ABSTRACT
Background
Malnutrition is common in patients with chronic heart failure (CHF) and is associated with adverse outcome, but few data exist.
Objectives
The objective of this study was to compare the agreement and classification performance of 6 malnutrition tools in patients with CHF.
Methods
We evaluated the performance of 6 malnutrition tools: COntrolling NUTritional Status Index (CONUT), Geriatric Nutritional Risk Index (GNRI), Prognostic Nutritional Index (PNI), Malnutrition Universal Screening Tool (MUST), Mini Nutritional Assessment–Short Form (MNA-SF), and Subjective Global Assessment (SGA), in 467 consecutive patients with CHF who attended our clinic for follow-up. We used Venn diagrams and Kappa statistics to study the agreement of different tools. Because there is no “gold standard” for malnutrition evaluation, for each of the malnutrition tools, we used the results of the other 5 tools to produce a standard combined index for evaluating at least moderate malnutrition. Subjects were considered as having at least moderate malnutrition if so identified by ≥3/5 tools. We evaluated the sensitivity, specificity, and predictive values of different tools in identifying significant malnutrition as defined by the combined index.
Results
Men comprised 67% of patients, median age was 76 years, and median N-terminal pro-B-type natriuretic peptide (NTproBNP) was 1156 ng/L. The prevalence of any degree and at least moderate malnutrition ranged between 6–60% and 3–9%, respectively, with CONUT classifying the highest proportion of subjects as malnourished. Malnourished patients tended to be older and have worse symptoms, higher NTproBNP, and more comorbidities. CONUT had the highest sensitivity (80%), MNA-SF and SGA had the highest specificity (99%), and MNA-SF had the lowest misclassification rate (2%) in identifying at least moderate malnutrition as defined by the combined index.
Conclusions
Malnutrition is common in patients with CHF. The prevalence of malnutrition varies depending on the tool used. Among the 6 malnutrition tools studied, MNA-SF has the best classification performance in identifying significant malnutrition as defined by the combined index.
Keywords: heart failure, malnutrition, screening, assessment, agreement, classification performance
This is a prospective observation study to compare the agreement and classification performance of 6 commonly used malnutrition tools in 467 consecutive outpatients with heart failure (HF) attending a routine follow-up visit. We evaluated the performance of 3 simple tools [COntrolling NUTritional Status Index (CONUT), Geriatric Nutritional Risk Index (GNRI), and Prognostic Nutritional Index (PNI)] and 3 multidimensional tools [Malnutrition Universal Screening Tool (MUST), Mini Nutritional Assessment–Short Form (MNA-SF), and Subjective Global Assessment (SGA)].
We found that 1) malnutrition is common in patients with HF and is associated with increasing age, comorbidities, and severity of HF; 2) the prevalence of malnutrition varies according to the malnutrition tool used; 3) the agreement among malnutrition tools varies from weak to moderate; and 4) among the 6 tools studied, MNA-SF gave the best performance in detecting significant malnutrition compared with a standard combined index. MNA-SF might be useful in screening for malnutrition in patients with HF.
Introduction
Patients with chronic heart failure (CHF) are at risk of developing malnutrition. CHF is a condition characterized by systemic venous congestion. Malnutrition in CHF might be related to right heart dysfunction and congestion, which predispose to bowel edema, inflammatory activation, and malabsorption, thereby leading to malnutrition and cachexia (1, 2). CHF and malnutrition also share common risk factors such as depression and smoking (3, 4). Once malnutrition develops, it might further contribute to progression of cardiac dysfunction, due to either lack of important nutrients or systemic inflammation (5, 6). Although it is common in patients with CHF, with a prevalence of up to 62%, and is associated with increased morbidity and mortality (7, 8), there is no standard method for evaluating malnutrition.
Several tools have been proposed to evaluate malnutrition and they can generally be categorized as simple or multidimensional tools (7). Simple tools screen for malnutrition by considering laboratory tests and anthropometric measures; on the other hand, multidimensional tools offer a more comprehensive assessment of nutrition status by assessing a variety of factors, including acute illness, mobility, comorbidities, and dietary intake. Multidimensional tools such as the Subjective Global Assessment (SGA) predict mortality in patients with heart failure (HF) (9), but they are unlikely to be used in routine practice because they are too complex and time-consuming. On the other hand, simple tools such as the Geriatric Nutritional Risk Index (GNRI) are also of prognostic value in patients with HF (10, 11); although rapid and easy to perform, they are also unlikely to be used in clinical practice if they do not offer the same information as the complex tools. It is therefore important to compare the 2 classes of tools to determine if the ideal solution of a “quick and useful” tool is realizable.
Previous studies have mostly evaluated malnutrition using individual tools in different populations and settings (7). Few studies have simultaneously evaluated different tools in the same cohort of patients. We have previously evaluated malnutrition using 3 simple tools: GNRI, Prognostic Nutritional Index (PNI), and COntrolling NUTritional Status Index (CONUT), in 2 cohorts of patients with acute or chronic HF. We found that worsening malnutrition using each tool was independently related to an adverse prognosis (3, 12).
To the best of our knowledge, no study has compared simple with multidimensional tools for evaluating malnutrition in patients with CHF. We therefore prospectively compared the prevalence of malnutrition, agreement, and classification performance of 3 simple and 3 multidimensional malnutrition tools in a well-characterized cohort of patients with CHF.
Methods
Study population
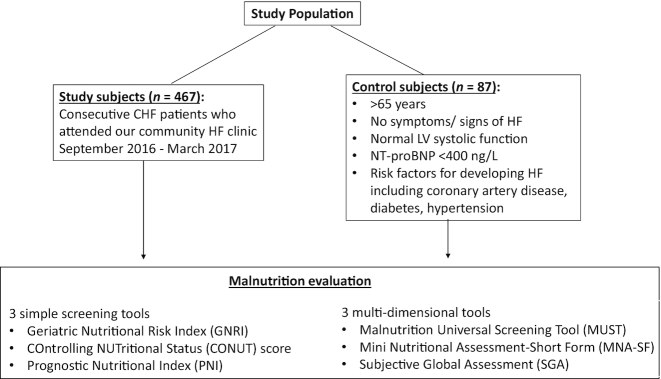
Consecutive ambulatory patients with CHF attending a community HF clinic were enrolled between September 2016 and March 2017 (Figure 1). All patients had a pre-existing (>1 year) clinical diagnosis of CHF. Patients had to have either a left ventricular ejection fraction (LVEF) <40% or at least moderate left ventricular systolic dysfunction by visual inspection if LVEF was not calculated, defined as heart failure with reduced ejection fraction (HeFREF); or normal left ventricular systolic function (LVEF >40% or mild or better left ventricular systolic dysfunction by visual inspection) and raised N-terminal pro-B-type natriuretic peptide (NTproBNP) of >400 ng/L, defined as heart failure with normal ejection fraction (HeFNEF) (13). All patients had already been initiated on HF treatment.
FIGURE 1.
Participant flowchart. CHF, chronic heart failure; HF, heart failure; LV, left ventricular; NTproBNP, N-terminal pro-B-type natriuretic peptide.
Individuals who had previously consented to take part in research were recruited as controls. Control subjects were >65 years, with no previous or current symptoms or signs of HF; with normal left ventricular systolic function on echocardiography and NTproBNP of <400 ng/L. They also had risk factors for development of HF, including coronary artery disease, diabetes mellitus, and hypertension (Figure 1).
All patients had a full medical history, physical examination, blood tests (full blood count, urea and electrolytes, and NTproBNP), an electrocardiogram, and a consultation with a HF specialist. The New York Heart Association (NYHA) functional classification was used to assess the severity of HF symptoms (14).
Malnutrition evaluation
All patients and controls were evaluated by the same researcher (SS) for malnutrition (Supplemental Table 1).
Simple screening tools
The simple screening tools used are discussed next. These tools only take into account laboratory tests and anthropometric measures and can be completed within 1 minute.
GNRI
GNRI was calculated using the following formula: [1.489 × albumin (g/L)] + [41.7 × current weight/ideal weight] (15). Ideal body weight was calculated using the following formula: 22 × square of height in meters (16). Subjects with GNRI >98 have normal nutritional status; those with GNRI 92–98, 82–91, and <82 have mild, moderate, and severe malnutrition, respectively (15). GNRI ≤98 is classified as malnourished.
CONUT score (scored between 0 and 12)
The CONUT score was developed by Ignacio de Ulibarri and colleagues in 2005 as a screening tool for assessment of nutritional status of inpatients (17). It uses serum albumin, cholesterol, and total lymphocyte count. Subjects with a CONUT score 0–1 have normal nutritional status; those with CONUT scores 2–4, 5–8, and 9–12 have mild, moderate, and severe malnutrition, respectively (17). Subjects with a CONUT score ≥2 are classified as malnourished.
PNI
PNI is calculated using the following formula: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (mm3) (18). Subjects with PNI >38 have normal nutritional status; those with PNI 35–38 and <35 have moderate and severe malnutrition, respectively (18). Subjects with PNI ≤38 are classified as malnourished.
Multidimensional tools
The multidimensional tools used are discussed next. These tools take into account different factors that affect nutritional status, including the effect of acute illness, mobility, comorbidities, and dietary intake. They are more time-consuming to perform (on average, 20 minutes for SGA).
Malnutrition Universal Screening Tool (scored between 0 and 2)
The Malnutrition Universal Screening Tool (MUST; Supplemental Figure 1) is a 3-step screening tool developed by the Multidisciplinary Malnutrition Advisory Group of the British Association for Parenteral and Enteral Nutrition (BAPEN) in 2003 to identify malnutrition in adults (19). MUST uses 3 simple steps—determining body mass index (BMI) in kg/m2, weight loss, and the effect of acute illness on food intake—to generate an overall risk of malnutrition. Subjects with MUST score 0 have normal nutritional status (low malnutrition risk); those with MUST score 1 and ≥2 have mild (medium-risk) and at least moderate (high-risk) malnutrition, respectively (19). Subjects with MUST ≥1 are classified as malnourished. The researcher who assessed nutrition status completed the “Nutritional Screening using MUST” BAPEN e-learning module available at https://www.bapen.org.uk/e-learning-portal.
Mini Nutritional Assessment–Short Form (scored between 0 and 14)
The Mini Nutritional Assessment (MNA; Supplemental Table 2) was developed in 1996 as a tool to identify malnutrition in elderly patients (20). MNA–short form (MNA-SF) (21), a shorter version of MNA, consists of 6 questions that assess food intake, weight loss, mobility, acute events, neuropsychological problems, and BMI. Subjects with MNA-SF score 12–14 have normal nutritional status; those with MNA-SF score 8–11 and ≤7 have mild and at least moderate malnutrition, respectively (21). Subjects with MNA-SF score ≤11 are classified as malnourished.
SGA (scored as A–C)
SGA is a nutritional assessment tool that is widely used in a variety of clinical settings (Supplemental Table 3) (22–24). It includes an assessment of medical history (weight loss, changes in dietary intake, gastrointestinal symptoms, and functional capacity) and a physical examination (wasting of large muscle groups as determined by low bulk that is detectable on palpation; low subcutaneous fat measured in the triceps, biceps, and periorbital region; and degree of sacral or ankle edema and ascites). The 4 features of the physical examination are scored as normal (A), mild to moderate (B), or severe (C) malnutrition. These measurements are not precise but, rather, merely a subjective impression. Subjects with SGA-A have normal nutritional status, those with SGA-B and -C have mild and at least moderate malnutrition, respectively (22). Subjects with SGA-B or -C are classified as malnourished.
Data analysis
During data analysis, it quickly became apparent that CONUT score was reporting a disproportionately large number of subjects as having malnutrition of some degree. We therefore performed detailed analyses to study subjects identified by different tools as having “any degree of malnutrition” and “at least moderate malnutrition.”
Comorbidities
Comorbidities were measured using the Charlson Comorbidity Index (25). Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or a pre-existing diagnosis (26). Anemia was defined as hemoglobin <13.0 g/dL in men and <12.0 g/dL in women (27). Diabetes mellitus was defined according to the Diabetes United Kingdom guideline (28). Patients consented to the use of electronic medical records to identify previous clinical history of myocardial infarction, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, dementia, rheumatological disease, peptic ulcer, hemiplegia/paraplegia, liver/renal disease, or malignancy. None of the patients had dementia sufficiently severe as to be lacking capacity.
Statistical analysis
Continuous data are expressed as a median with 25th to 75th centiles, and categorical data are expressed as n (%). Independent t tests and Mann–Whitney U tests were used to compare 2 continuous variables for normally and non-normally distributed data. The chi-square test was used to compare proportions between groups. Pearson and Spearman correlation coefficients were used to assess the relation between 2 variables.
We studied the prevalence of any degree of malnutrition and at least moderate malnutrition in subjects using the different malnutrition tools previously described in detail. We used Venn diagrams to illustrate the relation between malnutrition tools and Kappa statistics to study the agreement between simple and multidimensional malnutrition tools.
We then studied the classification performance of different malnutrition tools (simple and multidimensional tools). Because there is no gold standard in evaluating malnutrition in patients with CHF, for each of the tools, we used the results of the other 5 tools to produce a single combined malnutrition index, which we assumed to be the standard. This methodology has been previously suggested by Pablo et al. (29).
We created 2 sets of combined indices, one for evaluation of any degree of malnutrition and the other for evaluation of at least moderate malnutrition. The combined index for any degree of malnutrition classifies subjects into malnourished (any degree) and not malnourished: subjects were considered as malnourished (any degree) if so identified by at least 3 of the 5 tools. Similarly, the combined index for at least moderate malnutrition classifies subjects into ≤ mild malnutrition and at least moderate malnutrition: subjects were considered as having at least moderate malnutrition if so identified by at least 3 of the 5 tools.
In a separate analysis, in order to assess the value of single laboratory tests (albumin, lymphocyte count, and cholesterol) in defining any degree of malnutrition or at least moderate malnutrition, we compared each with 2 similar combined indices as described previously (one for evaluation of any degree of malnutrition and another for evaluation of at least moderate malnutrition) derived from the tools that did not contain the variable in question.
The sensitivity, specificity, and predictive values for each of the individual tools and single laboratory tests in identifying malnutrition as defined by the combined index were calculated.
To investigate the bias associated with SGA being a subjective malnutrition tool, in addition to the principal investigator (SS), a second investigator (JW) also completed the SGA for a random sample of 23 patients. Kappa statistic was used to determine the interoperator agreement.
All statistical analyses were performed using SPSS version 24 (SPSS) and Stata version 14 (StataCorp) statistical computer package. A 2-tailed P value of <0.05 was considered significant in all analyses.
The study conformed to the principles outlined in the Declaration of Helsinki and was approved by the Yorkshire and the Humber–South Yorkshire Research Ethics Committee (study reference number 03/02/044). All subjects gave their written informed consent for their data to be used for research.
Results
A total of 467 consecutive patients with CHF and 87 controls were studied. The agreement and classification performance of different malnutrition tools were evaluated and compared. Table 1 shows the baseline characteristics of the HF cohort compared with controls. The majority of patients and controls were men and elderly; 17% of those with CHF were >85 years (compared with 2% of controls). Most of the patients with CHF had HeFREF (62%) with a median NTproBNP >1100 ng/L; approximately one-fifth had severe symptoms (NYHA class III/IV).
TABLE 1.
Baseline characteristics of HF cohort compared with controls1
| Controls (n = 87) | HF (n = 467) | Wilcoxon test statistic | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y (median: 25th–75th centiles) | 73 (69–77) | 76 (69–82) | 19,952 | 0.11 |
| Male, n (%) | 69 (79) | 313 (67) | — | 0.02 |
| HR, bpm (median: 25th–75th centiles) | 61 (55–70) | 70 (60–80) | 16,193 | <0.001 |
| BP systolic, mm Hg (median: 25th–75th centiles) | 144 (130–152) | 139 (126–162) | 128,931 | 0.98 |
| BP diastolic, mm Hg (median: 25th–75th centiles) | 76 (70–82) | 75 (66–83) | 128,433 | 0.40 |
| NYHA III/IV, n (%) | — | 103 (22) | — | — |
| HeFREF, n (%) | — | 291 (62) | — | — |
| HeFNEF, n (%) | — | 176 (38) | — | — |
| Height, m (median: 25th–75th centiles) | 1.71 (1.63–1.75) | 1.68 (1.61–1.75) | 127,866 | 0.20 |
| Weight, kg (median: 25th–75th centiles) | 81 (73–92) | 83 (69–99) | 23,016 | 0.22 |
| BMI, kg/m2 (median: 25th–75th centiles) | 27.8 (25.2–30.8) | 29.0 (25.0–33.2) | 21,848 | 0.08 |
| Charlson score (median: 25th–75th centiles) | 6 (4–7) | 8 (6–10) | 12,643 | <0.001 |
| Medications | ||||
| BB, n (%) | 57 (66) | 392 (84) | — | <0.001 |
| ACEi/ARB, n (%) | 51 (59) | 389 (83) | — | <0.001 |
| MRA, n (%) | 1 (1) | 214 (46) | — | <0.001 |
| Digoxin, n (%) | 0 | 100 (21) | — | <0.001 |
| Loop diuretic, n (%) | 3 (3) | 347 (74) | — | <0.001 |
| Thiazide, n (%) | 8 (9) | 17 (4) | — | 0.02 |
| Statin, n (%) | 67 (77) | 290 (62) | — | 0.008 |
| ≥5 medications, n (%) | 58 (67) | 404 (87) | — | <0.001 |
| Blood tests | ||||
| NTproBNP, ng/L (median: 25th–75th centiles)2 | 170 (99–278) | 1156 (496–2463) | 7180 | <0.001 |
| Hb, g/dL (median: 25th–75th centiles) | 139 (127–147) | 131 (118–142) | 123,648 | 0.007 |
| Na, mmol/L (median: 25th–75th centiles) | 137 (136–139) | 137 (135–138) | 125,823 | 0.01 |
| K, mmol/L (median: 25th–75th centiles) | 4.4 (4.2–4.6) | 4.4 (4.2–4.7) | 22,212 | 0.11 |
| eGFR, mL/min per 1.73 m2 (median: 25th–75th centiles) | 77 (64–87) | 55 (40–73) | 119,721 | <0.001 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; BP, blood pressure; bpm, beats per minute; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HeFNEF, heart failure with normal ejection fraction; HeFREF, heart failure with reduced ejection fraction; HF, heart failure; HR, heart rate; K, potassium; MRA, mineralocorticoid receptor antagonist; Na, sodium; NTproBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
Two values are missing for NTproBNP.
Prevalence of malnutrition
Malnutrition of any degree
The prevalence of malnutrition of any degree in patients with CHF was highly variable, ranging from 6% to 60%, depending on the malnutrition tool used (Supplemental Figure 2). The CONUT score classified a much larger proportion of subjects (both patients with CHF and controls) as malnourished by any degree than other tools [patients: n = 279 (60%); controls: n = 43 (49%)].
Among the simple screening tools, CONUT score graded the greatest proportion and PNI graded the lowest proportion of patients as malnourished by any degree (Figure 2, Supplemental Figure 2). Only 3% (n = 15) of patients were classified as malnourished by any degree by all 3 simple screening tools (Figure 2, top right).
FIGURE 2.
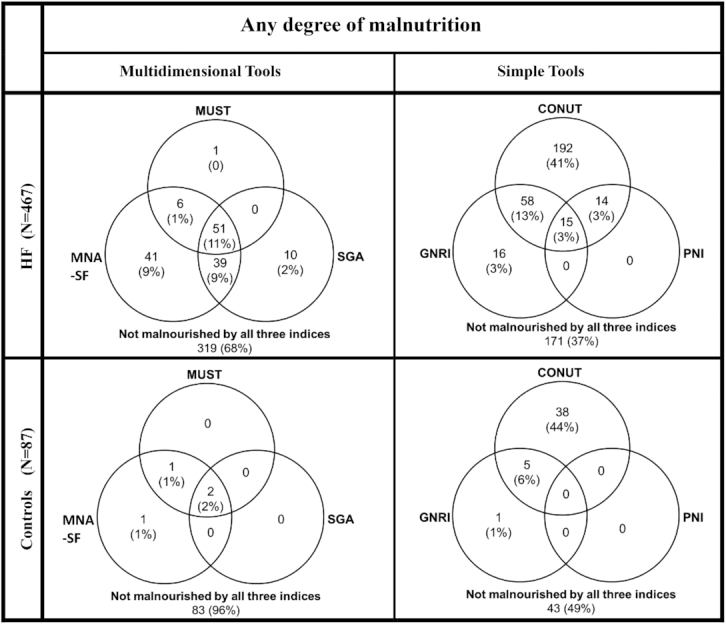
Venn diagrams showing the relation between different simple and multidimensional screening tools in detecting any degree of malnutrition in patients with HF and in controls CONUT, COntrolling NUTritional Status Index; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; MNS-SF, Mini Nutritional Assessment–Short Form; MUST, Malnutrition Universal Screening Tool; PNI, Prognostic Nutritional Index; SGA, Subjective Global Assessment.
Among the multidimensional tools, MNA-SF graded the greatest proportion and the MUST score graded the lowest proportion of patients as malnourished by any degree (Figure 2, Supplemental Figure 2). Only 11% (n = 51) of patients were classified as malnourished by any degree by all 3 multidimensional tools (Figure 2, top left).
The prevalence of malnutrition of any degree was similar in patients with HeFNEF compared to those with HeFREF but was generally more common in patients with atrial fibrillation (AF) compared to those with sinus rhythm (Supplemental Table 4). The prevalence of malnutrition of any degree increased with decreasing BMI and increasing NYHA class, age, and NTproBNP (Supplemental Table 4).
At least moderate malnutrition
The prevalence of at least moderate malnutrition in patients with CHF ranged from 3% to 9%, depending on the malnutrition tool used (Supplemental Figure 2). It was much more common in patients with CHF than in controls.
Among the simple screening tools, the CONUT score graded the greatest proportion of patients as having at least moderate malnutrition (Figure 3, Supplemental Figure 2). Only 2% (n = 9) of patients were classified as having at least moderate malnutrition by all 3 simple screening tools (Figure 3, top right).
FIGURE 3.
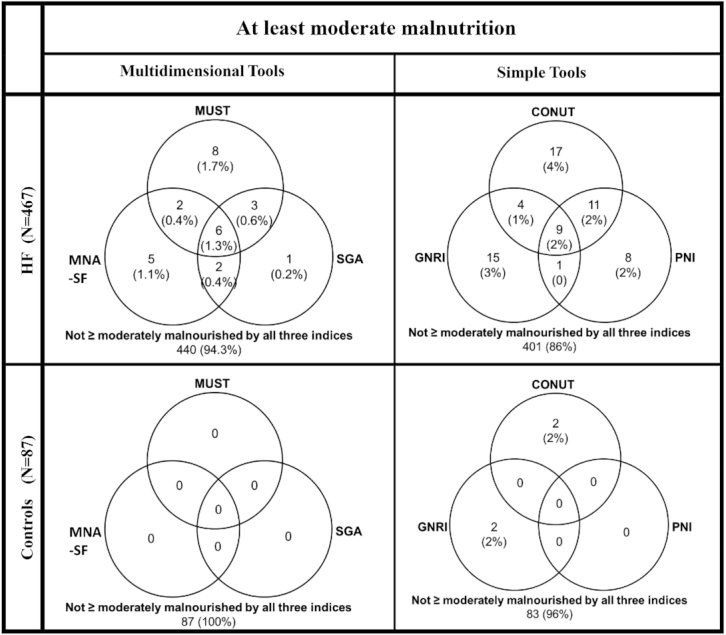
Venn diagrams showing the relation between different simple and multidimensional screening tools in detecting at least moderate malnutrition in patients with HF and in controls. CONUT, COntrolling NUTritional Status Index; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; MNS-SF, Mini Nutritional Assessment–Short Form; MUST, Malnutrition Universal Screening Tool; PNI, Prognostic Nutritional Index; SGA, Subjective Global Assessment.
Among the multidimensional tools, the MUST score graded the greatest proportion of patients as having at least moderate malnutrition (Figure 3, Supplemental Figure 2). Only 1.3% (n = 6) of patients were classified as having at least moderate malnutrition by all 3 multidimensional tools (Figure 3, top left).
The prevalence of at least moderate malnutrition was similar in patients with HeFNEF compared to those with HeFREF and in patients with AF compared to those with sinus rhythm (Table 2). The prevalence of at least moderate malnutrition increased with decreasing BMI and increasing NYHA class and NTproBNP (Table 2).
TABLE 2.
Prevalence of at least moderate malnutrition in different subgroups of patients with CHF1
| Multidimensional tools | Simple tools | |||||
|---|---|---|---|---|---|---|
| MUST (n = 19) | MNA-SF (n = 15) | SGA (n = 12) | GNRI (n = 29) | CONUT (n = 41) | PNI (n = 29) | |
| Heart rhythm | ||||||
| SR (n = 252) | 4% (n = 10) | 2% (n = 5) | 2% (n = 4) | 7% (n = 17) | 6% (n = 16) | 4% (n = 11) |
| AF (n = 215) | 4% (n = 9) | 5% (n = 10) | 4% (n = 8) | 6% (n = 12) | 12% (n =25) | 8% (n = 18) |
| P (SR vs. AF) | 0.91 | 0.10 | 0.15 | 0.60 | 0.04 | 0.07 |
| BMI categories, kg/m2 | ||||||
| <24.9 (n = 111) | 13% (n = 14) | 10% (n = 11) | 9% (n = 10) | 26% (n = 29) | 18% (n = 20) | 10% (n = 11) |
| 25.0–29.9 (n = 158) | 2% (n = 3) | 1% (n = 2) | 1% (n = 2) | 0 | 8% (n = 12) | 7% (n = 11) |
| ≥30 (n = 198) | 1% (n = 2) | 1% (n = 2) | 0 | 0 | 5% (n = 9) | 4% (n = 7) |
| P (BMI categories) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.07 |
| HF phenotype | ||||||
| HeFREF (n = 291) | 5% (n = 13) | 3% (n = 9) | 2% (n = 6) | 6% (n = 18) | 9% (n = 26) | 6% (n = 17) |
| HeFNEF (n = 176) | 3% (n = 6) | 3% (n = 6) | 3% (n = 6) | 6% (n = 11) | 9% (n = 15) | 7% (n = 12) |
| P (HeFREF vs. HeFNEF) | 0.58 | 0.85 | 0.37 | 0.98 | 0.89 | 0.67 |
| NYHA class | ||||||
| I/II (n = 364) | 3% (n = 12) | 2% (n = 7) | 1% (n = 5) | 6% (n = 21) | 6% (n = 22) | 4% (n = 16) |
| III/IV (n = 103) | 7% (n = 7) | 8% (n = 8) | 7% (n = 7) | 8% (n = 8) | 18% (n = 19) | 13% (n = 13) |
| P (I/II vs. III/IV) | 0.11 | 0.003 | 0.002 | 0.46 | <0.001 | 0.002 |
| NTproBNP (ng/L) | ||||||
| <1000 (n = 215) | 1% (n = 2) | 1% (n = 1) | 0 | 3% (n = 7) | 5% (n = 10) | 3% (n = 7) |
| 1000–2000 (n = 108) | 4% (n = 4) | 1% (n = 1) | 1% (n = 1) | 7% (n = 8) | 6% (n = 6) | 5% (n = 5) |
| >2000 (n = 144) | 9% (n = 13) | 9% (n = 13) | 8% (n = 11) | 10% (n = 14) | 18% (n = 25) | 12% (n = 17) |
| P (NTproBNP categories) | 0.001 | <0.001 | <0.001 | 0.04 | <0.001 | 0.003 |
| Age, y | ||||||
| <65 (n = 82) | 1% (n = 1) | 0 | 0 | 2% (n = 2) | 2% (n = 2) | 2% (n = 2) |
| 65–75 (n = 139) | 2% (n = 3) | 2% (n = 3) | 2% (n = 3) | 3% (n = 4) | 6% (n = 8) | 6% (n = 8) |
| >75 (n = 246) | 6% (n = 15) | 5% (n = 12) | 4% (n = 9) | 9% (n = 23) | 13% (n = 31) | 8% (n = 19) |
| P (age categories) | 0.06 | 0.07 | 0.18 | 0.01 | 0.006 | 0.22 |
AF, atrial fibrillation; CHF, chronic heart failure; CONUT, COntrolling NUTritional Status Index; GNRI, Geriatric Nutritional Risk Index; HeFREF, heart failure with reduced ejection fraction; HeFNEF, heart failure with normal ejection fraction; MNA-SF, Mini Nutritional Assessment–Short Form; MUST, Malnutrition Universal Screening Tool; NTproBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PNI, Prognostic Nutritional index; SGA, Subjective Global Assessment; SR, sinus rhythm.
Relation between malnutrition and clinical data
Malnutrition of any degree
Compared with those with normal nutritional status, patients with malnutrition of any degree were older and had a lower BMI, more comorbidities, worse symptoms, higher NTproBNP, and lower hemoglobin. They were also less likely to be on angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor antagonist (ARB) or statins (Supplemental Table 5).
At least moderate malnutrition
Compared with those with normal nutritional status or mild malnutrition, patients with at least moderate malnutrition were older and had a lower BMI, more comorbidities, worse symptoms, higher NTproBNP, and lower hemoglobin (Supplemental Table 6). They were also less likely to be on ACEi/ARB or statins.
Agreement between simple and multidimensional tools
Malnutrition of any degree
Of the simple screening tools, GNRI had the highest and CONUT score the lowest agreement with multidimensional tools in identifying malnutrition of any degree (Supplemental Table 7). There was a greater degree of agreement in identifying patients with any degree of malnutrition using the multidimensional tools compared with simple screening tools.
At least moderate malnutrition
Of the simple screening tools, GNRI had the highest and CONUT score the lowest agreement with multidimensional tools in identifying at least moderate malnutrition (Supplemental Table 8). There was a greater degree of agreement in identifying patients with at least moderate malnutrition using the multidimensional tools compared with simple screening tools.
Classification performance of different malnutrition tools according to the combined index
Malnutrition of any degree
Among the patients with CHF, the MNA-SF score had the greatest sensitivity and MUST and PNI had the highest specificity in identifying malnutrition of any degree defined by the combined index (Supplemental Table 9). SGA had the lowest and CONUT had the highest misclassification rate. Single tests generally had higher misclassification rates compared with either simple or multidimensional tools.
In nonobese patients (BMI <30), GNRI had a sensitivity of 73% in identifying malnutrition of any degree, but its sensitivity was 0 in obese patients (BMI ≥30) (Supplemental Tables 10 and 11). Similarly, in nonobese patients, SGA had a sensitivity of 94% in identifying malnutrition of any degree, but its sensitivity was 38% in obese patients (Supplemental Tables 10 and 11).
At least moderate malnutrition
Among the patients with CHF, the CONUT score had the greatest sensitivity and MNA-SF and SGA had the highest specificity in identifying at least moderate malnutrition defined by the combined index (Table 3). MNA-SF had the lowest and CONUT the highest misclassification rate. Single tests (serum albumin, cholesterol, or total lymphocyte levels) generally had higher misclassification rates compared with either simple or multidimensional tools.
TABLE 3.
Sensitivity, specificity, and misclassification rates of different malnutrition tools in identifying at least moderate malnutrition in patients with CHF as defined by the combined index (the assumed gold standard)1
| Malnutrition screening | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Simple | Multidimensional | Single tests | |||||||
| HF patients | CONUT | GNRI | PNI | MUST | MNA-SF | SGA | Lymphocyte <1.2 × 109/L | Albumin <30 g/L | Cholesterol <3.62 mmol/L |
| Sensitivity, % | 80 | 57 | 73 | 56 | 69 | 56 | 56 | 38 | 60 |
| Specificity, % | 94 | 95 | 96 | 98 | 99 | 99 | 84 | 98 | 68 |
| PPV, % | 29 | 28 | 38 | 47 | 73 | 75 | 7 | 42 | 6 |
| NPV, % | 99 | 99 | 99 | 98 | 99 | 98 | 99 | 98 | 98 |
| False positive, % | 6 | 5 | 4 | 2 | 1 | 1 | 15 | 2 | 31 |
| False negative, % | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 |
| Misclassification rate, % | 7 | 6 | 5 | 4 | 2 | 3 | 16 | 4 | 32 |
CHF, chronic heart failure; CONUT, COntrolling NUTritional Status Index; GNRI, Geriatric Nutritional Risk Index; MNA-SF, Mini Nutritional Assessment–Short Form; MUST, Malnutrition Universal Screening Tool; NPV, negative predictive value; PNI, Prognostic Nutritional Index; PPV, positive predictive value; SGA, Subjective Global Assessment.
In nonobese patients (BMI <30), GNRI had a sensitivity of 62% in identifying at least moderate malnutrition, but its sensitivity was 0 in obese patients (BMI ≥30) (Supplemental Tables 12 and 13). Similarly, in nonobese patients, SGA had a sensitivity of 60% in identifying at least moderate malnutrition, but its sensitivity was 0 in obese patients (Supplemental Tables 12 and 13).
Interoperator agreement of SGA
The agreement between the 2 operators’ judgments on degree of malnutrition in a random sample of subjects (n = 23) using the SGA had a kappa coefficient of 0.65 (95% CI: 0.59, 0.71; P = 0.001).
Discussion
Ours is the first study to compare directly several commonly used simple and multidimensional malnutrition tools in patients with CHF. We found that malnutrition is common, with a prevalence of malnutrition by any degree and moderate to severe malnutrition of 6–60% and 3–9%, respectively, depending on the tool used. Our findings are similar to those from a meta-analysis that evaluated the role of different malnutrition tools in patients with acute and chronic HF (7). The prevalence of malnutrition in patients with CHF was between 16% and 62% depending on the malnutrition tool used and the population studied.
Our results showed that the variation in prevalence of malnutrition (of any degree and at least moderate) is much greater among simple screening tools (any degree: 6–60%; at least moderate: 6–9%) compared with multidimensional tools (any degree: 12–29%; at least moderate: 3–4%). The CONUT score in particular suggested that many more patients were “malnourished” compared with GNRI or PNI. There was a greater degree of agreement in identifying malnourished patients using the multidimensional tools compared with simple screening tools. The agreement between the simple and multidimensional tools was weak for some tools, suggesting that the tools are measuring different aspects of malnutrition because they do not identify the same group of patients as being malnourished. The heterogeneity of the tools was further demonstrated by our finding that the prevalence of malnutrition was higher in patients with AF than in patients with sinus rhythm according to some malnutrition tools but not others.
We found that malnutrition was equally common in patients with HeFREF and in those with HeFNEF. Malnutrition was more common in patients with worse NYHA classes and higher natriuretic peptide concentrations, suggesting that malnutrition is more closely related to the severity of HF rather than to the HF phenotype.
Different tools have their own strengths and weaknesses. Among the simple screening tools, CONUT score has the highest sensitivity, but it also has the highest false-positive rate in identifying at least moderate malnutrition compared with the combined index. The CONUT score is confounded by the use of statins (62% of patients with CHF were on statins), which causes lower cholesterol concentrations irrespective of nutritional status. Furthermore, of the 3 components of CONUT score, cholesterol concentration and lymphocyte count treated as single measures misclassified a significant proportion of patients compared with the combined index. Further studies are needed to determine the optimal cutoffs for each component of the CONUT score to improve its classification performance.
PNI (although specific) has the highest false-negative rate in identifying malnutrition of any degree, hence underestimating malnutrition compared with other tools. This is because PNI does not have a mild malnutrition category and only identifies patients with at least moderate malnutrition. GNRI seems to be the best simple screening tool for malnutrition in patients with CHF, but only when BMI is <30.
The multidimensional tools offer a more comprehensive evaluation of nutritional status compared with the simple screening tools. They have more stringent criteria for identifying malnutrition compared with simple tools; although they classify a smaller proportion of subjects as malnourished, they are likely to be more accurate in detecting malnutrition. MUST score and MNA are both commonly used in different settings: hospital wards, clinics, general practice, and care homes (30, 31). MNA-SF, a shorter version of MNA, is quicker to complete and has similar validity and accuracy as the MNA in detecting malnutrition in older adults (15, 32, 33). In our study, among all the malnutrition tools studied, MNA-SF had the lowest misclassification rate in detecting at least moderate malnutrition compared with the combined index; therefore, it might be appropriate to use in patients with CHF. Compared with the MUST score, in addition to considering BMI, weight loss, and the effect of acute illness on nutritional intake, MNA-SF also takes into account the impact of mobility and neuropsychological problems.
SGA is the most comprehensive of the 3 multidimensional tools. It considers weight change, dietary changes, gastrointestinal symptoms, and functional capacity. In addition, a significant proportion of the assessment depends on the results of a comprehensive physical examination. Similar to MNA-SF, SGA also has a low misclassification rate in detecting significant malnutrition compared with the combined index. However, SGA is subjective and is not sensitive in detecting malnutrition in obese patients. It also requires significant time to perform (average of ∼20 minutes).
Biomarkers such as lymphocyte count, albumin, or cholesterol have long been used in isolation to evaluate nutritional status, but they might be affected by treatments, social conditions, or other diseases rather than malnutrition alone. They thus are unlikely to be able to evaluate nutritional status accurately (34, 35). We found that compared with simple and multidimensional tools, individual biomarkers had higher misclassification rates.
The double burden of malnutrition is a novel concept that emphasizes the coexistence of undernutrition and overnutrition (overweight and obesity) (36). Most of the malnutrition tools we studied regard malnutrition as “undernutrition without overnutrition”; classifying patients as “malnourished” based on factors such as low body weight or BMI, weight loss, decline in food intake, low cholesterol concentration, low muscle bulk, or subcutaneous fat on physical examination. GNRI and SGA focus on anthropometric measures; they have a much lower sensitivity in detecting malnutrition in obese compared with non-obese patients. Apart from anthropometric measures, MNA-SF also takes into account other factors affecting nutrition, such as acute illness, cognition, and mobility, and it is thus the only tool that is effective at identifying malnutrition in the obese [prevalence of malnutrition by any degree according to MNA-SF was 19% in patients with BMI ≥30, much higher than that determined by other tools apart from CONUT (Supplemental Table 4)]. The new malnutrition reality is that it has varied manifestations and should not be managed with a siloed approach.
Study limitations
This is a single-center study conducted in the United Kingdom with limited sample size and mainly enrolled Caucasians. External validation of our results in other populations is needed. However, our study is the largest one to directly compare several commonly used malnutrition tools in consecutive, unselected patients with CHF.
Second, we have only studied 6 of the most commonly used malnutrition tools in the literature. A large number of other malnutrition tools have been proposed.
Third, this study only focuses on studying the agreement and classification performance of different malnutrition tools. The prognostic role of these tools will be presented in a subsequent article due to the vastness of information already presented here. Furthermore, some might not agree with our approach of creating a combined index, invented for comparison of the different tools. However, given the fact that there is currently no consensus on how malnutrition should be evaluated in patients with CHF, we think this approach is a reasonable way to allow comparisons to be made. A consensus definition of malnutrition is needed in order to determine how best to measure it.
Last, aging is a risk factor for the development of malnutrition (37). In our cohort, old age might have partially contributed to the higher prevalence of malnutrition in patients with CHF compared with controls.
Conclusions
Malnutrition is common in patients with CHF and is associated with increasing age, comorbidities, and severity of HF. The prevalence is variable depending on the malnutrition tool used. The agreement among malnutrition tools varies from weak to moderate. Among the 6 tools studied, MNA-SF has the best classification performance in identifying significant malnutrition compared with the combined index and might be useful in screening for malnutrition in patients with CHF.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SS, PP, and ALC: designed the research; SS, PP, and JW: conducted the research; SS, PP, and JZ: analyzed the data; SS: wrote the manuscript; PP, JZ, JW, and ALC: reviewed the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–13 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor antagonist; BAPEN, British Association for Parenteral and Enteral Nutrition; BMI, body mass index; CHF, chronic heart failure; CONUT, COntrolling NUTritional Status Index; GNRI, Geriatric Nutritional Risk Index; HeFNEF, heart failure with normal ejection fraction; HeFREF, heart failure with reduced ejection fraction; HF, heart failure; JW, fourth author; LVEF, left ventricular ejection fraction; MNA, Mini Nutritional Assessment; MNA-SF, Mini Nutritional Assessment–Short Form; MUST, Malnutrition Universal Screening Tool; NTproBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PNI, Prognostic Nutritional Index; SGA, Subjective Global Assessment; SS, first author.
References
- 1. Sze S, Pellicori P, Zhang J, Clark AL. Malnutrition, congestion and mortality in ambulatory patients with heart failure. Heart. 2019;105:297–306. [DOI] [PubMed] [Google Scholar]
- 2. Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, Elsner S, Sliziuk V, Scherbakov N, Murín J et al.. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation and cachexia in chronic heart failure. Eur Heart J. 2016;37:1684–91. [DOI] [PubMed] [Google Scholar]
- 3. van Bokhorst-de van der Schueren MA, Lonterman-Monasch S, de Vries OJ, Danner SA, Kramer MH, Muller M. Prevalence and determinants for malnutrition in geriatric outpatients. Clin Nutr. 2013;32:1007–11. [DOI] [PubMed] [Google Scholar]
- 4. Sokoreli I, Pauws SC, Steyerberg EW, de Vries GJ, Riistama JM, Tesanovic A, Kazmi S, Pellicori P, Cleland JG, Clark AL. Prognostic value of psychosocial factors for first and recurrent hospitalisations and mortality in heart failure patients: insights from the OPERA-HF study. Eur J Heart Fail. 2018;20:689–96. [DOI] [PubMed] [Google Scholar]
- 5. Cascino TM, Hummel SL.. Nutrient deficiencies in heart failure: a micro problem with macro effects?. J Am Heart Assoc. 2018;7:e010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chien SC, Lo CI, Lin CF, Sung KT, Tsai JP, Huang WH, Yun CH, Hung TC, Lin JL, Liu CY et al.. Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Failure. 2019;6:953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev. 2016;21:549–65. [DOI] [PubMed] [Google Scholar]
- 8. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, Clark AL. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Failure. 2018;6:476–86. [DOI] [PubMed] [Google Scholar]
- 9. Guerra-Sánchez L, Martinez-Rincón C, Fresno-Flores M. Prevalence of undernutrition in hospital patients with unbalanced heart failure; subjective global assessment like prognosis sign. Nutr Hosp. 2015;31:1757–62. [DOI] [PubMed] [Google Scholar]
- 10. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric Nutritional Risk Index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77:705–11. [DOI] [PubMed] [Google Scholar]
- 11. Kaneko H, Suzuki S, Goto M, Yuzawa Y, Arita T, Yagi N, Murata N, Kato Y, Kano H, Matsuno S et al.. Geriatric Nutritional Risk Index in hospitalized heart failure patients. Int J Cardiol. 2015;181:213–5. [DOI] [PubMed] [Google Scholar]
- 12. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol. 2017;106:533–41. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA et al.. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 14. Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed.Boston: Little, Brown; 1994.. pp. 253–56. [Google Scholar]
- 15. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–83. [DOI] [PubMed] [Google Scholar]
- 16. Cereda E, Pedrolli G.. The Geriatric Nutritional Risk Index. Curr Opin Clin Nutr Metab Care. 2009;12:1–7. [DOI] [PubMed] [Google Scholar]
- 17. Ignacio de Ulibarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 18. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic Nutritional Index in gastrointestinal surgery. Am J Surg. 1980;139:160–7. [DOI] [PubMed] [Google Scholar]
- 19.Elia M.2003. Screening for malnutrition: a multi-disciplinary responsibility. Development and use of the “Malnutrition Universal Screening Tool” (“MUST”) for adults. MAG, a Standing Committee of BAPEN. ISBN: 1 899467 70 X.
- 20. Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54:59–65. [DOI] [PubMed] [Google Scholar]
- 21. Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J Gerontol. 2001;56:M366–72. [DOI] [PubMed] [Google Scholar]
- 22. Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? J Parenter Enteral Nutr. 1987;11:8–13. [DOI] [PubMed] [Google Scholar]
- 23. Makhija S, Baker J.. The Subjective Global Assessment: a review of its use in clinical practice. Nutr Clin Pract. 2008;23:405–9. [DOI] [PubMed] [Google Scholar]
- 24. da Silva Fink J, Daniel de Mello P, Daniel de Mello E. Subjective global assessment of nutritional status: a systematic review of the literature. Clin Nutr. 2015;34:785–92. [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 26. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr et al.. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 27. Janz TG, Johnson RL, Rubenstein SD. Anemia in the emergency department: evaluation and treatment. Emerg Med Pract. 2013;15:1–15. [PubMed] [Google Scholar]
- 28. Diabetes UK, Diagnostic criteria for diabetes. [Internet] [Cited 2018 Aug 8]. Available from: https://www.diabetes.org.uk/Professionals/Position-statements-reports/Diagnosis-ongoing-management-monitoring/New_diagnostic_criteria_for_diabetes.
- 29. Pablo AR, Izaga MA, Alda LA. Assessment of nutritional status on hospital admission: nutritional scores. Eur J Clin Nutr. 2003;57:824–31. [DOI] [PubMed] [Google Scholar]
- 30. Russell CA, Elia M. Nutrition screening surveys in hospitals in England, 2007–2011: a report based on the amalgamated data from the four Nutrition Screening Week surveys undertaken by BAPEN in 2007, 2008, 2010 and 2011. British Association for Parenteral and Enteral Nutrition. [Internet] 2014; [cited 2020 Feb 19] Available from:https://www.bapen.org.uk/pdfs/nsw/bapen-nsw-uk.pdf. [Google Scholar]
- 31. Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature—what does it tell us? J Nutr Health Aging. 2006;10:466–85. [PubMed] [Google Scholar]
- 32. Sieber CC. Nutritional screening tools: how does the MNA compare? Proceedings of the session held in Chicago May 2–3, 2006 (15 years of Mini Nutritional Assessment). J Nutr Health Aging. 2006;10:488–92. [PubMed] [Google Scholar]
- 33. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony P, Charlton KE, Maggio M et al.. Validation of the Mini Nutritional Assessment Short-Form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13:782–8. [DOI] [PubMed] [Google Scholar]
- 34. Mueller C, Compher C, Ellen DM. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. J Perenter Enteral Nutr. 2011;35:16–24. [DOI] [PubMed] [Google Scholar]
- 35. Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–64. [DOI] [PubMed] [Google Scholar]
- 36. Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fávaro-Moreira NC, Krausch-Hofmann S, Matthys C, Vereecken C, Vanhauwaert E, Declercq A, Bekkering GE, Duyck J. Risk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal data. Adv Nutr. 2016;7:507–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.