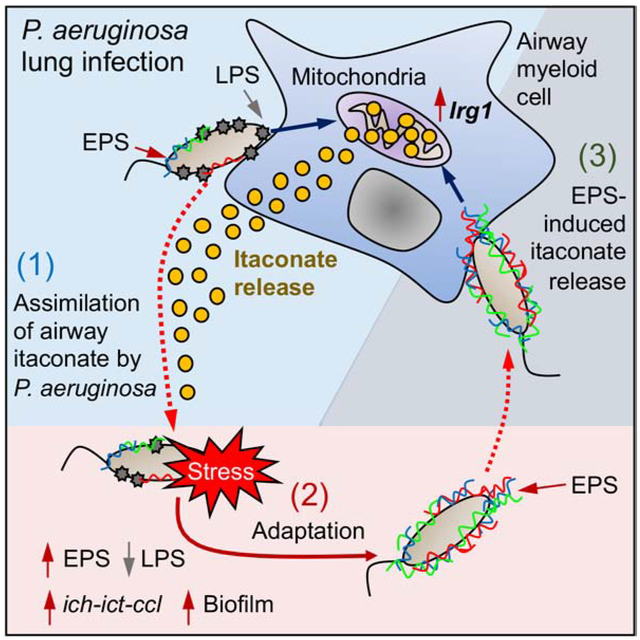
Abstract
The bacterium Pseudomonas aeruginosa is especially pathogenic, often being associated with intractable pneumonia and high mortality. How P. aeruginosa avoids immune clearance and persists in the inflamed human airway remains poorly understood. In this study, we show that P. aeruginosa can exploit the host immune response to maintain infection. Notably, unlike other opportunistic bacteria, we found that P. aeruginosa alters its metabolic and immunostimulatory properties in response to itaconate, an abundant host-derived immunometabolite in the infected lung. Itaconate induces bacterial membrane stress, resulting in downregulation of lipopolysaccharides (LPS) and upregulation of extracellular polysaccharides (EPS). These itaconate-adapted P. aeruginosa accumulate lptD mutations, which favor itaconate assimilation and biofilm formation. EPS, in turn, induces itaconate production by myeloid cells, both in the airway and systemically, skewing the host immune response to one permissive of chronic infection. Thus, the metabolic versatility of P. aeruginosa needs to be taken into account when designing therapies.
Graphical Abstract
eTOC blurb
The ability of Pseudomonas aeruginosa to chronically infect the lungs of immunocompetent individuals suggests these organisms might utilize aspects of the host defense system to their advantage. Riquelme et al demonstrate how P. aeruginosa exploits itaconate, a major mitochondrial metabolite produced by immune cells to thwart effective immune clearance, to promote biofilm formation.
Introduction
Metabolic activation of myeloid cells is a key component of innate immune clearance of airway infection. Upon encountering lipopolysaccharide (LPS), the major immunostimulatory component of Gram-negative pathogens, macrophages and monocytes increase aerobic glycolysis, generate and oxidize succinate resulting in the synthesis of the potent anti-bacterial cytokine IL-1β (Mills et al., 2016; Mills et al., 2017; Tannahill et al., 2013). The inflammation and release of oxidants damages both bacteria and local tissue. In response to oxidant stress, myeloid cells upregulate immune-response gene 1 (Irg1) expression (Mills et al., 2018), which synthetizes the carboxylate metabolite itaconate that suppresses both glycolysis (Qin et al., 2019) and succinate catabolism (Lampropoulou et al., 2016). Itaconate serves to recover tissue homeostasis by deactivating effector immune cells. Itaconate is one of the most abundant metabolites produced by myeloid cells in response to infection and a major activator of the host anti-oxidant response (Lampropoulou et al., 2016; Mills et al., 2018). However, accumulation of the immunometabolites itaconate and succinate may have additional consequences, by providing substrates and imposing selective pressure on infecting organisms with sufficient genomic and metabolic plasticity to adapt (Riquelme et al., 2019; Sasikaran et al., 2014).
Bacterial pneumonia remains a global problem, actually increasing in prevalence in some populations as a complication of increased longevity and expanded intensive-care unit (ICU) and health-care associated infections (Wunderink and Waterer, 2017). While the emergence of antibiotic resistance complicates treatment, not all antibiotic-resistant pathogens have emerged as global threats to health. Pseudomonas aeruginosa is a Gram-negative pathogen associated with high morbidity and mortality even in hosts with normal immune function, such as in individuals with health-care associated pneumonia (Fernandez-Barat et al., 2017; Wunderink and Waterer, 2017), chronic-obstructive pulmonary disease (COPD) (Garcia-Nunez et al., 2017) or cystic fibrosis (CF) (Deretic et al., 1995; Elborn, 2016; Winstanley et al., 2016). The bacterium has achieved a CDC and WHO designation as one of the most feared pathogens worldwide (Kubes and Fridkin, 2019; World-Health-Organization, 2017). Despite inducing a robust LPS-mediated acute inflammatory response, P. aeruginosa persists within the airways by forming intractable biofilms. Among many factors, the tremendous metabolic flexibility harbored in the large genome of P. aeruginosa enables this pathogen to readily adapt to conditions in the airway and especially to the immune metabolic program that is activated in response to infection.
The extraordinary success of antibiotic-resistant pathogens such as P. aeruginosa depends on their capacity to actively adapt to and efficiently consume available local nutrients, often in the presence of extensive myeloid cell recruitment. Activated monocytes and macrophages release substantial amounts of both succinate, a known P. aeruginosa substrate (Collier et al., 1996; Gorke and Stulke, 2008; Riquelme et al., 2019; Rojo, 2010), as well as itaconate, which it and other highly pathogenic airway bacteria, such as M. tuberculosis, can potentially metabolize in vivo (Wang et al., 2019). In this study, based upon human clinical isolates of P. aeruginosa, and in contrast to the standard laboratory strains, we demonstrate that itaconate selects metabolically-adapted P. aeruginosa strains with increased resistance to oxidant stress generated by altered bacterial metabolic activity and substantially increased synthesis of extracellular polysaccharides (EPS), as well as providing a mechanism outlining this process. Namely, we found that mature LPS, expected to be the major surface immunostimulatory component of P. aeruginosa, is replaced by EPS in the presence of itaconate. EPS not only protect the bacteria from oxidants, they trigger myeloid cell metabolic reprogramming, both locally and in circulating monocytes, to induce even greater itaconate release. This host and pathogen metabolic adaptation generates a self-perpetuating bacterial community and provides a steady supply of antibiotic-resistant pathogens.
RESULTS AND DISCUSSION
Clinical isolates of P. aeruginosa undergo an adaptive change in expression of LPS and EPS
P. aeruginosa exhibits a dynamic metabolic network that coordinates energy production and the generation of multiple macromolecules, such as LPS and EPS (FIGURE 1A, blue and red boxes). While TCA cycle activity is mostly associated with ATP generation (FIGURE 1A, yellow circle), glucose metabolism is bidirectional, and can be reprogramed to shunt carbohydrates either into synthesis of LPS, protective EPS (gluconeogenesis, FIGURE 1A red arrows) or to produce energy (Entner-Duodoroff and Embden-Meyerhof Pathways, FIGURE 1A orange and green boxes, respectively). EPS production is favored in response to bacterial outer-membrane stress, which is mainly triggered by pro-oxidants and bactericidal components of host immunity. Gluconeogenesis and EPS synthesis can be supported by the glyoxylate shunt, an abbreviated TCA cycle that under stress is primed by acetyl-coA (FIGURE 1A, purple box). Itaconate is also a source of acetyl-coA (FIGURE 1A, gray box), suggesting this immunometabolite might fuel EPS synthesis. We postulated that virtually all isolates of P. aeruginosa from chronically infected sites, such as wounds or the airways, must undergo metabolic changes to cope with the oxidants imposed by the recruited immune response. Thus, we analyzed published transcriptional data from P. aeruginosa isolates derived from several sites of infection in immunocompetent hosts to assess in vivo-acquired metabolic changes associated with outer-membrane stress; namely, changes in LPS and EPS synthesis (FIGURE 1B). These isolates included strains from sputum of individuals with CF and soft-tissue samples from subjects with chronic and burn wound infections (Cornforth et al., 2018). We compared these expression changes with those of P. aeruginosa isolates obtained from 40-h acute burn and 4-day non-diabetic chronic wound infections from mice (Turner et al., 2014). The most consistently upregulated genes were those involved in activating the EPS alginate operon, such as the outer membrane stress-related sigma factor algT (also known as algU) and its co-expressed regulator mucA (FIGURE 1B, red group). AlgW, which encodes a mucA protease, was partially downregulated. AlgA and algR were upregulated, as was algD, loci that are under the control of algT during surface perturbations. While alginate over-production is typically associated with CF-related strains of P. aeruginosa (Deretic et al., 1995), the genes regulating its expression are upregulated in many other clinical settings. Less consistent were the effects on the expression of the EPS genes pelA (Pel) and pslA (Psl), which varied depending on the source of the organism analyzed (FIGURE 1B, red group). In contrast, in most of the studies there was decreased expression of genes involved in endotoxin and O-antigen assembly, including many multi-operon genes, such as wzy, wbpA, wbpG, wbpY (FIGURE 1B, blue group). LptD, the outer membrane protein that translocates LPS to the bacterial surface was also decreased (FIGURE 1B, blue group) (Balibar and Grabowicz, 2016; Botos et al., 2016), resulting in accumulation of LPS in the periplasmic space. These findings suggest that, once in the host, P. aeruginosa from various sites decreases LPS synthesis and its trafficking, a response associated with outer-membrane stress, while also increasing algT expression, which would increase production of EPS (Lima et al., 2013).
Figure 1.
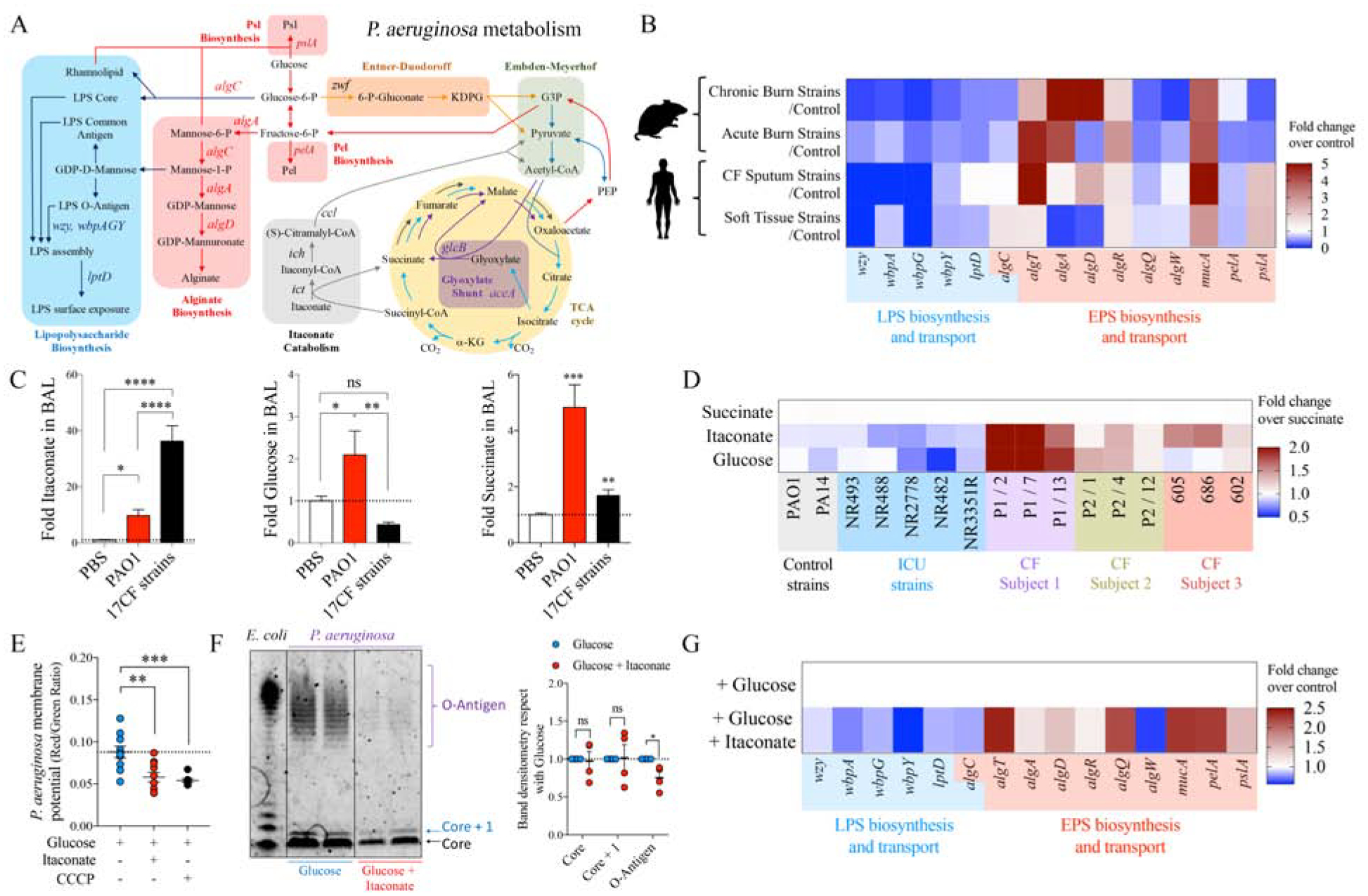
Metabolic preferences of P. aeruginosa in the airway.
A) Scheme showing how metabolism regulates extracellular polysaccharides (EPS) (red box) and lipopolysaccharide (LPS) (blue box) synthesis and transport in P. aeruginosa. Itaconate metabolism (gray box), TCA cycle activity (yellow circle) and glucose catabolism (orange-green boxes) are also shown. Gluconeogenic flux is shown as red arrows.
B) Compared with their respective controls (fold increase), expression of different genes involved in EPS and LPS production by P. aeruginosa isolates from mouse and human subjects.
C) Airway abundance of succinate, itaconate and glucose by Mass-Spec in mice infected with P. aeruginosa PAO1 or a collection of 17 CF host-adapted P. aeruginosa isolates. PBS-treated mice are controls. 4–5 mice pooled from n=2 independent experiments.
D) Fold overnight growth of each P. aeruginosa strain with respect to succinate: laboratory strains, ICU and CF isolates are from different subjects.
E) P. aeruginosa membrane potential as measured by flow cytometry with baclight DiOC2(3) dye after overnight glucose incubation with or without itaconate (1:1). Membrane potential uncoupler CCCP was used for 30min as positive control for dye specificity.
F) LPS extracts from PAO1 grown overnight in glucose or glucose and itaconate (1:1) and stained for O-antigen and core. E.coli LPS: positive control. Respect to glucose only, core, core + 1 and O-antigen LPS band intensities for PAO1 grown in glucose and itaconate were quantified with FIJI.
G) Fold increase of genes involved in EPS and LPS synthesis in PAO1 growth in glucose and itaconate, with respect to growth only in glucose.
C, E: One-Way ANOVA; F: Student’s t-test. Data are shown as average +/− SEM. D-G represent at least n = 3. ****: P < 0.0001; ***: P < 0.001; **: P < 0.01; *: P < 0.05; ns: non-significant. See also Figure S1.
P. aeruginosa consumes mitochondrial metabolites
These changes in the EPS and LPS pathways in P. aeruginosa isolates are likely induced by inflammatory cells at sites of infection and the presence of myeloid metabolites, such as succinate and itaconate (Lampropoulou et al., 2016; Tannahill et al., 2013). We used clinical isolates from individuals with CF to first establish whether P. aeruginosa induces accumulation of itaconate, succinate and glucose in the airway as compared to the laboratory strain WT PAO1 (referred to as simply PAO1 hereafter). Using a mouse model of pneumonia, we found host-adapted isolates induced significantly more itaconate secretion into the bronchoalveolar lavage (BAL), as compared with responses to PAO1, the latter of, in contrast, induced greater secretion of succinate and glucose into the BAL (FIGURE 1C). Thus, P. aeruginosa induces the production of immunometabolites, but the relative amounts of these correlates with the extent of the balance of bacterial EPS-to-LPS-related gene expression.
P. aeruginosa can use a variety of carbon sources depending on growth conditions; namely, aerobic or anaerobic conditions and exponential versus stationary phase growth (Arai, 2011). To evaluate the preferences of P. aeruginosa isolates we monitored growth in succinate, glucose or itaconate. As host-adapted strains from different subjects with CF exhibited variable growth rates, we focused on their growth in itaconate and glucose with respect to succinate, the last of which is expected to be the preferred P. aeruginosa carbon source (Collier et al., 1996; Gorke and Stulke, 2008; O’Toole et al., 2000; Rojo, 2010). We found that host-adapted strains reached stationary growth phase faster than PAO1 (Figure S1A) and produced more biomass in itaconate or glucose than succinate at later time points (FIGURE 1D). In this same time-frame P. aeruginosa strains isolated from acute infections in the ICU, as well as the laboratory control strains PAO1 and PA14, generally grew exponentially (Figure S1A) and preferred succinate or grew equally well on all three substrates (FIGURE 1D). These data indicate that airway-adapted P. aeruginosa strains, which gain EPS production over LPS synthesis, display a differential dynamic growth pattern from laboratory strains with the former exhibiting a specific preference for itaconate and glucose over succinate.
Itaconate promotes expression of anti-membrane stress EPS and suppresses LPS assembly
The preferential consumption of itaconate and glucose as primary carbon sources by the apparently LPS-deficient host-adapted P. aeruginosa isolates and their increased expression of algD, pelA and pslA, suggested that itaconate promotes EPS synthesis. Itaconate belongs to the group of electrophiles (Bambouskova et al., 2018), known to trigger the P. aeruginosa stress defense response (Juarez et al., 2017; Wongsaroj et al., 2018). We found that when added to glucose (1:1), itaconate induced substantial variation in P. aeruginosa size (FIGURE S1B) and surface granularity (FIGURE S1C), changes expected in response to superficial damage and membrane distress. By using the baclight DiOC2(3) membrane potential dye, we studied changes in bacterial surface homeostasis. This dye accumulates inside bacteria and emits green fluorescence in direct proportion with size. Under active oxidative phosphorylation (OXPHOS), this dye also emits red fluorescence, as it senses the transport of protons from the cytoplasm and their accumulation at the periplasmic space. The red-to-green color ratio can be used to track changes in bacterial membrane potential. We observed that despite accumulating more intrabacterial green dye due to their increased size (FIGURE S1D), itaconate-treated P. aeruginosa did not exhibit a proportional increase in red fluorescence (FIGURE S1E), which was interpreted as a reduced membrane potential (FIGURE 1E). This finding suggested that itaconate reduced OXPHOS in P. aeruginosa. However, these organisms produced the same amount of OXPHOS-derived anion superoxide (O2*−) with or without itaconate (FIGURE S1F), suggesting this immunometabolite also induces proton leakage from the periplasm into the extracellular space through a permissive outer membrane. This membrane potential diffusion, irregular surface granularity and the partial production of LPS core but not O-antigen in P. aeruginosa PAO1 (FIGURE 1F) is consistent with the selection of variants exhibiting membrane perturbation and rough LPS, as often seen in CF (Evans et al., 1994). Outer membrane stress and lack of O-antigen production was consistent with downregulation of genes involved in LPS biosynthesis and its trafficking by itaconate, including wbpA, wbpG, wbpY, and lptD (FIGURE 1G). In response to this pressure, itaconate-treated PAO1 induced overexpression of the membrane anti-stress algT locus, which corresponded with increased levels of algA, algD, algR and algQ (FIGURE 1G). MucA, which is co-transcribed with the EPS alginate operon, was also upregulated along with pelA and pslA expression (FIGURE 1G), confirming the positive role of itaconate inducing the protective EPS machinery instead of LPS synthesis due to membrane deregulation.
P. aeruginosa lacking surface LPS activates macrophage metabolism
Surface-exposed LPS is arguably the major immunostimulatory component of P. aeruginosa, inducing TLR4 signaling in macrophages that activates pro-inflammatory gene expression. Cytokine production is supported by profound metabolic changes in macrophages such as succinate production, its oxidation by mitochondria, HIF-1α induction and increased glycolysis (Mills et al., 2016; Mills et al., 2017; Tannahill et al., 2013). Rapid alterations in LPS biosynthesis or its surface display, such as those due to lack of lptD function in host-adapted P. aeruginosa, are expected to have major consequences on bacterial membrane-stress responses (Lima et al., 2013) and immune cell activation (Mills et al., 2016). We predicted that mutants lacking surface-exposed LPS would experience stress, outer membrane permeability and lose immunogenicity. We used a ΔlptD4213 PAO1 mutant, which harbors a 23 amino acid depletion (Figure S2A) in an extracellular loop that produces a truncated LptD protein (Balibar and Grabowicz, 2016; Lee et al., 2016) unable to traffic LPS to the bacterial surface, to evaluate the effects of periplasmic LPS accumulation on P. aeruginosa immunogenicity. These strains display external barrier destabilization (Balibar and Grabowicz, 2016), and antibodies that bind LPS-related sugars penetrate their outer membranes, allowing for the detection of increased levels of endotoxin components accumulated in the periplasmic region (Figure S2B). Using mouse bone marrow-derived macrophages (BMDMs) we observed that the ΔlptD4213 PAO1 mutant induced even higher extracellular acidification rates (ECAR, lactate production: glycolysis) than did the WT control (FIGURE 2A), suggesting that these strains were fully metabolo-stimulatory. Although to a lesser extent, mitochondrial ROS (O2*−) production in BMDMs was also induced by these mutant organisms (FIGURE 2B), and oxygen consumption rates (OCRs) were increased by both WT and ΔlptD4213 strains with respect to PBS-treated cells and only differed before glucose addition (FIGURE 2C). These data suggest that in metabolically active P. aeruginosa LPS surface expression is not necessarily the major inducer of macrophage metabolic changes.
Figure 2.
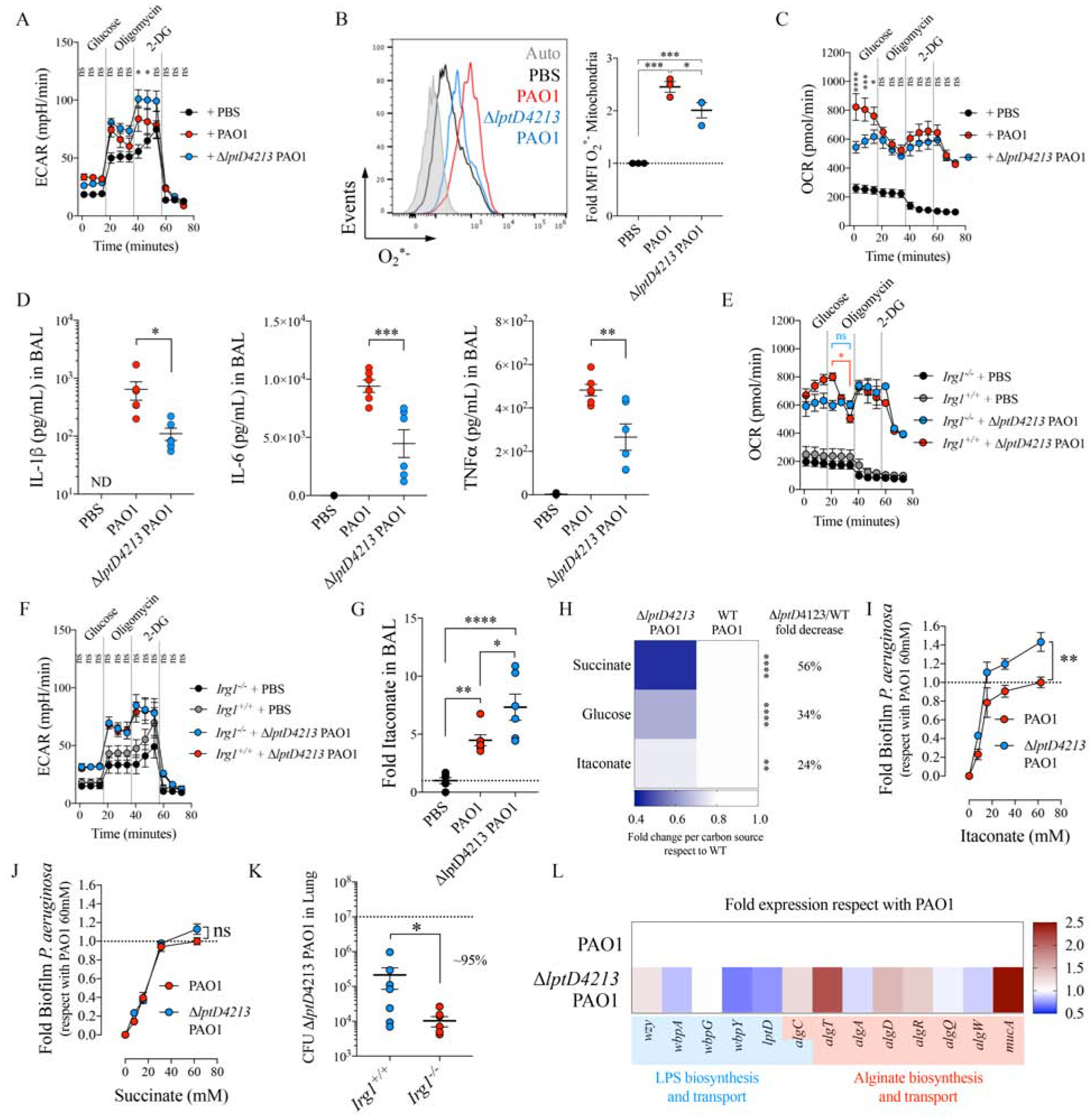
ΔlptD4213 PAO1 induces macrophage itaconate metabolism in the airway.
A-C) Extracellular acidification rates (ECAR) by Seahorse (A), mitochondrial ROS (O2*−) as determined by Mitosox and flow cytometry (B), and oxygen consumption rates (OCR) by Seahorse (C) in mouse BMDMs either uninfected (PBS) or infected with PAO1 or ΔlptD4213 PAO1.
D) Levels of the inflammatory cytokines IL-1β (left), IL-6 (middle) and TNFα (right) in the BAL from uninfected (PBS) or 16h-intranasally infected mice with PAO1 or ΔlptD4213 PAO1.
E-F) The OCR (E) and the ECAR (F) for Irg1+/+ and Irg1−/− BMDMs that were either uninfected (PBS) or infected with ΔlptD4213 PAO1.
G) Mice were infected as in D and itaconate was quantified by Mass Spec in BAL.
H) Respect with WT PAO1, ΔlptD4213 PAO1 fold overnight growth in different carbon sources.
I-J) Crystal violet biofilms produced by PAO1 and ΔlptD4213 PAO1 strains growth in M9 supplemented either with itaconate or succinate.
K) Bacterial burden (CFUs) measured in Irg1+/+ and Irg1−/− lungs of mice infected with ΔlptD4213 PAO1.
L) Fold increase of genes involved in EPS and LPS synthesis in ΔlptD4213 PAO1, in respect to PAO1. Data are shown as average +/− SEM. B, D, G: One-Way ANOVA; K: t-Student; A, C, E-F, I-J: Two-Ways ANOVA. In vivo data are from n = 2 (6–7 mice total). Seahorse were a minimal of n = 3. ****: P < 0.0001; ***: P < 0.001; **: P < 0.01; *: P < 0.05; ns: non-significant. See also Figure S2.
We next tested the immunostimulatory consequences of the ΔlptD4213 strain as compared with PAO1 in a mouse model of pneumonia. Both strains induced similar amounts of chemoattractants, such as Rantes, Eotaxin, CCL12, CCL17, CCL19, CCL20, CCL21, CCL22, MCP-1, GCS-F, GM-CSF and VGEF (FIGURE S2C). We found equivalent numbers of alveolar macrophages (FIGURE S2D–E) and Ly6C+/− Ly6G+ neutrophils and Ly6CHighLy6G− monocytes in the BAL (FIGURE S2F–G) and lungs (FIGURE S2H). Thus, lack of surface LPS did not inhibit signaling to attract phagocytes. However, due to lack of TLR4 signaling mice infected with the LPS-mutants had significantly less IL-1β, IL-6 and TNFα secretion into the airways, as compared with PAO1 (FIGURE 2D), indicating that lptD dysfunction in P. aeruginosa results in the activation of thwarted airway inflammatory response, as we predicted. Nevertheless, this qualitatively different response triggered by LPS-mutant P. aeruginosa conserved the metabolic changes associated with macrophage activation.
LptD-mutant P. aeruginosa stimulates itaconate production for bacterial metabolism
The induction of oxidative metabolism in macrophages by ΔlptD4213 PAO1 implied that these mutants could also trigger the mitochondrial anti-oxidant program. In response to oxidative metabolism, mitochondria upregulate Irg1 expression, which produces the anti-oxidant intermediate itaconate (Lampropoulou et al., 2016; Mills et al., 2018). We observed that Irg1 was required to suppress macrophages OCR (FIGURE 2E) but not glycolysis (FIGURE 2F) during ΔlptD4213 infection, and in a mouse model of pneumonia these ΔlptD4213 mutants induced greater amounts of itaconate accumulation in the BAL than the PAO1 control (FIGURE 2G). These findings suggest that despite lacking surface LPS, these lptD P. aeruginosa mutants induce Irg1 activity and itaconate production to suppress pro-oxidant mitochondrial activities.
The release of itaconate subjects P. aeruginosa to a major selective pressure as itaconate is bactericidal (Naujoks et al., 2016; Rittenhouse and McFadden, 1974), though the bacterium can consume itaconate by upregulating the ict-ich-ccl locus (Sasikaran et al., 2014). We found that both PAO1 and ΔlptD4213 responded to itaconate by upregulating these genes (FIGURE S2I), and both strains grew in itaconate to a similar extent during the exponential (no differences) and stationary growth phase (24% difference) (FIGURE 2H and FIGURE S2J). However, lack of lptD function caused a higher reduction in PAO1 growth in succinate (56%) and glucose (34%) at both early and late time points (FIGURE 2H and FIGURE S2J), suggesting that LPS deregulations are associated with preservation of itaconate as a major carbon source. Moreover, growth in itaconate resulted in increased production of biofilm (FIGURE 2I), more so than in succinate (FIGURE 2J), which was expected to be the preferred carbon source for PAO1 (Collier et al., 1996; Gorke and Stulke, 2008; Rojo, 2010). This preference for itaconate by mutant PAO1 was manifested in vivo. Pulmonary infection with this ΔlptD4213 strain was significantly greater in WT mice compared to mice unable to produce itaconate (i.e., Irg1−/− mice) as we saw a 95% decrease in the level of bacterial colony forming units (CFUs) with infection of the knockout mice vs. the WT mice (FIGURE 2K). However, the ΔlptD4213 strain elicited similar numbers of immune cells into the airway of WT and Irg1−/− mice (FIGURE S2K–L), suggesting the mutation did not interfere with initial immune recognition of the pathogen. In total these data suggest that lack of lptD function and thus reduced accumulation of LPS in the bacterial periplasm tunes both bacterial and immune cell activities as a consequence of itaconate metabolism.
EPS alginate activates macrophage immunometabolism
We were surprised to find that P. aeruginosa with substantial defects in LPS display, nonetheless, activates glycolysis and mitochondrial respiration in myeloid cells (FIGURE 2A–C and FIGURE 2G). Given that the ΔlptD4213 strain upregulated the cascade of pro-EPS genes, including algT and algD, as compared to PAO1 (FIGURE 2L), we postulated that EPS, especially alginate, might induce changes in macrophage metabolism. Though typically associated with CF strains, alginate production is a feature, to varying degrees, of P. aeruginosa infections in acute and chronic infections (Bragonzi et al., 2005; Franklin et al., 2011; Hentzer et al., 2001), as we observed (FIGURE 1B). The immunometabolic effects of alginate on macrophages were assessed by measuring changes in ECAR and OCR, and we found that soluble alginate increased ECAR but not mitochondrial respiration in treated BMDMs (FIGURE 3A–B), similar to what was observed with LPS. We demonstrated these alginate effects were specific, as neither polyethylene glycol (PEG) nor N-acetyl glucosamine, a major bacterial cell wall component (Dhar et al., 2018), induced ECAR or increased OCR in BMDMs (FIGURE S3A). We further found that alginate increased the mitochondrial membrane potential (ΔΨ) without augmenting ROS production by macrophages (FIGURE 3C–D), indicating that this EPS alone causes mitochondrial deregulation probably due to augmented glycolysis as seen with LPS (Mills et al., 2016).
Figure 3.
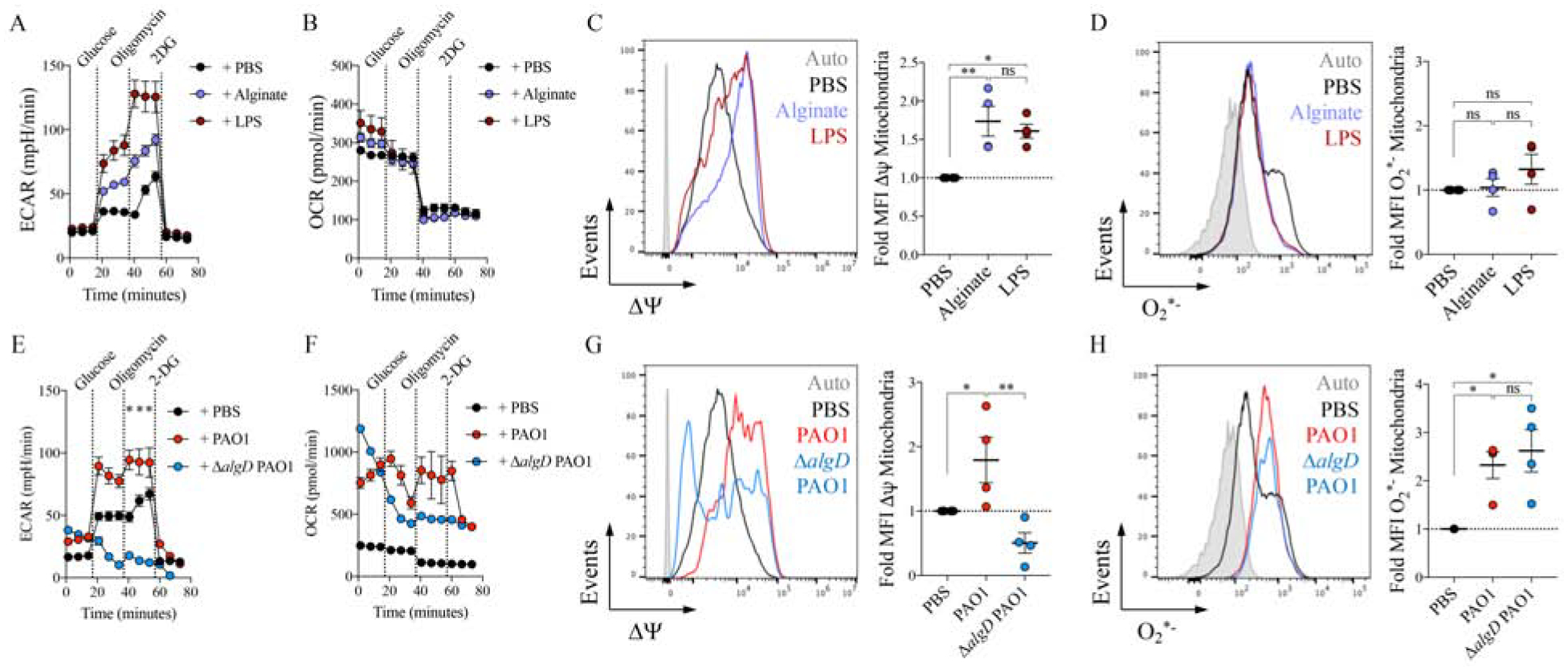
P. aeruginosa EPS alginate induces anti-oxidant itaconate metabolism.
A-D) Extracellular acidification rate (ECAR) (A) and oxygen consumption rate (OCR) (B) by Seahorse, and mitochondrial membrane potential ΔΨ (C) and mitochondrial ROS (O2*−) (D) by flow cytometry in mouse BMDMs either untreated (PBS) or treated with alginate or LPS.
E-H) The ECAR (E), the OCR (F), the ΔΨ (G), and the O2*− levels (H) in mouse BMDMs treated with PBS, WT PAO1 or ΔalgD PAO1.
Data are shown as average +/− SEM. C-D, G-H: One-Way ANOVA. Seahorse and flow cytometry experiments were a minimal of n=3. ****: P < 0.0001; ***: P < 0.001; **: P< 0.01; *: P < 0.05; ns: non-significant. See also Figure S3.
AlgD expression modulates macrophage metabolism and immunotoxicity of P. aeruginosa
To confirm the effects of alginate expressed by living organisms on host metabolic activity, we used an ΔalgD mutant in the PAO1 background, which cannot synthetize alginate. This ΔalgD and its WT PAO1 control generate the same amount of LPS (Goldberg et al., 1995). We observed that the ΔalgD strain was apparently toxic to mammalian macrophages in our attempts to monitor induced ECAR (FIGURE 3E). Macrophages treated with the ΔalgD PAO1 had higher initial OCR than those exposed to PAO1, which rapidly declined after glucose administration (FIGURE 3F). Mitochondrial dysfunction was confirmed after observing that ΔalgD readily reduced the ΔΨ independent of the amounts of O2*− induced (FIGURE 3G–H).
These results suggest that bacterial alginate metabolism might protect macrophages from both a glucose- and ΔΨ-dependent cell death pathway. We observed that lack of algD not only reduced algT, algR and mucA expression in PAO1, it also induced synthesis of translocons pcrV-popD and exoT-exoS toxins, both components of the type three secretion system (T3SS) (FIGURE S3B–C). The P. aeruginosa T3SS is known for inducing mitochondrial damage and a highly pro-inflammatory type of cell death called pyroptosis, which is associated with IL-1β production (Evavold et al., 2018; Faure et al., 2014; Jabir et al., 2015). To test this hypothesis in vivo, we infected mice intranasally with either ΔalgD or WT PAO1. Mice infected with the ΔalgD harbored greater numbers of dead alveolar macrophages in the BAL than those infected with the control PAO1 (FIGURE 4A and FIGURE S3D). Cell death was also reflected in reduced numbers of viable alveolar macrophages, Ly6CHighLy6G− monocytes and neutrophils in the BAL of ΔalgD-infected mice (FIGURE 4B and FIGURE S3E–F).
Figure 4.
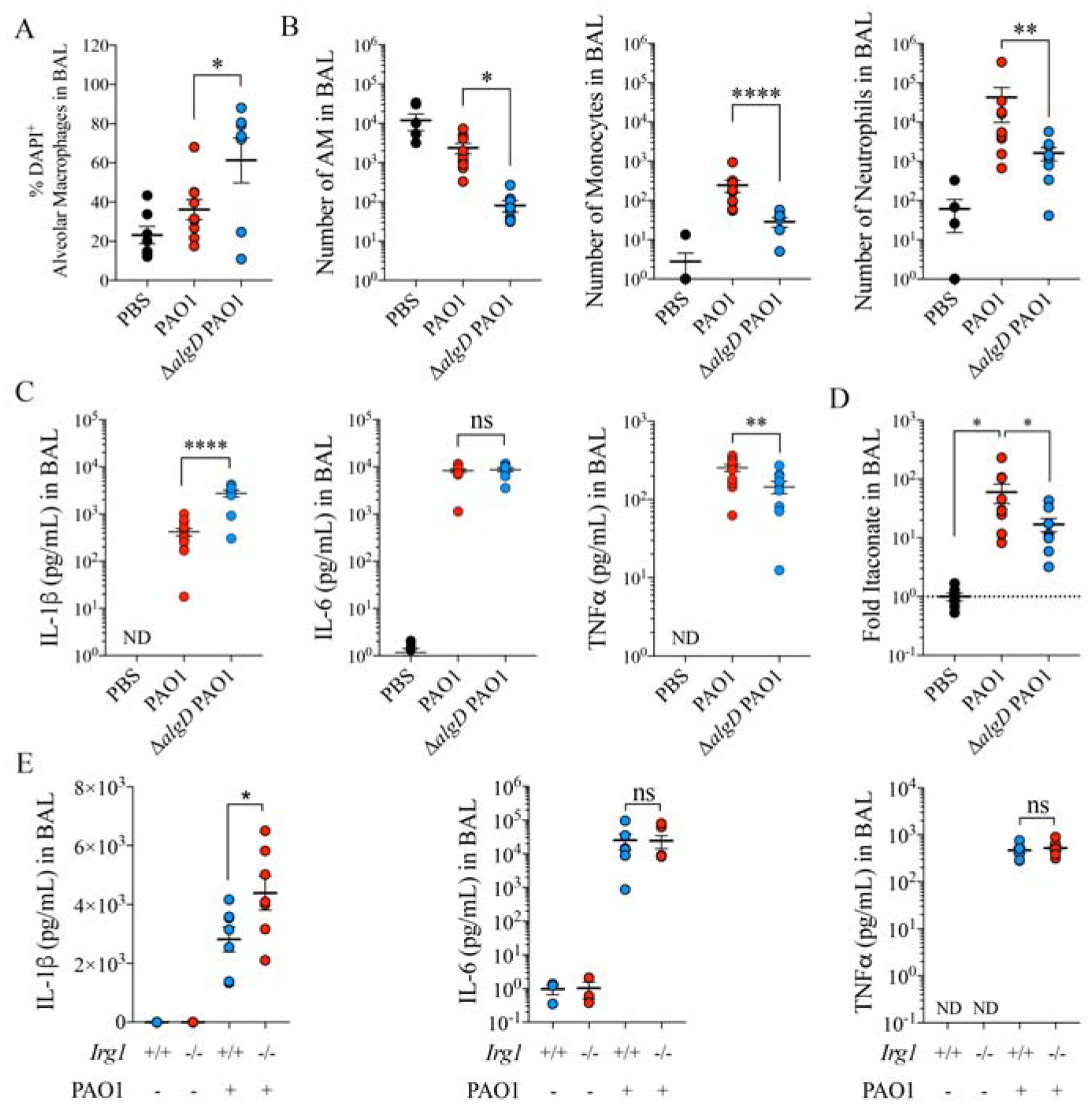
P. aeruginosa EPS alginate induces airway itaconate and prevents from cell death.
A-D) Percentage of DAPI+ alveolar macrophages (death cells) by flow cytometry (A), number of alveolar macrophages (left), Ly6CHighLy6G− monocytes (middle) and neutrophils (right) by flow cytometry (B), pro-inflammatory IL-1β (left), IL-6 (middle) and TNFα (right) cytokines (C) by ELISA and itaconate by Mass Spec (D) in BAL of mice left untreated (PBS) or infected with PAO1 WT or ΔalgD PAO1.
E) Pro-inflammatory IL-1β (left), IL-6 (middle) and TNFα (right) cytokines in BAL of Irg1+/+ and Irg1−/− mice treated with PBS or infected with PAO1.
Data are shown as average +/− SEM. A-E: One-Way ANOVA; Data are from n=2 (7–8 mice total). ****: P < 0.0001; ***: P < 0.001; **: P < 0.01; *: P < 0.05; ns: non-significant. See also Figure S3.
We also found that ΔalgD-infected animals secreted significantly (P < 0.0001) more IL-1β, but not more IL-6 or TNFα than controls (FIGURE 4C), consistent with the hypothesis that alginate suppresses the induction of pyroptosis in immune cells. We confirmed that alginate synthesis promoted more itaconate accumulation in the BAL (FIGURE 4D). In addition, this itaconate production correlated with reduced IL-1β release specifically (i.e., no change in IL-6 or TNFα) compared to that seen in the Irg1 knockout mouse (FIGURE 4E). These findings suggest that in the presence of LPS, alginate production by P. aeruginosa suppresses T3SS activity, IL-1β production and pyroptotic cell death, facilitating accumulation of itaconate-producing host immune cells.
Itaconate suppresses glucose utilization by P. aeruginosa and promotes monocyte accumulation
We next addressed how P. aeruginosa supports increased EPS production in the setting of itaconate utilization as a major substrate. In P. aeruginosa, glucose is mainly consumed by the Entner-Duodoroff pathway, with zwf (glucose-6-phosphate dehydrogenase: G6PDH) as the rate limiting step (Kim et al., 2008; Ma et al., 1998; Maleki et al., 2015) (FIGURE 1A, orange box). We analyzed a mouse and two human cohorts of P. aeruginosa isolates (Figure 1B) which indicated that in the acute and chronic setting of infections zwf and several downstream pro-glycolytic genes were downregulated (FIGURE 5A). When present, itaconate downregulated zwf expression in glucose-fed P. aeruginosa (FIGURE 5B), indicative of active catabolite repression and likely use of itaconate as main carbon source. When we analyzed a Δzwf PAO1 mutant and its complemented strain Δzwf Comp, we observed that lack of zwf function increased energy production with itaconate but not glucose or succinate (FIGURE 5C). Itaconate addition to Luria-bertani (LB) media increased growth of the Δzwf PAO1 strain but not the WT or complemented control (FIGURE 5D), which correlated with increased expression of ict, ich and ccl (FIGURE 5E), the three genes involved in itaconate metabolism in environmental P. aeruginosa. Together, these findings suggested that itaconate provides P. aeruginosa with energy while reducing zwf function and shunting glucose into generation of EPS.
Figure 5.
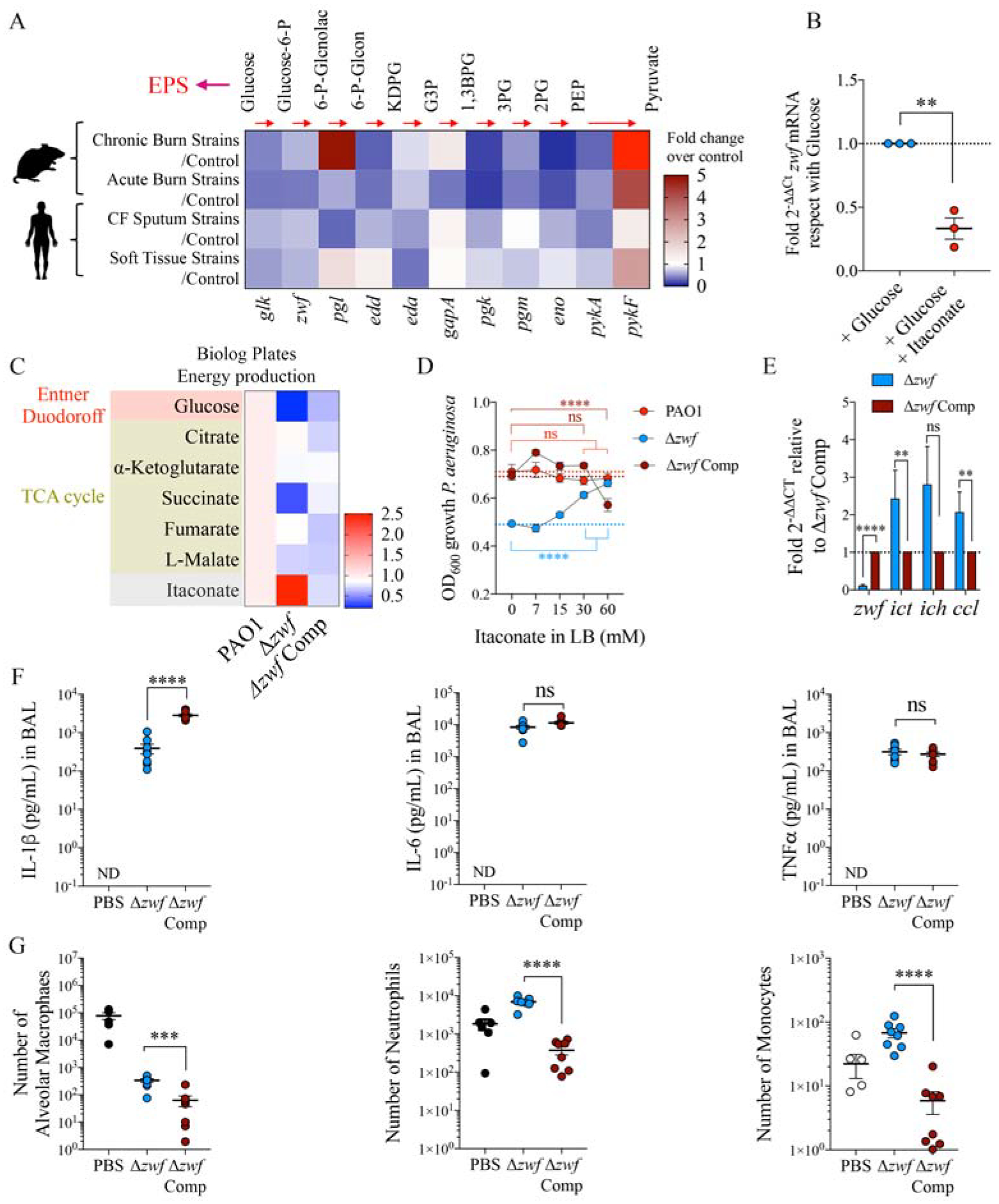
Reduced glycolytic flux facilitates itaconate assimilation by P. aeruginosa.
A) Compared with their respective controls (fold increase), expression of different genes involved in glucose catabolism in P. aeruginosa isolates from mouse and human subjects.
B) Zwf mRNA levels by qRT-PCR in M9 + glucose-fed PAO1 exposed or not to itaconate (n=3).
C-E) Energy production by Biolog plates (C), bacterial growth under different itaconate concentrations in LB (D) and zwf, ict, ich and ccl mRNA levels by qRT-PCR (E) in Δzwf PAO1 and its complement Δzwf PAO1 Comp control, respect with WT PAO1.
F-G) Pro-inflammatory IL-1β (left), IL-6 (middle) and TNFα (right) cytokines by ELISA (F), and alveolar macrophages (left), Ly6ChighLy6G− monocytes (middle) and Ly6Chigh/lowLy6G+ neutrophils (right) numbers by flow cytometry (G) in BAL of mice untreated (PBS) or 16h-infected with Δzwf PAO1 or its Δzwf PAO1 complement control.
Data are shown as average +/− SEM. B, E, F-G: t-Student. D: Two-Way ANOVA. In vivo data are from n=2 (8 mice total). ****: P < 0.0001; ***: P < 0.001; **: P < 0.01; ns: non-significant. See also Figure S4.
To test the impact of reduced glucose catabolism on immune signaling by P. aeruginosa, we infected mice with the Δzwf PAO1 mutant and the complemented control. We observed that lack of zwf function only reduced IL-1β BAL accumulation, as IL-6 and TNFα remained unchanged (FIGURE 5F). Infection due to the Δzwf P. aeruginosa was associated with greater numbers of alveolar macrophages (FIGURE 5G and FIGURE S4A) as it induced less cell death (FIGURE S4B). The Δzwf P. aeruginosa infection conserved more Ly6ChighLy6G− monocytes and Ly6Chigh/lowLy6G+ neutrophils in the BAL (FIGURE 5G and FIGURE S4C). These findings suggest that reduced glucose catabolism by P. aeruginosa induces less IL-1β-associated myeloid cell death and instead, results in a relative accumulation of phagocytes that might contribute to the release of itaconate into the airway.
P. aeruginosa clinical isolates have an unusual ability to assimilate itaconate and produce biofilm
Having found that itaconate regulates EPS-LPS dynamics on the P. aeruginosa surface and the relative preferences of P. aeruginosa from different stages of infection for myeloid metabolites, we evaluated whether host-adapted P. aeruginosa isolates displayed genetic and metabolic adaptation to itaconate. We reviewed the sequenced genomes of 17 CF-associated clinical isolates of P. aeruginosa collected over 5 years (Riquelme et al., 2019) (FIGURE 1C) and found they had accumulated non-synonymous mutations (NSM) in lptD, algW and pelA, but not in algT, algD, pslA, ict, ich and ccl (FIGURE 6A). In two representative isolates, mucoid 605 and small-colony variant (SCV) 686, these mutations correlated with differential mRNA expression profiles associated with outer membrane stress: lptD was almost undetectable, pelA and pslA were slightly upregulated and algD was substantially increased (FIGURE 6B). We noted that expression of the genes enabling P. aeruginosa to metabolize itaconate, ict, ich and ccl, increased in P. aeruginosa strains as a function of their relative adaptation to the airway: CF host-adapted strains, from additional, independent collections had substantial upregulation of these genes with respect to PAO1, whereas control acute ICU isolates, in general, did not (FIGURE 6C). These genes are at least 50% conserved at the levels of gene and protein identity in the majority of available P. aeruginosa genomes (FIGURE S5A–B). The P. aeruginosa itaconate-associated genes/proteins sequences were significantly less frequent in genomes of other common respiratory pathogens, such as Haemophilus influenzae, Klebsiella pneumoniae, Stenotrophomonas maltophilia, Burkholderia cepacia complex, Achromobacter xylosoxidans, Acinetobacter baumanni, Staphylococcus aureus, Streptococcus pneumoniae and Mycobacterium (FIGURE S5C–H). It appears that P. aeruginosa has a relatively unique genetic capability to induce and consume airway itaconate while modifying immunostimulatory surface components, which could be a factor in its high prevalence as a cause of major pulmonary infection.
Figure 6.
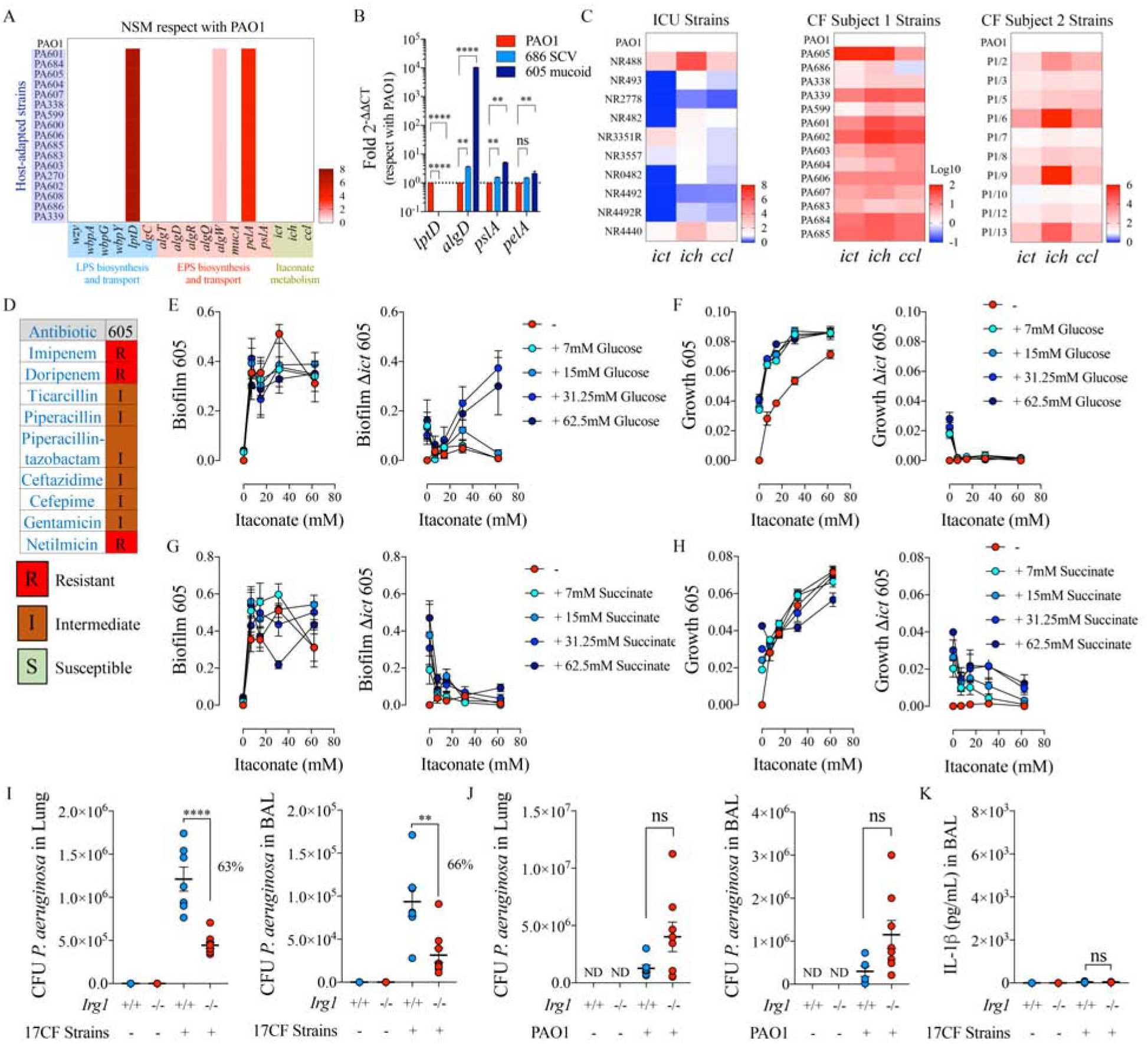
Host-adapted P. aeruginosa isolates exploit host itaconate to colonize the airway.
A) Numbers of non-synonymous SNPs found in a collection of 17 P. aeruginosa isolates from an individual with CF, as compared with PAO1 genome. Pathways analyzed: LPS-EPS biosynthesis-trafficking and itaconate catabolism.
B) Fold increase gene expression by qRT-PCR respect with PAO1 in two representative P. aeruginosa isolates from A: mucoid 605 and small colony variant 686.
C) Ict-ich-ccl locus expression by qRT-PCR in different ICU and CF P. aeruginosa isolates, in respect with PAO1.
D) Antibiogram of PA605 mucoid strain.
E-H) Itaconate-mediated both biofilm and growth in presence of glucose (E-F) or succinate (G-H) by mucoid 605 P. aeruginosa and its isogenic Δict mutant.
I-J) Bacterial burden (CFUs) found in Irg1+/+ and Irg1−/− lungs and BALs from mice left untreated (PBS) or infected with CF isolates (I) and PAO1 (J).
K) IL-1β levels in 17 CF isolates-infected Irg1+/+ and Irg1−/− BALs.
Data are shown as average +/− SEM. B: One-Way ANOVA; I-K: t-Student. In vivo data are from n=2 (7–8 mice total). ****: P < 0.0001; ***: P < 0.001; **: P < 0.01; *: P < 0.05; ns: non-significant. See also Figure S5 and Figure S6.
Host-adapted P. aeruginosa isolates become itaconate-dependent
As itaconate is considered an antibacterial metabolite produced by myeloid cells (Naujoks et al., 2016; Rittenhouse and McFadden, 1974), we predicted that P. aeruginosa must be obligated to metabolize it in order to persist. The role of itaconate in supporting growth and biofilm production was tested in the phenotypically mucoid P. aeruginosa strain 605, which acquired resistance to multiple antibiotics in the human airway (FIGURE 6D). The upregulation of the itaconate-catabolism genes was confirmed as a response to selective pressure, as a Δict 605 isogenic mutant did not grow in glucose when itaconate was present and failed to produce biofilm (FIGURE 6E–F). While unable to grow on itaconate, in the presence of both itaconate and succinate, planktonic growth by the Δict-mutant was slightly rescued, but not the ability to form biofilm (FIGURE 6G–H). Similar findings were observed with non-adapted PAO1, as succinate, but not glucose, rescued both biofilm formation and growth by its isogenic Δict mutant (FIGURE S6A–D). The ability of P. aeruginosa isolates to resist itaconate toxicity was demonstrated in vivo, as intranasal administration of a high 60 mM itaconate dose barely changed the CFUs levels and did not eradicate infection from the mouse airway (FIGURE S6E). These findings suggest that the metabolic adaptation of the clinical isolates to itaconate toxicity, and likely to Enter-Duodoroff pathway suppression, had generated itaconate dependency.
Adaptation to itaconate alters the ability of P. aeruginosa to infect the mouse airway
We anticipated that the accrual of metabolic changes in response to itaconate toxicity would impact the infectious phenotype of P. aeruginosa isolates in the host lung. More than growing exponentially as laboratory strains do, host-adapted P. aeruginosa variants are selected as proficient biofilm producers in response to environmental stress (Maurice et al., 2018). This particular bacterial community is known for their high degree of resistance to antibiotic therapy and penetrance of immune components (Taylor et al., 2014), thus preserving a chronic reservoir of lung-adapted mutants (Driffield et al., 2008). As compared with control PAO1, we recovered fewer CFUs from WT mouse airway infected with the host-adapted strain 605 (Figure S6F). We confirmed this phenotype correlated with long-term adaptation to itaconate, as ict deletion from 605 rescued the number of CFUs to a level similar to infection caused by PAO1 (Figure S6F). This strong association between in-host adaptation to itaconate, metabolic reprograming and acquisition of the biofilm lifestyle was not a property of PAO1, which has never been exposed to itaconate previously. Indeed, WT and Δict PAO1 exhibited similar CFUs in the mouse airway (Figure S6G), confirming that the in vivo metabolic changes associated with the itaconate-ict axis are displayed in P. aeruginosa strains that also harbor the rest of these adaptive changes, in lptD, algD and zwf. In this context, we recapitulated the 605 infectious phenotype in PAO1 mutants lacking either zwf function (Δzwf) (Figure S6H), lptD activity (ΔlptD4213) (Figure S6I) or expressing algD (Figure S6J), as each of these mutant strains caused decreased airway infection (as measured by differences in CFUs) in mice with respect to their corresponding controls. The cumulative effects of ict overexpression, lptD-algD-zwf deregulation and itaconate dependence predicted that such host-adapted P. aeruginosa isolates would exploit itaconate as major airway carbon source.
Itaconate-adapted P. aeruginosa exploit host Irg1 function to infect the airway
To assess the overall importance of host itaconate in pathogenesis, we infected Irg1−/− mice, with either host-adapted CF strains or with PAO1, the laboratory control strain. Consistent with their acquired dependence upon itaconate consumption in the human airways, we observed that in the absence of Irg1 there was significantly (P < 0.0001) decreased recovery of the host-adapted P. aeruginosa from lungs and the BAL (FIGURE 6I). In contrast, PAO1 infection in the Irg1−/− mice was slightly but not significantly greater than in the WT mice (P > 0.05) (FIGURE 6J). Correlating with their lack of lptD, augmented algD expression and substantial itaconate response in the airway (FIGURE 1C), the host-adapted strains failed to induce IL-1β in either WT or Irg1−/− mice (FIGURE 6K). Itaconate-adapted P. aeruginosa induced a highjacked inflammatory response that fuels infection.
Host-adapted P. aeruginosa induce accumulation of itaconate-producing monocytes in the mouse airway
Itaconate is one of the most abundant metabolites produced by myeloid cells in response to infection (Lampropoulou et al., 2016). Circulating human monocytes, such as peripheral CD14++ cells, are strong itaconate producers (Dominguez-Andres et al., 2019) and are also actively recruited into the lung during infection (Jardine et al., 2019). These CD14++ monocytes are represented in the mouse model in part by the Ly6ChighLy6G− population (Ingersoll et al., 2010; Narasimhan et al., 2019). Having found that the presence of alginate prevents phagocytes from IL-1β-dependent cell death and stimulates airway itaconate accumulation, we expected to see that mouse lungs infected with CF-associated P. aeruginosa isolates with increased algD expression would accumulate more Irg1-expressing Ly6ChighLy6G− cells. From the entire monocyte population found in the host-adapted P. aeruginosa infected BAL, 75% corresponded to Ly6ChighLy6G− and 25% to Ly6ClowLy6G− cells (FIGURE 7A–B). These values substantially differed from what was observed with PBS or PAO1-treated animals, in which the majority of monocytes corresponded to Ly6ClowLy6G− cells (FIGURE 7A–B). The importance of these differences was confirmed as we documented that 95% of the Ly6ChighLy6G− monocytes induced by the host-adapted strains expressed Irg1, as opposed to the 60% of the monocyte population recruited by PAO1 infection (FIGURE 7C–D). No differences in Irg1 intracellular levels were observed in the Ly6ClowLy6G− cells population for both type of bacteria (FIGURE S7A). These data suggest that host-adapted P. aeruginosa induce accumulation of specific airway itaconate-producing immune cells that augment the bacterium’s metabolic requirements.
Figure 7.
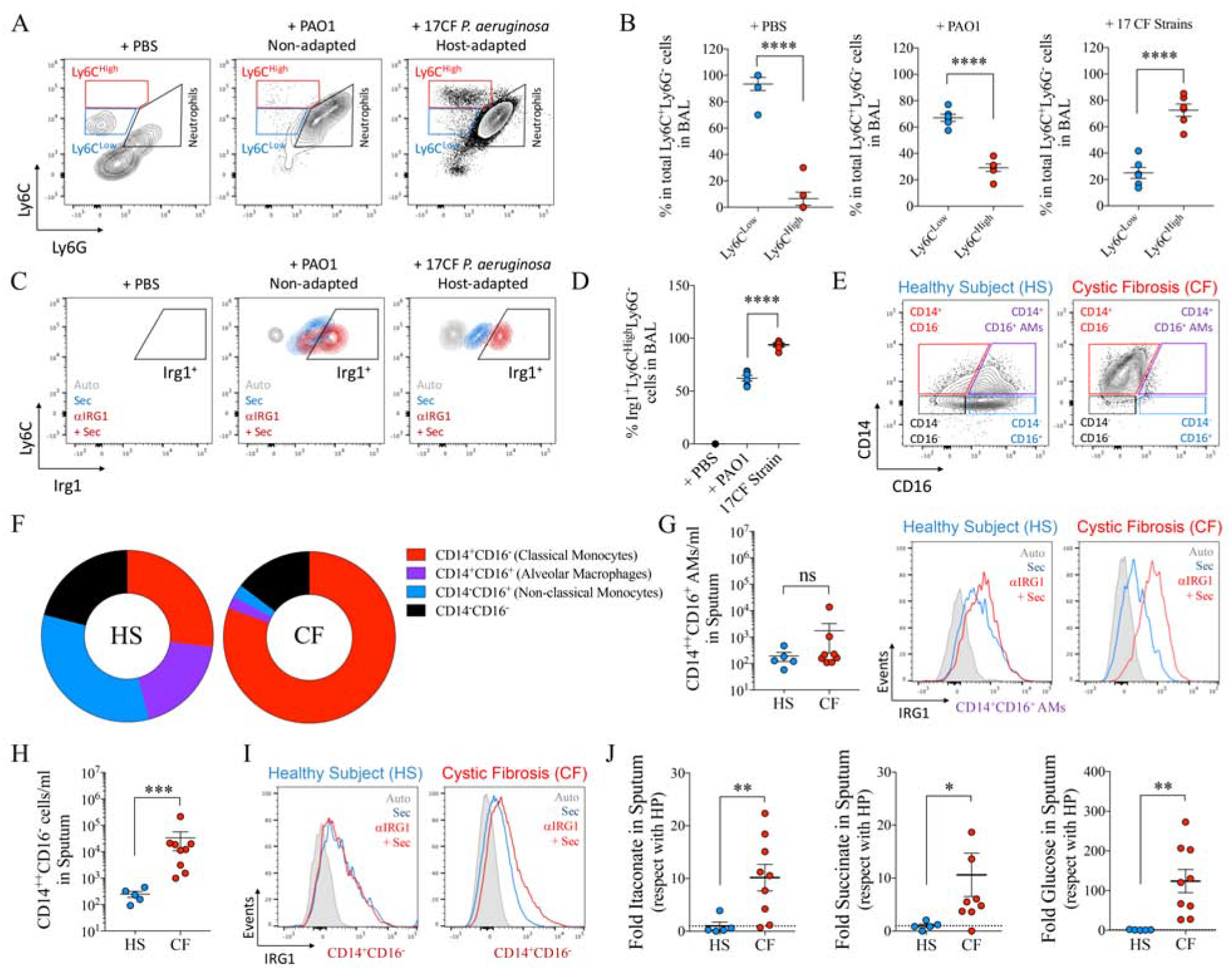
IRG1 producing airway myeloid cells feed P. aeruginosa isolates infection.
A-D) Representative density plots for Ly6ChighLy6G− and Ly6ClowLy6G− monocyte populations (A), their respective percentage in the total Ly6C+Ly6G− population (B) and the percentage of Irg1+Ly6ChighLy6G− population (C-D) in BAL of mice treated with PBS, PAO1 or a collection of 17 CF P. aeruginosa isolates.
E-F) Representative density plots (E) and frequency of cells (F) expressing CD14 and/or CD16 surface markers in sputum from healthy subjects (HS) and individuals with CF by flow cytometry.
G) Number of alveolar macrophages (CD14+CD16+ in E) found in sputum and their IRG1 expression.
H-I) Number of CD14+CD16− monocytes (shown in E) found in sputum and their IRG1 expression.
J) Sputum metabolomics from healthy subjects (HS) and individuals with CF.
Data are shown as average +/− SEM. B, D, G, H, I: t-Student. In vivo data are from n=2 (5 mice total). In G, H and J each point represents a single patient. ****: P < 0.0001; ***: P < 0.001; *: P < 0.05; ns: non-significant. See also Figure S7.
Subjects with CF harbor an increased population of peripheral and airway IRG1-expressing CD14+CD16− monocytes
We next sought to determine if the analogous population of CD14+ monocytes in humans is increased in response to P. aeruginosa infection. As most subjects with CF are infected with alginate-producing P. aeruginosa (albeit it to varying degrees), we predicted that these individuals would harbor increased numbers of circulating IRG1-expressing peripheral blood mononuclear cells (PBMCs). Analysis of monocytes among PBMCs from a third human cohort of healthy controls and adult subjects with CF confirmed a significantly increased frequency of CD14++CD16− (classical monocytes) and CD14++CD16+ (intermediate monocytes) cells expressing high levels of IRG1 in the CF group as compared with controls (FIGURE S7B–E). No major differences were observed between controls and subjects with CF in the frequency of the CD14−CD16− cells in PBMCs, which were found to be poor IRG1 producers (FIGURE S7C, FIGURE S7E–F). This increased frequency of circulating IRG1+CD14++ PBMCs in the majority of individuals with CF correlated with prior exposure to alginate-producing P. aeruginosa (FIGURE S7G).
In a fourth human cohort, we analyzed sputum from healthy subjects and individuals with CF and found that CD14+CD16− cells predominated among all airway myeloid cells (CD45+CD19−CD3− lineage cells) in the subjects with CF (FIGURE 7E–F and FIGURE S7H). Although total alveolar macrophage numbers, depicted as CD14+CD16+ in human lungs (Jardine et al., 2019), did not change between healthy subjects and individuals with CF (FIGURE 7G and FIGURE S7I), IRG1-producing CD14+CD16− monocytes were significantly increased in sputum from individuals with CF versus healthy controls (FIGURE 7H–I and FIGURE S7I). We found that accumulation of these IRG1+-cells correlated with increased itaconate levels in sputum from individuals with CF with respect to controls (FIGURE 7J), probably in response to accumulation of the pro-oxidants metabolites succinate and glucose also produced by active immune cells (FIGURE 7J). Thus, individuals with CF with chronic EPS-producing P. aeruginosa infection exhibit more itaconate-producing cells in the blood and the airways, cells that provide a substrate that can promote persistent infections.
Amidst the pressure to develop novel antimicrobial strategies to target infection caused by multiply drug resistant opportunistic infectious agents, such as P. aeruginosa, there is renewed interest in identifying novel targets to suppress bacterial proliferation. In the studies presented in this report, we demonstrate one of the major challenges in targeting P. aeruginosa; namely, its exceptional metabolic flexibility. By studying a variety of isolates that had adapted to their human hosts, we demonstrate that a highly conserved series of genetic changes enables this species, specifically, to adapt to the consumption of itaconate. The rapid switch in cell surface display from LPS to EPS and suppression of glucose catabolism provide a feed-forward mechanism to generate itaconate. By inducing and then consuming the itaconate, which serves to protect the host from oxidant damage, P. aeruginosa supports a self-sustaining community of bacteria. While it is disconcerting that these bacteria can exploit the basic elements of host immune defenses for their own benefit, controlling acute inflammation and the abundance of favored metabolites could reduce the risk of developing intractable long-term infection.
Alginate is synthetized in varying degrees in many clinical settings of P. aeruginosa infection (Bragonzi et al., 2005; Hentzer et al., 2001), in addition in the lungs of individuals with CF. Its production correlates with the intensity of the oxidative response mounted by the host. Unexpectedly, we found that alginate has important immunoregulatory functions for host immune cells. Far from an inert protective shell for the bacteria, alginate induces metabolic changes in myeloid cells, changes that are also dictated by LPS and associated with how cells produce energy during infection (Mills et al., 2016). Macrophage metabolic reprograming in response to LPS increases production of itaconate (Lampropoulou et al., 2016), which we confirmed here in vivo with alginate-producing bacteria. By studying a variety of clinical isolates, including those that have undergone selection for persistence in the setting of airway inflammation, as in our CF-associated strains, we noted the emergence of mutants that by limiting the generation of IL-1β, they prevented myeloid cell death and induced more airway itaconate. The CF-associated isolates provide the opportunity to determine the long-term effects of bacterial adaptation to the inflamed airway, changes that are ongoing but less obvious in other settings. While the acute LPS-TLR4-mediated inflammatory response is a key component of initial infection, the rapid endotoxin-TLR4 signaling suppression and active EPS-mediated oxidative macrophage metabolism provides a complex mechanism of pathogenesis that favors long-term infection by P. aeruginosa. The contribution of host immunity to infection should be considered in the future development of next-generation antibacterial therapies.
Increasing evidence suggests that during infection, immunometabolites induce key post-translational modifications in proteins and metabolites involved in host immunity. Whereas succinate produces protein lysine succinylation in several metabolic pathways primed by LPS (Tannahill et al., 2013), itaconate induces Keap1 alkylation (Mills et al., 2018) and glutathione electrophilic stress (Bambouskova et al., 2018), major regulators of the Nrf2 antioxidant response. The progressive accumulation of these metabolites in the airway and chronic exposure of P. aeruginosa to them likely induces clinically relevant modifications in bacterial gene products associated with intractable infection. LPS trafficking and its display on the outer membrane rely on key cysteine-to-cysteine interactions within the LptD core (Ruiz et al., 2010), and the potential of itaconate to modify these thiol groups might favor membrane stress and activation of the algT anti-stress response, as we observed here. The explicit role of immunometabolites on the modification of bacterial macromolecules remains to be evaluated.
The acute pro-inflammatory response evoked by environmental and laboratory P. aeruginosa strains compromises host survival and limits the long-term metabolic evolution predicted to occur in these bacteria (Riquelme et al., 2017; Riquelme et al., 2019; Winstanley et al., 2016). The use of host-adapted clinical isolates that have been selected over years of exposure to the oxidative environment in the inflamed human airway provides a more nuanced model of infection to study how P. aeruginosa exploits immune components to cause chronic disease. The accumulation of itaconate-producing CD14+ myeloid “feeder” cells into the infected lungs provides a reliable energy source for host-adapted bacteria. The availability of mouse models that elicit the same population of myeloid feeder cells might provide a useful tool to identify future therapeutic strategies to treat aggressive P. aeruginosa infections. Indeed, while there has been substantial alarm raised by the spectrum of multiply antibiotic resistant strains of bacteria, particularly P. aeruginosa, by identifying the mechanisms by which these organisms adapt and exploit host immune defenses it is conceivable that future therapies could be developed that target these very adaptations to combat such infectious agents and the devastating diseases they cause.
Limitations of Study
We acknowledge that the complex metabolic matrix of the inflamed human airway might not be fully represented by mouse models of pneumonia. In situ concentrations of metabolites in the airway of mice and in healthy individuals or individuals with CF might differ due to different degrees of dehydration, mucus accumulation, myeloid cell activities and phagocyte numbers. These variables must be better characterized in future work. Further, the intricate metabolic program exhibited by P. aeruginosa isolates suggests that metabolites other than itaconate might also be fueling their in-host selection. Our study does not consider the interactions between itaconate and other pathways, providing only a partial mechanism of the chronic CF-associated infectious pathology. Our studies are also limited by determining in vitro but not in vivo the gene expression program of P. aeruginosa in the presence of itaconate. We extrapolate data using laboratory P. aeruginosa and mouse strains to findings observed in P. aeruginosa mutants derived from chronically infected patients. A more comprehensive study with a further diverse collection of P. aeruginosa isolates and human cells would support and improve the conclusions of this manuscript.
STAR METHODS
● RESOURCE AVAILABILITY
Lead contact
● Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Alice Prince (asp7@cumc.columbia.edu).
Materials availability
● P. aeruginosa clinical isolates and mutants analyzed in this work are available from the Lead Contact without restriction.
Data and Code Availability
● The published article includes all datasets generated or analyzed during this study.
● EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Samples
All human blood samples from healthy individuals and subjects with CF were obtained from adults in the CF program at Columbia University under the IRB AAAR1395 approved protocol. Male and female samples were collected in an approximate 50%−50% ratio. Under the clinical settings of this study, age and gender are not expected to influence the variables tested. Sputum samples from healthy adult individuals and subjects with CF were provided by Dr. Clemente Britto-Leon from Yale University. In total, 23 CF and 19 healthy control samples were studied. An informed consent was signed by all subjects providing samples.
Mice experiments –
All animal experiments were performed following institutional guidelines at Columbia University. Animals were housed and maintained at Columbia University Irving Medical Center under regular rodent light/dark cycles, 18–23°C, as well as fed with regular irradiated chow diet (Purina Cat 5053, distributed by Fisher). Mice health was routinely checked by an institutional Veterinary. Mice studies were approved by protocol IACUC AAAR9403 and IACUC AABE8600. 6–8-week-old (20–25grs) WT C57bl/6 mice were obtained from The Jackson Laboratories. Irg1−/− (Acod1−/−) were also obtained from The Jackson Laboratories and bred in our facilities, Columbia University Irving Medical Center. These mice were used between 6–8-week-old. WT and Irg1−/− mice are immunocompetent animals, and they received neither medical nor drug treatments previous to infection experiments. Each in vivo experiment was performed using 50% female and 50% male animals. Sex was not expected to influence the final results of experiments. During studies, animals were randomly assigned to cages. For experiments using bone-marrow derived macrophages (BMDMs), 50% of experiments were done using males and the other 50% females.
P. aeruginosa strains–
Laboratory P. aeruginosa strains used in this study were: WT PA14, WT PAO1, Δzwf PAO1, Δzwf complemented PAO1, ΔalgD PAO1, ΔlptD4213 PAO1. Clinical P. aeruginosa strains used were: 17 host-adapted P. aeruginosa strains recovered between 2012–2015 from sputum of a chronic-infected CF patient (Riquelme et al., 2019) (“CF Patient 3”); 3 “CF Patient 1” strains collected from a 5 year-old chronically infected CF patient; 3 “CF Patient 2” strains collected from a 10 year-old chronically CF infected patient; 10 ICU isolates were obtained from 10 acutely infected patients at Columbia University Irving Medical Center facilities. All of these strains were plated in LB agar and their phenotypes were characterized regarding SCV and mucoid morphology. For overnight and kinetic growth studies in minimal media with different carbon sources, overnight LB-cultured P. aeruginosa strains were extensively washed and subcultured in complete M9 minimal media supplemented or not with 62.5mM itaconate, glucose or succinate (pH 7.0). For overnight growth studies, culture was performed under agitation in 96-well plate, and 16h later growth was measured at OD600nm. For kinetic studies, strains were inoculated in 96-well plates and growth was studied at 37°C. When mentioned, LB-cultured PAO1, Δzwf and Δzwf complemented PAO1 were growth overnight in LB supplemented or not with increasing itaconate concentrations. Overnight OD600 was measured as growth. For mice infection studies, all strains were culture overnight in LB, then subcultured until exponential growth, extensively washed and used to infect animals.
● METHOD DETAILS
Human PBMCs flow cytometry –
Fresh blood samples obtained from randomly selected individuals were centrifuged in BD Vacutainer CPT tubes and the PBMCs-containing buffy coat was recovered. Buffy coat was washed several times in PBS and resuspended in FACS buffer. Cells were stained extracellularly for CD14, CD15, CD11b, CD45, CD19, CD3 and CD16. Cells were also stained for viability using DAPI live/dead kit. Once stained for 30min on ice, cells were washed and fixed/permeabilized using the Invitrogen fix/perm kit. Then, cells were stained for IRG1 with a rabbit monoclonal antibody and then treated with a secondary antibody anti rabbit coupled to AF647. Staining was for 30 min each, and then cells were washed extensively and fixed with 2% PFA-PBS. Cells were analyzed by flow cytometry LSRII machine and analyzed with FlowJo X.
Human Sputum flow cytometry –
Sputum samples from randomly selected patients were digested with DTT 0.1 % 10mM Sodium Acetate for 30min on ice with intermittent vortexing. Samples were passed through a sterile gaze and filtered through a 40umts cell strainer. Single cell solutions were centrifuged and washed 3 times with RPMI. Then, cells were stained extracellularly for CD14, CD15, CD11b, CD45, CD19, CD3 and CD16 and also stained for viability using DAPI live/dead kit. Once stained for 30min on ice, cells were washed and fixed/permeabilized using the Invitrogen fix/perm kit. The, cells were stained for IRG1 with a rabbit monoclonal antibody and then treated with a secondary antibody anti rabbit coupled to AF647. Staining was for 30min each, and then cells were washed extensively and fixed with 2% PFA-PBS. Cells were analyzed by flow cytometry and analyzed with FlowJo X.
Itaconate P. aeruginosa mutants generation –
The itaconate mutant strains in this study were created in the Pseudomonas aeruginosa PAO1 and host-adapted 605 strain backgrounds using homologous recombination. Constructs for these deletions were generated using the yeast gap repair cloning method and employed the allelic-replacement vector pMQ30 and the yeast strain InvSc1. For deletion of a specific gene, ~1 kb flanking regions plus ~100 bp of each end of the coding region were amplified and cloned into the plasmid. Resultant plasmids were propagated in E. coli UQ950, transformed into the mating strain E. coli BW29427, and transferred into PAO1 and 605 strain by conjugation. Recombinants were plated onto selective media containing 100 μg/mL gentamicin. Candidate mutant strains were identified by counter-selection on LB agar containing 10% sucrose without NaCl. Verification of the final clones was obtained via PCR and sequencing.
LPS Studies –
To visualize the O-antigen and core ladder of bacteria, LPS was first isolated using a hot phenol extraction from overnight cultures normalized to OD600 of 0.5 after 1:10 dilution. Samples were treated with DNase and RNase for 30 min at 37°C and Proteinase K at 59°C overnight. After hot phenol extraction with Trizol, the isolated LPS was run on a Tricine 10–20% gel. The gel was then stained using Pro-Q Emerald 300 Lipopolysaccharide Gel Stain Kit and imaged using UV light on a Protein Simple imager.
LPS detection in ΔlptD4213 PAO1 strains –
Both WT and ΔlptD4213 PAO1 were grown overnight in LB, washed extensively with PBS and stained with two anti-LPS carbohydrates antibodies for 30min. Then, cells were washed and stained with anti H+L-mouse IgM AF 598. Cells were analyzed by flow cytometry.
Biofilms studies with P. aeruginosa strains –
Isolates and laboratory controls were growth in LB overnight, extensively washed in minimal media and then inoculated in 96-well plates containing different carbon sources in M9-Salts supplemented. Plates were incubated under agitation at 37°C overnight. Next day, bacterial growth was measured at OD600nm, supernatant discarded and then stained for biofilm with crystal violet (CV). Biofilm production was analyzed at OD540nm.
P. aeruginosa membrane potential –
PAO1 was grown overnight in LB under agitation. Then, bacteria were extensively washed in M9 media and added to M9 supplemented either with glucose or glucose + itaconate (60mM each). After overnight incubation, bacteria were washed and incubated with baclight DiOC2(3) dye and analyzed by flow cytometry as indicated by the manufacturer. Membrane potential was calculated as the ratio between red and green DiOC2(3) fluorescence in the green positive population. Forward (bacterial size) and Side (surface granularity) scatters were measured as well.
Carbon source assimilation by P. aeruginosa –
Either PAO1, Δzwf and its complemented counterpart were grown until exponential phase, OD600nm measured and applied at same concentration to a 96-well plate array where each well contained a single carbon source (PM1 and PM2A plates). For preparation of bacteria, solutions and plates the instructions of the manufacturer were followed. Plates were incubated at 37°C for 48h-72h.
Mice infection with P. aeruginosa strains –
When indicated, either PAO1, ΔlptD4213 PAO1, Δzwf PAO1, Δzwf complemented PAO1, ΔalgD PAO1, Δict PAO1, control 605, Δict 605, or a mix of all 17CF isolates were used to infect either WT and/or irg1−/− (Acod1−/−) C57bl/6 mice. Mice were infected with 50uL PBS containing total 107 CFU or PBS alone (non-infected) (in the mix of 17CF strains, equal parts of 5.9 × 105 for each were used). 16h later, mice were sacrificed and BAL and lungs were collected. CFU amounts (LB agar plating), as well as immune cells (flow cytometry) and cytokines (ELISA) were quantified in BAL and in lungs. Flow cytometry; alveolar macrophages (CD45+CD11blow/−SiglecFhighCD11c+CD193−Ly6G−Ly6C−), neutrophils (CD45+CD11bhighSiglecFlow/−CD11c−MHCII−CD193−Ly6G+Ly6Clow/−) and monocytes (CD45+CD11bhighSiglecFlow/−CD11c−MHCII−CD193−Ly6G−Ly6Clow/high). Samples were analyzed with FlowJo X. Cell viability was determined by using live/dead DAPI staining. When indicated, BAL were analyzed by Mass-Spec metabolomics for succinate, glucose and itaconate abundance in all treatments.
In vivo Itaconate administration –
WT C57bl/6 mice were intranasally infected with 107 CFU of the collection of 17 CF P. aeruginosa isolates (equal parts of 5.9 × 105 for each strain). 24h later, mice were anesthetized and treated intranasally with 50uL of 60mM itaconate, pH 7. Mice were sacrificed 24h later and CFUs were quantified in lungs and BALs.
Seahorse experiments –
Mouse BMDMs were infected for 3h at multiplicity of infection (MOI)=10 with either PAO1, ΔalgD or ΔlptD4213 strains. When indicated, cells were treated only with PBS, 50ug/ml LPS, 50ug/ml of soluble alginate, 50ug/ml of polyethylene glycol (PEG, 200, 8000, 20000 MW) or 50ug/ml N-acetyl Glucosamine. During the infection cells were analyzed in a Seahorse machine using commercially obtained plates as recommended by the manufacturer to evaluate glycolysis (ECAR) and the oxygen consumption rate (OCR).
Mitochondrial studies in macrophages –
Mouse BMDMs were infected at 37°C for 2h at multiplicity of infection (MOI)=10 with either PAO1, ΔalgD or ΔlptD4213 strains. When indicated, cells were treated only with PBS, 50ug/ml LPS or 50ug/ml of soluble alginate. Then, cells were extensively washed and stained for mitochondrial membrane potential with Dilc1(5) and mitochondrial ROS (O2*−) with Mitosox dyes. Cells were extensively washed, resuspended in PBS and analyzed in a FACs CantoII flow cytometry machine. Data was analyzed with Flowjo X.
Whole genome comparison for metabolic genes –
Three genes of interest in PAO1 were studied: ict (PA0878), ich (PA0882) and ccl (PA0883). Both gene and protein coverage and identity for each of these metabolic regulators were searched and compared using Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi) against the whole database of genomes published and available online. Taxid used for each pathogen was: P. aeruginosa (taxid 136841); H. influenza (taxid 727); B. cepacia complex (taxid 87882); S. maltophilia (taxid 995085); A. xylosoxidans (taxid 85698); A. baumanni (taxid 470); Mycobacterium (taxid 85007); K. pneumoniae (taxid 573); S. pneumoniae (taxid 1313); S. aureus (taxid 1280). Coverage and identity obtained were graphed and analyzed.
Airway metabolomics –
BAL from either non-infected or 16h-infected mice were collected with 3ml of sterile PBS. Samples were immediately placed on ice. Then, samples were diluted with 100% methanol in a 1:1 proportion, mixed and stored at −80C for future metabolomics analysis. Just prior to mass spectrometry, samples were thawed and dried under a stream of N2 and were resuspended in HPLC-grade water at a 4:1 dilution (relative to the original BAL volume). High-resolution mass spectrometry data were acquired on a Thermo Fisher Exactive Mass spectrometer in negative mode using 25 min reverse phase gradients and ion-pairing chromatography. Metabolites were identified using the known chromatographic retention times of standards, and metabolite signals were quantified using MAVEN. Metabolite signal intensities were used to quantify difference between treatments in BAL and respect to PBS-treated animals.
qRT-PCR for genes in P. aeruginosa –
PAO1 strains were grown ON in LB and then subcultured in LB, or M9 supplemented with succinate, glucose or itaconate (50 mM) until exponential phase. Total RNA was extracted using either the RNeasy Mini Kit or the E.Z.N.A. Total RNA Kit 1. The RNA was then treated with DNase (DNA-free kit). The RNA concentration was measured using a NanoDrop One. cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and qPCR was performed with a StepOnePlus Real-Time PCR System (Applied Biosystems) using POWER SYBR Green PCR Master Mix (Applied Biosystems). Sequences of the primers used for qRT-PCRs are listed in Star Methods Key Resource Table. Relative gene expression was calculated by the 2−ΔΔCT method. The rpsL gene was used as a reference housekeeping gene, and the P. aeruginosa PAO1 strain grown in LB was used as a calibrator.
● KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD45 AF700 | Biolegend | Cat 103128, RRID:AB_493715 |
| Anti-mouse CD11c Bv605 | Biolegend | Cat 117333, RRID:AB_11204262 |
| Anti-mouse SiglecF | Biolegend | Cat 562680, RRID:AB_2687570 |
| Anti-mouse CD11b AF594 | Biolegend | Cat 101254, RRID:AB_2563231 |
| Anti-mouse MHC II APC/Cy7 | Biolegend | Cat 107628, RRID:AB_2069377 |
| Anti-mouse Ly6C Bv421 | Biolegend | Cat 128032, RRID:AB_2562178 |
| Anti-mouse Ly6G PerCP/Cy5.5 | Biolegend | Cat 127616, RRID:AB_1877271 |
| Anti-mouse CD193 FITC | Biolegend | Cat 144509, RRID:AB_2561608 |
| Anti-mouse IRG1 | Abcam | ab22241 |
| Anti-mouse IRG1 | Abcam | ab222411 |
| Donkey anti-rabbit AF647 IgG H+L secondary antibody | Thermofisher | Cat A31573, RRID:AB_2536183 |
| Anti-human CD45 APC/Fire 750 | Biolegend | Cat 304061, RRID:AB_2629700 |
| Anti-human CD19 FITC | Biolegend | Cat 392508, RRID:AB_2750099 |
| Anti-human CD15 BV421 | Biolegend | Cat 323040, RRID:AB_2566520 |
| Anti-human CD3 PercP-Cy5.5 | Biolegend | Cat 317336, RRID:AB_2561628 |
| Anti-human CD14 AF700 | Biolegend | Cat 301822, RRID:AB_493747 |
| Anti-human CD16 PE/Cy7 | Biolegend | Cat 302016, RRID:AB_314216 |
| Anti-human CD11b BV605 | Biolegend | Cat 301332, RRID:AB_2562021 |
| Anti LPS O5 antigen | MediMabs | MM-76605-100 |
| Anti LPS A-band | MediMabs | MM-0264 |
| Biological Samples | ||
| Blood PBMCs | This Study | N/A |
| CF sputum samples | This Study | N/A |
| Chemicals, peptides and Recombinant Proteins | ||
| Itaconic acid | Sigma Aldrich | I29204 |
| Succinic acid | Sigma Aldrich | 14160 |
| α-D-Glucose | Sigma Aldrich | G8270 |
| Sodium alginate | Sigma Aldrich | W201502 |
| Polyethylene glycol 200 | Sigma Aldrich | 8.07483 |
| Polyethylene glycol 8000 | Sigma Aldrich | 1546605 |
| Polyethylene glycol 20000 | Sigma Aldrich | 8.18897 |
| N-acetyl glucosamine | Sigma Aldrich | A8625 |
| Trizol™ Reagent | Thermofisher | 15596026 |
| Novex™ 10–20% Tricine Protein Gels | Thermofisher | EC6625BOX |
| Pro-Q™ Emerald 300 Lipopolysaccharide Gel Stain Kit | Thermofisher | P20495 |
| Live/Dead DAPi dye | Thermofisher | Cat L34962 |
| MitoProbe DilC1 (5) | Thermofisher | Cat M34151 |
| Mitosox | Thermofisher | Cat M36008 |
| BacLight™ Bacterial Membrane Potential Kit | Thermofisher | B34950 |
| Critical Commercial Assays | ||
| Mouse Cytokine Array / Chemokine Array 31-Plex | Evetechnologies | MD31 |
| Seahorse XFe24 FluxPaks | Agilent Technologies | 102340-100 |
| RNeasy Mini Kit | Qiagen | Cat 74104 |
| E.Z.N.A.® Total RNA Kit I | Omega Biotek | R6834-01 |
| DNA free removal kit | Thermofisher | AM1906 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems | 4368813 |
| Power SYBR™ Green PCR Master Mix | Applied Biosystems | 4368577 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | Jackson Laboratories | JAX: 000664 |
| Mouse: C57BL/6J/N | Jackson Laboratories | JAX: 005304 |
| Mouse: C57BL/6 Irg1−/− (Acod1−/−) | Jackson Laboratories | JAX: 029340 |
| Bacterial and Virus Strains | ||
| P. aeruginosa WT PAO1 | Our laboratory | N/A |
| P. aeruginosa Δict PAO1 | This study | N/A |
| P. aeruginosa isolate 605 control | This study | N/A |
| P. aeruginosa isolate Δict 605 | This study | N/A |
| P. aeruginosa WT PAO1 | Provided by Dr. J. Goldberg; Limoli et al, 2017 | N/A |
| P. aeruginosa ΔalgD PAO1 | Provided by Dr. J. Goldberg; Limoli et al, 2017 | N/A |
| P. aeruginosa WT PAO1 | Provided by Dr. CJ Balibar; Balibar et al, 2015 | N/A |
| P. aeruginosa ΔlptD4213 PAO1 | Provided by Dr. CJ Balibar; Balibar et al, 2015 | N/A |
| P. aeruginosa Δzwf PAO1 | Provided by Dr. D.E. Ohman; Silu-Suh et al, 2005 | N/A |
| P. aeruginosa Δzwf PAO1 Complemented (CarbR) | Provided by Dr. D.E. Ohman; Silu-Suh et al, 2005 | N/A |
| CF subject 3 CF P. aeruginosaa isolates | Riquelme et al, 2019 | N/A |
| CF subject 1 P. aeruginosa isolates | Provided by Dr. Barbara Kahl | N/A |
| CF Subject 2 CF P. aeruginosa isolates | Provided by Dr. Barbara Kahl | N/A |
| ICU P. aeruginosa isolates | Provided by Dr. A.C. Uhlemman | N/A |
| Oligonucleotides | ||
| zwf - qzwf-F: GAAGGTATCTCCCTGCAAGT | Riquelme et al, 2019 | N/A |
| zwf - qzwf-R: GGTAGGTCTCGGAAAAACTC | Riquelme et al, 2019 | N/A |
| ict - qict-F: CCTACAGCAGCATCCTTT | This study | N/A |
| ict - qict-R: CTGGTAGGCGTAGTACATC | This study | N/A |
| ich - qich-F: AGCACGACATCGTCTACC | This study | N/A |
| ich - qich-R: ACATAGGGCCAGTCGTAGT | This study | N/A |
| ccl - qccl-F: AACAGATTCTCGGTCACG | This study | N/A |
| ccl - qccl-R: GATGGATGCACAGCAAC | This study | N/A |
| Recombinant DNA | ||
| Yeast strain InvSc1 | Thermofisher | C81000 |
| UQ950 E. coli DH5 λpir train for cloning | Provided by Dr. Lars Dietrich | N/A |
| BW29427 E. coli donor strain for conjugation | Provided by Dr. Lars Dietrich | N/A |
| pMQ30 7.5Kb mobilizable vector oriT, sacB, GmR | Provided by Dr. Lars Dietrich | N/A |
| Software and Algorithms | ||
| GraphPad Prism 8.3.1 | GraphPad Software | www.graphpad.com |
| FlowJo X Flow cytometry | FlowJo | https://www.flowjo.com |
| FIJI | FIJI | http://imagej.net |
| Deposited data | ||
| P. aeruginosa RNAseq from mice tissue | Turner et al, 2014 (doi: 10.1371/journal.pgen.1004518) | Accession Number SRP033652 |
| P. aeruginosa RNAseq from humans and mice tissue | Conforth et al, 2018 (doi: 10.1073/pnas.1717525115) | Accession Number SRP135669 |
| Other | ||
| RPMI 1640 1x with glutamine | Corning | 10-040-CV |
| Antibiotic Penicillin-Streptomycin | Corning | 30-002-CI |
| Fetal Bovine Serum | Gibco | 26140-079 |
| Biolog plates PM1 and PM2A | Biolog | 12111 |
| Irradiated Purina regular chow mice diet | Purina | 5053 |
● QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis –
We modelled the amounts of independent experiments required to reach significance between groups by using the computer program JMP. Also, we based this simulation on experimental design, preliminary data and past experience. These analyses were done assuming a 20% standard deviation, and equivalent variances within groups. Significance < 0.05 with power 0.8 was used. Experiments in this study were not performed in a blinded fashion. Exclusion criteria: Analysis of blood and sputum did not include children samples, as mostly adults experience chronic P. aeruginosa infection. Out of the total number of human samples analyzed, 100% corresponded to adults. All analyses and graphs were performed using the GraphPad Prism 7a and 8 software. Data in graphs are shown as average +/− SEM and data were assumed to follow a normal distribution. For comparison between average values for more than 2 groups, we performed One-Way ANOVA with a multiple posteriori comparison. When studying two or more group along time, data was analyzed using Two-Way ANOVA with a multiple posteriori comparison. Differences between two groups in samples’ average values were analyzed using analysis of variance (parametric) or Student’s t-test for normally distributed data or Mann-Whitney or Kruskal-Wallis test otherwise. Differences were considered significant when P value (two-sides) was under 0.05 (P < 0.05). Significances P values and amount of independent experiments and replicates are indicated in each Figure Legend.
● ADDITIONAL RESOURCES
This study does not have additional resources.
Supplementary Material
Highlights.
P. aeruginosa-infected macrophages produce itaconate
Itaconate generates membrane stress in P. aeruginosa
Itaconate leads to decreased LPS, but increased EPS, to promote biofilm formation
The EPS-itaconate axis thwarts immune clearance enabling chronic infection
Context and Significance.
Intractable respiratory infections remain a major public health threat. P. aeruginosa pneumonia, which is associated with both acute and especially chronic infections, is specially challenging and one that can acquire resistance to virtually all available antibiotics. Here, Sebastián Riquelme and colleagues describe how itaconate, a major macrophage metabolite, selects for a reservoir of highly adapted P. aeruginosa mutants capable of producing biofilm in the lung. By inducing key metabolo-structural changes on the surface of the bacteria, itaconate modifies the immunostimulatory properties of P. aeruginosa, resulting in host-adapted variants that evoke an itaconate-driven immune response permissive, and even supportive, of chronic infection. These findings indicate that host immunometabolism must be considered in future translational therapies addressing multidrug resistant pneumonia.
Acknowledgments:
We thank Drs. Ian Lewis and Ryan Groves for their support in metabolomics studies; Dr. Joanna Goldberg for providing the algD PAO1 mutant and her helpful discussions; Dr. Carl J. Balibar for providing us with the ΔlptD4213 PAO1 mutant strains; Dr. Dennis E. Ohman for sharing with us Δzwf and Δzwf Comp PAO1 strains; Dr. William Sieling for human blood sampling.
Funding: A.P. is supported by NIH 1R35HL135800 and by Integrating Special Populations (ISP) Resource, CTSA, Columbia University (GG011557-26); SR by a CFF Postdoctoral fellowship RIQUEL 17F0/PG008837; CL by S. B. Postdoct Contract CD15/00125 and M-AES mobility grant MV16/00053; This publication by the NCATS-NIH, UL1TR001873 and by FIS PI16/01381 from ISCIII and performed in the CCTI Flow Cytometry Core at CUMC, NIH (S10RR027050).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information: This study counts with 7 Supplemental Figures that can be found in the online version of this manuscript.
Competing Interest: Authors declare that no conflicts of interest exist.
References
- Arai H (2011). Regulation and Function of Versatile Aerobic and Anaerobic Respiratory Metabolism in Pseudomonas aeruginosa. Front Microbiol 2, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balibar CJ, and Grabowicz M (2016). Mutant Alleles of lptD Increase the Permeability of Pseudomonas aeruginosa and Define Determinants of Intrinsic Resistance to Antibiotics. Antimicrob Agents Chemother 60, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, Korenfeld D, Mathyer ME, Kim H, Huang LH, et al. (2018). Electrophilic properties of itaconate and derivatives regulate the IkappaBzeta-ATF3 inflammatory axis. Nature 556, 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botos I, Majdalani N, Mayclin SJ, McCarthy JG, Lundquist K, Wojtowicz D, Barnard TJ, Gumbart JC, and Buchanan SK (2016). Structural and Functional Characterization of the LPS Transporter LptDE from Gram-Negative Pathogens. Structure 24, 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragonzi A, Worlitzsch D, Pier GB, Timpert P, Ulrich M, Hentzer M, Andersen JB, Givskov M, Conese M, and Doring G (2005). Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J Infect Dis 192, 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DN, Hager PW, and Phibbs PV Jr. (1996). Catabolite repression control in the Pseudomonads. Res Microbiol 147, 551–561. [DOI] [PubMed] [Google Scholar]
- Cornforth DM, Dees JL, Ibberson CB, Huse HK, Mathiesen IH, Kirketerp-Moller K, Wolcott RD, Rumbaugh KP, Bjarnsholt T, and Whiteley M (2018). Pseudomonas aeruginosa transcriptome during human infection. Proc Natl Acad Sci U S A 115, E5125–E5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Schurr MJ, and Yu H (1995). Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol. 3, 351–356. [DOI] [PubMed] [Google Scholar]
- Dhar S, Kumari H, Balasubramanian D, and Mathee K (2018). Cell-wall recycling and synthesis in Escherichia coli and Pseudomonas aeruginosa - their role in the development of resistance. J Med Microbiol 67, 1–21. [DOI] [PubMed] [Google Scholar]
- Dominguez-Andres J, Novakovic B, Li Y, Scicluna BP, Gresnigt MS, Arts RJW, Oosting M, Moorlag S, Groh LA, Zwaag J, et al. (2019). The Itaconate Pathway Is a Central Regulatory Node Linking Innate Immune Tolerance and Trained Immunity. Cell Metab 29, 211–220 e215. [DOI] [PubMed] [Google Scholar]
- Driffield K, Miller K, Bostock JM, O’Neill AJ, and Chopra I (2008). Increased mutability of Pseudomonas aeruginosa in biofilms. J Antimicrob Chemother 61, 1053–1056. [DOI] [PubMed] [Google Scholar]
- Elborn JS (2016). Cystic fibrosis. Lancet 388, 2519–2531. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Pier GB, Coyne MJ Jr., and Goldberg JB (1994). The rfb locus from Pseudomonas aeruginosa strain PA103 promotes the expression of O antigen by both LPS-rough and LPS-smooth isolates from cystic fibrosis patients. Mol Microbiol 13, 427–434. [DOI] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, and Kagan JC (2018). The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48, 35–44 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E, Mear JB, Faure K, Normand S, Couturier-Maillard A, Grandjean T, Balloy V, Ryffel B, Dessein R, Chignard M, et al. (2014). Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am J Respir Crit Care Med 189, 799–811. [DOI] [PubMed] [Google Scholar]
- Fernandez-Barat L, Ferrer M, De Rosa F, Gabarrus A, Esperatti M, Terraneo S, Rinaudo M, Li Bassi G, and Torres A (2017). Intensive care unit-acquired pneumonia due to Pseudomonas aeruginosa with and without multidrug resistance. J Infect 74, 142–152. [DOI] [PubMed] [Google Scholar]
- Franklin MJ, Nivens DE, Weadge JT, and Howell PL (2011). Biosynthesis of the Pseudomonas aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front. Microbiol 2, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nunez M, Marti S, Puig C, Perez-Brocal V, Millares L, Santos S, Ardanuy C, Moya A, Linares J, and Monso E (2017). Bronchial microbiome, PA biofilm-forming capacity and exacerbation in severe COPD patients colonized by P. aeruginosa. Future Microbiol 12, 379–392. [DOI] [PubMed] [Google Scholar]
- Goldberg JB, Coyne MJ Jr., Neely AN, and Holder IA (1995). Avirulence of a Pseudomonas aeruginosa algC mutant in a burned-mouse model of infection. Infect Immun 63, 4166–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorke B, and Stulke J (2008). Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6, 613–624. [DOI] [PubMed] [Google Scholar]
- Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, and Parsek MR (2001). Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183, 5395–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, et al. (2010). Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115, e10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabir MS, Hopkins L, Ritchie ND, Ullah I, Bayes HK, Li D, Tourlomousis P, Lupton A, Puleston D, Simon AK, et al. (2015). Mitochondrial damage contributes to Pseudomonas aeruginosa activation of the inflammasome and is downregulated by autophagy. Autophagy 11, 166–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine L, Wiscombe S, Reynolds G, McDonald D, Fuller A, Green K, Filby A, Forrest I, Ruchaud-Sparagano MH, Scott J, et al. (2019). Lipopolysaccharide inhalation recruits monocytes and dendritic cell subsets to the alveolar airspace. Nat Commun 10, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez P, Jeannot K, Plesiat P, and Llanes C (2017). Toxic Electrophiles Induce Expression of the Multidrug Efflux Pump MexEF-OprN in Pseudomonas aeruginosa through a Novel Transcriptional Regulator, CmrA. Antimicrob Agents Chemother 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jeon CO, and Park W (2008). Dual regulation of zwf-1 by both 2-keto-3-deoxy-6-phosphogluconate and oxidative stress in Pseudomonas putida. Microbiology 154, 3905–3916. [DOI] [PubMed] [Google Scholar]
- Kubes JN, and Fridkin SK (2019). Factors affecting the geographic variability of antibiotic-resistant healthcare-associated infections in the United States using the CDC Antibiotic Resistance Patient Safety Atlas. Infect Control Hosp Epidemiol 40, 597–599. [DOI] [PubMed] [Google Scholar]
- Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, et al. (2016). Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab 24, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Xue M, Wzorek JS, Wu T, Grabowicz M, Gronenberg LS, Sutterlin HA, Davis RM, Ruiz N, Silhavy TJ, et al. (2016). Characterization of a stalled complex on the beta-barrel assembly machine. Proc Natl Acad Sci U S A 113, 8717–8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S, Guo MS, Chaba R, Gross CA, and Sauer RT (2013). Dual molecular signals mediate the bacterial response to outer-membrane stress. Science 340, 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Hager PW, Howell ML, Phibbs PV, and Hassett DJ (1998). Cloning and characterization of the Pseudomonas aeruginosa zwf gene encoding glucose-6-phosphate dehydrogenase, an enzyme important in resistance to methyl viologen (paraquat). J. Bacteriol 180, 1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki S, Maerk M, Valla S, and Ertesvag H (2015). Mutational Analyses of Glucose Dehydrogenase and Glucose-6-Phosphate Dehydrogenase Genes in Pseudomonas fluorescens Reveal Their Effects on Growth and Alginate Production. Appl Environ Microbiol 81, 3349–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice NM, Bedi B, and Sadikot RT (2018). Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am J Respir Cell Mol Biol 58, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Dabritz JHM, Gottlieb E, Latorre I, et al. (2016). Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 167, 457–470 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EL, Kelly B, and O’Neill LAJ (2017). Mitochondria are the powerhouses of immunity. Nat Immunol 18, 488–498. [DOI] [PubMed] [Google Scholar]
- Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa ASH, Higgins M, Hams E, et al. (2018). Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan PB, Marcovecchio P, Hamers AAJ, and Hedrick CC (2019). Nonclassical Monocytes in Health and Disease. Annu Rev Immunol 37, 439–456. [DOI] [PubMed] [Google Scholar]
- Naujoks J, Tabeling C, Dill BD, Hoffmann C, Brown AS, Kunze M, Kempa S, Peter A, Mollenkopf HJ, Dorhoi A, et al. (2016). IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog 12, e1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, Gibbs KA, Hager PW, Phibbs PV Jr., and Kolter R (2000). The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol 182, 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Qin K, Zhang Y, Jia W, Chen Y, Cheng B, Peng L, Chen N, Liu Y, Zhou W, et al. (2019). S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat Chem Biol 15, 983–991. [DOI] [PubMed] [Google Scholar]
- Riquelme SA, Hopkins BD, Wolfe AL, DiMango E, Kitur K, Parsons R, and Prince A (2017). Cystic Fibrosis Transmembrane Conductance Regulator Attaches Tumor Suppressor PTEN to the Membrane and Promotes Anti Pseudomonas aeruginosa Immunity. Immunity 47, 1169–1181 e1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme SA, Lozano C, Moustafa AM, Liimatta K, Tomlinson KL, Britto C, Khanal S, Gill SK, Narechania A, Azcona-Gutierrez JM, et al. (2019). CFTR-PTEN-dependent mitochondrial metabolic dysfunction promotes Pseudomonas aeruginosa airway infection. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse JW, and McFadden BA (1974). Inhibition of isocitrate lyase from Pseudomonas indigofera by itaconate. Arch Biochem Biophys 163, 79–86. [DOI] [PubMed] [Google Scholar]
- Rojo F (2010). Carbon catabolite repression in Pseudomonas : optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev 34, 658–684. [DOI] [PubMed] [Google Scholar]
- Ruiz N, Chng SS, Hiniker A, Kahne D, and Silhavy TJ (2010). Nonconsecutive disulfide bond formation in an essential integral outer membrane protein. Proc Natl Acad Sci U S A 107, 12245–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikaran J, Ziemski M, Zadora PK, Fleig A, and Berg IA (2014). Bacterial itaconate degradation promotes pathogenicity. Nat Chem Biol 10, 371–377. [DOI] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. (2013). Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PK, Yeung AT, and Hancock RE (2014). Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies. J Biotechnol 191, 121–130. [DOI] [PubMed] [Google Scholar]
- Turner KH, Everett J, Trivedi U, Rumbaugh KP, and Whiteley M (2014). Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet 10, e1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fedorov AA, Fedorov EV, Hunt DM, Rodgers A, Douglas HL, Garza-Garcia A, Bonanno JB, Almo SC, and de Carvalho LPS (2019). An essential bifunctional enzyme in Mycobacterium tuberculosis for itaconate dissimilation and leucine catabolism. Proc Natl Acad Sci U S A 116, 15907–15913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C, O’Brien S, and Brockhurst MA (2016). Pseudomonas aeruginosa Evolutionary Adaptation and Diversification in Cystic Fibrosis Chronic Lung Infections. Trends. Microbiol 24, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsaroj L, Saninjuk K, Romsang A, Duang-Nkern J, Trinachartvanit W, Vattanaviboon P, and Mongkolsuk S (2018). Pseudomonas aeruginosa glutathione biosynthesis genes play multiple roles in stress protection, bacterial virulence and biofilm formation. PLoS One 13, e0205815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World-Health-Organization (2017). Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. (Geneva: ). [PubMed] [Google Scholar]
- Wunderink RG, and Waterer G (2017). Advances in the causes and management of community acquired pneumonia in adults. BMJ 358, j2471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
● The published article includes all datasets generated or analyzed during this study.


