Abstract
Despite major improvements in treatment outcome with novel targeted therapies, such as the Bruton tyrosine kinase (BTK) inhibitor ibrutinib, chronic lymphocytic leukemia (CLL) remains incurable in the majority of patients. Activation of PI3K, NF-κB, and/or MYC has been linked to residual disease and/or resistance in ibrutinib-treated patients. These pathways can be targeted by inhibitors of bromodomain and extra-terminal (BET) proteins. Here we report about the preclinical activity of GS-5829, a novel BET inhibitor, in CLL. GS-5829 inhibited CLL cell proliferation and induced leukemia cell apoptosis through deregulation of key signaling pathways, such as BLK, AKT, ERK1/2, and MYC. IκBα modulation indicates that GS-5829 also inhibited NF-κB signaling. GS-5829-induced apoptosis resulted from an imbalance between positive (BIM) and negative regulators (BCL-XL) of the intrinsic apoptosis pathway. The anti-leukemia activity of GS-5829 increased synergistically in combinations with B cell receptor signaling inhibitors, the BTK inhibitor ibrutinib, the PI3Kδ inhibitor idelalisib, and the SYK inhibitor entospletinib. In co-cultures that mimic the lymph node microenvironment, GS-5829 inhibited signaling pathways within nurselike cells and their growth, indicating that BET inhibitors also can target the supportive CLL microenvironment. Collectively, these data provide a rationale for the clinical evaluation of BET inhibitors in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by expansion of monoclonal mature B lymphocytes that accumulate in the bone marrow, secondary lymphoid organs (lymph nodes, spleen), and peripheral blood [1]. CLL cell proliferation occurs in distinct areas of secondary lymphoid organs [2], so-called proliferation centers or pseudo-follicles, where the leukemia cells receive growth and survival signals from interactions with the microenvironment, including activation of B cell receptor (BCR) signaling [3]. Treatment of CLL has fundamentally changed during the last few years due to the success of kinase inhibitors that target BCR signaling [4], such as the Bruton tyrosine kinase (BTK) inhibitor ibrutinib. Ibrutinib induces high response rates and durable remissions in CLL patients, including patients with high-risk disease [5–7]. Treatment with ibrutinib inhibits the proliferation of CLL cells and accelerates leukemia cell death [8–10]. Importantly, ibrutinib also disrupts interactions between leukemia cells and the tissue microenvironment, resulting in redistribution lymphocytosis during the first months on therapy, caused by treatment-induced egress of tissue-resident CLL cells into the peripheral blood [10–14]. Ibrutinib is increasingly replacing chemotherapy-based CLL treatment based on superiority in several randomized clinical trials in the frontline and relapsed disease settings [15–17]. However, ibrutinib does not fully eradicate the disease and therefore currently is used as a long-term therapy, with associated toxicities and financial burden. Persistent activation of PI3K, NF-κB, and/or MYC during ibrutinib therapy has been linked to primary and/or secondary ibrutinib resistance [18–22]. We hypothesized that a bromodomain and extra-terminal protein inhibitor may target these pathways in CLL and could synergize with kinase inhibitors, such as ibrutinib, that target BCR signaling.
The bromodomain and extra-terminal (BET) proteins BRD2, BRD3, BRD4, and BRDT comprise a family of epigenetic “reader” proteins that recognize acetylated lysine residues in histones [23]. BET proteins recruit positive regulators of RNA polymerase-II-dependent transcription to promoters and enhancers of actively expressed genes [24, 25]. Although these proteins are ubiquitously present in human tissues, neoplastic cells are particularly sensitive to their inhibition [26]. This phenomenon can be explained by the fact that proliferation and survival of cancer cells depend heavily on the expression of several cancer-specific oncogenes that are controlled by BET protein-overloaded superenhancers [27–29]. Several BET inhibitors have preclinical and clinical activity in BCR-dependent lymphoma cells, including diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) [28, 30–36]. In these malignancies, BET inhibitors reduce MYC levels and other downstream components of BCR signaling, they down-regulate BCL2 transcription and suppress NF-κB signaling. Given the preclinical rationale and the clinical need for further improvement in CLL therapy by targeting, for example, signaling pathways that can remain active in patients treated with BCR signaling inhibitors, we investigated the preclinical activity of the BET inhibitor GS-5829 in CLL [37]. We demonstrate that GS-5829 can target both, CLL cells and nurselike cells (NLC), and has synergistic anti-CLL activity when used together with ibrutinib and other BCR signaling inhibitors.
Materials and Methods
Patient samples and cell lines
Peripheral blood samples were drawn from patients fulfilling diagnostic criteria for CLL at the Department of Leukemia, MD Anderson Cancer Center, after obtaining informed consent on protocols reviewed and approved by the Institutional Review Board at MD Anderson Cancer Center, and in accordance with the Declaration of Helsinki. The primary samples were preselected to have a white blood cell count over 50000 cells/μL, no other restrictions were applied and samples were used as they became available. Clinical and biological characteristics of the samples used for this study may be found in supplementary Table 1. For all of the experiments utilizing primary cells, the reported sample size (N) represent a number of independent repetitions. MEC-1, a CLL cell-derived cell line, was purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany); it was validated by short tandem repeat (STR) method and tested negative for mycoplasma contamination. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque PLUS (GE Healthcare) gradient centrifugation and maintained in the RPMI 1640 medium supplemented with 10% FBS (Gibco), 2.05 mM L-glutamine (HyClone Laboratories), and penicillin-streptomycin (Corning). To establish a co-culture with NLC, freshly isolated PBMC were plated into 24- or 12-well plates (Falcon), or in 100 mm Petri dishes (Corning) at 1.5×107 cells/mL and incubated for 14 days. Then nonadherent cells were collected by pipetting, washed with fresh medium and returned to the wells with NLC at 1×107 cells/mL for further experiments. Only samples with more than 85% viable nonadherent cells were used in the study.
Inhibitors
GS-5829, the PI3Kδ inhibitor idelalisib, and the SYK inhibitor entospletinib were provided by Gilead Sciences, Inc. (Foster city, CA, USA) as 10 mM solutions in dimethyl sulfoxide (DMSO). JQ1 and the BTK inhibitor ibrutinib were purchased from Selleck Chemicals (Houston, TX, USA).
Viability assay and cell counting
The percentage of viable cells was determined by staining with 3,3 dihexyloxocarbocyanine iodide (DiOC6; Molecular Probes) and propidium iodide (PI; Molecular Probes), as previously described [38]. Relative changes in cell numbers were measured by counting cells at high sample flow for 20 seconds on a BD FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA). The viability of CD3+ T cells was measured in CLL PBMC co-cultures with NLC by flow cytometry after staining with CD3-APC antibody (BD Pharmingen), Annexin-V-FITC, and 7-AAD (Biolegend). To assess the NLC numbers, nonadherent cells were removed by pipetting, the adherent NLC were fixed with absolute methanol for 5 minutes and then stained with modified Giemsa stain (diluted 1:20 in PBS) for 1 hour. NLC were visualized using a phase-contrast microscope (Model ELWD 0.3; Nikon, Melville, NY, USA) with a Ph1 Plan 10 DL/0.25 160/- objective lens. Images were captured with a Nikon D40 digital camera (Nikon Corp) with the help of digiCamControl ver. 1.2.0.0 (http://digicamcontrol.com/). Average numbers of NLC counted in 8 different visual fields are reported.
XTT viability/proliferation assay
TACS XTT Cell Proliferation/Viability Assay (Trevigen) was performed according to the manufacturer’s instructions on MEC-1 cells treated with GS-5829 or the BCR signaling inhibitors for 72 hours. Half-maximal inhibitory concentrations (IC50) were calculated using Prism 6 or 7 for Mac OS X (GraphPad Software; http://www.graphpad.com) based on technical triplicate measurements.
Inhibitor combinations
The degree of drug interaction between GS-5829 and the BCR signaling inhibitors was quantitatively measured by combination indexes (CI) [39]. The CIs were calculated using CompuSyn software (ComboSyn Incorporated; http://www.combosyn.com/). The CI gives quantitative definition for additive effect (CI=1), synergism (CI<1), and antagonism (CI>1) in drug combinations. The Fa-CI plots (fraction of affected cells vs. combination index) were generated based on the DiOC6/PI staining results for primary CLL and on XTT assay results for MEC-1 cells. The CI values reported here were calculated at the median-effect dose (ED50) of the drug combination unless otherwise specified.
Western blot
Western blot was performed according to the previously published protocol [40]. Briefly, PBMC from patients with CLL co-cultured with NLC in 12-well plates were treated with 1 μM ibrutinib, 400 nM GS-5829, or both for 24 hours. Then nonadherent cells were collected by gentle pipetting, washed once with PBS, and lysed in RIPA buffer (Sigma-Aldrich) containing 1x Complete Protease Inhibitor and 1x PhosSTOP (Roche Molecular Biochemicals). NLC for Western blot analysis were co-cultured with CLL cells in 100 mm Petri dishes. After 24 hours of treatment with 400 nM GS-5829, CLL cells were removed by gentle pipetting; adherent NLC were washed twice with PBS to remove any residual nonadherent cells. The NLC then were lysed by two freeze/thaw cycles and collected by scraping in RIPA buffer containing 1x Complete Protease Inhibitor and 1x PhosSTOP. Antibodies against the following proteins were used in this study: β-actin (catalog #4970), AKT (catalog #9272), pAKT (S473) (catalog #4060), BCL6 (catalog #14895), BCL-XL (catalog #2764), BIM (catalog #2933), BLK (catalog #3262), BTK (catalog #3533), CD19 (catalog #3574), cyclin D2 (catalog #3741), ERK1/2 (catalog #9102), pERK1/2 (T202/Y204) (catalog #9101), HEXIM1 (catalog #12604), pIκBα (S32/S36) (catalog #9246), MYC (catalog #5605), p21 (catalog #2947), p27 (catalog #3688), PARP (catalog #9542), STAT3 (catalog #4904), pSTAT3 (Y705) (catalog #9131), pSTAT3 (S727) (catalog #94994), all from Cell Signaling Technology; BCL2 (catalog #05–826, Millipore), BRD4 (catalog #A301–985A-M, Bethyl Laboratories), pBTK (Y223) (catalog #ab68217, Abcam), CD68 (catalog #sc-9139, Santa Cruz Biotechnology), IκBα (catalog #sc-371, Santa Cruz Biotechnology), IKZF3 (catalog #NBP2–24495, Novus Biologicals), MCL1 (catalog #ADI-AAP-240-D, Enzo Life Sciences). The results were quantified by densitometry using ImageJ software (https://imagej.nih.gov/ij/).
Data analysis and statistics
All statistical analyses were performed using Prism 6 or 7 for Mac OS X. Mean was chosen as a center value for all graphs. 95% confidence interval (CI), standard deviation (SD), or standard error of the mean (SEM) were used as measures of spread as indicated in figure legends and the Results section. After confirming that the data meet the assumptions of the statistical test, repeated measures one-way or two-way ANOVA, one sample t-test, and paired t-test were used for statistical analyses as appropriate. The tests were two-sided. P values presented in this work were adjusted for multiple comparisons if necessary. A P value of <0.05 was considered statistically significant.
Results
GS-5829 suppresses proliferation and induces apoptosis in CLL cells
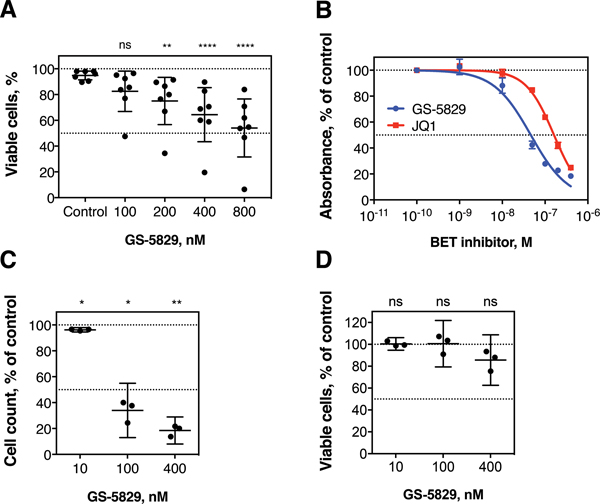
To investigate the effects of BET proteins inhibition in CLL, we treated primary CLL cells co-cultured with NLC with increasing concentrations of GS-5829 for 120 hours (information about the CLL samples used in this study can be found in supplementary Table 1). GS-5829 dose-dependently induced apoptosis of CLL cells: 400 nM GS-5829 reduced the percentage of viable cells from 94.8% (95% CI, 91.5% to 98.2%) to 64.4% (95% CI, 43.4% to 85.3%; P=0.0001) (Figure 1A). GS-5829 caused 11.6% (95% CI of difference, 4.2% to 18.9%; P=0.0118) more cell death than JQ1, another BET inhibitor, when used at the same concentration (supplementary Figure 1). Similarly, in XTT viability/proliferation assay GS-5829 demonstrated more potency against MEC-1 CLL cells compared to JQ1 with IC50 of 46.4 nM (95% CI, 38.1 to 56.6 nM) vs. 161.9 nM (95% CI, 153.3 to 171.0 nM) (Figure 1B). BET proteins inhibition resulted primarily in the suppressed proliferation of MEC-1 cells, rather than in induction of apoptosis. For instance, 100 nM GS-5829 reduced the total number of cells to 34.0% (95% CI, 13.0% to 55.0%; P=0.0162) without affecting the fraction of viable cells (100.5%, 95% CI, 79.3% to 121.8%; P>0.05) (Figure 1C–D).
Figure 1. GS-5829 induces apoptosis and suppresses proliferation of CLL cells.
(A) Primary CLL cells co-cultured with NLC were treated for 120 hours with increasing concentrations of GS-5829 that are indicated on the horizontal axis. The mean fraction of viable CLL cells with 95% CI is depicted for each experimental condition (N=7). (B) The BET inhibitors GS-5829 and JQ1 dose-dependently inhibit the metabolism/proliferation of MEC-1 cells, as measured in XTT assays. The tested concentration range is shown on the horizontal axis. Displayed is the mean (± SD) absorbance of the colored formazan product, based on triplicate measurements that were normalized to average control values. (C) GS-5829 significantly inhibits the proliferation of MEC-1 cells, based on cell counts after 96 hours of exposure to different GS-5829 concentrations (D). Inhibition of proliferation was not due to induction of MEC-1 cell death, given that cell viability under these conditions remained high and not significantly changed when compared to control cells. Displayed are the mean with 95% CI from 3 independent experiments.
GS-5829 combinations with BCR signaling inhibitors are synergistically toxic to CLL cells
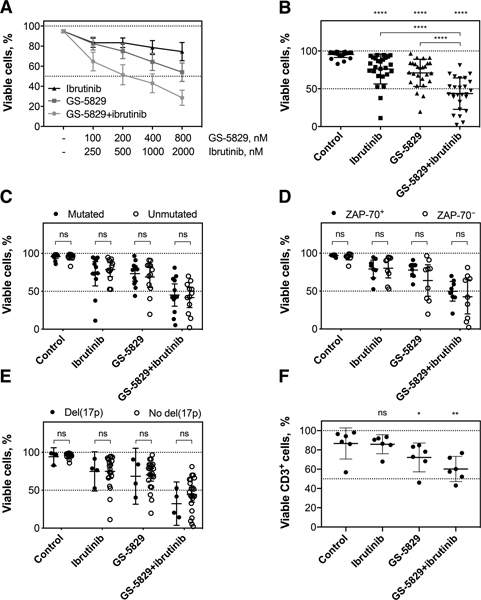
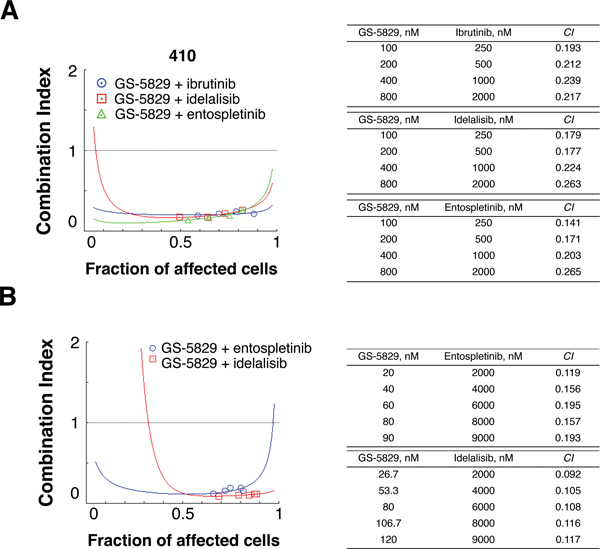
Next, we examined the interaction between GS-5829 and the BCR signaling inhibitors. Combining GS-5829 with ibrutinib significantly increased the level of CLL cell death in NLC co-culture at a range of different concentrations (Figure 2A). For instance, adding 1000 nM ibrutinib to 400 nM GS-5829 decreased the amount of viable CLL cells from 71.0% (95% CI, 63.7% to 78.3%) to 43.6% (95% CI, 35.2% to 51.9%; P<0.0001) (Figure 2B). We quantitatively characterized the drug interaction between GS-5829 and ibrutinib using combination indexes. The CIs calculated at the median-effect dose of the drug combination ranged from 0.036 to 0.615 in 7 individual samples indicating strong synergism between the two inhibitors (supplementary Figures 2 and 3). Interestingly, CLL cells were equally sensitive to GS-5829 and its combination with ibrutinib irrespective of prognostic markers such as IGHV mutational status, ZAP-70 expression, or the presence of del(17p)/TP53 mutation (Figure 2C–E). The results suggest that this combination may be beneficial to patients across different CLL risk subsets. GS-5829, alone or in combination with ibrutinib, significantly decreased the viability of CD3+ T cells in NLC co-cultures of CLL PBMC (Figure 2F). Importantly, GS-5829 combinations with idelalisib and entospletinib also were highly synergistic against CLL cells, both in CLL/NLC co-culture (N=3) (Figure 3A and supplementary Figure 3) and against MEC-1 cells (Figure 3B), indicating that this synergistic activity of GS-5829 applies to combination with the entire class of BCR signaling inhibitors.
Figure 2. GS-5829 and ibrutinib synergistically induce apoptosis of primary CLL cells irrespective of their IGHV mutational status and ZAP-70 expression.
(A) Displayed are the mean (± SEM) fraction of viable primary CLL cells in NLC co-cultures after 120 hours of treatment with increasing concentrations of GS-5829, ibrutinib, or both (N=7). The concentrations are shown on the horizontal axis. (B) Mean fractions with 95% CI of viable primary CLL cells in NLC co-culture after 120 hours of treatment with 400 nM GS-5829, 1 μM ibrutinib, or both (N=26). (C) Effects of ibrutinib and GS-5829 on primary CLL cell viability with mutated (N=12) or unmutated (N=12) IGHV. (D) Similarly, CLL cell viability after drug exposure was compared based on ZAP-70 expression status, in 8 ZAP-70+ and 9 ZAP-70− samples, (E) and in 4 samples carrying del(17p)/TP53 mutation and in 22 samples without these abnormalities. (F) The viability of CD3+ T cells in CLL PBMC co-cultured with NLC was determined by flow cytometry after 120 hours of treatment. Displayed are the mean (with 95% CI) percentages of viable T cells as measured in 6 samples.
Figure 3. GS-5829 and the BCR signaling inhibitors ibrutinib, idelalisib, and entospletinib synergistically induce CLL cell apoptosis.
(A) A representative Fa-CI plot indicates that GS-5829 and the BCR signaling inhibitors ibrutinib, idelalisib, and entospletinib synergistically induce apoptosis in primary CLL cells co-cultured with NLC. These calculations are based on exposure of CLL samples to the different concentrations of GS-5829 and the BCR signaling inhibitors that are listed in the tables next to the graph. (B) Synergistic interactions between GS-5829 and idelalisib or entospletinib were confirmed in MEC-1 cells.
BET inhibition attenuates pro-survival signaling in CLL
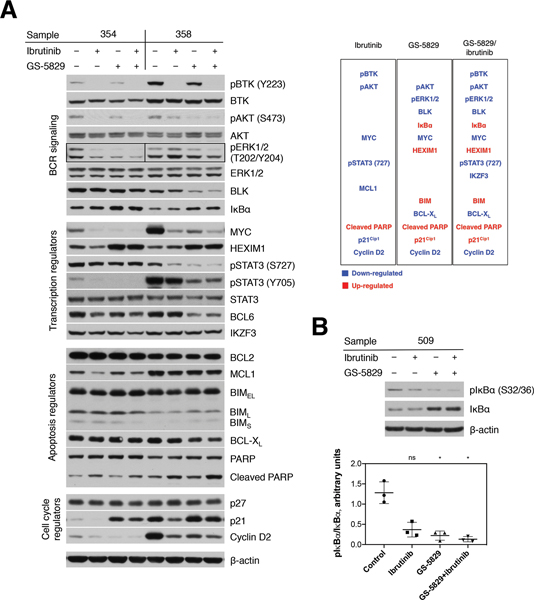
To find out the molecular mechanism underlying CLL cells’ sensitivity to GS-5829, we analyzed how it affects key signaling pathways for CLL cells’ proliferation and survival. In CLL cells co-cultured with NLC, GS-5829 induced a consistent increase in HEXIM1 protein (P<0.0001), a BET inhibitor pharmacodynamic marker [41], demonstrating on-target activity (Figure 4A and supplementary Figure 4). GS-5829 significantly decreased the activity of downstream mediators of BCR signaling, including BLK (P<0.0001), phospho-AKT (S473) (P=0.0080), phospho-ERK1/2 (T202/Y204) (P=0.0021), and MYC (P<0.0001). At the same time, it increased the levels of IκBα (P<0.0001), an inhibitor of NF-κB signaling. Accumulation of IκBα was associated with a significant decrease in its phosphorylation at S32/36 (Figure 4B), similar to what was reported in DLBCL [30]. Contrary to what was observed in other mature B-cell malignancies, in our experimental system the protein level of BTK remained stable after 24-hour exposure to GS-5829 [28, 31, 42]. Consistent with the anti-proliferative effect of GS-5829, we observed an increase in level of a cell cycle inhibitor p21Kip (P<0.0001) and a decrease in cyclin D2 (P<0.0001). In agreement with previous reports [10, 43, 44], ibrutinib inhibited BCR and STAT3 signaling in CLL cells through down-regulation of phospho-BTK (Y223) (P=0.0005), phospho-AKT (S473) (P=0.0006), phospho-ERK1/2 (T202/Y204) (P=0.0605), MYC (P<0.0001), and phospho-STAT3 (S727) (P=0.0075) (Figure 4A and supplementary Figure 4).
Figure 4. GS-5829 changes the levels of multiple proteins in CLL.
(A) Displayed are representative Western blot analyses of 2 CLL samples in NLC co-culture after 24 hours of treatment with GS-5829, ibrutinib, or both, as indicated on top of the blots. The functional categorization for each signaling molecule is shown on the left-hand side. A list of proteins that were significantly down- (blue) or up-regulated (red) by GS-5829 and ibrutinib (N=7) is presented on the right-hand side. (B) A representative Western blot analysis of the pIκBα (S32/36) and IκBα levels in a CLL sample after 24 hours of treatment and the pIκBα/IκBα ratio as measured by densitometry in 3 CLL samples.
GS-5829 induces apoptosis in CLL cells through changes in the intrinsic pathway
To evade cell death, CLL cells depend on high levels of the anti-apoptotic protein BCL2 which keeps the pro-apoptotic protein BIM in a bound, inactive state [45]. Both BIM and BCL2, as well as another anti-apoptotic protein BCL-XL, were previously shown to be regulated by BET proteins. Unlike in other hematologic malignancies [31, 33, 46, 47], the level of BCL2 did not change in CLL cells after 24 hours of treatment with GS-5829 (Figure 4A and supplementary Figure 4). However, the level of BIM significantly increased, while the level of BCL-XL decreased, likely tipping the balance towards apoptosis. Ibrutinib significantly reduced the amount of anti-apoptotic protein MCL1 (P=0.0010), which was unaffected by GS-5829. These findings provide additional mechanistic insight into the synergistic interaction between the two inhibitors, GS-5829 and ibrutinib.
GS-5829 targets CLL microenvironment
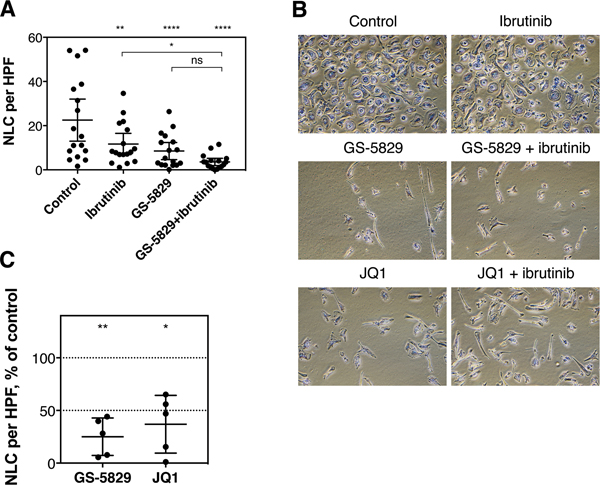
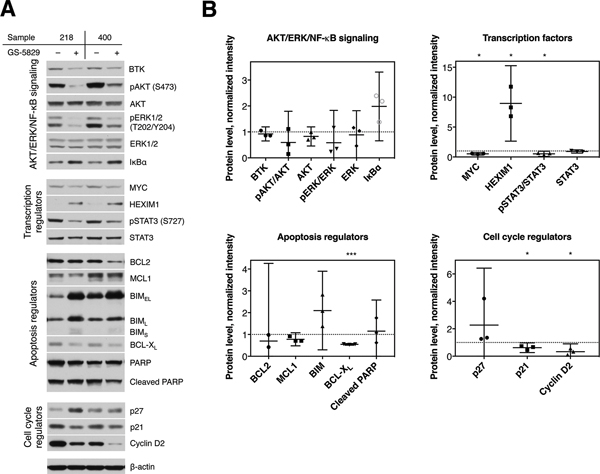
To analyze for potential effects of GS-5829 on CLL microenvironment-related cells, we monitored the fate of NLC in CLL/NLC co-cultures treated with GS-5829. After 120 hours of treatment, the number of adherent NLC significantly decreased from 22.5 NLC per visual field to 8.5 (95% CI of difference, 6.4 to 21.6; P<0.0001) (Figure 5A). Additionally, BET inhibition also resulted in a change in morphology of NLC, as they became spindle-shaped with two or more poles, rather than their normal round configuration, during GS-5829 treatment (Figure 5B). JQ1 induced similar changes in NLC number and morphology suggesting that these effects are specific to BET inhibitors (Figure 5B–C). Interestingly, ibrutinib also reduced the number of adherent NLC to 11.7 cells per field of view (95% CI of difference, 3.2 to 18.4; P=0.0019); however, it did not affect their morphology (Figure 5A–B). On the molecular level, changes in NLC protein levels after treatment with GS-5829 largely mirrored those changes seen in CLL cells. For example, the level of HEXIM1 increased (P=0.0324), while the levels of MYC (P=0.0103), BCL-XL (P=0.0004), and cyclin D2 (P=0.0371) considerably decreased (Figure 6A–B). GS-5829 significantly reduced the amount of phosphorylated STAT3 (S727) (P=0.0434) in NLC that is essential for their tumor-supporting activity [48–50].
Figure 5. GS-5829 also targets CLL nurselike cells in CLL/NLC co-cultures.
(A) The mean (with 95% CI) number of NLC per visual field was calculated in CLL/NLC co-cultures treated with 400 nM GS-5829, 1 μM ibrutinib or both for 120 hours (N=17). (B) Representative microphotographs from one CLL/NLC co-culture (sample 348). Modified Giemsa stain, original magnification ×100. (C) Comparison of the effects of 400 nM GS-5829 or JQ1 on NLC numbers. The mean with 95% CI of normalized cell numbers as measured in 5 samples.
Figure 6. Effects of GS-5829 on signaling in NLC.
(A) Displayed are representative Western blot analyses of NLC samples from two different patients after 24 hours of treatment with 400 nM GS-5829. The functional categorization for each signaling molecule is shown on the left-hand side, and the name of each specific molecule is depicted on the right-hand side. (B) Western blot results were quantified by densitometry, data were normalized to β-actin level in each sample and then to the untreated control. The mean with 95% CI of normalized intensity as measured in 3 NLC samples.
Discussion
Here we evaluated the anti-leukemia activity of a new BET inhibitor, GS-5829, in preclinical models of CLL. Our data indicate that GS-5829 inhibits CLL cell proliferation and triggers apoptosis in CLL cells through inhibition and deregulation of several signaling pathways. The most prominent pathways affected by GS-5829 are BCR and NF-κB signaling. CLL cells, when treated with GS-5829, demonstrated decreased levels of BLK, phospho-AKT, phospho-ERK1/2, and MYC and an increased level of IκBα. These findings are in agreement with data in DLBCL and MCL, suggesting a general mode of action of BET inhibitors in B cell malignancies with active BCR signaling [28, 30–32]. Down-regulation of BTK expression by BET inhibitors was reported in mature B cell lymphomas and in CLL [7, 28, 42]. In our experimental system, however, we did not observe significant changes in BTK levels during treatment with GS-5829, and we, therefore, conclude that down-regulation of BTK does not appear to be necessary for anti-CLL effect of GS-5829. We also demonstrate that GS-5829 changes the ratio of positive and negative regulators of the intrinsic pathway of apoptosis in CLL cells, with down-regulation of BCL-XL and up-regulation of BIM, contributing to the induction of CLL cell apoptosis. In other hematologic malignancies, down-regulation of BCL2 was identified as a primary mechanism by which BET inhibitors induce cell death [28, 31, 46]. However, BCL2 levels remained stable in CLL after exposure to GS-5829. Interestingly, recent analyses recognized high baseline expression of BCL2 and BIM as the most significant factors for predicting tumor cell sensitivity to BET inhibitors [33]. CLL fits this description of a BET inhibitor-sensitive malignancy well. As demonstrated by BH3 profiling, CLL cells rely on BCL2 to maintain their mitochondrial outer membrane intact [45]. BCL2 continuously sequesters apoptotic activator BIM, which makes CLL cells ‘primed for death’, i.e., exceptionally dependent on tonic anti-apoptotic function for survival [51]. In this situation, even a slight change in the balance between positive and negative apoptosis regulators can be sufficient to induce apoptosis.
In the light of its particular importance for disease pathogenesis and progression, targeting the CLL microenvironment is an attractive alternative therapeutic approach. Nurselike cells, which resemble M2 polarized macrophages, can be found in the lymph nodes of patients with CLL, and induce gene expression changes in CLL cells that indicate BCR and NF-κB activation and are identical to those found in CLL cells isolated from CLL lymph nodes [38, 52–54]. NLC support CLL cells by stimulating BCR signaling and protect them from spontaneous and drug-induced apoptosis [3, 55–57]. A recent study demonstrated that NLC, unlike normal macrophages, are present in CLL proliferation centers and that their relative abundance associates with an active disease requiring treatment [54]. Only a few approaches to target NLC and their interaction with CLL cells have been reported to date. A CXCL12-blocking antibody or CXCR4 peptide inhibitors partially diminish the protective function of NLC in ex vivo co-culture conditions [38, 57]. Lenalidomide modulates the phenotype of NLC and causes them to lose their CLL-nurturing abilities [58, 59]. The clinical efficacy of the BCR signaling inhibitors may be, at least partially, attributed to disrupting interactions between CLL cells and the microenvironment [60]. As we demonstrated here, BET inhibition presents yet another approach to target NLC and thereby the CLL microenvironment. The exposure of CLL/NLC co-culture to GS-5829 led to decreased numbers of NLC and caused changes in their morphology. On the molecular level, GS-5829 induced downregulation of signaling pathways in NLC that resembled those changes seen in CLL cells. For example, GS-5829 substantially reduced the level of BCL-XL and increased the level of BIM (the difference was not statistically significant likely due to a small number of observations) suggesting a mechanism of apoptosis induction similar to that in CLL cells. STAT3 phosphorylation is essential to macrophage M2 polarization and the cancer-protective function of tumor-associated macrophages [48–50]. GS-5829 treatment led to a significant decrease in STAT3 phosphorylation in NLC suggesting another mechanism by which BET protein inhibition may disrupt the CLL microenvironment. As CLL cells and NLC are interdependent in co-culture conditions, we cannot exclude the possibility that some of the observed effects were due to the disturbed CLL/NLC interaction.
Collectively, our findings demonstrate that BET proteins inhibition causes CLL cells death by intrinsic apoptosis and provide the rationale for clinical trials of BET inhibitors in CLL as single agents or in combination with the BCR signaling inhibitors.
Supplementary Material
Acknowledgments
This study was supported in part by MD Anderson’s CLL Moonshot program, MD Anderson’s Cancer Center Support grant P30 CA016672 and Gilead Sciences, Inc. E.t.H. is a Special Fellow of the Leukemia and Lymphoma Society. The BET inhibitor GS-5829 was provided by Gilead Sciences, Inc.
Competing Interests
The authors declare the following competing interests: A.C. is an employee of Gilead Sciences, Inc. and owns equity in the company; P.A.T. has served as a consultant for AbbVie and Pharmacyclics, an AbbVie company; N.J. has served as a consultant for AbbVie, Pharmacyclics, and Janssen Pharmaceuticals, Inc. and received research funding from Pharmacyclics; W.G.W. has received honoraria, served as a consultant and received research funding from Gilead Sciences, Inc.; J.A.B. has received research funding from Gilead Sciences and Pharmacyclics, travel expenses from Gilead Sciences and served as a consultant for Janssen Pharmaceuticals. The remaining authors declare no competing interests. The BET inhibitor GS-5829, idelalisib, and entospletinib were provided by Gilead Sciences, Inc.
References
- 1.Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers 2017; 3: 16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herndon TM, Chen SS, Saba NS, Valdez J, Emson C, Gatmaitan M et al. Direct in vivo evidence for increased proliferation of CLL cells in lymph nodes compared to bone marrow and peripheral blood. Leukemia 2017; 31(6): 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011; 117(2): 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger JA, O’Brien S. Evolution of CLL treatment - from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol 2018; 15(8): 510–527. [DOI] [PubMed] [Google Scholar]
- 5.Coutre SE, Furman RR, Flinn IW, Burger JA, Blum K, Sharman J et al. Extended Treatment with Single-Agent Ibrutinib at the 420 mg Dose Leads to Durable Responses in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Clin Cancer Res 2017; 23(5): 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood 2018; 131(17): 1910–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn IE, Farooqui MZH, Tian X, Valdez J, Sun C, Soto S et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 2018; 131(21): 2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burger JA, Li KW, Keating MJ, Sivina M, Amer AM, Garg N et al. Leukemia cell proliferation and death in chronic lymphocytic leukemia patients on therapy with the BTK inhibitor ibrutinib. JCI Insight 2017; 2(2): e89904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia 2014; 28(3): 649–657. [DOI] [PubMed] [Google Scholar]
- 10.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012; 119(5): 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012; 119(11): 2590–2594. [DOI] [PubMed] [Google Scholar]
- 12.Herman SE, Niemann CU, Farooqui M, Jones J, Mustafa RZ, Lipsky A et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia 2014; 28(11): 2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood 2013; 121(9): 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woyach JA, Smucker K, Smith LL, Lozanski A, Zhong Y, Ruppert AS et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood 2014; 123(12): 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. The New England journal of medicine 2018; 379(26): 2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanafelt TD, Wang V, Kay NE, Hanson CA, O’Brien SM, Barrientos JC et al. A Randomized Phase III Study of Ibrutinib (PCI-32765)-Based Therapy Vs. Standard Fludarabine, Cyclophosphamide, and Rituximab (FCR) Chemoimmunotherapy in Untreated Younger Patients with Chronic Lymphocytic Leukemia (CLL): A Trial of the ECOG-ACRIN Cancer Research Group (E1912). Blood 2018; 132(Suppl 1): LBA-4-LBA-4. [Google Scholar]
- 17.Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(1): 43–56. [DOI] [PubMed] [Google Scholar]
- 18.Moyo TK, Wilson CS, Moore DJ, Eischen CM. Myc enhances B-cell receptor signaling in precancerous B cells and confers resistance to Btk inhibition. Oncogene 2017; 36(32): 4653–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Zhang LL, Wu W, Guo H, Li Y, Sukhanova M et al. Activation of MYC, a bona fide client of HSP90, contributes to intrinsic ibrutinib resistance in mantle cell lymphoma. Blood Adv 2018; 2(16): 2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Lu P, Guo A, Cheng S, Zong H, Martin P et al. Characterization of ibrutinib-sensitive and -resistant mantle lymphoma cells. Br J Haematol 2014; 166(6): 849–861. [DOI] [PubMed] [Google Scholar]
- 21.Rahal R, Frick M, Romero R, Korn JM, Kridel R, Chan FC et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat Med 2014; 20(1): 87–92. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Lwin T, Silva A, Shah B, Tao J, Fang B et al. Unification of de novo and acquired ibrutinib resistance in mantle cell lymphoma. Nat Commun 2017; 8: 14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell 2014; 54(5): 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell 2005; 19(4): 535–545. [DOI] [PubMed] [Google Scholar]
- 25.Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell 2005; 19(4): 523–534. [DOI] [PubMed] [Google Scholar]
- 26.Chaidos A, Caputo V, Karadimitris A. Inhibition of bromodomain and extra-terminal proteins (BET) as a potential therapeutic approach in haematological malignancies: emerging preclinical and clinical evidence. Ther Adv Hematol 2015; 6(3): 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013; 153(2): 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapuy B, McKeown MR, Lin CY, Monti S, Roemer MG, Qi J et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer cell 2013; 24(6): 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA et al. Super-enhancers in the control of cell identity and disease. Cell 2013; 155(4): 934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceribelli M, Kelly PN, Shaffer AL, Wright GW, Xiao W, Yang Y et al. Blockade of oncogenic IkappaB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proceedings of the National Academy of Sciences of the United States of America 2014; 111(31): 11365–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun B, Shah B, Fiskus W, Qi J, Rajapakshe K, Coarfa C et al. Synergistic activity of BET protein antagonist-based combinations in mantle cell lymphoma cells sensitive or resistant to ibrutinib. Blood 2015; 126(13): 1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trabucco SE, Gerstein RM, Evens AM, Bradner JE, Shultz LD, Greiner DL et al. Inhibition of bromodomain proteins for the treatment of human diffuse large B-cell lymphoma. Clin Cancer Res 2015; 21(1): 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bui MH, Lin X, Albert DH, Li L, Lam LT, Faivre EJ et al. Preclinical Characterization of BET Family Bromodomain Inhibitor ABBV-075 Suggests Combination Therapeutic Strategies. Cancer Res 2017; 77(11): 2976–2989. [DOI] [PubMed] [Google Scholar]
- 34.Boi M, Gaudio E, Bonetti P, Kwee I, Bernasconi E, Tarantelli C et al. The BET Bromodomain Inhibitor OTX015 Affects Pathogenetic Pathways in Preclinical B-cell Tumor Models and Synergizes with Targeted Drugs. Clin Cancer Res 2015; 21(7): 1628–1638. [DOI] [PubMed] [Google Scholar]
- 35.Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol 2016; 3(4): e196–204. [DOI] [PubMed] [Google Scholar]
- 36.Forero-Torres A, Rosen S, Smith DC, Lesser G, Peguero J, Gupta S et al. Preliminary Results from an Ongoing Phase 1/2 Study of INCB057643, a Bromodomain and Extraterminal (BET) Protein Inhibitor, in Patients (pts) with Advanced Malignancies. Blood 2017; 130: 4048. [Google Scholar]
- 37.Sperandio D, Aktoudianakis V, Babaoglu K, Chen X, Elbel K, Chin G et al. Structure-guided discovery of a novel, potent, and orally bioavailable 3,5-dimethylisoxazole aryl-benzimidazole BET bromodomain inhibitor. Bioorg Med Chem 2019; 27(3): 457–469. [DOI] [PubMed] [Google Scholar]
- 38.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000; 96(8): 2655–2663. [PubMed] [Google Scholar]
- 39.Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem 1981; 115(1): 207–216. [DOI] [PubMed] [Google Scholar]
- 40.Kim E, Hurtz C, Koehrer S, Wang Z, Balasubramanian S, Chang BY et al. Ibrutinib inhibits pre-BCR(+) B-cell acute lymphoblastic leukemia progression by targeting BTK and BLK. Blood 2017; 129(9): 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin X, Huang X, Uziel T, Hessler P, Albert DH, Roberts-Rapp LA et al. HEXIM1 as a Robust Pharmacodynamic Marker for Monitoring Target Engagement of BET Family Bromodomain Inhibitors in Tumors and Surrogate Tissues. Mol Cancer Ther 2017; 16(2): 388–396. [DOI] [PubMed] [Google Scholar]
- 42.Ozer HG, El-Gamal D, Powell B, Hing ZA, Blachly JS, Harrington B et al. BRD4 Profiling Identifies Critical Chronic Lymphocytic Leukemia Oncogenic Circuits and Reveals Sensitivity to PLX51107, a Novel Structurally Distinct BET Inhibitor. Cancer Discov 2018; 8(4): 458–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014; 123(21): 3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo K, Shaim H, Thompson PA, Burger JA, Keating M, Estrov Z et al. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia 2018; 32(4): 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 2007; 117(1): 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 2011; 478(7370): 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shan X, Fung JJ, Kosaka A, Danet-Desnoyers G, Reproducibility Project: Cancer B. Replication Study: Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia L, Clear A, Liu FT, Matthews J, Uddin N, McCarthy A et al. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood 2014; 123(11): 1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007; 117(5): 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9(11): 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer cell 2006; 9(5): 351–365. [DOI] [PubMed] [Google Scholar]
- 52.Burgess M, Ellis JJ, Mapp S, Mollee P, Mazzieri R, Mattarollo SR et al. Transcriptomic analysis of monocytes and macrophages derived from CLL patients which display differing abilities to respond to therapeutic antibody immune complexes. Genom Data 2016; 7: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ysebaert L, Fournie JJ. Genomic and phenotypic characterization of nurse-like cells that promote drug resistance in chronic lymphocytic leukemia. Leukemia & lymphoma 2011; 52(7): 1404–1406. [DOI] [PubMed] [Google Scholar]
- 54.Boissard F, Laurent C, Ramsay AG, Quillet-Mary A, Fournie JJ, Poupot M et al. Nurse-like cells impact on disease progression in chronic lymphocytic leukemia. Blood cancer journal 2016; 6: e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood 2009; 113(13): 3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hacken ET, Gounari M, Back JW, Shimanovskaya E, Scarfo L, Kim E et al. Calreticulin as a novel B-cell receptor antigen in chronic lymphocytic leukemia. Haematologica 2017; 102(10): e394–e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood 2005; 106(5): 1824–1830. [DOI] [PubMed] [Google Scholar]
- 58.Schulz A, Durr C, Zenz T, Dohner H, Stilgenbauer S, Lichter P et al. Lenalidomide reduces survival of chronic lymphocytic leukemia cells in primary cocultures by altering the myeloid microenvironment. Blood 2013; 121(13): 2503–2511. [DOI] [PubMed] [Google Scholar]
- 59.Fiorcari S, Martinelli S, Bulgarelli J, Audrito V, Zucchini P, Colaci E et al. Lenalidomide interferes with tumor-promoting properties of nurse-like cells in chronic lymphocytic leukemia. Haematologica 2015; 100(2): 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burger JA, Wiestner A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer 2018; 18(3): 148–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.