Abstract
Introduction:
Genetic risk modifier testing (GRMT), an emerging form of genetic testing based on common single nucleotide polymorphisms and polygenic risk scores, has the potential to refine estimates of BRCA1/2 mutation carriers’ breast cancer risks. However, for women to benefit from GRMT, effective approaches for communicating this novel risk information are needed.
Objective:
To evaluate patient preferences regarding risk communication materials for GRMT.
Methods:
We developed four separate presentations (panel of genes, icon array, verbal risk estimate, graphical risk estimate) of hypothetical GRMT results, each using varying risk communication strategies to convey different information elements including number of risk modifier variants present, variant prevalence amongst BRCA1/2 carriers, and implications and uncertainties of test results for cancer risk. Thirty BRCA1/2 carriers evaluated these materials (randomized to low, moderate, or high breast cancer risk versions). Qualitative and quantitative data were obtained through in-person interviews.
Results:
Across risk versions, participants preferred the presentation of the graphical risk estimate, often in combination with the verbal risk estimate. Interest in GRMT was high; 76.7% of participants wanted their own GRMT. Participants valued the potential for GRMT to clarify their cancer susceptibility and provide actionable information. Many (65.5%) anticipated that GRMT would make risk management decisions easier.
Conclusions:
Women with BRCA1/2 mutations could be highly receptive to GRMT, and the minimal amount of necessary information to include in result risk communication materials includes graphical and verbal estimates of future cancer risk. Findings will inform clinical translation of GRMT in a manner consistent with patients’ preferences.
Keywords: Genetic testing, hereditary breast and ovarian cancer, risk communication, qualitative methods, polygenic risk score
Introduction
Women with pathogenic variants of BRCA1/2 (i.e., BRCA1/2 carriers) are at increased risk for hereditary breast and ovarian cancer compared to the general population. However, not all BRCA1/2 carriers will necessarily develop cancer over a lifetime (incomplete penetrance) and some will develop different types of cancer (variable expressivity). These variations in penetrance and expressivity are likely due to genetic and environmental factors. Incomplete penetrance results in wide-ranging cancer risk estimates; average breast cancer risk estimates for BRCA1/2 carriers by age 70 range from 44%−78% and 31%−56%, respectively [1], although breast cancer risks may be as high as 85% for carriers with especially strong family histories [2].
Despite this substantial uncertainty, female BRCA1/2 carriers are asked to consider risk management strategies with varying benefits, harms, and degrees of protection, including enhanced screening, chemoprevention, and prophylactic surgery [3,4]. As highlighted by recent media attention (e.g., “Angelina Jolie effect” [5–7]), the decision regarding prophylactic mastectomy is often most challenging. Although prophylactic mastectomy substantially reduces a BRCA1/2 carrier’s breast cancer risk [8], it is an invasive and irreversible strategy with physical and psychological ramifications. Thus, it is recommended that BRCA1/2 carriers treat adoption of prophylactic mastectomy as a preference-sensitive decision [4]. Many carriers have difficulty making a prophylactic mastectomy decision [9–15], in part because they must evaluate the benefits and harms of surgery with an incomplete understanding of their future breast cancer risk.
Recent research has demonstrated that multiple common genetic variants (i.e., single nucleotide polymorphisms; SNPs) modify the penetrance of BRCA1/2 mutations [16–19] and also contribute independently to breast cancer risk. These genetic risk modifiers each have a small quantitative effect on the subsequent risk of breast cancer, and could be used to generate a polygenic risk score reflecting a refined estimate of a BRCA1/2 mutation carrier’s future cancer susceptibility [20]. Existing data strongly suggest that these genetic risk modifiers can provide information to meaningfully discriminate between higher- and lower-risk BRCA1/2 carriers in a manner that may assist clinical decision-making [21,22]. Thus, the potential exists for incorporating genetic risk modifiers into a clinical genetic testing panel (i.e., a genetic risk modifier test; GRMT) to more accurately predict breast cancer risks among BRCA1/2 carriers.
A GRMT may have substantial clinical utility, because it could reduce some of the uncertainty surrounding cancer risk estimates for women with BRCA1/2 mutations and aid their decision-making regarding prophylactic mastectomy versus other less invasive risk management strategies. However, for women to benefit from GRMT, effective approaches for communicating this novel risk information are needed. Information about GRMT is complex and ambiguous in nature because it requires an understanding of gene-gene interactions, is supported by a small but growing body of scientific evidence, and the revised risk estimates remain probabilistic in nature. Recommended best practices in risk communication suggest that various strategies may be effective at promoting comprehension of this novel genetic risk information, including the use of numerical expressions such as percentages and frequencies, or visual presentations such as risk ladders, icon arrays, bar charts, and survival curves [23,24].
Basic cognitive psychological research on information processing demonstrates that images are especially effective at drawing attention to messages, facilitating understanding of health information, and increasing recall, suggesting particular value in pairing written, numerical risk information with graphical presentations [23,25–29]. In addition, using a visual aid to communicate risk can benefit vulnerable patient subgroups such as older adults, immigrant populations, and patients with low health literacy [23]. However, the effectiveness and patient acceptability of different graphical risk communication formats varies across studies and health contexts. For example, in one qualitative study evaluating strategies to communicate absolute lifetime risk across various health conditions including breast cancer, simple bar charts were found to be preferable over other images such as line graphs, thermometer graphs, icon arrays, and survival curves [30]. In another qualitative study to evaluate preferences for communicating genomic lifetime risk for melanoma, participants expressed the strongest preference for pie charts, followed by icon arrays, bar charts, scale diagrams, and box plots [31]. When the goal is to compare risks or convey an individual risk in context, bar charts, risk ladders, scales, and icon arrays appear quite effective [24]. Icon arrays – pictographs consisting of a field of 100 shapes with shading to depict the likelihood of an outcome – excel at portraying percentages as distinct visual units and part-to-whole ratios, work with individuals with varying levels of numeracy, and overcome some cognitive biases [32–35], and have been adopted in several commercial and research settings to convey genetic risk [36–39]. Although no single risk communication approach is universally recommended, it is advised that message developers consider the specific communication goal (e.g., present a comparison, show a time-based trend), minimize cognitive effort by providing less information not more, convey absolute risk rather than relative risk information, and provide comparative risk information when appropriate [40–43].
A related consideration involves the types of information that individuals may want to receive when obtaining results from GRMT, given that various risk communication strategies differ in the information they can potentially convey. Several recent studies suggest that there are limits on both the amount and complexity of information that users want to receive in the context of genetic testing. For example, a study comparing different risk communication strategies for presenting Oncotype DX genomic test results regarding risk for breast cancer recurrence found that participants preferred and had better comprehension of a simpler presentation that included less information (i.e., a recurrence risk stated in plain language, a risk continuum graphic, and confidence interval) than a standard report that included multiple information elements (i.e., a recurrence score, recurrence risk, graph, confidence interval, plain language risk categories, an assay description, and information about the test and company) [44]. Similarly, an analysis of verbal communication occurring in cancer genetic counseling sessions observed a mismatch between the types of information provided in the session and the information that patients preferred to receive [45]. Whereas the information provided frequently focused on details of risk assessment, biology, and technical aspects of testing, patients wanted to receive more information about their personal cancer risks and how test results directly applied to them. Thus, test users may not perceive all available information, or the same information that is prioritized by genetics experts, as being relevant or useful.
Before GRMT can be translated into patient care, it is critical to first develop and systematically evaluate GRMT risk communication materials to ensure that they are interpretable and consistent with the preferences of future test users. Thus, with the present study we sought to develop risk communication materials that would effectively convey results of this novel test to individuals. We developed materials using various risk communication strategies (e.g., icon arrays, bar charts) to present different types of potentially relevant information including the number of genetic risk modifier variants for which one had tested positive, prevalence of genetic risk modifiers in the population of BRCA1/2 carriers, implications of genetic risk modifiers for future breast cancer risks, and limitations and scientific uncertainties regarding GRMT. Then, using “gold standard” cognitive interviewing procedures [46], we obtained feedback from BRCA1/2 carriers about their perceptions, comprehension, and preferences regarding sample risk communication materials conveying hypothetical GRMT results to identify a refined approach to communicating about GRMT.
Materials and Methods
Participants
Eligible study participants were identified by their physician and included English-speaking women ages 25–80 years who had a BRCA1/2 mutation and were being treated at the Memorial Sloan Kettering Cancer Center (MSK) Special Surveillance Breast Clinic. The MSK Institutional Review Board deemed this work exempt research; therefore, all participants confirmed verbal agreement with their voluntary willingness to participate.
Phase 1: Development of GRMT educational material
Development and evaluation of GRMT risk communication materials proceeded in two phases.1 In Phase 1, educational material was designed by our study team of experts in clinical genetics, health psychology, risk communication, and qualitative methodology to describe how genetic risk modifiers affect breast cancer risk. This educational material explained how each genetic risk modifier has different variants that enhance, reduce, or have a neutral effect on a BRCA2 carrier’s overall risk for breast cancer. This material included multiple analogies to describe the process by which genetic risk modifiers affect breast cancer risk among women with BRCA2 mutations (analogies included a written description of how balls of different sizes could be added to a cup of water to raise the water level with illustrations, a written description of how small cracks and a hole created in a layer of ice could interact to cause ice to break apart on a frozen lake, and a written description of how smoking behavior could interact with genetic modifiers to cause lung cancer to develop in some smokers but not others). To determine whether the educational material was perceived as comprehensible by the target user population, a team member (JGH) conducted brief cognitive interviews with participants. Participants reviewed the educational material and provided verbal feedback including responses to true/false knowledge items and their perspectives about the clarity of the information. Interviews were audio-recorded, transcribed, and reviewed by members of the study team. The first round of cognitive interviews (n=7) indicated several areas for improvement including a preference for illustrations to accompany the analogies, confusion on several key points, and no clear preference for any of the analogies. The educational material was revised to include new analogies (including a written description of how weights of different sizes could be added to a scale/balance with illustrations, a written description of how cups of different sizes could be used to add or remove water from a larger cup to change the water level with illustrations, and a written description of how smoking behavior could interact with genetic modifiers to cause lung cancer to develop in some smokers but not others with illustrations) and then evaluated in a second round of cognitive interviews (n=6). Participant feedback indicated a strong preference for the analogy involving a scale/balance, a high level of knowledge following review of the material, and few suggestions for additional revision. The final educational material (see Online Supplementary Material) was used in Phase 2 of the study.
Phase 2: Development and evaluation of GRMT result materials
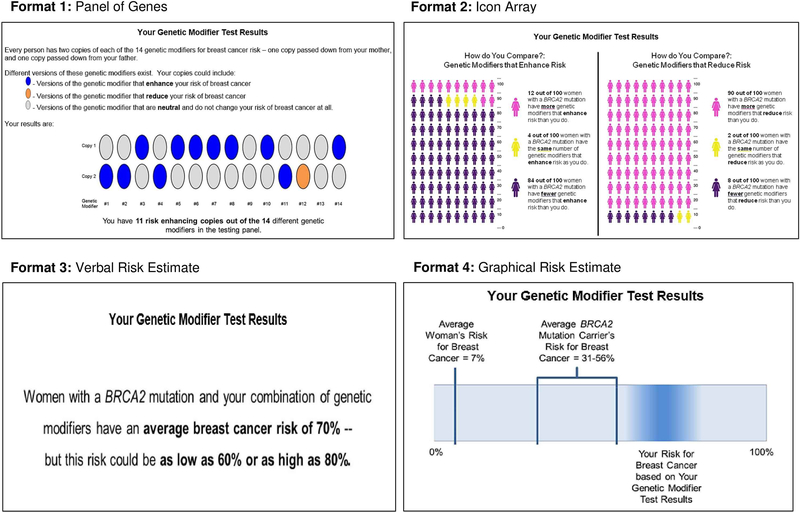
In Phase 2, GRMT result materials were developed to depict sample hypothetical results of a GRMT panel comprised of 14 genetic risk modifiers (consistent with the number of identified genetic risk modifiers at the time this study was developed [17,18]) for a woman with a BRCA2 mutation. We developed four sample GRMT result presentations, informed by risk communication best practices (see Figure 1). These presentations differed in the risk communication strategies used to depict the results, as well as the specific pieces of information conveyed by the presentation. Presentation 1 depicted a panel of genes to convey information about the specific number and types of genetic risk modifier variants carried by an individual. Presentation 2 used icon arrays to convey contextual social comparison information regarding how an individual’s GRMT result compared to those of other BRCA2 carriers (integrating “restroom gender” icons as per [47], with images created with [48]). Presentation 3 included a verbal risk estimate stating an individual’s average breast cancer risk and the upper and lower confidence intervals of the estimate based on her GRMT result. Presentation 4 involved a graphical risk estimate with a bar chart showing the breast cancer risks of the average woman and average BRCA2 carrier, as well as the estimated range of breast cancer risk for the individual given her GRMT result using a “blurred bar” feature to convey the uncertainty of the specific estimate (see [49]). Each presentation also included 2–5 relevant limitations of the GRMT results (e.g., “These estimates could change in the future as more information is learned about these genetic modifiers and cancer”). We created three versions of each presentation that reflected low, moderate, or high breast cancer risk GRMT results.
Figure 1.
Sample GRMT result presentations. All of the depicted presentations are for the high risk version. Each result presentation also included 2–5 relevant limitations of the GRMT results (not shown).
To evaluate these GRMT result materials, we recruited 30 participants (from a pool of 72 eligible patients, of whom 11 refused and 31 could not be contacted/scheduled) to complete an in-person individual cognitive interview with a study team member (JGH, MGG, ES). Participants were randomized to receive hypothetical GRMT results corresponding to one of the three levels of breast cancer risk (10 participants per version). Participants were first shown the educational material developed in Phase 1 and told to “imagine that as a woman with a BRCA2 mutation, your doctor has offered you a new test that checks for the 14 genetic modifiers for breast cancer risk. You take the test and get these results.” Participants were shown each of the four sample result presentations one at a time (in the order of Presentation 1: panel of genes, Presentation 2: icon array, Presentation 3: verbal risk estimate, Presentation 4: graphical risk estimate), and instructed to imagine each presentation was the only results that they had seen. For each presentation, participants were asked to provide qualitative feedback about the presentation and their comprehension of the results based on a semi-structured interview guide, and were also asked to indicate their perceived lifetime breast cancer risk based on the results using a 5-point Likert scale (1=very unlikely to 5=very likely). After reviewing all four presentations individually, participants were asked to examine them side-by-side and provide feedback about their overall impressions of the materials and their anticipated interest and utility of GRMT. Interviews lasted approximately 60 minutes and were audio-recorded and transcribed. Following the interview, participants completed a survey assessing demographics and numeracy (using the validated Subjective Numeracy Scale [50,51]). Participants received $50 for their contribution.
Transcripts were analyzed through thematic content analysis, an inductive qualitative data analysis method that seeks to identify and interpret recurring conceptual patterns directly from the data through intensive reading, coding, and interpretation [52–56]. This analytic approach [55,57] involved four coders (JGH, MGG, JCW, ES) and iterative rounds of consensus analysis to ensure reliability of the findings [58,59]. ATLAS.ti was used to facilitate analysis [60]. First, team members independently read the same transcripts, identified notable narrative content, created descriptive and interpretive codes capturing the essence of such content, and assigned the codes to the relevant narrative segments; team members subsequently met to reach consensus on codes, their meanings, and assignment to the narratives. Through this process a consensus codebook was developed. The team subsequently used the codebook to code future transcripts, which was refined and modified throughout this process of independent and collaborative coding. Upon coding of all 30 transcripts, the coders then engaged in a secondary analysis to interpret and synthesize the coded narrative content representing key themes related to risk communication material preferences, and perceived interest in and response to GRMT that had emerged during the first stage of consensus coding. The final stage involved team collaboration to identify commonalities across each coder’s thematic observations and reach agreement regarding salient themes and subthemes and how they should be best described. We selected the most illustrative participant quotations from the interview data to support our key findings. Additionally, participants’ responses were quantified in instances where the interview data allowed (e.g., Likert-scale responses regarding perceived breast cancer risk, yes/no or categorical response options to interview probes). Descriptive statistics were computed for demographic and numeracy data.
Results
Sample characteristics
Characteristics of Phase 2 participants appear in Table 1. Participants ranged in age from 28–74 years (M=46.3 years), and the majority were white (86.7%), non-Hispanic (93.3%), and had completed post-graduate education (63.3%). Numeracy level in this sample was generally high (M=4.8 out of 6).
Table 1.
Sample characteristics (N = 30)
| n (%) | |
|---|---|
| Age, years (M ± SD) | 46.3 ±13.6; range: 28–74 |
| Gender (Female) | 30 (100) |
| Race | |
| White/Caucasian | 26 (86.7) |
| Black/African American | 1 (3.3) |
| Multiple races | 2 (6.7) |
| Missing | 1 (3.3) |
| Ethnicity (Hispanic) | 2 (6.7) |
| Educational attainment | |
| Some college | 2 (6.7) |
| College graduate | 9 (30.0) |
| Post-graduate | 19 (63.3) |
| Income | |
| $35,000 to $49,999 | 1 (6.7) |
| $50,000 to $74,999 | 3 (10.0) |
| $75,000 to $99,999 | 1 (3.3) |
| $100,000 to $199,999 | 8 (26.7) |
| $200,000 or more | 12 (40.0) |
| Missing | 4 (13.3) |
| Previous cancer diagnosis (Yes) | 5 (13.7) |
| Subjective numeracy (scale range 1–6) | 4.8 ± 0.83; range:2.6–6.0 |
Risk communication preferences
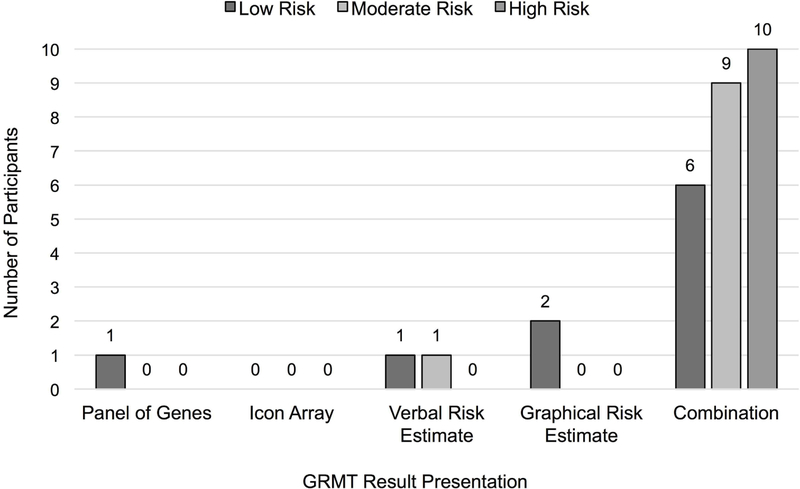
As shown in Figure 2, participants across all three hypothetical risk versions expressed a preference for the graphical risk estimate presentation (n=27, 90%). Analysis of the qualitative data (see Table 2 for all themes, subthemes, and participant quotations) indicated that participants perceived this risk communication presentation to be clear and straightforward. Furthermore, participants across all three risk versions noted that the graphical risk presentation provided comparative risk data that enables personalization of risk, including valuable contextual information that assists with comprehension of one’s personal breast cancer risk. A few participants shared their impressions about how uncertainty associated with the risk estimate was depicted in the graphical risk estimate presentation; whereas some found this feature to be a useful way to communicate the probabilistic nature of risk estimates, others found this to be confusing or unsatisfying because of a desire for a specific number.
Figure 2.
Participant preferences for the sample GRMT result presentations across the three hypothetical risk versions (N=30). As part of the interview, each participant was asked to identify her preferred result presentation. The “Combination” category reflects participant responses that involved combining one or more of the sample GRMT result presentations; in all instances, participants’ preferred combinations included the graphical risk estimate presentation. The most common combination across all risk versions was the graphical risk estimate plus the verbal risk estimate (preferred by 50%).
Table 2.
Patient perspectives about genetic risk modifier testing (GRMT) risk communication materials with illustrative participant quotes
| Theme: Risk communication preferences | |
| Graphical risk estimate presentation | |
| Clear and straightforward | “This one's also really clear. I like this little block with the colors. I think that that actually, visually I can understand it.” (#2004, high risk version) |
| “Just clear, concise, easy to understand." (#3015, moderate risk version) | |
| Provided comparative risk data that enables personalization of risk | “Helping me compare to what that means with risk factors, of how I compare with a population of women with BRCA2, and how I compare with a population of just women in general. And so that helps me feel like, okay, I know what this means for my health, because I have a comparison point.” (#3016, low risk version) |
| Impressions about how uncertainty associated with the risk estimate was depicted | “I think that having a visual tells a story, makes it, iťs right in front of you, iťs easy to understand. There's nothina confusing about it at all. I like how this is shaded. And even the way iťs like, it aoes from liaht to dark, versus like two hard lines, and iťs just a bar, sort of gives you the impression that it is a range." (#3014, high risk version) |
| “Of course I understand that you cannot estimate someone’s exact risk I mean nobody, no doctor can do that. And no patient can expect that. I understand that. But that is really vague. ‘We believe your breast cancer risk is most likely somewhere in the shaded area. ’ I mean that just can’t be more ambiguous.” (#3004, low risk version) | |
| Verbal risk estimate presentation | |
| Clear and succinct | “This is my favorite of all of them. I just find it clearer for me to comprehend.” (#1003, moderate risk version) |
| “I mean iťs more concrete. It lets you walk out of the room knowing something very concrete, that your risk is somewhere between 60 to 80%. Thaťs based on the BRCA mutation plus your genetic modifiers. It feels more personalized.” (#3013, high risk version) | |
| Requiring no cognitive interpretation to understand one’s personal breast cancer risk | “Iťs just very clear. It talks right down to the risk without you having to interpret or count up things; it did it for you. It gives you the take-home message, which is what I was sort of looking for. It sort of answers what it means." (#3018, moderate risk version) |
| Preference for combining graphical risk estimate and verbal risk estimate presentation | “I mean I like it visually how iťs in [graphical risk estimate presentation], but I think just seeing what this number is, ‘cause it doesn’t actually say on here 70%, or average or whatever. So I think it would be helpful to have this text from [verbal risk estimate presentation] on [graphical risk estimate presentation].” (#3013, high risk version) |
| Panel of genes presentation | |
| Uncertainty about what the results would mean for their personal breast cancer risk | “It doesn’t let you know how much the modifier affects your risk or decreases your risk.” (#2001, low risk version) |
| “I mean it looks pretty straightforward. Iťs a little bit busy, but once 1 read the surrounding text, it makes sense. And iťs actually not that busy at all. So I think iťs hard to know, there's nothing specific about what each of these modifiers turn on or turn off necessarily or how exactly they could affect my mutation. But it does feel straightforward. I understand what it says.” (2006, moderate risk version) | |
| Icon array presentation | |
| Confusion and frustration | “My brain is doing some gymnastics with this one. I don’t find it terribly clear...I cannot figure out if this is like good or bad information.” (#3009, moderate risk version) |
| Information overwhelming or irrelevant | “It’s not significant to me that two out of 100 women have the same number of genetic modifiers that reduce risk, and four have the same number of genetic modifiers that enhance risk. That’s useless information.”(#3001, low risk version) |
| “I mean it gives you sort of where you stack against other people, but what I’d still want to know is what that means.” (#3018, moderate risk version) | |
| Theme: Interest in GRMT | |
| Interested | “I would go right now. I would roll up my sleeve, take my blood. I would love this information.” (#1002, high risk version) |
| Ambivalent or uncertain | “I don’t know. Maybe if you did develop breast cancer, then you would get it after. But I don’t know if I would—if you already know that you’re [at] an increased risk with the BRCA, I don’t know if—of course you would love it to be at the lower, but get it, and get at the lower risk, but I don’t know.” (#1005, high risk version) |
| Uninterested | “I probably wouldnť go through the process, just to keep myself from having to go through the anxiety again.” (#2006, moderate risk version) |
| Theme: Perceived utility of GRMT | |
| Ability of GRMT to clarify understanding of breast cancer risk | “I personally believe that I want to be as informed about my care as possible. So I would want to know, you know, with BRCA2, family history, and now with the modifiers, the environmental and lifestyle behaviors, I would want to make sure I was taking care of myself in the best way and I think that these results, this genetic modifier does enhance that in some way.” (#3003, high risk version) |
| “I think I would have something to work with in terms of conversations with my doctor about what’s going on.I think there’s something reassuring about looking into the genetic code again and determining what can turn something on or turn it off and coming back with, to me what this is saying is we have a more solid understanding of what your risk is.So there’s something empowering about having data that feels more concrete.” (#2006, moderate risk version) | |
| GRMT would be valuable regardless of the specific results gained from testing | “Yeah, the truth is, all information is valuable to me. I need to be able to decide what to be able to do. If the results had been low, I think it would also be just as valuable for me to know that." (#3010, high risk version) |
| “I don’t think you could say just because the risk puts you in the average, that it’s less valuable. It just gives you more information. So I think it’s the same value." (#1001, low risk version) | |
| Perceived utility of GRMT was tied to the actionability, precision or accuracy, and personalization of the results | “Well, I think I would be interested if my doctor felt that it would give us better information on how the next 10 years of my life, what we do in terms of the screenings or surgeries or anything like that. If it was still too early, I'm sort of like I donť want to bother doing the testing, which doesnť really give us meaningful information unless iťs going to be used for research to help build the story about it. Then I would be interested." (#3018, moderate risk version) |
| “[With BRCA2] as far as the breast cancer side, the risk is really high. So even if you’re at the low end, unless you’re telling me that this is a modifier that means I will absolutely not get cancer, then I don’t know how helpful it is...If it’s not going to be something where I’m like at the population, with the 20 years of certainty that you have on the other parts of this, then I don’t know if it makes a difference." (#1001, low risk version) | |
| “Yeah, if this could tell me, it feels like iťs not like a super-definitive test in terms of like were more, if we knew more, I understand we're constantly working on that, and so iťs not a critique of like what science is already doing, but more just an acknowledgement of the fact that there is more, we have further to go. So I think iťs pretty valuable as it is. I think it would be more valuable if it were able to say concretely your risk is definitely this, or your risk is definitely that. Because as it is, we do have a bit of a window, right. Like we have something already. We know that compared to the average woman, the risk is this other amount, basically. Iťs still a big window, but at least we know iťs more, and iťs a significant amount more.ȝ (#2006, moderate risk version) | |
| Different GRMT results would have specific implications for risk management decisions | “I certainly wouldn’t think to myself, oh I should be doing more surveillance...I would feel like I was probably doing enough in terms of surveillance, because I am now being told that my risk is probably a little bit lower. That would be helpful.” (#3002, low risk version) |
| “Considering that chemoprevention and surgery can affect your quality of life, if you are to discover your incredibly low risk, then iťs not worth your while to move forward with those some mildly and some drastically life-altering surgeries. However, if your risk is incredibly high, those surgeries can be life-saving. And so, if you arenť sure, having a test that can clearly tell you, nope, you're at 80%, thaťs going to be a great tool to say, wow, I really need to move forward with one of these procedures.” (#2004, high risk version) | |
| Affective response to GRMT and family history of cancer | “Probably wouldnť change too much. I mean with the BRCA diagnosis, it is what it is, and I think you know genetics, my family history, my mother is one of four daughters, and all four have had breast cancer. But yet my mother is the only positive with the gene... and my cousins, their daughters have had breast cancer. But they're all negative on the gene. So in my experience, I've always thought there was something in additional to just the breast cancer gene, that there were other genetics going on that kind of played into it. So iťs not really kind of surprising, but obviously the more you know, I think it helps future generations. So I think this is easy, concise, and wouldn’t really change how I felt control- wise. Wouldnť really, I wouldnť feel like I had less control, even if it was tipped more, even though this one right here says iťs an average risk." (#3015, moderate risk version) |
| Affective response to GRMT and initial emotional response to receiving a pathogenic BRCA1/2 result | “No, no, I mean not any more than I’ve already been emotionally affected. You know, there is not, what more can you do? No. I’d probably feel sad if it’s true, but you know. Just have to figure out the best way to deal with it.” (#2002, low risk version) |
| “I mean, if I already know that I have a mutation, I'm kind of over the shock and horror of it, so this is just... Really, whaťs been lingering in my mind would be, well, how much of a risk? Is it 80? Is it 50? Is it 30? Where am I? I think that I would probably have been, well, I know for a fact I would be thinking... I primarily, I have the 80% chance depending on what happened with my family. It sounds like I would have a much higher risk, so this is not news to me. I think I would feel better just sort of knowing that I have, like, where I stand. So I donť think it would scare me." (#3010, high risk version) | |
Although most participants preferred the graphical risk estimate presentation, half of the sample suggested that it should be used in combination with the verbal risk estimate presentation (n=15; 50%). The verbal risk estimate presentation was described as having specific strengths, including being both clear and succinct, and requiring no cognitive interpretation to understand one’s personal breast cancer risk. Participants recommended combining these two presentations to provide the most comprehensive information regarding a woman’s personal breast cancer risk. Several participants also suggested labeling the bar graph used in the graphical risk estimate presentation with percentage gradations to improve comprehension (e.g., 0–100 with increments of 10).
In contrast, participants generally expressed dissatisfaction with the panel of genes presentation and icon array presentation. With the panel of genes presentation, participants often described uncertainty about what the results would mean for their personal breast cancer risk. Some participants were confused about how to interpret this presentation, raising questions about the size of the effect of a genetic risk modifier variant or whether interactions occurred between the copies of genetic risk modifiers inherited from each parent. For others, the information conveyed by the panel of genes presentation was understood but perceived to be unnecessary. In response to the icon array presentation, many participants felt confusion and frustration, and had difficulty in interpreting its meaning. Participants frequently described the presentation as incomplete or complex, which led to difficulty in interpreting the results. Other participants correctly interpreted the meaning of the icon array presentation but found the information overwhelming or irrelevant to their personal situation. Therefore, for both the panel of genes presentation and the icon array presentation, participant feedback suggested that these presentations were either difficult to understand or conveyed information deemed to be unimportant.
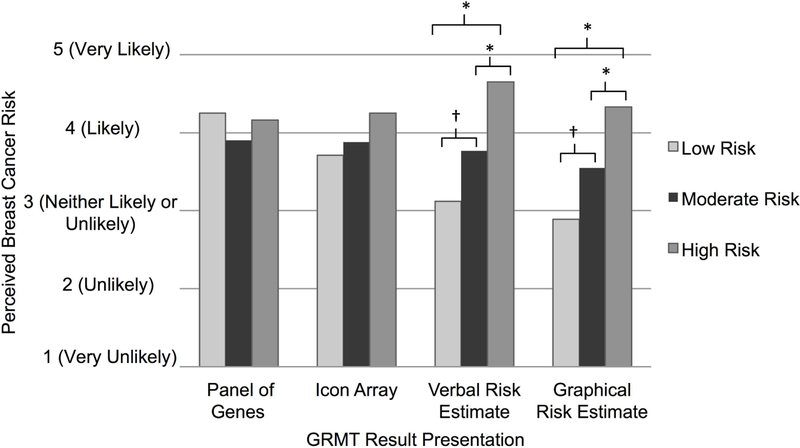
We explored how each of the different GRMT result presentations may have influenced participants’ perceived breast cancer risk. Specifically, for each GRMT result presentation we conducted ANOVAs to assess whether perceived breast cancer risk differed across the hypothetical risk versions (low vs. moderate vs. high). Post-hoc analyses were conducted using Fisher’s LSD test. As presented in Figure 3, the perceived lifetime risk of developing breast cancer significantly differed among participants shown the three hypothetical risk versions with the graphical risk estimate presentation, F(2, 26)=8.09, p=0.002. Specifically, in response to the graphical risk estimate presentation, participants shown the high risk version perceived greater risk for breast cancer than did participants shown the moderate risk version, who in turn perceived greater risk for breast cancer than did those shown the low risk version. Similarly, the perceived breast cancer risk significantly differed among participants shown the three risk versions with the verbal risk estimate presentation, F(2, 26)=10.65, p<0.001. However, no differences in perceived risk were observed across risk versions in response to either the panel of genes or icon array presentations.
Figure 3.
Perceptions of lifetime risk of developing breast cancer in response to the sample GRMT result presentations. Perceived risk differed significantly across the three hypothetical risk versions in response to the verbal risk estimate presentation (low risk M=3.06; moderate risk M=3.75; high risk M=4.65) and the graphical risk estimate presentation (low risk M=2.86; moderate risk M=3.55; high risk M=4.33). Significant differences between risk versions are indicated (* = p≤.04; † = p=0.06).
Interest in GRMT
During the interview, participants were asked how interested they would be in receiving GRMT if testing were available. Most (n=23; 76.7%) participants would be interested in having their own GRMT. A minority (n=4; 13.3%) were ambivalent or uncertain about their personal interest. Finally, a few participants (n=3; 10.0%) were uninterested in GRMT.
Perceived utility of GRMT
Several common themes regarding the utility of GRMT were described by participants across all three hypothetical risk versions. Participants frequently explained that as BRCA1/2 carriers, they are continuously negotiating the ambiguities of their cancer risks. Thus, the potential ability of GRMT to clarify their understanding of their breast cancer risk was highly valued. Participants viewed the prospect of having additional knowledge as empowering and valuable for informing risk management decisions and interactions with healthcare providers. Many expressed a belief that GRMT would be valuable regardless of the specific results gained from testing. Although participants explained that they may take different health-related actions upon knowing that they were at higher or lower risk, the inherent value of the GRMT itself would generally remain high.
Participants’ feedback suggested that the perceived utility of GRMT was tied to the actionability, precision or accuracy, and personalization of the results. In general, participants would ascribe higher value to GRMT if the results could provide clear direction to inform their next steps regarding health decisions, and could offer concrete, precise, and personalized risk information. If GRMT could not provide such information, then the value of the testing would be diminished. In general, participants recognized that scientific understanding is evolving as research is conducted over time, and that exact or definitive risk estimates are not likely to be attainable; yet, obtaining as much precision as possible was deemed to be important. Although expressed among participants across all three risk versions, such concerns about the specificity of GRMT results were most frequently raised by those viewing low or moderate risk results. Participants were queried about the impact that GRMT results would have on their cancer risk management decisions. A majority (65.5%) anticipated that GRMT would make their decision-making processes easier, some (31.0%) were unsure how this information would affect the difficulty of their decision-making, and only one participant (3.5%) anticipated that GRMT would make her decisions harder. Furthermore, participants believed different GRMT results would have specific implications for risk management decisions. Participants anticipated that learning low risk GRMT results would lead them to continue heightened breast cancer surveillance practices, and assist in making choices about foregoing more radical preventative measures (e.g., prophylactic mastectomy). Participants anticipated that learning moderate risk GRMT results would not significantly alter their present understanding of their risk. Thus, these results would have a limited impact on risk management practices, particularly surveillance, and would not likely lead them to pursue prophylactic surgery. Participants felt that learning high risk GRMT results would enhance interest in more invasive preventative measures, and may lead some to intensify screening.
As participants evaluated the risk communication materials, many anticipated that their previous experiences, including family history of cancer and initial emotional response to receiving a pathogenic BRCA1/2 result, would influence their affective reactions to GRMT results. When participants had strong family cancer histories, they often reported feeling underwhelmed by the GRMT results. These participants tended to perceive their breast cancer risk as high and felt the GRMT results did little to alter these beliefs. Nonetheless, most of these women ascribed value to the GRMT results since they regarded receiving more information as better. Across all risk versions and presentations, participants discussed how they felt that their emotional response to GRMT results would not be nearly as strong as their initial response to learning of their BRCA1/2 mutation. These individuals perceived that the GRMT results would offer additional insight into their cancer risk, but would unlikely carry the emotional weight or negative impact of their BRCA1/2 genetic test result.
Discussion/Conclusion
This study examined preferences and attitudes of BRCA1/2 carriers regarding newly-developed risk communication materials for a novel GRMT that has the potential to refine estimates of their future breast cancer risks. Participant feedback revealed a common preference for combining the graphical risk estimate presentation and verbal risk estimate presentation as a means of communicating GRMT results. Participants perceived these presentations as including clear and comprehensible risk communication strategies, as well as providing valuable, personalized information about their cancer susceptibility. This feedback is consistent with psychological research demonstrating the utility of pairing written, numerical information with graphical presentations of risk [23,25–29]. Furthermore, through analysis of participants’ quantitative ratings of their perceived lifetime breast cancer risks in response to each sample presentation and across risk versions, we observed that the graphical and verbal risk estimate presentations uniquely affected participants’ risk beliefs. Based on both the qualitative and quantitative data, we conclude that a refined risk communication approach integrating the graphical and verbal risk estimate presentations will not only meet future patients’ preferences, but may effectively convey the meaning of GRMT results.
In contrast to past research and recommended risk communication best practices supporting the utility of icon arrays [32–35,40], participants were particularly displeased with this presentation. It is notable that contrary to how icon arrays are typically used, which is to graphically convey a proportion or percentage reflecting the likelihood of a specific event (e.g., experiencing a side effect) for an individual, we attempted to use this strategy to convey the prevalence of genetic risk modifiers in the population of BRCA2 carriers. Our objective was to provide social comparison information about how an individual’s GRMT result compared to those of other BRCA2 carriers. Many participants found this application of the icon array difficult to understand, and even those who correctly understood the intended meaning were dissatisfied with the information. Participants were similarly dissatisfied with the panel of genes presentation, although a minority believed this information was interesting or could be offered in a supplemental nature to patients who wanted to learn more about their results. It is possible that participants’ preferences for specific ways of presenting GRMT results were guided by their preferences for specific pieces of information. Across presentations, varying risk communication strategies were used to convey distinct types of information that could be revealed by or be relevant to GRMT results. Ultimately, participants’ predominant concern was understanding their personal cancer risk; consequently, presentations that offered information about the number of SNPs identified through GRMT or the population prevalence of these results were deemed unsatisfactory since such information could not easily be converted into insight about their own susceptibility.
We observed substantial interest in the prospect of undergoing GRMT, with three-quarters of participants expressing interest in testing. This level is consistent with a recent study wherein 85% of interviewed BRCA1/2 carriers were interested in receiving refined cancer risk estimates [61]. Similarly, a study of breast cancer polygenic risk information among women from breast cancer families with uninformative genetic testing results found high interest (86.5%) and moderate actual uptake (42.1%−61.8%) of a polygenic risk score [62]. This finding is also consistent with a study examining interest in genetic testing for genes related to modest changes in breast cancer risk (e.g., moderate penetrance gene mutations) [63]; in this survey, 77% of women at moderate to high risk for breast cancer were interested in testing, with interest being greater for tests that conveyed more cancer risk. However, in the present study, high levels of interest existed across the hypothetical risk versions (i.e., low, moderate, and high risk). Participants’ interest in GRMT appears to have been largely shaped by the desire to gain a deeper understanding of their future cancer risks, a general belief in the empowering nature of information, and the potential for these results to inform, and possibly minimize the difficulty of, risk management decisions. Previous research has demonstrated that reducing uncertainty regarding cancer risk and decision-making is a key motivator for undergoing genetic testing, and patients might experience ongoing distress when their uncertainty is not reduced following testing [64–67]. This is not only true for individuals who receive uninformative genetic test results (e.g., variants of uncertain significance) [67–69], but also for individuals who receive pathogenic BRCA1/2 genetic test results indicating heightened cancer risks. These individuals can struggle with managing sustained, residual uncertainty regarding the prospect of cancer development and the potential efficacy of risk management options, and information seeking can be a common coping strategy [70]. Consequently, women with BRCA1/2 mutations could be very receptive to GRMT given its potential to provide information that can resolve at least one source of uncertainty, namely the probability of developing cancer in the future [71]. However, our results suggest that resolving probabilistic uncertainty is not entirely sufficient for establishing the utility of GRMT; study participants also placed high value on the ability of GRMT to produce risk information that is precise and accurate. Such concerns reflect another source of uncertainty, ambiguity (i.e., uncertainty regarding the strength, validity, consistency, or adequacy of risk estimates or risk information [71]), that patients contend with when interpreting genetic risk information.
In summary, these results suggest that women with BRCA1/2 mutations would be highly receptive to GRMT, and that the minimal amount of necessary information to include in GRMT result risk communication materials includes graphical and verbal estimates of future breast cancer risk. Communicating cancer risk through these presentations appears to be acceptable to patients, influences their breast cancer risk perceptions, and may assist in risk management decision-making. However, several study limitations must be acknowledged. This sample was small, racially and ethnically homogenous, and generally well educated with high numeracy. Further, participants were treated at one institution and recruited from a high-risk cancer surveillance clinic. Thus, results may not be generalizable to the broader population of BRCA1/2 carriers treated in other settings or who are less actively involved in managing their cancer risks. Though participants had direct experience with BRCA1/2 genetic testing, the GRMT results were hypothetical and were presented in the order described (i.e., panel of genes, then icon array, verbal risk estimate, and finally graphical risk estimate). This ordering of exposure to the result presentations may have influenced participants’ perspectives; for example, the significant difference observed in perceived risk for the graphic risk estimate presentation may be due to participants having seen the verbal risk estimate previously. Therefore, findings related to the impact of the result presentations on perceived risk should be interpreted cautiously. Nonetheless, each participant viewed all four presentations side-by-side toward the conclusion of the interview and was solicited for her overall preferences and impressions. We also did not assess additional individual characteristics that might have influenced both presentation preferences and overall interest in GRMT. For example, patient characteristics such as intolerance for uncertainty, a dispositional tendency to respond negatively on a cognitive, emotional, and behavioral level to an unknown outcome [72,73], have been associated with responses to genetic risk information [68], and may have contributed to patients’ perceptions of the GRMT result presentations. Future studies should investigate how patients’ attitudes toward and experiences with different sources of uncertainty affect not only their use of GRMT, but their subsequent reactions to the information provided by this testing. Overall, this study design allowed for an in-depth, thorough analysis of the perspectives of patients similar to those who are likely to be initial users of a future GRMT, a gold-standard initial step toward maximizing the comprehensibility and acceptability of novel risk communication strategies in research and clinical settings.
These findings can be used by our team and other investigators to inform ongoing efforts to translate GRMT into cancer care in a manner consistent with patient preferences and information needs [39,61,74]. Genomics research offers promise that GRMT and the calculation of polygenic risk scores can be leveraged to refine estimates of BRCA1/2 carriers’ cancer risks [16–19,21,22]; this possibility was a primary driver of participants’ interest in GRMT, with test results being deemed as valuable insomuch as they could help patients to contextualize and clarify uncertainty about their cancer susceptibility. However, as this testing moves into clinical practice, it will be crucial for future studies to investigate patients’ actual uptake of GRMT and explore how results subsequently shape patients’ cognitive perceptions of risk and emotional reactions, and to examine what influence GRMT ultimately has on medical decision-making.
Supplementary Material
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of any funder. We are extremely grateful to all participating patients.
Funding Sources
This research was supported by an award from the Breast Cancer Research Foundation (PI: Mark E. Robson), The Robert and Kate Niehaus Center for Inherited Cancer Genomics, the Andrew Sabin Family Foundation, and NCI P30 CA008748. Jada G. Hamilton was also supported by a Mentored Research Scholar Grants in Applied and Clinical Research, MRSG-16–020-01-CPPB, from the American Cancer Society and by the National Cancer Institute of the National Institutes of Health under Award Number R21 CA230879. The content is solely the responsibility of the authors and does not necessarily represent the official views of any funder.
Footnotes
To minimize variability, study risk communication materials (GRMT educational material and GRMT result materials) were developed in reference to a woman with a BRCA2 mutation.
Disclosure Statement
The authors have no conflicts of interest to declare.
Statement of Ethics
The Memorial Sloan Kettering Cancer Center Institutional Review Board deemed this work exempt research; therefore, all participants confirmed verbal agreement with their voluntary willingness to participate. All study procedures and materials were approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. : Average risks of breast and ovarian cancer associated with BRCA1 or BRC2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet 2003;72:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. : Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998;62:676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robson M, Offit K: Clinical practice. Management of an inherited predisposition to breast cancer. N Engl J Med 2007;357:154–162. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2019. Fort Washington, PA, National Comprehensive Cancer Network, Inc., 2018. [cited 2018 December 23]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf [Google Scholar]
- 5.Kamenova K, Reshef A, Caulfield T: Angelina Jolie’s faulty gene: Newspaper coverage of a celebrity’s preventive bilateral mastectomy in Canada, the United States, and the United Kingdom. Genet Med 2014;16:522–528. [DOI] [PubMed] [Google Scholar]
- 6.Jolie A: My medical choice: The New York Times, The New York Times, 2013. [Google Scholar]
- 7.Borzekowski DL, Guan Y, Smith KC, Erby LH, Roter DL: The Angelina effect: Immediate reach, grasp, and impact of going public. Genet Med 2014;16:516–521. [DOI] [PubMed] [Google Scholar]
- 8.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van ‘t Veer L, Garber JE, et al. : Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE study group. J Clin Oncol 2004;22:1055–1062. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MD, Valdimarsdottir HB, DeMarco TA, Peshkin BN, Lawrence W, Rispoli J, et al. : Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: Impact on measures of decision making and satisfaction. Health Psychol 2009;28:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard AF, Balneaves LG, Bottorff JL, Rodney P: Preserving the self: The process of decision making about hereditary breast cancer and ovarian cancer risk reduction. Qual Health Res 2011;21:502–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard AF, Bottorff JL, Balneaves LG, Kim-Sing C: Women’s constructions of the ‘right time’ to consider decisions about risk-reducing mastectomy and risk-reducing oophorectomy. BMC Womens Health 2010;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litton JK, Westin SN, Ready K, Sun CC, Peterson SK, Meric-Bernstam F, et al. : Perception of screening and risk reduction surgeries in patients tested for a BRCA deleterious mutation. Cancer 2009;115:1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan D, Sylvester H, Lucas FL, Miesfeldt S: Perceptions of high-risk care and barriers to care among women at risk for hereditary breast and ovarian cancer following genetic counseling in the community setting. J Genet Couns 2010;19:44–54. [DOI] [PubMed] [Google Scholar]
- 14.Klitzman R, Chung W: The process of deciding about prophylactic surgery for breast and ovarian cancer: Patient questions, uncertainties, and communication. Am J Med Genet A 2010;152A:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan MB, Bleiker EM, Menke-Pluymers MB, Van Gool AR, van Dooren S, Van Geel BN, et al. : Standard psychological consultations and follow up for women at increased risk of hereditary breast cancer considering prophylactic mastectomy. Hered Cancer Clin Pract 2009;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couch FJ, Wang X, McGuffog L, Lee A, Olswold C, Kuchenbaecker KB, et al. : Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genetics 2013;9:e1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudet MM, Kirchhoff T, Green T, Vijai J, Korn JM, Guiducci C, et al. : Common genetic variants and modification of penetrance of BRCA2-associated breast cancer. PLoS Genetics 2010;6:e1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudet MM, Kuchenbaecker KB, Vijai J, Klein RJ, Kirchhoff T, McGuffog L, et al. : Identification of a BRCA2-specific modifier locus at 6p24 related to breast cancer risk. PLoS Genetics 2013;9:e1003173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne RL, Antoniou AC: Genetic modifiers of cancer risk for BRCA1 and BRCA2 mutation carriers. Ann Oncol 2011;22 Suppl 1:i11–17. [DOI] [PubMed] [Google Scholar]
- 20.Sugrue LP, Desikan RS: What are polygenic scores and why are they important? JAMA 2019;321:1820–1821. [DOI] [PubMed] [Google Scholar]
- 21.Kuchenbaecker KB, McGuffog L, Barrowdale D, Lee A, Soucy P, Dennis J, et al. : Evaluation of polygenic risk scores for breast and ovarian cancer risk prediction in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 2017;109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavaddat N, Pharoah PD, Michailidou K, Tyrer J, Brook MN, Bolla MK, et al. : Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst 2015;107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Retamero R, Cokely ET: Communicating health risks with visual aids. Curr Dir Psychol Sci 2013;22:392–399. [Google Scholar]
- 24.Ancker JS, Senathirajah Y, Kukafka R, Starren JB: Design features of graphs in health risk communication: A systematic review. J Am Med Inform Assoc 2006;13:608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25 25.Vos SC, Cohen E: Using pictures in health and risk mssages: Oxford Research Encyclopedia of Communication, Oxford University Press, 2017. [Google Scholar]
- 26.Waldron CA, van der Weijden T, Ludt S, Gallacher J, Elwyn G: What are effective strategies to communicate cardiovascular risk information to patients? A systematic review. Patient Educ Couns 2011;82:169–181. [DOI] [PubMed] [Google Scholar]
- 27.Zipkin DA, Umscheid CA, Keating NL, Allen E, Aung K, Beyth R, et al. : Evidence-based risk communication: A systematic review. Ann Intern Med 2014;161:270–280. [DOI] [PubMed] [Google Scholar]
- 28.Nelson DL, Reed VS, Walling JR: Pictorial superiority effect. Journal of Experimental Psychology Human Learning and Memory 1976;2:523–528. [PubMed] [Google Scholar]
- 29.Paivio A: Dual coding theory: Retrospect and current status. Canadian Journal of Psychology/Revue canadienne de psychologie 1991;45:255–287. [Google Scholar]
- 30.Fortin JM, Hirota LK, Bond BE, O’Connor AM, Col NF : Identifying patient preferences for communicating risk estimates: A descriptive pilot study. BMC Med Inform Decis Mak 2001;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31 31.Smit AK, Keogh LA, Hersch J, Newson AJ, Butow P, Williams G, et al. : Public preferences for communicating personal genomic risk information: A focus group study. Health Expect 2016;19:1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A : The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns 2008;73:448–455. [DOI] [PubMed] [Google Scholar]
- 33.Galesic M, Garcia-Retamero R, Gigerenzer G: Using icon arrays to communicate medical risks: Overcoming low numeracy. Health Psychol 2009;28:210–216. [DOI] [PubMed] [Google Scholar]
- 34.Fagerlin A, Wang C, Ubel PA: Reducing the influence of anecdotal reasoning on people’s health care decisions: Is a picture worth a thousand statistics? Med Decis Making 2005;25:398–405. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Retamero R, Galesic M, Gigerenzer G: Do icon arrays help reduce denominator neglect? Med Decis Making 2010;30:672–684. [DOI] [PubMed] [Google Scholar]
- 36.Lautenbach DM, Christensen KD, Sparks JA, Green RC: Communicating genetic risk information for common disorders in the era of genomic medicine. Annu Rev Genomics Hum Genet 2013;14:491–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay JL, Berwick M, Zielaskowski K, White KA, Rodriguez VM, Robers E, et al. : Implementing an internet-delivered skin cancer genetic testing intervention to improve sun protection behavior in a diverse population: Protocol for a randomized controlled trial. JMIR Res Protoc 2017;6:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaphingst KA, McBride CM, Wade C, Alford SH, Brody LC, Baxevanis AD: Consumers’ use of web-based information and their decisions about multiplex genetic susceptibility testing. J Med Internet Res 2010;12:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forrest LE, Sawyer SD, Hallowell N, James PA, Young M-A: High-risk women’s risk perception after receiving personalized polygenic breast cancer risk information. J Community Genet 2019;10:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagerlin A, Zikmund-Fisher BJ, Ubel PA: Helping patients decide: Ten steps to better risk communication. J Natl Cancer Inst 2011;103:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipkus IM, Peters E: Understanding the role of numeracy in health: Proposed theoretical framework and practical insights. Health Educ Behav 2009;36:1065–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischoff B, Brewer NT, Downs J: Communicating risks and benefits: An evidence based user’s guide. Silver Spring, MD, Food and Drug Administration, U.S. Department of Health and Human Services, 2011. [Google Scholar]
- 43.Spiegelhalter D, Pearson M, Short I: Visualizing uncertainty about the future. Science 2011;333:1393–1400. [DOI] [PubMed] [Google Scholar]
- 44.Brewer NT, Richman AR, DeFrank JT, Reyna VF, Carey LA: Improving communication of breast cancer recurrence risk. Breast Cancer Res Treat 2012;133:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph G, Pasick RJ, Schillinger D, Luce J, Guerra C, Cheng JKY: Information mismatch: Cancer risk counseling with diverse underserved patients. J Genet Couns 2017;26:1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willis GB: Cognitive interviewing: A tool for improving questionnaire design. Thousand Oaks, CA, Sage, 2005. [Google Scholar]
- 47.Zikmund-Fisher BJ, Witteman HO, Dickson M, Fuhrel-Forbis A, Kahn VC, Exe NL, et al. : Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making 2014;34:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iconarray.com. University of Michigan, Risk Science Center and Center for Bioethics and Social Sciences in Medicine, [cited November 1, 2013]. Available from: http://www.iconarray.com/.
- 49.Han PK, Klein WM, Lehman T, Killam B, Massett H, Freedman AN: Communication of uncertainty regarding individualized cancer risk estimates: Effects and influential factors. Med Decis Making 2011;31:354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM: Measuring numeracy without a math test: Development of the Subjective Numeracy Scale. Med Decis Making 2007;27:672–680. [DOI] [PubMed] [Google Scholar]
- 51.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A: Validation of the Subjective Numeracy Scale: Effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making 2007;27:663–671. [DOI] [PubMed] [Google Scholar]
- 52.Boyatzis RE: Transforming qualitative information: Thematic analysis and code development, ed 5th Thousand Oaks, CA, Sage Publications, 2009. [Google Scholar]
- 53.Green J, Thorogood N: Qualitative methods for health research, ed 3rd London, UK, Sage Publications, 2014. [Google Scholar]
- 54.Miles MB, Huberman AM, Saldana J: Qualitative data analysis: A methods sourcebook. Thousand Oaks, CA, Sage Publications, 2014. [Google Scholar]
- 55.Patton MQ: Qualitative evaluation and research methods, ed 3rd Thousand Oaks, California, Sage Publications, 2002. [Google Scholar]
- 56.Saldana J: The coding manual for qualitative researchers, ed 2nd London, Sage Publications, 2013. [Google Scholar]
- 57.Brinkman S, Kvale S: InterViews: Learning the craft of qualitative research interviewing, ed 3rd Thousand Oaks, CA, Sage Publications, 2015. [Google Scholar]
- 58.Morse JM, Barrett M, Mayan M, Olsen K, Spiers J : Verification strategies for establishing reliability and validity in qualitative research. Int J Qual Methods 2002;1:1–19. [Google Scholar]
- 59.Denzin NK: The research act: A theoretical introduction to sociological methods ed 5th New Brunswick, NJ, Aldine Transaction, 2009. [Google Scholar]
- 60.Friese S: Qualitative data analysis with ATLAS.ti, ed 2nd London, UK, Sage Publications, 2014. [Google Scholar]
- 61.Hovick SR, Tan N, Morr L, Senter L, Kinnamon DD, Pyatt RE, et al. : Understanding BRCA mutation carriers’ preferences for communication of genetic modifiers of breast cancer risk. J Health Commun 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yanes T, Meiser B, Kaur R, Scheepers-Joynt M, McInerny S, Taylor S, et al. : Uptake of polygenic risk information among women at increased risk of breast cancer. Clin Genet 2019: 10.1111/cge.13687. [DOI] [PubMed] [Google Scholar]
- 63.Graves KD, Peshkin BN, Luta G, Tuong W, Schwartz MD: Interest in genetic testing for modest changes in breast cancer risk: implications for SNP testing. Public Health Genomics 2011;14:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baty BJ, Dudley WN, Musters A, Kinney AY: Uncertainty in BRCA1 cancer susceptibility testing. Am J Med Genet C Semin Med Genet 2006;142C:241–250. [DOI] [PubMed] [Google Scholar]
- 65.Baum A, Friedman AL, Zakowski SG: Stress and genetic testing for disease risk. Health Psychol 1997;16:8–19. [DOI] [PubMed] [Google Scholar]
- 66.Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E: Diagnostic genetic testing for hereditary breast and ovarian cancer in cancer patients: Women’s looking back on the pre-test period and a psychological evaluation. Genet Test 2004;8:13–21. [DOI] [PubMed] [Google Scholar]
- 67.O’Neill SC, Rini C, Goldsmith RE, Valdimarsdottir H, Cohen LH, Schwartz MD: Distress among women receiving uninformative BRCA1/2 results: 12-month outcomes. Psychooncology 2009;18:1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Neill SC, DeMarco T, Peshkin BN, Rogers S, Rispoli J, Brown K, et al. : Tolerance for uncertainty and perceived risk among women receiving uninformative BRCA1/2 test results. Am J Med Genet C Semin Med Genet 2006;142C:251–259. [DOI] [PubMed] [Google Scholar]
- 69.Vos J, Menko FH, Oosterwijk JC, van Asperen CJ, Stiggelbout AM, Tibben A: Genetic counseling does not fulfill the counselees’ need for certainty in hereditary breast/ovarian cancer families: an explorative assessment. Psychooncology 2013;22:1167–1176. [DOI] [PubMed] [Google Scholar]
- 70.Dean M, Davidson LG: Previvors’ uncertainty management strategies for hereditary breast and ovarian cancer. Health communication 2016;33:122–130. [DOI] [PubMed] [Google Scholar]
- 71.Han PKJ, Klein WMP, Arora NK: Varieties of uncertainty in health care: A conceptual taxonomy. Med Decis Making 2011;31:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koerner N, Dugas M: An investigation of appraisals in individuals vulnerable to excessive worry: The role of intolerance of uncertainty. Cognit Ther Res 2008;32:619–638. [Google Scholar]
- 73.Bredemeier K, Berenbaum H: Intolerance of uncertainty and perceived threat. Behav Res Ther 2008;46:28–38. [DOI] [PubMed] [Google Scholar]
- 74.Kaur R, Meiser B, Yanes T, Young M-A, Barlow-Stewart K, Roscioli T, et al. : Development and pilot testing of a leaflet informing women with breast cancer about genomic testing for polygenic risk. Fam Cancer 2019;18:147–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.