Abstract
Objectives:
To calculate the minimum fetal red blood cell concentration required to cause maternal Rh sensitization; validate the use of a flow cytometry protocol below that concentration; preliminarily assess the concentrations of fetal red blood cells in pregnant women before and after uterine aspiration.
Study Design:
Using pre-existing literature, we calculated the lowest concentration of fetal red blood cells found to cause sensitization within adult female circulation. We validated a two-color flow cytometry protocol using fluorescently labeled antibodies to Hemoglobin F (expressed by fetal red blood cells and adult F cells) and Carbonic Anhydrase (expressed in red blood cells during the third trimester and postnatally) by titrating second trimester cord blood into non-pregnant adult blood. We applied this flow cytometry protocol in a prospective cohort study of 42 pregnant women at 5 to 12 weeks gestational age undergoing uterine aspiration for induced or spontaneous abortion.
Results:
The calculated threshold for causing Rh sensitization was 250 fetal red blood cells per 10 million total red blood cells. We showed a linear relationship between observed and expected fetal red blood cell fractions in titrated samples. Fetal red blood cell counts were more reliable when samples acquired by flow cytometry contained at least 1 million red blood cells. All 37 subjects with evaluable paired samples demonstrated fetal red blood cell concentrations below the calculated threshold for Rh sensitization both pre- and post-procedure. The fetal RBC concentrations increased from a mean of 4.5 (median 0, range 0–57) fetal RBCS pre- to a mean of 8.6 (median 2, range 0–32) fetal RBCs post- per 10 million total RBCs (p<0.001).
Conclusions:
Flow cytometry was capable of separately quantifying fetal red blood cells and maternal F cells to very dilute concentrations. Fetal red blood cell exposure in the first trimester was well below the calculated threshold for maternal Rh sensitization in our cohort. Larger studies are warranted to confirm our pilot study findings, fill this evidence gap and inform universal guidelines for administering Rh immunoglobulin after first trimester uterine aspiration.
Keywords: Abortion, Flow cytometry, Kleihauer-Betke, Maternal F cells, Rh immunoglobulin, vaginal bleeding
1. Introduction:
International recommendations for provision of Rh immunoglobulin after first trimester bleeding events, such as induced, spontaneous and threatened abortion, vary by both gestational age and indication. Consensus is lacking because there is insufficient evidence to support or refute a need for immune-prophylaxis for Rhesus (Rh)-negative women in the first trimester.[1–4] The American College of Obstetricians and Gynecologists (ACOG) states that giving a 50mcg dose in early pregnancy loss “should be considered,” and recognizes the limited evidence we have to guide this clinical decision.[5] Indeed, we do not yet know the earliest gestational age at which Rhesus-negative pregnant women can become sensitized to the Rho(D) antigen. Approximately 15% of women in the United States are Rh negative and as many as 35% of pregnancies end in abortion or miscarriage annually, with an additional 15% of ongoing pregnancies being complicated by some vaginal bleeding in the first half of pregnancy.[6–8] Screening of patients and prophylaxis with Rh immunoglobulin impose large systemic burdens on clinics and emergency departments. Patients paying out of pocket for abortion care bear an added financial burden, whether directly or amortized, for testing and receipt of a treatment with unclear benefit.
Rh-immunoglobulin works by decreasing the maternal exposure to Rh-antigens present on fetal red blood cells that enter the maternal circulation during bleeding events. Both the minimum Rh-antigenic exposure required for sensitization and immunization, and the natural history of Rh-antigen expression on fetal red blood cells (RBCs) throughout fetal development remain as gaps in knowledge in part because technologies have been insufficiently sensitive to quantify fetal RBC exposure at very low concentrations. Early studies of fetomaternal hemorrhage in the first trimester relied on the Kleihauer-Betke Technique (K-B), with an inadequate lower limit of detection of 4,000 fetal RBCs per 10 million adult RBCs.[9] Additionally and critically, the K-B assay cannot distinguish between fetal RBCs and maternal F cells. Since maternal F cells are adult RBCs that express fetal hemoglobin, this limitation can lead to a false-positive K-B test result. Maternal F cell concentrations increase in patients with sickle cell disease, hemoglobinopathies and hereditary persistence of fetal hemoglobin, and during periods of physiologic stress, including early pregnancy. Maternal F cells do not cause Rh-sensitization because they are of maternal origin.[10,11]
In order to fill these persisting gaps in scientific knowledge, we calculated the threshold for Rh sensitization, tested a flow cytometry protocol below that threshold, and then piloted it in a cohort of women before and after first trimester uterine aspiration for spontaneous or induced abortion. Given that quantification of fetal RBCs through direct measurement in maternal blood is very challenging, we turned to earlier studies conducted on subjects injected with fetal RBCs to estimate a plausible threshold concentration of fetomaternal hemorrhage sufficient to cause maternal sensitization.[12] Extrapolating from these data, we estimated the level of sensitivity that an assay would be required to attain to detect fetomaternal hemorrhage during the first trimester. We then quantified the level of fetal RBCs in women undergoing uterine aspiration using a flow cytometry based assay, which is more sensitive and specific than the K-B assay. We relied on dual staining with hemoglobin F (HgbF) and carbonic anhydrase to distinguish fetal from adult RBCs [13, 14, 15], and further distinguished maternal F cells from fetal RBCs by the brightness of staining for HgbF. We further modified the RBC processing and staining conditions, cell input number and data analysis of the assay to improve its performance in the detection of rare events [16]. We then piloted this modified flow cytometry protocol in a cohort of women before and after first trimester uterine aspiration for spontaneous or induced bortion. We hypothesized that the concentration of fetal RBCs would not be clinically significantly increased following uterine aspiration in the first trimester.
2. Materials and Methods:
2.1. Threshold calculation.
We estimated the threshold for Rh sensitization based upon our analysis of the existing literature.[12,17,18] A study by Zipursky directly injected 15 nulliparous Rh-negative women with repeated doses of 0.1ml fetal RBCs.[12] Although 11 (73.3%) of these women did not develop antibodies, two of the fifteen women (13.3%) showed an antibody response after receiving two doses of 0.1ml given six weeks apart. Two more women developed antibodies after four and five total doses, respectively. Based on these data, we used 0.1ml as the minimum volume of fetomaternal hemorrhage capable of causing sensitization.[19,20] The average reported female blood volume in early pregnancy is 4,000ml.[21,22] Hence our target fetal RBC concentration threshold is 0.1/4,000 = 250 fetal RBCs per 10 million total RBCs.
2.2. Flow cytometry.
The detection of rare events is improved through the use of multiple antigens in flow cytometry. In order to distinguish maternal F cells from fetal RBCs and adult RBCs, we chose to use staining for both HgbF and carbonic anhydrase, using IQ Products’ Fetal Cell Count kit (IQ Products, Netherlands, Cat. No. IQP-363).[23,24] Red blood cells from a nulliparous adult female (control subject who had an elevated maternal F cell fraction) and from second trimester cord blood were collected in K2EDTA tubes and counted after washing twice with washing buffer provided in the Fetal Cell Count Kit (IQProducts, Cat# IQP-363). We modified the manufacturer’s protocol to adequately fix and permeabilize large numbers of cells needed to detect rare events of fetal RBCs. For the RBC experiments, 4.6×107 cells (control RBC or control RBC plus cord blood) were fixed for 45 minutes and permeabilized for 3 minutes and then stained with anti-human carbonic anhydrase conjugated to FITC and anti-human fetal hemoglobin conjugated to PE. In experiments with fetaltrol, commercially prepared fetal RBC + adult RBC mixture, R&D Systems Cat#FH101, FTL3, 1.8 × 107 fetaltrol cells were stained in a similar manner.
Next, we tested the modified manufacturer’s protocol for linearity and stability of fetal RBC and maternal F cell detection. For assay linearity, we serially titrated cord blood into adult nulliparous female blood in a 1:10 ratio to a concentration of 1:100,000. To test sample stability post-staining, the ratio between cord blood and control blood was adjusted to 1:10,000. The resulting cell mixtures were stained and then stored for 24, 48, and 72 hours prior to running flow cytometry. Negative controls included running control RBCs (nulliparous adult female) without cord blood at all three time points. We also ran flow cytometry on commercially prepared fetaltrol as a positive control. Next, we tested the stability of fetal RBCs in samples that were incubated for different lengths of time prior to mixing, fixation and staining. Cord blood and adult blood were stored at 4° C for 1, 3, 5, 7 and 13 days prior to staining. For the pre- and post-staining stability experiments, RBCs were kept at 4° C in the dark until flow cytometry was performed. Up to 5 million events per sample were acquired on an LSRII flow cytometer (BD Biosciences, San Diego, CA) and analyzed using FlowJo (Treestar Inc, v. 10.0.8). or analysis, singlets were gated based upon forward vs. side scatter area (using a stringent gate), followed by visualization of HgbF vs. carbonic anhydrase staining on bivariate plots.
2.3. In vivo cohort.
We performed an in vivo pilot study to assess the use of flow cytometry in a clinical cohort, approved by the University of Pennsylvania Institutional Review Board. We invited women undergoing uterine aspiration for induced or spontaneous abortion before 12 completed weeks gestation to participate. We excluded women with hemoglobinopathies or vaginal bleeding prior to enrollment. We collected demographic and clinical data on all participants. We performed phlebotomy before and after uterine aspiration. Rh status was determined for all participants and Rh-negative patients were treated with Rh immunoglobulin according to clinical protocol, following post-procedural phlebotomy. Samples were stained and run on the flow cytometer in batches within 72 hours of collection. We chose a sample size of convenience for this exploratory study, reflecting the lack of reliable published estimates of antigen exposure in early pregnancy and the levels needed to induce Rh-sensitization. A comparison of fetal RBC and maternal F cell fractions before versus after uterine aspiration was modeled using a repeated measures Poisson regression, including the total number of cells as an offset, and assuming a random intercept to account for clustering within each woman. All modeling was done in Stata v15 (STATA Corp., College Station TX). Data in figures were 180 visualized using Prism v.8 software (GraphPad, San Diego, CA).
3. Results:
3.1: Threshold calculation.
Based on existing literature, we calculated the lowest concentration of fetal RBCs found to cause sensitization within adult female circulation as 0.1ml/4000ml, which is equivalent to 250 fetal RBCs per 10 million total RBCs. Given this threshold, a flow cytometry assay capable of detecting concentrations below this level was required.
3.2. Flow cytometry
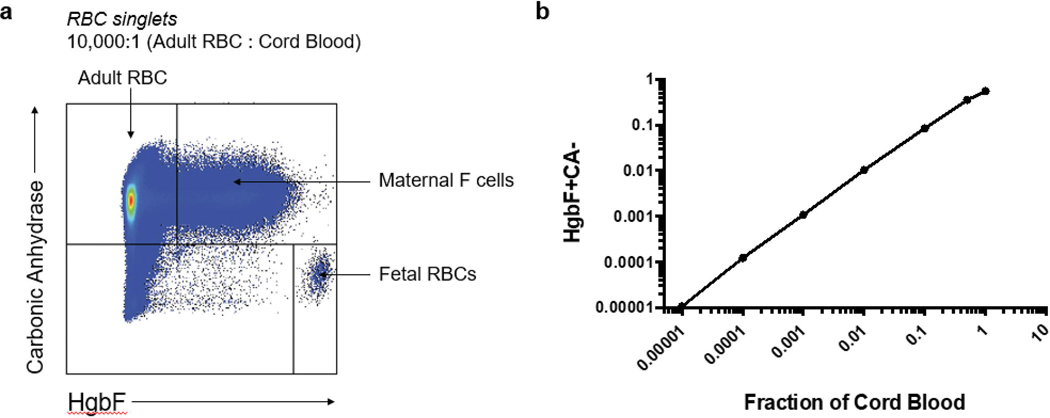
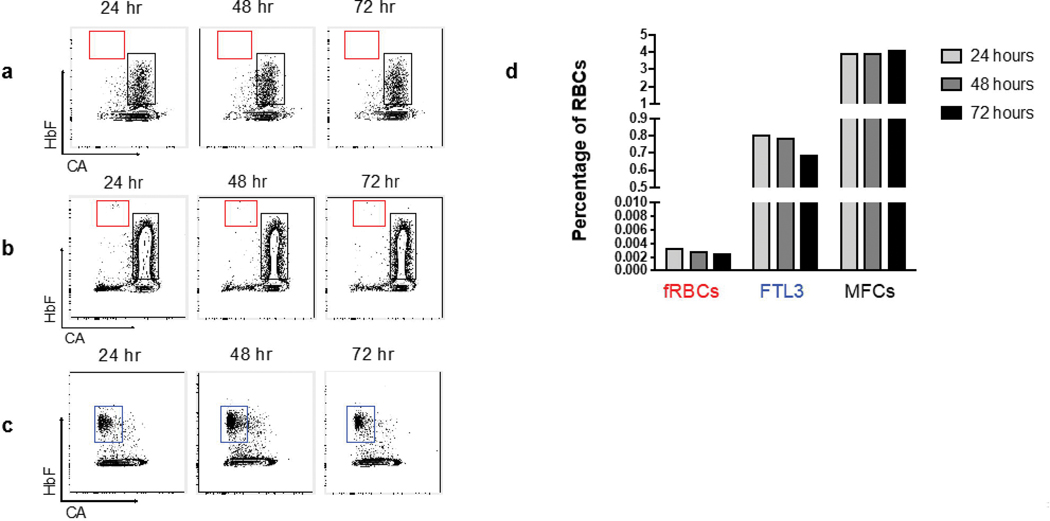
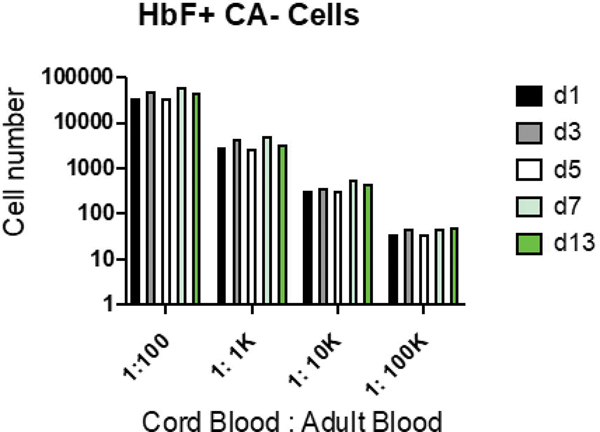
Flow cytometry gating revealed adult and fetal RBCs in the expected ratio of 10,000:1 (Fig. 1a). Maternal F cells outnumbered fetal RBCs at this ratio. In our linear titration experiment, the observed fraction of HgbF+CA- cells (fetal RBCs) correlated closely with the expected fraction of cord blood cells (Fig. 1b). Our negative control, nulliparous adult female blood without cord blood, showed an absence of fetal RBCs (Fig 2a), while the same sample containing second trimester cord blood (at a dilution of 1:10,000 fetal cells to adult cells) contained detectable fetal RBCs at all time points (Fig 2b). Commercially prepared fetaltrol exhibited the expected higher concentration of HgbF+CA- cells across time points (Fig 2c). Overall, the fetal RBCs, maternal F cells, and fetaltrol controls were fairly stable following staining, with a slight reduction in the fetal RBC levels at 72 hours (Fig. 2d). Samples stored for up to 13 days prior to staining also remained stable, even at a concentration as low as 1:100,000 (Fig. 3). We also carried out a series of bivariate analyses to study the effects of various pre-analytical variables on the results and observed that fetal RBC counts were more reliable when samples containing at least 1 million total RBCs were acquired by flow cytometry (data not shown).
Fig. 1: Titration of cord blood cells into nulliparous adult RBCs.
(a) Gates for detection of adult RBCs, fetal RBCs (fRBCs) and maternal F cells (MFCs). The ratio of adult blood to cord blood was 10,000:1. (b) Titration of cord blood (fetal RBCs) into adult RBCs. The measured fraction of fetal RBCs, which stain for fetal hemoglobin (HgbF+) and do not stain for carbonic anhydrase (CA-) is plotted versus the fraction of cord blood in the sample. The relationship between the observed and expected fetal RBC frequency was linear at 0.1–0.00001 (r2 = 0.995). Similar results were obtained in a second experiment (data not shown).
Fig. 2: Fetal RBC and maternal F cell stability at 24, 48 and 72 hours after staining.
(a) Adult nulliparous female RBCs showing the maternal F cell fraction (MFC, black gate) and no fetal RBCs (fRBCs, red gate). (b) Blood from the same adult subject plus cord blood (diluted at 1:10,000). (c) Fetaltrol (FTL3, blue gate) sample analyzed at 24, 48 and 72 hours after staining showed stability of fetal RBCs. (d) Quantitation of fetal RBC and maternal F cell from panel b and fetaltrol from panel c.
Fig. 3: Fetal RBC stability before staining.
Cord blood cells were stored at 4oC for 1, 3, 5, 7 and 13 days and then stained for hemoglobin F (HbF) and carbonic anhydrase (CA) as described in Methods. Data were analyzed for the number of HbF+, CA- cells. d=days.
3.2: In vivo cohort.
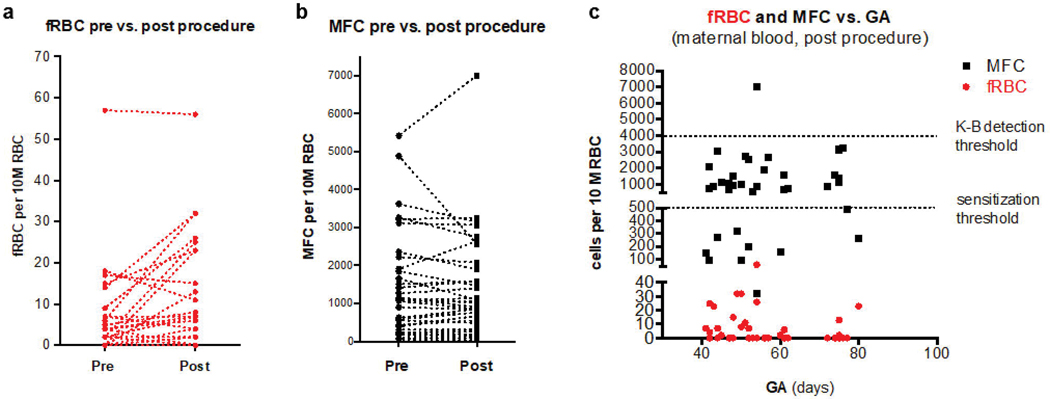
We then evaluated our flow cytometry methods with in vivo samples from study subjects obtained before and after uterine aspiration. Eighty-eight percent (37/42) of recruited participants had pre- and post- samples containing at least 1 million acquired cells by flow cytometry and were included in the analysis (Fig. 4). The characteristics of these 37 participants are in Table 1. All fetal RBC samples were well below our calculated threshold for Rh sensitization of 250 fetal RBCs per 10 million adult RBCs (Fig. 4). The fetal RBC concentrations statistically significantly increased from a mean 4.5 (median 0, range 0–57) pre- to a mean 8.6 (median 2, range 0–32) post- per 10 million total RBCs (Fig. 4a, p<0.001). There were no statistically significant differences in fetal RBC concentration by gestational age, use of sharp curettage, past pregnancy history or participant demographic characteristics (all p>.05), though we were underpowered to detect meaningful differences. Maternal F cell concentrations were much higher than fetal RBC concentrations, increasing from a mean 276.5 per 10 million total RBCs (median 135, range 4–2,411) pre-procedure to a mean 315.8 per 10 million total RBCs (median 127, range 6–5,079) post-procedure (Fig. 4b, p<0.001). Importantly, 2.7% (1/37) of samples showed maternal F cells above 4,000 cells per 10 million RBCs, the threshold for detection by K-B (Fig. 4c).
Figure 4. In vivo concentrations of fetal red blood cells and maternal F cells.
(a) fetal RBC number and (b) maternal F cell number per 10 million RBCs pre- vs. post-procedure in subjects undergoing first trimester uterine aspiration (n=37). Each line represents an individual subject. (c) Number of fetal RBCs (red) and maternal F cells (black) per 10 million in post-procedure samples (one mark of each color per subject). The lower dotted line is the calculated threshold for sensitization (250 fetal RBCs per 10 million total RBCs). The upper dotted line is the threshold for detection by the Kleihauer-Betke assay (4,000 fetal RBCs and/or maternal F cells per 10 million total RBCs). fRBC = fetal red blood cell. GA = gestational age. K-B = Kleihauer-Betke. MFC = maternal F cell.
Table 1.
Patient and procedure characteristics.
| Characteristic n=37 | |
|---|---|
| Participant demographics | |
| Age (years) | |
| Median (range) | 29 (19–43) |
| Race, n (%) | |
| Black | 25 (67.6) |
| White | 7 (18.9) |
| Asian | 1 (2.7) |
| Other | 4 (10.8) |
| Gravidity, n (%) | |
| 1 | 7 (18.9) |
| 2 | 7 (18.9) |
| 3+ | 23 (62.2) |
| Parity, n (%) | |
| 0 | 5 (13.5) |
| 1 | 9 (24.3) |
| 2+ | 16 (43.2) |
| Missing | 7 (18.9) |
| Gestational Age | |
| Median (range) | 7w3d (5w6d – 11w3d) |
| Mean | 7w6d |
| Indication, n (%) | |
| Spontaneous abortion | 5 (13.5) |
| Induced abortion | 32 (86.5) |
| ABO blood type, | |
| A | 6 (16.2) |
| B | 8 (21.6) |
| AB | 4 (10.8) |
| O | 19 (51.4) |
| Rh status, n (%) | |
| Positive | 34 (91.9) |
| Negative | 3 (8.1) |
| Procedure characteristics | |
| Any sharp curettage, | |
| Yes | 2 (5.4) |
| No | 35 (94.6) |
| Time to post-procedure | |
| phlebotomy (minutes) | |
| Mean (range) | 36 (8–76) |
SAB = spontaneous abortion. TAB = therapeutic abortion. Rh = Rhesus.
4. Discussion:
In March, 2019, the National Abortion Federation (NAF) released new guidance stating that its members may consider foregoing Rh testing and immunoglobulin administration for patients undergoing induced abortion by uterine aspiration up to 8w0d and by medication abortion up to 10w0d.[25] The American College of Obstetricians and Gynecologists (ACOG) recommends giving a 50mcg dose in early pregnancy loss, but reworded the recommendation from “should receive” to “should be considered” in the 2018 interim update, in recognition of the limited evidence we have to guide this clinical decision.[5] Other healthcare organizations continue to recommend Rh immune-globulin administration to Rh-negative women with bleeding in the first trimester, while acknowledging insufficient evidence to guide management.[1,20] This standard of care imposes large systemic burdens on clinics, emergency departments, and patients for an intervention with unclear benefit. The paucity of evidence to inform such recommendations is underscored by the variation in international obstetrical society guidelines for provision of Rh immunoglobulin at <12 weeks gestation for miscarriage, threatened abortion and molar pregnancies.[1] Epidemiologic data suggest that there are clinical situations in early pregnancy where Rh immunoglobulin is unnecessary. Wiebe and colleagues compared databases in Canada, where Rh immunoglobulin is routinely given for all vaginal bleeding events prior to 12 weeks gestation, and The Netherlands, where Rh immunoglobulin is not given before 10 weeks in early pregnancy loss nor before 7 weeks in induced abortion. [26] They found a statistically significantly higher rate of antibody detection in Canada 4.21 per 1,000 (95% CI: 4.12 to 4.30) versus The Netherlands 4.03 per 1,000 (95% CI: 3.93 to 4.12). Such indirect evidence underscores the importance of developing clinical metrics for evaluating the utility of Rh immunoglobulin in early pregnancy.
We tested a flow cytometry assay that is sensitive enough to quantify fetal RBC frequencies below 100 in 10 million total RBCs. Because there can be delays in getting samples processed by the lab (for example, if a sample arrives after hours or on a holiday or weekend), understanding the stability of the assay for clinical use is important. The fetal cells showed no decrease in concentration in samples stored prior to staining, even at 13 days, suggesting that fetal cells are stable in whole blood. Further laboratory studies that control specifically for ABO compatibility and type would strengthen these findings. Fetal RBCs are more resistant to degradation than adult RBCs, so overestimation of fetal RBCs is more likely than underestimation. However, multiple factors affect the clearance of fetal RBCs from maternal circulation, including the ABO blood type. The optimal timing of sample collection following fetometernal hemorrhage to capture maximum exposure to fetal RBCs is not known, but is likely within three hours.[27]
Dual-color flow cytometry has an additional advantage over the K-B and single-color immunophenotyping methods of being more specific, as it can distinguish fetal RBCs from maternal F cells based upon carbonic anhydrase staining.[12] Maternal F cells cannot cause maternal sensitization, so their concentrations are clinically irrelevant, but their inadvertent detection and misclassification can lead to falsely elevated results with the K-B method or a flow cytometry assay that lacks anti-carbonic anhydrase antibodies. A proportion of our samples revealed maternal F cells above the K-B detection threshold of 4000 per 10 million RBCs, and we surmise that earlier studies using K-B may have detected maternal F cells rather than fetal RBCs. This is plausible, given that distinguishing fetal RBCs from adult RBCs that express fetal hemoglobin has high interobserver variability and low reproducibility.[28] Additionally, K-B has historically been used to assess the F cell concentration in non-pregnant patients with sickle cell disease, hemoglobinopathies, hereditary persistence of fetal hemoglobin and their responses to medications, physiologic stress and pregnancy.[29]
We found a statistically significant increase in the concentration of fetal RBCs present in the maternal blood after uterine aspiration, suggesting that the procedure does in fact result in the transfer of low numbers of fetal RBCs into the maternal circulation and that our flow cytometry assay is sufficiently sensitive to quantify this change. In spite of the increase in fetal RBCs, this flow assay also shows that the concentration of fetal RBCs post-procedure was clinically insignificant as it remained below the reported threshold for sensitization (250 fetal RBCs per 10 million total RBCs) in all study participants.[11,19,28] In fact, even if we assumed that the level of fetal RBCs in the population were 195 per 10 million cells, the probability of seeing a woman with 250 or more fetal RBCs per 10 million cells is less than 0.0001 (0.01%) (see Methods for calculations). Nearly half of our participants exhibited fetal RBCs pre-procedure (18/37), consistent with Hollenbach et al., who found that 60% of their participants at gestational ages 6–22 weeks exhibited fetal RBCs pre-procedure.[30] We excluded women with any vaginal bleeding, while 26% of the Hollenbach cohort reported some prior bleeding; however, there was no correlation in their study between bleeding and presence of fetal RBCs. Additionally, we used a flow cytometry protocol capable of not just detecting fetal RBCs and 288 distinguishing them from maternal F cells, but also capable of separately quantifying maternal F cells.
There are several limitations to our study. The threshold calculation was made using small studies that would be logistically and ethically challenging to reproduce today. Human immune systems are complex and heterogenous, and individual responses to antigenic exposure may vary in subtle ways including by pregnancy status, ABO compatibility/incompatibility, genetic predisposition and timing and level of exposure. There are also limits to our understanding of fetal RBC development throughout gestation and the natural history of maternal F cell production in pregnancy. Additional antigens that distinguish fetal RBCs from adult RBCs would be helpful in the removal of various sources of noise and false positives that can obscure rare event detection by flow cytometry, and is an important future research direction. Additionally, our cohort study is limited by its small sample size. Larger studies powered to detect differences by gestational age, procedural and pregnancy characteristics, and patient demographics are warranted.
This study also has several strengths. The threshold calculation was performed using the most conservative published inputs. The flow cytometry protocol was tested using second trimester cord blood, which contains fetal RBCs that do not yet express carbonic anhydrase. The protocol is capable of separately quantifying fetal RBCs and maternal F cells to very dilute concentrations.
Our study suggests that fetal RBC exposure in the first trimester is insufficient for maternal Rh-sensitization. Larger studies of pregnant women are warranted to identify both the earliest gestational age and the clinical circumstances under which fetal RBC exposure warrants Rh immunoglobulin treatment. This evidence can then be used to inform universal guidelines for Rh immunoglobulin in the first trimester, reduce cost and improve care.
Supplementary Material
Implications.
Fetal red blood cell exposure following first trimester uterine aspiration is well below the calculated threshold for maternal Rh sensitization in our cohort.
Acknowledgements:
This research was conducted with a grant from the Society of Family Planning Research Fund [SFPRF17-6]. The funder had no role in conducting the research, preparing or submitting the report. The study was also supported by the National Institutes of Health [R01-HD0719-20] and the Society of Family Planning Research Fund Midcareer Mentor Award (Courtney A. Schreiber).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sperling JD, Dahlke JD, Sutton D, Gonzalez JM, Chauhan SP. Prevention of RhD alloimmunization: a comparison of four national guidelines. American journal of perinatology. 2018. January;35(02):110–9. [DOI] [PubMed] [Google Scholar]
- [2].Chávez GF, Mulinare J, Edmonds LD. Epidemiology of Rh hemolytic disease of the newborn in the United States. Jama. 1991. June 26;265(24):3270–4. [DOI] [PubMed] [Google Scholar]
- [3].Karanth L, Jaafar SH, Kanagasabai S, Nair NS, Barua A. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews. 2013. March 28;(3):CD009617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jabara S, Barnhart KT. Is Rh immune globulin needed in early first-trimester abortion? A 365 review. American journal of obstetrics and gynecology. 2003. March 1;188(3):623–7. [DOI] [PubMed] [Google Scholar]
- [5].American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132(5):e197–207. [DOI] [PubMed] [Google Scholar]
- [6].Garratty G, Glynn SA, McEntire R. Retrovirus Epidemiology Donor Study. ABO and Rh (D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004. May;44(5):703–6. [DOI] [PubMed] [Google Scholar]
- [7].Jatlaoui TC. Abortion Surveillance—United States, 2016. MMWR. Surveillance Summaries. 2019;68. [DOI] [PubMed] [Google Scholar]
- [8].Hasan R, Baird DD, Herring AH, Olshan AF, Funk ML, Hartmann KE. Association between first-trimester vaginal bleeding and miscarriage. Obstetrics and gynecology. 2009. October;114(4):860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pelikan DM, Mesker WE, Scherjon SA, Kanhai HH, Tanke HJ. Improvement of the Kleihauer-Betke test by automated detection of fetal erythrocytes in maternal blood. Cytometry Part B: Clinical Cytometry: The Journal of the International Society for Analytical Cytology. 2003. July;54(1):1–9. [DOI] [PubMed] [Google Scholar]
- [10].Dover GJ, Boyer SH, Charache S, Heintzelman K. Individual variation in the production and survival of F cells in sickle-cell disease. New England Journal of Medicine. 1978. December 28;299(26):1428–35. [DOI] [PubMed] [Google Scholar]
- [11].Pembrey ME, Weatherall DJ, Clegg JB. Maternal synthesis of haemoglobin F in pregnancy. The Lancet. 1973. June 16;301(7816):1350–5. [DOI] [PubMed] [Google Scholar]
- [12].International journal of laboratory hematology. 2013. June;35(3):344–50.Zipursky A, Pollock J. Transplacental isoimmunization by fetal red blood cells. National Foundation-March of Dimes; 1965. [Google Scholar]
- [13].Gielezynska A, Stachurska A, Fabijanska-Mitek J, Debska M, Muzyka K, Kraszewska E. Quantitative fetomaternal hemorrhage assessment with the use of five laboratory tests. International journal of laboratory hematology. 2016. August;38(4):419–25. [DOI] [PubMed] [Google Scholar]
- [14].Umazume T, Yamada T, Morikawa M, Ishikawa S, Kojima T, Cho K, et al. Occult fetomaternal hemorrhage in women with pathological placenta with respect to permeability. Journal of Obstetrics and Gynaecology Research. 2016. June;42(6):632–9. [DOI] [PubMed] [Google Scholar]
- [15].Akbar SA, Brown PR. Measurement of human erythrocyte CAI and CAII in adult, newborn, and fetal blood. Clinical biochemistry. 1996. April 1;29(2):157–64. [DOI] [PubMed] [Google Scholar]
- [16].Hedley BD, Keeney M. Technical issues: flow cytometry and rare event analysis. International journal of laboratory hematology. 2013. June;35(3):344–50. [DOI] [PubMed] [Google Scholar]
- [17].Jakobowicz R, Williams L, Silberman F. Immunization of Rh-negative volunteers by repeated injections of very small amounts of Rh-positive blood. Vox sanguinis. 1972. October;23(4):376–81. [DOI] [PubMed] [Google Scholar]
- [18].Krevans JR, Woodrow J, Nosenzo C, Finn R. Patterns of Rh Immunization. International 402 Society of Blood Transfusion 1965. (Vol. 23, pp. 781–781). Karger Publishers. [Google Scholar]
- [19].Holmgren C, Porter TF. Management of Alloimmunization During Pregnancy. Obstetrics and Gynecology. 2018. March 1;131(3):E82–90. [DOI] [PubMed] [Google Scholar]
- [20].American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 181: Prevention of Rh D alloimmunization. Clinical management guidelines for obstetrician gynecologists. Obstet Gynecol. 2017;130(2):e57–70. [DOI] [PubMed] [Google Scholar]
- [21].Zipursky A, Pollack J, Neelands P, Chown B, Israels LG. The Transplacental Passage Of Foetal Red Blood Cells And The Pathogenesis Of Rh Immunisation During Pregnancy. Obstetrical & Gynecological Survey. 1964. April 1;19(2):233–5. [DOI] [PubMed] [Google Scholar]
- [22].Hytten F Blood volume changes in normal pregnancy. Clinics in haematology, 1985. 14(3): p.601–612. [PubMed] [Google Scholar]
- [23].Porra V, Bernaud J, Gueret P, Bricca P, Rigal D, Follea G, et al. Identification and quantification of fetal red blood cells in maternal blood by a dual-color flow cytometric method: evaluation of the Fetal Cell Count kit. Transfusion. 2007. July;47(7):1281–9. [DOI] [PubMed] [Google Scholar]
- [24].IQ Products Fetal Cell Count Kit. https://www.iqproducts.nl/wp-content/uploads/PI-IQP-363-INT-1.pdf Accessed December 1, 2018.
- [25].Mark A, Foster AM, Grossman D, Prager SW, Reeves M, Velásquez CV, et al. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019. May 1;99(5):265–6. [DOI] [PubMed] [Google Scholar]
- [26].Wiebe ER, Campbell M, Aiken AR, Albert A. Can we safely stop testing for Rh status and immunizing Rh-negative women having early abortions? Acomparison of Rh alloimmunization in Canada and the Netherlands. Contraception: X. 2019. January 1;1:100001. [Google Scholar]
- [27].Klein HG, Anstee DJ. Mollison’s blood transfusion in clinical medicine. John Wiley & Sons; 2014. February 3. [Google Scholar]
- [28].Nance SJ, Nelson JM, Arndt PA, Lam HT, Garratty G. Quantitation of fetal–maternal hemorrhage by flow cytometry: a simple and accurate method. American journal of clinical pathology. 1989. March 1;91(3):288–92. [DOI] [PubMed] [Google Scholar]
- [29].Kohne E. 50 Years Kleihauer-Betke Test. Klinische Pädiatrie. 2007. September;219(05):252–3. [DOI] [PubMed] [Google Scholar]
- [30].Hollenbach SJ, Cochran M, Harrington A. “Provoked” feto-maternal hemorrhage may represent insensible cell exchange in pregnancies from 6 to 22 weeks gestational age. Contraception. 2019. August 1;100(2):142–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.