Abstract
Due to the dangers associated with psychotropic medications, there is an urgent need for non-pharmacologic therapies to treat behavioral and psychological symptoms of dementia. Acupuncture and acupressure are safe and well-tolerated non-pharmacologic therapies for this population, but currently no review has explored acutherapy for management of distressing dementia symptoms. This review synthesizes research on acupuncture and acupressure for behavioral and psychological symptoms of dementia. Upon searching 5 databases, 15 studies met inclusion/exclusion criteria. Nine examined acupressure, 6 acupuncture and eight were randomized controlled trials. The percent of studies demonstrating statistically significant improvements in symptoms were: activities of daily living (75%), agitation (100%), anxiety (67%), depression (100%), mood (100%), neuropsychological disturbances (67%) and sleep disturbances (100%). Variations in study design, intervention procedures and outcomes limit interpretations about effectiveness. It is recommended that further research be done to examine the efficacy of these therapies and promote generalizability.
Keywords: Dementia, acupuncture, acupressure, acutherapy, behavioral and psychological symptoms of dementia
Nearly 47 million people around the world are currently diagnosed with dementia. It is expected that nearly 10 million more people will be diagnosed each year due to the aging population (World Health Organization, 2018). Dementia is marked by progressively deteriorating cognitive function, as well as worsening physical, functional, behavioral, psychological and emotional health (Graham, Emery, & Hodges, 2004; Kalaria & Ballard, 1999). Behavioral and psychological symptoms of dementia (BPSD) have been shown to affect approximately 60–80% of people with dementia (Lyketsos et al., 2002; Margallo-Lana et al., 2001). These symptoms result in negative effects on families, caregivers, healthcare providers and society. Pharmacologic treatments are often ineffective at managing BPSD, and can be harmful to older adults with dementia (Jeste et al., 2008; Maust et al., 2015), thus practice guidelines highly encourage the use of non-pharmacologic interventions as first line treatment of BPSD. Based on the high level of safety and tolerance of non-pharmacological therapies among those with dementia, acupuncture and acupressure present as potential treatment options for BPSD. Furthermore, acutherapy is less complex to deliver compared to other non-pharmacologic, multifaceted psychosocial and behavioral interventions designed to improve BPSD (Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010; Waldorff et al., 2012). This review aims to examine the research relevant to acupuncture and acupressure specifically for managing these distressing symptoms of dementia.
Management of Behavioral and Psychological Symptoms of Dementia
BPSD are defined as signs and symptoms of disturbed perception, thought content, mood, or behavior that frequently occur in patients with dementia (Finkel, Silva, Cohen, Miller, & Sartorius, 1997). Examples of BPSD include anxiety, sleep disturbance, depressed mood, hallucinations and delusions, aggression, restlessness, agitation, wandering, culturally inappropriate behaviors, screaming, wandering, sexual disinhibition, hoarding, cursing and shadowing (Finkel et al., 1997; Kar, 2009).
The majority of people diagnosed with dementia are likely to experience at least one specific behavioral or psychological symptom during the disease. Specific symptoms are often episodic in nature, while BPSD as a whole are present throughout the lifetime of the diagnosis (Aalten, de Vugt, Jaspers, Jolles, & Verhey, 2005; Steinberg et al., 2004). The causes of BPSD are extremely diverse, complex, and related to multiple factors. Some individuals with dementia may be more at risk than others to experience certain types and degrees of BPSD. Research has shown that females may be more at risk for depressive symptoms (Buchanan, Wang, Ju, & Graber, 2004; Lövheim, Sandman, Karlsson, & Gustafson, 2009; Steinberg et al., 2006), while men may be more at risk for aggressive type behaviors (Buchanan et al., 2004; Hall & O’Connor, 2004; Lövheim et al., 2009). BPSD are most common in the middle stages of the disease, with an estimated prevalence of 61%−88% (Lövheim, Sandman, Karlsson, & Gustafson, 2008; Steffens, Maytan, Helms, & Plassman, 2005). Specific BPSD have been shown to vary depending on dementia type. For example, depressive and anxious type symptoms have consistently shown to be more prevalent among individuals with vascular type dementia compared to Alzheimer’s dementia, although a substantial overlap can exist between these two types (Cerejeira, Lagarto, & Mukaetova-Ladinska, 2012; Steinberg et al., 2006). Meanwhile, worsening general health and serious comorbidities are associated with increased risk for a number of BPSD, including agitation/aggression, aberrant motor behavior disinhibition and irritability (Steinberg et al., 2006).
BPSD further complicate the diagnosis and have been shown to be a leading factor of worsening caregiver burden (Campbell et al., 2008). BPSD negatively impact the quality of life of the individual with dementia, as well as family caregivers (Banerjee et al., 2006; Hurt et al., 2008). Similarly, these symptoms have been associated with increased stress and burden in nursing staff members in geriatric-acute care and nursing home settings (Cocco, Gatti, de Mendonça Lima, & Camus, 2003). These difficult behaviors can ultimately result in early institutionalization, or placement in long term care settings, and increased healthcare utilization (Gaugler, Yu, Krichbaum, & Wyman, 2009). BPSD are also incredibly costly to society, as they account for 30% of the $277 billion total health costs for Alzheimer’s disease in the United States (Alzheimer’s Association, 2018; Schnaider Beeri, Werner, Davidson, & Noy, 2002).
Many pharmacologic treatments for BPSD have been shown to be ineffective and in some cases, dangerous to people with dementia. For example, antipsychotics continue to be used as a primary treatment for severe agitation and psychosis in dementia; however, these medications have been shown to have limited efficacy and have been linked to increased mortality rates in this population (Maust et al., 2015). Current FDA black box warnings caution against the use of all antipsychotics in older adults with dementia due to the increased risk of death (Jeste et al., 2008). Anti-anxiety medications such as benzodiazepines, which are also commonly used for BPSD management, are associated with worsening cognitive function and increased falls and fractures in older adults (Defrancesco, Marksteiner, Fleischhacker, & Blasko, 2015; Van Strien, Koek, Van Marum, & Emmelot-Vonk, 2013). Additionally, use of anti-depressant medications have been linked to increased risk for suicide in this population (Seyfried, Kales, Ignacio, Conwell, & Valenstein, 2011).
Due to the dangers associated with psychotropic medications in older adults with dementia, recent practice guidelines and recommendations encourage the use of non-pharmacologic interventions for BPSD (Austrom, Boustani, & LaMantia, 2018; Reus et al., 2016). BPSD not only impact the individual with dementia, but also their family, friends, healthcare providers and society. There is an urgent need for safe and effective alternative treatments for BPSD for family members, caregivers, nurses and clinicians to better manage these burdensome symptoms in people with dementia. Acupuncture and acupressure present as possible non-pharmacologic therapies for BPSD management.
Acupuncture is a non-pharmacologic treatment that is based in Traditional Chinese Medicine and emerged in China as early as the first century B.C. The traditional theory of acutherapy is based on scientifically non-detectable energy pathways called meridians that are interconnected throughout the body and thousands of acupoints that are along these pathways can be stimulated (using needles, manual hand pressure, or electrical stimulation) to correct various disturbances in the harmony of the body (Kaptchuk, 2002). Acupuncture uses thread-like needles to stimulate acupoints, while acupressure uses hands or objects to apply manual pressure to stimulate acupoints. Acutherapy has been integrated into Western medicine throughout the late 20th century (Vickers, 1998) and has been found to be a safe and effective treatment option for a number of symptoms/diseases such as chronic pain, migraines, depression, anxiety and weight loss. (Amorim et al., 2018; Kim, Shin, & Park, 2018; Lee & Ernst, 2011; Wu, Yeung, Schnyer, Wang, & Mischoulon, 2012).
The understanding of acutherapy has been transformed to a less mysterious, more quantifiable science based on replicable randomized controlled trials and functional magnetic resonance imaging (fMRI) studies. MRI studies have demonstrated how the stimulation of certain acupoints result in the activation of corresponding areas of the brain (Fang, Krings, Weidemann, Meister, & Thron, 2004). The exact mechanism of action of acutherapy is not fully understood, but is thought to stimulate the central nervous system through the release of specific neurotransmitters and hormones. It is speculated that the specific pathways, neurotransmitters and hormones involved are distinct for different symptoms and disease states (Han, 2003; Kou et al., 2017; Li et al., 2013).
Acupuncture and acupressure have been studied in persons with Alzheimer’s and vascular type dementias. A systematic review by Zhou, Peng, Xu, Li, and Liu (2015) supports that acupuncture is a safe and reliable non-pharmacologic therapy for improving cognitive function in people with dementia. This systematic review supports that acupuncture is a safe intervention for people with dementia with few adverse effects and that participants often report high satisfaction with the treatments. In addition, MRI studies demonstrate activation of areas of the brain responsible for cognitive function in people with dementia with the use of acupuncture (Shan et al., 2018; Z. Wang et al., 2012).
Purpose
Despite these encouraging findings, the effects of acupuncture and acupressure on BPSD specifically remain understudied. To date, no review exists on acupuncture and/or acupressure specifically for BPSD. The purpose of this review was to identify, examine and summarize the science relating to the effects of acupuncture and acupressure therapy on BPSD. The research question guiding this review was, “What are the effects of acupuncture and acupressure therapy on the behavioral and psychological symptoms in persons with dementia?”.
Methods
This review was guided by the scoping review methodology outlined by the Joanna Briggs Institute Reviewers’ Manual (Peters, McInerney, Soares, Khalil, & Parker, 2015), which is based on the scoping review frameworks of Arksey and O’Malley (2005) and Levac, Colquhoun, and O’Brien (2010).
A scoping review framework uses a systematic approach to identify, examine and summarize available evidence and to identify gaps in knowledge (Arksey & O’Malley, 2005). Prior to this review, it was unknown as to what evidence was available regarding acutherapies for managing BPSD and what their effectiveness was. A scoping review is useful when the state of the science is emerging, as it allows for a broad view of the nature and range of evidence on the given topic area (Munn et al., 2018). Unlike a systematic review, a scoping review framework would allow for greater breadth and inclusion of all pertinent research regarding acupuncture and/or acupressure for BPSD, irrespective of quality (Peters, Godfrey, et al., 2015). This approach also enables identification and examination of gaps in the evidence, which would be useful to this emerging area of study.
Eligibility Criteria
Studies were included that consisted of participants with a diagnosis of dementia. Alzheimer’s disease and vascular dementia are the two most common types of dementia and were expected to be the most prevalent dementia diagnoses in this review; however, some studies did not specify the type of dementia of the participants in the study. As the characteristics of the participants and settings were similar among all studies, despite whether they reported dementia type or not, studies were not excluded for not specifying dementia type.
The included studies examined the use of acupuncture and/or acupressure therapy. The studies could use multi-modal interventions, but acupuncture or acupressure treatment had to be included. Because the aim of this review was to explore the effects of acupuncture or acupressure therapy on BPSD rather than cognitive function, at least one behavioral or psychological symptom needed to be included as an outcome of interest in the studies to be included in this review. Studies evaluating activities of daily living (ADLs) as an outcome were also included, as BPSD are often marked by increased ADL dependence and poor hygiene (Kar, 2009). Studies that only evaluated cognitive function or no BPSD were excluded.
Included studies had to be published in the English language, as the reviewer was only proficient in English. Studies were excluded that only contained partial English translations. As acupuncture therapy is rooted in Traditional Chinese Medicine, studies could be conducted within or outside of the United States. No exclusion criteria were applied to the setting of the studies, for example, they could be conducted in acute care, primary care and/or community settings.
Due to the scope of the project and the need for feasibility, gray literature such as dissertations/theses, conference reports and proceedings, and white papers were excluded. In addition, commentaries, opinion pieces and letters to the editor were also excluded as they provided little support relevant to the research question. Reviews and meta-analyses that did not include BPSD were also excluded.
A broad range of study designs was included, due to the potential paucity of studies and unknown state of the science on acupuncture and acupressure therapy for BPSD in those with dementia. Studies were also not excluded based on the date of publication, as to not limit studies that may add historical value to the research question.
Search Strategy
An initial search was done using the MEDLINE database to briefly review the topic of interest, as to ensure no review had been done on the topic already. This was also done to gain a better understanding of keywords to be used in a more systematic search.
A systematized search strategy was then created and conducted. University of Michigan Health Science Library informationalists were involved and provided expertise in creating and conducting the search. Five databases were used in the search strategy and included: PubMed, CINAHL, Embase, PsychINFO and AgeLine. Search terms and keywords, along with how they were combined for the database searches are outlined in Table 1.
Table 1.
Search Terms and Combinations
| Boolean operator | Field | Keywords |
|---|---|---|
| Title, abstract, medical subject headings (in PubMed), author defined keywords | ‘dementia’, ‘dementia, vascular’, ‘Alzheimer disease’ and keywords and synonyms of dementia such as ‘senile’, ‘Alzheimers’, ‘Alzheimer’, ‘Alzheimer’s’, ‘dementia’ and ‘amentia’ | |
| AND | Title, abstract, medical subject headings (in PubMed), author defined keywords | like ‘acupuncture’, ‘acupuncture therapy’, ‘acupuncture, ear’, ‘acupuncture points’ and ‘acupressure’ |
| OR | Title, abstract, medical subject headings (in PubMed), author defined keywords | ‘shiatsu’, ‘pharmacopuncture’, ‘acupuncture’, ‘electroacupuncture’, ‘acupoints’, ‘meridians’, ‘acupressure’, ‘acupotomy’, ‘auriculotherapy’, ‘intradermal needling’, ‘Zhi Ya’, ‘Chih Ya’ and ‘Tui Na’ |
BPSD were not included in the database search, as this concept involves a wide range of examples and could result in excluding relevant studies if a specific BPSD or synonym was not included in the database search. For this reason, the inclusion and exclusion criteria were applied to this concept during the review of titles/abstracts and of full text articles.
Data Management
Review of titles/abstracts and full text articles was conducted by a primary reviewer (M. Harris). Studies that seemed uncertain in regards to inclusion and exclusion criteria were discussed with authors L. Struble and M. Titler until a consensus was reached. Data was extracted pertaining to reference title, author, year, sample characteristics (size, dementia types, dementia severity), study location, study design, acutherapy intervention procedures, BPSD examined and other outcomes, adverse effects of interventions, and significant results relating to BPSD. Data extraction was conducted by the primary author (M. Harris). These data were charted in an ongoing, iterative manner in tables, which were then shared and discussed among the review team to synthesize the findings.
Results
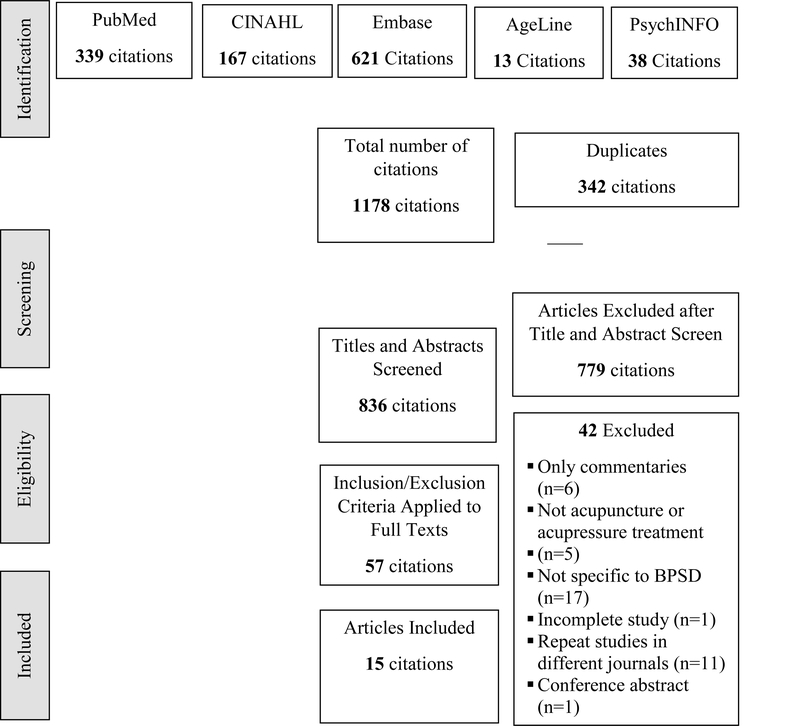
Figure 1. provides a PRISMA diagram outlining the results of the search process. Database searches were conducted between October 2 through October 19, 2018. All article citations were downloaded to EndNote X8. The total number of citations from the database search was 1178. EndNote X8 was used to filter duplications, which left a total of 836 citations to be reviewed by title and abstract. Common reasons for exclusion at the title and abstract screening phase were studies that evaluated alternative treatments but did not include acupuncture or acupressure therapy and studies that did include a BPSD.
Figure 1.
Prisma Flow Diagram of Acupuncture and Acupressure Therapy Studies for BPSD
Fifty-seven articles were then reviewed by full-text. Articles were excluded for the same reasons as the title and abstract review, in addition, some studies were only commentaries (n=6), one study was a report on a not yet completed study, one study was a conference abstract and several articles reported on the same study, but in different journals (n=11). Ultimately, 15 articles were included based on the inclusion and exclusion criteria. The final step of the search was a review of the references of the included studies, no new studies were found.
Summarized Findings
The final 15 studies from the search are summarized in Tables 2 and 3. Categories used for data extraction of articles include authors, sample size, study location and design, acutherapy intervention procedures used, BPSD outcomes examined and findings related to the targeted BPSD. Data specific to cognitive function and cognitive impairment severity are included in Table 4.
Table 2.
Acupuncture Treatment Studies for BPSD
| Authors (Year) | Sample Size |
Location/ Design (Arm Specification) |
Acutherapy Intervention |
Outcomes (Measurements) |
Significant Findings |
|---|---|---|---|---|---|
| Jia et al. (2017) | 79 | China In the home RCT, 2 arms (Acupuncture vs. daily oral donepezil) |
Acupuncture treatments 3x/week for 12 weeks | Cognitive function (ADAS-Cog) Overall clinician impression of change (CIBIC-Plus) ADLs (ADCS-ADL) Neuropsychiatric disturbances (NPI) |
Improved cognitive function and clinican impression of change in acupuncture group |
| Kwok et al. (2013) | 19 | China Community centers Repeated measures design, 1 arm |
6-week control followed by treatments 2x/week for 6 weeks | Sleep disturbances (Wrist actigraphy) Sleep parameters Cognitive function (ADAS-Cog) |
Improved sleep time and quality, and cognitive function with acupuncture therapy |
| Lombardo et al. (2001) | 11 | United States Setting not specified Repeated measures design, 1 arm |
Acupuncture treatments 3x/week for 2 weeks, then 2–3x/week for 7–10 weeks | Depression (CSDD) Anxiety (State-trait anxiety inventory) Cognitive impairment (Medical Outcomes Survey) Mood (POMS) |
Improved depression, anxiety and mood with acupuncture therapy |
| Shi et al. (2012) | 16 | China Hospital Repeated measures design, 1 arm |
Every other day treatment for 6 weeks | Cognitive impairment (MMSE) Quality of life (DEMQOL) ADLs (ADL scales) Oxidative DNA damage (Oxidative marker detection through urine samples) |
Increased cognitive function and quality of life with acupuncture therapy |
| Shi et al. (2015) | 63 | China Hospital RCT, 2 arms (randomized vs. non-randomized) |
Every other day treatment for 6 weeks | Cognitive impairment (MMSE) ADLs (ADL scales) QOL (DEMQOL) |
Increased cognitive function and ADL function in acupuncture group |
| Y. Wang et al. (2014) | 55 | China Inpatient and outpatient neurology units RCT, 2 arms (daily oral donepezil vs. daily oral donepezil + acupuncture) |
Combined donepezil therapy with once-daily acupuncture treatments for 20 days | Cognitive impairment (MMSE) ADL’s (ADAS-ADL) Brain activity (Electroencephalogram) |
Improved cognitive function and ADL function in acupuncture group |
Note. ADLs activities of daily living, ADCS-ADL Alzheimer’s disease cooperative study activities of daily living scales, ADAS-Cog Alzheimer’s Disease Assessment Scale-Cognitive subscale, BPSD behavioral and psychological symptoms of dementia, CSDD Cornell scale for depression in dementia, DEMQOL Dementia Quality of Life Scale, MMSE Mini-Mental Status Exam, NPI Neuropsychiatric Inventory, PMS Profile of Mood States PWD person with dementia, RCT randomized controlled trial
Table 3.
Acupressure Treatment Studies for BPSD
| Authors (Year) | Sample Size |
Location/ Design (Arm Specification) |
Acutherapy Intervention |
Outcomes (Measurements) |
Significant Findings |
|---|---|---|---|---|---|
| Fung et al. (2018) | 60 | China 3 RCH RCT, 3 arms (aroma-massage with acupressure + exercise vs. cognitive training + exercise vs. aroma-massage with acupressure + cognitive training) |
Aroma massage with acupressure 2x/week for 3 weeks | Agitation (CCMAI) Neuropsychiatric disturbances (NPI) Cognitive function (CMMSE) ADLs (Barthel Index) |
Reduced neuropsychiatric symptom distress and improved ADL function in aroma-massage/acupressure groups compared to non-acupressure group |
| Kwan et al. (2016) | 24 | China 3 LTC Repeated measures, non-randomized design, 8 dosage-combo groups |
1–4 weeks, 1–2x per day acupressure treatments, depending on dosage group | Agitation (CMAI) Stress (Salivary cortisol levels) |
Reduced agitation and stress. Optimal dosage was determined to be 2x/day for 2 weeks |
| Kwan et al. (2017) | 119 | China 12 RCHs RCT, 3 arms (acupressure vs. sham vs. usual care) |
5 days/week acupressure treatments for 2 weeks with 6-week follow up | Agitation (CMAI) Stress (Salivary cortisol levels) |
Reduced stress and agitation in acupressure group (agitation was reduced at 5th week follow up) |
| Lin et al. (2009) | 133 | China Dementia units RCT, cross-over design, 3 arms (acupressure vs. Montessori methods vs. presence therapy) |
Four weeks of the each of the three therapies (implemented in 3 different sequences) with weeklong pre/post measures and 2-week wash out periods between therapy types | Agitation (CMAI) Ease of care with ADLs (Ease of care inventory) Emotional expression (Apparent Affect rating scale) Need for restraint (Unit log) Number of family visits (Unit log) |
Reduced agitation and improved ease of care and ADLs in acupressure and Montessori group compared to presence group |
| Rodríguez-Mansilla et al. (2015) | 111 | Spain RCHs RCT, 3 arms (usual care vs. ear acupressure vs. massage therapy) |
Continuous ear acupressure for 3 months | Pain (DOLOPLUS2 scale) Depression (CSDD) Anxiety (Campbell scale) Cognitive impairment (MMSE) |
Reduced depression, anxiety and pain seen in acupressure group with greater improvement in pain and depression with ear acupressure compared to massage therapy |
| Sutherland et al. (1999) | 10 | Location not specified Dementia unit Quasi-experimental, 2 arms (foot acupressure vs. usual care) |
Foot acupressure treatments, 10 minutes/day for 10 days | Wandering (Behavioral documentation instrument created by researchers) Pulse Respirations Quiet time behaviors |
Decreased wandering in acupressure group |
| Simoncini et al. (2015) | 129 | Location not specified Dementia nursing homes Longitudenal prospective study, 1 arm |
Continuous 8-hour overnight stimulation of the HT7 acupoint applied for 8 weeks | Cognitive impairment (MMSE) Anxiety (State-trait anxiety inventory) Neuropsychiatric disturbances (NPI) ADLs (ADL scales) Disease stage QOL (Global health quality of life scale) Subjective sleep quality (Pittsburgh sleep quality index) |
Neuropsychiatric symptoms, health quality ADL, and sleep improved with acupressure therapy |
| Yang et al. (2007) | 20 | China LTF dementia unit Repeated measures, 1 arm |
2x/day acupressure treatments for 5 days/week for 4 weeks | Agitation (CMAI, daily records of agitated behaviors) Ease of care with ADLs (Ease of care inventory) |
Reduced agitation and improved ease of care with ADLs with acupressure therapy |
| Yang et al. (2015) | 186 | China Dementia care facilities RCT, 3 arms (aroma-acupressure vs. aromatherapy only vs. usual care) |
Aroma–acupressure 1x/day, 5 days/week for 4 weeks | Agitation (CCMAI, heart rate variability) | Reduced agitation in acupressure group |
Note. ADL activities of daily living, ADAS-Cog Alzheimer’s Disease Assessment Scale-Cognitive subscale, BPSD behavioral and psychological symptoms of dementia, CCMAI Chinese version of Cohen-Mansfield Agitation Inventory, CMAI Cohen-Mansfield Agitation Inventory, CSDD Cornell Scale for Depression in Dementia, LTF long-term care facilities, MMSE Mini-Mental Status Exam, NPI Neuropsychiatric Inventory, PWD person with dementia, RCHs residential care homes, RCT randomized controlled trial
Table 4.
Cognitive Measures and Cognitive Impairment Severity of Study Samples
| Author (Year) | Measurement Tool | Baseline CF Mean (SD) |
Sample (n) | Cognitive Impairment Severity |
|---|---|---|---|---|
| Fung et al. (2018) | Chinese version MMSE | 14.6 (1.90) | Aroma-message with acupressure + exercise group (20) | Moderate |
| 13.85 (1.93) | Aroma-message with acupressure + cognitive training group (20) | |||
| Jia et al. (2017) | ADAS-Cog | 29.38 (9.43) | Acupuncture group (43) | Moderate |
| Kwan et al. (2016) | MMSE | 6.61 (6.32) | Total study sample, excluding 1 participant who declined (23) | Severe |
| Kwan et al. (2017) | MMSE | 7.4 (5.8) | Acupuncture group (39) | Severe |
| Kwok et al. (2013) | Chinese version ADAS-Cog | 27.28 (10.93) | Total study sample (19) | Moderate |
| Lin et al. (2009) | MMSE | 6.9 (6.1) | Sequence 1 Acupressure-Presence-Montessori (42) | Severe |
| 7.1 (6.5) | Sequence 2 Montessori-Acupressure-Presence (39) | |||
| 8.0 (6.1) | Sequence 3 Presence-Montessori-Acupressure (52) | |||
| Lombardo et al. (2001) | MMSE | 21.9 (5.9) | Total study sample (11) | Mild |
| Rodríguez-Mansilla et al. (2015) | MMSE | Mean scores not reported | Ear acupressure group (40) | 50% of participants in acupressure group had moderate dementia, 50% had severe dementia |
| Shi et al. (2012) | MMSE | 18.24 (0.91) | Total study sample (16) | Moderate |
| Shi et al. (2015) | MMSE | 18.27 (4.08) | Randomized group (22) Non-randomized group (19) |
Moderate |
| 17.74 (3.33) | Non-randomized group (19) | |||
| Simoncini et al. (2015) | MMSE | 18.0 (4.6) | Total study sample (129) | Moderate |
| Y. Wang et al. (2014) | MMSE | 18.4 (2.9) | Combined group (27) | Moderate |
Note. CF cognitive function, ADAS-Cog Alzheimer’s Disease Assessment Scale-Cognitive subscale, MMSE Mini-Mental Status Exam
The 6 studies in Table 2 examined the effects of acupuncture in persons with dementia (Jia et al., 2017; Kwok et al., 2013; Lombardo et al., 2001; Shi et al., 2015; Shi, Liu, Li, Zhu, & Wang, 2012; Y. Wang, Qin, & Yu, 2014). The 9 articles in Table 3 include studies of acupressure therapy (Fung & Tsang, 2018; Kwan, Leung, & Lai, 2016; Kwan, Leung, & Lai, 2017; Lin et al., 2009; Rodríguez-Mansilla et al., 2015; Simoncini et al., 2015; Sutherland, Reakes, & Bridges, 1999; Yang, Lin, Wu, Chiu, Wang, & Lin, 2015; Yang, Wu, Lin, & Lin, 2007). The publication years of the articles range from 1999–2018. The sample sizes of all the studies range from 10–186, with an average of 69 participants.
Participants, Diagnoses and Severity
A total of 1154 participants were included in the 15 studies. Four studies included participants with Alzheimer’s disease (Jia et al., 2017; Simoncini et al., 2015; Sutherland et al., 1999; Y. Wang et al., 2014), while two studies included participants with vascular type dementia (Shi et al., 2012, 2015) and the remainder of the articles did not specify the type of dementia. Fifty-seven percent of the participants were female, although two studies did not report the gender of participants (Simoncini et al., 2015; Sutherland et al., 1999). The reported age range of participants in all studies was 50–96.
Table 4. summarizes severity of cognitive impairment and measures for the 12 studies reporting cognitive function of participants at baseline (Fung & Tsang, 2018; Jia et al., 2017; Kwan et al., 2016, 2017; Kwok et al., 2013; Lin et al., 2009; Lombardo et al., 2001; Rodríguez-Mansilla et al., 2015; Shi et al., 2012, 2015; Simoncini et al., 2012, 2015; Y. Wang et al., 2014). The Mini-Mental Status Exam (MMSE) and the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) were the tools used across the studies with the MMSE used most frequently. The MMSE scores range from 0–30. MMSE scores greater than 25 indicate no cognitive impairment, 19–23 mild cognitive impairment, 10–18 moderate cognitive impairment and ≤ 9 severe cognitive impairment (Folstein, Folstein, & McHugh, 1975). The ADAS-Cog subscale scores range from 0–70, with higher scores indicative of greater impairment (Rosen, Mohs, & Davis, 1984).
Cognitive impairment severity across the 12 studies varied with seven studies reflecting mild cognitive impairment (Fung & Tsang, 2018; Jia et al., 2017; Kwok et al., 2013; Shi et al., 2015; Shi et al., Simoncini et al., 2012, 2015; Y. Wang et al., 2014), and three had severe cognitive impairment (Kwan et al., 2016, 2017; Lin et al., 2009). One study reported 50% of participants with moderate, and 50% with severe impairment (Rodríguez-Mansilla et al., 2015). and one study excluded individuals with severe dementia (Lombardo et al., 2001). No studies reported on the duration of dementia diagnosis for participants.
Location, Setting and Design
The majority of the studies were conducted in China (n = 11). (Fung & Tsang, 2018; Jia et al., 2017; Kwan et al., 2016, 2017; Kwok et al., 2013; Lin et al., 2009; Shi et al., 2012, 2015; Y. Wang et al., 2014; Yang et al., 2007, 2015). One study was conducted in Spain (Rodríguez-Mansilla et al., 2015). Only one study was conducted in the United States and it was of small sample size with only 11 participants (Lombardo et al., 2001). Two studies did not specify the location (Simoncini et al., 2015; Sutherland et al., 1999).
The majority of the studies took place in settings such as nursing homes, specialized dementia and residential care homes and long-term care facilities (n=10) (Fung & Tsang, 2018; Kwan et al., 2016, 2017; Kwok et al., 2013; Lin et al., 2009; Rodríguez-Mansilla et al., 2015; Simoncini et al., 2015; Sutherland et al., 1999; Yang et al., 2015; Yang et al., 2007). Two studies took place in a hospitalized setting (Shi et al., 2012, 2015). One study was conducted in the home (Jia et al., 2017), and one included participants from an inpatient and outpatient neurology department (Y. Wang et al., 2014). One study did not specify the setting where the study was conducted (Lombardo et al., 2001)
Most of the studies used a randomized controlled trial design with 2 or 3 arms (n=8) (Fung & Tsang, 2018; Jia et al., 2017; Kwan et al., 2017; Lin et al., 2009; Rodríguez-Mansilla et al., 2015; Shi et al., 2015; Y. Wang et al., 2014; Yang et al., 2015). Four of these studies were double-blinded (Kwan et al., 2017; Rodríguez-Mansilla et al., 2015; Y. Wang et al., 2014; Yang et al., 2015). Six studies used single group designs such as repeated measures, time-serial and longitudinal prospective designs (Kwan et al., 2016; Kwok et al., 2013; Lombardo et al., 2001; Shi et al., 2012; Simoncini et al., 2015; Yang et al., 2007). One study used a quasi-experimental design with purposive sampling and random assignment of participants to the experimental and control groups (Sutherland et al., 1999).
Intervention Procedures
Intervention procedures varied widely among the 15 studies (See Tables 2 and 3). Two studies used acupressure or acupuncture treatments in combination with medication (donepezil) for improving memory in dementia (Jia et al., 2017; Y. Wang et al., 2014). Three studies used multi- modal treatments that involved acupressure therapy in combination with another alternative treatment, such as massage, Montessori-based activities, and aromatherapy. (Fung & Tsang, 2018; Lin et al., 2009; Yang et al., 2015).
Duration of treatment sessions differed throughout the studies, while some studies did not report duration. There was also a variation in the acupuncture points selected for each intervention method. Some studies reported the specific acupoints used, while others did not. Only one study examined optimal dosage of acutherapy for BPSD by evaluating what dosage and frequency yielded the most significant effect on agitation in people with dementia. The optimal dosage of acupressure therapy for agitation was determined to be twice a day for two weeks (Kwan et al., 2016). The findings of this pilot study were then used in a larger RCT (Kwan et al., 2017).
Six of the studies included interventions performed by professional acupuncturists (Jia et al., 2017; Kwok et al., 2013; Lombardo et al., 2001; Rodríguez-Mansilla et al., 2015; Shi et al., 2012, 2015). Four of the studies demonstrated that non-professional and non-healthcare trained individuals could feasibly implement acupressure therapy in people with dementia (Fung & Tsang, 2018; Kwan et al., 2016, 2017; Lin et al., 2009). One study included nurse implemented acupressure (Simoncini et al., 2015).
Symptoms and Measurements
Table 5 summarizes the BPSD outcomes that were targeted in the 15 studies (See tables 2 and 3 for measures used in each study) including those for which there was significant improvement. Agitation and ADL function were the most commonly measured outcomes. Four studies included multiple BPSD outcome measures (Fung & Tsang, 2018; Jia et al., 2017; Lombardo et al., 2001; Simoncini et al., 2015).
Table 5.
Behavioral and Psychological Symptoms of Dementia Evaluated in Acupuncture and Acupressure Studies
| BPSD Outcomes | Number of Studies that Evaluated Outcome (N=15) |
Number of Studies with Statistically Significant Improvement in Outcomes (%) |
|---|---|---|
| ADL’s | 8 | 6 (75%) |
| Agitation | 6 | 6 (100%) |
| Anxiety | 3 | 2 (67%) |
| Depression | 2 | 2 (100%) |
| Mood | 1 | 1 (100%) |
| Neuropsychological disturbances | 3 | 2 (67%) |
| Sleep disturbance | 2 | 2 (100%) |
| Total | 25 | 21 (84%) |
Eight studies included ADL function as an outcome variable (Fung & Tsang, 2018; Jia et al., 2017; Lin et al., 2009; Shi et al., 2012, 2015; Simoncini et al., 2015; Y. Wang et al., 2014; Yang et al., 2007). Six of these studies showed statistically significant improvement in ADL scores post-intervention (Fung & Tsang, 2018; Lin et al., 2009; Shi et al., 2015; Simoncini et al., 2015; Y. Wang et al., 2014; Yang et al., 2007). The ADCS-ADL and Barthel Index were used to measure ADLs, both instruments have been validated and demonstrated good reliability in older adults with dementia (Galasko et al., 1997; Sheehan, 2012).
Six studies included agitation as an outcome measure and all demonstrated significant improvements in agitation scores after the acupuncture or acupressure therapies (Fung & Tsang, 2018; Kwan et al., 2016, 2017; Lin et al., 2009; Yang et al., 2015; Yang et al., 2007). The Cohen-Mansfield Agitation Inventory was used as a measurement tool for agitation in five studies and has good reliability in people with dementia (Cohen-Mansfield, 1986).
Three studies evaluated anxiety as an outcome (Lombardo et al., 2001; Rodríguez-Mansilla et al., 2015; Simoncini et al., 2015) and two found significant improvement. The study that did not demonstrate improvement in anxiety attributed it to many participants with low anxiety at baseline (Simoncini et al., 2015). The Spielberger State‐Trait Anxiety Inventory was used in two studies; it is known to have a demonstrated bias of increased anxiety for geriatric adults, which may be confounded by a decreased well-being in this population (Kvaal, Laake, & Engedal, 2001). The Campbell scale was used in one study (Rodríguez-Mansilla et al., 2015); the validity and reliability of this measure in the geriatric or dementia population is unknown.
The outcome of depression was measured in two studies (Lombardo et al., 2001; Rodríguez-Mansilla et al., 2015), both of which reported significant improvement in depression scores after the acupuncture or acupressure intervention. The Cornell Scale for Depression in Dementia (CSDD) was used to measure depression – a tool that is commonly used to measure depression in people with dementia and validated for this population (Alexopoulos, Abrams, Young, & Shamoian, 1988).
Mood was an uncommon outcome measure and was only examined in one study. This variable was measured using the Profile of Mood States (POMS) instrument (Lombardo et al., 2001). This study did see an improvement in the POMS scores. The validity of this tool has been established in an older adult sample; however, this sample did not include people with cognitive impairment (Kaye et al., 1988).
Neuropsychologic disturbances were examined in three studies (Fung & Tsang, 2018; Jia et al., 2017; Simoncini et al., 2015). The study by Jia et al. did not demonstrate significant improvement in this outcome variable; however, did demonstrate significant improvement in cognitive function measures after the intervention (2017). The Neuropsychiatric Inventory was used to measure neuropsychological disturbances in all three studies. This tool is widely used for persons with dementia and has been shown to have high inter-rater reliability and consistency (Cummings et al., 1994). This scale measures a wide array of symptoms including hallucinations, delusions, agitation/aggression, dysphoria/depression, anxiety, irritability, disinhibition, euphoria, apathy, aberrant motor behavior.
Finally, sleep disturbance was included as an outcome in two studies (Kwok et al., 2013; Simoncini et al., 2015). Both studies demonstrated improvement in the sleep post-intervention. Wrist actigraphy was used in one study (Kwok et al., 2013), while the Pittsburgh Sleep Quality Index (PSQI) was used in the other (Simoncini et al., 2015). The PSQI has been used in a nursing home population and showed to have good reliability scores; however, the sample included only cognitively intact residents (Gentili, Werner, Kuchibhatla, & Edinger, 1995). Limited information was found on the reliability in people with dementia.
Six studies included physiologic outcome measures. Kwok et al. included wrist actigraphy to measure sleep/rest cycles (2013). The 2012 study by Shi et al. included urine samples to detect an oxidative marker indicative of DNA damage. Kwan et al. (2016/2017). used salivary cortisol samples to evaluate stress. The results of these studies indicated the acupressure treatment group had a significant reduction in salivary cortisol levels, suggesting a reduction in stress.
Safety
No adverse effects were reported in the 15 studies. One acupuncture study reported that 10.8% of participants experienced punctate hemorrhage when acupuncture needles were withdrawn (bleeding stopped within 5–10 seconds of holding dry, sterile cotton to the sites) (Jia et al., 2017). Another study stated that 25% of participants reported mild discomfort at the acupoint sites during treatments and 20% experienced mild bruising (Shi et al., 2015). These side effects were reported as non-significant in both studies.
Discussion
The results of this review suggest acupuncture and acupressure therapy have the potential to improve BPSD. All 15 of the studies indicated the safety and overall satisfaction of acupuncture and acupressure treatments in individuals with dementia. All of the studies demonstrated statistically significant (p<0.05) improvements in at least one targeted BPSD outcome, except for two of the acupuncture studies. The two studies that did not demonstrate improvements in the BPSD of interest did report significant improvements in other important outcomes, including cognitive function outcomes and quality of life (Jia et al., 2017; Shi et al., 2012). There is an ongoing need for safe and effective non-pharmacologic treatment options for BPSD for individuals in all stages of the disease. Findings relating to cognitive impairment severity across studies suggest that these therapies are feasible and safe for individuals with mild, moderate and severe cognitive impairment. The significant BPSD findings suggest that these interventions may be reasonable options for BPSD management. Acupuncture and acupressure offer an area for further scientific and practical examination for treating BPSD.
Although the findings were encouraging, due to variations in study designs and measures, as well as incomplete explanations of intervention procedures (such as use of specific acupoints, acutherapy dose, and who carried out the interventions) it is difficult to determine exact conclusions about the efficacy of these therapies. Given the methodological flaws in these studies including the limited theoretical basis for the interventions and symptoms, differences in measures, variations in study designs and intervention dosage, and overall limitations in internal and external validity, there remains a gap in the knowledge and quality of evidence needed to use acupuncture and acupressure for BPSD.
There is a need for additional research evaluating the use of acupuncture and acupressure, specifically in countries with a Westernized approach to medical care where acutherapy is not as widely used. The majority of the studies were conducted in China, as acutherapy is rooted in theories of Traditional Chinese Medicine; however, acupuncture and acupressure have the potential to expand the understanding and treatment of many diagnoses and symptoms in Western society. Therefore, additional studies conducted in western healthcare systems are needed to support the generalizability of acupuncture and acupressure treatments for BPSD.
Many families, caregivers and friends of diagnosed individuals strive to keep the person with dementia living in the home setting, as this is where they feel most safe, secure and comfortable. In fact, the majority of individuals diagnosed with dementia reside in the home with family caregivers (Alzheimer’s Association, 2018). BPSD are often distressing to family members and friends and can become unmanageable, which can lead to early placement to long-term care settings. Despite the impact that BPSD have on home-dwelling individuals with dementia and their family caregivers, the majority of the studies (n=10/15) were conducted in long-term care facilities. Additional research is needed to evaluate the feasibility of applying these interventions in the home. Furthermore, as family caregivers often play a pivotal role in directing care for their loved ones with dementia, it is important for future research to address the perceptions of family caregivers regarding use of acutherapy as a complementary symptom management strategy. To our knowledge, no studies have examined family caregivers’ perceptions regarding use of these therapies. Acupuncture and acupressure have the potential to improve the quality of life of those with dementia and their family caregivers’, which could ultimately reduce the risk of long-term care placement.
Acupuncture is often performed by licensed acupuncturists and state level regulations may require acupuncturists to work under the supervision of a medical doctor, which can be a limitation for applying this intervention in the home. Alternatively, this review supports that acupressure can feasibly be performed by a non-professional given adequate training and education. Acupressure presents as an opportunity to support informal caregivers (such as family members and friends) in having complementary treatment options for better managing BPSD.
Due to the limited efficacy of psychotropic medications in this population, there is an ever-increasing need for complementary treatment options for nursing staff and healthcare providers to better care for people with dementia. Nurses play a critical role in being educated on and educating others on these alternative treatments for distressing symptoms of dementia, especially due to the vulnerability of this population that has few other effective pharmacologic treatment options. Acupuncture and acupressure therapies represent promising treatment options for nursing staff to implement in a number of settings including long-term care facilities, memory care units, inpatient geriatric-psych units and medical units that provide care to people with dementia. There is a need to examine the feasibility of formal caregivers in providing acutherapy to people with dementia. Additionally, an evaluation of the costs and benefits is needed to further support the organizational use of these interventions in such settings. This is especially significant as nursing staff are in a meaningful position to incorporate these interventions into many areas of practice.
From an organizational perspective, many national and state level entities exist to educate healthcare professionals, family members, friends and those diagnosed with dementia regarding BPSD management techniques. These organizations can empower healthcare professionals and families with knowledge regarding these complementary therapies in times when other, more traditional approaches, such as psychotropic medications fail, or when they cause more harm than healing. Organizations such as the Alzheimer’s Association and the Alzheimer’s Foundation of America present as significant opportunities to promote education for informal and formal caregivers alike regarding alternative and complementary approaches to dementia symptom management.
The policy implications of this work are based on the fact that national programs, such as Medicare, currently cover pharmacologic interventions for dementia care; however, there is limited and/or conditional coverage for non-pharmacologic therapies and psychiatric care. As the evidence for non-pharmacologic treatments continues to grow, there will be a need for policy makers to advocate for better coverage for these treatments.
Behavioral and psychological symptoms are highly prevalent in persons with dementia. They can be physically and mentally devastating to the person with dementia, as well as the caregiver. BPSD ultimately threatens a person’s ability to remain in the home, resulting in negative effects on society due to increased healthcare utilization and cost. Due to the increased susceptibility to adverse effects of pharmacologic treatment in this population, there is an ever-increasing need to include complementary therapies in the management of BPSD.
In conclusion, this review evaluated the current research relevant to acupuncture and acupressure treatments for BPSD. Fifteen studies were included in the final review and findings tended to be in support of acupuncture and acupressure for symptoms such as agitation, anxiety, ADL function, sleep, mood, depression, and neuropsychological disturbances. Limitations in study designs, intervention procedures and outcome measures limit the interpretations regarding efficacy of acupuncture and acupressure for BPSD. Additionally, concerns relating to generalizability limit the ability to make broad statements in support of the use of acutherapy in practice. It is recommended that additional rigorous research and randomized controlled trials be conducted to further examine the efficacy of these therapies in Western healthcare systems and in settings other than long term care. Nurses play a critical role in promoting the use of non-pharmacologic treatments for BPSD management. Having multiple treatment options is necessary for improving the health and wellness in patients with dementia. Acupuncture and acupressure present as promising therapies for improving distressing BPSD; however, further research is needed to determine efficacy and promote generalizability.
Acknowledgments
Funded by the Predoctoral Fellowship Training Grant (T32 NR016914. Program Director: Titler) Complexity: Innovations in Promoting Health and Safety. 7/1/2017 - 6/30/2022.
Appreciation and acknowledgement are extended to Dr. Barbara L. Brush, PhD, ANP-BC, FAAN for sharing her knowledge and support on the scoping review process, as well as for the support and guidance from the University of Michigan Taubman Library informationalists, Kate Saylor and Jackie Freeman, in designing and conducting the database search.
References
- Aalten P, de Vugt ME, Jaspers N, Jolles J, & Verhey FRJ (2005). The course of neuropsychiatric symptoms in dementia. Part I: Findings from the two-year longitudinal Maasbed study. International Journal of Geriatric Psychiatry, 20(6), 523–530. 10.1002/gps.1316 [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Abrams RC, Young RC, & Shamoian CA (1988). Cornell scale for depression in dementia. Biological Psychiatry, 23(3), 271–284. 10.1016/0006-3223(88)90038-8 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2018). Alzheimer’s disease facts and figures. https://www.alz.org/media/HomeOffice/Facts%20and%20Figures/facts-and-figures.pdf
- Amorim D, Amado J, Brito I, Fiuza SM, Amorim N, Costeira C, & Machado J (2018). Acupuncture and electroacupuncture for anxiety disorders: A systematic review of the clinical research. Complementary Therapies in Clinical Practice, 31, 31–37. 10.1016/j.ctcp.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Arksey H, & O’Malley L (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- Austrom MG, Boustani M, & LaMantia MA (2018). Ongoing medical management to maximize health and well-being for persons living with dementia. The Gerontologist, 58(Suppl. 1), S48–S57. 10.1093/geront/gnx147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Smith SC, Lamping DL, Harwood RH, Foley B, Smith P, . . . Knapp M(2006). Quality of life in dementia: More than just cognition. An analysis of associations with quality of life in dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 77(2), 146–148. 10.1136/jnnp.2005.072983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RJ, Wang S, Ju H, & Graber D (2004). Analyses of gender differences in profiles of nursing home residents with Alzheimer’s disease. Gender Medicine, 1(1), 48–59. 10.1016/S1550-8579(04)80010-X [DOI] [PubMed] [Google Scholar]
- Campbell P, Wright J, Oyebode J, Job D, Crome P, Bentham P, . . . Lendon C (2008). Determinants of burden in those who care for someone with dementia. International Journal of Geriatric Psychiatry, 23(10), 1078–1085. 10.1002/gps.2071 [DOI] [PubMed] [Google Scholar]
- Cerejeira J, Lagarto L, & Mukaetova-Ladinska E (2012). Behavioral and psychological symptoms of dementia. Frontiers in Neurology, 3(73). 10.3389/fneur.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco E, Gatti M, de Mendonça Lima CA, & Camus V (2003). A comparative study of stress and burnout among staff caregivers in nursing homes and acute geriatric wards. International Journal of Geriatric Psychiatry, 18(1), 78–85. 10.1002/gps.800 [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J (1986). Agitated Behaviors in the Elderly: II. Preliminary results in the cognitively deteriorated. Journal of the American Geriatrics Society, 34(10), 722–727. 10.1111/j.1532-5415.1986.tb04303.x [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, & Gornbein J (1994). The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308–2308. 10.1212/WNL.44.12.2308 [DOI] [PubMed] [Google Scholar]
- Defrancesco M, Marksteiner J, Fleischhacker WW, & Blasko I (2015). Use of benzodiazepines in Alzheimer’s Disease: A systematic review of literature. International Journal of Neuropsychopharmacology, 18(10), pyv055. 10.1093/ijnp/pyv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JL, Krings T, Weidemann J, Meister IG, & Thron A (2004). Functional MRI in healthy subjects during acupuncture: Different effects of needle rotation in real and false acupoints. Neuroradiology, 46(5), 359–362. 10.1007/s00234-003-1125-7 [DOI] [PubMed] [Google Scholar]
- Finkel S, Silva JCE, Cohen G, Miller S, & Sartorius N (1997). Behavioral and psychological signs and symptoms of dementia: A consensus statement on current knowledge and implications for research and treatment. International Journal of Geriatric Psychiatry, 12(11), 1060–1061. [DOI] [PubMed] [Google Scholar]
- Fung J, & Tsang H (2018). Management of behavioural and psychological symptoms of dementia by an aroma‐massage with acupressure treatment protocol: A randomised clinical trial. Journal of Clinical Nursing, 27(9/10), 1812–1825. 10.1111/jocn.14101 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, & Ferris S (1997). An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Disease & Associated Disorders, 11, 33–39. [PubMed] [Google Scholar]
- Gaugler JE, Yu F, Krichbaum K, & Wyman JF (2009). Predictors of nursing home admission for persons with dementia. Medical Care, 47(2), 191–198. [DOI] [PubMed] [Google Scholar]
- Gentili A, Werner DK, Kuchibhatla M, & Edinger JD (1995). Test-retest reliability of the Pittsburgh Sleep Quality Index in nursing home residents Journal of the American Geriatrics Society, 43(11), 1317–1318. 10.1111/j.1532-5415.1995.tb07415.x [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Dennis MP, Hodgson N, & Hauck WW (2010). Targeting and managing behavioral symptoms in individuals with dementia: A randomized trial of a nonpharmacological intervention. Journal of the American Geriatrics Society, 58(8), 1465–1474. 10.1111/j.1532-5415.2010.02971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NL, Emery T, & Hodges JR (2004). Distinctive cognitive profiles in Alzheimer’s disease and subcortical vascular dementia. Journal of Neurology, Neurosurgery & Psychiatry, 75(1), 61–71. [PMC free article] [PubMed] [Google Scholar]
- Hall KA, & O’Connor DW (2004). Correlates of aggressive behavior in dementia. International Psychogeriatrics, 16(2), 141–158. 10.1017/S1041610204000286 [DOI] [PubMed] [Google Scholar]
- Han J-S (2003). Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends in Neurosciences, 26(1), 17–22. 10.1016/S0166-2236(02)00006-1 [DOI] [PubMed] [Google Scholar]
- Hurt C, Bhattacharyya S, Burns A, Camus V, Liperoti R, Marriott A, . . . Byrne EJ (2008). Patient and caregiver perspectives of quality of life in Dementia. Dementia and Geriatric Cognitive Disorders, 26(2), 138–146. 10.1159/000149584 [DOI] [PubMed] [Google Scholar]
- Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, . . . Yaffe K. (2008). ACNP white paper: Update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology, 33(5), 957–970. 10.1038/sj.npp.1301492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhang X, Yu J, Han J, Yu T, Shi J, . . . Nie K. (2017). Acupuncture for patients with mild to moderate Alzheimer’s disease: a randomized controlled trial. BMC Complemententary and Alternative Medicine, 17(1), 556 10.1186/s12906-017-2064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN, & Ballard C (1999). Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Disease and Associated Disorders, 13(Suppl. 3), S115–S123. 10.1097/00002093-199912003-00017 [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ (2002). Acupuncture: Theory, efficacy, and practice. Annals of Internal Medicine, 136(5), 374–383. 10.7326/0003-4819-136-5-200203050-00010 [DOI] [PubMed] [Google Scholar]
- Kar N (2009). Behavioral and psychological symptoms of dementia and their management. Indian Journal of Psychiatry, 51(Suppl. 1), S77–S86. [PMC free article] [PubMed] [Google Scholar]
- Kaye JM, Lawton MP, Gitlin LN, Kleban MH, Windsor LA, & Kaye D (1988). Older people’s performance on the Profile of Mood States (POMS). Clinical Gerontologist, 7(3–4), 35–56. 10.1300/J018v07n03_05 [DOI] [Google Scholar]
- Kim SY, Shin IS, & Park YJ (2018). Effect of acupuncture and intervention types on weight loss: a systematic review and meta-analysis. Obesity Reviews, 19(11), 1585–1596. 10.1111/obr.12747 [DOI] [PubMed] [Google Scholar]
- Kou R. z., Chen H, Yu M. l., Xu T. c., Fu S. p., & Lu S. f. (2017). Acupuncture for behavioral changes of experimental depressive disorder: A systematic review and meta-analysis. Scientific Reports, 7, 9669 10.1038/s41598-017-09712-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal K, Laake K, & Engedal K (2001). Psychometric properties of the state part of the Spielberger State-Trait Anxiety Inventory (STAI) in geriatric patients. International Journal of Geriatric Psychiatry, 16(10), 980–986. 10.1002/gps.458 [DOI] [PubMed] [Google Scholar]
- Kwan R, Leung M, & Lai C (2016). The effect of acupressure on agitation and salivary cortisol in people with dementia: A pilot study. Journal of Alternative and Complementary Medicine, 22(11), 903–910. 10.1089/acm.2016.0062 [DOI] [PubMed] [Google Scholar]
- Kwan R, Leung M, & Lai C (2017). A randomized controlled trial examining the effect of acupressure on agitation and salivary cortisol in nursing home residents with dementia. Dementia and Geriatric Cognitive Disorders, 44(1–2), 92–104. 10.1159/000478739 [DOI] [PubMed] [Google Scholar]
- Kwok T, Leung PC, Wing YK, Ip I, Wong B, Ho DWH, . . . Ho F (2013). The effectiveness of acupuncture on the sleep quality of elderly with dementia: A within-subjects trial. Clinical Interventions in Aging, 8, 923–929. 10.2147/CIA.S45611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, & Ernst E (2011). Acupuncture for pain: An overview of Cochrane reviews. Chinese Journal of Integrative Medicine, 17(3), 187–189. 10.1007/s11655-011-0665-7 [DOI] [PubMed] [Google Scholar]
- Levac D, Colquhoun H, & O’Brien KK (2010). Scoping studies: Advancing the methodology. Implementation Science, 5(1), 69 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q-Q, Shi G-X, Xu Q, Wang J, Liu C-Z, & Wang L-P (2013). Acupuncture effect and central autonomic regulation. Evidence-based Complementary and Alternative Medicine, 267959. 10.1155/2013/267959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-C, Yang M-H, Kao C-C, Wu S-C, Tang S-H, & Lin J-G (2009). Using Acupressure and Montessori-based activities to decrease agitation for residents with dementia: A cross-over trial. Journal of the American Geriatrics Society, 57(6), 1022–1029. 10.1111/j.1532-5415.2009.02271.x [DOI] [PubMed] [Google Scholar]
- Lombardo N, Dresser M, Malivert M, McManus C, Vehvilainen L, Ooi WL, . . . Perry K. (2001). Acupuncture as treatment for anxiety and depression in persons with dementia: Results of a feasibility and effectiveness study. Alzheimer’s Care Quarterly, 2(4), 28–41. [Google Scholar]
- Lövheim H, Sandman P-O, Karlsson S, & Gustafson Y (2008). Behavioral and psychological symptoms of dementia in relation to level of cognitive impairment. International Psychogeriatrics, 20(4), 777–789. 10.1017/S1041610208006777 [DOI] [PubMed] [Google Scholar]
- Lövheim H, Sandman P-O, Karlsson S, & Gustafson Y (2009). Sex differences in the prevalence of behavioral and psychological symptoms of dementia. International Psychogeriatrics, 21(3), 469–475. 10.1017/S1041610209008497 [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, & DeKosky S (2002). Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA, 288(12), 1475–1483. 10.1001/jama.288.12.1475 [DOI] [PubMed] [Google Scholar]
- Margallo-Lana M, Swann A, O’Brien J, Fairbairn A, Reichelt K, Potkins D, . . . Ballard C (2001). Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. International Journal of Geriatric Psychiatry, 16(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Maust DT, Kim H, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, & Kales HC (2015). Antipsychotics, other psychotropics, and the risk of death in patients with dementia: Number needed to harm. JAMA Psychiatry, 72(5), 438–445. 10.1001/jamapsychiatry.2014.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, & Aromataris E (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology, 18(1), 143 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, & Soares CB (2015). Guidance for conducting systematic scoping reviews. International Journal of Evidence-Based Healthcare, 13(3), 141–146. 10.1097/xeb.0000000000000050 [DOI] [PubMed] [Google Scholar]
- Peters MD, McInerney P, Soares CB, Khalil H, & Parker D (2015). Chapter 11. Methodology for JBI scoping reviews In Aromataris E & Munn Z (Eds.) Joanna Briggs Institute Reviewers’ Manual: 2015 edition. Australia: The Joanna Briggs Institute. [Google Scholar]
- Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, . . . Yager J (2016). The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. American Journal of Psychiatry, 173(5), 543–546. 10.1176/appi.ajp.2015.173501 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Mansilla J, González López-Arza MV, Varela-Donoso E, Montanero-Fernández J, González Sánchez B, & Garrido-Ardila EM (2015). The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: A randomized controlled trial. Clinical Rehabilitation, 29(7), 683–693. 10.1177/0269215514554240 [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, & Davis KL (1984). A new rating scale for Alzheimer’s disease. American Journal of Psychiatry, 141(11), 1356–1364. 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- Schnaider Beeri M, Werner P, Davidson M, & Noy S (2002). The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. International Journal of Geriatric Psychiatry, 17(5), 403–408. 10.1002/gps.490 [DOI] [PubMed] [Google Scholar]
- Seyfried LS, Kales HC, Ignacio RV, Conwell Y, & Valenstein M (2011). Predictors of suicide in patients with dementia. Alzheimer’s & Dementia, 7(6), 567–573. 10.1016/j.jalz.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Wang JJ, Wang ZQ, Zhao ZL, Zhang M, Xu JY, . . . Lu J. (2018). Neuronal specificity of acupuncture in Alzheimer’s disease and nild cognitive impairment patients: A functional MRI study. Evidence-Based Complementary and Alternative Medicine, 2018. 10.1155/2018/7619197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan B (2012). Assessment scales in dementia. Therapeutic advances in neurological disorders, 5(6), 349–358. 10.1177/1756285612455733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GX, Li QQ, Yang BF, Liu Y, Guan LP, Wu MM, . . . Liu CZ. (2015). Acupuncture for vascular dementia: A pragmatic randomized clinical trial. Scientific World Journal, 2015, 1–8. 10.1155/2015/161439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GX, Liu CZ, Li QQ, Zhu H, & Wang LP (2012). Influence of acupuncture on cognitive function and markers of oxidative DNA damage in patients with vascular dementia. Journal of Traditional Chinese Medicine, 32(2), 199–202. [DOI] [PubMed] [Google Scholar]
- Simoncini M, Gatti A, Quirico PE, Balla S, Capellero B, Obialero R, . . . Pernigotti LM. (2015). Acupressure in insomnia and other sleep disorders in elderly institutionalized patients suffering from Alzheimer’s disease. Aging Clinical and Experimental Research, 27(1), 37–42. 10.1007/s40520-014-0244-9 [DOI] [PubMed] [Google Scholar]
- Steffens DC, Maytan M, Helms MJ, & Plassman BL (2005). Prevalence and clinical correlates of neuropsychiatric symptoms in dementia. American Journal of Alzheimer’s Disease & Other Dementias, 20(6), 367–373. 10.1177/153331750502000611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M, Corcoran C, Tschanz JT, Huber C, Welsh-Bohmer K, Norton MC, . . . Lyketsos CG. (2006). Risk factors for neuropsychiatric symptoms in dementia: The Cache County Study. International Journal of Geriatric Psychiatry, 21(9), 824–830. 10.1002/gps.1567 [DOI] [PubMed] [Google Scholar]
- Steinberg M, Tschanz JT, Corcoran C, Steffens DC, Norton MC, Lyketsos CG, & Breitner JCS (2004). The persistence of neuropsychiatric symptoms in dementia: The Cache County Study. International Journal of Geriatric Psychiatry, 19(1), 19–26. 10.1002/gps.1025 [DOI] [PubMed] [Google Scholar]
- Sutherland JA, Reakes J, & Bridges C (1999). Clinical scholarship -- abstract. Foot acupressure and massage for patients with Alzheimer’s disease and related dementias. Journal of Nursing Scholarship, 31(4), 347–348. [Google Scholar]
- Van Strien AM, Koek HL, Van Marum RJ, & Emmelot-Vonk MH (2013). Psychotropic medications, including short acting benzodiazepines, strongly increase the frequency of falls in elderly. Maturitas, 74(4), 357–362. 10.1016/j.maturitas.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Vickers AJ (1998). Bibliometric analysis of randomized trials in complementary medicine. Complementary Therapies in Medicine, 6(4), 185–189. 10.1016/S0965-2299(98)80026-5 [DOI] [Google Scholar]
- Waldorff FB, Buss DV, Eckermann A, Rasmussen MLH, Keiding N, Rishøj S, . . . Waldemar G. (2012). Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: The multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ (Clinical research ed.), 345, e4693-e4693. 10.1136/bmj.e4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qin WG, & Yu CD (2014). Clinical observation on effect of cranial suture acupuncture combined with donepezil hydrochloride tablets for Alzheimer’s disease. World Journal of Acupuncture - Moxibustion, 24(2), 19–24. 10.1016/S1003-5257(14)60020-9 [DOI] [Google Scholar]
- Wang Z, Nie B, Li D, Zhao Z, Han Y, Song H, . . . Li K. (2012). Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. Public Library of Science, 7(8), e42730-e42730. 10.1371/journal.pone.0042730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018). Towards a dementia plan: A WHO guide. https://www.who.int/mental_health/neurology/dementia/en/
- Wu J, Yeung AS, Schnyer R, Wang Y, & Mischoulon D (2012). Acupuncture for depression: A review of clinical applications. The Canadian Journal of Psychiatry, 57(7), 397–405. 10.1177/070674371205700702 [DOI] [PubMed] [Google Scholar]
- Yang MH, Lin L, Wu S, Chiu J, Wang P, & Lin J (2015). Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complementary & Alternative Medicine, 15, 93 10.1186/s12906-015-0612-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Wu S, Lin J, & Lin L (2007). The efficacy of acupressure for decreasing agitated behaviour in dementia: A pilot study. Journal of Clinical Nursing, 16(2), 308–315. 10.1111/j.1365-2702.2006.01428.x [DOI] [PubMed] [Google Scholar]
- Zhou J, Peng W, Xu M, Li W, & Liu Z (2015). The effectiveness and safety of acupuncture for patients with Alzheimer disease: A systematic review and meta-analysis of randomized controlled trials. Medicine, 94(22), e933 10.1097/MD.0000000000000933 [DOI] [PMC free article] [PubMed] [Google Scholar]