Abstract
An immunosuppressive microenvironment promoting leukemia cell immune escape plays an important role in the pathogenesis of AML. Through its interaction with cereblon, a substrate receptor for the E3 ubiquitin ligase complex, pomalidomide leads to selective ubiquitination of transcription factors Aiolos and Ikaros thereby promoting immune modulation. In this phase I trial, 51 newly diagnosed non-favorable risk AML and high-risk MDS patients were enrolled and treated with AcDVP16 (cytarabine 667 mg/m2/day IV continuous infusion days 1–3, daunorubicin 45 mg/m2 IV days 1–3, etoposide 400 mg/m2 IV days 8–10) induction therapy followed by dose- and duration-escalation pomalidomide beginning at early lymphocyte recovery. Forty-three patients (AML: n = 39, MDS: n = 4) received pomalidomide. The maximum tolerated dose of pomalidomide was 4 mg for 21 consecutive days. The overall complete remission (CR + CRi) rate, median overall survival, and disease-free survival were 75%, 27.1 and 20.6 months, respectively. Subset analyses revealed 86% CR/CRi rate in AML patients with unfavorable-risk karyotype treated with pomalidomide. Pomalidomide significantly decreased Aiolos expression in both CD4+ and CD8+ peripheral blood and bone marrow T cells, promoted T cell differentiation, proliferation, and heightened their cytokine production. Finally, pomalidomide induced distinct gene expression changes in immune function-related ontologies in CD4+ and CD8+ T cells.
Introduction
Overall survival (OS) has improved steadily for patients with acute myeloid leukemia (AML) over the past several decades, due in large part to advancements in supportive care and refinement of allogeneic stem cell transplantation (alloSCT) [1]. Even so, induction chemotherapy consisting of continuous infusion (CI) cytarabine plus an anthracycline, with or without additional chemotherapeutics, produces durable complete remission (CR) in less than half of AML patients [2]. Timed sequential induction therapy (TST) has been associated with improved disease-free survival (DFS) in the newly diagnosed younger AML patients [3–6]. However, OS following TST appears to be comparable to other conventional induction chemotherapy regimens, suggesting that chemotherapy intensification alone cannot effectively address leukemia cell persistence following treatment [6].
Innate and adaptive immune system aberrations that promote immune escape of AML cells occur at diagnosis and persist through disease progression [7, 8]. CD8+ T cells in AML express co-inhibitory and senescence markers suggesting multiple pathways of T cell dysfunction [9–11]. Our recent studies demonstrate the reversibility of the phenotypic and transcriptional signatures of CD8+ T cells in patients who achieve CR in contrast to nonresponders following induction chemotherapy [9]. Regulatory T cells (Tregs) are also increased in the peripheral blood (PB) and the bone marrow (BM) of AML patients [12–14], exhibit potent immunosuppressive effects on T-effector cells [14], and are minimally affected by chemotherapy [15]. Moreover, increased levels of Tregs appear to be associated with worse outcomes in AML [13]. In the context of induction TST, our group demonstrated that early lymphocyte recovery (ELR), which customarily occurs between days 14 and 21 of TST, is dominated by an expanded oligoclonal population of peripherally-derived Tregs [16]. These data support the idea that modulating immune environment early after treatment might be of benefit in promoting response to induction chemotherapy.
Immunomodulatory drugs have been shown to have protean effects on the immune system such as inhibiting the proliferation and function of Tregs [17], potentiating T cell activity [18, 19], and repairing defective immune synapse formation on T cells [20–22]. Pomalidomide is a potent IMiD with established efficacy in multiple myeloma (MM), including in those patients who progressed on lenalidomide therapy [23]. Through interaction with cereblon, a substrate receptor for the E3 ubiquitin ligase complex, pomalidomide leads to the selective ubiquitination and degradation of two transcription factors, Ikaros (IKZF1) and Aiolos (IKZF3), thereby increasing IL-2 production [24]. Our observations of temporally predictable Treg expansion following TST coupled with the recognition of multifaceted immune dysfunction in AML led us to the hypothesis that pomalidomide during ELR might modulate the immune environment in AML and synergize with induction chemotherapy. To explore our hypothesis, we conducted a phase 1 dose escalation clinical-translational trial to investigate the safety and activity of pomalidomide at ELR through early hematopoietic recovery after induction TST in newly diagnosed AML.
Methods
Study population
Adult patients 18–65 years with pathologically confirmed untreated AML or high-risk MDS (>10% blasts and Intermediate-2/high-risk) were eligible for this study (ClinicalTrials.gov NCT02029950). Patients with favorable-risk cytogenetics (i.e., t(8;21); inv(16); t(16;16)) and acute promyelocytic leukemia were excluded. Detailed eligibility criteria are outlined in Supplementary Information. The study was conducted in accordance with the Declaration of Helsinki after approval by the ethics committee of each participating center. Informed consent was obtained from all subjects prior to participation.
Treatment plan
All patients received induction therapy with AcDVP16 (cytarabine 667mg/m2/day CI IV days 1–3, daunorubicin 45 mg/m2 IV days 1–3 (or idarubicin 8 mg/m2 during daunorubicin shortage), and etoposide 400 mg/m2 IV days 8–10). Pomalidomide was administered orally at the time of ELR, after day 14 of induction therapy and within 3 days of WBC > 0.2 × 109/L above nadir, but no later than day 30 of induction therapy (Fig. 1a). All patients received antimicrobial prophylaxis according to institutional standards. Intrathecal prophylaxis was administered according to the institutional standards and was required to be >3 days prior to initiating pomalidomide. Growth factors were not permitted during induction.
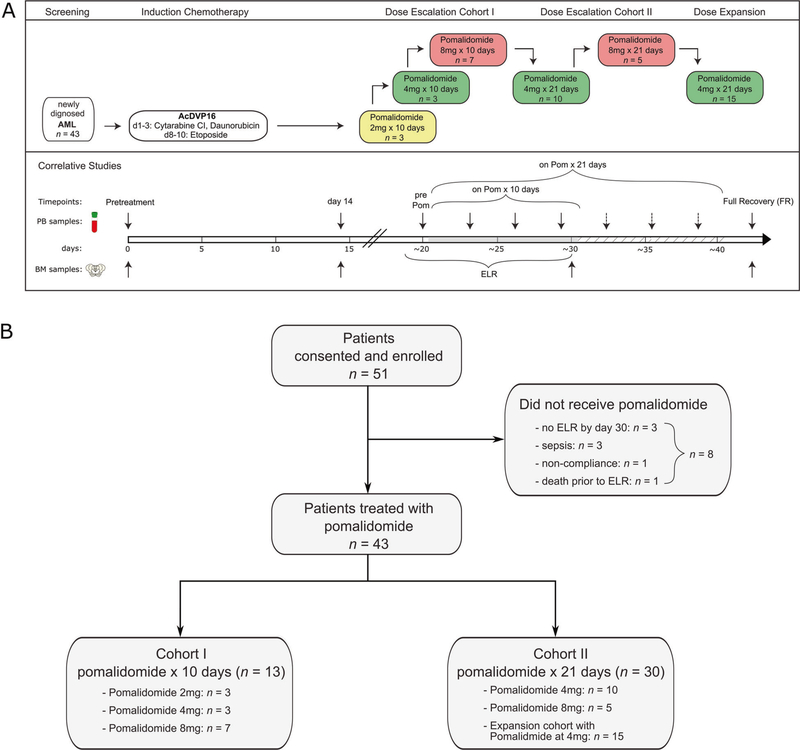
Fig. 1. Study schema and survival outcomes.
a All patients received induction therapy AcDVP16, followed by pomalidomide (POM) at the time of early lymphocyte recovery (ELR), after day 14 and within 3 days of WBC > 0.2 × 109/L above nadir, but no later than day 30 of induction therapy. Dose escalation of pomalidomide during ELR occurred in two cohorts. Cohort 1 consisted of pomalidomide duration of 10 consecutive days and Cohort 2 consisted of pomalidomide duration of 21 consecutive days. PB and BM samples for laboratory-correlative studies were collected at pretreatment, on day 14 after receiving induction chemotherapy, at the time of ELR (before start of pomalidomide treatment), every 3–4 days during pomalidomide treatment and at full recovery after treatment (FR). b Consort Diagram. A total of 51 patients were consented and enrolled on this study while 43 patients received planned pomalidomide. Cohort 1 (n = 13) consisted of pomalidomide in dose escalation for 10 consecutive days, while Cohort 2 (n = 30) consisted of pomalidomide in dose escalation for 21 consecutive days with planned dose expansion of 15 patients.
Patients who achieved CR/CRi were eligible to receive consolidation therapy with high dose cytarabine (HiDAC: ≤60 years: cytarabine 3 gm/m2 IV every 12 h days 1, 3, and 5; >60 years: cytarabine 1.5 gm/m2 IV every 12 h days 1, 3, and 5) for 1–4 cycles; or TST AcDAc (cytarabine 667 mg/m2/day IV CI days 1–3 and 10–12, daunorubicin 45 mg/m2 IV days 1–3) for 1 cycle. Maintenance phase consisted of pomalidomide 4 mg orally daily for 21 days every 4–6 weeks for 4 cycles in those who achieved CR/CRi in induction, completed consolidation therapy, and had absolute neutrophil count (ANC) ≥ 1.0 × 109/L and platelet count ≥ 50 × 109/L without transfusion support. Patients who received alloSCT were not eligible for maintenance phase.
Study design and safety assessments
Dose escalation of pomalidomide (2–8 mg) during ELR occurred in two cohorts (10 days versus 21 days), Fig. 1a. Dose escalation parameters are outlined in Supplementary Information. An expansion cohort of 15 patients was planned at the maximum tolerated dose (MTD) in cohort 2. AEs were graded by NCI Common Terminology Criteria for AEs version 4.0. Dose-limiting toxicity (DLT) definitions are outlined in Supplementary Information.
Response assessment
A BM aspirate and biopsy was performed at the time of hematologic recovery but no later than day 52 from the start of induction therapy. Response criteria were consistent with standard guidelines [25]. In the maintenance phase, BM aspirate and biopsy was performed prior to cycle 1, day 14 of cycle 4, and every 3–4 months until 1 year from the start of therapy.
Correlative studies and methods (Supplementary Table S1) are described in the Supplementary Information.
Statistical analysis
The primary objective of this phase 1 study was to determine the MTD of pomalidomide during ELR following AcDVP16 induction therapy. Secondary objectives included safety, tolerability, and toxicity of maintenance phase pomalidomide, DFS, as defined by time from CR/CRi to relapse, death or last follow up, and event-free survival (EFS), as defined by time from treatment to no response, relapse, death, or last follow up, and OS. Time to event outcomes were estimated with the Kaplan–Meier method. Median follow-up for the whole cohort was estimated using the reverse Kaplan–Meier method. Differences in time to event outcomes according to patient subgroups were explored using logrank tests. Statistical analysis plan of correlative studies is presented in Supplementary Information.
Results
Patient characteristics
Between January, 2014 and December, 2017, 51 patients were enrolled and 43 were treated with escalating doses of pomalidomide (2–8 mg) for 10 (Cohort 1) or 21 days (Cohort 2) at the time of ELR following TST with AcDVP16. Eight (16%) patients were consented and treated with AcDVP16 induction but did not receive pomalidomide due to no ELR by day 30 (n = 3), sepsis (n = 3), noncompliance with treatment (n = 1), and death prior to ELR (n = 1) (Fig. 1b). Clinical demographics and patient characteristics of the 51 enrolled patients are shown in Table 1. Overall, four (8%) patients had high-risk MDS (refractory anemia with excess blasts), one (2%) had chronic myelomonocytic leukemia-2 (CMML-2) with 18% blasts and did not receive treatment with pomalidomide due to no ELR, and 46 (90%) had AML. Twenty-one (41%) and seventeen (33%) patients had unfavorable and adverse-risk based on SWOG cytogenetic classification [26] and European Leukemia Net (ELN) Classification [27], respectively. Targeted next-generation sequencing was performed locally on PB or BM in 31/43 (72%) patients treated with pomalidomide (Supplementary Fig. S1).
Table 1.
Demographics and baseline characteristics.
| Patient characteristics | High-risk MDSa | AMLa | Overall pom-treated | Not treated with pom | Overall evaluable |
|---|---|---|---|---|---|
| (n = 4) | (n = 39) | (n = 43) | (n = 8) | (n = 51) | |
| Age-median (range) | 50 (35–63) | 54 (21–65) | 54 (21–65) | 61 [46–64] | 54 (21–65) |
| Age > 60 years | 1 (25%) | 11 (28%) | 12 (28%) | 5 (63%) | 17 (33%) |
| Male | 2 (50%) | 16 (41%) | 18 (42%) | 1 (13%) | 19 (37%) |
| Female | 2 (50%) | 23 (59%) | 25 (58%) | 7 (88%) | 32 (63%) |
| Bone marrow blast %-median (range) | 17% (15–18%) | 55% (11b–95%) | 54% (11–95%) | 53% (18c–70%) | 54% (11–95%) |
| Peak WBC-median (range) | 3.1 (0.9–5.3) | 12.7 (1.0–227.3) | 6.1 (0.9–227.3) | 14.7 (2.6–64.9) | 6.9 (0.9–227.3) |
| Secondary AML | N/A | 14 (36%) | 14 (33%) | 3 (38%) | 17 (33%) |
| AML with MDS-related changesd | N/A | 13 (33%) | 13 (33%) | 4 (50%) | 17 (37%) |
| ELN classification | |||||
| Favorable | N/A | 9 (23%) | 9 (23%) | 1 (13%) | 10 (20%) |
| Intermediate | N/A | 17 (44%) | 17 (44%) | 2 (25%) | 19 (37%) |
| Adverse | N/A | 13 (33%) | 13 (33%) | 4 (50%)e | 17 (33%) |
| SWOG cytogenetics Classification |
|||||
| Favorable-risk | 0 | 0 | 0 | 0 | 0 |
| Intermediate-risk | 1 (25%) | 23 (59%) | 24 (56%) | 3 (38%) | 27 (53%) |
| Unfavorable-risk | 3 (75%) | 14 (36%) | 17 (40%) | 4 (50%) | 21 (41%) |
| Unknown | 0 | 2 (5%) | 2 (5%) | 1 (13%) | 3 (6%) |
| NPM1 mutation | 0 | 8 (21%) | 8 (19%) | 1 (13%) | 9 (18%) |
| FLT3-ITD mutation | 0 | 4 (10%) | 4 (9%) | 1 (13%) | 5 (10%) |
| FLT3-TKD mutation | 1 (25%) | 4 (10%) | 5 (12%) | 1 (13%) | 6 (12%) |
| Site | |||||
| Johns Hopkins | 1 (25%) | 23 (59%) | 24 (56%) | 2 (25%) | 26 (51%) |
| University of North | 1 (25%) | 10 (26%) | 11 (26%) | 6 (75%) | 17 (33%) |
| Carolina | |||||
| Yale | 2 (50%) | 6 (15%) | 8 (19%) | 0 | 8 (16%) |
Pomalidomide-treated patients
1 patient had 11% blasts in the bone marrow but >20% blasts in the peripheral blood
1 patient had CMML-2 who was not treated with Pomalidomide due to not achieving ELR
AML with MDS-Related Changes were defined by preexisting MDS and/or MDS-related cytogenetic abnormalities [28]
1 patient with CMML-2 was not classified by ELN AML Risk
Dose determination and safety
Pomalidomide was initiated at the time of ELR, median day 21, of AcDVP16 induction therapy (range: 15–30 days). Grade ≥3 non-hematologic toxicities related to pomalidomide across each dose level are shown in Table 2. There were no DLTs seen in the first 3 dose levels of cohort 1 (2 mg, 4 mg, and 8 mg for 10 days) and dose level 2 of cohort 2 (4 mg for 21 days) in the first stage. Upon escalation to 8 mg for 21 days, there were 2 DLTs: grade 3 ALT/AST increase, and grade 4 respiratory failure. Thus, 4 mg for 21 days was considered the MTD and dose expansion for 15 patients occurred at this dose level. The most common non-hematologic grade 1–2 toxicities related to pomalidomide (Supplementary Table S2) were rash (16%), increased ALT/AST (14%), mucositis (14%), and fever (12%). Overall, 13 (30%) patients developed a rash (predominantly maculopapular; grade 3: n = 6, grade 2: n = 4, grade 1: n = 3) that was temporally associated with pomalidomide exposure. Overall 30-day and 60-day mortality was 2% (one patient died of acute respiratory distress syndrome (ARDS) after AcDVP16 and never received pomalidomide) and 4% (one patient died due to residual disease), respectively.
Table 2.
Non-hematologic Grade ≥3 toxicities possibly related to Pomalidomide.
| Adverse event | 2 mg × 10 days (n = 3) |
4 mg × 10 days (n = 3) |
8 mg × 10 days (n = 7) |
4 mg × 21 days (n = 25) |
8 mg × 21 days (n = 5) |
|---|---|---|---|---|---|
| Infectious | |||||
| Febrile neutropenia | 1 | 3 | 5 | 1 | |
| Lung infection | 1 | ||||
| Sepsis | 1 | ||||
| Electrolyte abnormalities | |||||
| Hypokalemia | 1 | ||||
| Hepatic | |||||
| ALT elevation | 1 | 1 | |||
| AST elevation | 1 | ||||
| Pulmonary | |||||
| Hypoxia | 1 | ||||
| Respiratory failure | 1 | ||||
| Renal | |||||
| Acute kidney injury | 1 | ||||
| General | |||||
| Fatigue | 1 | ||||
| Maculo-papular rash | 5 | 1 |
Pomalidomide was discontinued early in 14 (33%) patients due to: disease progression (n = 4), grade 3 rash (n = 3), grade 2 acute kidney injury, and grade 3 lung infection (n = 1), DLT (n = 2: grade 4 respiratory failure, grade 3 ALT/AST increase), patient decision (n = 2), falling blood counts with fever (n = 1), and grade 4 acute kidney injury (n = 1).
In those achieving CR/CRi with AcDVP16 followed by pomalidomide, median time to ANC ≥ 1.0 × 109/L and platelet ≥ 100 × 109/L recovery was 38 days (range: 26–86 days) and 33 days (range: 24–75 days) of AcDVP16 induction, respectively. In contrast, median time to full neutrophil and platelet recovery was 42 days (range: 26–54 days) and 33 days (range: 26–48 days), respectively, for those who achieved CR/CRi with AcDVP16 alone and did not receive pomalidomide on study.
Clinical activity
Overall, 38 (75%) patients achieved an overall CR (CR: n = 35, CRi: n = 3) as outlined in Table 3. Among those treated with pomalidomide, 77% (MDS: 3/4 = 75%; AML: 30/39 = 77%) achieved CR/CRi. Five out of eight (63%) patients who did not receive pomalidomide achieved CR/CRi. Of the 33 CR/CRi patients who received pomalidomide, 19 (58%) were determined to have no evidence of disease by institutional minimal residual disease testing (i.e., flow cytometry, FISH, cytogenetics, and/or PCR). Overall CR/CRi rates among adverse-risk by ELN [27] (AML-only) and SWOG [26] criteria were 71 and 76%, respectively. Overall CR/CRi rates were similar between <60 versus ≥60 years (74 and 76%, respectively). Overall CR/CRi rate among those with AML with MDS-related changes (MRC), as defined by the 2016 World Health Organization [28], was 76% (85% among those treated with pomalidomide).
Table 3.
Summary of efficacy data, predictors of response, and comparison to historical controls.
| Response characteristics | MDS (n = 4) | AML (n = 39) | Overall pom-treated (n = 43) | Not treated with pom (n = 8) | Overall evaluable (n = 51) |
|---|---|---|---|---|---|
| CR | 3 (75%) | 28 (72%) | 31 (72%) | 4 (50%) | 35 (69%) |
| CRi | 0 | 2 (5%) | 2 (5%) | 1 (13%) | 3 (6%) |
| Overall CR | 3 (75%) | 30 (77%) | 33 (77%) | 5 (63%) | 38 (75%) |
| Overall CR subgroups | |||||
| Secondary AML | N/A | 10/14 (71%) | 10/14 (71%) | 2/3 (67%) | 12/17 (71%) |
| AML with MDS-related changes | N/A | 11/13 (85%) | 11/13 (85%) | 2/4 (50%) | 13/17 (76%) |
| <60 years | 2/3 (67%) | 21/28 (75%) | 23/31 (74%) | 2/3 (67%) | 25/34 (74%) |
| >60 years | 1/1 (100%) | 9/11 (82%) | 10/12 (83%) | 3/5 (60%) | 13/17 (76%) |
| ELN-risk (2017) [27] | |||||
| Favorable | N/A | 9/9 (100%) | 9/9 (100%) | 1/1 (100%) | 10/10 (100%) |
| Intermediate | N/A | 12/17 (71%) | 12/17 (71%) | 1/2 (50%) | 13/19 (68%) |
| Adverse | N/A | 9/13 (69%) | 9/13 (69%) | 3/4 (75%) | 12/17 (71%) |
| SWOG cytogenetics risk [26] | |||||
| Favorable | N/A | N/A | N/A | N/A | N/A |
| Intermediate | 1/1 | 16/23 (70%) | 17/25 (68%) | 3/3 (100%) | 20/28 (71%) |
| Unfavorable | 2/3 (67%) | 12/14 (86%) | 14/17 (82%) | 2/4 (50%) | 16/21 (76%) |
| Unknown | N/A | 2/2 (100%) | 2/2 (100%) | 0/1 (0) | 2/3 (67%) |
| 4 mg × 21 days (MTD) | 3/4 (75%) | 15/21 (71%) | 18/25 (72%) | N/A | 18/25 (72%) |
| AML patient characteristics | AcDVP16 historical controls n = 301 (6) |
AcDVP16 + pomalidomide n = 39 |
|||
| Median age (range) | 52 (20–74) | 54 (21–65) | |||
| Overall CR/CRi | 205/301 (68%) | 30/39 (77%) | |||
| Age ≥ 60 years | 45/79 (57%) | 9/11 (82%) | |||
| Secondary AML | 51/96 (53%) | 10/14 (71%) | |||
| Cytogenetic classification | |||||
| Intermediate | 133/180 (74%) | 16/23 (70%) | |||
| Unfavorable | 49/94 (52%) | 12/14 (86%) | |||
| Non-favorable | 182/274 (66%) | 28/37 (76%)a | |||
| Allogeneic SCT | 98/301 (33%) | 23/39 (59%) | |||
| Median OS | 17.2 months | 33.8 months | |||
| Median OS-unfavorable-risk cytogenetics | 8.2 months | 19.7 months | |||
| Median DFS | 15.0 months | 27.1 months | |||
| Median DFS-unfavorable-risk cytogenetics | 9.2 months | 8.2 months | |||
2 patients had unknown cytogenetic risk per SWOG classification and were thus excluded for comparison to overall non-favorable risk
HiDAC consolidation was administered to 25/38 (66%) CR/CRi patients (median = 2 cycles; range: 1–4 cycles). One patient received AcDAc consolidation chemotherapy and died during aplasia due to disseminated fusarium. Twenty-eight (55%) patients (23/38 CR/CRi patients) received an alloSCT after HiDAC consolidation (n = 13), directly after induction therapy (n = 9), or after subsequent salvage therapy (n = 6; including one patient who never received pomalidomide) with median time from last day of pomalidomide to alloSCT 100 days (range: 34–372 days).
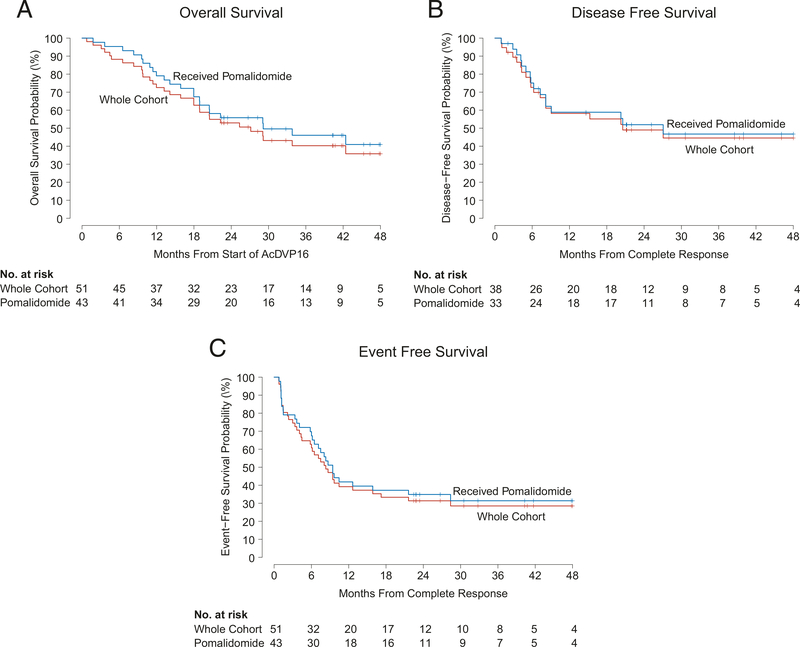
The median OS, DFS, and EFS were 27.1 months (Fig. 2a), 20.6 months (Fig. 2b), and 8.3 months (Fig. 2c), respectively, with a median follow-up of 41.7 months for all patients enrolled. Two-year OS, DFS, and EFS were 53%, 49%, and 31%, respectively. Of the patients treated with AcDVP16 + pomalidomide (n = 43), median OS, DFS, and EFS were 33.8 months, 27.1 months, and 9.4 months, respectively. Overall, 19 (50%) patients relapsed by the last database cut-off. Thirty (57%) patients died due to: leukemia-related complications (n = 25), infection post-alloSCT (n = 2), infection during consolidation (n = 2), and ARDS during induction (n = 1).
Fig. 2. Survival outcomes.
a Median overall survival (OS) was 27.1 months (95% CI: 18.9, N/A) versus 33.8 months (95% CI: 20.5, N/A) for the whole cohort and pomalidomide-treated patients, respectively. b Median disease-free survival (DFS) was 20.6 months (95% CI: 8.2, NA) versus 27.1 months (95% CI: 8.2, N/A) for the whole cohort and pomalidomide-treated patients, respectively. c median event-free survival (EFS) was 8.3 months (95% CI: 6.0, 17.2) versus 9.4 months (95% CI: 7.1, NA) for the whole cohort and pomalidomide-treated patients, respectively.
Five patients were found to have persistent AML (median: day 28 of induction; range: day 27–34; median BM blasts: 16%; range: 5–49%) while receiving pomalidomide prior to hematologic recovery and ultimately achieved a CR (n = 4) or CRi (n = 1) at full recovery after completing pomalidomide 4 mg for 21 days without additional therapy. Three of these patients relapsed after median 190 days, whereas the other two patients remained in CR for 1.8 years with ongoing CR, and 14.9 months (died of septic shock post-alloSCT), respectively.
Maintenance pomalidomide
Seven (16%) patients received maintenance pomalidomide after completing consolidation chemotherapy (median = 4 cycles; range: 1–4; Supplementary Table S3). Dose reductions occurred in 4/7 (57%) patients, 1 of whom began at a dose reduction (2 mg) due to pomalidomide-induced grade 3 AST/ALT during induction. Dose reductions were due to: grade 3 neutropenia (n = 2), grade 3 thrombocytopenia (n = 1), and grade 3 ALT during induction (n = 1). One patient required two dose reductions to 1 mg daily due to grade 3 and subsequently grade 4 neutropenia. Pomalidomide was discontinued early due to: relapsed disease (n = 2), periorbital edema (n = 1), and lower extremity edema (n = 1).
Pharmacodynamics and biomarker correlates
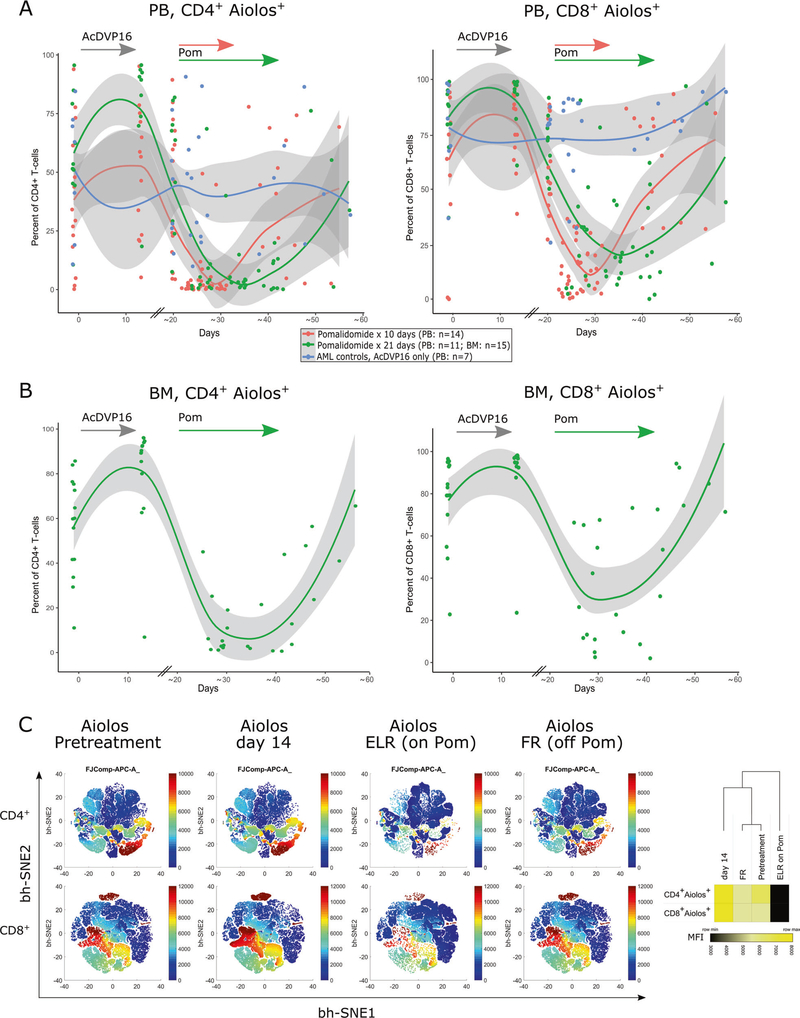
Pomalidomide has direct effects on T cells by promoting the ubiquitination and degradation of IL-2 transcriptional repressors such as Aiolos and Ikaros [24, 29]. We observed a significant decrease in Aiolos expression in both PB CD4+ and CD8+ T cells by flow cytometry (n = 25) during pomalidomide administration at ELR, compared with AML controls receiving chemotherapy only (n = 7) (p < 0.0001) (Fig. 3a). The Aiolos expression returned to baseline within a week after pomalidomide was stopped. Similar kinetics were observed in BM CD4+ and CD8+ T cells of pomalidomide-treated patients (p < 0.0001) (Fig. 3b, c) suggesting that measurement of Aiolos expression could serve as a pharmacodynamic biomarker of pomalidomide’s in vivo effect. Aiolos expression in T cells at diagnosis or at the time of ELR (before pomalidomide administration) was not predictive of response. The degree of Aiolos inhibition during pomalidomide treatment also did not significantly differ between subsequent responders and nonresponders to therapy though both analyses were limited by small numbers of nonresponders (data not shown).
Fig. 3. Aiolos expression in T cells as a pharmacodynamic biomarker of in vivo pomalidomide effect.
Aiolos staining and analysis by flow cytometry was performed on paired CD4+ or CD8+ T cell samples (PB, n = 25; BM, n = 15). Fixed timepoints included a pretreatment, day 14 and a pre-pomalidomide sample at beginning of ELR (around day 20, PB only). PB samples during pomalidomide were drawn every 3–4 days thereafter (for BM only once) until full recovery. The data were summarized in dot plot and nonlinear regression was calculated in R, using a LOESS curve fitting model. a PB samples, 14 patients received pomalidomide for 10 days (red dots/line) and 11 patients for 21 days (green dots/line). PB samples from 7 AML controls who received induction chemotherapy (AcDVP16) only, but not pomalidomide (blue dots/line). AML controls are lacking day 14 sample and modeled curves have to be interpreted accordingly. b For BM samples, only the AML patient group receiving pomalidomide for 21 days was analyzed (n = 15). c bidimensional maps obtained from flow cytometric data using the bh-SNE algorithm depicting serial Aiolos expression on BM CD4+ and CD8+ T cells of the 15 patients presented in (b). Each point in the map represents an individual cell, and the cells are colored according to the intensity of expression of individual markers, as indicated on the color scale to the right of individual maps. Heatmap (right) represents the median fluorescence intensity (MFI) of Aiolos expression in CD4+ and CD8+ T cells at different timepoints. Heatmap and clustering was done using Morpheus software (Broad Institute) using Euclidean distance and average linkage method.
We next examined the effects of pomalidomide on T cell differentiation, activation/proliferation, and ability to produce cytokines. We used CD45RA and CCR7 to distinguish between the four major T cell maturation states: naïve (TN), central memory (TCM), effector memory (TEM) and terminally differentiated EM (TEMRA) T cells. Since we did not observe any significant pomalidomide dose-dependent effect on T cells and due to limited number of samples at 2 mg and 8 mg dose, we combined all pomalidomide flow cytometric measurements together in a multivariable linear regression model. During pomalidomide treatment the percentage of TEM significantly increased in both PB and BM CD4+ and CD8+ T cells (Supplementary Fig. S2A–B). The increased percentage of TN and TCM was only observed among BM T cells. Notable was the significant decrease in the percentages of both PB and BM CD4+ and CD8+ TEMRA cells in pomalidomide-treated patients, populations known to be enriched for senescent and exhausted T cells [9]. Further, pomalidomide treatment was associated with significant increase in Ki67 expression on both PB and BM CD4+ and CD8+ T cells, suggesting that pomalidomide promotes T cell proliferation.
In order to functionally characterize T cells, we examined cytokine production upon PMA/ionomycin in vitro stimulation of serially collected PB and BM T cells. The changes in cytokine expression while on pomalidomide were most significant for BM CD4+ T cells, specifically increase in IL-2 (p = 0.006) and IFN-γ production (p = 0.003) (Supplementary Fig. S3A). Similar trends of increase in IL-2 and TNFα production were observed for BM CD8+ T cells, and TNFα for PB CD4+ and CD8+ T cells (Supplementary Fig. S3B). However, the analysis of the simultaneous cytokine expression revealed that PB CD4+ T cells had heightened polyfunctionality during pomalidomide treatment compared with PB CD4+ T cells collected from AML chemotherapy controls during ELR (Supplementary Fig. S3C). At the time of full recovery, the cytokine pattern of CD4+ T cells was similar to baseline for both groups. The bidimensional map on Aiolos and cytokine expression by PB CD4+ T cells (Supplementary Fig. S3D) using the bh-SNE algorithm shows that during pomalidomide treatment there is a decrease in Aiolos and increase in cytokine expression; but T cell clusters secreting Aiolos and individual cytokines differ.
Further, assessment of total numbers of respective flow cytometric T cell subsets corroborated these data whereby significantly higher numbers of Ki67+ and IL-2+ T cells and lower numbers of Aiolos+ T cells and Tregs were seen in pomalidomide-treated patients during ELR compared with AML controls (Supplementary Fig. S4). Overall, our data indicate that pomalidomide impacts T cell differentiation, proliferation, and cytokine production during ELR.
Effect of pomalidomide on T cell gene expression profiles
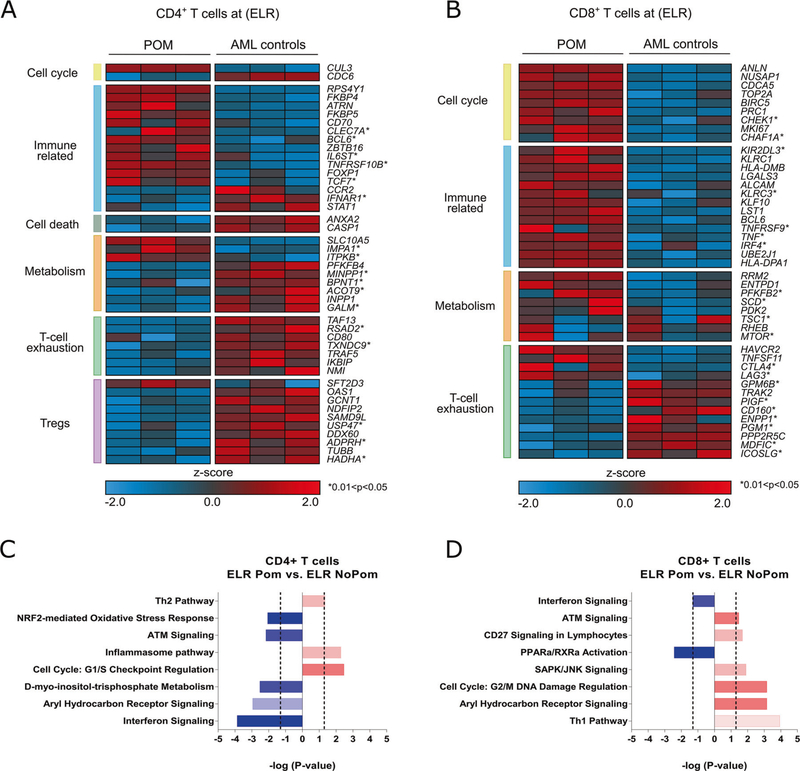
Testing our hypothesis that pomalidomide may also induce unique changes in T cell gene expression in vivo, we compared transcriptional profiles of sorted PB CD4+ and CD8+ T cells collected from AML patients while receiving pomalidomide to age-matched AML chemotherapy controls during ELR. We used the Human Prime View Gene Expression Array (Affymetrix) and selected top differentially expressed genes (DEGs) based on >2 fold change (FC) between pomalidomide-treated patients (n = 3) versus AML controls (n = 3). Using P < 0.01, we identified a total of 248 and 374 DEGs, in CD4+ and CD8+ T cells, respectively (Supplementary Table S4; Supplementary Fig. S5A–B). When classifying the DEGs into Gene Ontology (GO) terms, we found that genes involved in the biological and metabolic processes were overrepresented in both CD4+ and CD8+ T cells (Supplementary Fig. S6A–B). In addition, in CD8+ T cells we also found enrichment of GO terms associated with the regulation of cell cycle, cell organization, and biogenesis. Gene set enrichment analysis (GSEA) probing Molecular Signatures Database was consistent with GO analysis (Supplementary Table S5). Further in-depth analysis of the hand curated DEGs from landmark studies characterizing T cell transcriptional signatures [30–33] also revealed the upregulation of multiple genes involved in immune-related processes and cell cycle for both CD4+ and CD8+ T cells. Genes associated with T cell exhaustion and Tregs (CD4+ T cells only) were enriched within the top downregulated DEGs, while those involved in metabolism were found both within up- and downregulated DEGs (Fig. 4a, b). Given our previous findings of expanded Tregs in AML patients during ELR [16], we conducted GSEA using published gene sets from a landmark study characterizing suppressive Tregs from the tumor microenvironment [33]. This analysis revealed that a suppressive Treg signature was negatively enriched in CD4+ T cells of pomalidomide-treated patients compared with AML chemotherapy controls (normalized enrichment score;NES of −2.06) (Supplementary Fig. S6C) confirming our phenotypic data (Supplementary Fig. S7), suggesting that pomalidomide may be able to inhibit suppressive tumor Tregs in vivo. Finally, the Ingenuity Pathway Analysis further confirmed previous observations of differential effect of pomalidomide on CD4+ and CD8+ T cells, with metabolic pathways predominantly affected in CD4+ and those associated with effector differentiation in CD8+ T cells, respectively (Fig. 4c, d, Supplementary Table S6). The cell cycle pathway was activated by pomalidomide in both T cell subpopulations, consistent with flow cytometric finding of Ki67 upregulation (Supplementary Fig. 2), implying the relevance of the proliferative circuit as a major effect of pomalidomide on T cells.
Fig. 4. Pomalidomide induces unique changes in T cell gene expression in vivo.
Gene expression analysis of highly purified (>98%) PB CD4+ and CD8+ T cells at ELR comparing pomalidomide-treated AML patients (n = 3) to age-matched AML patients receiving induction chemotherapy alone (n = 3). a, b Heatmap of select DEGs for CD4+ and CD8+ T cells, respectively, grouped into key biological categories (log2FC > 1 and <−1; P < 0.01; *0.01 < P < 0.05). Every row represents a gene, and every column a patient sample. Red indicates an increase over the mean (Z > 0) and blue a decrease (Z < 0). c, d Ingenuity pathway analysis of the differentially expressed genes (log2FC > 1 and <−1; P < 0.05). Pathways were selected according to P < 0.01 and availability of a predictive Z score. Activated pathways (positive Z score) are colored red; inhibited pathways (negative Z score) are colored blue.
Discussion
This is the first study investigating pomalidomide in AML. Our findings reveal that pomalidomide can be safely added to intensive induction TST at the time of ELR. The MTD of pomalidomide was determined to be 4 mg daily for 21 consecutive days. Despite being initiated during a state of profound cytopenias, overall toxicities were manageable. Time to full hematologic recovery (median time to ANC ≥ 1.0 × 109/L = 38 days) appeared similar to historical controls receiving AcDVP16 alone (median time to partial neutrophil recovery: ANC ≥ 0.5 × 109/L = 34 days) [6], those on study who received AcDVP16 but did not receive pomalidomide (median time to ANC ≥ 1.0 × 109/L = 42 days), and those receiving other TST backbones (median time to ANC ≥ 1.0 × 109/L = 37 days with alvocidib, cytarabine, mitoxantrone: FLAM) [4], suggesting that pomalidomide did not result in substantial delays in hematologic recovery. Moreover, time to hematologic recovery in this study appears to be similar to 1 cycle of CPX-351 induction (median time to ANC ≥ 0.5 × 109/L = 35 days); [34] however, the CPX-351-treated patient population was an older subgroup (60–75 years) of AML with MRC which may have a higher risk of prolonged myelosuppression post induction compared with the patient population on this study. There was no treatment-related mortality seen during pomalidomide administration though one patient died of ARDS after AcDVP16 and did not receive pomalidomide. Our correlative studies suggest that pomalidomide given during ELR effectively suppresses Aiolos expression in PB and BM T cells and modulates T cell composition, proliferation, cytokine production, and gene expression.
The most frequent pomalidomide-related toxicities included skin rash, liver function abnormalities, mucositis, and fever, with liver function abnormalities and respiratory failure being DLTs at the 8 mg × 21 days pomalidomide dose. Overall, the toxicities were similar to those observed in MM, with the exception of peripheral neuropathy and thromboembolism, which were not observed on this study [22, 35]. Nine patients discontinued pomalidomide early at the MTD including three patients who stopped early as investigator discretion due to persistent leukemia detected in an early BM biopsy or PB prior to full hematologic recovery. Pomalidomide may have been discontinued too early in these patients as ongoing antileukemic activity was demonstrated in five patients with increased BM blasts while on pomalidomide whom ultimately achieved an overall CR/CRi after completion of therapy. Our findings of potential antileukemic activity of pomalidomide are supported by a recent study suggesting that beside immunomodulatory activity, pomalidomide has direct antileukemic activity against primary AML cells in vitro and in vivo in a mouse leukemia xenograft model [36]. Thus, for future trials, every attempt should be made to continue pomalidomide therapy and defer response assessment until its completion.
The addition of pomalidomide to TST led to an overall CR/CRi rate of 77% which compared favorably to responses in AML historical controls who received AcDVP16 at JH 2004–2013 (n = 301; Table 3), particularly in patients ≥60 years (82 versus 57%), those with secondary AML (71 versus 53%), and unfavorable-risk cytogenetics (86 versus 52%) [6]. The median DFS and OS of the AML patient cohort also compared favorably to historical controls (27.1 versus 15.0 months and 33.8 versus 17.2 months, respectively), but these outcomes may be impacted by a higher proportion of patients undergoing alloSCT on our study. A direct comparison of the results of the present study to TST alone and other induction AML regimens is not possible given the small numbers of patients and the phase I nature of our current study. However, overall outcomes with conventional induction regimens are relatively poor in patients with secondary AML (CR rates 45% with 7 + 3) [37], AML with MRC (CR rates 48% with CPX-351) [34] and adverse-risk disease (CR rates 57% with 7 + 3 using high dose daunorubicin) [38]. The clinical activity seen with the addition of pomalidomide to induction therapy in these poor-risk subsets warrants a larger randomized phase 2 study to test the hypothesis that the addition of pomalidomide could augment both response and depth of CR after induction chemotherapy.
The IMiD compound lenalidomide has been studied in AML as a single agent and in combination with chemotherapy at various doses with mixed results in terms of tolerability and activity [22, 39, 40]. A phase II study revealed potential added efficacy of lenalidomide (10–25 mg, day 1–21) in combination with 7 + 3 induction chemotherapy (CR rates = 46%) in patients with del[5q] AML and high-risk MDS, particularly those patients having a very poor-risk cytogenetics [41]. Increased toxicity was seen in several studies with lenalidomide doses of 25 mg or higher in combination with chemotherapy agents [41, 42]. Whether pomalidomide and lenalidomide have different efficacy or tolerability when combined with chemotherapy in AML requires further study.
Durable response to lenalidomide in AML patients has been associated with BM T cell infiltration and differential sensitivity of subclones, but not with the clearance of driver mutations [43]. We performed exploratory analysis examining the correlation of pretreatment mutation profile with response to therapy for 31 patients with available data (Supplementary Fig. S1) but small sample size and heterogeneity of tested mutations precluded the identification of statistically relevant patterns of response. Future studies with larger patient numbers and serial mutation profiles will be required to better delineate patterns of response in relation to the presence of molecular mutations.
This study was built on the premise that immune modulation during ELR after induction chemotherapy might be of benefit in promoting and maintaining an antileukemic immune milieu. Our correlative studies suggest that pomalidomide has direct effects on both PB and BM T cells. First, we observed that pomalidomide caused a significant decrease in the expression of Aiolos in immune cells during ELR. The effect was rapid but Aiolos expression in T cells restored to baseline soon after pomalidomide was stopped. Second, the treatment with pomalidomide promoted changes in T cell subsets, most notably a decrease in TEMRA cells in both PB and BM. We recently reported that newly diagnosed AML patients have an increase in CD8+ TEMRA T cells that are enriched for a senescent immunophenotype. The achievement of CR is associated with a significant decrease in CD8+ TEMRA, whereas they remained unchanged or increased in nonresponders to induction chemotherapy [9]. Whether pomalidomide-mediated decrease in TEMRA cells may have led to the downregulation of immunosuppressive cellular circuits is unclear. Third, treatment with pomalidomide was also paralleled by changes in T cell cytokine production and their in vivo proliferation. These observations are consistent with prior lenalidomide studies in MM and MDS [44, 45]. Finally, our gene expression studies suggest that pomalidomide primarily affects pathways associated with T cell proliferation, differentiation, and metabolism, but differently in CD4+ and CD8+ T cells. Our earlier studies demonstrated that ELR is dominated by an expanded oligoclonal population of peripherally-derived Tregs [16]. While we have not observed significant changes in the frequency of Tregs during pomalidomide treatment (Supplementary Fig. S7), total numbers of Tregs were significantly reduced during pomalidomide treatment (Supplementary Fig. S4) and by applying GSEA we found that the genes overexpressed in the suppressive Tregs in several tumors are negatively enriched in bulk CD4+ T cells of pomalidomide-treated patients (Supplementary Fig. S6C). This observation suggests that Treg function rather than number may be modulated by pomalidomide in vivo.
While our clinical data compared favorably with historical controls and more contemporaneous induction regimens, there are important limitations that must be considered. Patients could have consented to this study up to 14 days after induction therapy rather than prospectively prior to induction therapy. Eight patients (16%) did not receive pomalidomide after enrolling on this study including three patients who did not reach ELR based on eligibility criteria. Given the safety of pomalidomide and no significant effect on hematologic recovery, it would be critical for future studies to assess the role of pomalidomide after induction chemotherapy for all patients irrespective of achievement of ELR and underlying infections. Pomalidomide, similar to other IMiDs, when administered in vivo gives rise to pleiotropic biological effects, making it difficult to tease apart the effect that most significantly contributes to the clinical activity. Despite these limitations, this study provides a template for further exploration of diverse immunomodulatory strategies including the immune checkpoint inhibition and vaccines at the time of ELR and as a maintenance approach to optimize immune-based antileukemic efficacy.
In conclusion, pomalidomide given at the time of ELR following TST is well tolerated in newly diagnosed AML and HR-MDS with particularly high CR rate in patients with unfavorable-risk cytogenetics. The exact mechanism of potential benefit from pomalidomide administration after chemotherapy is uncertain, but our data raise the possibility that pomalidomide can induce an immunostimulatory milieu that overcomes net drug resistance and promotes the achievement of CR. These findings warrant the design of a phase II study of pomalidomide given sequentially at the time of ELR after TST and other conventional induction regimens to further define its efficacy and biology behind the observed responses.
Supplementary Material
Acknowledgements
This data was presented at the annual American Society of Hematology conference in 2015 in Orlando, FL (poster), 2016 in San Diego, CA (poster), and 2018 in San Diego, CA (oral presentation). This study was supported in part by research funding from UM1-CA186691 and P30 CA006973 (Johns Hopkins), 5-UM1-CA186704 (University of North Carolina), and P30CA016359/5-UM1-CA186689-5 (Yale). JFZ was supported and funded by Leukemia and Lymphoma Society Special Fellow in Clinical Research Award. HAK was supported by ASBMT New Investigator Award/ Gabrielle’s Angel Foundation and by the Vienna Fund for Innovative Cancer Research. AMZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). The authors would like to thank the research support staff at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, University of North Carolina, Lineberger Comprehensive Cancer Center, and Yale Cancer Center. We would also like to thank all of the patients and their families who trusted us for their care. Without these partnerships, this trial could not have been performed.
Funding This study was supported in part by research funding from UM1-CA186691 and P30 CA006973 (Johns Hopkins), 5-UM1-CA186704 (University of North Carolina), and P30CA016359/5-UM1-CA186689-5 (Yale). JFZ was supported and funded by Leukemia and Lymphoma Society Special Fellow in Clinical Research Award. HAK was supported by ASBMT New Investigator Award/Gabrielle’s Angel Foundation and by the Vienna Fund for Innovative Cancer Research. AMZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA).
Conflict of interest JFZ reports receiving research funding from Celgene, Merck, Takeda, and Tolero Pharmaceuticals, honoraria from AbbVie, Agios, Celgene, Daiichi Sankyo, Genentech, Pfizer, and Tolero Pharmaceuticals, and has served as a consultant for AsystBio Laboratories, Celgene, Covance, and Takeda. AMZ reports receiving research funding (institutional) from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics, consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Jazz, Celgene, Ariad, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, Beyond-Spring, and Takeda. None of these relationships were related to the development of this manuscript. BDS reports being on Data Safety Monitoring Board for Celgene and has served as a consultant for Agios, Bristol-Myers Squibb, Jazz, Novartis, and Pfizer. MJL reports receiving research funding from Astellas, FujiFilm, and Novartis, and has served as a consultant for Agios, Amgen, Astellas, Daiichi Sankyo, FujiFilm, Karyopharm, Menarini, and Novartis. CCC has received honoraria from Abbvie, Loxo, Pharmacyclics, Octapharma, and H3 Biomedicine, has served as a consultant for Abbvie, Covance, and Cowen & Co., and has received institutional funding from Incyte, Gilead, AROG, Loxo, and H3 Biomedicine. LL has received research support from Genentech and Merck and has a patent licensed to WindMIL Therapeutics (WO2014085437A2). IG received research funding from Merck and Amgen, and participated in the advisory board meetings for Amgen, Jazz, Novartis and Abbvie. None of these relationships were related to the development of this manuscript. All other authors declare no conflict of interest.
Footnotes
Compliance with ethical standards
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information The online version of this article (https://doi.org/10.1038/s41375-019-0693-4) contains supplementary material, which is available to authorized users.
References
- 1.Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematol Am Soc Hematol Educ Program. 2012; 2012:1–6. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karp JE, Garrett-Mayer E, Estey EH, Rudek MA, Smith BD, Greer JM, et al. Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica. 2012;97:1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeidner JF, Foster MC, Blackford AL, Litzow MR, Morris LE, Strickland SA, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100:1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castaigne S, Chevret S, Archimbaud E, Fenaux P, Bordessoule D, Tilly H, et al. Randomized comparison of double induction and timed-sequential induction to a “3 + 7” induction in adults with AML: long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104:2467–74. [DOI] [PubMed] [Google Scholar]
- 6.Norsworthy KJ, DeZern AE, Tsai HL, Hand WA, Varadhan R, Gore SD, et al. Timed sequential therapy for acute myelogenous leukemia: Results of a retrospective study of 301 patients and review of the literature. Leuk Res. 2017;61:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knaus HA, Kanakry CG, Luznik L, Gojo I. Immunomodulatory drugs II: immune checkpoint agents in acute leukemia. Curr Drug Targets. 2017;18:315–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson-Moncada J, Viboch E, Church SE, Warren SE, Rutella S. Dissecting the Immune Landscape of Acute Myeloid Leukemia. Biomedicines. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knaus HA, Berglund S, Hackl H, Blackford AL, Zeidner JF, Montiel-Esparza R, et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 2018;3:e120974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong Y, Zhang J, Claxton DF, Ehmann WC, Rybka WB, Zhu L, et al. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. 2015;5:e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, et al. T cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res. 2016;22:3057–66. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Zheng J, Liu J, Yao J, He Y, Li X, et al. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75:468–76. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Dong Y, Yang Q, Xu W, Jiang S, Yu Z, et al. Acute myeloid leukemia cells express ICOS ligand to promote the expansion of regulatory T cells. Front Immunol. 2018;9:2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 2010;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15:3325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood. 2011;117:608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187:1885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–90. [DOI] [PubMed] [Google Scholar]
- 20.Shanafelt TD, Ramsay AG, Zent CS, Leis JF, Tun HW, Call TG, et al. Long-term repair of T cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL). Blood. 2013;121:4137–41. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay AG, Evans R, Kiaii S, Svensson L, Hogg N, Gribben JG. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T cell motility by altering Rho GTPase signaling that is reversible with lenalidomide. Blood. 2013;121:2704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeidner JF, Foster MC. Immunomodulatory drugs: IMiDs in acute myeloid leukemia (AML). Curr Drug Targets. 2017;18:304–14. [DOI] [PubMed] [Google Scholar]
- 23.Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009–02. Blood. 2013;121:1968–75. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21: 4642–9. [DOI] [PubMed] [Google Scholar]
- 26.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83. [PubMed] [Google Scholar]
- 27.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. [DOI] [PubMed] [Google Scholar]
- 31.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Investig. 2011; 121:2350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachireddy P, Hainz U, Rooney M, Pozdnyakova O, Aldridge J, Zhang W, et al. Reversal of in situ T cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123:1412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–85. [DOI] [PubMed] [Google Scholar]
- 34.Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clinical Oncol. 2018:Jco2017776112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson PG, Siegel D, Baz R, Kelley SL, Munshi NC, Laubach J, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121: 1961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Roy A, Prebet T, Castellano R, Goubard A, Riccardi F, Fauriat C, et al. Immunomodulatory drugs exert anti-leukemia effects in acute myeloid leukemia by direct and immunostimulatory activities. Front Immunol. 2018;9:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone RM, Mazzola E, Neuberg D, Allen SL, Pigneux A, Stuart RK, et al. Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J Clin Oncol. 2015;33:1252–7. [DOI] [PubMed] [Google Scholar]
- 38.Luskin MR, Lee JW, Fernandez HF, Abdel-Wahab O, Bennett JM, Ketterling RP, et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeidan AM, Smith BD, Carraway HE, Gojo I, DeZern A, Gore SD. A phase 2 trial of high dose lenalidomide in patients with relapsed/refractory higher-risk myelodysplastic syndromes and acute myeloid leukaemia with trilineage dysplasia. Br J Haematol. 2017;176:241–7. [DOI] [PubMed] [Google Scholar]
- 40.Medeiros BC, McCaul K, Kambhampati S, Pollyea DA, Kumar R, Silverman LR, et al. Randomized study of continuous high-dose lenalidomide, sequential azacitidine and lenalidomide, or azacitidine in persons 65 years and over with newly-diagnosed acute myeloid leukemia. Haematologica. 2018;103:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ades L, Prebet T, Stamatoullas A, Recher C, Guieze R, Raffoux E, et al. Lenalidomide combined with intensive chemotherapy in acute myeloid leukemia and higher-risk myelodysplastic syndrome with 5q deletion. Results of a phase II study by the Groupe Francophone Des Myelodysplasies. Haematologica. 2017;102:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis M, Culligan D, Karamitros D, Vyas P, Johnson P, Russell NH, et al. Lenalidomide monotherapy and in combination with cytarabine, daunorubicin and etoposide for high-risk myelodysplasia and acute myeloid leukaemia with chromosome 5 abnormalities. Leuk Res Rep. 2013;2:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bansal D, Vij K, Chang GS, Miller CA, DiPersio JF, Vij R, et al. Lenalidomide results in a durable complete remission in acute myeloid leukemia accompanied by persistence of somatic mutations and a T cell infiltrate in the bone marrow. Haematologica. 2018;103:e270–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clave E, Douay C, Coman T, Busson M, Bompoint C, Moins-Teisserenc H, et al. Lenalidomide consolidation and maintenance therapy after autologous stem cell transplant for multiple myeloma induces persistent changes in T cell homeostasis. Leuk lymphoma. 2014;55:1788–95. [DOI] [PubMed] [Google Scholar]
- 45.McDaniel JM, Zou JX, Fulp W, Chen DT, List AF, Epling-Burnette PK. Reversal of T cell tolerance in myelodysplastic syndrome through lenalidomide immune modulation. Leukemia. 2012;26:1425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.