Abstract
OBJECTIVE
To investigate if capnometry-assisted anti-hyperventilation respiratory training, successful in treating panic, and sleep hygiene instructions would reduce posttraumatic stress disorder (PTSD) hyperarousal symptoms in U.S. military veterans.
METHOD
We conducted a parallel, non-blinded clinical trial and randomized 80 veterans with PTSD hyperarousal into treatment or waitlist. Primary treatment outcomes from baseline to first follow-up were analyzed using mixed modeling. Baseline physiological measures were compared between the PTSD hyperarousal group and a no-PTSD group (n = 68).
RESULTS
Baseline respiration rate but not partial-pressure of end-tidal carbon dioxide (PCO2) were higher in the PTSD hyperarousal group than the no-PTSD group during three minutes of quiet sitting, indicating no difference in baseline hyperventilation. There was no significant effect of the intervention on PTSD hyperarousal symptoms or hyperventilation compared to waitlist, but treatment did lower respiratory rate.
CONCLUSION
This intervention did not reduce PTSD hyperarousal symptoms, perhaps due to differences between underlying mechanisms of PTSD hyperarousal and panic disorder or to differences between veteran and civilian populations.
Keywords: PTSD, hyperarousal, hyperventilation, breathing training
Hyperarousal in posttraumatic stress disorder (PTSD) consists of “marked alterations in arousal and reactivity” according to DSM-5 (APA, 2013). When faced with a stressor, humans and other animals exhibit a distinct pattern of behavioral and physiological responses (Belda, Fuentes, Daviu, Nadal, & Armario, 2015). Among these are changes in autonomically controlled variables, such as increased heart rate (Bertram et al., 2014), hyperventilation (Alpers, Wilhelm, & Roth, 2005), and sleep disturbances (Spoormaker & Montgomery, 2008). Physiological activation drives this pattern of responses, which are typically accompanied by feelings of anxiety.
Hyperventilation is both a cause and an effect of anxiety, producing body sensations of anxiety and higher arousal in a positive feedback loop. End-expiratory partial pressure of carbon dioxide (PCO2) quantifies hyperventilation. Breathing rapidly and/or deeply (i.e., hyperventilating) reduces PCO2 levels, which can lead to symptoms like lightheadedness and tingling in the limbs. Such sensations are often accompanied by anxiety, which can exacerbate those sensations and may result in a panic attack (Meuret, Wilhelm, Ritz, & Roth, 2008).
PTSD and panic disorder often overlap, with as many as 35% of PTSD patients reporting panic attacks (Cougle, Feldner, Keough, Hawkins, & Fitch, 2010). A proposed mechanism for the relationship between panic and posttraumatic reactions is that internal or external cues associated with the trauma become conditioned as triggers for panic symptoms and physiological activation (Nixon, Resick, & Griffin, 2004).
A successful treatment for panic disorder is Capnometry-Assisted Respiratory Training (CART; Meuret et al., 2008), which teaches patients to raise their levels of PCO2 as fed back to them on a capnometer screen. Patients learn how to counteract hyperventilation and prevent the exacerbation of anxiety and unpleasant physical sensations. We hypothesized that if PTSD hyperarousal involved overbreathing (Alpers et al., 2005), this intervention would reduce subjective anxiety and physiological activation, and thus hyperarousal symptoms.
Other PTSD treatments have included breathing modification components, but none has given subjects PCO2 feedback (e.g., Sudarshan Kriya yoga; Seppälä et al., 2014).
We report here a non-blinded randomized clinical trial of CART in veterans with PTSD hyperarousal symptoms. Since one such symptom, poor sleep, has been particularly resistant to PTSD treatments (Gutner, Casement, Gilbert, & Resick, 2013), we supplemented CART with stimulus control and sleep hygiene instructions, like those in cognitive-behavior therapy for insomnia (CBT-I; Manber & Kuo, 2002).
Our primary hypothesis was that after treatment, participants with PTSD would show decreased hyperarousal and other symptoms compared to untreated participants. Our primary outcome analysis compared intent-to-treat immediate treatment (IT) with waitlist (WL) from baseline to first follow-up. Our secondary outcome analysis compared within-group changes across all time points for the treatment group.
Method
Participants
Eligible participants were U.S. military veterans ages 18–65 with current or recent PTSD. Recent PTSD was operationalized as meeting full DSM-IV-TR diagnostic criteria for PTSD within the previous five years, and with at least two current hyperarousal symptoms (required to meet hyperarousal cluster criteria). It should be noted that DSM-5 PTSD hyperarousal symptom criteria are not substantially different from those in DSM-IV-TR.
We included veterans with recent PTSD because it is not uncommon for this population to have unremitting symptoms, including hyperarousal, even after trauma-focused treatment (Steenkamp, Litz, Hoge, & Marmar, 2015). Exclusion criteria were substance abuse within previous three months, schizophrenia, bipolar I disorder, severe traumatic brain injury, currently receiving trauma-focused treatment (e.g., prolonged exposure or cognitive processing therapy), or in another mental health clinical trial. For the no-PTSD group, participants could not have ever met criteria for a full PTSD diagnosis and could not currently meet criteria for the hyperarousal symptom cluster. This group was deliberately heterogeneous to compare age-, sex- and measurement-setting-matched physiological values, particularly those for respiration, to the PTSD group. The no-PTSD group did not receive any intervention and thus did not serve as a control group for the effects of treatment.
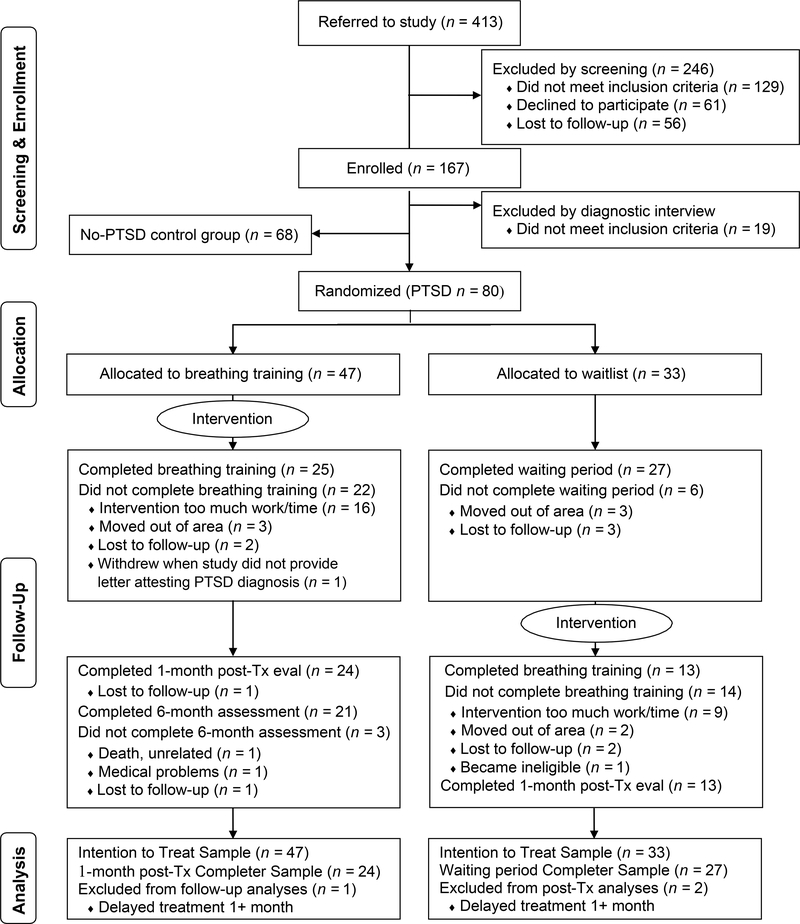
80 veterans with either current PTSD (n = 68), or recent PTSD and current hyperarousal (n = 12) were randomized to study intervention (n = 47) or waitlist (n = 33; see Figure 1). There were 68 participants (veterans and non-veterans) in the no-PTSD group. Demographic and clinical characteristics of the participants are in Table 1.
Figure 1.
Diagram summarizing participant flow through the study protocol and analyses. Tx = treatment; eval = evaluation
Table 1.
Demographic and Clinical Characteristics
| PTSD Groupa | no-PTSD Group | ||||
|---|---|---|---|---|---|
|
n = 80 |
n = 68 |
||||
| M or | SD or | M or | SD or | Test | |
| n | % | n | % | ||
| Demographics | |||||
| Age (years) | 53.14 | 10.80 | 53.63 | 9.45 | U = 2700.5 |
| Female | 10 | 12.5 | 6 | 8.8 | χ2(1) = 0.52 |
| Race | χ2(3) = 9.21* | ||||
| Caucasian | 35 | 43.8 | 41 | 60.3 | |
| African-American | 18 | 22.5 | 8 | 11.8 | |
| Asian/Pacific Islander | 6 | 7.5 | 10 | 14.7 | |
| Multi-Racial/Other | 21 | 26.3 | 9 | 13.2 | |
| Married/Living with Partner | 30 | 38.0 | 25 | 36.8 | χ2(1) = 0.02 |
| Military Veteran | 80 | 100 | 47 | 69.1 | χ2(1) = 28.79*** |
| OEF/OIF Veteran | 13 | 22.8 | 3 | 6.4 | χ2(1) = 2.08 |
| VA compensation for PTSD: | |||||
| Receiving, pending, or planning to apply | 55 | 69.6 | 0 | 0 | — |
| Current mental health treatment | χ2(2) = 22.40*** | ||||
| Outpatient | 41 | 54.7 | 13 | 19.1 | |
| Domiciliary | 8 | 10.7 | 4 | 5.9 | |
| None | 26 | 34.7 | 9 | 72.1 | |
| Trauma and Clinical Characteristics | |||||
| Trauma Exposed | 80 | 100 | 31 | 45.6 | χ2(1) = 58.04*** |
| Current PTSD | 68 | 85.0 | 0 | 0 | — |
| Index Trauma | χ2(3) = 27.49*** | ||||
| Combat | 36 | 45.0 | 1 | 3.2 | |
| Physical Assault (non-combat) | 12 | 15.0 | 7 | 22.6 | |
| Sexual Assault | 16 | 20.0 | 3 | 9.7 | |
| Other | 16 | 20.0 | 20 | 29.4 | |
| Years since Traumatic Event | 27.32 | 16.10 | 22.31 | 17.03 | U = 1002.5 |
| CAPS total | 69.78 | 24.30 | 10.85 | 11.32 | U = 52.0*** |
| CAPS hyperarousal | 24.46 | 6.76 | 5.21 | 4.81 | U = 55.5*** |
| Baseline respiration (3 mins quiet sitting in laboratory) | |||||
| End-tidal PCO2 (mmHg) | 38.42 | 4.01 | 37.13 | 4.54 | t(142) = 1.81 |
| Respiration rate (breaths per min) | 16.23 | 4.00 | 14.76 | 4.43 | t(141) = 2.07* |
| Sleep qualityb | 11.77 | 4.02 | 6.07 | 4.01 | U = 602.0*** |
| PTSD-specific sleep qualityb | 7.85 | 5.22 | 0.74 | 1.05 | U = 193.0*** |
| Reported Sleep Apnea diagnosis | 24.0 | 30.8 | 10.0 | 14.7 | χ2(1) = 5.04* |
| Beck Depression Inventory-II | 20.08 | 11.16 | 5.80 | 6.84 | U = 584.0*** |
| Beck Anxiety Index | 22.00 | 14.52 | 3.90 | 6.43 | U = 598.5*** |
| Aggression Questionnaire | 79.93 | 22.05 | 55.20 | 16.73 | U = 977.0*** |
| Reported mild Traumatic Brain Injury | 38 | 49.4 | 9 | 13.2 | χ2(1) = 9.63** |
| Major Depressive Disorder diagnosis | 17 | 21.3 | 3 | 4.4 | χ2(1) = 5.70* |
| Past Alcohol or Substance Use Disorder diagnosis | 59 | 73.8 | 32 | 47.1 | χ2(1) = 2.27 |
Note. ES = effect size; OEF/OIF = Operation Enduring Freedom/Operation Iraqi Freedom; CAPS = Clinician-Administered PTSD Scale; PCO2 = partial pressure of carbon dioxide; mmHg = millimeters of mercury. Group differences analyzed with Student’s t-test for normally distributed data, Mann-Whitney U test for not normally distributed data, or chi-squared test for categorical data. Due to missing data, subjects in specific comparisons varied from 66 – 80 in PTSD group and 61 – 68 in no-PTSD group.
Group differences reported between combined PTSD group (Immediate treatment + Waitlist, n = 80) and no-PTSD group. There were no significant demographic or clinical differences between the Immediate treatment and Waitlist PTSD groups.
Five no-PTSD and four PTSD participants reporting untreated sleep apnea were excluded from sleep analyses.
p ≤ .05
p ≤ .01
p ≤ .001.
Procedure
Participants were recruited from February 2010 to May 2013 with flyers and media advertisements. After telephone screening, interested participants were invited to our office to complete questionnaires, a diagnostic interview, and a physiological assessment.
Diagnostic interviews and the study intervention were conducted by a clinical psychologist or supervised psychology graduate students. Eligible participants were assigned to either the PTSD hyperarousal group or no-PTSD group by the supervising psychologist and then randomly assigned to either IT or WL by the research assistant or study coordinator. A weighted randomization procedure was 35% more likely to assign new participants to the IT than WL condition because of differing withdrawal rates.
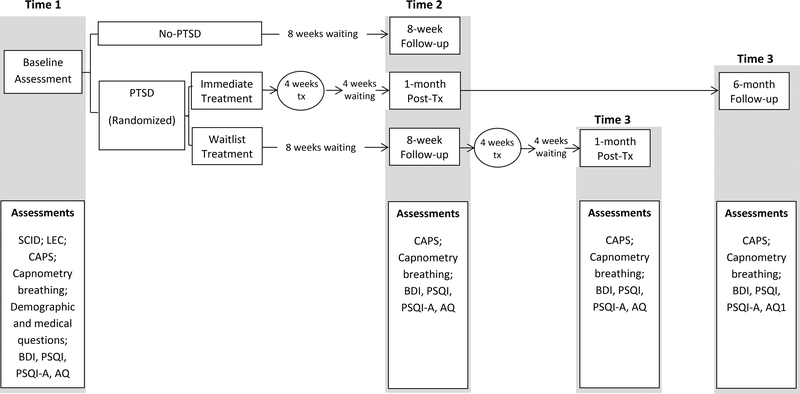
A full assessment and treatment timeline for participants is available in Figure 2. Participants in the IT group began the study intervention within two weeks of baseline assessment, completed a second assessment four weeks after completing CART, and then a six-month follow-up assessment. Participants in the WL group completed their second assessment eight weeks after baseline and the third assessment four weeks after completing CART. Diagnostic raters were not blind to group membership because participants themselves typically revealed their treatment status during the CAPS interview. Participants were compensated for each completed assessment but not for the treatment sessions. Verbal and written informed consent were obtained prior to study enrollment. This study was approved by the Stanford University Institutional Review Board and registered as clinical trial NCT00855816.
Figure 2.
Assessment timeline. Tx = treatment; SCID = Structured Clinical Interview for DSM-IV Disorders (First, Spitzer, Gibbon, & Williams, 2002); LEC = Life Events Checklist (Gray, Litz, Hsu, & Lombardo, 2004); CAPS = Clinician-Administered PTSD Scale for DSM-IV (Blake et al., 1995); BDI = Beck Depression Inventory-II (Beck, Steer, & Brown, 1996); PSQI = Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989); PSQI-A = Pittsburgh Sleep Quality Index-Addendum for PTSD (Germain, Hall, Krakow, Shear, & Buysse, 2005); AQ = Aggression Questionnaire (Buss & Perry, 1992)
Assessment
At baseline, we administered the Structured Clinical Interview for DSM-IV Disorders (SCID; First, Spitzer, Gibbon, & Williams, 2002), the Clinician-Administered PTSD Scale for DSM-IV (CAPS; Blake et al., 1995), and the Life Events Checklist (Gray, Litz, Hsu, & Lombardo, 2004). The CAPS total and CAPS hyperarousal subscale were highly reliable in our sample, with Cronbach alphas of .97 and .92 respectively. Diagnostic interviewers attained 100% agreement on all SCID diagnoses and CAPS inter-rater reliability coefficients were greater than .90 on training cases prior to interviewing new participants.
Participants completed demographic and medical questionnaires created by the investigators, and standardized questionnaires about depression, sleep, and aggression (Figure 2). They also evaluated the credibility of the intervention and their expectancy of improvement (Borkovec et al., 1987), and participants and therapists completed the Working Alliance Inventory (Hatcher & Gillaspy, 2006).
Respiration was assessed during five minutes of quiet sitting in our treatment room during which the participant’s exhaled breaths were drawn through a nasal cannula into a capnograph (Tidal Wave Sp, Model 715; Philips Healthcare, Andover, Massachusetts). Capnometry data were downloaded after each assessment, and respiration rate and PCO2 values were recorded. At the pre-treatment assessment, participants were told to breathe normally. Post-treatment, those who had completed CART were told to “breathe as you were taught in treatment.”
Treatment
CART comprised five sessions over four weeks of biofeedback-assisted respiratory training and sleep hygiene instructions, as well as twice-daily breathing exercises and daily self-monitoring of sleep. Breathing exercises required listening to a 17-minute recording of paced tones while breathing into the portable capnometer. The capnometer displayed the individual’s respiration rate and PCO2 in nearly real-time, and participants wrote down these data in logs. Treatment details for each session are in Appendix A. The treatment rationale was presented in session one. Participants were told that PTSD hyperarousal symptoms could both exacerbate and be exacerbated by hyperventilation, even if they were not hyperventilating while calm. Regardless of their baseline PCO2, participants were provided information about maintaining target levels of 40 millimeters of mercury (mmHg) and respiration at nine breaths per minute. In a previous study in our lab, we had found that CART with a respiration rate of nine breaths per minute successfully treated episodic anxiety (Kim, S., Wollburg, E., & Roth, W. T. (2012). Clinical experience had taught us that it was more achievable than the goal of six breaths per minutes for the intended demographic. Participants were told that they would learn to maintain safe PCO2 levels while calm, so they would not hyperventilate when PTSD symptoms were triggered.
Sleep hygiene included behavioral recommendations to increase the likelihood of better sleep. Stimulus-control instructions were to minimize time spent awake in the sleep environment. Participants tracked how well they followed these instructions and their sleep/wake times in daily sleep logs.
Data Analyses
Demographic and baseline clinical variables were compared between the combined PTSD groups and the no-PTSD group, as well as between IT and WL PTSD groups. For these analyses, missing data were excluded pairwise or analysis by analysis. Missing treatment outcome data were replaced using restricted maximum likelihood estimation in mixed modeling.
Means and standard deviations of capnometer data were calculated for the last three minutes of quiet sitting in the lab. PCO2 levels less than 10 or greater than 50 mmHg and respiratory rates less than 3 or greater than 30 breaths per minute were presumed measurement artifacts and discarded.
Primary treatment outcomes from baseline to first follow-up in the intent-to-treat PTSD sample were analyzed by mixed modeling using restricted maximum likelihood estimation. Fixed effects were between-subject Group (IT, WL), within-subject Time (1, 2) and Group*Time interaction, with Subjects as a random effect. Cohen’s d effect sizes were calculated for changes within each group over time and between groups at follow-up. Piecewise linear mixed modeling was also used to detect any delayed effects in only the IT group because the waitlist group did not complete a 6-month follow-up assessment. Exploratory analyses included repeated-measure analyses of variance and covariance (ANOVA and ANCOVA) for completer analyses and multiple regressions for moderator and attrition analyses. A priori power analyses estimated that power to detect treatment effects ranged from 0.74 to 0.90 if there were 25 participants in each condition, with a target of .80.
Results
No baseline demographic and clinical differences were found in the IT and WL PTSD groups, so their data are combined in Table 1 (“PTSD Group”). Missing data differed by variable, ranging from 0–23%, with the greatest data loss for sleep variables. This was due to excluding participants with untreated sleep apnea from the sleep analyses. Details on missing data are reported in Table 1 footnotes and Appendix B.
Baseline Respiratory Measures
Baseline respiration rate was higher in the PTSD group (M = 16.23 breaths per minute, SD = 4.00) than in the no-PTSD group (M = 14.77, SD = 4.43) during three minutes of quiet sitting in the laboratory (t(141) = −2.07, p = .040, d = 0.35). Mean PCO2 was not significantly different.
Study Attrition
Fifty-one participants across the PTSD groups (63.8%) completed assessment time point 2, which was used for the primary outcome analyses. All three assessment time points were completed by 34 (42.5%) PTSD participants (Figure 1). The most common reasons cited for study withdrawal were inconvenience and study burden.
Treatment Adherence and Fidelity
Of the 80 participants in the PTSD group, 38 (47.5%) completed all treatment sessions. Most withdrawals (n = 18) occurred prior to treatment onset or after the first treatment session (n = 9). No differences were found in pre-treatment credibility, expectancies or working alliance ratings between treatment completers and non-completers. For participants who attended at least one treatment session, 51.6% of the breathing exercises assigned for homework were completed weekly per capnometer data. For treatment completers, the weekly average was 70.7%. Weekly adherence to the nine daily sleep instructions was moderate (M = 60.9%, SD = 18.2%). There was no significant change from week 1 to week 4 in either PCO2 or respiration rate with or without tones (p values ranged from .085 to .217). A random selection of 33% of treatment cases rated on therapy adherence by other study therapists and trained research assistants found high fidelity. These data are available in Appendix C.
Primary Outcome: Intent-to-treat IT vs. WL from Time 1 to Time 2
Outcome variable means and standard deviations for both PTSD groups across all time points are in Table 2. Linear mixed modeling found no treatment effects of CART on any tested outcome variables except respiration rate, which indicated that those who received treatment reduced their respiration rate as instructed (Table 3). There were no significant changes in physiologically measured hyperventilation (PCO2). There was a within-group decrease in CAPS hyperarousal scores at Time 2 in the WL group only, but this decrease was not significantly different from the change in the IT group. Similarly, there was a significant decrease in self-reported sleep onset latency in the IT group, but no interaction effect.
Table 2.
Means and Standard Deviations of Outcomes at Three Time Points for PTSD Groups
| Immediate Treatment |
Waitlist (Delayed Treatment) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time 1: | Time 2: | Time 3: | Time 1: | Time 2: | Time 3: | |||||||
| Baseline | 1-month | 6-month | Baseline | 8-week | 1-month | |||||||
| Post-Treatment | Follow-up | Follow-up | Post-Treatment | |||||||||
| n = 47 | n = 23 | n = 20 | n = 33 | n = 27 | n = 11 | |||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Respiration measures: | ||||||||||||
| End-tidal pCO2 (mmHg) | 38.53 | 3.45 | 37.10 | 6.22 | 39.33 | 5.14 | 38.27 | 4.72 | 37.26 | 4.75 | 35.81 | 4.11 |
| Respiration rate (breaths per min) | 16.22 | 4.11 | 10.21 | 4.06 | 10.42 | 4.55 | 16.24 | 3.91 | 16.56 | 4.28 | 11.75 | 3.30 |
| CAPS total | 70.17 | 22.89 | 59.65 | 26.80 | 57.70 | 30.63 | 69.24 | 26.51 | 62.70 | 24.25 | 50.82 | 26.90 |
| CAPS hyperarousal | 24.19 | 6.80 | 20.70 | 7.92 | 20.20 | 9.53 | 24.85 | 6.79 | 20.70 | 8.60 | 18.45 | 7.93 |
| Beck Depression Inventory | 20.14 | 11.04 | 19.50 | 14.13 | 16.58 | 12.64 | 20.0 | 11.50 | 17.0 | 11.98 | 18.27 | 12.83 |
| Aggression Questionnaire | 80.82 | 22.86 | 72.25 | 21.55 | 70.53 | 21.43 | 78.72 | 21.17 | 73.81 | 20.35 | 73.82 | 26.22 |
| Sleep measuresa: | ||||||||||||
| Sleep quality | 10.77 | 3.71 | 10.00 | 3.98 | 9.83 | 4.24 | 12.41 | 4.28 | 12.32 | 4.00 | 10.80 | 4.73 |
| PTSD-specific sleep quality | 7.65 | 4.66 | 6.31 | 5.61 | 7.05 | 5.17 | 7.77 | 5.69 | 5.00 | 4.42 | 6.00 | 5.27 |
| Self-reported sleep onset latencyb (mins) | 39.01 | 40.32 | 25.31 | 25.94 | 23.88 | 17.76 | 40.64 | 31.15 | 39.60 | 22.50 | 32.73 | 23.49 |
| Self-reported total sleep timeb (hours) | 5.75 | 1.66 | 5.50 | 1.33 | 5.56 | 1.66 | 5.17 | 1.62 | 5.36 | 1.70 | 5.80 | 1.84 |
Note: pCO2 = partial pressure of carbon dioxide; mmHg = millimeters of mercury; CAPS = Clinician-Administered PTSD Scale.
Three Immediate treatment and one Waitlist subject were excluded from sleep analyses due to reported untreated sleep apnea.
Derived from Pittsburgh Sleep Quality Index.
Table 3.
Mixed Modeling Effects of Immediate Treatment versus Waitlist Groups from Baseline (Time 1) to First Follow-up (Time 2)
| Immediate Treatment |
Waitlist (Delayed Treatment) |
Treatment Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Within Group | Within Group | Group*Time | Between | |||||||||
| Time 1 to Time 2 | Time 1 to Time 2 | Groups | ||||||||||
| (Time 2) | ||||||||||||
|
|
|
|
||||||||||
| Est. | SE | 95% CI | d a | Est. | SE | 95% CI | d a | Est. | SE | 95% CI | d a | |
|
|
|
|
||||||||||
| Respiration measures: | ||||||||||||
| End-tidal pCO2 (mmHg) | 14.93 | 8.42 | −2.89, −1.05 | 0.22 | −1.01 | 0.92 | −2.85, 0.83 | 0.20 | −0.09 | 1.25 | 2.57, 2.39 | 0.04 |
| Respiration rate (breaths per min)a | −5.55*** | 0.74 | −7.03, −4.07 | 1.40 | 0.47 | 0.69 | −0.92, 1.85 | 0.11 | 6.01*** | 0.97 | 4.09, 7.94 | 1.56 |
| CAPS total | −5.77 | 3.18 | −12.15, 0.60 | 0.25 | −4.98 | 2.95 | −10.89, −0.59 | 0.20 | 0.79 | 4.26 | −7.74, 9.31 | 0.00 |
| CAPS hyperarousal | −1.99 | 1.09 | −4.18, 0.18 | 0.27 | −3.61** | 1.01 | −5.64, 1.59 | 0.51 | −1.61 | 1.45 | 4.51, 1.28 | 0.17 |
| Beck Depression Inventory | 0.09 | 1.76 | −3.44, 3.62 | 0.01 | −2.26 | 1.58 | −5.43, 0.91 | 0.19 | −2.35 | 2.32 | −6.99, 2.29 | 0.23 |
| Aggression Questionnaire | −1.55 | 4.03 | −4.61, 6.51 | 0.09 | −4.10 | 3.52 | −11.16, 2.95 | 0.17 | −2.55 | 5.16 | −12.86, 7.54 | 0.18 |
| Sleep measuresb: | ||||||||||||
| Sleep quality | −1.26 | 0.77 | −2.82, 0.30 | 0.26 | −0.08 | 0.67 | −1.44, 1.27 | 0.08 | 1.17 | 0.99 | −0.82, 3.16 | 0.60 |
| PTSD-specific sleep quality | −0.66 | 0.95 | −2.56, 1.23 | 0.12 | −2.64** | 0.83 | −4.31, −0.98 | 0.54 | −1.98 | 1.21 | −4.41, 0.44 | 0.38 |
| Self-reported sleep onset latencyc (mins) | −18.61** | 7.01 | −32.56, −4.65 | 0.58 | −1.91 | 6.10 | −14.09, 10.27 | 0.07 | 16.70 | 8.76 | −0.72, 34.11 | 0.56 |
| Self-reported total sleep timec (hours) | 0.06 | 0.33 | −0.61, 0.73 | 0.04 | 0.08 | 0.29 | −0.50, 0.66 | 0.13 | 0.02 | 0.42 | −0.82, 0.86 | 0.16 |
|
| ||||||||||||
Note: Est. = estimate of fixed effect; SE = standard error; CI = confidence interval; pCO2 = partial pressure of carbon dioxide; mmHg = millimeters of mercury; CAPS = Clinician-Administered PTSD Scale.
Effect sizes calculated on estimated marginal means and pooled variance within group at both time points, and between group at Time 2.
Three Immediate treatment and One Waitlist subject were excluded from sleep analyses due to reported untreated sleep apnea.
Derived from Pittsburgh Sleep Quality Index.
p ≤ .05
p ≤ .01
p ≤ .001.
Secondary Outcome: Within-Group Changes across all Time Points for the IT group
An unstructured covariance structure was used for modeling the intent-to-treat IT group’s data across three time points: pre-treatment, post-treatment, and six-month follow-up. Results replicate the findings of the primary outcome analyses in that the only treatment effect was on respiration rate (F(2, 20.45) = 23.52, p < .001), which remained at six-month follow-up: Time 1 to Time 2 estimate = 5.69, SE = 1.04, 95% CI = 3.53 to 7.85, d = 1.37; Time 2 to Time 3 estimate = 0.08, SE = 0.66, 95% CI = −1.29 to 1.46, d = 0.02.
Exploratory Analyses
Repeated-measures ANOVAs for all participants who completed the first and second assessments (n = 51) found a main time effect on CAPS hyperarousal scores (F(1, 48) = 9.58, p = .003) and PSQI sleep onset latency (F(1, 40) = 6.73, p = .013). There was an overall improvement in CAPS hyperarousal and PSQI sleep onset latency at Time 2, though the WL participants did not receive the intervention during this interval. The significant treatment effect on respiration rate remained (F(1, 46) = 26.13, p < .001, η2p = 0.36). Repeated-measures ANCOVAs for combined IT and WL treatment completers (n = 38), with PCO2, respiration rate, number of completed homework breathing exercises, and pre-post change in PCO2 as covariates, found no treatment effect on any primary outcome variable.
Variables that theoretically could predict outcome were regressed onto CAPS hyperarousal scores at Time 2. A model that included baseline CAPS hyperarousal (β = .84, p < .001), baseline PCO2 (β = −.20, p = .380), BDI depression (β = .12, p = .157), age (β = .13, p = .183), and concurrent other mental health treatment (β = 2.28, p = .149), accounted for 68% of the variance (R2 = .68, F(5, 31) = 13.22, p < .001). Baseline hyperarousal was the only significant individual predictor. Group (received or did not receive treatment) did not add to the model (R2 change < .001) and was removed.
Change in PCO2 was not associated with change in hyperarousal symptoms (r = .21, p = .242). Number of completed homework exercises was not associated with change in PCO2 (r = .04, p = .831). To assess for moderation, we did a backwards regression of group (IT or WL), age, BDI depression, expectancy of improvement, concurrent other mental health treatment, number of completed homework exercises, pre-post change in PCO2, and pretreatment PCO2, respiration rate, and hyperarousal on post-treatment hyperarousal, in the combined IT and WL treatment completers group. “Post-treatment hyperarousal” consisted of combined CAPS hyperarousal scores from Time 2 for the IT group and Time 3 for the WL group. All variables were removed except group (β = −5.62, p = .004), pretreatment hyperarousal (β = .92, p < .001), and pretreatment PCO2 (β = −.61, p = .043), accounting for 68% of the variance (R2 = .68, F(1, 25) = 18.16, p < .001).
Baseline PCO2 did not predict PCO2 change (β = −.45, t(32) = −1.33, p = .192), credibility (β = .37, t(32) = .90, p = .373), working alliance (β = −.02, t(32) = −.388, p = .701), or homework adherence (β = .24, t(32) = .27, p = .788)
Demographic and clinical variables that were individually associated with study withdrawal were entered into a logistic regression. Being in the IT group (β = 1.78, p = .035), being an Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veteran (β = −2.72, p = .012), and currently receiving other mental health treatment (β = .92, p = .025), significantly predicted study withdrawal by Time 2 (χ2 (3, N = 48) = 20.16, p < .001).
Discussion
Treating PTSD hyperarousal symptoms in U.S. veterans with CART was not successful, nor did the addition of stimulus control and sleep hygiene instructions result in improved self-reported sleep. Perhaps our premise that PTSD hyperarousal and the anxiety of panic disorder are both based on hyperventilation-related anxiety is false. We did not find lower PCO2 pre-treatment levels that would have indicated hyperventilation during quiet sitting among our PTSD patients, nor was there a correlation between PCO2 change and CAPS hyperarousal symptoms. Perhaps CART works better for acute episodes of anxiety as opposed to the more chronic nature of PTSD hyperarousal. Furthermore, the therapeutic effects of CART in panic disorder may have less to do with hyperventilation than with interoceptive exposure to respiratory sensations during training (Meuret, Ritz, Wilhelm, Roth, & Rosenfield, 2018). Individuals with PTSD hyperarousal may not have the same fear specifically of respiratory symptoms often found in panic (e.g., Meuret, Rosenfield, Hofmann, Suvak, & Roth, 2009), thereby making CART ineffective in this population.
As in previous CART trials, our PTSD participants did decrease their respiratory rate after treatment. However, they did not increase PCO2 levels. Individuals commonly take deeper breaths to compensate for slower breathing rates (Meuret et al., 2008) despite our instructions to the contrary. Inability to increase PCO2 may have reduced self-efficacy, and subsequent treatment adherence and expectancies. Hyperarousal scores at Time 2 were predicted by baseline hyperarousal, with some contribution of baseline PCO2, depression, concurrent other mental health treatment and age, but not by actually receiving our treatment. Those whose baseline PCO2 was already in the normal range may have experienced no therapeutic benefit from reaching the target of 40 mmHg, and may have put forth less effort or discontinued treatment. When we combined all treatment completers to investigate moderation, only being in the immediate treatment group, having higher pretreatment hyperarousal, and having lower pretreatment PCO2 predicted increased post-treatment hyperarousal scores. Though overall there was no treatment effect, being in the WL group predicted relatively greater decrease in hyperarousal symptoms, perhaps because they were individuals motivated enough to wait eight weeks and still fully engage in treatment.
Another reason that CART was ineffective in this study could be that too many participants dropped out or practiced too little. The number of completed homework exercises was not a significant predictor of any outcome, but its restricted range may limit our ability to detect an effect. Our dropout rate, mostly at treatment onset, was high compared to previous CART trials, although lower than in other treatment studies of veterans (e.g., Garcia, Kelley, Rentz & Lee, 2011). Most of the withdrawals occurred before the first treatment session. At that point, participants were aware of the tasks and time required for the study, but not the treatment rationale. We did not assess treatment credibility until after the first treatment session, so we can only speculate that some participants may have discontinued beforehand, believing the treatment approach was implausible. Perhaps the veterans, especially those already receiving mental health treatment, decided after receiving compensation for the initial assessment that our treatment was not worth the additional effort.
While patients in CART panic trials have been almost all female civilians, our patients were mostly male veterans with multiple medical and mental health comorbidities, which likely contributed to both attrition and lack of treatment response. Veterans and service members with PTSD have been relatively resistant to the standard PTSD treatments, showing low treatment engagement and retention especially among OEF/OIF veterans (Steenkamp et al., 2015) and more modest treatment effect sizes (Foa et al., 2018).
The failure of stimulus control and sleep hygiene instructions to improve self-reported sleep may be because adherence to sleep instructions was only moderate. Our sleep instructions have since been found to be less potent components of the gold standard sleep treatment for PTSD, CBT-I, as compared to sleep restriction (Taylor & Pruiksma, 2014).
Because of our relatively small sample size after dropouts, we were limited in our ability to test exploratory hypotheses about why treatment failed, or whether certain variables predicted therapeutic benefit. Always giving two interventions targeting sleep (CART and sleep instructions) meant that we could not disentangle their effects on outcome. We did not assess the potential biases of our diagnostic raters, who were not blind to the participant’s group assignment. Setting respiration goals to six breaths per minute might have made a difference. Possibly reduced respiration rates might have health benefits we did not assess, such as reduced blood pressure (Russo, Santarelli, & O’Rourke, 2017).
In spite of these limitations, our results discourage application of CART to military PTSD. Perhaps PTSD hyperarousal symptoms are not components of a unitary biopsychological arousal factor that includes the increase in physiological arousal characteristic of other anxiety disorders. That most of our participants’ baseline PCO2 was in the normal range and that baseline PCO2 was one of the predictors of treatment outcome suggests that the arousal targeted by CART (e.g., hypocapnia) may be different from the physiological arousal of PTSD. Incorporating physiological measures of respiration could elucidate whether respiratory changes are driving the therapeutic changes in other breathing treatments for PTSD. Mediocre engagement and retention has plagued many efforts to improve PTSD treatment for veterans. Testing CART in civilian PTSD could clarify if it is not effective for PTSD or specifically not for veterans with PTSD. Overall, these results highlight the need for finding new interventions in which veterans with PTSD are willing and able to engage.
Supplementary Material
Clinical Impact Statement.
This study shows that an anti-hyperventilation biofeedback therapy successful in treating panic in civilians, failed to alleviate the PTSD hyperarousal symptoms of military veterans, even when combined with sleep hygiene instructions. Thus, a physiological intervention targeting hyperarousal failed to relieve the “hyperarousal symptoms” diagnostic of PTSD. Our results, like those of many other studies attempting to treat military PSTD, highlight the need for discovering new effective interventions for this population and the importance of understanding mechanisms of action in existing treatments.
Acknowledgements
Julia Branch, Lillith Poppe, and Myriam Leupin assisted in data collection. Shivani Sharma, Miriam Thiel, Tanja Mueller, and Marius Keller assisted with data entry and management. Booil Jo advised data analyses.
This project was funded by Veterans Affairs Clinical Science Research and Development Merit Review award (MHBA-005-08F) to Walton T. Roth. The Clinical Trials Registry number is NCT00855816.
References
- Alpers GW, Wilhelm FH, & Roth WT (2005). Psychophysiological assessment during exposure in driving phobic patients. Journal of Abnormal Psychology, 114(1), 126–139. doi: 10.1037/0021-843X.114.1.126 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington DC: Author. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1, 82. dx.doi.org/10.3109/10253890.2015.1067678 [Google Scholar]
- Belda X, Fuentes S, Daviu N, Nadal R, & Armario A (2015). Stress-induced sensitization: the hypothalamic–pituitary–adrenal axis and beyond. Stress, 18(3), 269–279. [DOI] [PubMed] [Google Scholar]
- Bertram F, Jamison AL, Slightam C, Kim S, Roth HL, & Roth WT (2014). Autonomic arousal during actigraphically estimated waking and sleep in male veterans with PTSD. Journal of Traumatic Stress, 27(5), 610–617. doi: 10.1002/jts.21947 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. doi: 10.1007/BF02105408 [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Mathews AM, Chambers A, Ebrahimi S, Lytle R, & Nelson R (1987). The effects of relaxation training with cognitive or nondirective therapy and the role of relaxation-induced anxiety in the treatment of generalized anxiety. Journal of Consulting and Clinical Psychology, 55(6), 883–888. 10.1037/0022-006X.55.6.883 [DOI] [PubMed] [Google Scholar]
- Buss AH, & Perry M (1992). The aggression questionnaire. Journal of Personality and Social Psychology, 63(3), 452–459. 10.1037/0022-3514.63.3.452 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Cougle JR, Feldner MT, Keough ME, Hawkins KA, & Fitch KE (2010). Comorbid panic attacks among individuals with posttraumatic stress disorder: Associations with traumatic event exposure history, symptoms, and impairment. Journal of Anxiety Disorders, 24(2), 183–188.doi: 10.1016/j.janxdis.2009.10.006 [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, & Williams J (2002). Structured Clinical Interview for DSM- IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, New York State Psychiatric Institute. Biometrics Research. [Google Scholar]
- Foa EB, McLean CP, Zang Y, Rosenfield D, Yadin E, Yarvis JS, … & Fina BA (2018). Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: A randomized clinical trial. JAMA: Journal of the American Medical Association, 319(4), 354–364.doi: 10.1001/jama.2017.21242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MW (2016). Transdiagnostic mechanisms of change and cognitive-behavioral treatments for PTSD. Current Opinion in Psychology. doi.org/10.1016/j.copsyc.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Garcia HA, Kelley LP, Rentz TO, & Lee S (2011). Pretreatment predictors of dropout from cognitive behavioral therapy for PTSD in Iraq and Afghanistan war veterans. Psychological Services, 8(1), 1–11. doi: 10.1037/a0022705 [DOI] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, & Lombardo TW (2004). Psychometric properties of the life events checklist. Assessment, 11, 330–341. doi: 10.1177/1073191104269954 [DOI] [PubMed] [Google Scholar]
- Gutner CA, Casement MD, Gilbert KS, & Resick PA (2013). Change in sleep symptoms across cognitive processing therapy and prolonged exposure: a longitudinal perspective. Behaviour Research and Therapy, 51(12), 817–822. doi.org/10.1016/j.brat.2013.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher RL, & Gillaspy JA (2006). Development and validation of a revised short version of the Working Alliance Inventory. Psychotherapy Research, 16(1), 12–25. 10.1080/10503300500352500 [DOI] [Google Scholar]
- Kim S, Wollburg E, & Roth WT (2012). Opposing breathing therapies for panic disorder: Randomized controlled trial of lowering vs raising end-tidal PCO₂. The Journal of Clinical Psychiatry, 73(7), 931–939. 10.4088/JCP.11m07068 [DOI] [PubMed] [Google Scholar]
- Manber R, & Kuo TF (2002). Cognitive-behavioral therapies for insomnia. In: Lee-Choing L, Sateia J & Carskadon MA (Eds.), Sleep Medicine (pp. 177–185). Philadelphia, PA: Hanley & Belfus. [Google Scholar]
- Meuret AE, Ritz T, Wilhelm FH, Roth WT, & Rosenfield D (2018). Hypoventilation therapy alleviates panic by repeated induction of dyspnea. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 539–545. 10.1016/j.bpsc.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Hofmann SG, Suvak MK, & Roth WT (2009). Changes in respiration mediate changes in fear of bodily sensations in panic disorder. Journal of Psychiatric Research, 43(6), 634–641. 10.1016/j.jpsychires.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, & Roth WT (2008). Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research, 42(7), 560–568. doi: 10.1016/j.jpsychires.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MA, Santarelli DM, & O’Rourke D (2017). The physiological effects of slow breathing in the healthy human. Breathe, 13(4), 298–309. doi: 10.1183/20734735.009817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä EM, Nitschke JB, Tudorascu DL, Hayes A, Goldstein MR … Davidson RJ. (2014). Breathing‐based meditation decreases posttraumatic stress disorder symptoms in US military veterans: A randomized controlled longitudinal study. Journal of Traumatic Stress, 27(4), 397–405. 10.1002/jts.21936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, & Montgomery P (2008). Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Medicine Reviews, 12(3), 169–184. [DOI] [PubMed] [Google Scholar]
- Steenkamp MM, Litz BT, Hoge CW, & Marmar CR (2015). Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA, 314(5), 489–500. doi: 10.1001/jama.2015.8370 [DOI] [PubMed] [Google Scholar]
- Taylor DJ, & Pruiksma KE (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. International Review of Psychiatry, 26(2), 205 213. 10.3109/09540261.2014.902808 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.