Abstract
Objective:
To assess the impact of a granular measure of socioeconomic deprivation on pancreatic surgical and cancer-related outcomes at a high-volume cancer center that employs a standardized clinic pathway.
Summary Background Data:
Prior research has shown that low socioeconomic status leads to less treatment and worse outcomes for pancreatic adenocarcinoma. However, these studies employed inconsistent definitions and categorizations of socioeconomic status, aggregated individual socioeconomic data using large geographic areas, and lacked detailed clinicopathologic variables.
Methods:
We conducted a retrospective cohort study of 1,552 pancreatic adenocarcinoma patients between 2008 and 2015. Patients were stratified using the Area Deprivation Index, a validated dataset that ranks census block groups based on socioeconomic deprivation (SED). Multivariable models were used in the curative surgery cohort to predict the impact of SED on 1) grade 3/4 Clavien-Dindo complications, 2) initiation of adjuvant therapy 3) completion of adjuvant therapy, and 4) overall survival
Results:
Patients from high SED neighborhoods constituted 29.9% of the cohort. Median overall survival was 28 months. The rate of Clavien-Dindo grade 3/4 complications was 14.2% and completion of adjuvant therapy was 65.6%. There was no evidence that SED impacted surgical evaluation, receipt of curative-intent surgery, postoperative complications, receipt of adjuvant therapy or overall survival.
Conclusions:
While nearly one-quarter of curative-intent surgery patients were from high SED neighborhoods, this factor was not associated with measures of treatment quality or survival. These observations suggest that treatment at a high-volume cancer center employing a standardized clinical pathway may in part address socioeconomic disparities in pancreatic cancer.
Keywords: pancreatic cancer, socioeconomic status, socioeconomic deprivation, cancer disparities, area deprivation index
Mini-Abstract
This study used a granular measure of socioeconomic deprivation (SED) to assess rates of surgical evaluation and post-operative outcomes for pancreatic cancer (PDAC). SED was not associated with treatment quality or survival. Treatment of PDAC at a high-volume center employing a standardized clinical pathway may positively impact socioeconomic disparities.
Introduction
Surgical resection is the only potentially curative option for pancreatic cancer and remains a vital treatment to improve survival in patients with localized disease. With receipt of high-quality, multimodality treatment at high-volume centers there have been significant improvements in pancreatic cancer survival.1 The distribution of these improvements, however, has been unequal; several studies show that low socioeconomic status leads to less treatment and worse outcomes for pancreatic cancer.2–6
The social determinants of cancer play a major role in the course of care for patients across the spectrum of oncologic disease.7–9 Emphasizing the social context in which disease and treatment occur, this approach asserts that political and socioeconomic structures influence the factors that lead to disease and impact subsequent outcomes.10 However, for pancreatic and other cancers, the measurement of social determinants has proved a challenge for multiple reasons. First, myriad definitions and categorizations of socioeconomic status/position have been deployed in the literature with socioeconomic status functioning as an umbrella term for various dimensions of social disadvantage (including race, insurance status, income, education level, poverty, housing/rent, and employment status).11 This has resulted in difficulties making meaningful comparisons across studies and has the potential for information bias and misclassification of risk.12 Second, reliance on national administrative datasets, which contain varying degrees of socioeconomic variables and often lack detailed clinicopathologic and treatment data that may serve as potential confounders, can also lead to bias.13, 14 Finally, the paucity of individual-level socioeconomic data has led to large geographic units (zip codes or counties) being used for aggregate analysis of socioeconomic variables. While census tract-level or smaller units for socioeconomic status geocoding is recommended, the use of larger area geocoding continues despite warnings about imprecision.1, 4, 6, 15, 16
Given these challenges, new and standardized approaches are needed to better assess the role of socioeconomic status in cancer outcomes. One detailed measure of socioeconomic deprivation (SED), the area deprivation index (ADI), has recently been updated and made publicly available. The ADI is a validated dataset that ranks census block groups (neighborhoods) on socioeconomic disadvantage based on US census and American Community Survey data that includes 17 variables such as income, education, employment, and housing quality data.17 This index measure of SED allows for convenient comparison across populations and uses census block groups for aggregate socioeconomic data, avoiding the limitations of zip code or larger geocoding. To date no studies have assessed the impact of SED at the census block group level on pancreatic adenocarcinoma (PDAC) outcomes controlling for detailed clinicopathologic data and geographic distance from the treating facility. We therefore employed the ADI to 1) determine the degree of SED among PDAC patients treated at a high-volume comprehensive cancer center and 2) explore the relationship between SED and PDAC outcomes, including post-operative complications, initiation and completion of adjuvant therapy, and overall survival.
Methods
Study Cohort, Setting and Standardized Pathway
Moffitt Cancer Center (MCC) is the only National Cancer Institute designated Comprehensive Cancer Center in the state of Florida. Florida has the third largest population in the US and is ranked 49th in the US in overall health disparities with approximately 36% of the population living below 200% of the Federal Poverty Level.18 Of the 29,488 incident cases of pancreatic tumors captured by the Florida Cancer Data System from 2008–2015, approximately 10% (2,867) were evaluated at MCC.
We performed a retrospective cohort study of patients diagnosed with PDAC from January 1, 2008 to December 31, 2015 at MCC. Patients were excluded if no ADI ranking was available, i.e., patients with PO box addresses, or if there was only a single visit (second opinion), identifying 1,552 patients. Surgical evaluation was defined as a completed surgical clinic appointment or documented presentation at multidisciplinary tumor board. Curative-intent surgical patients were then analyzed; patients who did not undergo a curative resection, e.g., palliative bypass, were excluded from the final analysis.
Of 307 curatively-resected PDAC patients who underwent surgery from 2008–2015, 289 patients had ADI data for analyses (289 had complication data, 283 had initiation of adjuvant therapy, 282 had completion of adjuvant therapy, 288 had overall survival and 256 had recurrence information). Treatment was provided in conjunction with a standardized clinical pathway based on multidisciplinary diagnosis and staging agreement (Supplemental Figure 1).19 Patients with resectable disease underwent surgery as a first treatment and patients with borderline resectable disease underwent neoadjuvant treatment followed by surgery.20 Surgery types included pancreatoduodenectomy, distal pancreatectomy with or without splenectomy and total pancreatectomy; robotic-assisted and open cases were included. The institutional review board of MCC approved this protocol.
Predictor Variable: Area Deprivation Index
The ADI is a validated dataset that ranks census block groups (neighborhoods) on socioeconomic disadvantage, which comprises 17 variables, including income, education, employment, and housing quality data based on the 2013 American Community Survey (ACS).21 US census block groups typically contain between 600 to 3,000 people and are statistical subdivisions within census tracts, which themselves are small, relatively permanent statistical groupings designed to be homogenous in demographic and socioeconomic characteristics.22 Given the relative permanence of socioeconomic conditions, geographic-based measures of SED were developed and validated using US census measures through empirical linkage to mortality.23 Using 2013 ACS data, the ADI was updated and made publicly available.17
For our cohort, state-level ADI decile was determined by patient address at diagnosis and divided into terciles of disadvantage (low, comprising rankings 1–3; moderate, comprising rankings 4–6; high comprising rankings 7–10) as previously described.24 To visualize census block group data, Figure 1B shows the ADI by block group for Hillsborough and Pinellas Counties, FL alongside approximation of patient geographic residence at diagnosis according to zip code (Figure 1C). To highlight the greater sensitivity of census block group socioeconomic estimates, within the zip code 33602 which comprises downtown Tampa Bay, ADI rankings range from 1 to 10 with a mean of 4.27 and median of 3. While 9.1% of the zip code’s population have a rank of 1, the lowest rank of socioeconomic disadvantage, 8.2% of the zip code’s population has a rank of 10, indicating the highest level of socioeconomic disadvantage. For census block groups with a rank of 1, the range of median household income based on the 2013 ACS was US$ 116,146 to 117,542. For census block groups with a rank of 10, the range of median household income was US$ 9,515 to 20,500.
Figure 1.
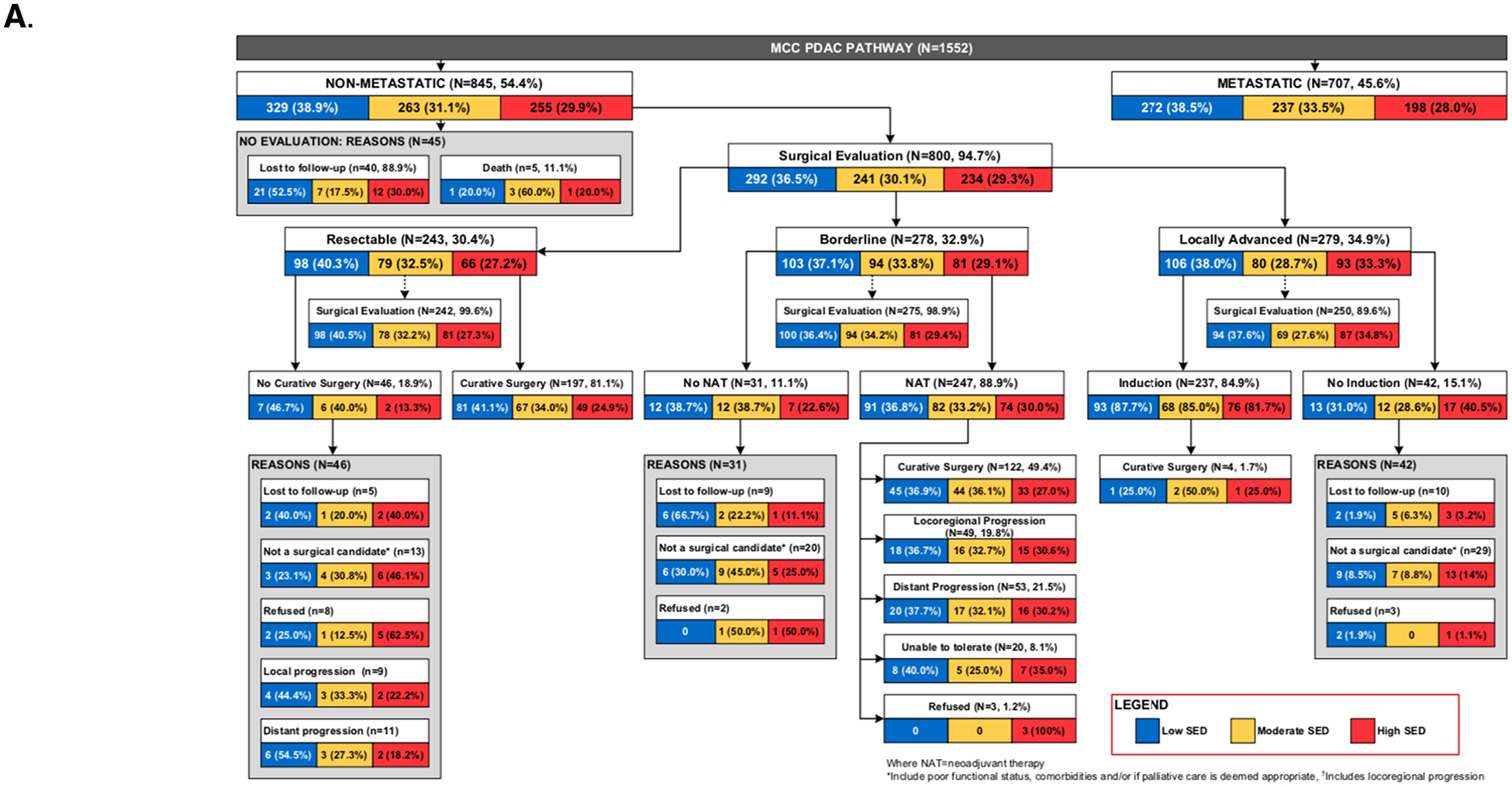
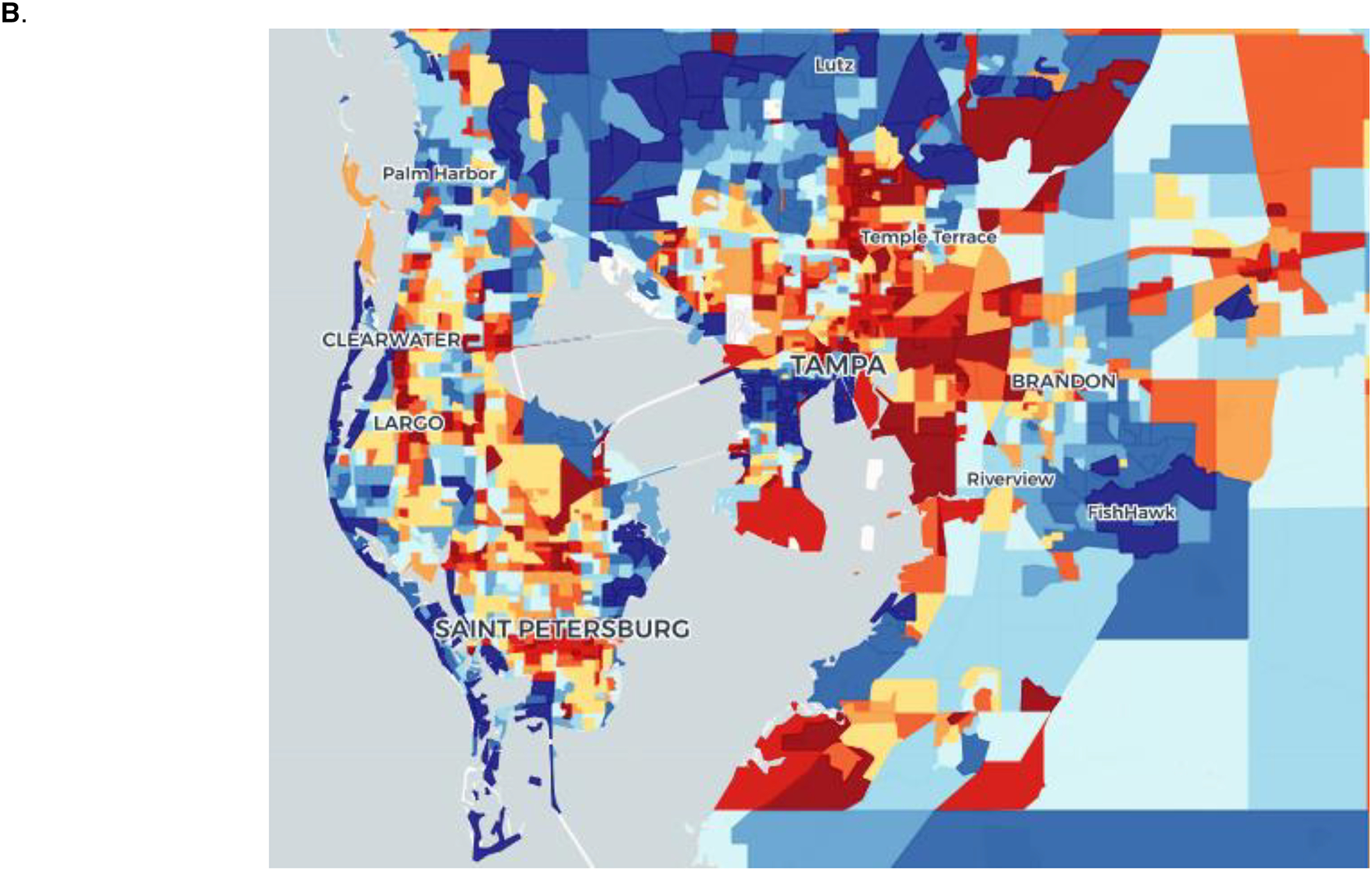
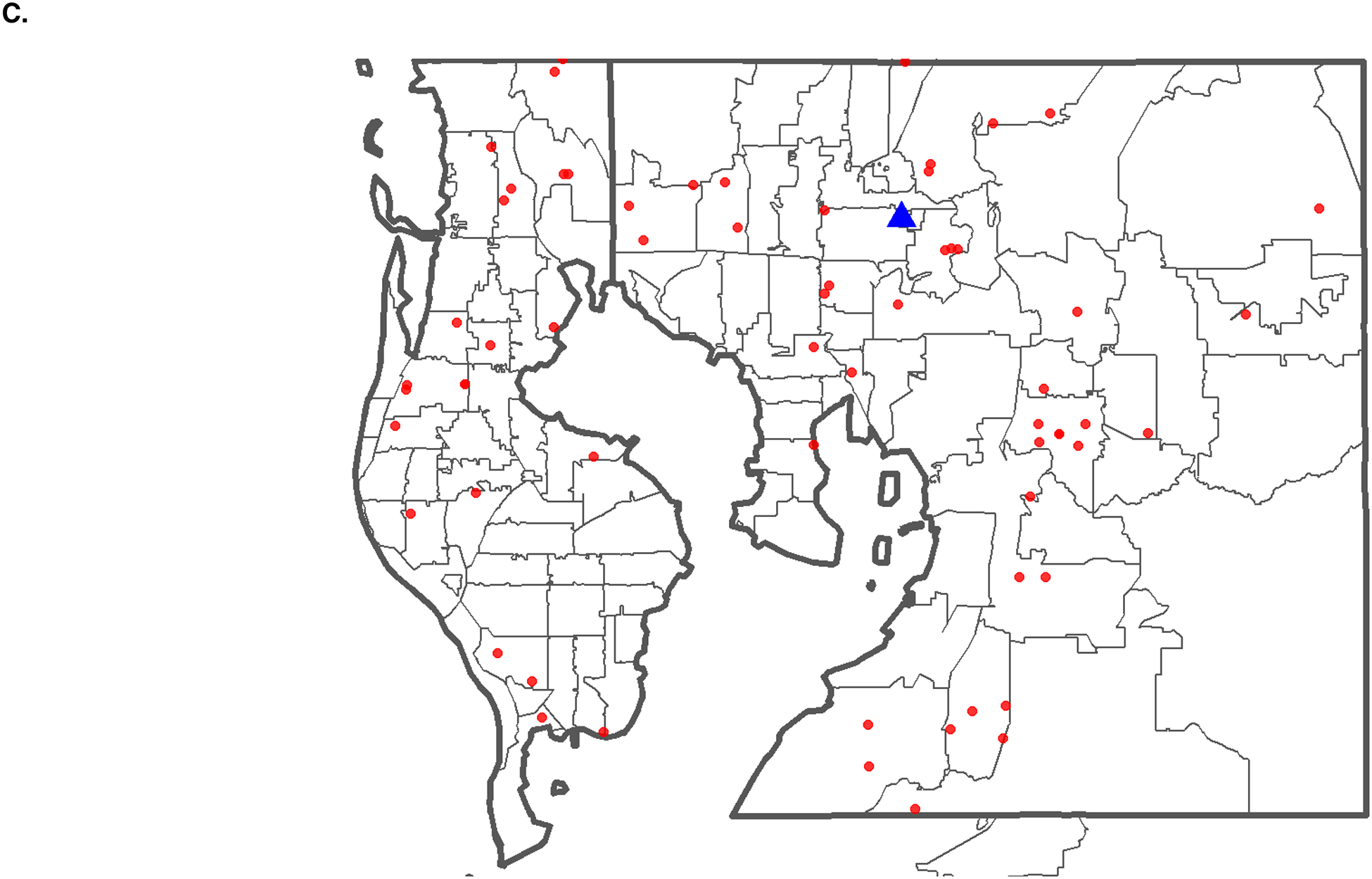
(A) Flowchart of PDAC patients entering the MCC standardized clinical pathway by socioeconomic deprivation (B) Density map of Hillsborough and Pinellas counties, FL showing SED decile (1–10) scores at the census block group level with the least disadvantaged block groups in blue and the most disadvantaged block groups in red. (C) Zip code level map of Hillsborough and Pinellas counties, FL, showing the geographic distribution of patients (red dots) from the treating facility (blue triangle). (D) County-level map of Florida, showing the geographic distribution of patients (red dots) from the treating facility (blue triangle).
Outcome Variables
The outcome variables assessed in this study were 1) grade 3/4 Clavien-Dindo complication, 2) initiation of adjuvant therapy 3) completion of adjuvant therapy, and 4) overall survival. Clavien-Dindo grade 3/4 complications have been previously defined.25 Complications assessed as possible grade 3/4 Clavien-Dindo complications included pancreatic-specific surgery complications (fistula, hemorrhage, delayed gastric emptying, biliary, and chyle leak) and non-pancreatic-specific surgical complications (surgical site infection, fascial dehiscence, pneumonia, deep vein thrombosis, pulmonary embolism, urinary tract infection, renal failure, cardiac arrest, sepsis, septic shock, Clostridium difficile infection, as well as mortality). Initiation of adjuvant therapy was defined as receipt of one or more cycles of chemotherapy with or without radiation. Completion of adjuvant therapy was defined as completion of intended adjuvant therapy course. The Moffitt clinical pathway for pancreatic cancer calls for all patients to receive adjuvant therapy regardless of receipt of neoadjuvant therapy. Therefore, the adjuvant therapy outcomes were analyzed as a separate covariate. Overall survival was defined as time from first treatment to death or last follow-up.
Covariates
Potential confounders were included as covariates. Preoperative variables included age, sex, race, insurance status (grouped as private, Medicare with private supplement, and government insurance, which included Medicare, Medicaid, and uninsured patients), BMI, pre-operative diagnosis of diabetes mellitus, Charlson Comorbidity Index (CCI), American Society of Anesthesiologists (ASA) score, presence of a biliary stent, and neoadjuvant treatment approach (defined as chemotherapy with or without chemoradiation). The Charlson age comorbidity index (CACI) was calculated to offer a comparison of comorbidity in our cohort to that of other published studies; CCI and age, however, were analyzed independently in regression models.26 Distance to MCC was calculated in miles using the patient’s address at diagnosis. Operative data included operation type, vascular resection, estimated blood loss, and operative time. Pathologic variables assessed included tumor stage, margin status, lymph node stage, presence of lymphovascular and perineural invasion. Additional postoperative variables included length of stay, 30-day mortality, and 90-day readmission.
Statistical Analyses
Patient characteristics were summarized using descriptive statistics including median and range for continuous measures and proportions and frequencies for categorical measures. When comparing characteristics to ADI the median and 25th and 75th percentiles are shown for continuous variables. The association between continuous variables and ADI were assessed using Kruskal-Wallis tests. The associations between categorical variables and ADI were evaluated using Chi-squared tests or Chi-squared permutation tests when the expected frequencies were less than 5 in some cells. Trend tests were also used when comparing variables to ADI (low to moderate to high). Categorical variable levels for overall survival were compared using the Log-rank test.
Logistic regression models were fit for the outcomes Grade 3 or 4 Clavien complication (no/yes), initiation of adjuvant therapy (no/yes), and completion of adjuvant therapy (no/yes), and Cox proportional hazard models were fit for overall survival. Unadjusted and adjusted (multivariable) models were run for ADI and covariates. Odds ratios (or hazard ratios for Cox models), with 95% confidence intervals, and p values are presented. For categorical variables with more than two levels, p values are presented for each level compared to a referent level, and also an overall p value using the type-III analysis-of-variance result for the respective model. For comparing ADI tercile group trends to the outcomes, the variable was converted to ordinal and Cox models fit. The proportional hazards assumption was assessed with Schoenfeld residuals. Due to the exploratory nature of this analysis p-values were not adjusted for multiple comparisons. All analyses were performed with R version 3.5.1
Results
Characteristics of the Study Population
Figure 1 provides a flowchart of MCC PDAC patients. The majority of patients with non-metastatic PDAC received surgical evaluation (94.7%). Of surgically treatable patients (resectable and borderline cohorts), 99.6% and 98.9%, respectively, received surgical evaluation. There was no difference in surgical evaluation by SED group (p=0.144). Of surgically treatable patients, 33 (6.3%) were not surgical candidates. There was no difference in failure to receive curative-intent surgery by SED group (p=0.353).
Baseline characteristics of the 289 curative-intent surgical patients by SED tercile are shown in Table 1. Patients with low, moderate, and high SED comprised 117 (40.5%), 101 (34.9%) and 71 (24.6%) of the cohort, respectively. Preoperative resectability status showed that 189 (65.4%) were designated resectable and 100 (34.6%) were designated borderline resectable. Neoadjuvant therapy was given to 101 (34.9%) of patients, including all borderline resectable patients. Vascular venous reconstruction was performed in 36 (12.5%) patients in the cohort. The median time to recurrence was 13.3 months, with 185 recurrences (70.1%). The liver was the most common site of recurrence, occurring in 64 (24.4%) patients; 28 (10.7%) of patients recurred locally. We found no difference between SED and CCI at presentation or ASA score. We observed that the median CACI for our cohort was 6 [25th and 75th percentiles, 5;7] and was not associated with ADI in our cohort (p=0.718).
Table 1:
Baseline Characteristics of Patients with Resected PDAC at Moffitt Cancer Center Between 2008 and 2015 by SED
| Low SED (n=117) | Moderate SED (n=101) | High SED (n=71) | Overall p | T rend p | |
|---|---|---|---|---|---|
| Age | 68.0 [64.0; 76.0] | 68.0 [61.0; 75.0] | 68.0 [61.0; 72.5] | 0.465 | 0.220 |
| Age (binary) | 0.639 | 0.361 | |||
| <70 | 66 (56.4%) | 59 (58.4%) | 45 (63.4%) | ||
| ≥70 | 51 (43.6%) | 42 (41.6%) | 26 (36.6%) | ||
| Sex | 0.203 | 0.124 | |||
| Female | 54 (46.2%) | 46 (45.5%) | 24 (33.8%) | ||
| Male | 63 (53.8%) | 55 (54.5%) | 47 (66.2%) | ||
| Race | 0.748 | 0.184 | |||
| White | 110 (94.0%) | 91 (90.1%) | 63 (88.7%) | ||
| Black | 4 (3.42%) | 5 (4.95%) | 4 (5.63%) | ||
| Other / unknown | 3 (2.56%) | 5 (4.95%) | 4 (5.63%) | ||
| Insurance | 0.237 | 0.083 | |||
| Private | 41 (35.0%) | 25 (24.8%) | 19 (26.8%) | ||
| Government with private supplement | 46 (39.3%) | 48 (47.5%) | 26 (36.6%) | ||
| Government | 30 (25.6%) | 28 (27.7%) | 26 (36.6%) | ||
| Distance from Moffitt (miles) | 56.5 [24.4; 128] | 48.9 [20.1; 97.3] | 54.8 [33.2; 104] | 0.062 | 0.460 |
| Distance from Moffitt (binary) | 0.195 | 0.412 | |||
| < 85 miles | 71 (60.7%) | 73 (72.3%) | 46 (64.8%) | ||
| ≥ 85 miles | 46 (39.3%) | 28 (27.7%) | 25 (35.2%) | ||
| Charlson comorbidity index | 0.355 | 0.339 | |||
| ≤3 | 31 (26.5%) | 23 (22.8%) | 13 (18.3%) | ||
| 4–5 | 60 (51.3%) | 47 (46.5%) | 42 (59.2%) | ||
| ≥6 | 26 (22.2%) | 31 (30.7%) | 16 (22.5%) | ||
| BMI | 26.0 [23.7; 28.8] | 25.8 [23.5; 29.6] | 27.1 [23.1; 31.8] | 0.399 | 0.281 |
| Diabetes mellitus | 0.193 | 0.102 | |||
| No | 91 (77.8%) | 69 (68.3%) | 48 (67.6%) | ||
| Yes | 26 (22.2%) | 32 (31.7%) | 23 (32.4%) | ||
| Preoperative biliary stent | 0.433 | 0.335 | |||
| No | 55 (47.0%) | 39 (38.6%) | 29 (40.8%) | ||
| Yes | 62 (53.0%) | 62 (61.4%) | 42 (59.2%) | ||
| Preoperative resectability | 0.550 | 0.649 | |||
| Resectable | 80 (68.4%) | 62 (61.4%) | 47 (66.2%) | ||
| Borderline resectable | 37 (31.6%) | 39 (38.6%) | 24 (33.8%) | ||
| Neoadjuvant therapy | 0.456 | 0.632 | |||
| No | 80 (68.4%) | 61 (60.4%) | 47 (66.2%) | ||
| Yes | 37 (31.6%) | 40 (39.6%) | 24 (33.8%) | ||
| ASA class | 0.144 | 0.197 | |||
| 2 | 57 (48.7%) | 36 (35.6%) | 29 (40.8%) | ||
| 3 | 60 (51.3%) | 65 (64.4%) | 42 (59.2%) | ||
| Surgery | 0.590 | 0.336 | |||
| Whipple/total pancreatectomy | 90 (76.9%) | 79 (78.2%) | 59 (83.1%) | ||
| Distal pancreatectomy/splenectomy | 27 (23.1%) | 22 (21.8%) | 12 (16.9%) | ||
| Vascular Reconstruction | 0.226 | 0.130 | |||
| No | 105 (89.7%) | 90 (89.1%) | 58 (81.7%) | ||
| Venous | 12 (10.3%) | 11 (10.9%) | 13 (18.3%) | ||
| Estimated blood loss | 300 [200;525] | 300 [200;500] | 300 [150;500] | 0.551 | 0.830 |
| Operative Time (hours) | 6.33 [4.67;8.00] | 6.92 [5.03;8.47] | 7.32 [5.46;9.24] | 0.074 | 0.022 |
| Pathologic tumor stage | 0.202 | 0.106 | |||
| CR or in situ | 11 (9.40%) | 9 (8.91%) | 1 (1.41%) | ||
| T1 | 9 (7.69%) | 6 (5.94%) | 9 (12.7%) | ||
| T2 | 59 (50.4%) | 47 (46.5%) | 31 (43.7%) | ||
| T3 | 38 (32.5%) | 39 (38.6%) | 30 (42.3%) | ||
| Positive lymph nodes | 0.252 | 0.111 | |||
| N0 (0) | 51 (43.6%) | 48 (47.5%) | 24 (33.8%) | ||
| N1 (1–3) | 47 (40.2%) | 37 (36.6%) | 28 (39.4%) | ||
| N2 (4+) | 19 (16.2%) | 16 (15.8%) | 19 (26.8%) | ||
| Margin Status | 0.066 | 0.304 | |||
| R0 | 100 (85.5%) | 96 (95.0%) | 63 (88.7%) | ||
| R1 | 17 (14.5%) | 5 (4.95%) | 8 (11.3%) | ||
| Lymphovascular invasion | 0.191 | 0.229 | |||
| No | 29 (25.0%) | 36 (36.4%) | 22 (31.9%) | ||
| Yes | 87 (75.0%) | 63 (63.6%) | 47 (68.1%) | ||
| Perineural invasion | 0.143 | 0.208 | |||
| No | 15 (12.9%) | 23 (23.2%) | 13 (18.8%) | ||
| Yes | 101 (87.1%) | 76 (76.8%) | 56 (81.2%) | ||
| Clavien complication (grade 3/4) | 0.412 | 0.920 | |||
| No | 102 (87.2%) | 83 (82.2%) | 63 (88.7%) | ||
| Yes | 15 (12.8%) | 18 (17.8%) | 8 (11.3%) | ||
| Pancreatic fistula (grade B/C) | 0.891 | 0.639 | |||
| None | 101 (86.3%) | 88 (87.1%) | 63 (88.7%) | ||
| Yes | 16 (13.7%) | 13 (12.9%) | 8 (11.3%) | ||
| Length of stay | 10.0 [8.00; 13.0] | 11.0 [9.00; 14.0] | 10.0 [8.00; 15.0] | 0.361 | 0.230 |
| Readmission by 90 days | 0.082 | 0.406 | |||
| No | 98 (83.8%) | 78 (77.2%) | 64 (90.1%) | ||
| Yes | 19 (16.2%) | 23 (22.8%) | 7 (9.86%) | ||
| Initiated adjuvant therapy | 0.504 | 0.958 | |||
| No, failed to initiate | 19 (16.7%) | 22 (22.0%) | 11 (15.9%) | ||
| Yes, initiated | 95 (83.3%) | 78 (78.0%) | 58 (84.1%) | ||
| Completed adjuvant therapy | 0.636 | 0.983 | |||
| No, failed to complete | 37 (32.7%) | 38 (38.0%) | 22 (31.9%) | ||
| Yes, completed | 76 (67.3%) | 62 (62.0%) | 47 (68.1%) | ||
| Overall survival | |||||
| Alive | 29 (24.8) | 27 (26.7) | 18 (25.4) | 0.946 | 0.705 |
| Dead | 88 (75.2) | 74 (73.3) | 53 (74.6) |
As shown in Table 1, we did not find evidence of a difference between SED and race, insurance status, comorbidity, resectability, pathology, post-operative complications, initiation of adjuvant therapy, completion of adjuvant therapy, or site of recurrence. Additionally, we did not find evidence of a difference between SED and geographic distance from MCC.
Grade 3 or 4 Clavien Complication
Grade 3 or 4 Clavien complications were identified in 41 (14.2%) of patients and grade B or C ISGPS pancreatic fistulae were identified in 37 patients (12.8%), similar to previously published rates from retrospective analyses and clinical trials.27–29 After adjustment for potential confounders, moderate or high SED did not predict Grade 3 or 4 Clavien complications relative to low SED (Table 2). In the multivariable model, an ASA score of 3 increased the relative odds of a Grade 3 or 4 Clavien complication (OR, 3.52; 95% CI 1.36–10.05).
Table 2:
Relative Odds of Clavien Grade 3/4 Complication: Univariable and Multivariable Logistic Regression Models, Odds Ratios (95% CI)
| Univariable | P value | Overall P value | Multivariable | P value | Overall P value | |
|---|---|---|---|---|---|---|
| SED (reference: Low SED) | 0.416 | 0.657 | ||||
| Moderate SED | 1.48 (0.70, 3.14) | 0.306 | 1.13 (0.48, 2.72) | 0.777 | ||
| High SED | 0.86 (0.33, 2.11) | 0.753 | 0.70 (0.23, 1.97) | 0.511 |
The multivariable model was adjusted for age, sex, race, insurance status, distance from MCC, Charlson comorbidity index, diabetes mellitus, BMI, presence of a preoperative biliary stent, receipt of neoadjuvant therapy, ASA class, type of resection, venous reconstruction, estimated blood loss, operative time, pathologic tumor stage, pathological nodal stage, and margin status.
Initiation of Adjuvant Therapy
Adjuvant therapy was initiated in 231 (81.6%) patients. In univariable analysis, government insurance predicted the lowest relative odds of initiating adjuvant therapy (OR, 0.31; 95% CI, 0.12–0.72); higher age, CCI, and blood loss as well as ASA class 3 and Clavien grade 3/4 complication predicted decreased odds of initiating adjuvant therapy (Table 3). After adjustment for potential confounders, moderate or high SED did not drive initiation of adjuvant therapy relative to low SED. In the multivariable model, grade 3 or 4 Clavien complication conferred the lowest odds of initiating adjuvant treatment (OR, 0.22; 95% CI, 0.08–0.57). Male sex and increasing age were also associated with decreased relative odds of initiating adjuvant therapy.
Table 3:
Relative Odds of Initiation of Adjuvant Therapy: Univariable and Multivariable Logistic Regression Models, Odds Ratios (95% CI)
| Univariable | P value | Overall P value | Multivariable | P value | Overall P value | |
|---|---|---|---|---|---|---|
| SED (reference: Low SED) | 0.506 | 0.286 | ||||
| Moderate SED | 0.71 (0.36, 1.40) | 0.324 | 0.55 (0.22, 1.32) | 0.184 | ||
| High SED | 1.06 (0.48, 2.44) | 0.898 | 1.12 (0.39, 3.31) | 0.837 | ||
| Age | 0.92 (0.89, 0.96) | <0.001 | 0.88 (0.83, 0.94) | <0.001 | ||
| Male sex (reference: female) | 0.58 (0.30, 1.09) | 0.096 | 0.35 (0.15, 0.77) | 0.011 | ||
| Race (reference: White) | 0.403 | 0.416 | ||||
| Black | 2.89 (0.55, 53.16) | 0.314 | 1.34 (0.18, 27.69) | 0.803 | ||
| Other / unknown | 2.64 (0.50, 48.89) | 0.357 | 5.39 (0.61, 136.18) | 0.194 | ||
| Insurance (reference: private) | 0.031 | 0.167 | ||||
| Government with private supplement | 0.48 (0.19, 1.10) | 0.094 | 0.97 (0.30, 2.98) | 0.962 | ||
| Government | 0.31 (0.12, 0.72) | 0.009 | 0.46 (0.13, 1.44) | 0.193 | ||
| Distance from Moffitt (miles) | 1.00 (0.99, 1.00) | 0.502 | 1.00 (0.99, 1.01) | 0.715 | ||
| Charlson comorbidity index | 0.77 (0.63, 0.95) | 0.015 | 1.36 (0.94, 2.01) | 0.113 | ||
| Diabetes mellitus (reference: no) | 0.61 (0.33, 1.17) | 0.127 | 0.66 (0.28, 1.54) | 0.330 | ||
| BMI | 0.99 (0.94, 1.05) | 0.720 | 0.98 (0.92, 1.05) | 0.559 | ||
| Preoperative biliary stent (reference: none) | 1.00 (0.54, 1.82) | 0.988 | 1.21 (0.47, 3.00) | 0.681 | ||
| Neoadjuvant Therapy (reference: none) | 1.02 (0.55, 1.95) | 0.951 | 0.91 (0.34, 2.47) | 0.843 | ||
| ASA class 3 (reference: ASA 2) | 0.34 (0.16, 0.67) | 0.003 | 0.58 (0.22, 1.45) | 0.252 | ||
| Distal pancreatectomy +/− splenectomy (reference: Whipple/total pancreatectomy) | 1.32 (0.63, 3.05) | 0.488 | 1.27 (0.34, 4.86) | 0.728 | ||
| Venous reconstruction (reference: none) | 0.92 (0.40, 2.41) | 0.859 | 1.68 (0.50, 6.45) | 0.423 | ||
| Estimated blood loss | 1.00 (1.00, 1.00) | 0.021 | 1.00 (0.99, 1.00) | 0.060 | ||
| Operative time | 0.90 (0.80, 1.02) | 0.100 | 0.87 (0.72, 1.04) | 0.131 | ||
| Pathologic tumor stage (reference: CR/in situ) | 0.602 | 0.691 | ||||
| T1 | 2.80 (0.48, 22.14) | 0.268 | 2.02 (0.21, 28.58) | 0.565 | ||
| T2 | 1.23 (0.33, 3.76) | 0.729 | 1.06 (0.22, 4.54) | 0.939 | ||
| T3 | 1.02 (0.27, 3.14) | 0.977 | 0.68 (0.13, 3.08) | 0.622 | ||
| Pathologic nodal stage (reference: N0) | 0.262 | 0.157 | ||||
| N1 | 1.78 (0.90, 3.63) | 0.102 | 2.71 (0.99, 7.70) | 0.055 | ||
| N2 | 1.19 (0.54, 2.79) | 0.669 | 1.78 (0.53, 6.30) | 0.361 | ||
| R1 Margin status (reference: R0) | 1.09 (0.43, 3.37) | 0.868 | 0.39 (0.11, 1.53) | 0.159 | ||
| Lymphovascular invasion (reference: none) | 1.15 (0.59, 2.16) | 0.682 | 0.85 (0.27, 2.62) | 0.784 | ||
| Perineural invasion (reference: none) | 1.00 (0.43, 2.14) | 0.997 | 0.99 (0.30, 3.14) | 0.986 | ||
| Clavien grade 3/4 complication (reference: none) | 0.31 (0.15, 0.66) | 0.002 | 0.22 (0.08, 0.57) | 0.002 |
Completion of Adjuvant Therapy
Adjuvant therapy was completed in 185 (65.6%) of patients, which is comparable to prior reports and clinical trials.30 In univariable analysis, venous reconstruction was associated with the lowest relative odds of completing adjuvant therapy (OR, 0.42; 95% CI, 0.20–0.84); higher age, CCI and estimated blood loss as well as ASA class 3 and Clavien grade 3/4 complication predicted decreased odds of completing adjuvant therapy (Table 4). After adjustment for potential confounders, moderate or high SED did not predict completion of adjuvant therapy relative to low SED. In the multivariable model, Clavien grade 3/4 complication conferred the lowest odds of completing adjuvant treatment (OR, 0.33; 95% CI 0.14–0.77). Increasing age was also associated with decreased relative odds of initiating adjuvant therapy and other/unknown race predicted increased odds of completing adjuvant therapy.
Table 4:
Relative Odds of Completion of Adjuvant Therapy: Univariable and Multivariable Logistic Regression Models, Odds Ratios (95% CI)
| Univariable | P value | Overall P value | Multivariable | P value | Overall P value | |
|---|---|---|---|---|---|---|
| SED (reference: Low SED) | 0.637 | 0.462 | ||||
| Moderate SED | 0.79 (0.45, 1.40) | 0.423 | 0.64 (0.32, 1.29) | 0.215 | ||
| High SED | 1.04 (0.55, 1.99) | 0.904 | 0.82 (0.37, 1.86) | 0.634 | ||
| Age | 0.94 (0.91, 0.97) | <0.001 | 0.93 (0.88, 0.97) | 0.002 | ||
| Male sex (reference: female) | 0.79 (0.48, 1.30) | 0.360 | 0.68 (0.37, 1.25) | 0.220 | ||
| Race (reference: White) | 0.146 | 0.088 | ||||
| Black | 1.89 (0.56, 8.59) | 0.343 | 1.03 (0.25, 5.38) | 0.968 | ||
| Other / unknown | 6.24 (1.19, 114.92) | 0.082 | 14.14 (1.92, 310.59) | 0.028 | ||
| Insurance (reference: private) | 0.112 | 0.982 | ||||
| Government with private supplement | 0.56 (0.30, 1.04) | 0.071 | 0.99 (0.42, 2.32) | 0.987 | ||
| Government | 0.52 (0.26, 1.00) | 0.052 | 0.93 (0.37, 2.34) | 0.882 | ||
| Distance from Moffitt (miles) | 1.00 (0.99, 1.00) | 0.189 | 1.00 (0.99, 1.00) | 0.091 | ||
| Charlson comorbidity index | 0.80 (0.67, 0.95) | 0.012 | 1.03 (0.76, 1.40) | 0.848 | ||
| Diabetes mellitus (reference: no) | 0.69 (0.41, 1.19) | 0.179 | 0.78 (0.38, 1.60) | 0.497 | ||
| BMI | 1.02 (0.98, 1.07) | 0.346 | 1.06 (0.99, 1.12) | 0.076 | ||
| Preoperative biliary stent (reference: none) | 1.64 (0.99, 2.70) | 0.051 | 1.84 (0.88, 3.86) | 0.104 | ||
| Neoadjuvant Therapy (reference: none) | 0.88 (0.53, 1.47) | 0.609 | 0.75 (0.35, 1.60) | 0.451 | ||
| ASA class 3 (reference: ASA 2) | 0.47 (0.28, 0.79) | 0.005 | 0.89 (0.44, 1.82) | 0.747 | ||
| Distal pancreatectomy +/− splenectomy (reference: Whipple/total pancreatectomy) | 0.78 (0.43, 1.42) | 0.405 | 0.52 (0.18, 1.45) | 0.213 | ||
| Venous reconstruction (reference: none) | 0.42 (0.20, 0.84) | 0.015 | 0.64 (0.25, 1.66) | 0.354 | ||
| Estimated blood loss | 1.00 (1.00, 1.00) | 0.015 | 1.00 (0.99, 1.00) | 0.103 | ||
| Operative time | 0.94 (0.85, 1.04) | 0.213 | 0.88 (0.75, 1.02) | 0.082 | ||
| Pathologic tumor stage (reference: CR/in situ) | 0.275 | 0.443 | ||||
| T1 | 1.70 (0.38, 7.98) | 0.485 | 0.79 (0.13, 4.81) | 0.792 | ||
| T2 | 0.60 (0.19, 1.67) | 0.354 | 0.51 (0.12, 1.88) | 0.324 | ||
| T3 | 0.64 (0.19, 1.82) | 0.423 | 0.36 (0.08, 1.42) | 0.154 | ||
| Pathologic nodal stage (reference: N0) | 0.574 | 0.191 | ||||
| N1 | 1.34 (0.78, 2.32) | 0.300 | 2.11 (0.95, 4.76) | 0.069 | ||
| N2 | 1.21 (0.61, 2.45) | 0.586 | 1.69 (0.63, 4.69) | 0.306 | ||
| R1 Margin status (reference: R0) | 0.84 (0.39, 1.92) | 0.673 | 0.36 (0.13, 1.03) | 0.056 | ||
| Lymphovascular invasion (reference: none) | 0.94 (0.55, 1.60) | 0.826 | 0.48 (0.18, 1.21) | 0.126 | ||
| Perineural invasion (reference: none) | 1.24 (0.65, 2.33) | 0.505 | 2.20 (0.87, 5.64) | 0.096 | ||
| Clavien grade 3/4 complication (reference: none) | 0.49 (0.25, 0.97) | 0.038 | 0.33 (0.14, 0.77) | 0.011 |
Overall Survival
The median overall survival was 27.6 months with 215 deaths (74.4%). The 30-day mortality rate was 1.7% and the 90-day mortality rate was 3.1%, which are comparable to other high-volume centers.31 There was no difference in survival probability by SED tercile rank (Figure 2). As shown in Table 5, in univariable analysis, the greatest decrease in hazard of death was predicted by completion of adjuvant therapy (HR, 0.42; 95% CI 0.32–0.56). Neoadjuvant therapy (HR, 0.59; 95% CI 0.44–0.79) also predicted decreased hazard of death; increasing age, higher T stage, and higher N stage predicted increased hazard of death. After adjustment for potential confounders, moderate or high SED did not predict hazard of death relative to low SED. In the multivariable model, completion of adjuvant therapy conferred the greatest decrease in hazard of death (HR, 0.38; 95% CI 0.25–0.58). Neoadjuvant therapy was also predictive of decreased hazard of death (HR, 0.54; 95% CI 0.36–0.80). Increasing N stage as well as T2 and T3 stage relative to CR/in situ disease were the greatest drivers of increased hazard of death. Increased operative time and length of stay also predicted increased mortality.
Figure 2.
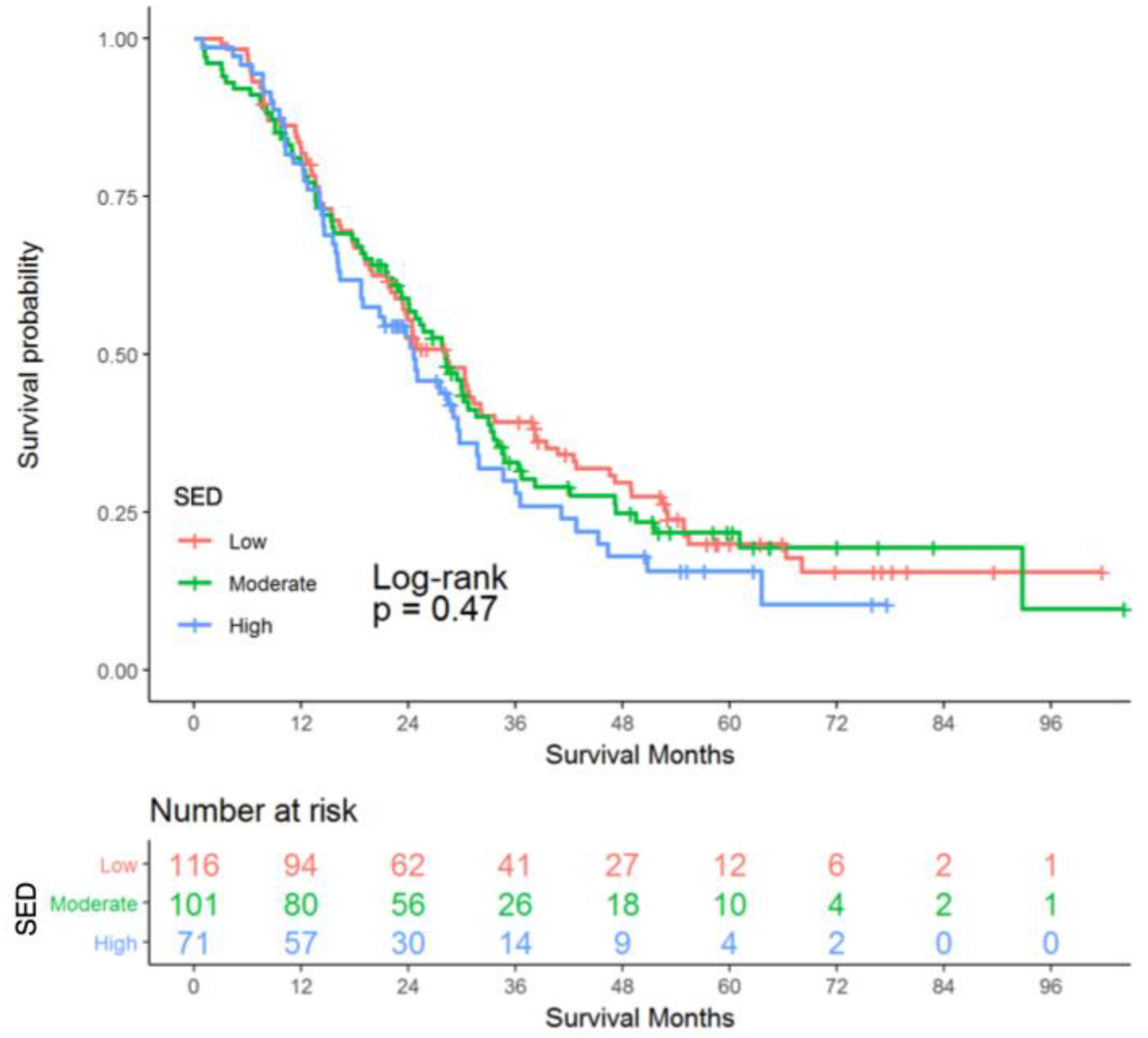
Kaplan-Meier Curve of the Probability of Overall Survival for Resected Pancreatic Adenocarcinoma Patients
Table 5:
Relative Hazard of Death: Univariable and Multivariable Cox Proportional Hazards Regression Model, Hazard Ratios (95% CI)
| Univariable | P value | Overall P value | Multivariable | P value | Overall P value | |
|---|---|---|---|---|---|---|
| SED (reference: Low SED) | 0.471 | 0.953 | ||||
| Moderate SED | 1.05 (0.77, 1.43) | 0.772 | 1.02 (0.72, 1.46) | 0.897 | ||
| High SED | 1.24 (0.88, 1.74) | 0.228 | 1.07 (0.72, 1.58) | 0.757 | ||
| Age | 1.02 (1.00, 1.03) | 0.016 | 0.99 (0.97, 1.01) | 0.408 | ||
| Male sex (reference: female) | 1.25 (0.95, 1.65) | 0.108 | 1.21 (0.88, 1.68) | 0.248 | ||
| Race (reference: White) | 0.497 | 0.749 | ||||
| Black | 0.75 (0.37, 1.52) | 0.422 | 0.84 (0.37, 1.88) | 0.664 | ||
| Other / unknown | 0.69 (0.31, 1.56) | 0.373 | 0.76 (0.32, 1.80) | 0.529 | ||
| Insurance (reference: private) | 0.153 | 0.096 | ||||
| Government with private supplement | 0.91 (0.65, 1.26) | 0.556 | 0.67 (0.43, 1.03) | 0.066 | ||
| Government | 1.24 (0.88, 1.76) | 0.221 | 0.94 (0.60, 1.47) | 0.787 | ||
| Distance from Moffitt (miles) | 1.00 (0.99, 1.00) | 0.526 | 1.00 (0.99, 1.00) | 0.123 | ||
| Charlson comorbidity index | 1.17 (1.07, 1.28) | 0.001 | 1.02 (0.86, 1.21) | 0.835 | ||
| Diabetes mellitus (reference: no) | 1.08 (0.80, 1.46) | 0.631 | 1.12 (0.75, 1.66) | 0.585 | ||
| BMI | 1.01 (0.99, 1.02) | 0.278 | 1.00 (0.97, 1.03) | 0.835 | ||
| Preoperative biliary stent (reference: none) | 1.19 (0.90, 1.56) | 0.216 | 1.19 (0.79, 1.79) | 0.411 | ||
| Neoadjuvant Therapy (reference: none) | 0.59 (0.44, 0.79) | 0.001 | 0.54 (0.36, 0.80) | 0.002 | ||
| ASA class 3 (reference: ASA 2) | 1.37 (1.04, 1.81) | 0.024 | 1.36 (0.95, 1.93) | 0.092 | ||
| Distal pancreatectomy +/− splenectomy (reference: Whipple/total pancreatectomy) | 0.93 (0.66, 1.31) | 0.677 | 1.52 (0.86, 2.69) | 0.150 | ||
| Venous reconstruction (reference: none) | 1.16 (0.77, 1.74) | 0.474 | 0.99 (0.58, 1.68) | 0.967 | ||
| Estimated blood loss | 1.00 (1.00, 1.00) | 0.150 | 1.00 (1.00, 1.00) | 0.602 | ||
| Operative time | 1.06 (1.00, 1.12) | 0.035 | 1.12 (1.03, 1.21) | 0.006 | ||
| Pathologic tumor stage (reference: CR/in situ) | <0.001 | 0.004 | ||||
| T1 | 1.77 (0.76, 4.15) | 0.189 | 1.23 (0.45, 3.38) | 0.689 | ||
| T2 | 3.49 (1.76, 6.92) | <0.001 | 2.86 (1.26, 6.49) | 0.012 | ||
| T3 | 4.19 (2.10, 8.35) | <0.001 | 3.42 (1.52, 7.73) | 0.003 | ||
| Pathologic nodal stage (reference: N0) | <0.001 | 0.001 | ||||
| N1 | 2.14 (1.57, 2.91) | <0.001 | 2.34 (1.51, 3.63) | 0.001 | ||
| N2 | 2.56 (1.77, 3.69) | <0.001 | 2.36 (1.41, 3.96) | 0.001 | ||
| R1 Margin status (reference: R0) | 1.37 (0.88, 2.11) | 0.161 | 1.07 (0.62, 1.84) | 0.802 | ||
| Lymphovascular invasion (reference: none) | 2.22 (1.60, 3.08) | <0.001 | 1.01 (0.61, 1.68) | 0.967 | ||
| Perineural invasion (reference: none) | 1.85 (1.25, 2.74) | 0.002 | 1.17 (0.72, 1.91) | 0.531 | ||
| Clavien grade 3/4 complication (reference: none) | 1.51 (1.04, 2.20) | 0.032 | 1.27 (0.77, 2.07) | 0.350 | ||
| Length of stay (days) | 1.04 (1.01, 1.06) | 0.001 | 1.03 (1.00, 1.06) | 0.041 | ||
| Readmission by 90 days | 0.95 (0.66, 1.37) | 0.796 | 0.64 (0.41, 1.01) | 0.053 | ||
| Initiation of adjuvant therapy | 0.47 (0.34, 0.65) | <0.001 | 0.71 (0.41, 1.23) | 0.220 | ||
| Completion of adjuvant therapy | 0.42 (0.32, 0.56) | <0.001 | 0.38 (0.25, 0.58) | <0.001 |
Discussion
This study used a publically-available, validated dataset at the census block group level to examine the impact of SED on PDAC post-operative outcomes and overall survival. Use of the ADI overcomes several obstacles in measuring and comparing SED and has broad applicability in surgical and oncologic research. We observed that nearly one-third of non-metastatic patients are from high SED neighborhoods. There was no evidence that SED was associated with receipt of surgical evaluation, curative-intent surgery or that SED predicts post-operative Clavien grade 3/4 complications, initiation or completion of chemotherapy, or overall survival. These results are in contrast to several studies that have observed low socioeconomic status is associated with worse post-operative outcomes and survival.2, 3, 5, 6, 32–34
In studies using national registries, low socioeconomic status has been predictive of higher operative mortality, worse post-operative outcomes, and decreased receipt of adjuvant therapy.4, 35, 36 Reames (2014) observed that patients in the lowest quintile of socioeconomic status had increased rates of complications, failure to rescue, and mortality for pancreatic cancer. The higher odds of failure to rescue persisted when controlling for patient characteristics, however, they were attenuated when adjusting for hospital characteristics, including volume. Low socioeconomic status has also been associated with decreased receipt of adjuvant therapy.36 Dimou (2016) observed that patients with government insurance had decreased odds of multimodality PDAC treatment compared to those with private insurance.1
Several studies have evaluated the relationship between socioeconomic status and PDAC survival. Studies from the California Cancer Registry from the 1990s and 2000s at the census block group level found conflicting results; one study showed that high socioeconomic status was associated with improved survival while another did not, though the categorization of SES differed between the studies making comparison a challenge.2, 32 Using the Florida Cancer Data System, Cheung (2010) observed that higher poverty predicted decreased overall survival after adjustment for potential confounders.6 In a more contemporary cohort of resected patients, higher median income was predictive of improved survival.3
One explanation offered for these disparities is that high-volume centers are predisposed to do well because they treat higher socioeconomic status patients with better baseline comorbidity status.4, 37 While we did not identify a difference in Charlson comorbidity score by SED in curative-intent surgery patients, the median CACI was 6 and not associated increased early mortality. These findings are in contrast with published data from other high-volume centers that observed a median CACI of 3 for resected PDAC patients and found that a score of 6 or higher led to increased early mortality.38, 39
Another potential explanation for our findings is that high-volume care in conjunction with adherence to clinical guidelines/pathways not only improves outcomes but may reduce socioeconomic disparities.40–42 While the impact of high-volume care has been well-documented, few studies have explored the impact of guideline/pathway adherence. Visser (2012) showed that after controlling for facility volume, compliance with NCCN guidelines for pancreatic cancer decreased mortality. Furthermore, high-volume centers with NCCN compliant PDAC care had a greater than 10-month improvement in median survival compared to non-compliant high-volume centers. However, others have shown that disparities persist with pathway use.43 While promising, clinical pathway implementation alone is unlikely to serve as a widely-applicable panacea and more research is needed to identify interventions and policy measures to reduce socioeconomic disparities.43–45
Finally, compared to national data and Florida demographics, our cohort was significantly more homogenous in terms of race/ethnicity and insurance status, which may contribute to the results observed. Our cohort consisted of 4.5% African-Americans and 4.5% had Medicaid or were uninsured compared to national rates of 9.3% for African Americans and 8.0% for Medicaid/uninsured patients undergoing surgery, respectively.46 These elisions of patient diversity limit the external validitity of these findings. Consequently, generalizing from these homogenous data is fraught with challenges as they do not account for the potentially compounding vulnerabilities that exist at the intersection of SED, race, and insurance status.
There are additional limitations to consider when interpreting these findings. Although the majority of patients are referred from other providers, our sample may have increased health-seeking behaviors and/or characteristics which may lead to improved outcomes. While we showed that our patients have similar characteristics across SED strata, we cannot exclude unmeasured selection bias that may have impacted our findings. Additionally, this measure of SED is a geocoded, aggregate measure that relies on census block group data and therefore has the potential for ecological fallacy, though this error is decreased compared to zip code level analysis. Finally, while we did not find a statistical difference between SED and outcomes, we cannot exclude that the study was underpowered.
Despite these limitations, our findings suggest that a granular measure of SED can be applied to studies of complex cancer care. Noting the revealing racial and insurance status disparities between MCC and more representative populations, our findings suggest that treatment of localized PDAC at a high-volume cancer center employing a standardized pathway can offer high rates of surgical evaluation, curative-surgery, and superior post-operative outcomes. While we have suggested that high fidelity to a standardized clinical pathway may contribute to these findings, this was not directly tested and it remains uncertain what factor or combination of factors (cohort homogeneity, high-volume center, teaching facility, standardized clinical pathway, clinical and social support services) contributed to these results. It is also unknown if these outcomes would persist if the number of patients from neighborhoods of high SED was increased at our institution, however, other studies have not shown that centralization diminishes outcomes.47
There have been prior calls for centralization of complex cancer care to improve outcomes.48 Increasing the number of patients from areas of high SED receiving treatment at high-volume cancer centers with adherence to clinical guidelines may improve outcomes for this group. However, this remains a challenge particularly for low SED patients. Nearly 40% of US patients receive care at low-volume facilities and compliance with NCCN guideline care is less than 35%.42, 49, 50 If the field of surgical oncology hopes to move toward achieving equity for cancer patients, it will involve critical examination of the social determinants of health and moving beyond descriptive studies of disparities. This necessitates accurate measurement of SED that allows for convenient comparison across populations. This transition to a more local/regional analytic approach, focused on actionable efforts to improve access and quality of care, may lead to improved outcomes for patients from even the most socioeconomically deprived neighborhoods.
Supplementary Material
Acknowledgements
The authors thank Yanbing Dong for assistance with data acquisition, Leigha Vilen at the Neighborhood Atlas for assistance with the Area Deprivation Index, and Diana Castillo for assistance with figure preparation.
Funding: H. Lee Moffitt Cancer Center & Research Institute NCI Cancer Center Support Grant (P30-CA076292)
Footnotes
The authors declare no conflicts of interest.
References
- 1.Dimou F, Sineshaw H, Parmar AD, et al. Trends in Receipt and Timing of Multimodality Therapy in Early-Stage Pancreatic Cancer. J Gastrointest Surg 2016; 20(1):93–103; discussion 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zell JA, Rhee JM, Ziogas A, et al. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev 2007; 16(3):546–52. [DOI] [PubMed] [Google Scholar]
- 3.Moaven O, Richman JS, Reddy S, et al. Healthcare disparities in outcomes of patients with resectable pancreatic cancer. Am J Surg 2018. [DOI] [PubMed] [Google Scholar]
- 4.Reames BN, Birkmeyer NJ, Dimick JB, et al. Socioeconomic disparities in mortality after cancer surgery: failure to rescue. JAMA Surg 2014; 149(5):475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riall TS, Townsend CM Jr., Kuo YF, et al. Dissecting racial disparities in the treatment of patients with locoregional pancreatic cancer: a 2-step process. Cancer 2010; 116(4):930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung MC, Yang R, Byrne MM, et al. Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? Cancer 2010; 116(3):723–33. [DOI] [PubMed] [Google Scholar]
- 7.Collaborators USBoD, Mokdad AH, Ballestros K, et al. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA 2018; 319(14):1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health 2017; 2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiatt RA, Breen N. The social determinants of cancer: a challenge for transdisciplinary science. Am J Prev Med 2008; 35(2 Suppl):S141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N. Epidemiology and the people’s health : theory and context. New York: Oxford University Press, 2011. [Google Scholar]
- 11.Berkman LF, Kawachi I, Glymour MM. Social epidemiology. Second edition. ed. Oxford: Oxford University Press, 2014. [Google Scholar]
- 12.Nuru-Jeter AM, Michaels EK, Thomas MD, et al. Relative Roles of Race Versus Socioeconomic Position in Studies of Health Inequalities: A Matter of Interpretation. Annu Rev Public Health 2018; 39:169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miura JT, Evans DB, Pappas SG, et al. Borderline resectable/locally advanced pancreatic adenocarcinoma: improvements needed in population-based registries. Ann Surg Oncol 2013; 20(13):4338–47. [DOI] [PubMed] [Google Scholar]
- 14.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol 2008; 15(2):415–23. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol 2002; 156(5):471–82. [DOI] [PubMed] [Google Scholar]
- 16.Grubesic TH, Matisziw TC. On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int J Health Geogr 2006; 5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible -The Neighborhood Atlas. N Engl J Med 2018; 378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David C Radley DM, Hayes Susan L.. The Commonwealth Fund: 2018 Scorecard on State Health System Performance. 2018.
- 19.Center MC. Available at: https://moffitt.org/for-healthcare-providers/clinical-pathways/.
- 20.Rashid OM, Pimiento JM, Gamenthaler AW, et al. Outcomes of a Clinical Pathway for Borderline Resectable Pancreatic Cancer. Ann Surg Oncol 2016; 23(4):1371–9. [DOI] [PubMed] [Google Scholar]
- 21.Area Deprivation Index [University of Wisconsin School of Medicine and Public Health web site]. Available at: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed December 12, 2018.
- 22.US Census Block Groups [United States Census Bureau web site]. 2018. Available at: https://www.census.gov/geo/reference/webatlas/blockgroups.html. Accessed December 29, 2018.
- 23.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health 2003; 93(7):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akwo EA, Kabagambe EK, Harrell FE Jr., et al. Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States. Circ Cardiovasc Qual Outcomes 2018; 11(1):e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 27.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 2006; 244(6):931–7; discussion 937–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013; 258(4):554–9; discussion 559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen PJ, Gonen M, Brennan MF, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med 2014; 370(21):2014–22. [DOI] [PubMed] [Google Scholar]
- 30.Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 2014; 32(6):504–12. [DOI] [PubMed] [Google Scholar]
- 31.Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017; 265(1):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control 2006; 17(4):403–9. [DOI] [PubMed] [Google Scholar]
- 33.Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection: a key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer 2009; 115(17):3979–90. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro M, Chen Q, Huang Q, et al. Associations of Socioeconomic Variables With Resection, Stage, and Survival in Patients With Early-Stage Pancreatic Cancer. JAMA Surg 2016; 151(4):338–45. [DOI] [PubMed] [Google Scholar]
- 35.Birkmeyer NJ, Gu N, Baser O, et al. Socioeconomic status and surgical mortality in the elderly. Med Care 2008; 46(9):893–9. [DOI] [PubMed] [Google Scholar]
- 36.Abraham A, Al-Refaie WB, Parsons HM, et al. Disparities in pancreas cancer care. Ann Surg Oncol 2013; 20(6):2078–87. [DOI] [PubMed] [Google Scholar]
- 37.Bliss LA, Yang CJ, Chau Z, et al. Patient selection and the volume effect in pancreatic surgery: unequal benefits? HPB (Oxford) 2014; 16(10):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dias-Santos D, Ferrone CR, Zheng H, et al. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery 2015; 157(5):881–7. [DOI] [PubMed] [Google Scholar]
- 39.Xia BT, Habib DA, Dhar VK, et al. Early Recurrence and Omission of Adjuvant Therapy after Pancreaticoduodenectomy Argue against a Surgery-First Approach. Ann Surg Oncol 2016; 23(13):4156–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 1999; 126(2):178–83. [PubMed] [Google Scholar]
- 41.Fong Y, Gonen M, Rubin D, et al. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg 2005; 242(4):540–4; discussion 544–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visser BC, Ma Y, Zak Y, et al. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB (Oxford) 2012; 14(8):539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leeds IL, Alimi Y, Hobson DR, et al. Racial and Socioeconomic Differences Manifest in Process Measure Adherence for Enhanced Recovery After Surgery Pathway. Dis Colon Rectum 2017; 60(10):1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hisam B, Zogg CK, Chaudhary MA, et al. From understanding to action: interventions for surgical disparities. J Surg Res 2016; 200(2):560–78. [DOI] [PubMed] [Google Scholar]
- 45.Torain MJ, Maragh-Bass AC, Dankwa-Mullen I, et al. Surgical Disparities: A Comprehensive Review and New Conceptual Framework. J Am Coll Surg 2016; 223(2):408–18. [DOI] [PubMed] [Google Scholar]
- 46.Tohme S, Kaltenmeier C, Bou-Samra P, et al. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann Surg Oncol 2018. [DOI] [PubMed] [Google Scholar]
- 47.Swan RZ, Niemeyer DJ, Seshadri RM, et al. The impact of regionalization of pancreaticoduodenectomy for pancreatic Cancer in North Carolina since 2004. Am Surg 2014; 80(6):561–6. [PubMed] [Google Scholar]
- 48.Anaya DA, Malafa M. Outcome Disparities in Pancreatic Cancer: Need for Improved Regionalization of Care. JAMA Surg 2016; 151(4):345–6. [DOI] [PubMed] [Google Scholar]
- 49.Al-Refaie WB, Muluneh B, Zhong W, et al. Who receives their complex cancer surgery at low-volume hospitals? J Am Coll Surg 2012; 214(1):81–7. [DOI] [PubMed] [Google Scholar]
- 50.Gage-Bouchard EA, Rodriguez EM, Saad-Harfouche FG, et al. Factors influencing patient pathways for receipt of cancer care at an NCI-designated comprehensive cancer center. PLoS One 2014; 9(10):e110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


