Abstract
The nervous and immune systems use bi-directional communication to control host responses against microbial pathogens. Recent studies at the interface of the two systems have highlighted important roles of the nervous system in the regulation of both microbicidal pathways and pathogen avoidance behaviors. Studies on the neural circuits in the simple model host Caenorhabditis elegans have significantly improved our understanding of the roles of conserved neural mechanisms in controlling innate immunity. Moreover, behavioral studies have advanced our understanding of how the nervous system may sense potential pathogens and consequently elicit pathogen avoidance, reducing the risk of infection. In this review, we discuss the neural circuits that regulate both behavioral immunity and molecular immunity in C. elegans.
Introduction
Metazoans have developed multiple strategies to deal with pathogenic microbes, including pathogen avoidance, resistance, and tolerance [1]. Different animals, ranging from simple nematodes to chimpanzees and humans, engage in many behaviors that reduce their exposure to pathogens [2–4]. These avoidance behaviors that protect against pathogen infections are referred to as the behavioral immune system [5]. In addition, microbial sensing mechanisms activate molecular immune pathways that provide resistance to pathogens by reducing pathogen burden and clearing the infection. Increasing evidence also indicates that those mechanisms involved in the initial activation of defense pathways also regulate the immune system. Thus, immune regulatory mechanisms play a critical role in maintaining immune homeostasis because disproportionate activation of immune pathways could be detrimental to the host.
The nervous system maintains control of homeostasis through bi-directional communication with peripheral physiological systems. Recent research at the interface of the nervous system and the immune system identified neural circuits that are triggered by and control immune pathways [6–10]. While the understanding of neuro-immune communications holds great therapeutic potential [11], the complex nervous and immune systems of higher organisms have limited our understanding of these connections. Studies in the model nematode Caenorhabditis elegans have advanced our understanding of neuro-immune connections. In this review, we describe these recent advances in C. elegans.
Neural circuits involved in behavioral immunity
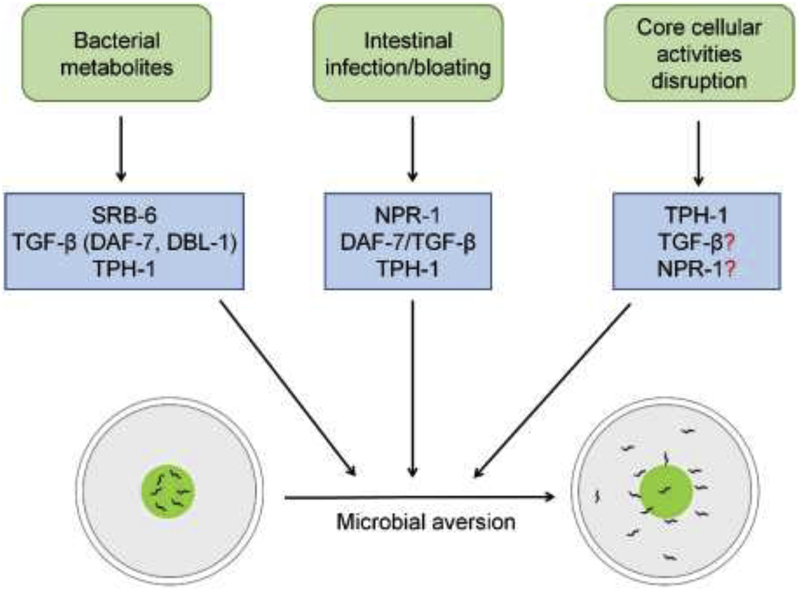
Physical avoidance of pathogens is a critical defense strategy used by hosts to reduce pathogen infections [2,4]. In mammals, olfactory chemosensory neurons and nociceptor sensory neurons detect various bacterial products, such as toxins, quorum-sensing molecules, formyl peptides, and lipopolysaccharides, through distinct molecular mechanisms that lead to rapid avoidance behaviors [6,12–16]. Similarly, in the fruit fly Drosophila melanogaster, olfactory and gustatory neurons have been reported to detect geosmin (the smell of mold), phenol, and lipopolysaccharides via distinct molecular mechanisms, allowing the organism to avoid food contaminated with bacteria [17,18]. The simple and well-described nervous system of C. elegans has made it an important model for understanding how animals learn to avoid pathogens. Behavioral pathogen avoidance is a crucial defense response employed by C. elegans that significantly improves the survival of animals [19–22]. The animals are capable of sensing and avoiding pathogenic microbes by multiple mechanisms (Figure 1). C. elegans shows innate as well as learned avoidance of pathogenic microbes. In innate avoidance behavior, animals rapidly avoid a pathogen without any prior exposure. C. elegans shows innate aversion to several species of Streptomyces [23], which produce various nematicides. Streptomyces secreted dodecanoic acid is sensed by a C. elegans G-protein-coupled receptor (GPCR), SRB-6, which is expressed in five types of amphid and phasmid chemosensory neurons, leading to a rapid avoidance behavior that happens within seconds. This rapid detection of and escape from Streptomyces species, that secrete powerful toxins, is potentially important for the survival of nematodes [23]. In contrast to innate avoidance, in the learned avoidance behavior, animals are initially attracted towards a given pathogen and through a learning process eventually develop an aversive behavior [24].
Figure 1.
Different mechanisms of elicitation of microbial aversion behavior in C. elegans
Sensation of bacterial metabolites, intestinal infection leading to bloating, and disruption of core cellular components activate various neuroendocrine signals required for microbial aversion.
The neurotransmitter serotonin is known to play an important role in the associative learning of pathogenic bacteria in C. elegans [24]. Exposure to pathogenic bacteria increases serotonin in ADF chemosensory neurons by transcriptional and post-transcriptional mechanisms. The tph-1 gene encodes the tryptophan hydroxylase that is required for a rate-limiting step in serotonin biosynthesis. C. elegans tph-1 mutants were found to be defective in avoidance of pathogenic bacteria [25]. In addition, tph-1 mutants were also found to be defective in avoidance of non-pathogenic bacteria under various forms of animal physiological perturbations [26,27]. However, some studies showed that TPH-1 might not be required for the avoidance of pathogens [28,29]. This suggests that the role of TPH-1 in eliciting avoidance behavior might be context-dependent. Some studies also suggest that the lack of pathogen avoidance in tph-1 mutant animals could be because of background mutations [28].
Transforming growth factor-β (TGF-β) superfamily ligands are secreted molecules that play critical roles in cell-to-cell communication, cell growth, differentiation, and death [30]. Two of the five TGF-β ligands in C. elegans, DBL-1 and DAF-7, signal through a canonical receptor-Smad signaling pathway [31]. Whereas DAF-7 regulates diverse functions such as the dauer developmental decision, foraging and aggregation behaviors, quiescence, metabolism, and longevity, DBL-1 controls body morphology, innate immunity, and reproductive aging [31]. In addition to the regulation of the aforementioned functions, both of these TGF-β ligands are also known to regulate pathogen avoidance behavior. Expression of DBL-1 and its receptor SMA-6 in the AVA command interneurons and hypodermis, respectively, is required for the avoidance behavior [32]. Similarly, expression of DAF-7 in the ASI and ASJ chemosensory neurons is important for the avoidance behavior [33]. Recently, it was shown that DAF-7/TGF-β is also required for transgenerational memory of pathogen avoidance [34]. Interestingly, expression of DAF-7 in the ASI neurons, but not in ASJ neurons, was found to be important for this transgenerational memory, suggesting that the circuits for pathogen avoidance might be different from those involved in transgenerational memory.
The GPCR NPR-1 is related to mammalian neuropeptide Y receptors. Along with its ligands, the FMRF-like peptides FLP-18 and FLP-21, NPR-1 controls several behavioral and physiological functions such as behavioral quiescence and social feeding [35,36]. NPR-1 is expressed in the body fluid neurons AQR, PQR, and URX and regulates innate immunity non-cell-autonomously [22,37]. In addition to the above functions, NPR-1 and its ligands, FLP-18 and FLP-21, also regulate the pathogen avoidance behavior [19,21,22,29]. The expression of NPR-1 in the neurons AQR, PQR, and URX is required for the avoidance behavior [38]. Various stimuli, such as mechanosensation via OLL neuron pair [39] or intestinal bloating caused by pathogen infection [29], modulate NPR-1 activity and thus, avoidance behavior.
The NPR-1 and DAF-7/TGF-β pathways are required for aerotaxis behavior and regulate hyperoxia avoidance in C. elegans. Inhibition of the NPR-1 and DAF-7/TGF-β pathways elicit avoidance of high oxygen (O2), while higher activity of these pathways induces avoidance of low O2 [33,36,40,41]. Recent studies have shown that C. elegans pathogen avoidance could also be mediated by avoidance of nitric oxide (NO) [42] and carbon dioxide (CO2) [43]. The chemosensory neurons ASJ and BAG were found to be required for avoidance against NO and CO2, respectively. Interestingly, both of these neurons are also important for sensing O2 and, therefore, it is possible that animals integrate information about different gases to sense their environment. Because bacterial metabolism influences the local concentration of gases, these studies highlight that C. elegans microbial avoidance behavior is influenced by the animal’s ability to respond to different levels of gases. Consistent with this idea, animals lacking ASI neurons, that are involved in sensing O2, were found to have dampened pathogen avoidance behavior [44].
It was recently shown that bloating of the intestine caused by pathogen infection induces microbial avoidance behavior [27,29,45]. Intestinal bloating also induced multiple neuroendocrine pathways that are known to regulate avoidance behavior, including the serotonin biosynthesis pathway, the DAF-7/TGF-β pathway, and the NPR-1/GPCR pathway [29]. These studies suggest that modulation of neuroendocrine pathways that lead to avoidance behavior might be driven by the sensation of physiological changes induced by pathogen infection, such as intestinal bloating, rather than direct chemosensation of pathogenic microbes. Therefore, physiological changes induced by pathogen infection might play an important role in the learning of pathogen avoidance. Indeed, intestinal expression of the neuropeptide INS-11, that is induced by Pseudomonas aeruginosa infection, modulates the pathogen avoidance behavior [28].
Neural regulation of molecular immunity
While the activities of immune pathways reduce pathogen burden and are important to resist infections, uncontrolled activation of immune pathways could be detrimental to the host. Therefore, activation of the immune system must be controlled to minimize the changes in organismal homeostasis that occur during the host’s response to pathogen attack. The nervous system of C. elegans seems to play an important role during the host response to infections as it can both activate and suppress the immune system through multiple circuits (Figure 2).
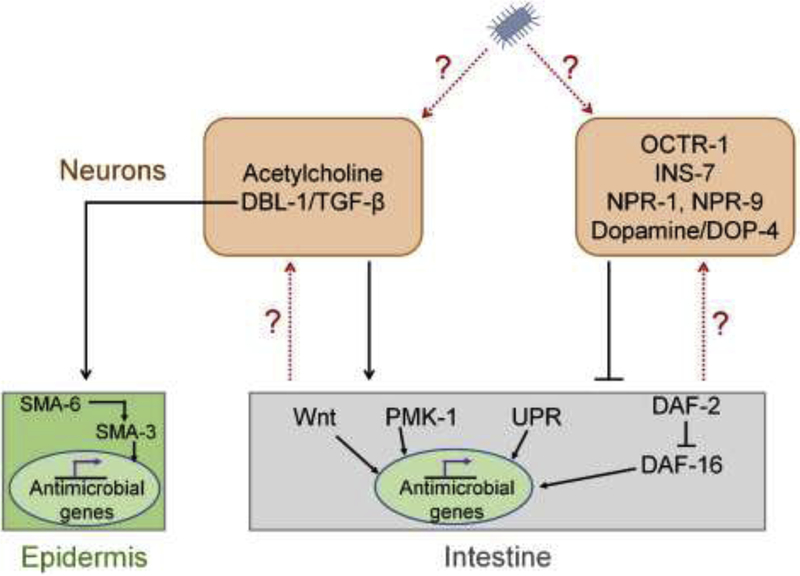
Figure 2.
Neural regulation of immunity in C. elegans
Different neural circuits either activate or inhibit immune responses in non-nervous tissues. Signals upstream of the neural circuits activating immune responses remain poorly defined.
Neural activation of immune pathways
Neural circuits that activate immune pathways in different tissues have been identified in C. elegans and are involved in defense responses against different pathogenic microbes. Infections of the fungus Drechmeria coniospora lead to increased production of antimicrobial peptides of the Caenacin family in the epidermis of C. elegans. The expression of caenacin genes is controlled by the neural TGF-β analog DBL-1 via its receptor SMA-6 and the cytosolic Smad SMA-3 in hypodermis [46]. In addition to its importance in defense against the fungus D. coniospora, DBL-1 signaling is also required for protection against bacterial pathogens that cause intestinal infections [47–49]. Because DBL-1 is expressed only in the nervous system [31], it is likely that additional circuits involving DBL-1 control the expression of antimicrobial peptides in the intestine. It will be interesting to study whether the DBL-1 receptors and downstream signaling components such as the receptor SMA-6 and the cytoplasmic Smad SMA-3 play any roles in intestinal immune regulation or whether other novel receptors are involved in this regulation.
In a recent study, it was shown that infection of the Gram-positive pathogen Staphylococcus aureus leads to the release of acetylcholine from neurons [50]. The infection-induced release of acetylcholine stimulated muscarinic signaling in the epithelium, driving downstream induction of Wnt expression in the same tissue. Wnt induction activated the epithelial canonical Wnt pathway, resulting in the expression of C-type lectin and lysozyme genes that enhanced host defense. Because infection with several pathogens as well as bloating of the intestine induce the same C-type lectin and lysozyme genes [29,51–53], it is important to delineate whether the release of acetylcholine is specific to S. aureus or is generally related to infection-induced damage in the host.
Neural suppression of immune pathways
Several neural circuits have been identified that suppress immune pathways in C. elegans. Animals defective in neural secretion, such as unc-13 and unc-31 mutants, were more resistant to P. aeruginosa infection [54]. This suggested that the nervous system secretes cues that are immunoinhibitory. Indeed, treatments that resulted in a sustained increase in neural secretion increased sensitivity to pathogens. One of the immunoinhibitory cues that is released by neurons is the insulin-like peptide INS-7. INS-7 activates insulin/IGF-1-receptor/DAF-2 signaling in the intestinal cells, resulting in cytoplasmic retention of the FOXO transcription factor DAF-16 and decreased expression of antimicrobial genes [54]. Interestingly, it appears that pathogens have evolved mechanisms to subvert this pathway to suppress host immunity [55]. Infection of C. elegans by P. aeruginosa induces ins-7 expression, which in turn suppresses immune gene expression by accelerating the exit of the FOXO transcription factor DAF-16 from nuclei in intestinal cells.
One of the first neural circuits identified that suppresses immune pathways involves NPR-1. C. elegans deficient in NPR-1 exhibit enhanced susceptibility to infections by P. aeruginosa, Salmonella enterica, and Enterococcus faecalis [22]. Expression of NPR-1 in the oxygen-sensing neurons AQR, PQR, and URX is sufficient to rescue the enhanced susceptibility to these pathogens. In addition, lack of AQR, PQR, and URX neurons partially rescued the enhanced susceptibility to pathogens of npr-1 loss of function animals, indicating that NPR-1 inhibits the activity of these neurons. Importantly, gene expression studies on NPR-1 lacking animals demonstrated that NPR-1 regulates expression of genes that are controlled by a conserved PMK-1/p38 mitogen-activated protein kinase signaling pathway in the intestine [22,37].
The catecholamine GPCR OCTR-1 suppresses PMK-1/p38 mitogen-activated protein kinase signaling as well as canonical and non-canonical unfolded protein responses (UPR) in the ER, which are important for innate immunity [56,57]. OCTR-1 activity in the ASH neuron suppresses the AIA interneuron, which is required for release of NLP-20, a neuropeptide that regulates the induction of UPR and innate immune genes controlled by PMK-1 signaling [44]. The neurotransmitter octopamine, that is produced by RIC neurons, is the endogenous ligand for OCTR-1 and is involved in the suppression of innate immunity [58]. Interestingly, the RIC neurons are deactivated in the presence of pathogens but transiently activated by nonpathogenic bacteria. Thus, the activation of the octopaminergic pathway on nonpathogenic bacteria potentially suppresses unwanted innate immune responses. On the other hand, the inactivation of octopaminergic pathway on pathogenic bacteria likely helps in the activation of appropriate immune responses.
Pharmacological inhibition of dopamine signaling activates the PMK-1/p38 mitogen-activated protein kinase signaling pathway in the intestine [59,60]. The immunoinhibitory effects of the dopamine signaling are mediated via the D1-like dopamine receptor, DOP-4, in C. elegans. This function of dopamine originates in CEP neurons and requires active DOP-4 in downstream ASG neurons [60]. It will be interesting to study whether the dopaminergic signaling is differentially active on pathogenic vs. nonpathogenic bacteria.
The gastrin-releasing peptide receptor homolog, NPR-9, inhibits innate immune response [61]. Animals lacking NPR-9 show reduced colonization, enhanced survival on pathogen, and enhanced immune gene expression. NPR-9 is expressed in the interneuron AIB and antagonizes the activity of this neuron. Channelrhodopsin-2(ChR2)-mediated activation of the AIB interneuron enhances immune response that is suppressed by overexpression of NPR-9. However, it remains to be studied how pathogens modulate the NPR-9 pathway and the activity of the AIB interneurons.
Intersection of behavioral and molecular immune regulation
Several neural regulators that control molecular immunity also modulate avoidance behavior. For example, NPR-1 in the AQR, PQR, and URX neurons not only regulates innate immunity but also controls the avoidance behavior [22,38]. Similarly, DBL-1 controls both of these defense responses via a single circuit involving the TGF-β receptor SMA-6 and the cytoplasmic Smad SMA-3 in the hypodermis [32,46]. The modulation of both molecular immunity and avoidance behavior by the same neural circuits could be because of the common role of these mechanisms in improving the defense and survival of animals against pathogens. It is also likely that animals use common upstream signals to activate both avoidance behavior and molecular immunity. Indeed, bloating of the intestine caused by pathogen infection or microbial colonization enhances expression of immune genes and elicits microbial avoidance behavior [27,29]. Moreover, disruption of core cellular activities induces both avoidance behavior and immune gene expression [26]. These studies suggest that common upstream signals can modulate both behavioral and molecular immunity via either the same or different neural circuits.
Conclusion and future perspectives
Studies on the neural control of immunity in C. elegans have greatly improved our understanding of conserved neuro-immune connections. The roles of several conserved neurotransmitters such as serotonin, dopamine, octopamine, and acetylcholine in regulating immunity have been described. In addition, the roles of different TGF-β pathways as well as conserved neuropeptides in controlling immunity have been delineated. Despite these advances, it is still not understood if and how the neural circuits are activated by bacterial components and/or signals from the non-nervous tissues. Moreover, the signals from the nervous system that modulate immunity in non-nervous tissues remain to be understood. Developing an understanding of different signals involved in the communication across tissues, as well as signals from pathogens, would improve our knowledge of the mechanisms involved in the maintenance of organismal homeostasis. Because C. elegans neural circuits have both stimulatory and inhibitory actions on immunity, it is important to determine how the different neural circuits are fine-tuned to obtain an optimal immune response upon pathogen infection.
Highlights.
Neural circuits regulate immune pathways in non-nervous tissues.
Neural circuits control behavioral and molecular immunity in C. elegans.
Conserved neuromodulators inhibit or activate immune responses in C. elegans.
Acknowledgement
Work in the Aballay laboratory is funded by the NIH (grants GM070977 and AI117911, www.nigms.nih.gov).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Medzhitov R, Schneider DS, Soares MP: Disease Tolerance as a Defense Strategy. Science 2012, 335:936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarabian C, Curtis V, McMullan R: Evolution of pathogen and parasite avoidance behaviours. Philos Trans R Soc B 2018, 373:20170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacheco-López G, Bermúdez-Rattoni F: Brain-immune interactions and the neural basis of disease-avoidant ingestive behaviour. Philos Trans R Soc B 2011, 366:3389–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavaliers M, Ossenkopp K-P, Choleris E: Social neuroscience of disgust. Genes, Brain Behav 2019, 18:e12508. [DOI] [PubMed] [Google Scholar]
- 5.Schaller M: The Behavioral Immune System. In The Handbook of Evolutionary Psychology.. 2015:206–224. [Google Scholar]
- 6.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, et al. : Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shaanan TL, Azulay-Debby H, Dubovik T, Starosvetsky E, Korin B, Schiller M, Green NL, Admon Y, Hakim F, Shen-Orr SS, et al. : Activation of the reward system boosts innate and adaptive immunity. Nat Med 2016, 22:940–944. [DOI] [PubMed] [Google Scholar]
- 8.Fung TC, Olson CA, Hsiao EY: Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 2017, 20:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas-ballina M, Olofsson PS, Ochani M, Valdés-ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, et al. : Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science 2011, 334:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, et al. : Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell 2018, 173:1083–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Chavan SS, Pavlov VA, Tracey KJ: Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 2017, 46:927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review on mechanisms of peripheral sensory neuronal function in response to immune challenges, the neural regulation of immunity and inflammation, and the therapeutic implications of those mechanistic insights.
- 12.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn J-A, Fernandez-Pena C, Talavera A, Kichko T, et al. : TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun 2014, 5:3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill MEA, Silver WL, Kinnamon SC, Finger TE: Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci 2010, 107:3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang NJ, Chiu IM: Bacterial Signaling to the Nervous System through Toxins and Metabolites. J Mol Biol 2017, 429:587–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivière S, Challet L, Fluegge D, Spehr M, Rodriguez I: Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 2009, 459:574–577. [DOI] [PubMed] [Google Scholar]
- 16.Boillat M, Challet L, Rossier D, Kan C, Carleton A, Rodriguez I: The Vomeronasal System Mediates Sick Conspecific Avoidance. Curr Biol 2015, 25:251–255. [DOI] [PubMed] [Google Scholar]
- 17.Mansourian S, Corcoran J, Enjin A, Lo C, Stensmyr MC, Dacke M, Stensmyr MC: Fecal-Derived Phenol Induces Egg-Laying Aversion in Drosophila Report Fecal-Derived Phenol Induces Egg-Laying Aversion in Drosophila. Curr Biol 2016, 26:2762–2769. [DOI] [PubMed] [Google Scholar]
- 18.Soldano A, Alpizar YA, Boonen B, Franco L, Lopez-Requena A, Liu G, Mora N, Yaksi E, Voets T, Vennekens R, et al. : Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila. Elife 2016, 5:e13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin N, Singh J, Aballay A: Natural Genetic Variation in the Caenorhabditis elegans Response to Pseudomonas aeruginosa. G3 2017, 7:1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meisel JD, Kim DH: Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol 2014, 35:465–470. [DOI] [PubMed] [Google Scholar]
- 21.Reddy KC, Andersen EC, Kruglyak L, Kim DH: A Polymorphism in npr-1 Is a Behavioral Determinant of Pathogen Susceptibility in C. elegans. Science 2009, 323:382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A: Innate Immunity in Caenorhabditis elegans Is Regulated by Neurons Expressing NPR-1/GPCR. Science 2008, 322:460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Tran A, Tang A, Loughlin CTO, Balistreri A, Chang E, Villa DC, Li J, Varshney A, Jimenez V, Pyle J, et al. : C. elegans avoids toxin-producing Streptomyces using a seven transmembrane domain chemosensory receptor. Elife 2017, 6:e23770. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported innate avoidance of toxin-producing bacteria in C. elegans. The authors showed that Streptomyces secreted dodecanoic acid is sensed by a C. elegans GPCR, SRB-6, which is expressed in five types of amphid and phasmid chemosensory neurons, leading to a rapid avoidance behavior that happens within seconds.
- 24.Zhang Y, Lu H, Bargmann CI: Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005, 438:179–84. [DOI] [PubMed] [Google Scholar]
- 25.Shivers RP, Kooistra T, Chu SW, Pagano DJ, Kim DH: Tissue-Specific Activities of an Immune Signaling Module Regulate Physiological Responses to Pathogenic and Nutritional Bacteria in C. elegans. Cell Host Microbe 2009, 6:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo JA, Ruvkun G: Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell 2012, 149:452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Kumar S, Egan BM, Kocsisova Z, Schneider DL, Murphy JT, Diwan A, Kornfeld K: Lifespan Extension in C. elegans Caused by Bacterial Colonization of the Intestine and Subsequent Activation of an Innate Immune Response. Dev Cell 2019, 49:100–117.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors reported that bacterial colonization of the intestine induces microbial avoidance behavior.
- 28•.Lee K, Mylonakis E: An Intestine-Derived Neuropeptide Controls Avoidance Behavior in Caenorhabditis elegans. Cell Rep 2017, 20:2501–2512. [DOI] [PubMed] [Google Scholar]; This report demonstrated that intestinally produced neurpeptide INS-11 suppresses pathogen avoidance behavior.
- 29••.Singh J, Aballay A: Microbial colonization activates an immune fight-and-flight response via neuroendocrine signaling. Dev Cell 2019, 49:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that intestinal bloating caused by pathogen infection modulates neuroendocrine pathways that control the bacterial avoidance behavior.
- 30.Massagué J: TGFβ signalling in context. Nat Rev Mol Cell Biol 2012, 13:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumienny TL, Savage-Dunn C: TGF-β signaling in C. elegans. WormBook 2013, doi: 10.1895/wormbook.1.22.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Zhang Y: DBL-1, a TGF-, is essential for Caenorhabditis elegans aversive olfactory learning. Proc Natl Acad Sci 2012, 109:17081–17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH: Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell 2014, 159:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Moore RS, Kaletsky R, Murphy CT: Piwi/PRG-1 Argonaute and TGF-β Mediate Transgenerational Learned Pathogenic Avoidance. Cell 2019, 177:1827–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that pathogen avoidance learning can be transmitted transgenerationally up to four generations. Piwi/PRG-1 argonaute-regulated expression of DAF-7/TGF-β in the ASI neurons was required for the transgenerational learning of pathogen avoidance.
- 35.Choi S, Chatzigeorgiou M, Taylor KP, Schafer WR, Kaplan JM: Analysis of NPR-1 reveals a circuit mechanism for behavioral quiescence in C.elegans. Neuron 2013, 78:869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers C, Reale V, Kim K, Chatwin H, Li C, Evans P, de Bono M: Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat Neurosci 2003, 6:1178–85. [DOI] [PubMed] [Google Scholar]
- 37.Nakad R, Snoek LB, Yang W, Ellendt S, Schneider F, Mohr TG, Rösingh L, Masche AC, Rosenstiel PC, Dierking K, et al. : Contrasting invertebrate immune defense behaviors caused by a single gene, the Caenorhabditis elegans neuropeptide receptor gene npr-1. BMC Genomics 2016, 17:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy KC, Hunter RC, Bhatla N, Newman DK, Kim DH: Caenorhabditis elegans NPR-1-mediated behaviors are suppressed in the presence of mucoid bacteria. Proc Natl Acad Sci 2011, 108:12887–12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang HC, Paek J, Kim DH: Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour. Nature 2011, 480:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Bono M, Bargmann CI: Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 1998, 94:679–689. [DOI] [PubMed] [Google Scholar]
- 41.Chang AJ, Chronis N, Karow DS, Marletta M a, Bargmann CI: A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol 2006, 4:e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Hao Y, Yang W, Ren J, Hall Q, Zhang Y, Kaplan JM: Thioredoxin shapes the C. elegans sensory response to Pseudomonas produced nitric oxide. Elife 2018, 7:e36833. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports that P. aeruginosa-produced nitric oxide (NO) elicits avoidance behavior in C. elegans. P. aeruginosa and NO avoidance are mediated by the chemosensory neuron ASJ and these responses require receptor guanylate cyclases and cyclic nucleotide gated ion channels.
- 43.Brandt JP, Ringstad N: Toll-like Receptor Signaling Promotes Development and Function of Sensory Neurons Required for a C. elegans Pathogen-Avoidance Behavior. Curr Biol 2015, 25:2228–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Cao X, Kajino-Sakamoto R, Doss A, Aballay A: Distinct Roles of Sensory Neurons in Mediating Pathogen Avoidance and Neuropeptide-Dependent Immune Regulation. Cell Rep 2017, 21:1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified distinct neural circuits in C. elegans that control pathogen avoidance and molecular immunity.
- 45.Lee YT, Wang MC: The Bacterivore’s Solution: Fight and Flight to Promote Survival. Dev Cell 2019, 49:7–9. [DOI] [PubMed] [Google Scholar]
- 46.Zugasti O, Ewbank JJ: Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 2009, 10:249–256. [DOI] [PubMed] [Google Scholar]
- 47.Portal-celhay C, Bradley ER, Blaser MJ: Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol 2012, 12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon G, Lee J, Koh JH, Lim YH: Lifespan Extension of Caenorhabditis elegans by Butyricicoccus pullicaecorum and Megasphaera elsdenii with Probiotic Potential. Curr Microbiol 2018, 75:557–564. [DOI] [PubMed] [Google Scholar]
- 49.Berg M, Monnin D, Cho J, Nelson L, Crits-Christoph A, Shapira M: TGFβ/BMP immune signaling affects abundance and function of C. elegans gut commensals. Nat Commun 2019, 10:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Labed SA, Wani KA, Jagadeesan S, Hakkim A, Najibi M, Irazoqui JE: Intestinal Epithelial Wnt Signaling Mediates Acetylcholine-Triggered Host Defense against Infection. Immunity 2018, 48:963–978.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports that S. aureus infection-induced release of acetylcholine stimulated muscarinic signaling in the epithelium, driving downstream induction of Wnt expression in the same tissue. Wnt induction activated the epithelial canonical Wnt pathway, resulting in the expression of C-type lectin and lysozyme genes that enhanced host defense.
- 51.Irazoqui JE, Troemel ER, Feinbaum RL, Luhachack LG, Cezairliyan BO, Ausubel FM: Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog 2010, 6:e1000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong D, Bazopoulou D, Pujol N, Tavernarakis N, Ewbank JJ: Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol 2007, 8:R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuen GJ, Ausubel FM: Both live and dead Enterococci activate Caenorhabditis elegans host defense via immune and stress pathways. Virulence 2018, 9:683–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawli T, Tan M-W: Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol 2008, 9:1415–1424. [DOI] [PubMed] [Google Scholar]
- 55.Evans EA, Kawli T, Tan MW: Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 2008, 4:e1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun J, Singh V, Kajino-Sakamoto R, Aballay A: Neuronal GPCR Controls Innate Immunity by Regulating Noncanonical Unfolded Protein Response Genes. Science 2011, 332:729–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun J, Liu Y, Aballay A: Organismal regulation of XBP-1-mediated unfolded protein response during development and immune activation. EMBO Rep 2012, 13:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Sellegounder D, Yuan C-H, Wibisono P, Liu Y, Sun J: Octopaminergic Signaling Mediates Neural Regulation of Innate Immunity in Caenorhabditis elegans. MBio 2018, 9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors identified the neurotransmitter octopamine as an endogenous ligand for the GPCR, OCTR-1 in immune regulation and showed that the octopamine-producing RIC neurons function in the OCTR-1 neural circuit to suppress innate immunity.
- 59.Cai Y, Cao X, Aballay A: Whole-animal chemical screen identifies colistin as a new immunomodulator that targets conserved pathways. MBio 2014, 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Cao X, Aballay A: Neural Inhibition of Dopaminergic Signaling Enhances Immunity in a Cell-Non-autonomous Manner. Curr Biol 2016, 26:2329–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrated that domaminergic signaling mediated via DOP-4 suppresses the PMK-1/p38 mitogen-activated protein kinase signaling pathway in the intestine.
- 61.Yu Y, Zhi L, Wu Q, Jing L, Wang D: NPR-9 regulates the innate immune response in Caenorhabditis elegans by antagonizing the activity of AIB interneurons. Cell Mol Immunol 2018, 15:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]