Abstract
The establishment of correlates of protection is particularly relevant in the context of rare, highly lethal pathogens. We previously demonstrated that an Ebola glycoprotein virus-like particle (VLP) vaccine, when given as two intramuscular doses, conferred protection from challenge in a murine challenge model. Adjuvants were key to protection and the protection was dependent on a combination of cellular and humoral immunity. In this study, we compared the ability of novel delta inulin-based adjuvant formulations (Advax1-4) to enhance protection mediated by our Ebola VLP vaccine in mice. After two immunizations, Advax-adjuvants that included a TLR9 agonist component induced high IgG responses to the Ebola vaccine, with complete protection against Ebola virus challenge. Although anti-Ebola GP IgG levels waned slowly overtime, protection was durable and was still evident 150 days post-immunization. Mice were protected after just a single VLP immunization with Advax-2 or -4 adjuvants. Advax-adjuvanted VLPs induced a stronger IFN-γ, TNF and IL-12 signature post-immunization. Serum transferred from Advax-adjuvanted vaccinees was able to transfer protection to naïve animals, showing that Ebola protection can be achieved by humoral immunity in the absence of cellular immunity. By contrast, serum from pICLC vaccinees did not transfer protection despite high anti-GP antibody levels on ELISA. These data highlight the importance of adjuvant selection for development of successful Ebola VLP vaccines.
Introduction
The expansion of human development into previously rural or uninhabited ecosystems; increase in international movement of people through mass transit; and changes in the environment due to global warming all increase the threat of emerging and re-emerging infectious disease [1]. Defining an approach for rapid production of pathogen-specific vaccines would help public health officials safeguard against emerging threats. Many different vaccine platforms including nucleic acid-based vaccines, vector-based vaccines and protein-based vaccines are in development to try and counter such threats [2].
Ebola virus disease has a rapid disease course characterized by gross dysregulation and activation of the innate and adaptive immune systems causing a “cytokine storm” of inflammatory and inhibitory cytokines and chemokines; vascular dysfunction and epithelial cell damage; and organ damage and failure [3]. Survivors of Ebola virus infection tend to have lower peak and/or detected viremia, which correlates with lower inflammation and immune activation, and the emergence of an anti-Ebola IgG response [3]. Due to the rapid progression of infection, a robust and durable antibody response will likely be critical for effective protection against Ebola virus infection while cellular T cell immunity may also be important. Our VLP protein-based vaccine has demonstrated efficacy in murine and nonhuman primate models of Ebola virus infection [4–9]. The VLP vaccine when combined with appropriate adjuvants conferred both rapid-onset and durable immunity in mice. That protection was dependent on a combination of cellular and humoral immune responses and was specifically associated with a robust CD4 T cell response and IgG2c antibody response in mice [7]. Advax adjuvants arose from the NIH Adjuvant Development Program and are derived from inulin polysaccharide formulated into microcrystalline particles known as delta inulin [10–12]. The addition of Advax adjuvants to a broad range of vaccines results in significant benefits including enhanced protection associated with higher antibody titers, increased B cell receptor affinity maturation, IgG subtype diversification, enhanced memory CD4 and CD8 T cell responses and antigen dose sparing [13–18]. A particular advantage of Advax adjuvants is that they have already been shown to be safe and well tolerated in human clinical trials in combination with a variety of different antigens [19–21], thereby facilitating rapid translation of successful vaccines from preclinical studies to human trials. In more recently developed Advax formulations, the delta inulin component has been complemented by addition of toll-like receptor (TLR) agonists to enhance vaccine immunity [13, 14, 22].
In this study, we investigated the efficacy of Advax adjuvant formulations to enhance the ability of a VLP vaccine to protect in the mouse model of Ebola virus disease.
RESULTS
Advax adjuvants enhance protection of Ebola VLP vaccine
As previously reported, Ebola VLP formulated with a TLR3 agonist adjuvant provided 100% survival after two intramuscular doses [6]. Out of the four Advax formulations, Advax-2 and -4, which both contained a TLR9 agonist component, resulted in the strongest protection, achieving 100% survival after two intramuscular doses as compared to only 50% survival observed with VLP alone (Figure 1A). Animals injected with any of the adjuvants alone succumbed to infection between 6 and 9 days after vaccination, indicating protection was VLP-specific (data not shown).
Figure 1.
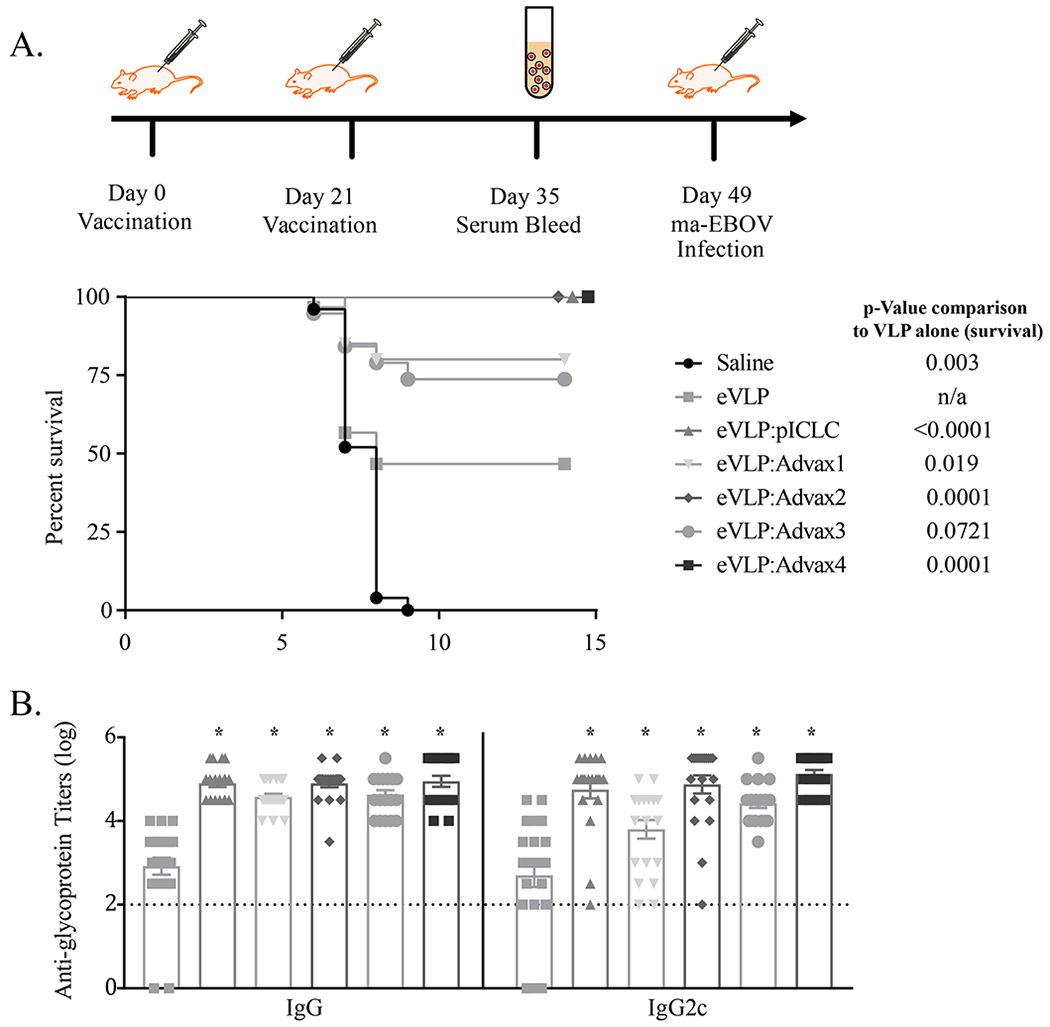
Vaccination with adjuvanted VLP GP antigen confers protection from Ebola virus challenge in mice. C57BL/6 mice were vaccinated with VLP (1.25 μg based on glycoprotein (GP) content) in combination with various adjuvants (1mg Advax-1, -2, -3, or -4 or 10 μg pICLC) on Day 0 and 21, and then challenged with 1000 pfu of mouse adapted (ma)-Ebola virus IP on day 49 (p-value based on Log-rank Mantel-Cox test) (A). Sera was collected from animals seven days after the second vaccination to assess anti-GP antibody titers; p value in comparison to eVLP alone indicated with asterisks (* = p<0.0001, one-way ANOVA comparison) (B). For saline, eVLP, eVLP + (pICLC, Advax-1, Advax-2, Advax-3, Advax-4) the number of animals was 20, 25, 20, 20, 20, 19, and 20, respectively, from combined studies. Animals vaccinated with adjuvant alone all succumbed to infection (data not shown).
Advax adjuvants enhance anti-Ebola antibody response
We had previously demonstrated that the protective VLP and adjuvant combinations elicited strong CD4 T cell responses and IgG2c against Ebola virus antigens [4]. We therefore evaluated the serum anti-Ebola total IgG and IgG2c response in immunized animals. The groups with the highest IgG2c titers pre-challenge (Advax-2 and -4, and PolyIC:LC) had the highest levels of Ebola protection (Figure 1B). This suggests an IgG2c-skewed immune response might be relevant to protection from Ebola virus challenge in mice. Advax-1 contained delta inulin alone and Advax-3 contained delta inulin plus murabutide, whereas Advax-2 and -4 both contained both delta inulin and CpG (and in Advax-4 murabutide as a third component). As Advax-1 and Advax-3 showed a trend to slightly lower antibody responses and levels of Ebola protection, only Advax-2 and Advax-4 were used in subsequent experiments.
Advax adjuvants enable single dose VLP vaccine protection
Previous studies have relied upon a traditional prime and boost VLP vaccine schedule with the assumption that two or more vaccine doses were needed to induce sufficiently strong cellular and humoral immunity for Ebola protection. As Advax adjuvants have previously been shown to provide single dose vaccine protection [23, 24], including against a highly lethal H5N1 avian influenza virus [25], we next asked whether this would also be true for the Ebola VLP vaccine. Mice were vaccinated a single time with the VLP vaccine formulated with the various adjuvants and then challenged 28 days after the single immunization. A single immunization with VLP and either Advax-2, Advax4, or pICLC comparator adjuvant conferred high levels of protection in all the adjuvanted vaccine groups, whereas no protection was seen against Ebola challenge in control mice and only low levels of protection in mice immunized with a single dose of VLP alone (Figure 2A).
Figure 2.
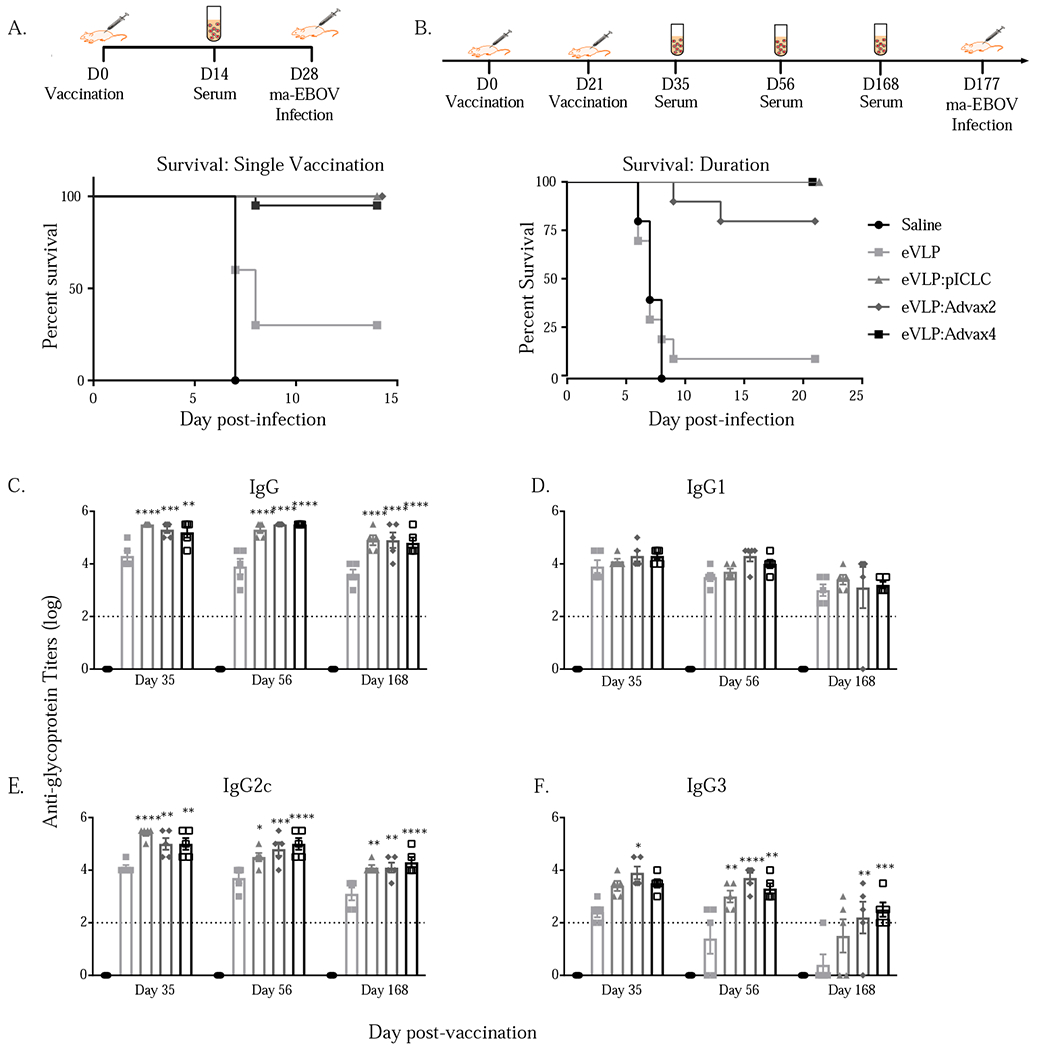
Impact of vaccination with VLP and adjuvants on Ebola vims challenge after a single vaccination and after two immunizations in response to delayed challenge. C57BL/6 mice were vaccinated on Day 0 with VLP (1.25 μg GP) in combination with adjuvants (1 mg Advax-2 or -4 or 10 μg pICLC) and then challenged with 1000 pfu of mouse adapted (ma)-Ebola vims IP on day 28 (p-value based on Log-rank Mantel-Cox test, <0.005 for all adjuvants in comparison to eVLP alone) (A). C57BL/6 mice were vaccinated on Day 0 and 21 with VLP (1.25 μg GP) with adjuvants (Advax-2 or -4 or pICLC) and then challenged with 1000 pfu of mouse adapted (ma)-Ebola vims IP on day 177 (p-value based on Log-rank Mantel-Cox test, <0.005 for all adjuvants in comparison to eVLP alone) (B). Anti-GP titers from samples collected at various time points after vaccination and boost (p-value based on 2 way ANOVA in comparison to eVLP alone, * = p<0.05, ** = p<0.005, *** = p< 0.0005, **** = p<0.0001) (C-F).
Advax adjuvants provide durable VLP vaccine protection
It is theoretically possible that some adjuvants may enhance short term immune responses at the expense of long-term protective immunity. Hence, to assess the longevity of vaccine protection, mice were vaccinated on day 0 and day 21 with VLP and the selected adjuvants, and then rested for an additional 156 days prior to challenge. Differences in survival between the three adjuvant groups were not significant, with 100% survival in mice immunized with VLP with Advax-4 or pICLC adjuvant, 80% in mice with Advax-2 and only 10% in mice immunized with VLP alone (Figure 2B). Sera from animals that received either of the three adjuvants exhibited consistently higher IgG titers over the course of the durable vaccination schedule when compared to animals that received VLP alone, with significantly higher titers of IgG2c and IgG3 antibodies in the adjuvanted groups (Figure 2C–F).
Advax adjuvant enhances serum cytokine responses
We next examined the serum cytokine and chemokine response 6 hours and 24 hours after a single immunization. The VLP vaccine alone exhibited minimal effects on serum cytokine and chemokines at either 6 hours or 24 hours, with only significant increases in serum IL-6 and KCGRO at 6-hours. By contrast the 3 adjuvanted vaccine groups exhibited significant elevations in IFN-y, TNFα, IL6 and KCGRO. With respect to IFNγ, the pICLC adjuvant was associated with an increase at 6 hours whereas increases for Advax-2 and Advax-4 were seen at 24 hours, suggesting different kinetics of the cytokine responses with these different adjuvants. Advax-2 was the only adjuvant that exhibited significant increase in IL12 at 24 hours, mirroring the increased IFN-γ at the same time point in this group. We have previously observed peak serum cytokine response at 6 hours with pICLC and other adjuvant combinations [6], suggesting the Advax adjuvants might have a delayed effect on immune activation that does not occur till 24 hours after vaccination (Figure 3).
Figure 3.
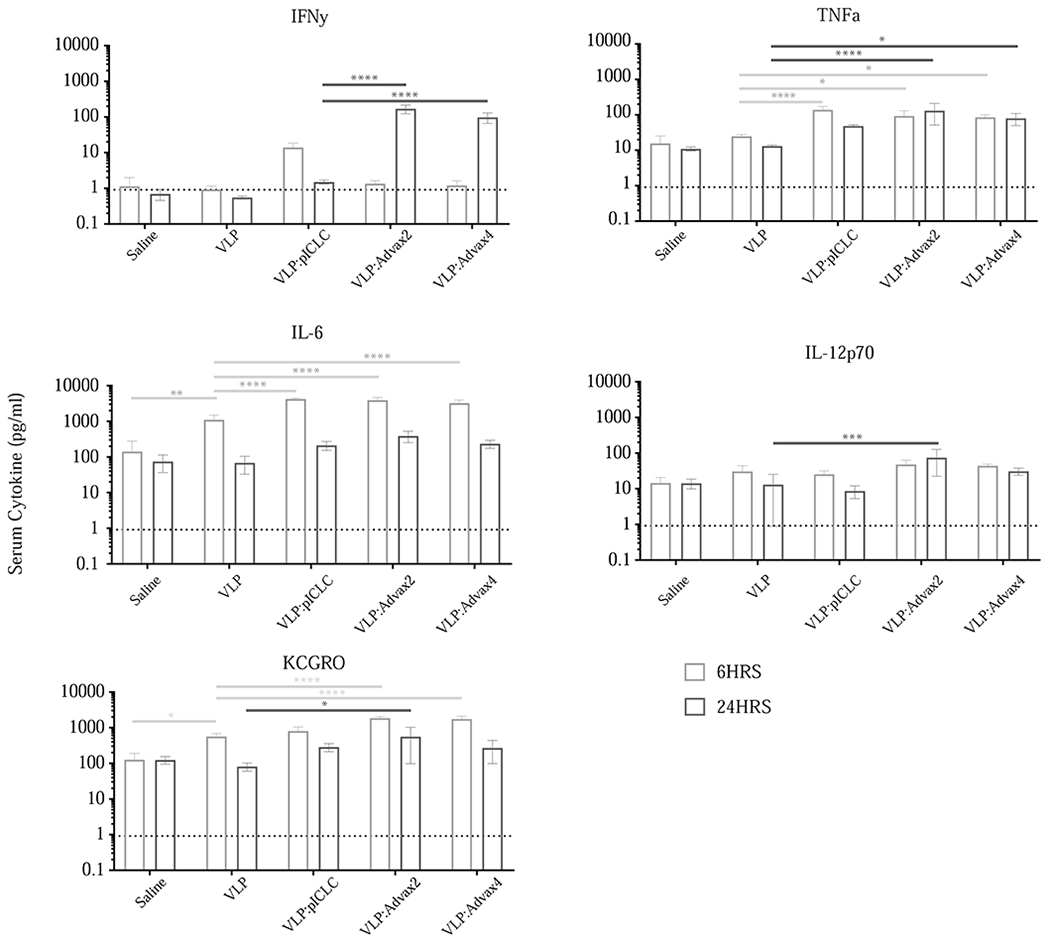
Cytokine and chemokine levels in sera 6 hours and 24 hours after vaccination. Replicates include at least 5 animals per group; 2 way ANOVA used to compare VLP alone with other treatment groups (* = p<0.05, ** = p<0.005, *** = p<0.0005, **** = p<0.0001).
Advax adjuvant increases the frequency of T follicular helper cells
Considering the robust antibody response seen after vaccination with all three adjuvants, we explored the impact of the adjuvants on T follicular cell (Tfh) production and germinal B cell frequency. We previously showed that VLP formulated with pICLC adjuvant elicited a strong induction of Tfh cell populations seven days after vaccination in the draining (popliteal) lymph node [7]. Similarly, in this study we observed that immunization with Advax-2 and -4 adjuvants just like pICLC significantly increased the frequency of Tfh cells in the draining popliteal lymph node when compared to vaccination with VLP alone (Figure 4). Germinal center B cells were measured in the draining popliteal and inguinal lymph nodes day 10 post-immunization and showed significant increases of germinal center B cells in animals immunized with Advax-4 and pICLC but not Advax-2, when compared to vaccination with VLP alone. Despite equally high serum antibody titers in animals receiving each of the 3 adjuvants (Figure 2), and equivalent levels of protection, the frequency of germinal center B cells was higher in the pICLC group than in either of the Advax-adjuvanted groups.
Figure 4.
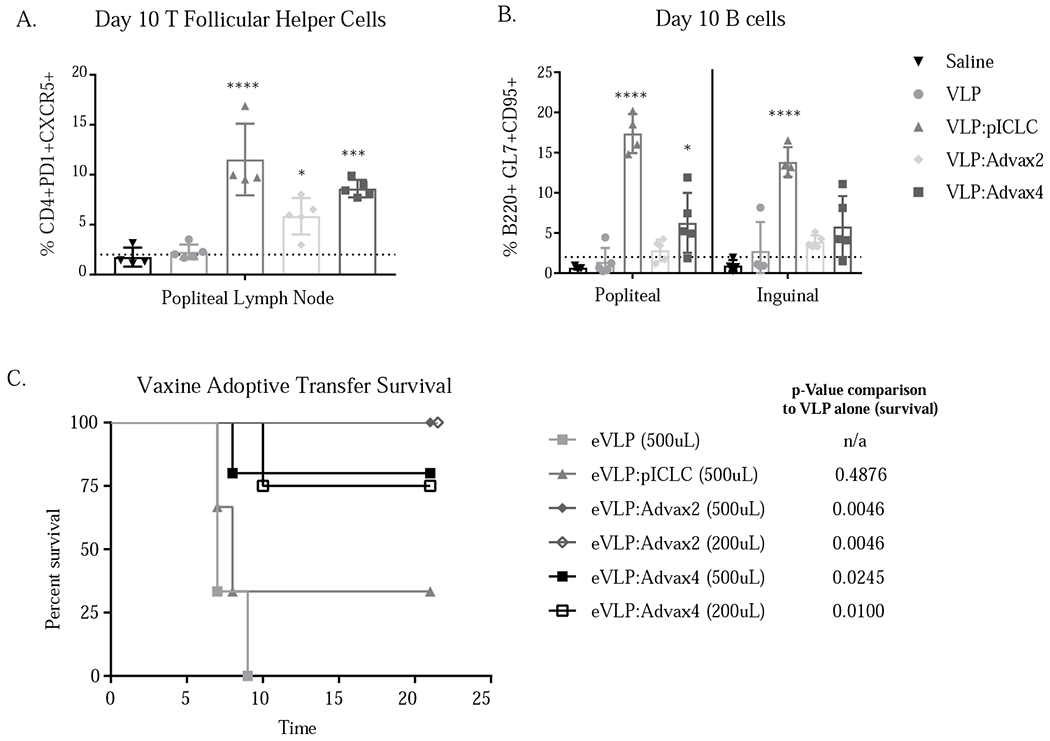
Adjuvants enhance development of T follicular helper cells (A) and germinal center B cells (B) after vaccination. One or two way ANOVA with multiple comparisons was used to compare adjuvanted groups to vaccination with eVLP alone (* = p<0.05, ** = p<0.005, *** = p<0.0005, **** = p<0.0001). Protection after adoptive transfer of sera from vaccinated animals. 500ul or 200ul of immune sera from Advax-2 or -4 or pICLC immunized animals was transferred to naïve C57BL/6 mice 24 hours prior to challenge with ma-EBOV; p-value based on Log-rank Mantel-Cox test (C).
Immune sera from Advax but not pICLC immunized mice transfers protection
A combination of CD4 T cells and immune sera from mice vaccinated with VLP and pICLC was shown to be required to transfer protection in a previous study [7]; in this study we sought to test whether immune sera alone from any of the groups was capable of conferring protection against Ebola virus. Remarkably, transfer of immune sera alone from animals vaccinated with VLP plus either Advax-2 or Advax-4 conferred protection from challenge in a dose-dependent fashion, with 500 μL or even 200 μL of sera from Advax-2 immunized mice providing 100% protection and 200-500 μL sera from Advax-4 immunized mice providing 75-80% protection. By contrast, even 500 μL sera from pICLC immunized mice did not transfer protection.
DISCUSSION
This study demonstrated that the combination of an Ebola VLP vaccine with Advax-2 or -4 adjuvant formulations resulted in a rapid and durable immune response that protected against a lethal Ebola challenge. Just a single dose of VLP vaccine when combined with Advax-2 or -4 adjuvant was sufficient to confer Ebola protection. While all three adjuvants provided similar levels of Ebola protection, interesting differences in their mechanisms of action emerged when the phenotypes of the vaccine responses were explored in more detail. All adjuvants induced high IgG titers with IgG2c as the dominant subtype. Similarly, all adjuvants were associated with a strong induction of T follicular helper (Tfh) cell populations seven days after vaccination in the draining lymph node. However, the frequency of germinal center B cells 10 days post-immunization was higher in the pICLC group than the Advax-4 group and was only marginally raised in the Advax-2 group. It was surprising, therefore, to find that immune sera from the Advax-2 immunized group was the most potent in transferring protection against Ebola to naïve mice, although sera from the Advax-4 immunized group also transferred high levels of protection. By contrast, immune sera from the pICLC immunized group conferred no protection, despite similar anti-Ebola GP antibody titers.
We can currently only speculate as to the immune mechanism underlying this difference between adjuvants. Notably the two Advax-adjuvanted vaccines that contained delta inulin plus CpG oligonucleotide, a TLR9 agonist, induced the highest levels of IFN-γ, indicating stimulation of Th1 immunity. By contrast the addition of the NOD2 agonist, murabutide, to Advax-4 did not appear to give any additional benefit. Advax adjuvant has previously been shown in human clinical trial subjects to increase B cell receptor affinity maturation in day 7 post immunization plasmablasts, which was associated with increased expression of activation induced cytidine deaminase[17], the enzyme controlling B cell affinity maturation. Advax-1 and -2 adjuvants were shown to enhance heterologous protection with a single dose of inactivated Japanese encephalitis virus vaccine protecting mice against a lethal West Nile virus challenge [18]. This enhanced heterologous protection was able to be transferred by memory B cells from the Advax-immunized mice. It therefore appears that Advax may help induce a memory B cell population that expresses B cell receptors better able to provide protection. Induction of extrafollicular memory B cells/plasmablasts by Advax formulations could help explain the high serum antibody levels seen in VLP immunized mice despite their low observed levels of germinal center B cells.
However, total antibody titers are only a crude measure and do not take account of differences in antibody quality, as reflected by avidity, epitope specificity and depth of the BCR repertoire against the relevant antigen. Notably, the pICLC adjuvant while inducing a similar quantity of antibody as the Advax adjuvants, failed to induce antibodies able to transfer Ebola protection. Previous studies showed pICLC-mediated VLP protection was dependent on the combination of both CD4 T cells and antibody [7]. A systematic analysis of monoclonal antibodies to GP antibodies showed neutralization and induction of multiple immune effector functions correlated most strongly with Ebola protection [26]. This suggests that the antibodies induced by the Advax-2 and -4 formulated vaccine that mediated protection did so via neutralization and/or by immune effector functions. Notably the two most effective adjuvant formulations contained a TLR9 agonist in addition to delta inulin. It is surprising that the antibodies induced by poly IC:LC adjuvanted VLP vaccine were not able to mediate protection, suggesting that the TLR3 agonist was inducing different types of anti-GP antibody.
Is a VLP vaccine against Ebola still needed? rVSV-ZEBOV is a vector based live, replication competent, attenuated, recombinant vesicular stomatitis virus (rVSV) chimeric virus vaccine in which the VSV glycoprotein gene has been replaced with the glycoprotein gene of Zaire Ebola virus. Over 20,000 people have received the rVSV-ZEBOV vaccine in clinical trials, which appeared to be highly protective when trialled in 2015 during the West African Ebola epidemic [27]. Whilst the rVSV-ZEBOV vaccine is a welcome advance, nevertheless, many questions remain, including the durability of its protection, its effectiveness in boosting protection in pre-immune individuals, and the vaccine’s safety in immune-suppressed individuals, infants and pregnant women. Although adverse reactions to date, including fever, headache, myalgia, arthralgia, rash and vesicular mucosal lesions have been considered acceptable, the possibility of more serious rare adverse events cannot be excluded until many more people have received the vaccine [28]. Hence, given the continued threat posed by Ebola and these ongoing unknowns with the rVSV-ZEBOV vaccine, it would seem sensible to continue with development of promising non-vector based vaccines, such as the Advax-adjuvanted VLP vaccine described herein, to provide alternative options should future problems be encountered with the rVSV-ZEBOV vaccine.
This study once again highlights the importance of availability of new adjuvants to ensure vaccines that provide maximal protection. Previously, it was reported a EBOVgp-Fc vaccine formulated with TLR3 agonist adjuvant provided 100% protection of guinea pigs against Ebola lethal challenge whereas the same vaccine formulated with QS-21 or alum adjuvants provided only partial protection [5]. This highlights that not all adjuvants provide the same benefits. It was previously shown that the most effective adjuvant for Ebola VLP vaccine elicited a Th1-skewed antibody response and strong CD4 T cell responses, including an increase in Tfh frequency [7]. Notably, this was also a feature for the successful Advax-2 and Advax-4 adjuvants, which both elicited Th1-skewed antibody, IFN-γ and Tfh, responses.
Notably, in the current study only the serum from the Advax-2 or -4 immunised mice was able to transfer protection, indicating not all adjuvants are the same. Adjuvant formulations equivalent to Advax-2 have already been produced under cGMP (current Good Manufacturing Practices), and shown to be safe in GLP toxicology studies and are currently being tested in an multicentre clinical trial of adjuvanted influenza vaccines (https://clinicaltrials.gov/ct2/show/NCT03945825). This advanced stage of development facilitates development of a VLP vaccine incorporating Advax-2 adjuvant for use in prevention of Ebola infection.
A deficiency in this study was our inability to directly measure the ability of immune sera to neutralise Ebola virus in vitro, with assays only available to measure GP antibody binding by ELISA. Nevertheless, the demonstration that immune sera from Advax-2 and Advax-4 immunized mice was able to directly transfer protection to naïve mice indicates that these antibodies had neutralizing or immune effector activity against Ebola in vivo.
In summary, this study showed that adjuvant-dependent factors shape Ebola VLP vaccine protection. Advax adjuvant formulations containing delta inulin and CpG uniquely induced production of antibodies able to transfer protection to naïve animals. This highlights the importance of study of the mechanisms of protection for each individual vaccine/adjuvant formulation. This study shows that adjuvants can shape and re-define the correlates of immunity, highlighting that careful adjuvant selection is vital to deliver the best protection.
Materials and Methods
Animals
C57BL/6 mice were obtained from Charles River (CR strain 027). Mice between 8 and 12 weeks of age were vaccinated with 100 μl via the intramuscular (IM) route in the caudal thigh. All mice in each study were female and age-matched and therefore were inherently randomized. C57BL/6J (Jackson Laboratory strain 000664) mice were used as controls. Animals were monitored at least once daily by technical staff members who were blinded to the study aims. Animal status was evaluated according to an Intervention Scoresheet approved by USAMRIID IACUC. Monitoring increased to at least two times daily at the onset of disease. Euthanasia was by CO2 inhalation followed by confirmatory cervical dislocation. Surviving animals were euthanized on day 14 post infection. For all survival studies, control groups included animals vaccinated with saline and/or adjuvant alone
Ethics Statement
Research was conducted under an IACUC approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. The IACUC committee approving this protocol is the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) IACUC. The facility where this research was conducted, USAMRIID, is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the 8th Edition of the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
Virus-like-particles (VLPs)
VUPs were produced by transfecting HEK293E cells with Ebola Zaire (Kikwit) virus glycoprotein (GP) and viral protein 40 (VP40) genes in pWRG expression vectors as previously described [29]. In brief, VLP supernatants were collected 3 days post transfection, purified via sucrose gradient and total protein concentration determined by BCA and western blot. VLPs were irradiated at 1e6 rad to guarantee sterility and contained less than 25 EU/ml endotoxin and less than 10 colony forming units of bacteria per vaccination. The VLPs GP content for these studies was determined via western blot and fixed to either a 1.25ug or 10μg GP dose per vaccination. VLPs were maintained at −80°C and diluted in sterile saline and/or combined with Advax adjuvants prior to vaccination.
Adjuvants
Advax adjuvants were provided by Vaxine Pty Ltd, Adelaide, Australia. Advax-1 comprised delta inulin alone (1mg/dose), Advax-2 comprised 1mg delta inulin plus 10μg CpG oligonucleotide (CpG), Advax-3 comprised 1mg delta inulin and 10μg murabutide and Advax-4 comprised 1mg delta inulin, 10μg CpG and 10μg murabutide. PolyIC/LC adjuvant was provided by Oncovir, Inc. Adjuvants were diluted with sterile saline and combined with VLPs prior to vaccination.
Vaccination and Infection
Vaccines were administered IM in the hind leg using prime (D0 vaccination with 1.5 μg VLP - D28 challenge), prime-boost (D0/D21 vaccination with 1.5 μg VLP - D49 Challenge) and durable (D0/D21 vaccination with 10 μg VLP– D177 Challenge) vaccination models. An infectious dose of 1000 pfu of mouse-adapted (ma-) Ebola virus was administered via the intraperitoneal (IP) route. The mouse model of Ebola virus challenge is a well-documented small animal model that has been used to evaluate various vaccines and therapeutics developed against fdoviruses [30]. In brief, the Zaire subtype of Ebola virus was serially passaged in progressively older suckling mice, eventually obtaining a plaque-purified virus that was lethal for mature, immunocompetent BALB/c and C57BL/6 inbred and ICR (CD-1) outbred mice. [30]
Adoptive Transfer Studies
C57BL/6 mice were vaccinated IM using a prime-boost model, with three weeks between vaccinations. Four weeks after the second vaccination, mice were deeply anesthetized, and serum was collected via cardiac puncture. Serum was injected IP into naïve recipient mice 24 hours before infection. Recipient mice were infected IP with 1000 pfu of ma-EBOV.
Enzyme-linked Immunosorbent Assays (ELISA)
ELISAs were performed as previously described [6]. In brief, blood was collected in BD Vacutainer serum-separating tubes at indicated time points. Plates were coated over night with 2 μg/ml of recombinant mammalian cell-expressed Ebola virus GP(rGP). Plates were incubated with blocking buffer (5% milk, 0.05% Tween in PBS) for 2 hours. Serum was added to plates starting at a 1:100 dilution, diluting the sample down the plate in half log dilutions. After 2 hours, plates were washed with PBS + 0.05% Tween and secondary antibody was added at 0.6 μg/ml. Secondary antibodies included goat anti-mouse IgG-HRP (Southern Biotech 1030–05) and IgG2c-HRP (Southern Biotech 1079–05). One hour later, plates were washed and exposed using Sure Blue TMB 1-component substrate and stop solution (KPL), the absorbance was read at 450 nm on a SpectraMax M5 (Molecular Devices). Serum from unvaccinated animals was used to establish background and titers were defined as the background plus 0.2. Pooled convalescent serum from previous studies was included in each assay as a positive control.
Serum Cytokine Analysis
Serum was evaluated using VPLEX Plus Proinflammatory Panel 1 (MSD K15048G) according to the manufacturer’s instructions. Briefly, cytokines IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, KCGRO, IL-10, IL-12p70, and TNF-α were measured using peripheral blood collected 3 days prior to vaccination, 6 hours and 24 hours post vaccination. VPLEX plates were washed three times prior to the addition of samples. Serum was diluted 2-fold, plated, and incubated at room temp with shaking for two hours. Plates were washed again, detection antibody solution was added, and plates were incubated at room temperature with shaking for two hours. Plates were washed, read buffer was added, and plates were analysed on the MSD Sector Imager.
Highlights for review.
Advax-2 or Advax-4 delta inulin/CpG combination adjuvants enhanced VLP vaccine protection of mice against lethal Ebola infection
Protection correlated with a robust Th1 T cell robust as well as high antibody titers
Immune sera from Advax immunised mice transferred robust protection to naïve recipients
The mechanism of filovirus vaccine protection varies dependent on the adjuvant used
Advax/CpG combination adjuvants represent promising clinical stage products for use in developing filovirus vaccines
Acknowledgments
Funding Sources
DTRA JSTO-CBD (CB10208). Development of Advax adjuvants was supported by funding from National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Contracts. HHS-N272201400053C, HHS-N272200800039C and U01-AI061142.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
NP is affiliated with Vaxine which holds commercial rights in Advax adjuvants. The remaining authors declare no conflict of interest. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Department of the Army or the US Department of Defense or the National Institutes of Health.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
NP is affiliated with Vaxine which holds proprietary interests in Advax adjuvants.
REFERENCES
- [1].Vignier N, Bouchaud O. Travel, Migration and Emerging Infectious Diseases. EJIFCC. 2018. November 7;29(3):175–179 [PMC free article] [PubMed] [Google Scholar]
- [2].Bernasconi V, Kristiansen PA, Whelan M, Román RG, Bettis A, Yimer SA, Gurry C,Andersen SR, Yeskey D, Mandi H, Kumar A, Holst J, Clark C, Cramer JP, Røttingen JA, Hatchett R, Saville M, Norheim G. Developing vaccines against epidemic-prone emerging infectious diseases. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020. January;63(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wing K, Oza S, Houlihan C, Glynn JR, Irvine S, Warrell CE, et al. Surviving Ebola: A historical cohort study of Ebola mortality and survival in Sierra Leone 2014-2015. PLoS One. 2018;13:e0209655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cooper CL, Martins KA, Stronsky SM, Langan DP, Steffens J, Van Tongeren S, et al. T-cell-dependent mechanisms promote Ebola VLP-induced antibody responses, but are dispensable for vaccine-mediated protection. Emerg Microbes Infect. 2017;6:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Konduru K, Shurtleff AC, Bradfute SB, Nakamura S, Bavari S, Kaplan G. Ebolavirus Glycoprotein Fc Fusion Protein Protects Guinea Pigs against Lethal Challenge. PLoS One. 2016;11:e0162446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Martins KA, Steffens JT, van Tongeren SA, Wells JB, Bergeron AA, Dickson SP, et al. Toll-like receptor agonist augments virus-like particle-mediated protection from Ebola virus with transient immune activation. PLoS One. 2014;9:e89735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martins KAO, Cooper CL, Stronsky SM, Norris SLW, Kwilas SA, Steffens JT, et al. Adjuvant-enhanced CD4 T Cell Responses are Critical to Durable Vaccine Immunity. EBioMedicine. 2016;3:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Warfield KL, Dye JM, Wells JB, Unfer RC, Holtsberg FW, Shulenin S, et al. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PLoS One. 2015;10:e0118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196 Suppl 2:S430–7. [DOI] [PubMed] [Google Scholar]
- [10].Cooper PD, Barclay TG, Ginic-Markovic M, Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23:1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology. 2011;21:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Petrovsky N, Cooper PD. Advax, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33:5920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Counoupas C, Pinto R, Nagalingam G, Britton WJ, Petrovsky N, Triccas JA. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci Rep. 2017;7:8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89:2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Honda-Okubo Y, Saade F, Petrovsky N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Larena M, Prow NA, Hall RA, Petrovsky N, Lobigs M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody. J Virol. 2013;87:4395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li L, Honda-Okubo Y, Li C, Sajkov D, Petrovsky N. Delta Inulin Adjuvant Enhances Plasmablast Generation, Expression of Activation-Induced Cytidine Deaminase and B-Cell Affinity Maturation in Human Subjects Receiving Seasonal Influenza Vaccine. PLoS One. 2015;10:e0132003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petrovsky N, Larena M, Siddharthan V, Prow NA, Hall RA, Lobigs M, et al. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87:10324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gordon D, Kelley P, Heinzel S, Cooper P, Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine. 2014;32:6469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gordon DL, Sajkov D, Honda-Okubo Y, Wilks SH, Aban M, Barr IG, et al. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax delta inulin adjuvant. Vaccine. 2016;34:3780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heddle R, Smith A, Woodman R, Hissaria P, Petrovsky N. Randomized controlled trial demonstrating the benefits of delta inulin adjuvanted immunotherapy in patients with bee venom allergy. J Allergy Clin Immunol. 2019;144:504–13 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davtyan H, Zagorski K, Rajapaksha H, Hovakimyan A, Davtyan A, Petrushina I, et al. Alzheimer’s disease Advax(CpG)-adjuvanted MultiTEP-based dual and single vaccines induce high-titer antibodies against various forms of tau and Abeta pathological molecules. Sci Rep. 2016;6:28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Honda-Okubo Y, Kolpe A, Li L, Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32:4651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Honda-Okubo Y, Ong CH, Petrovsky N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine. 2015;33:4892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, Donart N, et al. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29:6242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saphire EO, Schendel SL, Fusco ML, et al. Viral Hemorrhagic Fever Immunotherapeutic Consortium. Systematic Analysis of Monoclonal Antibodies against Ebola Virus GP Defines Features that Contribute to Protection. Cell. 2018. August 9;174(4):938–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet. 2017;389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Monath TP, Fast PE, Modjarrad K, Clarke DK, Martin BK, Fusco J, et al. rVSVDeltaG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine X. 2019;1:100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Swenson DL, Warfield KL, Kuehl K, Larsen T, Hevey MC, Schmaljohn A, et al. Generation of Marburg virus-like particles by co-expression of glycoprotein and matrix protein. FEMS Immunol Med Microbiol. 2004;40:27–31. [DOI] [PubMed] [Google Scholar]
- [30].Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis. 1998;178:651–61. [DOI] [PubMed] [Google Scholar]


