Abstract
It is unknown whether the HPV vaccine is effective in immunocompromised women during catch-up ages. We performed a case-control study of 4,357 women with incident CIN2+ (cases) and 5:1 age-matched, incidence-density selected controls (N=21,773) enrolled in an integrated health care system from 2006 to 2014. Vaccine effectiveness was estimated from multivariable conditional logistic regression models, with results stratified by immunosuppression history, defined as prior HIV infection, solid organ transplant history, or recently prescribed immunosuppressive medications. HPV vaccination resulted in a 19% reduction in CIN2+ rates for women without an immunosuppression history but a nonsignificant 4% reduction for women with an immunosuppression history. Further research is needed to evaluate whether catch-up HPV vaccine effectiveness varies by immunosuppression status, especially given the recent approval of the HPV vaccine for adults up to 45 years of age.
Keywords: vaccination, human papillomavirus, HIV, immunosuppression
BACKGROUND
Human papillomavirus (HPV) vaccination for the prevention of cervical and other cancers is currently recommended at 11 or 12 years of age for boys and girls with catch-up vaccination through age 26, and shared clinical decision-making for those aged 27–45. [1] Recommendations apply to eligible individuals regardless of immune status, although data on effectiveness in immunocompromised populations are lacking. Current data indicate that the HPV vaccine is safe and immunogenic in immunocompromised individuals including persons with HIV, those with autoimmune disorders including systemic lupus erythematosus and inflammatory bowel disease, and those taking immunosuppressive therapies, such as solid organ transplant recipients.[2–4] While studies have consistently shown that immunocompromised individuals develop serologic immune responses following HPV immunization, most report that HPV antibody titers or persistence are lower among these individuals compared with healthy controls[2, 5]. Also, persons with HIV on antiretroviral therapies and those with higher CD4 count at the time of first dose of HPV vaccine tend to have a better immune responses.[2, 6–8] Finally, two small randomized clinical trials (RCTs) among HIV-infected populations suggest reduced effectiveness against clinical effectiveness with catch-up HPV vaccination, but did not evaluate histological outcomes.[7, 9]
Here, our objective was to evaluate the effectiveness of the HPV vaccine among girls and women in the HPV catch-up age range comparing those with and without pre-existing immunocompromising conditions. We leveraged data from a previous study of women enrolled in a large integrated healthcare system, which evaluated the association of HPV vaccination history[10] and immunocompromising conditions (HIV, solid organ transplant, immunosuppressive medications)[11] on the risk of cervical precancer or cancer.
METHODS
The study was conducted among women enrolled in Kaiser Permanente Northern California (KPNC), a large integrated health care delivery system providing comprehensive care for over 3.9 million members in the greater San Francisco Bay Area. The study population was a subset of participants in a previously described[10, 11] nested case-control study. Briefly, cases included women with an incident (i.e., new within past 18 months) diagnosis of cervical intraepithelial neoplasia grade 2, 2/3, 3, adenocarcinoma in situ or cancer (CIN2+) proven by cervical biopsy during 2006–2014. We only included women eligible for catch-up HPV vaccine since its availability in 2006 and who were screen-eligible during follow-up, corresponding with those aged 18–26 years at some point between 2006 and 2014. For each case, we randomly selected 5 controls without CIN2+ by incidence-density sampling, with matching by age (within one year), time since first cytology in the health system (within one year), and years of continuous prior health plan membership (within one year). Cases and controls were excluded if they had a prior hysterectomy. The index date for cases was the diagnosis date, and controls were assigned the same index date as the case to which they were matched. Cases and controls were also required to have a cytology test performed within 12 months prior to their assigned index date. A secondary study population of interest was CIN3+ and their matched controls.
The primary data source for the current study was the electronic health record with comprehensive clinical and administrative data available since 1995, including inpatient and outpatient medical encounters, pharmacy, laboratory, procedures (including vaccination), health plan membership, and demographic information. Histopathology results of cervical biopsies were ascertained by natural language processing of pathology reports. Since 2006, KPNC offered the quadrivalent HPV vaccine; the nonavalent HPV vaccine was introduced in August 2015, after the end of the study period.
The primary exposures of interest were ever HPV vaccination history (i.e., ≥1 HPV vaccine dose) and prior immunosuppression history both assessed at the index date. As described in detail previously,[11] immunocompromising conditions included ever HIV-infection (i.e., any time prior to index) ascertained from the Kaiser Permanente Northern California HIV registry; (2) ever solid organ transplantation (i.e., any time prior to index) defined as two or more diagnosis or procedure codes at any time before the index date; and (3) recent (i.e., within 18 months prior to the index date) use of 17 non-topical immunosuppressive medication drug classes ascertained from pharmacy records. Other CIN2+ risk factors collected were race/ethnicity, recent smoking, high parity (3 or more live births), recent sexually transmitted infections (herpes, gonorrhea, syphilis and chlamydia), and recent hormonal contraceptive use. We also collected recent outpatient visits as a proxy for healthcare engagement.
Conditional logistic regression was used to estimate odds ratios, which represent unbiased estimates of rate ratios (RR) in a nested case-control study with incidence density sampling.[12] Adjusted models for outcomes of CIN2+ and CIN3+ included terms for vaccine history, immunocompromising conditions, vaccine/immunocompromised interaction term, and covariates listed above. We also performed two sensitivity analyses, including (1) limiting to subjects with continuous membership since 2006 to minimize misclassification of HPV vaccine history; and (2) replacing all “recent” versions of covariates with “ever” versions (i.e., any time in past as a proxy for lifetime exposure) to minimize potential for residual confounding. Analyses were conducted using the LOGISTIC procedure in SAS, Version 9.3 (Cary, North Carolina).
The institutional review board at Kaiser Permanente Northern California approved this study with a waiver of written informed consent.
RESULTS
The study population included 4,357 CIN2+ cases and 21,773 controls. Of 4,357 CIN2+ cases, 874 (20%) were CIN2, 1,634 (38%) were CIN2/3, 1,744 (40%) were CIN3, 82 (2%) were adenocarcinoma in situ and 23 (<1%) were cancer (9 adenocarcinoma, 13 squamous cell carcinoma and 1 other cancer). As shown previously,[10] cases and controls were similar with respect to matching parameters of age (mean age 26.3 for both cases and controls), index year (mean 2010.4 for both cases and controls) and mean years of prior health plan membership (mean 7.4 and 7.5 years for cases and controls). Compared with controls, cases were more likely to be non-Hispanic White (49% vs. 44%), had a higher mean number of outpatient visits per year (7.5 vs. 6.9), higher recent smoking history (24% vs. 17%), higher recent hormonal contraceptive use (66% vs. 58%), and were more likely to have had a recent sexually transmitted infection (7% vs. 4%). Among cases, 506 women (11.6%) had a history of immunosuppression, including (not mutually exclusive) 470 with one recent (<18 months) immunosuppressive medication, 30 with two or more medications, six with prior solid organ transplantation and four with HIV infection. Among controls, 2,672 women (12.3%) had a history of immunosuppression, including 2,565 with one recent immunosuppressive medication, 100 with two or more medications, 11 with prior solid organ transplantation and five with HIV infection. Four hundred and twenty-nine (10%) of cases and 2,408 (11%) of controls had any prior HPV vaccination. The youngest age at first vaccination was 14 years. Among vaccinated cases and controls in the immunosuppressed group, 20 (47%) and 226 (53%) had 3+ vaccine doses and 15 (35%) and 63 (29%) had first vaccine dose at <18 years of age. Among vaccinated cases and controls in the non-immunosuppressed group, 194 (50%) and 1,197 (55%) had 3+ vaccine doses and 62 (16%) and 453 (21%) had first vaccine dose at <18 years of age.
Among 4,357 CIN2+ cases and 21,773 controls, the crude CIN2+ RRs for vaccination among women with and without prior immunosuppression were 1.00 (95% CI 0.71–1.42) and 0.84 (95% CI 0.74–0.95), respectively (Table). As shown in the Figure, the adjusted CIN2+ RRs for vaccination among women with and without prior immunosuppression were 0.96 (0.68–1.37) and 0.81 (0.71–0.92), respectively (p-value for test of interaction = 0.35). Among women with continuous membership since 2006 (2,495 cases and 12,514 controls), differences by immunosuppression status were more pronounced with adjusted RRs of 0.99 (0.64–1.55) and 0.66 (0.56–0.78), respectively (p-interaction = 0.09). Among 1,849 CIN3+ cases and 9,242 controls, RRs were similar for all women and for those with continuous membership since 2006, but with wider CIs compared with results for CIN2+ cases and controls (Figure). Replacing recent versions of smoking, sexually transmitted infections and hormonal contraceptive use had negligible impacts on findings (data not shown).
Table.
Human papillomavirus vaccine history and unadjusted rate ratios among cervical intraepithelial neoplasia grade 2 or worse (CIN2+) cases, CIN3+ cases, and matched controls
| Cases | Controls | ||||
|---|---|---|---|---|---|
| N | (%) | N | (%) | RR (95% CI)1 | |
| CIN2+ | |||||
| Women with prior immunosuppression2 | 506 | (100) | 2,672 | (100) | |
| Prior vaccination (≥1 dose) | 43 | (8.5) | 221 | (8.3) | 1.00 (0.71, 1.42) |
| No prior vaccination | 463 | (91.5) | 2,451 | (91.7) | 1 (reference) |
| Women with no prior immunosuppression2 | 3,851 | (100) | 19,101 | (100) | |
| Prior vaccination (≥1 dose) | 386 | (10.0) | 2,187 | (11.4) | 0.84 (0.74, 0.95) |
| No prior vaccination | 3,465 | (90.0) | 16,914 | (88.6) | 1 (reference) |
| CIN3+ | |||||
| Women with prior immunosuppression2 | 215 | (100) | 1,142 | (100) | |
| Prior vaccination (≥1 dose) | 16 | (7.4) | 81 | (7.1) | 1.02 (0.58, 1.80) |
| No prior vaccination | 199 | (92.6) | 1,061 | (92.9) | 1 (reference) |
| Women with no prior immunosuppression2 | 1,634 | (100) | 8,100 | (100) | |
| Prior vaccination (≥1 dose) | 138 | (8.5) | 812 | (10.0) | 0.80 (0.65, 0.98) |
| No prior vaccination | 1,496 | (91.5) | 7,288 | (90.0) | 1 (reference) |
Based on bivariate conditional logistic regression models
Ever prior HIV-infected, ever prior solid organ transplant, or immunosuppressive therapy in prior 18 months
Figure. Human papillomavirus catch-up vaccine effectiveness to prevent CIN2+ and CIN3+ by immunosuppression status.
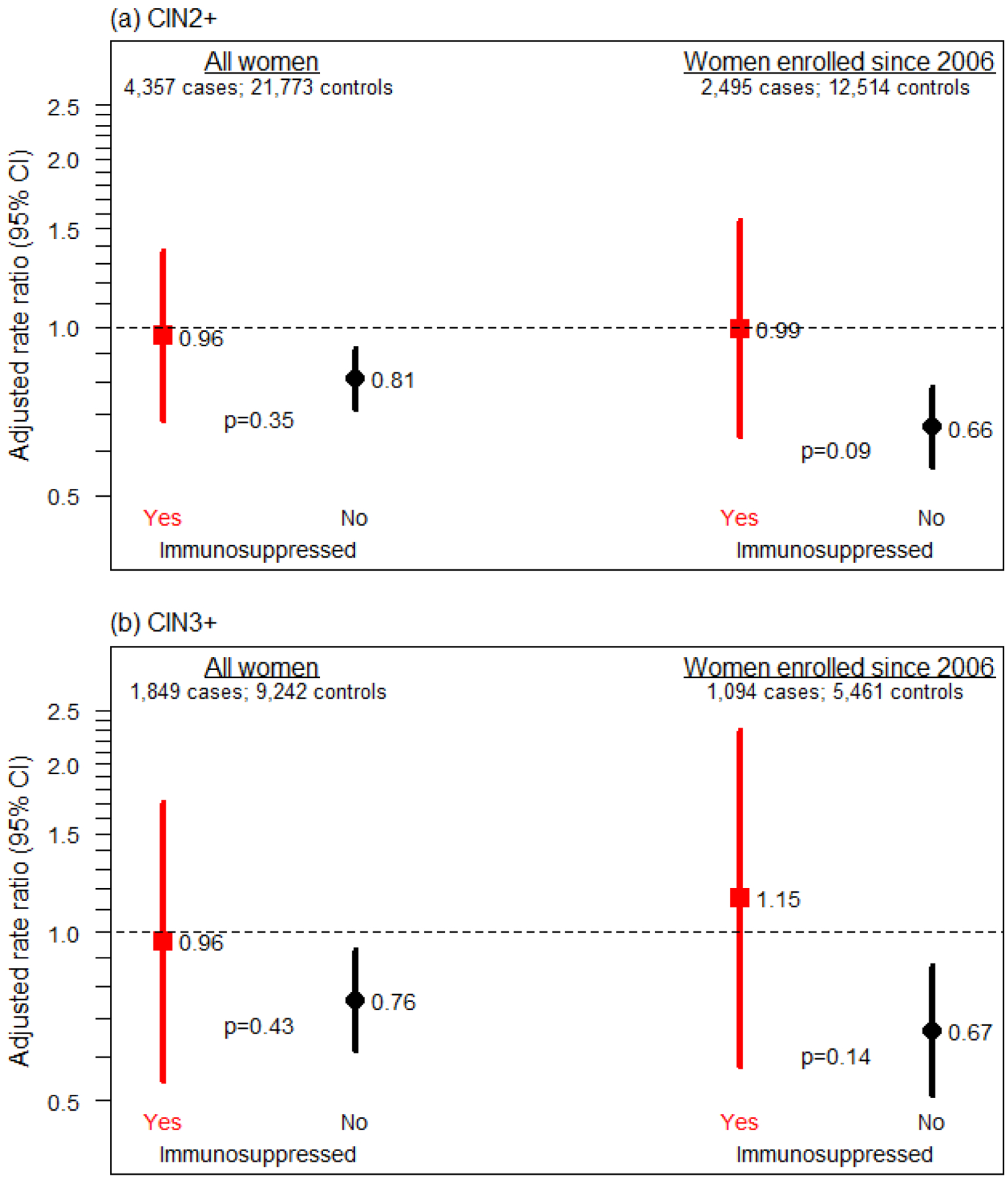
Adjusted rate ratios (RR) and 95% CI for (a) CIN2+ and (b) CIN3+ comparing women with and without (reference) human papillomavirus catch-up vaccination history (≥1 prior dose). Results are stratified by immunosuppression history. Results are shown for all women and for the subset of women with continuous enrollment in the healthplan since 2006. RRs from conditional logistic regression models with terms for vaccine history, immunosuppression history, vaccination*immunosuppression, smoking, hormone therapy or oral contraceptives, race and ethnicity, recent sexually transmitted infections, parity, and prior number of outpatient visits. The p-values presented in the figure represent tests of the HPV vaccination*immunosuppression interaction.
DISCUSSION
We found that HPV vaccination, when provided during catch-up ages to women in an integrated healthcare system was associated with a significant 19% reduction in CIN2+ rates for women without an immunosuppression history and a nonsignificant 4% reduction in CIN2+ for women with an immunosuppression history, although differences comparing vaccine effectiveness by immunosuppression status were not statistically significant. Results were consistent for CIN2+ and CIN3+ outcomes, and in sensitivity analyses.
The few studies that have evaluated clinical endpoints have been conducted in HIV populations.[7–9] The first such study[7] reported the results of a single arm RCT of 279 women (median age 39 years) with HIV who received the quadrivalent HPV vaccine. HPV infection and genital warts were low over two years and the authors noted their observed rates were higher than published rates for vaccinated women without HIV, but lower than unvaccinated HIV-infected women. Another RCT[9] enrolled 575 adults (>80% men) 27 years of age or older randomized to HPV vaccine or placebo. The study was stopped early due to statistical futility with no evidence of vaccine efficacy for preventing anal HPV or high-grade squamous intraepithelial lesions. Finally, a prospective cohort study of 310 perinatally HIV-infected and 148 uninfected adolescents who received the vaccine noted lower antibody titers in the HIV-infected group[8]. Our results are consistent with the reduced efficacy shown in trials evaluating catch-up vaccination in HIV-positive setting[7, 9] and add to the literature given our extended follow-up, evaluation of histological endpoints, and expanded definition of immunocompromised women.
There are several limitations of the study. First, despite adjustment for several known risk factors for CIN2+, we cannot exclude the possibility of residual confounding. For example, one explanation of findings is that immunosuppressed women were more likely to have prior HPV exposure. In anticipation of this, our study only evaluated incident CIN2+ (i.e., no CIN2+ in prior 18 months), but it is possible that subjects had asymptomatic HPV or CIN2+ more than 18 months in the past. A second limitation was that few immunosuppressed women had HIV infection or history of solid organ transplantation, and we did not evaluate duration of immunosuppression, or timing with respect to vaccination. Our prior work[11] indicated that the associations of immunosuppressive medications on CIN2+ risk may be stronger for specific drug classes, such as anti-proliferative agents and calcineurin inhibitors, but the sub-analyses here were not powered to evaluate effectiveness separately by drug class. Third, vaccine history may be incomplete in electronic health records. However, our sensitivity analyses restricting to women with continuous membership demonstrated even greater differences in effectiveness by immunosuppression status. The small numbers of vaccinated women also precluded a detailed analysis of age at first vaccination or number of vaccine doses. Descriptively, the percentage of women with 3+ doses and first vaccination <18 years of age was generally similar by case/control status, with some small differences. For example, among immunosuppressed women, cases had a slightly reduced proportion with 3+ doses, but higher proportion vaccinated before 18 years of age. We conclude that these small differences with opposite potential confounding effects were unlikely to completely explain the lack of vaccine effectiveness among immunosuppressed women. Future studies are also needed to evaluate effectiveness with the more recently approved nonavalent HPV vaccine.
In summary, we found reduced effectiveness of the catch-up quadrivalent HPV vaccine among women with immunosuppression, but differences in vaccine effectiveness by immunosuppression status were not statistically significant. While our results are suggestive of differential effectiveness by immunosuppression status, the number of immunocompromised women in the sample was relatively small and therefore these results should be viewed as provisional pending replication. Research is needed in other settings with a higher prevalence of HIV infection or solid organ transplant recipients and with greater vaccine coverage. In addition, it will be important to evaluate reasons for the potential reduced effectiveness among immunosuppressed women such as a higher prevalence of prior exposure to HPV infections. Nevertheless, findings may be particularly relevant given a recent change in the guidelines[1] recommending shared clinical decision-making regarding HPV vaccine initiation for those aged 27–45, an age group that may have a higher prevalence of both immunocompromising conditions and prior HPV exposure.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Financial support. This work was supported by a research grant (1 R01 CA169093) from the National Cancer Institute at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Meites E, Szilagyi PG, Chesson HW, et al. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019;68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Garland SM, Brotherton JML, Moscicki AB, et al. HPV vaccination of immunocompromised hosts. Papillomavirus Res 2017;4:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mok CC, Ho LY, To CH. Long-term immunogenicity of a quadrivalent human papillomavirus vaccine in systemic lupus erythematosus. Vaccine 2018;36:3301–3307. [DOI] [PubMed] [Google Scholar]
- [4].Jacobson DL, Bousvaros A, Ashworth L, et al. Immunogenicity and tolerability to human papillomavirus-like particle vaccine in girls and young women with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lacey CJ. HPV vaccination in HIV infection. Papillomavirus Res 2019;8:100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Toft L, Tolstrup M, Storgaard M, et al. Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives. Sex Health 2014;11:511–23. [DOI] [PubMed] [Google Scholar]
- [7].McClymont E, Lee M, Raboud J, et al. The Efficacy of the Quadrivalent Human Papillomavirus Vaccine in Girls and Women Living With Human Immunodeficiency Virus. Clin Infect Dis 2019;68:788–794. [DOI] [PubMed] [Google Scholar]
- [8].Moscicki AB, Karalius B, Tassiopoulos K, et al. Human Papillomavirus Antibody Levels and Quadrivalent Vaccine Clinical Effectiveness in Perinatally Human Immunodeficiency Virus-infected and Exposed, Uninfected Youth. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wilkin TJ, Chen H, Cespedes MS, et al. A Randomized, Placebo-Controlled Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Adults Aged 27 Years or Older: AIDS Clinical Trials Group Protocol A5298. Clin Infect Dis 2018;67:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Silverberg MJ, Leyden WA, Lam JO, et al. Effectiveness of catch-up human papillomavirus vaccination on incident cervical neoplasia in a US health-care setting: a population-based case-control study. Lancet Child Adolesc Health 2018;2:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Silverberg MJ, Leyden WA, Chi A, et al. Human Immunodeficiency Virus (HIV)- and Non-HIV-Associated Immunosuppression and Risk of Cervical Neoplasia. Obstet Gynecol 2018;131:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Langholz B. Case-control studies = odds ratios: blame the retrospective model. Epidemiology 2010;21:10–2. [DOI] [PubMed] [Google Scholar]


