Abstract
Naturally occurring human genetic variants that are predicted to inactivate protein-coding genes provide an in vivo model of human gene inactivation that complements knockout studies in cells and model organisms. Here we report three key findings regarding the assessment of candidate drug targets using human loss-of-function variants. First, even essential genes, in which loss-of-function variants are not tolerated, can be highly successful as targets of inhibitory drugs. Second, in most genes, loss-of-function variants are sufficiently rare that genotype-based ascertainment of homozygous or compound heterozygous ‘knockout’ humans will await sample sizes that are approximately 1,000 times those presently available, unless recruitment focuses on consanguineous individuals. Third, automated variant annotation and filtering are powerful, but manual curation remains crucial for removing artefacts, and is a prerequisite for recall-by-genotype efforts. Our results provide a roadmap for human knockout studies and should guide the interpretation of loss-of-function variants in drug development.
Subject terms: Target validation, Genomics
Analysis of predicted loss-of-function variants from 125,748 human exomes and 15,708 whole genomes in the Genome Aggregation Database (gnomAD) provides a roadmap for human ‘knockout’ studies and a guide for future research into disease biology and drug-target selection.
Main
Human genetics is an increasingly crucial source of evidence guiding the selection of new targets for drug discovery1. Most new clinical drug candidates eventually fail for lack of efficacy2, and although in vitro, cell culture and animal model systems can provide preclinical evidence that the compound engages its target, too often the target itself is not causally related to human disease1. Candidates targeting genes with human genetic evidence for disease causality are more likely to reach approval3,4, and identification of humans with loss-of-function (LoF) variants, particularly two-hit (homozygous or compound heterozygous) genotypes, has, for several genes, correctly predicted the safety and phenotypic effect of pharmacological inhibition5. Although these examples demonstrate the value of human genetics in drug development, important questions remain regarding strategies for identifying individuals with LoF variants in a gene of interest, interpretation of the frequency—or lack—of such individuals, and whether it is wise to pharmacologically target a gene in which LoF variants are associated with a deleterious phenotype.
Public databases of human genetic variation have catalogued predicted loss-of-function (pLoF) variants—nonsense, essential splice site, and frameshift variants expected to result in a non-functional allele. This presents an opportunity to study the effects of pLoF variation in genes of interest and to identify individuals with pLoF genotypes to understand gene function or disease biology, or to assess potential for therapeutic targeting. Although many variants initially annotated as pLoF do not, in fact, abolish gene function6, rigorous automated filtering can remove common error modes7. True LoF variants are generally rare, and show important differences between outbred, bottlenecked8 and consanguineous9 populations6,10. Counting the number of distinct pLoF variants in each gene in a population sample allows the quantification of gene essentiality in humans through a metric named ‘constraint’10–13. Specifically, the rate at which de novo pLoF mutations arise in each gene is predicted on the basis of rates of DNA mutation10,12, and the ratio of the count of pLoF variants observed in a database to the number expected based on mutation rates—obs/exp, or constraint score—measures how strongly purifying natural selection has removed such variants from the population. The annotation of pLoF variants remains imperfect, and continued improvements are being made14, but constraint usefully measures gene essentiality, as demonstrated by agreement with cell culture and mouse knockout experiments7, by overlap with human disease genes7,10 and genes depleted for structural variation15, and by the power of constraint to enrich for deleterious variants in neurodevelopmental disorders7,16.
Building on these insights, here we leverage pLoF variation in the Genome Aggregation Database (gnomAD)7 v2 dataset of 141,456 individuals to answer open questions in the interpretation of human pLoF variation in disease biology and drug development.
Constraint in human drug targets
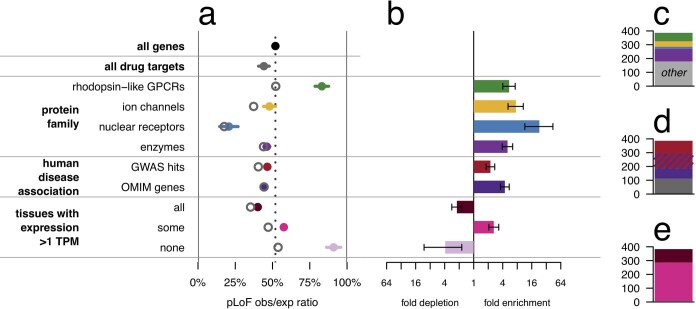
We compared constraint in the targets of approved drugs extracted from DrugBank17 (n = 383) versus all protein-coding genes (n = 17,604). Drug targets were, on average, just slightly more constrained than all genes (mean 44% versus 52%, nominal P = 0.00028, D = 0.11, two-sided Kolmogorov–Smirnov test), but the two gene sets had a qualitatively similar distribution of scores, ranging from intensely constrained (0% obs/exp) to not at all constrained (≥100% obs/exp) (Fig. 1a). Constraint scores showed clear divergence between categories of genes (Extended Data Table 1) expected to be more or less tolerant of inactivation (Fig. 1b), as previously reported7,10, validating the usefulness of constraint as a measure of gene essentiality. Nonetheless, when drug targets were stratified by drug effect (Fig. 1b), modality, or indication (Extended Data Fig. 1), no statistically significant differences between subsets of drug targets were observed.
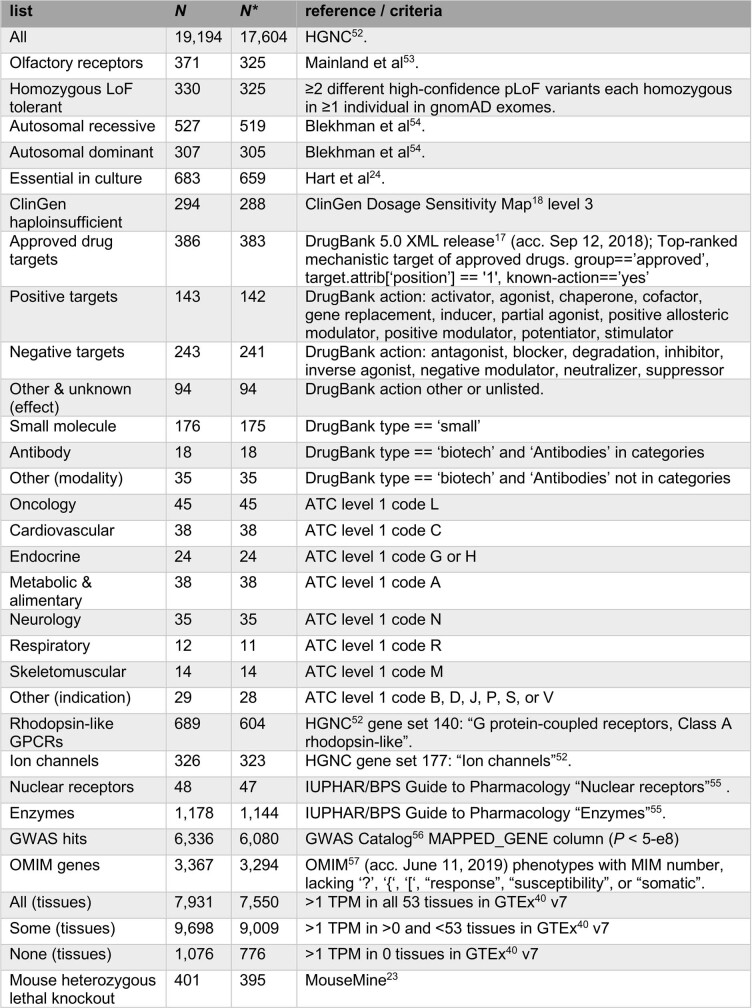
Fig. 1. pLoF constraint in drug targets.
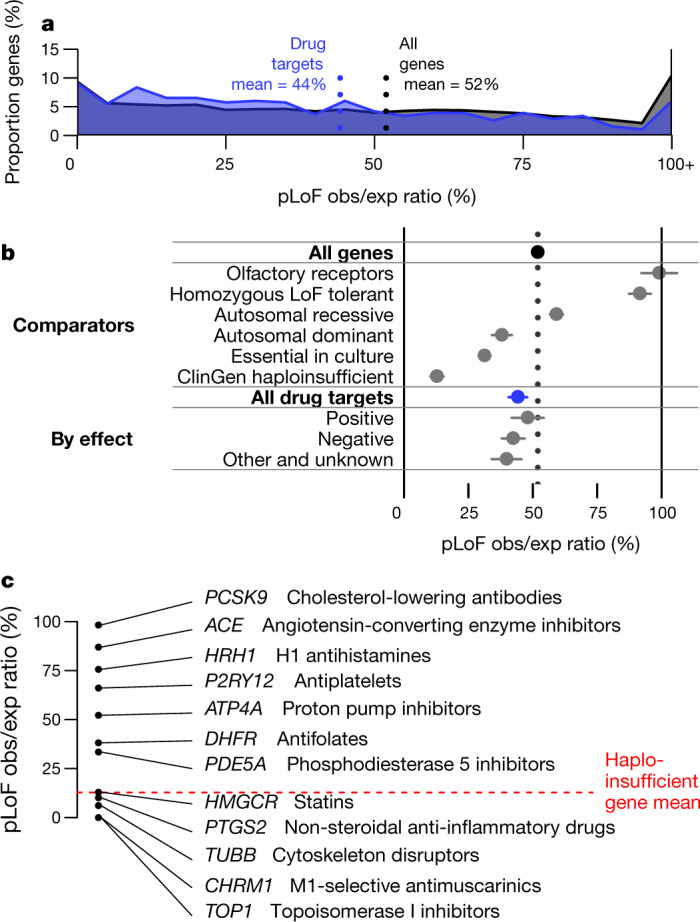
a, Histogram of pLoF obs/exp values for all genes (black, n = 17,604) versus drug targets (blue, n = 383). b, Forest plot of means (dots) and 95% confidence intervals of the mean (line segments), for constraint in the indicated gene sets (data sources and n values in Extended Data Table 1). For drug effect, ‘positive’ indicates agonist, activator or inducer, whereas negative indicates antagonist, inhibitor or suppressor, for example. c, Examples of drug targets and corresponding drug classes from across the constraint spectrum. Details in Extended Data Table 2.
Extended Data Table 1.
Data sources for gene lists used in this study
For analysis, only protein-coding genes with unambiguous mapping to current approved gene symbols were used; numbers in the table reflect this. Values in the N column indicate totals from the full universe of 19,194 genes; values in the N* column indicate the subset of genes with non-missing constraint values, used for Fig. 1 and Extended Data Figs. 1, 2. The following references are cited in the table: refs. 17,18,23,24,40,52–57.
Extended Data Fig. 1. Drug target constraint by modality and indication.
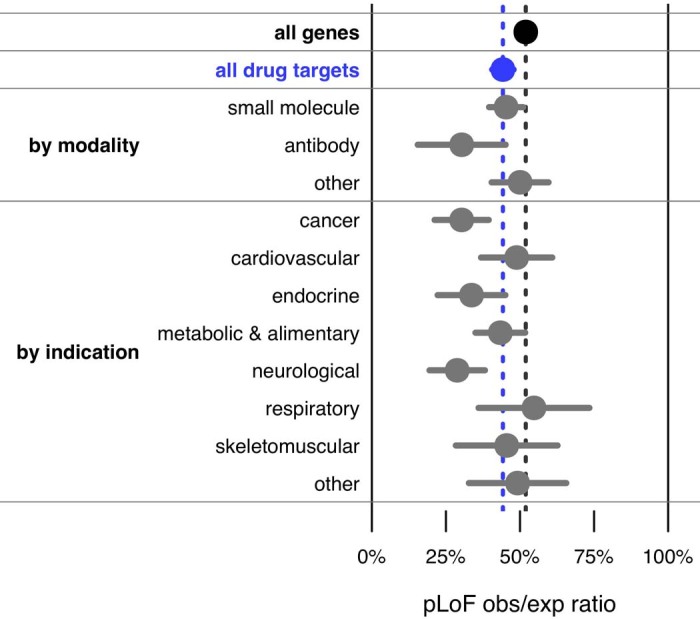
Mean (dots) and 95% confidence interval (line segments) for constraint in subsets of drug-targets sets (data sources and number of genes for each list are provided in Extended Data Table 1). Modality information was extracted from DrugBank and indication information from ATC codes; see Extended Data Table 1 for details.
The slightly but significantly lower obs/exp value among drug targets may superficially appear to provide evidence that constrained genes make superior drug targets. Stratification of drug targets by protein family, human disease association, and tissue expression, however, argues against this interpretation. Drug targets are strongly enriched for a few canonically ‘druggable’ protein families, for genes known to be involved in human disease, and for genes with tissue-restricted expression; each of these properties is in turn correlated with either significantly stronger or weaker constraint (Extended Data Fig. 2). Although controlling for these correlations does not abolish the trend of stronger constraint among drug targets, the correlation of so many observed variables with the status of a gene as a drug target argues that many unobserved variables probably also confound interpretation of the lower mean obs/exp value among drug targets.
Extended Data Fig. 2. Drug-target gene set confounding.
a, Forest plot of means (dots) and 95% confidence intervals of the mean (line segments) for gene sets evaluated for confounding with drug-target status. Data sources and number of genes for each list are provided in Extended Data Table 1. LoF obs/exp ratios differ significantly from the set of all genes for four canonically druggable protein families (top), human disease-associated genes (middle), and genes by broadness of tissue expression (bottom). Within each class, the genes that are drug targets have a lower mean obs/exp ratio (hollow grey circles) than the class overall. b, The druggable protein families, disease-associated genes, and genes expressed in some tissues but not others are enriched several-fold among the set of drug targets. Bars indicate fold enrichment and error bars indicate 95% confidence intervals. c–e, Composition of drug targets when broken down by protein family (c), disease association (d), or broadness of tissue expression (e). The enriched classes account for most drug targets. In a linear model, after controlling for protein family, disease association status, and number of tissues with expression >1 transcript per million (TPM), drug targets are still more constrained than other genes (−8.0% obs/exp, nominal P = 0.00011, t = −3.9, df = 17,325 for the contribution of drug_target in the linear regression obs/exp ~ drug_target + family + dz_assoc + n_tissues), but the probable existence of additional unobserved confounders cautions against over-interpretation of this observation (see main text).
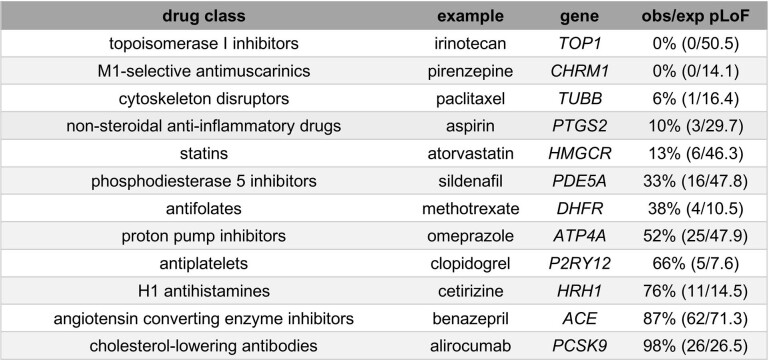
The overall constraint distribution of drug targets (Fig. 1a) also argues against the view that a gene in which LoF is associated with a deleterious phenotype cannot be successfully targeted. Indeed, 19% of drug targets (n = 73), including 52 targets of inhibitors, antagonists or other ‘negative’ drugs, have lower obs/exp values than the average (12.8%) for genes known to cause severe diseases of haploinsufficiency18 (ClinGen level 3). To determine whether this finding could be explained by a particular class or subset of drugs, we examined constraint in several well-known example drug targets (Fig. 1c, Extended Data Table 2). Some heavily constrained genes are targets of cytotoxic chemotherapy agents such as topoisomerase inhibitors or cytoskeleton disruptors, a set of drugs intuitively expected to target essential genes. However, genes with near-complete selection against pLoF variants also include HMGCR and PTGS2, the targets of highly successful, chronically used inhibitors—statins and aspirin.
Extended Data Table 2.
Spectrum of tolerance to genetic inactivation among human drug targets
Example targets are arranged from the most intolerant (top) to the most tolerant (bottom) of inactivation.
These human in vivo data further the evidence from other species and models that essential genes can be good drug targets. Homozygous knockout of Hmgcr and Ptgs2 are lethal in mice19–21. Drug targets exhibit higher inter-species conservation than other genes22. Targets of negative drugs include 14 genes with lethal heterozygous knockout mouse phenotypes reported23 and 6 reported as essential in human cell culture24.
Prospects for finding human ‘knockouts’
Athough constraint alone is not adequate to nominate or exclude drug targets, the study of individuals with single hit (heterozygous) or two-hit (‘knockout’) LoF genotypes in a gene of interest can be highly informative about the biological effect of engaging that target5. To assess prospects for ascertaining knockout individuals, we computed the cumulative allele frequency (CAF) of pLoF variants in each gene (Methods), and then used this to estimate the expected frequency of two-hit individuals under different population structures (Fig. 2) in the absence of natural selection.
Fig. 2. Prospects for discovery of human knockouts.
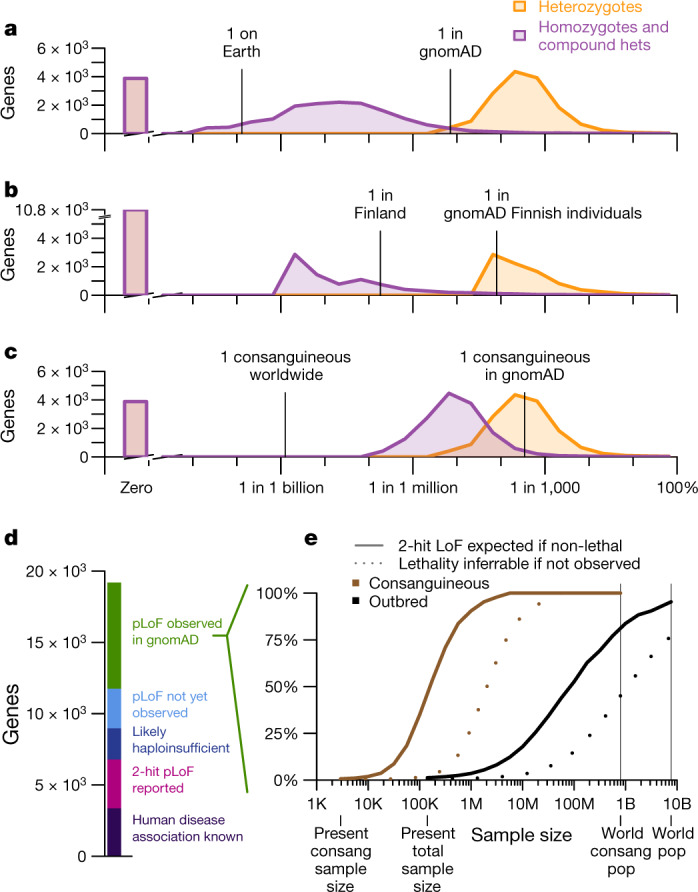
a–c, Histograms (a–c): genes by expected heterozygote frequency (orange), and two-hit homozygote and compound heterozygote frequency (purple). a, Outbred populations. b, Finnish individuals; an example of a bottlenecked population. c, Consanguineous individuals. d, Current status of pLoF or disease association discovery for all protein-coding genes. e, Projected sample sizes required for discovery of two-hit individuals (solid lines) and for statistical inference that a two-hit genotype is lethal if no such individuals are observed (dashed lines), for ‘pLoF observed in gnomAD’ genes (d) for consanguineous and outbred individuals.
Whereas gnomAD is now large enough to include at least one pLoF heterozygote for most (15,317 out of 19,194; 79.8%) genes, ascertainment of total knockout individuals in outbred populations will require 1,000-fold larger sample sizes for most genes: the median expected two-hit frequency of a gene is just six per billion (Fig. 2a). Even if every human on Earth were sequenced, there are 4,728 genes (24.6%) for which identification of even one two-hit individual would not be expected in outbred populations. Intuitively, because the sample size of gnomAD today is larger than the square root of the world population, variants so far seen in zero or only a few heterozygous individuals are not likely to ever be seen in a homozygous state in outbred populations, except where variants prove common in populations not yet well-sampled by gnomAD.
Because population bottlenecks can result in very rare variants present in a founder rising to an unusually high frequency, we also considered knockout discovery in bottlenecked populations, using Finnish individuals in gnomAD as an example8. Although this population structure can enable well-powered association studies for the small fraction of genes in which pLoF variants drifted to high frequency due to the bottleneck, overall, identification of two-hit pLoF individuals for a pre-specified gene of interest appears equally or more difficult in Finnish individuals than in outbred populations (Fig. 2b, Extended Data Fig. 3), because rare variants not present in a founder have been effectively removed from the population.
Extended Data Fig. 3. Expected frequency of individuals with one or two null alleles for every protein-coding gene across different population models, with sample size held constant.
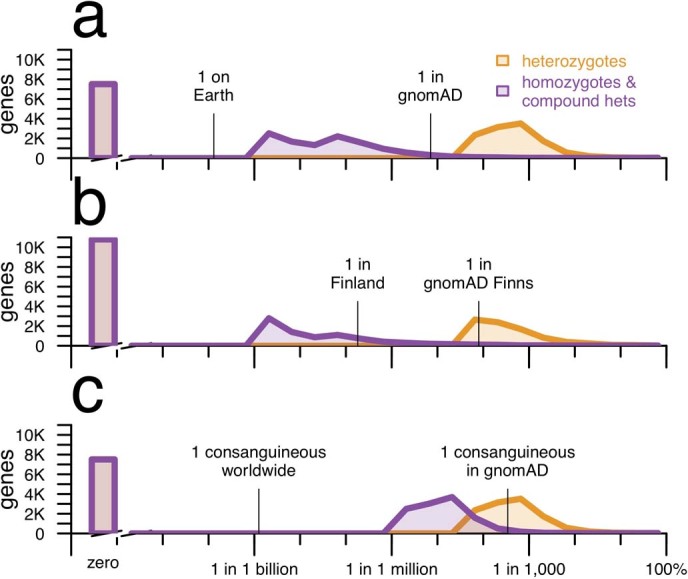
This is identical to Fig. 2 except as follows. As noted in the Methods, one caveat about Fig. 2 is that the sample size is larger for the plots using all gnomAD exomes (Fig. 2a, c) than for Finnish exomes (Fig. 2b). This figure shows the same analysis, but with the global gnomAD population downsampled to 10,824 randomly chosen exomes so that the same size is identical to that of Finnish exomes. Computation of P = 1 − sqrt(q) as described in the Methods is computationally expensive for downsampled datasets because it requires individual-level genotypes. Instead, this analysis uses ‘classic’ CAF, which is simply the sum of allele frequencies of all high-confidence pLoF variants each at allele frequency <5%, capped at a total of 100%, for both global and Finnish exomes. The results show that even when the sample size is held constant, the number of genes with zero pLoF variants observed is higher in a bottlenecked population than in a mostly outbred population. A constant y axis with no axis breaks is used in this figure to make this difference more clearly visible.
In consanguineous individuals, parental relatedness greatly increases the frequency of homozygous pLoF genotypes. The n = 2,912 individuals in the East London Genes & Health (ELGH) cohort25 who report having parents who are second cousins or closer have on average 5.8% of their genomes autozygous. Here, the expected frequency of two-hit individuals is many times higher than in outbred populations, at five per million for the median gene (Fig. 2c).
These projections allow us to draft a roadmap for discovery of human knockouts across 19,194 genes (Fig. 2d, e). Online Mendelian Inheritance in Man (OMIM) already describes human disease association for 3,367 genes (18%), although the discovery of LoF individuals in population databases will still be valuable for assessing penetrance and identifying LoF syndromes of known gain-of-function genes. Another 3,421 genes (18%) without known human disease association have two-hit pLoF genotypes reported in gnomAD7, ELGH26, PROMIS27, deCODE28 or UK Biobank29, which suggests that this genotype may be tolerated. An additional 2,190 genes (11%) appear intolerant of heterozygous inactivation (pLI score > 0.9) in gnomAD—a set expected to be enriched for genes with severe heterozygous and lethal homozygous LoF phenotypes. Another 2,781 genes (14%) have no pLoF variants observed in gnomAD, but our sample size is not yet large enough to robustly infer LoF intolerance. For these genes, observation of outbred two-hit individuals is not expected, and we cannot yet assess the feasibility of identifying consanguineous two-hit individuals because we lack an estimate of pLoF allele frequency.
This leaves 7,435 genes (39%) for which one or more pLoFs are observed in gnomAD, but strong LoF intolerance cannot be determined, two-hit genotypes have not been observed, and a human disease phenotype is not known. We projected the sample sizes required to identify knockout individuals for these genes (Fig. 2e). In outbred populations, current sample sizes would need to increase by approximately 1,000-fold before ascertainment of a single two-hit LoF individual would be expected for the typical gene. By contrast, around a 10- to 100-fold increase from current consanguineous sample size, meaning hundreds of thousands of individuals in absolute terms, would identify at least one two-hit LoF individual for the typical gene. Among other simplifying assumptions (Methods), these projections presume that complete knockout is tolerated. When only one or a few two-hit individuals are expected in a dataset, the absence of any such individuals can be due to either early lethality, a severe clinical phenotype incompatible with inclusion in gnomAD, or simply chance. Thus, the ability to infer lethality of the two-hit genotype based on statistical evidence will lag behind the identification of two-hit individuals where they do exist (Fig. 2e). For some genes, inference of lethality will always remain impossible in outbred populations, though it may be feasible in consanguineous individuals.
Curation of pLoF variants
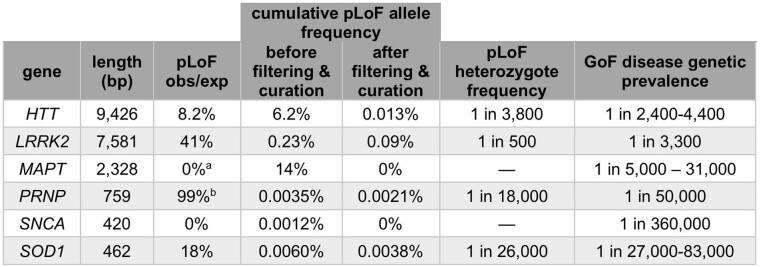
Where pLoF variants can be identified, they are a valuable resource for assessing the effect of lifelong reduction in gene dosage. To highlight the challenges and opportunities of identifying such variants, we manually curated gnomAD data and the scientific literature for six genes associated with gain-of-function (GoF) neurodegenerative diseases, for which inhibitors or suppressors are under development30–35: HTT (Huntington's disease), MAPT (tauopathies), PRNP (prion disease), SOD1 (amyotrophic lateral sclerosis), and LRRK2 and SNCA (Parkinson's disease). The results (Fig. 3, Extended Data Table 3) illustrate four points about pLoF variant curation.
Fig. 3. Insights from non-random positional distributions of pLoF variants.
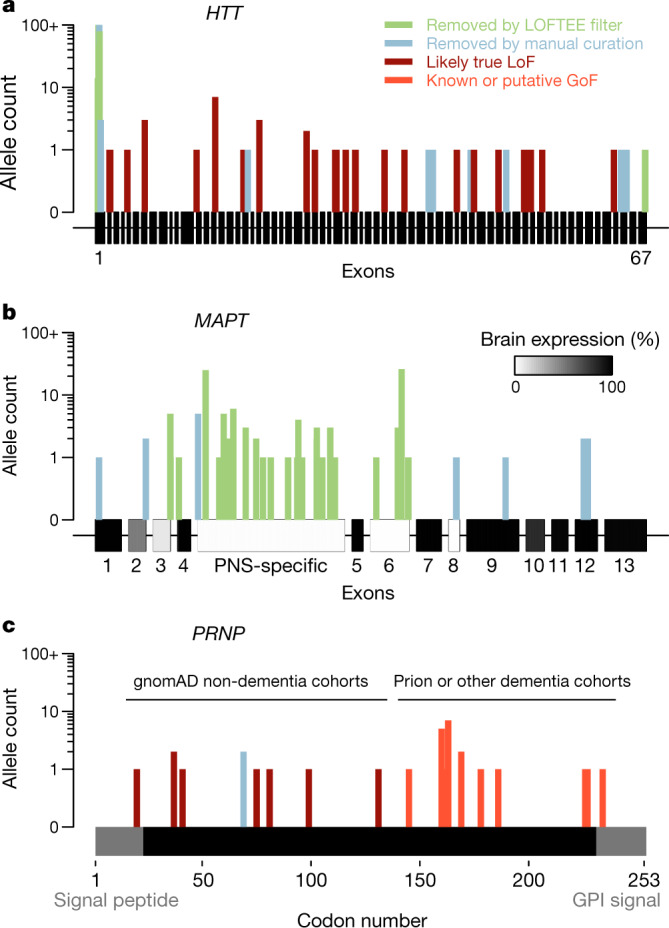
a–c, HTT (a), MAPT, with brain expression data from GTEx40 (b) and PRNP, a single protein-coding exon with domains removed by post-translational modification in grey (c), showing previously reported variants41 and those newly identified in gnomAD and in the literature (Extended Data Table 5). GPI, glycosylphosphatidylinositol. Detailed variant curation results are provided in Supplementary Table 1.
Extended Data Table 3.
Curation of pLoF variation in six neurodegenerative disease genes
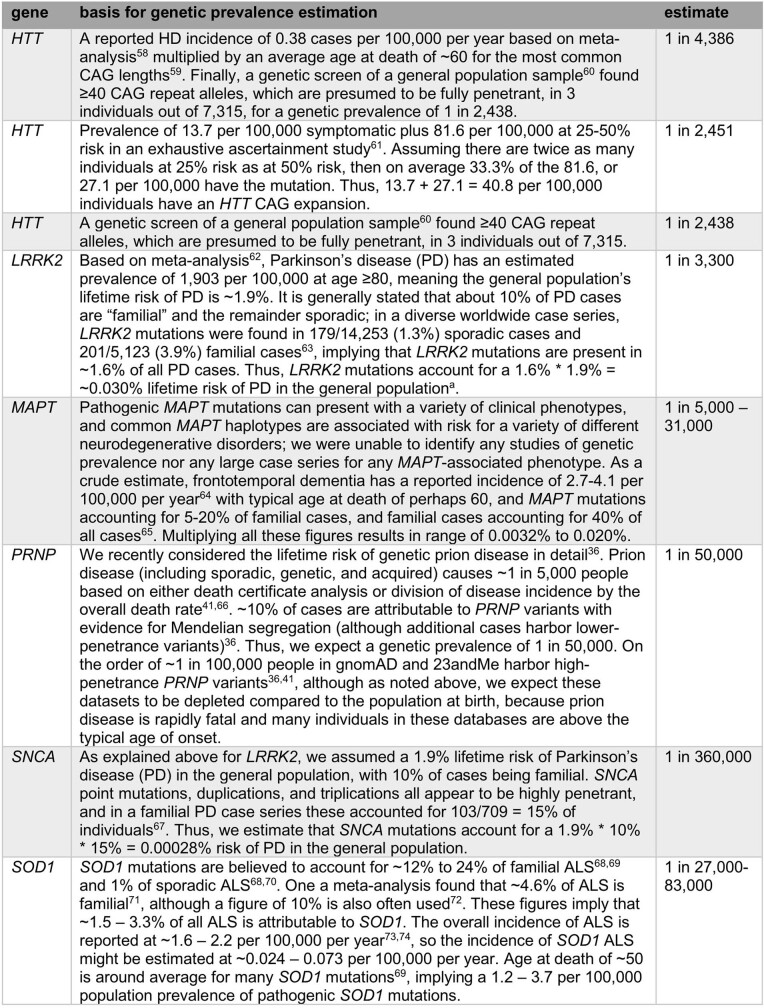
Shown are the coding sequence length (base pairs, bp), constraint value (pLoF obs/exp) after filtering and curation, cumulative allele frequency before and after filtering and manual curation, estimated frequency of true pLoF heterozygotes in the population, and genetic prevalence (population frequency including pre-symptomatic individuals) of the GoF disease associated with the gene. Genetic prevalence calculations are described in Extended Data Table 4, and variant curation details are provided in Supplementary Table 1, except for LRRK2, which is described in detail elsewhere49.
aConstitutive brain-expressed exons only.
bPRNP codons 1–144; see Fig. 3c for rationale.
First, other things being equal, genes with longer coding sequences offer more opportunities for LoF variants to arise, and so tend to have a higher cumulative frequencies of LoF variants, unless they are heavily constrained. Ascertainment of LoF individuals is thus harder for shorter and/or more constrained genes, even though these may be good targets.
Second, many variants annotated as pLoF are false positives6, and these are enriched for higher allele frequencies, so that both filtering and curation have an outsized effect on the cumulative allele frequency of LoF. Studies of human pLoF variants lacking stringent curation can therefore easily dilute results with false pLoF carriers.
Third, after careful curation, cumulative LoF allele frequency is sometimes sufficiently high to place certain bounds on what heterozygote phenotype might exist. For example, GoF mutations causing genetic prion disease have a genetic prevalence of approximately 1 in 50,00036 and have been known for three decades, with thousands of cases identified, making it unlikely that a comparably severe and penetrant haploinsufficiency syndrome associated with PRNP would have gone unnoticed to the present day despite being more than twice as common (roughly 1 in 18,000). Similar arguments can be made for HTT, LRRK2 and SOD1 genes (Extended Data Tables 3, 4). Of course, this does not rule out a less severe or less penetrant heterozygous LoF phenotype.
Extended Data Table 4.
Estimation of genetic prevalence for GoF genetic neurodegenerative diseases
Data sources were identified by PubMed and Google Scholar searches. Genetic prevalence was defined as the proportion of the population at birth carrying a mutation and destined to later develop disease, and estimated as described for each gene. The following references are cited in the table: refs. 36,41,58–74.
aIt is important to consider how this figure relates to the penetrance of LRRK2 mutations, as LRRK2 variants appear to occupy a spectrum of penetrance75. Some variants exhibit Mendelian segregation with disease76,77, implying high risk; the G2019S variant is estimated to have approximately 32% penetrance78; and other common variants are risk factors with odds ratios of only around 1.2 estimated through genome-wide association studies (GWAS)79. The GWAS-implicated common variants were not included in the case series on which our estimate is based63, but G2019S does account for most cases in that series. Because the 0.03% estimate here is based on counting symptomatic cases rather than asymptomatic individuals, it will appropriately underestimate the number of G2019S carriers. In essence, in this calculation each G2019S carrier in the population only counts as 1/3 of a person, because they have only a 1/3 probability of developing a disease. It is therefore appropriate that our estimate of genetic prevalence (0.03%) is actually lower than double the allele frequency of G2019S in gnomAD (0.1%).
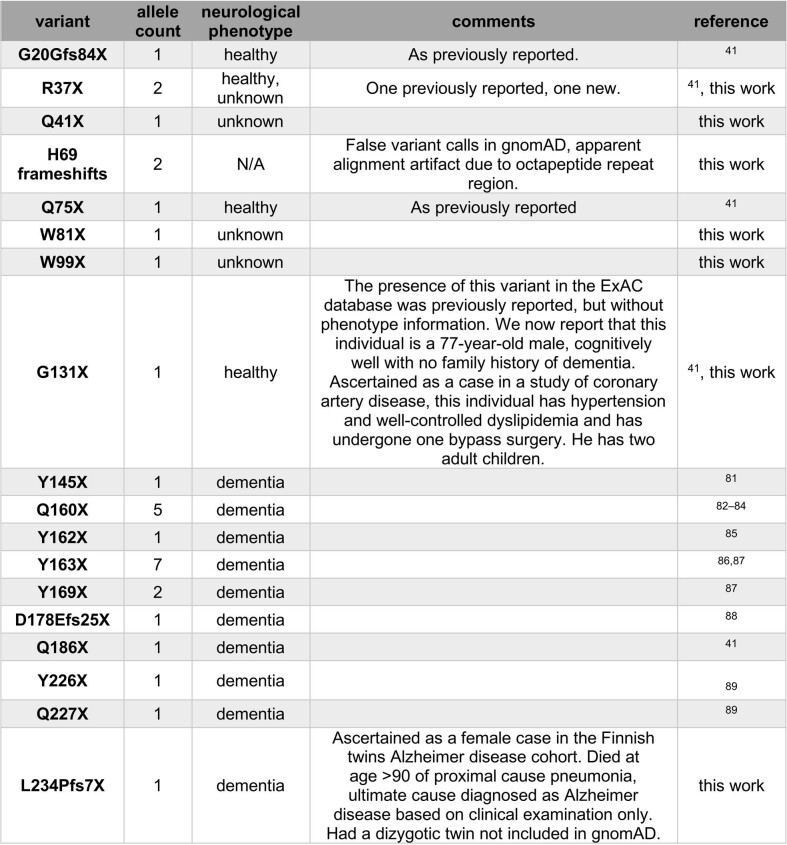
Finally, careful inspection of the distributions of pLoF variants can reveal important error modes or disease biology. HTT, MAPT and PRNP genes each have different non-random positional distributions of pLoF variants (Fig. 3). High-frequency HTT pLoF variants cluster in the polyglutamine/polyproline repeat region of exon 1 and appear to be alignment artefacts (Fig. 3a). True HTT LoF variants are rare and the gene is highly constrained, which might suggest some fitness effect in a heterozygous state in addition to the known severe homozygous phenotype37,38, although the frequency of LoF carriers still argues against a penetrant syndromic illness, consistent with the lack of phenotype reported in heterozygotes identified so far38,39. High-frequency MAPT pLoF variants cluster in exons not expressed in the brain in GTEx data14,40, and all remaining pLoFs appear to be alignment or annotation errors (Fig. 3b). No true LoFs are observed in MAPT, although our sample size is insufficient to prove that MAPT LoF is not tolerated—among constitutive brain-expressed exons, we expect 12.6 LoFs and observe 0, giving a 95% confidence interval upper bound of 23.7% for obs/exp values. PRNP-truncating variants in gnomAD cluster in the N terminus; the sole C-terminal truncating variant in gnomAD is a dementia case (Extended Data Table 5), consistent with variants at codon ≥145 causing a pathogenic gain-of-function through change in localization (Fig. 3c). Within codons 1–144, PRNP is unconstrained (Extended Data Table 3), and no neurological phenotype has been identified in individuals with truncating variants so far, consistent with the hypothesis that N-terminal truncating variants are true LoF and are tolerated in a heterozygous state41.
Extended Data Table 5.
Details of PRNP-truncating variants
Allele count for variants from the literature in Fig. 3c is the total number of definite or probable cases with sequencing performed in the studies cited in this table. The L234Pfs7X variant changes the C-terminal GPI signal of prion protein from SMVLFSSPPVILLISFLIFLIVGX to SMVPSPLHLX. This new sequence does not adhere to the known rules of GPI anchor attachment80: GPI signals must contain a 5–10-polar-residue spacer followed by 15–20 hydrophobic residues. Thus, this frameshifted prion protein would be predicted to be secreted and thus may be pathogenic, explaining the Alzheimer’s disease diagnosis in this individual. However, it is also possible that the new C-terminal sequence found here interferes with prion formation, and/or that this variant is incompletely penetrant, and that the diagnosis of Alzheimer’s disease in this individual is merely a coincidence. The following references are cited in the table: refs. 41,81–89.
Discussion
Studying human gene inactivation can illuminate human biology and guide the selection of drug targets, complementing mouse knockout studies42, but analysis of any one gene requires genome-wide context to set expectations and guide inferences. Here we have used gnomAD data to provide context to aid in the interpretation of human LoF variants.
Targets of approved drugs range from highly constrained to completely unconstrained. There may be several reasons why some genes apparently tolerate pharmacological inhibition but not genetic inactivation. LoF variants in constitutive exons should affect all tissues for life, whereas drugs differ in tissue distribution and timing and duration of use. Many drugs known or suspected to cause fetal harm are tolerated in adults43, and might target developmentally important genes. Constraint is thought to primarily reflect selection against heterozygotes13, the effective gene dosage of which may differ from that achieved by a drug. Constraint measures natural selection over centuries or millennia; the environment of our ancestors presented different selective pressures from what we face today. Actions of small-molecule drugs may not map one-to-one onto genes44–47. Regardless, these human in vivo data show that even a highly deleterious knockout phenotype is compatible with a gene being a viable drug target.
For most genes, the lack of total knockout individuals identified so far does not yet provide statistical evidence that this genotype is not tolerated. Indeed, for many genes, such evidence may never be attainable in outbred populations. Bottlenecked populations, individually, are unlikely to yield two-hit individuals for a pre-specified gene of interest, although the sequencing of many different, diverse bottlenecked populations will certainly expand the set of genes accessible by this approach. Identification of two-hit individuals will be most greatly aided by increased investment in consanguineous cohorts, in which the sample size required for any given gene is often orders of magnitude lower than in outbred populations. Our analysis is limited by sample size, insufficient diversity of sampled populations, and simplifying assumptions about population structure and distribution of LoF variants, so our calculations should be taken as rough, order-of-magnitude estimates. Nonetheless, this strategic roadmap for the identification of human knockouts should inform future research investments and rationalize the interpretation of existing data.
Recall-by-genotype efforts are only valuable if the variants in question are correctly annotated. Automated filtering7 and transcript expression-aware annotation14 are powerful tools, but we demonstrate the continued value of manual curation for excluding further false positives, assessing and interpreting the cumulative allele frequency of true LoF variants, and identifying error modes or biological phenomena that give rise to non-random distributions of pLoF variants across a gene. Such curation is essential before any recontact efforts, and establishing methods for high-throughput functional validation48 of LoF variants is a priority. Our curation of pLoF variants in neurodegenerative disease genes is limited by a lack of functional validation and detailed phenotyping; a companion paper demonstrates a deeper investigation of the effects of LoF variants in the LRRK2 gene49.
Drug development projects may increasingly be accompanied by efforts to phenotype human carriers of LoF variants. With the cost of drug discovery driven overwhelmingly by failure50, successful interpretation of LoF data to select the right targets and right clinical pathways will yield outsize benefits for research productivity and, ultimately, human health.
Methods
No statistical methods were used to predetermine sample size. The experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
Data sources
pLoF analyses used the gnomAD dataset of 141,456 individuals7. For data consistency, all genome-wide constraint and CAF analyses used only the 125,748 gnomAD exomes. Curated analyses of individual genes used all 141,456 individuals including 15,708 whole genomes. Gene lists used in this study were extracted from public data sources between September 2018 and June 2019. Data sources and criteria for gene list extraction are shown in Extended Data Table 1. This study was performed under ethical approval from the Partners Healthcare Institutional Research Board (2013P001339/MGH) and the Broad Institute Office of Research Subjects Protection (ORSP-3862). All research participants provided informed consent.
Calculation of pLoF constraint
The calculation of constraint values for genes has been described in general elsewhere10,12 and for this dataset specifically by Karczewski et al.7. Constraint calculations used LOFTEE-filtered (‘high confidence’) single-nucleotide variants (which for pLoF means nonsense and essential splice site mutations) found in gnomAD exomes with minor allele frequency <0.1%. Only unique canonical transcripts for protein-coding genes were considered, yielding 17,604 genes with available constraint values. For curated genes (Extended Data Table 2), the number of observed variants passing curation was divided by the expected number of variants to yield a curated constraint value. For PRNP, the expected number of variants was adjusted by multiplying by the ratio of the sum of mutation frequencies for all possible pLoF variants in codons 1–144 to the sum of mutation frequencies for all possible pLoF variants in the entire transcript, yielding 6 observed out of 6.06 expected. For MAPT, the expected number of variants was taken from Ensembl transcript ENST00000334239, which includes only the exons identified as constitutively brain-expressed in Fig. 3b (exon numbering previously described51).
Calculation of pLoF heterozygote and homozygote/compound heterozygote frequencies
LOFTEE-filtered high-confidence pLoF variants with minor allele frequency <5% in 125,748 gnomAD exomes were used to compute the proportion of individuals without a loss-of-function variant (q); the CAF was computed as p = 1 − sqrt(q). This approach conservatively assumes that, if an individual has two different pLoF variants, they are in cis to each other and count as only one pLoF allele.
For outbred populations (Fig. 2a), we used the value of p from all 125,748 gnomAD exomes, as this allows the largest possible sample size. This includes some individuals from bottlenecked populations, for which the distribution of p does differ from outbred populations, but these individuals are a small proportion of gnomAD exomes (12.6%). This also includes some consanguineous individuals, but these are an even smaller proportion of gnomAD exomes (2.3%), and any difference in the value of p between consanguineous and outbred populations is expected to be very small. Heterozygote frequency was calculated as 2p(1 −p) and homozygote and compound heterozygote frequency was calculated as p2. Lines indicate the size of gnomAD (141,456 individuals) and the world population (6.69 billion).
For bottlenecked populations (Fig. 2b), we used the value of p from the 10,824 Finnish exomes only. Lines indicate the number of Finnish individuals in gnomAD (12,526) and the population of Finland (5.5 million).
For consanguineous individuals (Fig. 2c), we again used the value of p from all gnomAD exomes, because p is not expected to differ greatly in consanguineous versus outbred populations. We used the mean proportion of the genome in runs of autozygosity (a) from individuals self-reporting second cousin or closer parents in East London Genes & Health, a = 0.05766 (rounded to 5.8%). Heterozygote frequency was calculated as 2p(1 − p) and homozygote and compound heterozygote frequency was calculated as (1 − a)p2 + ap. Lines indicate the number of consanguineous South Asian individuals in gnomAD (n = 2,912, by coincidence the same number as report second cousin or closer parents in ELGH) based on F > 0.05 (a conservative estimate, because second cousin parents are expected to yield F = 0.015625), and the estimated number of individuals in the world with second cousin or closer parents (10.4% of the world population)9.
Several caveats apply to our CAF analysis. First, our approach naively treats genes with no pLoFs observed as having P = 0, even though pLoFs might be discovered at a larger sample size. Second, we naively group all populations together, even though the distribution of populations sampled in gnomAD does not reflect the world population7; we believe that this is reasonable because CAF for many genes is driven by singletons and other ultra-rare variants for which frequency is not expected to differ appreciably by continental population10. (It is important to note that the histograms shown in Fig. 2 reflect the expected frequency of heterozygotes and homozygotes/compound heterozygotes, based on gnomAD allele frequency, rather than the actual observed frequency of individuals with these genotypes in gnomAD.) Third, we use only protein-truncating variants annotated as pLoF in gnomAD. Structural and non-coding variation resulting in a loss of function may be missed in exomes, and missense variants resulting in a loss of function cannot be rigorously annotated. Fourth, we naively treat genes with one pLoF allele observed as having P = 1/(2 × 125,748), even though on average singleton variants have a true allele frequency lower than their nominal allele frequency10. Fifth, the variants included in this analysis are filtered but have not been manually curated or functionally validated, so some will ultimately prove not to be true LoF. These false positives tend to be more common and will have disproportionately contributed to the cumulative LoF allele frequency. Sixth, as described in the main text, our calculations assume that complete knockout is tolerated, which will not be true for some genes. We therefore also include a projection of the sample size needed to infer lethality from the absence of two-hit knockout individuals (Fig. 2e). Points one to three will tend to lead to underestimation of the true complete knockout frequency, whereas points four to six will tend to lead to overestimation. On balance, our calculations may reflect an upper bound of complete knockout frequency for most genes owing to the strong influence of factors five and six. Finally, as a matter of comparison between population structures, the sample size for all gnomAD exomes (Fig. 2a, c) is larger than for only Finnish exomes (Fig. 2b). For a version of Fig. 2 with the global gnomAD population downsampled to the same sample size as the gnomAD Finnish population, see Extended Data Fig. 2.
Knockout roadmap
For the knockout ‘roadmap’ (Fig. 2d, e), we classified genes according to the current status of human disease association and LoF ascertainment. Genes were classified as having a Mendelian disease association if they were present in OMIM with the filters described in Extended Data Table 1.
Remaining genes were classified as ‘2-hit LoF reported’ based on presence in one or more of the following gene lists: homozygous LoF genotypes in gnomAD curated as previously described7; filtered homozygous LoF genotypes in runs of autozygosity with minor allele frequency <1% in canonical transcripts in the Bradford, Birmingham and ELGH25 cohorts (total n = 8,925); observed number of imputed homozygotes >1 or number of compound heterozygous carriers where minor allele frequency <2% (for both variants) in deCODE28; homozygous LoF reported in PROMIS27; homozygous LoF with minor allele frequency <1% in UK Biobank29.
The remainder of genes were sequentially classified as ‘likely haploinsufficient’ if pLI >0.9 in gnomAD, ‘pLoF not yet observed’ if CAF = 0 in gnomAD, and, finally, ‘pLoF observed in gnomAD’ if CAF >0 in gnomAD.
Genetic prevalence estimation
Here, we define ‘genetic prevalence’ for a given gene as the proportion of individuals in the general population at birth who have a pathogenic variant in that gene that will cause them to later develop disease. Genetic prevalence has not been well-studied or estimated for most disease genes.
In principle, it should be possible to estimate genetic prevalence simply by examining the allele frequency of reported pathogenic variants in gnomAD. In practice, three considerations usually preclude this approach. First, the present gnomAD sample size of 141,456 exomes and genomes is still too small to permit accurate estimates for very rare diseases. Second, the mean age of gnomAD individuals is approximately 55, which is above the age of onset for many rare genetic diseases, and individuals with known Mendelian disease are deliberately excluded, so pathogenic variants will be depleted in this sample relative to the whole birth population. Third and most importantly, a large fraction of reported pathogenic variants lack strong evidence for pathogenicity and are either benign or low penetrance10,41, so without careful curation of pathogenicity assertions, summing the frequency of reported pathogenic variants in gnomAD will in most cases vastly overestimate the true genetic prevalence of a disease.
Instead, we searched the literature and very roughly estimated genetic prevalence based on available data. In most cases, we took disease incidence (new cases per year per population), multiplied by proportion of cases due to variants in a gene of interest, and multiplied by average age at death in cases. In some cases, estimates of at-risk population or direct measures of genetic prevalence were available. Details of the calculations undertaken for each gene are provided in Extended Data Table 4.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-020-2267-z.
Supplementary information
This supplementary table contains details of curated variants in neurodegenerative disease genes.
Reviewer reports and authors’ response from the peer review of this Article at Nature.
Acknowledgements
This study was performed under ethical approval from the Partners Healthcare Institutional Research Board (2013P001339/MGH) and the Broad Institute Office of Research Subjects Protection (ORSP-3862) in compliance with all relevant ethical regulations; written informed consent was obtained from all research participants. We thank all of the research participants for contributing their data. E.V.M. acknowledges support from the National Institutes of Health (NIH) (F31 AI122592) and an anonymous organization. gnomAD data aggregation was supported primarily by the Broad Institute, gnomAD analysis was supported in part by NIDDK U54 DK105566, and development of LOFTEE by NIGMS R01 GM104371. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. E.L.G.H. is funded by the Wellcome Trust (102627, 210561), the Medical Research Council (M009017), Higher Education Funding Council for England Catalyst, Barts Charity (845/1796), Health Data Research UK (for London substantive site), and research delivery support from the NHS National Institute for Health Research Clinical Research Network (North Thames). N.W. is supported by a Rosetrees and Stoneygate Imperial College Research Fellowship. The results published here are in part based upon data: (1) generated by The Cancer Genome Atlas managed by the NCI and NHGRI (accession: phs000178.v10.p8). Information about TCGA can be found at http://cancergenome.nih.gov; (2) generated by the Genotype-Tissue Expression Project (GTEx) managed by the NIH Common Fund and NHGRI (accession: phs000424.v7.p2); (3) generated by the Exome Sequencing Project, managed by NHLBI; (4) generated by the Alzheimer’s Disease Sequencing Project (ADSP), managed by the NIA and NHGRI (accession: phs000572.v7.p4). We thank J. Kaprio and M. Kurki (Finnish Twins AD cohort) and Academy of Finland grant 312073, and Ruth McPherson (Ottawa Genomics Heart Study) for providing information on individuals with PRNP-truncating variants. We thank J. B. Carroll, K. Heilbron, J. Fah Sathirapongsasuti, and L. C. Francioli for comments and suggestions. A subset of the analyses reported here originally appeared as a blog post on CureFFI.org (http://www.cureffi.org/2018/09/12/lof-and-drug-safety/).
Extended data figures and tables
Author contributions
Conceived and designed the study: E.V.M., S.L.S., D.G.M. Performed analysis: E.V.M., K.J.K., H.C.M., B.B.C., N.W., D.R. Supervised the research: J.A., R.C.T., D.A.v.H., M.J.D., S.L.S., D.G.M. Provided data: gnomAD consortium (Genome Aggregation Database Production Team and Genome Aggregation Database Consortium), H.C.M., R.C.T., D.A.v.H. Wrote the paper: E.V.M. Edited and approved the final manuscript: all authors
Data availability
The gnomAD v2 data are available via the gnomAD browser (https://gnomad.broadinstitute.org).
Code availability
Additional data and the R 3.5.1 and Python 2.7.10 source code for this study are available via GitHub (https://github.com/ericminikel/drug_target_lof).
Competing interests
E.V.M. has received research support in the form of charitable contributions from Charles River Laboratories and Ionis Pharmaceuticals, and has consulted for Deerfield Management. K.J.K. is a shareholder of Personalis. H.C.M., B.B.C., M.W., D.R. and J.A. have no competing interests to declare. R.C.T. serves on the Scientific Advisory Board of Ipsen Ltd and has current funding from the Wellcome Trust and the National Institute for Health Research UK. D.A.v.H. is a shareholder of Nexpep Pty Ltd; has current or recent research funding from Wellcome Trust, Medical Research Council UK, National Institute for Health Research UK, Alnylam Pharmaceuticals; and serves on the Population & Systems Medicine Board of the Medical Research Council UK. MJD is a founder of Maze Therapeutics. S.L.S. serves on the Board of Directors of the Genomics Institute of the Novartis Research Foundation (‘GNF’); is a shareholder and serves on the Board of Directors of Jnana Therapeutics; is a shareholder of Forma Therapeutics; is a shareholder and advises Decibel Therapeutics and Eikonizo Therapeutics; serves on the Scientific Advisory Boards of Eisai Co., Ltd., Ono Pharma Foundation, Exo Therapeutics, and F-Prime Capital Partners; and is a Novartis Faculty Scholar. D.G.M. is a founder with equity in Goldfinch Bio, and has received research support from AbbVie, Astellas, Biogen, BioMarin, Eisai, Merck, Pfizer, and Sanofi-Genzyme.
Footnotes
Peer review information Nature thanks Paul de Bakker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lists of authors and their affiliations appear at the end of the paper
Deceased: Pamela Sklar
Change history
2/3/2021
A Correction to this paper has been published: 10.1038/s41586-020-03177-5
Contributor Information
Eric Vallabh Minikel, Email: eminikel@broadinstitute.org.
Daniel G. MacArthur, Email: d.macarthur@garvan.org.au
Genome Aggregation Database Production Team:
Jessica Alföldi, Irina M. Armean, Eric Banks, Louis Bergelson, Kristian Cibulskis, Ryan L. Collins, Kristen M. Connolly, Miguel Covarrubias, Beryl B. Cummings, Mark J. Daly, Stacey Donnelly, Yossi Farjoun, Steven Ferriera, Laurent Francioli, Stacey Gabriel, Laura D. Gauthier, Jeff Gentry, Namrata Gupta, Thibault Jeandet, Diane Kaplan, Konrad J. Karczewski, Kristen M. Laricchia, Christopher Llanwarne, Eric V. Minikel, Ruchi Munshi, Benjamin M. Neale, Sam Novod, Anne H. O’Donnell-Luria, Nikelle Petrillo, Timothy Poterba, David Roazen, Valentin Ruano-Rubio, Andrea Saltzman, Kaitlin E. Samocha, Molly Schleicher, Cotton Seed, Matthew Solomonson, Jose Soto, Grace Tiao, Kathleen Tibbetts, Charlotte Tolonen, Christopher Vittal, Gordon Wade, Arcturus Wang, Qingbo Wang, James S. Ware, Nicholas A. Watts, Ben Weisburd, and Nicola Whiffin
Genome Aggregation Database Consortium:
Carlos A. Aguilar Salinas, Tariq Ahmad, Christine M. Albert, Diego Ardissino, Gil Atzmon, John Barnard, Laurent Beaugerie, Emelia J. Benjamin, Michael Boehnke, Lori L. Bonnycastle, Erwin P. Bottinger, Donald W. Bowden, Matthew J. Bown, John C. Chambers, Juliana C. Chan, Daniel Chasman, Judy Cho, Mina K. Chung, Bruce Cohen, Adolfo Correa, Dana Dabelea, Mark J. Daly, Dawood Darbar, Ravindranath Duggirala, Josée Dupuis, Patrick T. Ellinor, Roberto Elosua, Jeanette Erdmann, Tõnu Esko, Martti Färkkilä, Jose Florez, Andre Franke, Gad Getz, Benjamin Glaser, Stephen J. Glatt, David Goldstein, Clicerio Gonzalez, Leif Groop, Christopher Haiman, Craig Hanis, Matthew Harms, Mikko Hiltunen, Matti M. Holi, Christina M. Hultman, Mikko Kallela, Jaakko Kaprio, Sekar Kathiresan, Bong-Jo Kim, Young Jin Kim, George Kirov, Jaspal Kooner, Seppo Koskinen, Harlan M. Krumholz, Subra Kugathasan, Soo Heon Kwak, Markku Laakso, Terho Lehtimäki, Ruth J. F. Loos, Steven A. Lubitz, Ronald C. W. Ma, Daniel G. MacArthur, Jaume Marrugat, Kari M. Mattila, Steven McCarroll, Mark I. McCarthy, Dermot McGovern, Ruth McPherson, James B. Meigs, Olle Melander, Andres Metspalu, Benjamin M. Neale, Peter M. Nilsson, Michael C. O’Donovan, Dost Ongur, Lorena Orozco, Michael J. Owen, Colin N. A. Palmer, Aarno Palotie, Kyong Soo Park, Carlos Pato, Ann E. Pulver, Nazneen Rahman, Anne M. Remes, John D. Rioux, Samuli Ripatti, Dan M. Roden, Danish Saleheen, Veikko Salomaa, Nilesh J. Samani, Jeremiah Scharf, Heribert Schunkert, Moore B. Shoemaker, Pamela Sklar, Hilkka Soininen, Harry Sokol, Tim Spector, Patrick F. Sullivan, Jaana Suvisaari, E. Shyong Tai, Yik Ying Teo, Tuomi Tiinamaija, Ming Tsuang, Teresa Dan Turner, Teresa Tusie-Luna, Erkki Vartiainen, Marquis P. Vawter, James. S. Ware, Hugh Watkins, Rinse K. Weersma, Maija Wessman, James G. Wilson, and Ramnik J. Xavier
Extended data
is available for this paper at 10.1038/s41586-020-2267-z.
Supplementary information
is available for this paper at 10.1038/s41586-020-2267-z.
References
- 1.Plenge, R. M., Scolnick, E. M. & Altshuler, D. Validating therapeutic targets through human genetics. Nat. Rev. Drug Discov. 12, 581–594 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Hay, M., Thomas, D. W., Craighead, J. L., Economides, C. & Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 32, 40–51 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015). [DOI] [PubMed] [Google Scholar]
- 4.King, E. A., Davis, J. W. & Degner, J. F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 15, e1008489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musunuru, K. & Kathiresan, S. Genetics of common, complex coronary artery disease. Cell177, 132–145 (2019). [DOI] [PubMed] [Google Scholar]
- 6.MacArthur, D. G. et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science335, 823–828 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature10.1038/s41586-020-2308-7 (2020). [DOI] [PMC free article] [PubMed]
- 8.Lim, E. T. et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 10, e1004494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittles, A. H. & Black, M. L. Evolution in health and medicine Sackler colloquium: consanguinity, human evolution, and complex diseases. Proc. Natl Acad. Sci. USA107 (Suppl. 1), 1779–1786 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature536, 285–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrovski, S., Wang, Q., Heinzen, E. L., Allen, A. S. & Goldstein, D. B. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 9, e1003709 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samocha, K. E. et al. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 46, 944–950 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller, Z. L., Berg, J. J., Mostafavi, H., Sella, G. & Przeworski, M. Measuring intolerance to mutation in human genetics. Nat. Genet. 51, 772–776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings, B. B. et al. Transcript expression-aware annotation improves rare variant discovery and interpretation. Nature10.1038/s41586-020-2329-2 (2020). [DOI] [PMC free article] [PubMed]
- 15.Collins, R. L. et al. A structural variation reference for medical and population genetics. Nature10.1038/s41586-020-2287-8 (2020). [DOI] [PMC free article] [PubMed]
- 16.Kosmicki, J. A. et al. Refining the role of de novo protein-truncating variants in neurodevelopmental disorders by using population reference samples. Nat. Genet. 49, 504–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46 (D1), D1074–D1082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehm, H. L. et al. ClinGen—the clinical genome resource. N. Engl. J. Med. 372, 2235–2242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morham, S. G. et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell83, 473–482 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Ohashi, K. et al. Early embryonic lethality caused by targeted disruption of the 3-hydroxy-3-methylglutaryl-CoA reductase gene. J. Biol. Chem. 278, 42936–42941 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Nagashima, S. et al. Liver-specific deletion of 3-hydroxy-3-methylglutaryl coenzyme A reductase causes hepatic steatosis and death. Arterioscler. Thromb. Vasc. Biol. 32, 1824–1831 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Lv, W. et al. The drug target genes show higher evolutionary conservation than non-target genes. Oncotarget7, 4961–4971 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motenko, H., Neuhauser, S. B., O’Keefe, M. & Richardson, J. E. MouseMine: a new data warehouse for MGI. Mamm. Genome26, 325–330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart, T. et al. Evaluation and design of genome-wide CRISPR/SpCas9 knockout screens. G3 (Bethesda)7, 2719–2727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finer, S. et al. Cohort profile: East London genes & health (ELGH), a community-based population genomics and health study of British Bangladeshi and British Pakistani people. Int. J. Epidemiol. 49, 20–21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narasimhan, V. M. et al. Health and population effects of rare gene knockouts in adult humans with related parents. Science352, 474–477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleheen, D. et al. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature544, 235–239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulem, P. et al. Identification of a large set of rare complete human knockouts. Nat. Genet. 47, 448–452 (2015). [DOI] [PubMed] [Google Scholar]
- 29.DeBoever, C. et al. Medical relevance of protein-truncating variants across 337,205 individuals in the UK Biobank study. Nat. Commun. 9, 1612 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabrizi, S. J. et al. Targeting Huntingtin expression in patients with Huntington’s disease. N. Engl. J. Med. 380, 2307–2316 (2019). [DOI] [PubMed] [Google Scholar]
- 31.DeVos, S. L. et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med. 9, eaag0481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minikel, E. V. et al. Prion protein lowering is a disease-modifying therapy across prion strains, disease stages, and endpoints. Preprint at 10.1101/2020.03.27.011940 (2020). [DOI] [PMC free article] [PubMed]
- 33.McCampbell, A. et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J. Clin. Invest. 128, 3558–3567 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, J., Chen, Y. & Pu, J. Leucine-rich repeat kinase 2 in Parkinson’s disease: updated from pathogenesis to potential therapeutic target. Eur. Neurol. 79, 256–265 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Cole, T. A. et al. Alpha-synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson’s disease. Preprint at 10.1101/830554 (2019). [DOI] [PMC free article] [PubMed]
- 36.Minikel, E. V. et al. Age at onset in genetic prion disease and the design of preventive clinical trials. Neurology93, e125–e134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duyao, M. P. et al. Inactivation of the mouse Huntington’s disease gene homolog Hdh. Science269, 407–410 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Rodan, L. H. et al. A novel neurodevelopmental disorder associated with compound heterozygous variants in the huntingtin gene. Eur. J. Hum. Genet. 24, 1826–1827 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambrose, C. M. et al. Structure and expression of the Huntington’s disease gene: evidence against simple inactivation due to an expanded CAG repeat. Somat. Cell Mol. Genet. 20, 27–38 (1994). [DOI] [PubMed] [Google Scholar]
- 40.GTEx Consortium. Genetic effects on gene expression across human tissues. Nature550, 204–213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minikel, E. V. et al. Quantifying prion disease penetrance using large population control cohorts. Sci. Transl. Med. 8, 322ra9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zambrowicz, B. P. & Sands, A. T. Knockouts model the 100 best-selling drugs—will they model the next 100? Nat. Rev. Drug Discov. 2, 38–51 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Uhl, K., Kennedy, D. L. & Kweder, S. L. Risk management strategies in the Physicians’ Desk Reference product labels for pregnancy category X drugs. Drug Saf. 25, 885–892 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Haggarty, S. J., Koeller, K. M., Wong, J. C., Grozinger, C. M. & Schreiber, S. L. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl Acad. Sci. USA100, 4389–4394 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, B. W. et al. T cell responses in calcineurin A alpha-deficient mice. J. Exp. Med. 183, 413–420 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacinto, E. et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Hoshi, N., Langeberg, L. K., Gould, C. M., Newton, A. C. & Scott, J. D. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol. Cell37, 541–550 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Findlay, G. M. et al. Accurate classification of BRCA1 variants with saturation genome editing. Nature562, 217–222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiffin, N. et al. Characterising the loss-of-function impact of 5′ untranslated region variants in whole genome sequence data from 15,708 individuals. Nat. Commun.10.1038/s41467-019-10717-9 (2020). [DOI] [PMC free article] [PubMed]
- 50.DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47, 20–33 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Andreadis, A. Tau splicing and the intricacies of dementia. J. Cell. Physiol. 227, 1220–1225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yates, B. et al. Genenames.org: the HGNC and VGNC resources in 2017. Nucleic Acids Res. 45 (D1), D619–D625 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mainland, J. D., Li, Y. R., Zhou, T., Liu, W. L. L. & Matsunami, H. Human olfactory receptor responses to odorants. Sci. Data2, 150002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blekhman, R. et al. Natural selection on genes that underlie human disease susceptibility. Curr. Biol. 18, 883–889 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harding, S. D. et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 46 (D1), D1091–D1106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacArthur, J. et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 45 (D1), D896–D901 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamosh, A., Scott, A. F., Amberger, J. S., Bocchini, C. A. & McKusick, V. A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 33, D514–D517 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pringsheim, T. et al. The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov. Disord. 27, 1083–1091 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Keum, J. W. et al. The HTT CAG-expansion mutation determines age at death but not disease duration in huntington disease. Am. J. Hum. Genet. 98, 287–298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kay, C. et al. Huntington disease reduced penetrance alleles occur at high frequency in the general population. Neurology87, 282–288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisher, E. R. & Hayden, M. R. Multisource ascertainment of Huntington disease in Canada: prevalence and population at risk. Mov. Disord. 29, 105–114 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Pringsheim, T., Jette, N., Frolkis, A. & Steeves, T. D. L. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 29, 1583–1590 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Healy, D. G. et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 7, 583–590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Onyike, C. U. & Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry25, 130–137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bang, J., Spina, S. & Miller, B. L. Frontotemporal dementia. Lancet386, 1672–1682 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maddox, R. A. et al. Prion disease incidence in the United States, 2003–2015. Neurology94, e153–e157 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Trinh, J., Guella, I. & Farrer, M. J. Disease penetrance of late-onset parkinsonism: a meta-analysis. JAMA Neurol. 71, 1535–1539 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Chiò, A. et al. Prevalence of SOD1 mutations in the Italian ALS population. Neurology70, 533–537 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Cudkowicz, M. E. et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann. Neurol. 41, 210–221 (1997). [DOI] [PubMed] [Google Scholar]
- 70.Renton, A. E., Chiò, A. & Traynor, B. J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byrne, S. et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry82, 623–627 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Rowland, L. P. & Shneider, N. A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1688–1700 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Hirtz, D. et al. How common are the “common” neurologic disorders? Neurology68, 326–337 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Logroscino, G. et al. Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry81, 385–390 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hernandez, D. G., Reed, X. & Singleton, A. B. Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J. Neurochem. 139 (Suppl. 1), 59–74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Funayama, M. et al. A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 51, 296–301 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Zimprich, A. et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron44, 601–607 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Goldwurm, S. et al. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counseling in Parkinson disease. Neurology68, 1141–1143 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Do, C. B. et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 7, e1002141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kinoshita, T. & Fujita, M. Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J. Lipid Res. 57, 6–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitamoto, T., Iizuka, R. & Tateishi, J. An amber mutation of prion protein in Gerstmann–Sträussler syndrome with mutant PrP plaques. Biochem. Biophys. Res. Commun. 192, 525–531 (1993). [DOI] [PubMed] [Google Scholar]
- 82.Finckh, U. et al. High prevalence of pathogenic mutations in patients with early-onset dementia detected by sequence analyses of four different genes. Am. J. Hum. Genet. 66, 110–117 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jayadev, S. et al. Familial prion disease with Alzheimer disease-like tau pathology and clinical phenotype. Ann. Neurol. 69, 712–720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fong, J. C. et al. Genetic prion disease caused by PRNP Q160X mutation presenting with an orbitofrontal syndrome, cyclic diarrhea, and peripheral neuropathy. J. Alzheimers Dis. JAD55, 249–258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bommarito, G. et al. A novel prion protein gene-truncating mutation causing autonomic neuropathy and diarrhea. Eur. J. Neurol. 25, e91–e92 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Mead, S. et al. A novel prion disease associated with diarrhea and autonomic neuropathy. N. Engl. J. Med. 369, 1904–1914 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Capellari, S. et al. Two novel PRNP truncating mutations broaden the spectrum of prion amyloidosis. Ann. Clin. Transl. Neurol. 5, 777–783 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsuzono, K. et al. A novel familial prion disease causing pan-autonomic-sensory neuropathy and cognitive impairment. Eur. J. Neurol. 20, e67–e69 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Jansen, C. et al. Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP. Acta Neuropathol. 119, 189–197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This supplementary table contains details of curated variants in neurodegenerative disease genes.
Reviewer reports and authors’ response from the peer review of this Article at Nature.
Data Availability Statement
The gnomAD v2 data are available via the gnomAD browser (https://gnomad.broadinstitute.org).
Additional data and the R 3.5.1 and Python 2.7.10 source code for this study are available via GitHub (https://github.com/ericminikel/drug_target_lof).