groeneveld A., Bagshaw S., Joannidis M., editors. Acute Nephrology for the Critical Care Physician. Springer International Publishing; Switzerland: 2015. pp. 203–216. [Google Scholar]
- 12.Jonckheer J., Vergaelen K., Spapen H., Malbrain M., De Waele E. Modification of nutrition therapy during continuous renal replacement therapy in critically ill pediatric patients: a narrative review and recommendations. Nutr Clin Pract. 2019;34(1):37–47. doi: 10.1002/ncp.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culley C.M., Bernardo J.F., Gross P.R. Implementing a standardized safety procedure for continuous renal replacement therapy solutions. Am J Health Syst Pharm. 2006;63(8):756–763. doi: 10.2146/ajhp050402. [DOI] [PubMed] [Google Scholar]
- 14.Burgner A., Ikizler T.A., Dwyer J.P. COVID-19 and the inpatient dialysis unit: managing resources during contingency planning pre-crisis. Clin J Am Soc Nephrol. 2020;15(5):720–722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malard B., Lambert C., Kellum J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med Exp. 2018;6(1):12. doi: 10.1186/s40635-018-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan H.K., Kaushik M., Tan C.W. Augmented adsorptive blood purification during continuous veno-venous haemodiafiltration in a severe septic, acute kidney injury patient: use of oXiris(R): a single centre case report. Blood Purif. 2019;47(Suppl 3):1–6. doi: 10.1159/000499633. [DOI] [PubMed] [Google Scholar]
- 17.Yagi N., Leblanc M., Sakai K., Wright E.J., Paganini E.P. Cooling effect of continuous renal replacement therapy in critically ill patients. Am J Kidney Dis. 1998;32(6):1023–1030. doi: 10.1016/s0272-6386(98)70078-2. [DOI] [PubMed] [Google Scholar]
- 18.Parienti J.J., Thirion M., Megarbane B. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA. 2008;299(20):2413–2422. doi: 10.1001/jama.299.20.2413. [DOI] [PubMed] [Google Scholar]
- 19.Chua H.R., Schneider A.G., Sherry N.L. Initial and extended use of femoral versus nonfemoral double-lumen vascular catheters and catheter-related infection during continuous renal replacement therapy. Am J Kidney Dis. 2014;64(6):909–917. doi: 10.1053/j.ajkd.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Goettler C.E., Pryor J.P., Hoey B.A., Phillips J.K., Balas M.C., Shapiro M.B. Prone positioning does not affect cannula function during extracorporeal membrane oxygenation or continuous renal replacement therapy. Crit Care. 2002;6(5):452–455. doi: 10.1186/cc1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clase C.M., Crowther M.A., Ingram A.J., Cina C.S. Thrombolysis for restoration of patency to haemodialysis central venous catheters: a systematic review. J Thromb Thrombolysis. 2001;11(2):127–136. doi: 10.1023/a:1011272632286. [DOI] [PubMed] [Google Scholar]
- 22.Hong W.Z., Haroon S., Lau T. Transitional care program to facilitate recovery following severe acute kidney injury. J Nephrol. 2019;32(4):605–613. doi: 10.1007/s40620-019-00616-z. [DOI] [PubMed] [Google Scholar]
- 23.Joannidis M., Forni L.G., Klein S.J. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020;46(4):654–672. doi: 10.1007/s00134-019-05869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendu M.L., May M.F., Kaze A.D. Non-tunneled versus tunneled dialysis catheters for acute kidney injury requiring renal replacement therapy: a prospective cohort study. BMC Nephrol. 2017;18(1):351. doi: 10.1186/s12882-017-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Rifai A., Sukul N., Wonnacott R., Heung M. Safety of arteriovenous fistulae and grafts for continuous renal replacement therapy: the Michigan experience. Hemodial Int. 2018;22(1):50–55. doi: 10.1111/hdi.12550. [DOI] [PubMed] [Google Scholar]
- 26.Bellomo R., Cass A., Cole L. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 27.Connor M.J., Jr., Karakala N. Continuous renal replacement therapy: reviewing current best practice to provide high-quality extracorporeal therapy to critically ill patients. Adv Chronic Kidney Dis. 2017;24(4):213–218. doi: 10.1053/j.ackd.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Bellomo R., Lipcsey M., Calzavacca P. Early acid-base and blood pressure effects of continuous renal replacement therapy intensity in patients with metabolic acidosis. Intensive Care Med. 2013;39(3):429–436. doi: 10.1007/s00134-012-2800-0. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda H., Uchino S., Uji M. The lower limit of intensity to control uremia during continuous renal replacement therapy. Crit Care. 2014;18:539. doi: 10.1186/s13054-014-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan H.K., Baldwin I., Bellomo R. Continuous veno-venous hemofiltration without anticoagulation in high-risk patients. Intensive Care Med. 2000;26(11):1652–1657. doi: 10.1007/s001340000691. [DOI] [PubMed] [Google Scholar]
- 31.Chua H.R., Baldwin I., Bailey M., Subramaniam A., Bellomo R. Circuit lifespan during continuous renal replacement therapy for combined liver and kidney failure. J Crit Care. 2012;27(6):744.e7–744.e15. doi: 10.1016/j.jcrc.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Poh C.B., Tan P.C., Kam J.W. Regional citrate anticoagulation for continuous renal replacement therapy - a safe and effective low-dose protocol. Nephrology (Carlton) 2020;25(4):305–313. doi: 10.1111/nep.13656. [DOI] [PubMed] [Google Scholar]
- 33.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhai Z., Li C., Chen Y. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(6):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shum H.P., Kwan A.M., Chan K.C., Yan W.W. The use of regional citrate anticoagulation continuous venovenous hemofiltration in extracorporeal membrane oxygenation. ASAIO J. 2014;60(4):413–418. doi: 10.1097/MAT.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 38.Brain M., Winson E., Roodenburg O., McNeil J. Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): a systematic review and meta-analysis. BMC Nephrol. 2017;18(1):69. doi: 10.1186/s12882-017-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fealy N., Aitken L., du Toit E., Lo S., Baldwin I. Faster blood flow rate does not improve circuit life in continuous renal replacement therapy: a randomized controlled trial. Crit Care Med. 2017;45(10):e1018–e1025. doi: 10.1097/CCM.0000000000002568. [DOI] [PubMed] [Google Scholar]
- 40.Gashti C.N., Salcedo S., Robinson V., Rodby R.A. Accelerated venovenous hemofiltration: early technical and clinical experience. Am J Kidney Dis. 2008;51(5):804–810. doi: 10.1053/j.ajkd.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Allegretti A.S., Endres P., Parris T. Accelerated venovenous hemofiltration as a transitional renal replacement therapy in the intensive care unit. Am J Nephrol. 2020;51:318–326. doi: 10.1159/000506412. [DOI] [PubMed] [Google Scholar]
- 42.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:107–110. [Google Scholar]
- 43.Chionh C.Y., Soni S.S., Finkelstein F.O., Ronco C., Cruz D.N. Use of peritoneal dialysis in AKI: a systematic review. Clin J Am Soc Nephrol. 2013;8(10):1649–1660. doi: 10.2215/CJN.01540213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chionh C.Y., Soni S., Cruz D.N., Ronco C. Peritoneal dialysis for acute kidney injury: techniques and dose. Contrib Nephrol. 2009;163:278–284. doi: 10.1159/000223811. [DOI] [PubMed] [Google Scholar]
- 45.Henderson S., Brown E., Levy J. Safety and efficacy of percutaneous insertion of peritoneal dialysis catheters under sedation and local anaesthetic. Nephrol Dial Transplant. 2009;24(11):3499–3504. doi: 10.1093/ndt/gfp312. [DOI] [PubMed] [Google Scholar]
- 46.Cullis B., Abdelraheem M., Abrahams G. Peritoneal dialysis for acute kidney injury. Perit Dial Int. 2014;34(5):494–517. doi: 10.3747/pdi.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabriel D.P., Caramori J.T., Martim L.C., Barretti P., Balbi A.L. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int Suppl. 2008;108:S87–S93. doi: 10.1038/sj.ki.5002608. [DOI] [PubMed] [Google Scholar]
- 48.Ponce D., Brito G.A., Abrao J.G., Balb A.L. Different prescribed doses of high-volume peritoneal dialysis and outcome of patients with acute kidney injury. Adv Perit Dial. 2011;27:118–124. [PubMed] [Google Scholar]
- 49.Chionh C.Y., Ronco C., Finkelstein F.O., Soni S.S., Cruz D.N. Acute peritoneal dialysis: what is the 'adequate' dose for acute kidney injury? Nephrol Dial Transplant. 2010;25(10):3155–3160. doi: 10.1093/ndt/gfq178. [DOI] [PubMed] [Google Scholar]
- 50.Dimkovic N., Aggarwal V., Khan S., Chu M., Bargman J., Oreopoulos D.G. Assisted peritoneal dialysis: what is it and who does it involve? Adv Perit Dial. 2009;25:165–170. [PubMed] [Google Scholar]
- 51.Brown E.A., Wilkie M. Assisted peritoneal dialysis as an alternative to in-center hemodialysis. Clin J Am Soc Nephrol. 2016;11(9):1522–1524. doi: 10.2215/CJN.07040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldwin I., Fealy N. Clinical nursing for the application of continuous renal replacement therapy in the intensive care unit. Semin Dial. 2009;22(2):189–193. doi: 10.1111/j.1525-139X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 53.Lim C.C., Tan C.S., Chia C.M. Long-term risk of progressive chronic kidney disease in patients with severe acute kidney injury requiring dialysis after coronary artery bypass surgery. Cardiorenal Med. 2015;5(3):157–163. doi: 10.1159/000381068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chua H.R., Wong W.K., Ong V.H. Extended mortality and chronic kidney disease after septic acute kidney injury. J Intensive Care Med. 2020;35(6):527–535. doi: 10.1177/0885066618764617. [DOI] [PubMed] [Google Scholar]