Abstract
Purpose:
To compare disease severity between pre-perimetric primary open-angle glaucoma (POAG) patients with and without deep-layer microvasculature dropout.
Methods:
Ninety-four eyes of 94 pre-perimetric POAG patients with β-zone parapapillary atrophy (βPPA) were categorized according to the presence of deep-layer microvasculature dropout defined as a complete loss of microvasculature within the choroid or scleral flange on optical coherence tomography angiography. Parameters representing disease severity i.e., VF mean deviation (MD), global and sectoral (6-sector) retinal nerve fiber layer (RNFL) thickness, as well as other factors including age, focal lamina cribrosa (LC) defect, width of βPPA with and without Bruch’s membrane (BM) (βPPA+BM and βPPA-BM), and optic disc hemorrhage (DH) were compared between eyes with and without dropout.
Results:
Deep-layer microvasculature dropout was observed in 33 pre-perimetric POAG eyes (35.1%). Eyes with dropout had significantly thinner RNFL in all areas except the inferonasal sector, worse VF MD, as well as higher prevalence of focal LC defect, and larger βPPA-BM (P<0.05), whereas the two groups did not differ in age, DH or βPPA+BM width (P>0.05). In the multivariable logistic regression, worse VF MD (odds ratio [OR], 1.485; P = 0.045), thinner RNFL (OR, 1.141; P<0.001), and higher prevalence of focal LC defect (OR, 6.673; P<0.001) were significantly associated with dropout.
Conclusions:
Deep-layer microvasculature dropout was observed in a considerable number of pre-perimetric POAG eyes, and worse disease severity was associated with dropout. Future studies elucidating the pathogenic role of deep-layer microvasculature dropout in the development and progression of glaucoma are warranted.
Keywords: Microvasculature dropout, Pre-perimetric glaucoma
Introduction
Glaucoma is known to be a multifactorial disease, and vascular mechanisms have been proposed to contribute to its development and progression. In this regard, the recent development of optical coherence tomography angiography (OCT-A) has enabled non-invasive visualization of both superficial and deep-layer microvasculature damage.1–4 Moreover, deep-layer microvasculature dropout, defined as complete loss of the parapapillary choriocapillaris and sclera, has been reported to be associated with development and progression of glaucomatous optic nerve head (ONH) damage.2, 5–12 Specifically, recent studies have suggested that the deep-layer microvasculature serves as a marker indicating worse glaucoma severity (i.e., thinner retinal nerve fiber layer (RNFL) and worse visual field (VF) damage).2, 7, 8, 10 However, prior investigations of microvasculature dropout have focused mainly on perimetric glaucoma, and have reported that the dropout was often observed in moderate-to-advanced glaucoma.2, 7, 8 Whether the dropout is also observed in early glaucoma, especially with normal VF, has not been reported. Delineating this relationship is relevant to determining whether deep-layer microvasculature loss is an epiphenomenon of the glaucomatous ONH damage or it can serve as a potential sign of early glaucoma. Moreover, comparison of the disease severity between pre-perimetric glaucomatous eyes with and without dropout is of clinical relevance since it is often difficult to grade disease severity in eyes with normal standard VF testing.
Therefore, the purpose of the present study was to determine whether deep-layer microvasculature damage also develops in early glaucomatous eyes with normal VF, and we also ascertain whether they have worse disease severity than those without dropout.
Materials and Methods
Study Subjects
This study consecutively included pre-perimetric primary open-angle glaucoma (POAG) patients who had visited the Haeundae Paik Hospital Glaucoma Clinic between January 2017 and September 2018. It was approved by the Institutional Review Board at Haeundae Paik Hospital. Informed consent was obtained from all of the subjects.
Pre-perimetric POAG was defined as signs of glaucomatous optic nerve damage (i.e., excavation of the optic disc, neuroretinal rim thinning, or RNFL loss that preferentially involve the superior and inferior area of the ONH) based on assessment of stereo photographs by two independent graders (M.H.S. and J.H.N.), the presence of an open angle, and normal VF results. A normal VF result was defined as a normal glaucoma hemifield test and mean deviation (MD) and pattern standard deviation (PSD) within the 95% confidence limits on at least two consecutive VF examinations.11
For inclusion in this study, pre-perimetric POAG patients were required to have visible β-zone parapapillary atrophy (βPPA) without retinal pigment epithelium (RPE) on fundus imaging with a temporal width ≥ 100 μm on at least 1 radial scan measured by the built-in caliper of spectral-domain (SD) OCT, and visual acuity ≥ 20/40.5, 8, 12,15,16 Subjects with a history of ocular intervention (except for uncomplicated cataract or glaucoma surgery) or systemic or intraocular disease that could influence the study results were excluded. Those with systemic hypertension and diabetes mellitus were included unless they had been diagnosed with diabetic or hypertensive retinopathy.5, 8, 14
All of the subjects underwent complete ophthalmic examinations, including best-corrected visual acuity, refraction, slit-lamp biomicroscopy, intraocular pressure (IOP) by Goldmann applanation tonometry, gonioscopy, pachymetry measured by the Pentacam Scheimpflug imaging system (Oculus Optikgeräte GmbH, Germany), axial length measured with the intraocular lens (IOL) Master (Carl Zeiss Meditec, Dublin, CA), dilated fundus examination with simultaneous color and red-free fundus photography (TRC-NW8; Topcon, Tokyo, Japan), standard automated perimetry (SAP) (Humphrey Field Analyzer; 30–2 Swedish interactive threshold algorithm; Carl-Zeiss Meditec), SD-OCT and OCT-A (Spectralis; Heidelberg Engineering GmbH, Heidelberg, Germany). All of the imaging tests and perimetry were conducted within a 3-month period. Systolic and diastolic blood pressures (BPs) were measured at the height of the heart with an automatic BP instrument (Model Easy X 800 (R/L), JAWON Medical Co. Ltd., Kyungsan, Korea). Mean ocular perfusion pressure (MOPP) was calculated as the difference between 2/3 of the mean arterial pressure (calculated as 1/3 systolic BP + 2/3 diastolic BP) and the IOP. Based on standardized review of fundus photographs, optic disc hemorrhage (DH) was defined as an isolated splinter or flame-shaped hemorrhage on the ONH.
Spectral-Domain Optical Coherence Tomography Imaging
Circumpapillary RNFL thickness, Bruch’s membrane opening (BMO) area, and βPPA microstructure were assessed by Spectralis OCT2 Glaucoma Module Premium Edition (GMPE) software (version 1.9.17.0) (Spectralis; Heidelberg Engineering GmbH, Heidelberg, Germany) based on the ONH Radial Circle scan pattern and 24 consecutive radial B scans aligned according to the fovea-to-BMO center axis. RNFL thickness was calculated at each point on a set-diameter (3.5 mm) circle in a global area and in the 6 sectors (superotemporal (TS), inferotemporal (TI), temporal (T), superonasal (NS), inferonasal (NI), nasal (N)) (Figs A4 and B4). The temporal width of βPPA+BM defined as an area without the RPE but with intact Bruch’s membrane (BM) and βPPA−BM as an area without RPE and BM were measured by the two observers (M.H.S. and J.H.N.) at 6 radial scans of which the center was located at the fovea-to BMO center axis using built-in caliper tool of the Spectralis OCT.9,15,16
Figure.
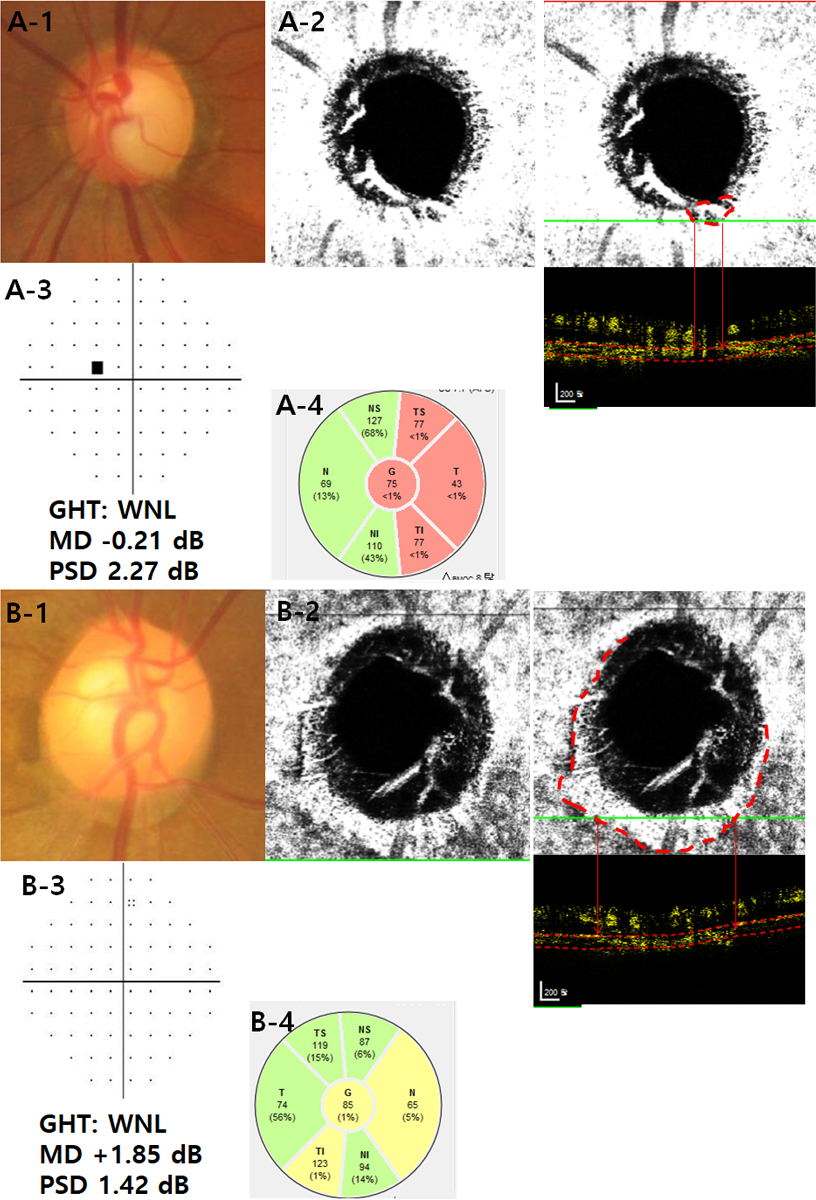
Representative cases showing differing RNFL thickness and VF sensitivity according to optical coherence tomography angiography (OCT-A)-derived parapapillary deep-layer microvasculature dropout in preperimetric glaucomatous eyes. The left eye of a 46-year-old male had neuroretinal rim loss (A-1) and complete dropout of the choriocapillaris (red dashed lines and red arrows) (A-2) in the inferotemporal (TI) sector. The right eye of a 51-year-old female had narrowing of the neuroretinal rim area (B-1) and a well-preserved choriocapillaris (red dashed lines and red arrows) (B-2). Both eyes had normal visual field (VF) results (A-3 and B-3), a well preserved anterior laminar surface, and no noticeable ß-zone parapapillary atrophy without Bruch’s membrane. Note that an eye with choriocapillaris dropout in the TI sector (A-2) had worse mean deviation (MD) and pattern standard deviation (PSD) on the VF (A-3) and thinner RNFL on the OCT (A-4) than did an eye without dropout (B-2, B-3, and B-4).
Based on a 20° × 20° high-resolution scan pattern that includes 48 radial B-scans in the enhanced depth imaging (EDI) mode, focal lamina cribrosa (LC) defects defined as laminar disinsertion or holes violating the normal U- or W-shaped contour of the anterior laminar surface,4, 8, 17,18 and juxtapapillary choroidal thickness (JPCT) defined as the choroidal area within 500 μm of the border tissue of Elsching14,19 were determined by the 2 masked observers (J.H.N. and H.R.K.). The JPCT was calculated as the average of values measured by the 2 observers at 12 clock-hour meridians on the 6 radial scans.19 The configuration of the border tissue of Elschnig at the temporal disc margin was categorized into 3 groups: nonoblique, internal oblique, or external oblique.19–21 In eyes with oblique border tissue, the innermost margin was determined at which the perpendicular distance between BM and choroidoscleral interface could be discerned.19–21
Deep-Layer Microvasculature Dropout in Parapapillary Atrophy
The Spectralis OCT Angiography Module (Spectralis; Heidelberg Engineering GmbH, Heidelberg, Germany) incorporated into the OCT2 platform was used for visualization of the deep-layer microvasculature within the ßPPA.5, 8 This technology has a central wavelength of 880 nm, an acquisition speed of 85 kHz, as well as lateral and axial resolutions of 5.7μm and 3.9 μm per pixel, respectively. The 15° × 10° scan pattern consisting of 256 clusters of 5 repeated B-scans (taken at ~12μm intervals) was used in this study. To minimize the projection artifacts of the superficial retinal vessels, projection-resolved (PR) technique was applied to the current OCT-A device.22 OCT-A images were excluded according to the following criteria: 1) a quality score < 25; 2) poor clarity; 3) local weak signal due to posterior vitreous detachment or floater; 4) residual motion artifacts visible as an irregular vessel pattern or disc boundary on the enface angiogram; 5) choroidal-layer segmentation errors.9
Details on OCT-A-derived parapapillary deep-layer microvasculature dropout can be found elsewhere.2, 5, 8 Briefly, dropout defined as complete loss of the choriocapillaris or microvasculature contained in the scleral flange within the βPPA on OCT-A vessel-density maps were determined by 2 masked observers (M.H.S. and J.H.N). Discrepancies between the 2 examiners were resolved by consensus, or in cases where consensus could not be reached, the subjects were excluded.2, 5, 8 Microvasculature within the scleral flange, as well as choriocapillaris, were assessed since it was observed in most healthy eyes and the dropout within the scleral flange was related to worse glaucoma severity.9,11,12 The dropout was required to be ≥ 200 μm in diameter on at least one scan and also to be present on at least 4 consecutive horizontal scans. The location of microvasculature dropout was determined as either the superior or the inferior area of the ONH (Figs A2 and B2). If both eyes had dropout, one eye was randomly selected. If one eye had dropout and the other eye did not, the eye with dropout was selected according to subject scarcity.
Standard Automated Perimetry
In addition to the MD and PSD, which are automatically provided by the SAP printout, the retinal sensitivity and total deviation (TD) sensitivity were calculated by averaging the measured thresholds from each test point in the superior and inferior hemifields, respectively.8
Data Analysis
Structural and functional ONH parameters on global and hemispheric areas, as well as baseline characteristics, were compared between eyes with and without deep-layer microvasculature dropout. Independent Student t−test and Mann Whitney test were performed for continuous variables according to the normality test results, and chi-squared test was performed for categorical variables. The kappa coefficient was calculated to evaluate the inter-observer agreements for dropout, focal LC defect, DH, and ßPPA.19 Bland-Atman plots were used to calculate inter-observer reproducibility in the measured widths of ßPPA+BM and ßPPA−BM and JPCT. MedCalc (MedCalc, Inc., Mariakerke, Belgium) was used to perform the statistical analyses. The α level (type I error) was set at 0.05.
Results
Among the total of 135 pre-perimetric POAG eyes meeting the eligibility criteria, 22 (16.3%) without βPPA, 5 (3.7 %) with poor-quality OCT-A images, and 14 (10.4%) with partial deep-layer microvasculature dropout not meeting the inclusion criteria for complete dropout were excluded, thus leaving 94 eyes for the subsequent analysis. There was excellent inter-observer agreement on determination of deep-layer microvasculature dropout (Kappa = 0.88), focal LC defect (Kappa = 0.86), DH (Kappa = 0.86), and presence of ßPPA (Kappa = 0.94) and ßPPA−BM (Kappa = 0.91). There was good inter-observer reproducibility for measurement of JPCT (Bland-Altman 95% LOA, −24.5 to 37.2μm), ßPPA+BM width (Bland-Altman 95% LOA, −55.2 to 38.7 μm), and ßPPA−BM width (Bland-Altman 95% LOA, −35.4 to 48.2 μm).
Thirty-three eyes (35.1%) of 94 pre-perimetric POAG patients had parapapillary deep-layer microvasculature dropout. Among them, dropout was observed in the inferior area in 23 eyes (69.7%), in the superior area in 4 eyes (12.1%), and in both the superior and inferior areas in 6 eyes (18.2 %).
Table 1 compares the demographics for eyes with and without deep-layer microvasculature dropout. Eyes with dropout had a significantly thinner central corneal thickness (CCT) (535.4±26.9 vs. 549.1±31.4μm) than did those without dropout (P = 0.037). The two groups did not differ for any of the other demographics including age, gender, AXL, presence/medication of diabetes and hypertension, untreated IOP, IOP at scan time, systolic and diastolic BP, and MOPP (P > 0.1 for all comparisons).
Table 1.
Comparison of demographics and test results between preperimetric primary open-angle glaucoma patients according to presence of parapapillary deep-layer microvasculature dropout
| Variables | Eyes with dropout (33 eyes, 33 patients) | Eyes without dropout (61 eyes, 61 patients) | P−value |
|---|---|---|---|
| Age (years) | 52.1 ± 16.0 | 49.3 ± 13.0 | 0.360* |
| Gender (male/female) | 19/14 | 32/29 | 0.636† |
| Spherical equivalent (D) | −2.4 ± 4.3 | −2.9 ± 3.6 | 0.386‡ |
| Axial length (mm) | 25.2 ± 2.0 | 25.0 ± 1.7 | 0.585‡ |
| Central corneal thickness (μm) | 535.4 ± 26.9 | 549.1 ± 31.4 | 0.037* |
| Self-reported history of diabetes, n (%) | 5 (15.2 %) | 6 (9.8 %) | 0.430† |
| Self-reported history of hypertension, n (%) | 1 (3.0 %) | 1 (1.6 %) | 0.657† |
| Diabetes medication, n (%) | 5 (15.2 %) | 6 (9.8 %) | 0.430† |
| Antihypertensive medication, n (%) | 1 (3.0 %) | 1 (1.6 %) | 0.657† |
| Number of topical glaucoma medications | 0.657† | ||
| 0 | 12 | 29 | |
| 1 | 10 | 12 | |
| >1 | 11 | 20 | |
| Topical medications, n | 0.932† | ||
| Prostaglandin analogues | 10 | 17 | |
| Beta-antagonists | 9 | 20 | |
| Carbonic anhydrase inhibitors | 11 | 20 | |
| Alpha-1 agonist | 3 | 4 | |
| Untreated intraocular pressure (mmHg) | 15.9 ± 5.2 | 16.2 ± 4.4 | 0.583‡ |
| Intraocular pressure at scan time (mmHg) | 13.0 ± 3.3 | 13.4 ± 2.9 | 0.454‡ |
| Systolic blood pressure (mmHg) | 122.6 ± 13.2 | 122.8 ±15.1 | 0.946* |
| Diastolic blood pressure (mmHg) | 73.3 ± 11.6 | 72.0 ± 8.6 | 0.712‡ |
| Mean ocular perfusion pressure (mmHg) | 46.8 ± 7.9 | 45.9 ± 6.8 | 0.526‡ |
Values are shown in mean ± standard deviation.
Statistically significant values are shown in bold.
The comparison was performed by using independent samples t-test.
The comparison was performed by using Chi-squared test.
The comparison was performed by using Mann-Whitney test.
In Table 2, the structural and functional ONH parameters between pre-perimetric POAG eyes with and without deep-layer microvasculature dropout are compared. Eyes with dropout had significantly worse VF MD (−1.52±1.67 vs. −0.53±1.26 dB; P = 0.002), worse PSD (1.84±0.65 vs. 1.65±0.41 dB; P < 0.001), worse superior and inferior retinal sensitivity (27.88±2.27vs. 29.04±1.94 dB; P = 0.020 for superior retinal sensitivity and 28.78±2.12 vs. 30.37±2.15 dB; P = 0.002 for inferior retinal sensitivity), and worse superior and inferior TD sensitivity (−1.56±2.03 vs. −0.57±1.59 dB; P = 0.010 for superior TD sensitivity and −1.89±1.80 vs. −0.61±1.45 dB; P < 0.001 for inferior TD sensitivity). RNFL was significantly thinner in eyes with dropout than in those without dropout in the global area (80.1±10.7 vs. 90.6±9.7 ㎛; P < 0.001) and in all sectors except the NI sector (92.7±21.5 vs. 100.3±19.5μm; P = 0.085) with largest differences in the TI sector (99.5±24.0 vs. 125.7±25.1μm; P < 0.001). Eyes with dropout had a significantly higher prevalence of focal LC defect (57.6 vs. 21.3%; P < 0.001) and larger βPPA−BM (240.9±306.7 vs. 128.0±186.1μm; P = 0.029) compared with those without dropout. Larger βPPA+BM was observed in eyes with dropout, but with marginal significance (199.7±105.5 vs. 164.5±76.6 μm; P = 0.066). Prevalence of DH, JPCT, and BMO area did not differ between the two groups (all P > 0.10).
Table 2.
Comparison of structural and functional parameters of optic nerve head between preperimetric primary open-angle glaucoma patients according to presence of parapapillary deep-layer microvasculature dropout
| Variables | Eyes with MvD_P (33 eyes, 33 patients) | Eyes without MvD_P (61 eyes, 61 patients) | P−value |
|---|---|---|---|
| Visual field parameters (dB) | |||
| Mean deviation | −1.52 ± 1.67 | −0.53 ± 1.26 | 0.002* |
| Pattern standard deviation | 1.84 ± 0.65 | 1.65 ± 0.41 | <0.001‡ |
| Superior retinal sensitivity | 27.88±2.27 | 29.04±1.94 | 0.020‡ |
| Inferior retinal sensitivity | 28.78±2.12 | 30.37±2.15 | 0.002‡ |
| Superior total deviation sensitivity | −1.56±2.03 | −0.57±1.59 | 0.010* |
| Inferior total deviation sensitivity | −1.89±1.80 | −0.61±1.45 | <0.001* |
| Circumpapillary RNFL thickness (μm) | |||
| Global area | 80.1 ± 10.7 | 90.6 ± 9.7 | <0.001* |
| Temporal | 63.4 ± 12.2 | 72.4 ± 12.0 | 0.001* |
| Nasal | 65.5 ± 12.7 | 71.5 ± 13.4 | 0.038* |
| Superotemporal | 106.6 ± 23.9 | 117.2 ± 23.7 | 0.043* |
| Superonasal | 98.7 ± 24.2 | 111.7 ± 23.9 | 0.014* |
| Inferonasal | 92.7 ± 21.5 | 100.3 ± 19.5 | 0.085* |
| Inferotemporal | 99.5 ± 24.0 | 125.7 ± 25.1 | <0.001* |
| Focal lamina cribrosa defect, n (%) | 19 (57.6 %) | 13 (21.3 %) | <0.001† |
| Disc Hemorrhage, n (%) | 4 (12.1 %) | 3 (4.9 %) | 0.207† |
| JPCT (μm) | 122.9 ± 37.6 | 127.2 ± 28.1 | 0.531* |
| BMO area (mm2) | 2.66 ± 0.80 | 2.60 ± 0.72 | 0.893‡ |
| βPPA+BM width (μm) | 199.7 ± 105.5 | 164.5 ± 76.6 | 0.066* |
| βPPA−BM width (μm) | 240.9 ± 306.7 | 128.0 ± 186.1 | 0.029* |
Values are shown in mean ± standard deviation.
Statistically significant values are shown in bold.
The comparison was performed by using independent samples t-test.
The comparison was performed by using Chi-squared test.
The comparison was performed by using Mann-Whitney test.
RNFL = retinal nerve fiber layer; JPCT = Juxtapapillary choroidal thickness; BMO = Bruch’s membrane opening; βPPA+BM = ß-zone parapapillary atrophy with Bruch’s membrane; PPA−BM = ß-zone parapapillary atrophy without Bruch’s membrane.
Supplemental Table S1 shows the results of the univariable and multivariable logistic regression analyses investigating the association of deep-layer microvasculature dropout with the subjects’ clinical characteristics. Based on the univariable logistic regression analysis, dropout was significantly associated with more severe glaucoma as assessed by worse VF MD (OR, 1.62 P = 0.003), and thinner global RNFL (OR, 1.106; P <0.001), as well as thinner CCT (OR, 1.106; P = 0.034), higher prevalence of focal LC defect (OR, 5.011; P < 0.001), and larger βPPA−BM (OR, 1.002; P = 0.031). Dropout was associated with worse VF PSD (OR, 2.009; P = 0.096) and larger βPPA+BM (OR, 1.005; P = 0.066), but with marginal significance. Other factors including age, untreated IOP, IOP at scan time, and JPCT were not significantly associated with dropout (all P > 0.10). Since VF MD and RNFL thickness were significantly associated with each other (P = 0.001), they were separately included in the multivariable model to avoid multicollinearity. In the multivariable logistic regression with VF MD included, worse VF MD (OR, 1.485; P = 0.045) and higher prevalence of focal LC defect (OR, 6.673; P < 0.001) remained as factors associated with dropout. Similarly, in the multivariable logistic regression with global RNFL thickness included, global RNFL thickness (OR, 1.141; P < 0.001), higher prevalence of focal LC defect (OR, 13.369; P < 0.001), and larger βPPA−BM (OR, 1.004; P = 0.011) remained as significant factors (Supplemental Table S1).
The figure shows an eye with pre-perimetric POAG having deep-layer microvasculature dropout in the TI sector (Fig A2) with thinner RNFL in the corresponding TI sector (Fig A4) than the eye without dropout (Fig B4). Although the two eyes had normal VF,11 the eye with dropout had worse MD and PSD (Fig A3) relative to the eye without dropout (Fig B3).
Discussion
In the present study, parapapillary deep-layer microvasculature dropout was observed in a considerable number of eyes with pre-perimetric POAG. Additionally, dropout was associated with thinner RNFL and worse VF sensitivity, even after adjusting for possible confounding factors including βPPA width and focal LC defect. These findings suggest that deep-layer microvascular dropout in the choroid and sclera is a characteristic sign of early glaucoma in eyes with normal VF.
Recently, deep-layer microvasculature dropout was reported to be rarely observed in healthy subjects and to be a marker of worse disease severity in POAG.2, 7, 8, 10 However, these studies focused on eyes with perimetric glaucoma, and reported that microvasculature dropout was often observed in moderate-to-advanced glaucoma.2, 7, 8 Under these circumstances, the current study showed that dropout can be observed in a considerable number of pre-perimetric glaucomatous eyes without VF damage (33/94). These findings imply that dropout is not a simple epiphenomenon of the disease process, but may be a clinically meaningful finding detectable in early glaucomatous ONH damage. Moreover, eyes with dropout had worse disease severity than those without dropout. As a large number of nerve fibers can be damaged prior to the appearance of VF damage, and given also the wide range of disease severity in pre-perimetric glaucoma,23,24 the current results suggest that loss of OCT-A-derived deep-layer microvasculature is a sign of early glaucoma in eyes with pre-perimetric glaucoma.
Recent studies have reported higher frequencies of deep-layer microvasculature dropout in progressive OAG eyes; thus, dropout might serve as an important marker of glaucoma progression.5,6 Since pre-perimetric glaucoma with worse VF severity and RNFL thickness has been reported to have a higher probability of glaucoma progression,25,26 the current findings that eyes with dropout had worse VF and RNFL results add to increasing evidence that dropout can also serve as a maker suggestive of disease. Moreover, a recent study reported that enlargement of the microvasculature dropout was associated with progressive RNFL thinning in OAG.27 However, this speculation is limited by the present study’s retrospective nature and small subject cohort. Also, it still remains to be elucidated, by both experimental and longitudinal clinical studies, whether deep-layer microvasculature damage serves as a causative factor for glaucoma progression.
Previous studies have reported that microvasculature dropout was observed mainly in the inferior area followed by the superior area.2,8,10 Similarly, the current study showed that 69.7% of eyes (23/33) with pre-perimetric glaucoma had dropout only in the inferior area, while only 12.1% (4/33) had dropout only in the superior area. In addition, in accordance with the location of dropout, RNFL thinning was most remarkable in the inferior area, with compatible VF damage in the superior area. Moreover, eyes with dropout had thinner RNFL and worse VF sensitivity in an area without dropout. These results concur with those of a recent study observing VF progression not only in the hemifield corresponding to deep-layer microvasculature dropout but also in the opposite hemifield.5 Also, Jo et al. recently reported that eyes with the dropout had significantly thinner RNFL and ganglion cell-inner plexiform layer than those without dropout in the perimetrically-intact hemiretinae.28 Further studies are needed in order to elucidate whether focal deep-layer microvasculature loss can propagate to an area wherein complete microvasculature dropout is not observed.
Similar to previous studies,5,11 this study assessed the hemispheric location of the dropout instead of its sectoral location. This is because the dropout was observed at > 1 adjacent Garway-Heath sectors in 51.5 % (17/33). Also, sectors that include the largest portion of dropout were mostly TI (75.8 % (27/33)) and TS (27.3 % (9/33)) sectors rather than lower-temporal (18.2 % (6/33)) or upper-temporal (3.0 % (1/33)) sectors.
The current results that eyes with dropout had significantly larger ßPPA without BM and a higher prevalence of focal LC defect correspond well with previous investigations.2,8, 9 Meanwhile, larger ßPPA with BM and thinner CCT were related to dropout in the univariable regression analysis but not in the multivariable regression analysis. It is unclear whether this is a meaningful result or an incidental finding. In the meantime, certainly, studies with larger subject cohorts are warranted to follow-up on these suggestive findings.
In clinical practice, it should be noted that the prevalence of deep-layer microvasculature dropout would be lower than in the present study (35.1% (33/94)) since an eye with dropout was preferentially selected if the contralateral eye did not have it. When all of the pre-perimetric POAG eyes were included, 27.5 % (33/120) had dropout.
The present study has several limitations. First, deep-layer microvasculature dropout was determined based on the qualitative assessments of two observers; thus, a relatively large number of eyes (n = 18) were excluded from the study due to partial reduction of the microvasculature and lack of agreement between graders. Improvement of the OCT-A techniques that enable user-defined regional quantitative analysis of deep-layer microvasculature would help resolve this limitation. Second, the cross-sectional nature of the current study prevents elucidation of the causative role of the deep-layer microvasculature in the pathogenesis of glaucoma. Third, the current OCT-A is limited in evaluating deep-layer microvasculature outside the ßPPA even after applying the PR technique.2,3,5,8–12,14,16,22,29,30 In this study, none of 22 eyes without ßPPA had the dropout, thus were excluded from the study. Although it is still unclear how to explain this phenomenon, a possible explanation is that projection artifacts of inner retinal vessels on the highly reflective RPE hinder the accurate analysis of the choroidal vasculature.30 It is likely that the current PR technique is not satisfactory in removing these artifacts. Finally, the current results are limited in their generalizability, due to the relatively young age of the study population since pre-perimetric POAG patients tend to be younger than perimetric POAG patients. The homogenous Asian population also limits the generalizability of this study to other racial groups.
In conclusion, a considerable number of eyes with pre-perimetric POAG had parapapillary deep-layer microvasculature dropout. Moreover, dropout was related to the extent of RNFL thinning and VF loss even after adjusting for possible confounding factors including βPPA width and focal LC defect. Longitudinal studies elucidating the pathogenic role of deep-layer microvasculature damage in the development and progression of glaucoma are needed.
Supplementary Material
Acknowledgments
Supported in part by National Eye Institute (R01EY029058) and an unrestricted grant by Research to Prevent Blindness (New York, NY)
Financial Disclosure(s): Min Hee Suh: none; Jeoung Ho Na: none; Linda M. Zangwill: CCarl Zeiss Meditec (F), Heidelberg Engineering (F), Merck (C), National Eye Institute (F), Optovue (F), Topcon (F). Robert N. Weinreb: Aerie Pharmaceuticals (C), Allergan (C), Bausch & Lomb (C), Carl Zeiss Meditec (F), Eyenovia (C), Heidelberg Engineering (F), National Eye Institute (F), Optos (F), Optovue (F), Unity Biotechnology (C).
Footnotes
The authors have no proprietary or commercial interest in any of the materials discussed in this article.
References
- 1.Yarmohammadi AZL, Diniz-Filho A, Suh MH, Malastas PI, Fatehee N, Yousefi S, Belghith A, Medeiros FA, Huang D, Weinreb RN. OCT Angiography Vessel Density in Healthy, Glaucoma Suspects, and Glaucoma. Invest Ophthalmol Vis Sci;2018;125:578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh MH, Zangwill LM, Manalastas PI, et al. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 2016;123(:2509–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee EJ, Lee KM, Lee SH, Kim TW. Parapapillary Choroidal Microvasculature Dropout in Glaucoma: A Comparison between Optical Coherence Tomography Angiography and Indocyanine Green Angiography. Ophthalmology 2017;124:1209–1217. [DOI] [PubMed] [Google Scholar]
- 4.Suh MH, Zangwill LM, Manalastas PI, et al. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology 2016;123:2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon JM, Weinreb RN, Zangwill LM, Suh MH. Parapapillary Deep-Layer Microvasculature Dropout and Visual Field Progression in Glaucoma. Am J Ophthalmol 2019;200:65–75. [DOI] [PubMed] [Google Scholar]
- 6.Park HL, Kim JW, Park CK. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology 2018;125:1003–1013. [DOI] [PubMed] [Google Scholar]
- 7.Akagi T, Iida Y, Nakanishi H, et al. Microvascular Density in Glaucomatous Eyes With Hemifield Visual Field Defects: An Optical Coherence Tomography Angiography Study. Am J Ophthalmol 2016;168:237–249. [DOI] [PubMed] [Google Scholar]
- 8.Suh MH, Park JW, Kim HR. Association Between the Deep-layer Microvasculature Dropout and the Visual Field Damage in Glaucoma. J Glaucoma 2018;27:543–551. [DOI] [PubMed] [Google Scholar]
- 9.Suh MH, Zangwill LM, Manalastas PIC, et al. Deep-Layer Microvasculature Dropout by Optical Coherence Tomography Angiography and Microstructure of Parapapillary Atrophy. Invest Ophthalmol Vis Sci 2018;59:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EJ, Lee SH, Kim JA, Kim TW. Parapapillary Deep-Layer Microvasculature Dropout in Glaucoma: Topographic Association With Glaucomatous Damage. Invest Ophthalmol Vis Sci 2017;58:3004–3010. [DOI] [PubMed] [Google Scholar]
- 11.Lee EJ, Kim TW, Kim JA, Kim JA. Parapapillary Deep-Layer Microvasculature Dropout in Primary Open-Angle Glaucoma Eyes With a Parapapillary γ-Zone. Invest Ophthalmol Vis Sci. 2017;58:5673–5680. [DOI] [PubMed] [Google Scholar]
- 12.Lee EJ, Kim TW, Lee SH, Kim JA. Underlying Microstructure of Parapapillary Deep-Layer Capillary Dropout Identified by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2017;58:1621–1627. [DOI] [PubMed] [Google Scholar]
- 13.Kim KE, Jeoung JW, Kim DM, et al. Long-term follow-up in preperimetric open-angle glaucoma: progression rates and associated factors. Am J Ophthalmol 2015;159:160–168 e1–2. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Suh MH, Agrawal R, Khandelwal N. Peripapillary Choroidal Vascularity Index in Glaucoma-A Comparison Between Spectral-Domain OCT and OCT Angiography. Invest Ophthalmol Vis Sci 2018;59:3694–3701. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Kim T-W, Weinreb RN, Lee EJ. Differentiation of Parapapillary Atrophy Using Spectral-Domain Optical Coherence Tomography. Ophthalmology 2013;120:1790–1797. [DOI] [PubMed] [Google Scholar]
- 16.Suh MH, Park JW, Khandelwal N, Agrawal R. Peripapillary Choroidal Vascularity Index and Microstructure of Parapapillary Atrophy. Invest Ophthalmol Vis Sci. 2019;60:3768–3775. [DOI] [PubMed] [Google Scholar]
- 17.Kiumehr S, Park SC, Dorairaj S, et al. In Vivo Evaluation of Focal Lamina Cribrosa Defects in Glaucoma. Archives of Ophthalmology 2012;130:552–559. [DOI] [PubMed] [Google Scholar]
- 18.Faridi OS, Park SC, Kabadi R, et al. Effect of Focal Lamina Cribrosa Defect on Glaucomatous Visual Field Progression. Ophthalmology. 2014;121:1524–1530. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Lee EJ, Kim TW. Topographic Correlation Between Juxtapapillary Choroidal Thickness and Microstructure of Parapapillary Atrophy. Ophthalmology 2016;123(9):1965–1973. [DOI] [PubMed] [Google Scholar]
- 20.Reis AS, Sharpe GP, Yang H, et al. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology 2012;119:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strouthidis NG, Yang H, Reynaud JF, et al. Comparison of clinical and spectral domain optical coherence tomography optic disc margin anatomy. Invest Ophthalmol Vis Sci 2009;50:4709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takusagawa HL, Liu L, Ma KN, et al. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology. 2017;124:1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 1989;107:453–464. [DOI] [PubMed] [Google Scholar]
- 24.Kerrigan-Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci 2000;41:741–748. [PubMed] [Google Scholar]
- 25.Meira-Freitas D, Tatham AJ, Lisboa R, et al. Predicting progression of glaucoma from rates of frequency doubling technology perimetry change. Ophthalmology 2014;121:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol 2006;142:576–582. [DOI] [PubMed] [Google Scholar]
- 27.Kim JA, Lee EJ, Kim TW. Evaluation of Parapapillary Choroidal Microvasculature Dropout and Progressive Retinal Nerve Fiber Layer Thinning in Patients With Glaucoma. JAMA Ophthalmol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo YH, Kwon J, Shon K, Jeong D, Kook MS. Greater Severity of Glaucomatous Damage in Eyes With Than Without Choroidal Microvasculature Dropout in Open-Angle Glaucoma. Invest Ophthalmol Vis Sci. 2019;60:901–912. [DOI] [PubMed] [Google Scholar]
- 29.Lee EJ, Kim S, Hwang S, Han JC, Kee C. Microvascular Compromise Develops Following Nerve Fiber Layer Damage in Normal-Tension Glaucoma Without Choroidal Vasculature Involvement. J Glaucoma. 2017;26(3):216–222 [DOI] [PubMed] [Google Scholar]
- 30.Gao SS, Jia Y, Zhang M, Su JP, et al. Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


