Abstract
Background
Evidence-based needs assessments for novel antibiotics against highly-resistant Gram-negative infections (GNIs) are scarce. We aimed to use real-world data from an electronic health record repository to identify treatment opportunities in US hospitals for GNIs resistant to all first-line drugs.
Methods
For this retrospective cohort study, population estimates with an unmet need for novel Gram-negative antibiotics were quantified using the Cerner Health Facts database (2009–15), aggregating episodes of infection in US hospitals with pathogens displaying difficult-to-treat resistance (DTR; resistance to carbapenems, other β-lactams, and fluoroquinolones) and episodes involving empirical coverage with reserve drugs (colistin or polymyxin B and aminoglycosides). Episodes displaying extended-spectrum cephalosporin resistance (ECR) were also estimated. Episodes were multiplied by site-specific and fixed 14-day treatment durations for conservative and liberal days-of-therapy (DOT) estimates and stratified by site and taxon. Hospital type-specific DOT rates were reliability adjusted to account for random variation; cluster analyses quantified contribution from outbreaks.
Findings
Across 2 996 271 inpatient encounters and 134 hospitals, there were 1352 DTR-GNI episodes, 1765 episodes involving empirical therapy with colistin or polymyxin B, and 16 632 episodes involving aminoglycosides. Collectively, these yielded 39·0 (conservative estimate) to 138·2 (liberal estimate) DOT per 10 000 encounters for a novel DTR-GNI-targeted drug, whereas greater treatment opportunities were identified for ECR (six times greater) and β-lactam susceptible GNIs (70 times greater). The most common DTR-GNI site and pathogen was lower respiratory (14·3 [43·3%] of 33 DOT per 10 000 encounters) and Pseudomonas aeruginosa (522 [38·1%] of 1371 episodes), whereas Enterobacteriaceae urinary-tract infections dominated the ECR or carbapenem-sparing niche (59·0% [5589 of 9535 episodes]) equating to 210·7 DOT per 10 000 encounters. DTR Stenotrophomonas maltophilia, Burkholderia spp, and Achromobacter spp represented less than 1 DOT per 10 000 encounters each. The estimated need for DTR-GNI-targeted antibiotics saw minor contributions by outbreaks and varied from 0·5 to 73·1 DOT per 10 000 encounters by hospital type.
Interpretation
Suspected or documented GNIs with no or suboptimal treatment options are relatively infrequent. Non-revenue-based strategies and innovative trial designs are probably essential to the development of antibiotics with improved effectiveness for these GNIs.
Funding
Center for Drug Evaluation and Research, US Food and Drug Administration; Intramural Research Program, National Institutes of Health Clinical Center and the National Institute of Allergy and Infectious Diseases and the National Cancer Institute.
Introduction
Global dissemination of antibiotic resistance is associated with increased mortality, resulting in national and global calls for the development of new antibiotics. A growing number of antibiotics for Gram-negative infections (GNIs) have gained approval in recent years, but the projected pipeline lacks paradigm-shifting innovation.1 Private sector investment has dwindled owing primarily to lack of return on investment, in part attributed to the relative rarity of drug-resistant infections.2 Furthermore, as of 2020, the sales-based revenue model seems misaligned with the ethos of antibiotic stewardship programmes aimed at slowing resistance and minimising cost.3
Aggregate antibiotic vial sales inform estimates of the number of patients who might need treatment for potentially resistant infections in real-world inpatient settings.4 For example, the 2018 US patient population with carbapenem-resistant infections has been estimated to be 53 300 treatment courses.4 However, aggregate antibiotic use data does not provide a complete picture. It remains unclear whether these estimates reflect actual case counts or represent artifacts of slow hospital formulary uptake secondary to cost and provider ambivalence due to scarce evidence of safety and effectiveness.5 Focusing on potential treatment opportunities rather than administered treatments alone could enable a more granular and realistic assessment of the need for novel antibiotics, and thereby better align incentives and investments.
Research in context.
Evidence before this study
Assessment of real-world needs for novel Gram-negative antibiotics with efficacy against highly resistant Gram-negative infections (GNI) could inform development and allocation of non-revenue-based incentives. We searched PubMed with the search terms “market”, “size”, and “antibiotics” on Feb 9, 2020. We evaluated all relevant articles and found one study that quantified the US market size for new antibiotics against carbapenem-resistant Enterobacteriaceae at $289 million (range, $169 to $439 million) based on aggregate sales data but no published studies with patient-level data quantifying treatment opportunities for novel antibiotics against the spectrum of highly resistant Gram-negative pathogens.
Added value of this study
We estimated the inpatients who might benefit from novel Gram-negative antibiotics by searching electronic health records generated at 134 well distributed US hospitals. Instead of aggregating sales data, we analysed patient-level data; microbiology, in-vitro susceptibility, and pharmacy data were merged to identify hypothetical treatment opportunities among those admitted to hospital. Infection episodes due to Gram-negative pathogens displaying difficult-to-treat resistance (DTR), a recently introduced metric signifying resistance to all first-line, high-efficacy, low-toxicity antibiotics combined with theoretical empirical therapy episodes yielded population estimates that ranged from 39·0 to 138·2 days of therapy per 10 000 encounters for a novel DTR-active drug. The study identified that DTR targeted-treatment opportunities varied considerably by hospital region, bed-capacity, and teaching status (ranging from 0·5 to 73·1 days of therapy per 10 000 encounters) and saw a minor contribution by outbreaks. Compared with infections displaying DTR, the study identified nearly six times greater infections due to extended-spectrum cephalosporin-resistant pathogens, representing a potential group for more effective carbapenem-sparing drugs, and 70 times greater infections due to β-lactam susceptible infections.
Implications of all the available evidence
The relatively low number of inpatients identified for novel Gram-negative antibiotics without first-line therapeutic options indicates the need for public sector investment and novel reimbursement strategies to adequately incentivise improved antibiotic research and development. Antibiotic developers should target efforts to high-priority bacterial infections identified in this study with limited treatment options and future trials should attempt to fulfil unmet needs for evidence in inpatients with bacteraemia and pneumonia. New carbapenem-sparing drugs might mitigate carbapenem-selective pressure but is likely contingent on reimbursement strategies.
In this study, we used real-world data from an electronic health record (EHR)-based repository to identify inpatient encounters with an indication for novel antibiotics active against resistant GNIs by quantifying and aggregating empirical and targeted treatment opportunities. We hypothesised that aggregate treatment opportunities to treat GNIs resistant to all first-line drugs would be low across US hospitals.
Methods
Data source and study population
We did a retrospective cohort study using inpatient data from 2009 to 2015, using the Cerner Health Facts database, a de-identified clinical data repository from US hospitals using Cerner EHR systems (North Kansas City, MO, USA; appendix p 2). Institutional review board evaluation was waived by the National Institutes of Health Office of Human Subjects Research because analyses were restricted to de-identified data. Our study conforms to Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for observational studies.6
Treatment opportunities were defined as episodes of suspected or confirmed GNIs due to selected resistant pathogens where a novel antibiotic not limited by safety, tolerability, effectiveness, pharmacokinetic or pharmacodynamic characteristics, formulary, or cost could potentially be used. Gram-negative species of interest were selected on the basis of known tendencies for resistance to currently available, high-efficacy, low-toxicity treatments (appendix p 4).
Resistance definitions
Difficult-to-treat resistance (DTR), a recently introduced resistance metric, is defined as in-vitro non-susceptibility to all first-line, high-efficacy, low-toxicity drugs, including β-lactams, carbapenems, and fluoroquinolones (appendix p 5).7, 8 A subset of this population with bacteraemia has been previously described.8 DTR Stenotrophomonas maltophilia was defined as intermediate or resistant to all tested non-carbapenem β-lactams, fluoroquinolones, and trimethoprim–sulfamethoxazole (co-trimoxazole). The prevalence of DTR S maltophilia bloodstream isolates was also reported using a modified DTR definition foregoing the need for co-trimoxazole resistance, given speculation that co-trimoxazole might not represent a high-efficacy drug against bloodstream isolates.9 Carbapenem-resistant (CR) and extended-spectrum cephalosporin-resistant (ECR) were defined on the basis of the 2015 US Centers for Disease Control (CDC) definitions (appendix p 5).10
Derivation of treatment opportunities for confirmed or suspected DTR-GNI
Episodes of targeted therapy were defined as growth of DTR Gram-negative pathogens along with an antibiotic administration, used to identify presumed infection (appendix p 6). To establish episodes within encounters, a hierarchical algorithm was implemented based on identification of index cultures and 14-day episode intervals (appendix p 7). Index cultures were classified on the basis of site and were thought to represent the source of a secondary bloodstream infection if the same organism and resistance phenotype was identified by blood culture within 3 days of the index culture, a concept adapted from CDC National Healthcare Safety Network surveillance definitions for health-care-associated infections (appendix p 7).11 Repeat treatment episodes were commenced 14 days after the index culture. Targeted days of therapy (DOT) were calculated by multiplying the number of episodes by site-specific DOT based on guidelines or consensus treatment recommendations (conservative estimate) or 14-day treatment periods for all sites (liberal estimate).
For species-level estimates, the hierarchical algorithm was applied to 14 individual taxon-specific datasets. These datasets included the Acinetobacter baumannii complex, Pseudomonas aeruginosa, and Enterobacteriaceae spp, three taxa labelled critical on the WHO priority pathogens list for research and development, along with less prevalent but clinically unique Gram-negative taxa often displaying resistance, including S maltophilia, Burkholderia spp, and Achromobacter spp.12
Given the widespread familiarity with the term CR, GNI episodes with Gram-negative pathogens displaying CR where one or more first-line drugs were active (ie, not meeting DTR criteria) were also quantified to highlight the fraction of CR infections less likely to receive candidate novel antibiotics if first-line alternatives are available. β-lactam susceptible infection episodes and DOT were also estimated (appendix p 2).
Algorithms identifying empirical therapy episodes had two requirements: receipt of either 1 or 2 consecutive days of colistin or polymyxin B (conservative estimate) or colistin, polymyxin B, or aminoglycosides (liberal estimate); along with any clinical culture obtained on the day of or day before the first antibiotic administration displaying either no growth, growth of any Gram-positive organism, or non-resistant Gram-negative pathogens (ie, not displaying DTR, CR, or ECR). Actual days (1 or 2 days) of empirical therapy were summed to generate the aggregate empirical DOT. Encounters receiving 3 days of colistin and polymyxin B, or aminoglycosides were calculated but not included in the analysis as we sought to not overestimate treatment opportunities. Similarly, empirical courses for intravenous tigecycline were also quantified but not included in the aggregate empirical therapy estimate, acknowledging that tigecycline might have been used for US Food and Drug Administration (FDA) approved indications, such as antibiotic-susceptible abdominal infections.7, 13
The real-world candidate population with an unmet need for a DTR panactive (ie, active across all Gram-negative species) antibiotic was estimated using the DOT sum of empirical and targeted DTR treatment opportunities. Henceforth, all reported site-specific and taxon-specific estimates are based on conservative targeted therapy counts.
Derivation of targeted treatment opportunities for ECR-GNI
ECR infections (not classified as DTR or CR) were examined to estimate the potential treatment opportunities for an ECR-active antibiotic with hypothetical carbapenem-sparing capacity. Given the high empirical carbapenem use in US hospitals, much of which is adjudicated as being inappropriate, it is difficult to aggregate empirical carbapenem use that would be considered to make up an idealised empirical therapy estimate.14 Therefore, empirical therapy for suspected ECR pathogens was not evaluated.
Contribution of outbreaks to DTR-GNI burden
To understand the contribution of potential hospital outbreaks to the DTR-GNI population, a cluster analysis was done to evaluate quarterly counts of DTR episodes at each hospital. A post-hoc cutoff point of ten or more DTR episodes per species per hospital quarter was set to represent a high probability for a local or hospital DTR-pathogen outbreak.
Statistical analysis
To estimate targeted treatment opportunities, microbiology and susceptibility datasets were merged to identify positive cultures for select taxa from sites of interest. Pharmacy data was then merged with the microbiology and susceptibility data to identified presumed infections. Using cultures that were presumed to represent infection, the hierarchical algorithm was applied to determine unique targeted treatment episodes. Pharmacy data were used to evaluate empirical episodes for a DTR active drug. Targeted and empirical DTR estimates were merged to determine the overall market size.
To assess variation in overall treatment opportunities by hospital, all 120 hospitals (number of hospitals with a DTR-GNI) were categorised into 19 groups based on hospital characteristics including bed capacity (ie, <100, 100–299, or ≥300 hospital beds), census region, and teaching status. DOT rates and overall encounters for DTR-GNI-targeted antibiotics were calculated for each hospital type. Reliability-adjusted estimates were derived to account for random hospital-level variation using random effects logistic regression.15, 16
Recursive partition analysis was used to construct decision trees to predict the probability of encountering DTR-GNIs by hospital type (appendix p 3). All the hospitals that reported at least one select Gram-negative isolate were included; combinations of available hospital-level variables (bed capacity, teaching status, urban vs rural, and geographical region) were used in the decision tree.
For the overall DTR population, mortality estimates were compared with prevalence of DTR infection by site and to the number of novel antibiotics FDA approved since 2014 (as of January, 2020) for each site. Antibiotics approved since 2014 were: ceftolozane–tazobactam, ceftazidime–avibactam, meropenem–vaborbactam, plazomicin, imipenem–relebactam, and cefiderocol for complicated urinary tract infections; ceftolozane–tazobactam, ceftazidime–avibactam, eravacycline, and imipenem–relebactam for complicated intra-abdominal infections; ceftazidime–avibactam and ceftolozane–tazobactam for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia; omadacycline for community-acquired pneumonia; and omadacycline for acute bacterial skin and skin structure infections.
Analyses were either done using the JMP software (SAS version 14.0.0) or R (version 3.6.0; Development Core Team).
Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
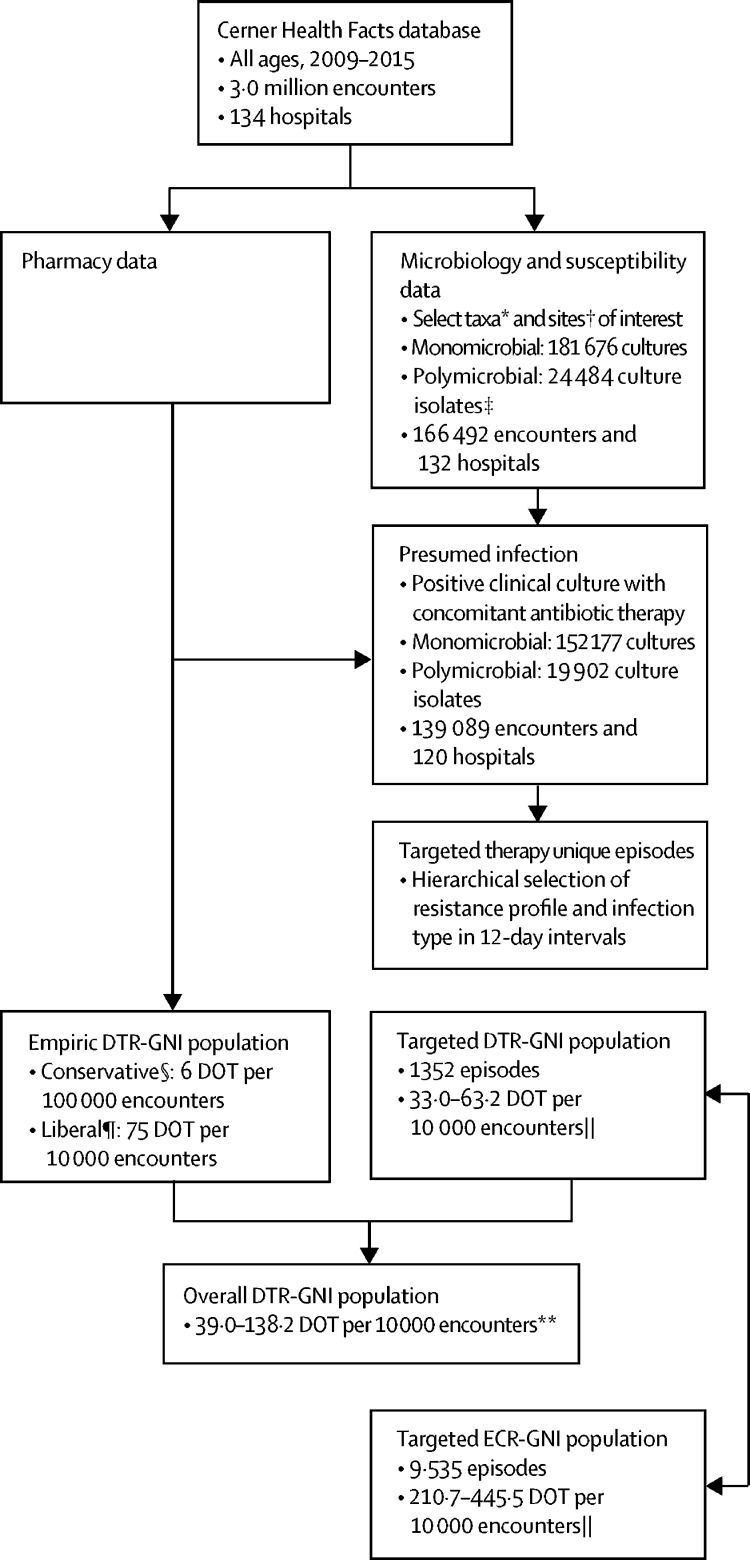
Between 2009 and 2015, there were 2 996 271 in-patient encounters at 134 hospitals included in the analysis (figure 1 ). The hierarchical selection algorithm identified 1352 total episodes warranting targeted DTR therapy, representing 9879 DOT over 7 years (table 1 ). This number of episodes averaged to 193 episodes per year over 7 years with a conservative targeted treatment estimate of 33·0 DOT per 10 000 encounters. Conservative empirical therapy analysis resulted in 1765 episodes for a total of 1802 DOT (6·0 DOT per 10 000 encounters; appendix p 8). Combining conservative targeted and empirical estimates yielded an overall conservative needs estimate of 39·0 DOT per 10 000 encounters (figure 1). Applying treatment durations of 14 days for each site for the targeted therapy population and estimating empirical therapy using empirical colistin, polymyxin B, or aminoglycosides use, the overall estimate more than tripled to 138·2 DOT per 10 000 (figure 1). Although the targeted therapy estimates for DTR-GNI represent 1352 episodes and a range of 33·0–63·2 DOT per 10 000 encounters, over the same time period there were 100 022 β-lactam susceptible GNIs (ie, neither DTR, CR, nor ECR), ranging from 2315·7 to 4673·5 DOT per 10 000 encounters, a 70 times greater market size (appendix p 9).
Figure 1.
Selection of empirical and targeted therapy episodes
DTR=difficult-to-treat resistance. GNI=Gram-negative infections. DOT=days of therapy. *See appendix p 4 for list of organisms of interest. †Sites of interest: blood, gastrointestinal, urine, intra-abdominal, skin and soft tissue, respiratory, secondary bacteraemia, and other. ‡Polymicrobial counts represent each isolate identified in a culture individually. §Conservative estimate based on 1–2 days of colistin or polymyxin B. ¶Liberal estimates based on 1–2 of colistin or polymyxin B and aminoglycosides (amikacin, tobramycin, and gentamicin). ||Lower bound determined with site-specific DOT multiplier and upper bound limited based on 14-day multiplier for all sites. **Overall market is the summation of conservative empirical market and targeted DTR (site-specific multiplier) and upper bound limited based on summation of liberal empirical market and targeted DTR (14-day multiplier for all sites).
Table 1.
DTR, CR, and ECR episodes and days of therapy for site-specific conservative and liberal treatment durations
| DTR episodes | DTR DOT | CR episodes | CR DOT | ECR episodes | ECR DOT | |
|---|---|---|---|---|---|---|
| Conservative treatment duration* | ||||||
| Urinary (5 DOT) | 421 | 2105 | 953 | 4765 | 5774 | 28 870 |
| Intra-abdominal (7 DOT) | 43 | 301 | 105 | 735 | 264 | 1848 |
| Lower respiratory (8 DOT) | 537 | 4296 | 1152 | 9216 | 1396 | 11 168 |
| Skin and soft tissue (5 DOT) | 151 | 755 | 316 | 1580 | 730 | 3650 |
| Other (5 DOT) | 42 | 210 | 97 | 485 | 179 | 895 |
| Primary bloodstream (14 DOT) | 124 | 1736 | 207 | 2898 | 835 | 11 690 |
| Secondary bloodstream (14 DOT) | 34 | 476 | 29 | 406 | 357 | 4998 |
| Total | 1352 | 9879 | 2859 | 20 085 | 9535 | 63 119 |
| DOT per 10 000 encounters | 4·5 | 33·0 | 9·5 | 67·0 | 31·8 | 210·7 |
| Liberal treatment duration† | ||||||
| Urinary (14 DOT) | 421 | 5894 | 953 | 13 342 | 5774 | 80 836 |
| Intra-abdominal (14 DOT) | 43 | 602 | 105 | 1470 | 264 | 3696 |
| Lower respiratory (14 DOT) | 537 | 7518 | 1152 | 16 128 | 1396 | 19 544 |
| Skin and soft tissue (14 DOT) | 151 | 2114 | 316 | 4424 | 730 | 10 220 |
| Other (14 DOT) | 42 | 588 | 97 | 1358 | 179 | 2506 |
| Primary bloodstream (14 DOT) | 124 | 1736 | 207 | 2898 | 835 | 11 690 |
| Secondary bloodstream (14 DOT) | 34 | 476 | 29 | 406 | 357 | 4998 |
| Total | 1352 | 18 928 | 2859 | 40 026 | 9535 | 133 490 |
| DOT per 10 000 encounters | 4·5 | 63·2 | 9·5 | 133·6 | 31·8 | 445·5 |
Data are n or n per 10 000 encounters. All categories (DTR, CR, and ECR) are mutually exclusive groups (CR category excludes episodes classified as DTR and ECR excludes episodes classified as DTR or CR); the term CR specifically refers to the subset of CR pathogens against which at least one first-line drug is active and hence not DTR. DTR=difficult-to-treat resistance. DOT=days of therapy. CR=carbapenem-resistant. ECR=extended-spectrum cephalosporin-resistant.
Conservative treatment duration as per guideline review and clinical practice recommendations (appendix p 9); since no guidelines exist for bacteraemia, a 14-day treatment course was selected arbitrarily.
Treatment duration of 14 days for all sites.
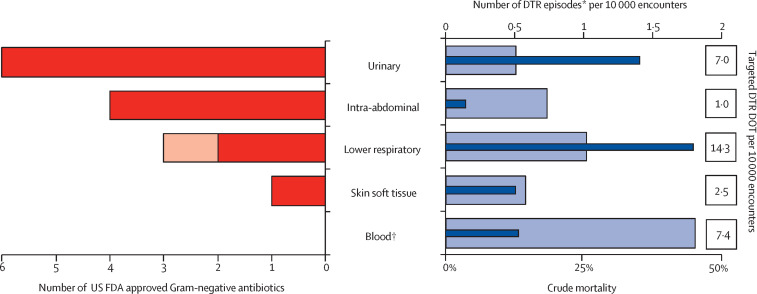
The most common site of infection for targeted therapy was the lower respiratory tract representing 14·3 (43·3%) of 33·0 DOT per 10 000 encounters encompassing the conservative estimate (figure 2 ). The median culture day Sequential Organ Failure Assessment score for patients with bloodstream infections was 4 (IQR 3–5), twice that compared with all other sites combined at 2 (IQR 0–5), p<0·0001; appendix p 10). Patients with DTR bloodstream and lower respiratory tract infections displayed greater need for intensive care unit (ICU) admission compared with patients who had with urinary, abdominal, and other infections. Overall crude mortality for DTR infections (including discharges to hospice) was 22·0% with DTR bloodstream infections displaying the highest site-specific mortality (39·2%; figure 2).
Figure 2.
DTR-GNI episodes, targeted treatment opportunities, and crude mortality versus number of US FDA approved Gram-negative active antibiotics by site since December, 2014
Thick bars on the left represent the number of FDA-approved Gram-negative active antibiotics by site. Both hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (dark red) and community-acquired pneumonia (light red) are displayed as lower respiratory. Thin dark blue bars on the right represent DTR episodes per 10 000 encounters by site and the light blue bars represent associated DTR mortality. FDA=Food and Drug Administration. DTR=difficult-to-treat resistance. DOT=days of therapy. *Includes all infection types per site; “other” infection site not included. †34 of 158 (21·5%) bloodstream infections are secondary to respiratory (n=17), urinary (n=14), skin and soft tissue (n=2), and lower respiratory (n=1) sites.
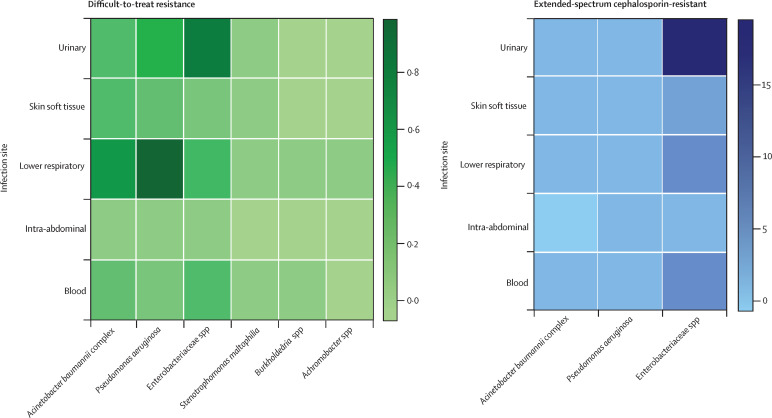
P aeruginosa was the most common DTR pathogen at 522 episodes, corresponding to 12·8 DOT per 10 000 encounters (38·1% of the species-specific DTR population). DTR A baumannii complex accounted for 393 episodes and 9·8 DOT per 10 000 encounters. DTR Enterobacteriaceae spp accounted for 456 episodes and 10·9 DOT per 10 000 encounters (table 2 ). The most common DTR Enterobacteriaceae was Klebsiella spp accounting for 8·9 DOT per 10 000 encounters (82·6% of the Enterobacteriaceae population), whereas all other Enterobacteriaceae spp episodes individually represented less than 1 DOT per 10 000 encounters (appendix p 11). The most common site for DTR A baumannii complex and P aeruginosa was lower respiratory, whereas for DTR Enterobacteriaceae spp, the most common site was urinary (figure 3 ).
Table 2.
DTR, CR, and ECR episodes and site-specific DOT by species*
| DTR episodes | DTR DOT | CR episodes | CR DOT | ECR episodes | ECR DOT | |
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | ||||||
| Urinary (5 DOT) | 127 | 635 | 715 | 3575 | 244 | 1220 |
| Intra-abdominal (7 DOT) | 17 | 119 | 74 | 518 | 16 | 112 |
| Lower respiratory (8 DOT) | 278 | 2224 | 1028 | 8224 | 396 | 3168 |
| Skin and soft tissue (5 DOT) | 48 | 240 | 233 | 1165 | 125 | 625 |
| Other (5 DOT) | 13 | 65 | 74 | 370 | 31 | 155 |
| Primary bloodstream (14 DOT) | 33 | 462 | 137 | 1918 | 38 | 532 |
| Secondary bloodstream (14 DOT) | 6 | 84 | 20 | 280 | 6 | 84 |
| Total | 522 | 3829 | 2281 | 16 050 | 856 | 5896 |
| DOT per 10 000 encounters | .. | 12·8 | .. | 53·6 | .. | 19·7 |
| Acinetobacter baumannii complex | ||||||
| Urinary (5 DOT) | 76 | 380 | 39 | 195 | 80 | 400 |
| Intra-abdominal (7 DOT) | 11 | 77 | 5 | 35 | 9 | 63 |
| Lower respiratory (8 DOT) | 173 | 1384 | 102 | 816 | 183 | 1464 |
| Skin and soft tissue (5 DOT) | 64 | 320 | 54 | 270 | 88 | 440 |
| Other (5 DOT) | 21 | 105 | 9 | 45 | 27 | 135 |
| Primary bloodstream (14 DOT) | 40 | 560 | 16 | 224 | 44 | 616 |
| Secondary bloodstream (14 DOT) | 8 | 112 | 4 | 56 | 4 | 56 |
| Total | 393 | 2938 | 229 | 1641 | 435 | 3174 |
| DOT per 10 000 encounters | .. | 9·8 | .. | 5·5 | .. | 10·6 |
| Enterobacteriaceae spp† | ||||||
| Urinary (5 DOT) | 222 | 1110 | 219 | 1095 | 5589 | 27 945 |
| Intra-abdominal (7 DOT) | 14 | 98 | 34 | 238 | 249 | 1743 |
| Lower respiratory (8 DOT) | 99 | 792 | 73 | 584 | 915 | 7320 |
| Skin and soft tissue (5 DOT) | 39 | 195 | 35 | 175 | 573 | 2865 |
| Other (5 DOT) | 10 | 50 | 16 | 80 | 134 | 670 |
| Primary bloodstream (14 DOT) | 52 | 728 | 56 | 784 | 774 | 10 836 |
| Secondary bloodstream (14 DOT) | 20 | 280 | 6 | 84 | 357 | 4998 |
| Total | 456 | 3253 | 439 | 3040 | 8591 | 56 377 |
| DOT per 10 000 encounters | .. | 10·9 | .. | 10·1 | .. | 188·2 |
Data are n or n per 10 000 encounters. DTR=difficult-to-treat resistance. DOT=days of therapy. CR=carbapenem resistant. ECR=extended-spectrum cephalosporin-resistant.
Hierarchical algorithm for generating market size estimates was applied to a dataset that included only the organism of interest individually; all categories (DTR, CR, and ECR) are mutually exclusive groups (CR category excludes episodes classified as DTR and ECR excludes episodes classified as DTR or CR); the term CR specifically refers to the subset of CR pathogens against which at least one first-line drug is active and hence not DTR.
Enterobacteriaceae spp is a summation of eight species evaluated individually (appendix p 11).
Figure 3.
Heat map of DTR and ECR GNI episodes per 10 000 overall inpatient encounters by species and site
The prevalence of DTR and ECR episodes were stratified by site and by species. Boxes represent species-specific days of therapy. The need for DTR-targeted antibiotics is concentrated around Acinetobacter baumannii and Pseudomonas aeruginosa lower respiratory tract infections and Enterobacteriaceae spp urinary tract infections. ECR Gram-negative infections for which novel carbapenem-sparing antibiotics might have a role were concentrated around Enterobacteriaceae spp urinary tract infections. For Stenotrophomonas maltophilia, Burkholderia spp, and Achromobacter spp only DTR treatment opportunities were evaluated. DTR=difficult-to-treat resistance. ECR=extended-spectrum cephalosporin resistance. GNI=Gram-negative infections.
We identified 19 DTR S maltophilia episodes (ie, resistance to all tested non-carbapenem β-lactams, fluoroquinolones, and co-trimoxazole), contributing to 0·5 DOT per 10 000 encounters and representing 0·5% of all 3652 S maltophilia isolates (before hierarchical algorithm; appendix p 13). This modified DTR definition for S maltophilia bloodstream isolates (that forgoes the need for co-trimoxazole resistance) identified an additional eight qualifying bloodstream infection episodes (0·87 DOT per 10 000 encounters). The DTR population for Burkholderia spp represented 0·51 DOT per 10 000 encounters and that of Achromobacter spp represented 0·29 DOT per 10 000 encounters, all but one of which were from lower respiratory sources (appendix p 13).
When defining DTR and CR as mutually exclusive groups, the targeted DTR-GNI treatment opportunities are about half as many as those of CR-GNI (33 vs 67 DOT per 10 000 encounters). At the species level, DTR-A baumannii complex and DTR-Enterobacteriaceae spp isolates are more common than CR isolates (Table 1, Table 2). Conversely, for P aeruginosa, CR-GNI predominate representing 53·6 DOT per 10 000 encounters compared with 12·8 DOT per 10 000 encounters for DTR only (appendix p 12). The crude mortality for CR-GNI was 15·7%, with bloodstream infections displaying the highest site-specific mortality (33·2%).
ECR-GNIs totalled 9535 episodes and 63 119 DOT over 7 years, representing a targeted treatment opportunity estimate ranging between 211 and 446 DOT per 10 000 encounters (table 1). Notably, Enterobacteriaceae spp predominated the ECR–GNI population at 86·1% (56 377 episodes and 188·2 DOT per 10 000 encounters; table 2). As with the DTR-GNIs, the most common site for ECR P aeruginosa and A baumannii complex was lower respiratory. The most common site for ECR Enterobacteriaceae spp remained urinary. At 11·9%, crude mortality for overall ECR-GNIs was just over half that of DTR-GNIs. However, unlike DTR-GNIs and CR-GNI, for ECR-GNIs, the lower respiratory tract site displayed the highest site-specific morality at 22·3% (appendix p 14).
There was considerable variation in the reliability-adjusted DOT rate and absolute burden of DTR-GNI targeted treatment opportunities by hospital type. Although teaching hospitals with 300 or more hospital beds in the west were found to have the highest DOT rate (73·1 DOT per 10 000 encounters), the greatest absolute burden of treatment opportunities was seen for teaching hospitals in the south (absolute count 3678 DOT) and northeast (absolute count 2024 DOT; appendix pp 15–16). Of 120 hospitals included in the recursive partitioning analysis, 73 (61%) displayed at least one DTR episode over 7 years and the hospital characteristic with the highest association with DTR-GNIs was hospital size (appendix p 17).
Although incomplete reporting by quarter and scarce data on transmission precluded precise identification of outbreaks using individual species estimates, six of the hospital quarterly reports across two hospitals met outbreak criteria for DTR A baumannii complex, as did four hospital quarters across two hospitals for DTR Klebsiella spp. None of the hospital quarters at any of the hospitals met outbreak criteria for DTR P aeruginosa despite having the greatest overall count of DTR episodes (appendix p 17). A baumannii complex and Klebsiella spp episodes that occurred during an outbreak quarter accounted for 22·4% (88 of 393) and 14·6% (54 of 371) of the individual species episodes.
Discussion
This study is the first patient-level, real-world needs assessment for novel antibiotics to treat GNIs with scarce or no routine treatment options. Inpatient Gram-negative clinical isolates at 134 US hospitals were identified along with concurrent antibiotic administration to increase the likelihood of true infection. DTR and empirical colistin or polymyxin B served as separate pragmatic markers for confirmed and suspected highly resistant isolates with few treatment options during the study period and provided a conservative estimate of the opportunity for more effective drugs that could potentially replace existing drugs.7, 8 In our large cohort of US hospitals, treatment opportunities for a hypothetical, novel antibiotic active against highly-resistant GNIs was relatively small, ranging between 39·0 and 138·2 DOT per 10 000 encounters, which is just over 1% of all GNI treatment opportunities. This finding closely mirrors our previous DTR estimate at 1% of Gram-negative bloodstream infections in a disparate cohort of 173 US hospitals in the Premier Healthcare Database, enhancing the external validity of our findings to other US hospitals and an estimate that is likely to shrink further as new antibiotics fill unmet therapeutic niches.7 Nonetheless, the considerable mortality burden of DTR-GNIs and the ongoing emergence of resistance erodes the effectiveness of even recently approved drugs, necessitating the maintenance of robust antibiotic development programmes.7, 17, 18
Our study offers complimentary evidence to a recently published US market size estimate. Decreasing trends in the use of polymyxins and increasing use of new carbapenem-resistant Enterobacteriaceae (CRE)-active drugs observed in US hospitals has been reported, using aggregate sales and patient-level data.19, 20 However, the new CRE-active drugs (ie, ceftazidime–avibactam, meropenem–vaborbactam, and plazomicin) were collectively found be used for only 35% of all CRE infections. Although new CRE-active drugs are being used more frequently, their use has remained low overall yielding approximately $101 million in annual sales in the USA.21 Projecting this sales estimate from 35% use to a hypothetical 100%, the potential annual US market size for new anti-CRE drugs was reported to be approximately $289 million (range $169 to $439 million). However, the denominator of annual CRE infections in the USA of 34 000 used in these studies was derived from a modelling study and is nearly three times the 2019 CDC antibiotic resistance threat report estimate of 13 100 CRE infections per year derived using EHR data from a nationally-weighted estimate of over 700 US hospitals.22, 23 As such, market size estimates hinged on externally derived denominators are subject to considerable variability. However, numerator and denominator data for our study estimates are derived from a single real-world data source of US hospitals. Furthermore, our study quantifies the universe of treatment opportunities for a new antibiotic unrestricted by availability, toxicity, and activity that spans the entire spectrum of DTR Gram-negative pathogens.
Several measures to enhance the use of safer, more effective drugs have been proposed, including optimising antibiotic access, susceptibility testing, hospital reimbursement, and generating a stronger evidence-based practice guideline.24, 25 However, even if barriers to prescribing antibiotics are overcome and the preferential use of new drugs is maximised, the universe of treatment opportunities for DTR Gram-negative pathogens is still likely to remain small, and as per our study, specifically amounts to only one in 70 of all treatment opportunities when compared with β-lactam susceptible infections. These rare treatment opportunities indicate that in addition to overcoming prescribing barriers, the antibiotic development industry will need to be adequately supported and incentivised to ensure that highly safe and effective antibiotics against DTR pathogens will remain available.
Since 2014, the FDA has approved eight new drugs with in-vitro activity against Gram-negative bacterial taxa on the WHO critical pathogen list.26, 27, 28 Current analysis of the antibiotic pipeline reveals 30 new chemical entities in development, but ten of 11 new candidate antibiotics with activity against GNIs are in pre-existing antibiotic classes.29 Re-establishing a robust, innovative antibiotic pipeline to treat and prevent infections will require public and private partnerships and substantial, forward-thinking investments in basic, preclinical, and clinical research along with expanding the research portfolio beyond antibiotics to other non-antibiotic therapies including rapid point-of-care diagnostics.30
Our study showed a disconnect between infection sites evaluated in trials for regulatory approval versus real-world need. Although the overall episodes of DTR P aeruginosa, A baumannii complex, and Enterobacteriaceae spp are comparable, lower respiratory tract (pneumonia) is the top DTR-GNI infection site at 43·3% (figure 2), indicating that DTR-targeted drugs with improved effectiveness in pulmonary infections should be prioritised. However, only two of the eight recently FDA approved GNI-active antibiotics (ceftazidime–avibactam and ceftolozane–tazobactam) have labelled indications for hospital-acquired bacterial pneumonia or ventilator-associated pneumonia, whereas a third (omadacycline) has approval for community-acquired pneumonia. Moreover, only two of eight newly approved drugs (meropenem–vaborbactam and plazomicin) have been evaluated in published randomised controlled trials of patients with carabepenem-resistant infections, whereas a study of cefiderocol for carbapenem-resistant Gram-negatives has been reported to the FDA but has not yet been published.31, 32, 33, 34 By site, bloodstream infections have the highest illness severity and need for critical care among DTR-GNIs with nearly half of these patients dying and therefore, antibiotics for this specific indication represent a serious unmet need. One could hypothesise that a novel drug that is able to decrease overall DTR mortality by half (ie, from 22% to 11%) would save approximately 2000 lives per year in the USA.
In addition to WHO critical priority pathogens,12 we also included less common Enterobacteriaceae genera such as Citrobacter spp, Serratia marcescens, Pantoea spp, and Providencia spp that can display DTR and also separately analysed other less frequent taxa such as S maltophilia, Burkholderia spp, and Achromobacter spp. 35 Although these taxa represent a small portion of GNIs, high-level resistance is common warranting inclusion. S maltophilia is an important cause of ICU-acquired infections comprising 2·1% of all GNI in our study cohort, 0·5% of which were found to display DTR. Intrinsically resistant to carbapenems, routine empirical therapy in ICUs often do not have any activity against S maltophilia. Co-trimoxazole is the generally accepted first-line drug for S maltophilia infections; however, its use is often limited owing to hypersensitivity and marrow suppression, and efficacy in bloodstream infections remains unclear.9 Hence, although our study reveals that S maltophilia resistant to all β-lactams, fluoroquinolones, and co-trimoxazole is exceedingly rare, there are other reasons why new antibiotics specifically targeting S maltophilia would be beneficial.
The treatment opportunities active against ECR-GNIs was six times larger than the DTR-GNI market. More effective carbapenem-sparing drugs could represent an important opportunity for drug development, but the treatment opportunities will be distributed amongst other effective treatment options. Such drugs with activity against ECR-GNIs might mitigate selective pressure globally on an antibiotic class that has seen increased use.36 A recent randomised controlled trial showed better outcomes when using meropenem versus piperacillin–tazobactam for bloodstream infections due to ceftriaxone-resistant Klebsiella pneumoniae and Escherichia coli, a result that is likely to further drive the use of carbapenems. 37 As such, showing added benefit in well controlled trials rather than relying on preclinical data alone will be needed to validate the role of any new Gram-negative antibiotic.
The DOT rate signifying targeted treatment opportunities for novel antibiotics against DTR-GNIs varied by region, bed capacity, and teaching status of US hospitals. Large teaching hospitals from US regions other than the Midwest are most likely to encounter DTR-GNIs, whereas outbreaks appear to provide a minor contribution to targeted treatment opportunities for DTR-GNIs. Our estimates were generated using inpatient data from a single EHR system comprised of US hospitals constrained to the years 2009–15, limiting our ability to extrapolate national and global needs estimates. Additional studies on the prevalence of DTR are emerging. A study38 of data from over 300 hospitals in California showed a trend towards DTR declining among Klebsiella spp health-care-associated infections from 2·2% in 2014 to 1·6% in 2016 (p=0·06). Notably, hospitals in our cohort were well distributed across a number of centre-level characteristics, albeit with some differences in geographical distribution and a greater contribution of large hospitals compared with overall US non-federal acute care hospitals. One could surmise that if our study sample was truly nationally representative, the estimate of aggregate treatment opportunities across US hospitals for novel drugs against suspected or confirmed DTR-GNI would approximate to 80 times our sample estimate, at a lower-bound estimate of approximately 113 000 DOT per year based on American Hospital Association data. Although our estimates are not generalisable to other countries, validations of DTR's prognostic use from Italy and Korea report rates of DTR among Gram-negative bloodstream infections of 11% (Italy) and 12% (Korea), which provide evidence that treatment opportunities in a number of regions globally might exceed those in the USA.39, 40 Ongoing surveillance of DTR could enable national or global indices between resistance and available treatment options at any time and potentially inform future market estimates.
Our study has some important limitations. First, conservative estimates based on site-specific treatment durations and empirical courses limited to colistin or polymyxin B use for 2 days or less might have underestimated overall DTR-GNI treatment opportunities. The duration of targeted therapy for highly resistant pathogens and GNIs involving bone and endovascular sources often exceed routinely recommended treatment courses.41 Accordingly, our liberal targeted estimates of DTR treatment opportunities (63 DOT per 10 000 encounters) might be a more realistic estimate. Second, we are unable to definitively determine whether a positive culture truly represents infection or colonisation despite our requirement for concomitant antibiotic administration. Finally, for several hospitals that reported intermittently or that were late to enter the dataset, data are missing for several quarters, precluding trend analyses and projections. Unfortunately, missing data are a problem common to many real-world data repositories where data elements are retrospectively collected.7 Additionally, real-world datasets contain further limitations in their ability to provide specific antibiotic dosage, precise start and stop times or institutional differences in how susceptibility reporting is performed and reported, all of which can confound results.
In conclusion, patient encounters with a need for novel antibiotics against DTR-GNIs remains small compared with non-DTR-GNIs in the USA. With DTR having found to occur nearly ten times as frequently in some countries outside of the USA (vs US regions) and continuing to display an unacceptably high associated mortality rate, and as de-novo resistance continues to emerge to newly introduced antibiotics, the possibility and repercussions of a diminished antibiotic industry seem ominous.28, 38, 39 Furthermore, implications of the COVID-19 pandemic on the incidence of resistant bacterial infections, empiric antibacterial use, and on the production, availability, and market for novel antibiotics are still unclear. Innovation in antibiotic development, non-antibiotic alternatives, rapid diagnostics, reimbursement strategies, and clinical trial design to better reflect real-world needs, as well as non-revenue-based incentives are likely essential to keep pace with the evolving threat posed by antibiotic resistance.42, 43
Acknowledgments
Acknowledgments
This work was done as part of the National Institute of Health Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI). We thank Edward M Cox (Former Director), John Farley (Director), and Thushi Amini (Associate Director for Research) from the Office of Antimicrobial Products within the Center for Drug Evaluation and Research at the US Food and Drug Administration for project funding; Amber Jessup (senior economist in the Office of Science and Data Policy for the Assistant Secretary for Planning and Evaluation in the US Department of Health and Human Services) for project input; David Fram and Huai-Chen Chun of Commonwealth Informatics for their assistance in data curation and mapping; and Michael Klompas and Dean Follman for their valuable input. The study was funded in part by the Center for Drug Evaluation and Research of the US Food and Drug Administration (interagency agreement number: 224–18–3008S) and the Intramural Research Program of the National Institutes of Health Clinical Center and the National Institute of Allergy and Infectious Diseases and the National Cancer Institute (contract number: HHSN261200800001E). The opinions expressed in this Article are those of the authors and do not represent any position or policy of the National Institutes of Health, the US Department of Health and Human Services, or the US Government.
Contributors
JRS and SSK conceptualised the study. JRS, SW, YLL, and SSK contributed to the study design. JRS and SSK obtained funding. JRS, SW, YLL, and SSK acquired the data. SW and CYD analysed the data. JRS, SW, RLD, and JHP interpreted the data. JRS and SSK drafted the manuscript. All authors critically reviewed the manuscript and approved it for publication.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Talbot GH, Jezek A, Murray BE. The Infectious Diseases Society of America's 10 × ‘20 initiative (ten new systemic antibacterial agents FDA-approved by 2020): is 20 × ’20 a possibility? Clin Infect Dis. 2019;69:1–11. doi: 10.1093/cid/ciz089. [DOI] [PubMed] [Google Scholar]
- 2.Rex JH, Outterson K. Antibiotic reimbursement in a model delinked from sales: a benchmark-based worldwide approach. Lancet Infect Dis. 2016;16:500–505. doi: 10.1016/S1473-3099(15)00500-9. [DOI] [PubMed] [Google Scholar]
- 3.Towse A, Hoyle CK, Goodall J, Hirsch M, Mestre-Ferrandiz J, Rex JH. Time for a change in how new antibiotics are reimbursed: development of an insurance framework for funding new antibiotics based on a policy of risk mitigation. Health Policy. 2017;121:1025–1030. doi: 10.1016/j.healthpol.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Stringer J. Biotechnology: antibiotic and antifungal update September 2019. https://needham.bluematrix.com/sellside/EmailDocViewer?encrypt=06ec2018-46a9-47e8-9e52-c0be3e6a0c9c&mime=pdf&co=Needham&id=tmaloney@needhamco.com&source=libraryView
- 5.Mazuski JE, Gasink LB, Armstrong J. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 7.Kadri SS, Adjemian J, Lai YL. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadri SS, Lai YLE, Ricotta EE. External validation of difficult-to-treat resistance prevalence and mortality risk in gram-negative bloodstream infection using electronic health record data from 140 US hospitals. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echols RM, Tillotson GS. DTR, do we need a new definition? Clin Infect Dis. 2019;69:1641–1642. doi: 10.1093/cid/ciz184. [DOI] [PubMed] [Google Scholar]
- 10.CDC Patient safety atlas. https://www.cdc.gov/hai/data/portal/patient-safety-atlas.html
- 11.Centers for Disease Control and Prevention CDC/NHSN surveillance definitions for specific types of infections. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf
- 12.Tacconelli E, Carrara E, Savoldi A. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 13.Eckmann C, Montravers P, Bassetti M. Efficacy of tigecycline for the treatment of complicated intra-abdominal infections in real-life clinical practice from five European observational studies. J Antimicrob Chemother. 2013;68(suppl 2):ii25–ii35. doi: 10.1093/jac/dkt142. [DOI] [PubMed] [Google Scholar]
- 14.Janssen J, Kinkade A, Man D. CARBapenem utilizatiON evaluation in a large community hospital (CARBON): a quality improvement study. Can J Hosp Pharm. 2015;68:327–331. doi: 10.4212/cjhp.v68i4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C, Jentzsch MS, Kadri SS. Variation in identifying sepsis and organ dysfunction using administrative versus electronic clinical data and impact on hospital outcome comparisons. Crit Care Med. 2019;47:493–500. doi: 10.1097/CCM.0000000000003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45:1614–1629. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadri SS, Strich JR, Swihart BJ. Attributable mortality from extensively drug-resistant gram-negative infections using propensity-matched tracer antibiotic algorithms. Am J Infect Control. 2019;47:1040–1047. doi: 10.1016/j.ajic.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen TB, Brass EP, Gilbert DN, Bartlett JG, Spellberg B. Sustainable discovery and development of antibiotics–is a nonprofit approach the future? N Engl J Med. 2019;381:503–505. doi: 10.1056/NEJMp1905589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clancy CJ, Potoski BA, Buehrle D, Nguyen MH. Estimating the treatment of carbapenem-resistant enterobacteriaceae infections in the United States using antibiotic prescription data. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strich JR, Ricotta E, Warner S. Pharmacoepidemiology of ceftazidime-avibactam use: a retrospective cohort analysis of 210 US hospitals. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa061. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clancy CJ, Nguyen MH. Estimating the size of the United States market for new antibiotics with activity against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01733-19. AAC.01733-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC Antibiotic resistance threats in the United States. 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- 23.Temkin E, Fallach N, Almagor J, Gladstone BP, Tacconelli E, Carmeli Y. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: a modelling study. Lancet Glob Health. 2018;6:e969–e979. doi: 10.1016/S2214-109X(18)30278-X. [DOI] [PubMed] [Google Scholar]
- 24.Outterson K, Powers JH, Daniel GW, McClellan MB. Repairing the broken market for antibiotic innovation. Health Aff (Millwood) 2015;34:277–285. doi: 10.1377/hlthaff.2014.1003. [DOI] [PubMed] [Google Scholar]
- 25.Humphries RM, Ferraro MJ, Hindler JA. Impact of 21st century cures act on breakpoints and commercial antimicrobial susceptibility test systems: progress and pitfalls. J Clin Microbiol. 2018;56:56. doi: 10.1128/JCM.00139-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theuretzbacher U, Paul M. Developing a new antibiotic for extensively drug-resistant pathogens: the case of plazomicin. Clin Microbiol Infect. 2018;24:1231–1233. doi: 10.1016/j.cmi.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Chambers HF. Omadacycline–the newest Tetracycline. N Engl J Med. 2019;380:588–589. doi: 10.1056/NEJMe1900188. [DOI] [PubMed] [Google Scholar]
- 28.Shields RK, Chen L, Cheng S. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61:61. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theuretzbacher U, Gottwalt S, Beyer P. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect Dis. 2019;19:e40–e50. doi: 10.1016/S1473-3099(18)30513-9. [DOI] [PubMed] [Google Scholar]
- 30.Cox E, Nambiar S, Baden L. Needed: antimicrobial development. N Engl J Med. 2019;380:783–785. doi: 10.1056/NEJMe1901525. [DOI] [PubMed] [Google Scholar]
- 31.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKinnell JA, Dwyer JP, Talbot GH. Plazomicin for infections caused by carbapenem-resistant enterobacteriaceae. N Engl J Med. 2019;380:791–793. doi: 10.1056/NEJMc1807634. [DOI] [PubMed] [Google Scholar]
- 33.Echols R, Ariyasu M, Nagata TD. Pathogen-focused clinical development to address unmet medical need: cefiderocol targeting carbapenem resistance. Clin Infect Dis. 2019;69(suppl 7):S559–S564. doi: 10.1093/cid/ciz829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shionogi I. FDA briefing document, meeting of the Antimicrobial Drugs Advisory Committee (AMDAC) 2019. https://www.fdagov/media/131703/download
- 35.Walters MS, Witwer M, Lee YK. Notes from the field: carbapenemase-producing carbapenem-resistant enterobacteriaceae from less common enterobacteriaceae genera–United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2018;67:668–669. doi: 10.15585/mmwr.mm6723a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laxminarayan R, Duse A, Wattal C. Antibiotic resistance–the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 37.Harris PNA, Tambyah PA, Lye DC. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with e coli or klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzo K, Horwich-Scholefield S, Epson E. Carbapenem and cephalosporin resistance among enterobacteriaceae in healthcare-associated infections, California, USA. Emerg Infect Dis. 2019;25:1389–1393. doi: 10.3201/eid2507.181938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannella M, Bussini L, Pascale R. Prognostic utility of the new definition of difficult-to-treat resistance among patients with gram-negative bloodstream infections. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh K, Chung DR, Young EH. Impact of difficult-to-treat resistance in gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa084. published online January 29. [DOI] [PubMed] [Google Scholar]
- 41.Athans V, Neuner EA, Hassouna H. Meropenem-vaborbactam as salvage therapy for ceftazidime-avibactam-resistant Klebsiella pneumoniae bacteremia and abscess in a liver transplant recipient. Antimicrob Agents Chemother. 2018;63:63. doi: 10.1128/AAC.01551-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanini S, Ioannidis JPA, Vairo F. Non-inferiority versus superiority trial design for new antibiotics in an era of high antimicrobial resistance: the case for post-marketing, adaptive randomised controlled trials. Lancet Infect Dis. 2019;19:e444–e451. doi: 10.1016/S1473-3099(19)30284-1. [DOI] [PubMed] [Google Scholar]
- 43.Verma S. Aligning payment and prevention to drive antibiotic innovation for Medicare beneficiaries. 2019. https://www.healthaffairs.org/do/10.1377/hblog20190802.505113/full/?linkId=71474777&
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.