Abstract
The plant defensin HsAFP1 is characterized by broad-spectrum antifungal activity and induces apoptosis in Candida albicans. In this study, we performed a transcriptome analysis on C. albicans cultures treated with HsAFP1 to gain further insight in the antifungal mode of action of HsAFP1. Various genes coding for cell surface proteins, like glycosylphosphatidylinositol (GPI)-anchored proteins, and proteins involved in cation homeostasis, autophagy and in cell cycle were differentially expressed upon HsAFP1 treatment. The biological validation of these findings was performed in the model yeast Saccharomyces cerevisiae. To discriminate between events linked to HsAFP1’s antifungal activity and those that are not, we additionally used an inactive HsAFP1 mutant. We demonstrated that (i) HsAFP1-resistent S. cerevisiae mutants that are characterized by a defect in processing GPI-anchors are unable to internalize HsAFP1, and (ii) moderate doses (FC50, fungicidal concentration resulting in 50% killing) of HsAFP1 induce autophagy in S. cerevisiae, while high HsAFP1 doses result in vacuolar dysfunction. Vacuolar function is an important determinant of replicative lifespan (RLS) under dietary restriction (DR). In line, HsAFP1 specifically reduces RLS under DR. Lastly, (iii) HsAFP1 affects S. cerevisiae cell cycle in the G2/M phase. However, the latter HsAFP1-induced event is not linked to its antifungal activity, as the inactive HsAFP1 mutant also impairs the G2/M phase. In conclusion, we demonstrated that GPI-anchored proteins are involved in HsAFP1’s internalization, and that HsAFP1 induces autophagy, vacuolar dysfunction and impairment of the cell cycle. Collectively, all these data provide novel insights in the mode of action of HsAFP1 as well as in S. cerevisiae tolerance mechanisms against this peptide.
Keywords: yeast, GPI-anchored proteins, autophagy, vacuolar dysfunction, cell cycle impairment
Graphical Abstract
1. Introduction
Fungal pathogens can be detrimental for all kind of organisms, leading to severe infections as well as die-offs and extinctions of wild species [1, 2]. To effectively treat fungal infections, relatively few classes of fungicides and antifungal drugs are available [1, 3]. Hence, there is a need for the development of novel antifungal compounds with a novel mode of action. In this context, great potential is attributed to antimicrobial peptides, such as plant defensins [4, 5].
Plant defensins are small (45–54 amino acids), antimicrobial, cysteine-rich peptides that are structurally homologous to defensins from insects and invertebrates [6]. They have a broad-spectrum antifungal activity [7, 8] and are generally not toxic for plants and animals [9–11]. Plant defensins contain 4–5 disulphide bridges, which increase their stability and make these peptides resistant to extreme conditions, such as exposure to high temperatures [12] and serum proteases [9]. In addition, plant defensins have been shown to reduce fungal infections in plant and animal infection models [9, 13, 14]. Understanding their mode of action is, however, crucial for their rational use. Fungal targets as well as fungal tolerance mechanisms have been identified for various plant defensins in the past decades (as reviewed in [4]).
This study focuses on the plant defensin HsAFP1, originating from the seeds of coral bell (Heuchera sanguinea). This peptide is characterized by a low in vitro frequency of resistance occurrence [10, 12]. Moreover, it affects a broad range of yeast/fungal species, including the model yeast Saccharomyces cerevisiae and the prominent human fungal pathogen Candida albicans by inhibiting biofilm formation [11]. HsAFP1 specifically binds to high-affinity binding sites on fungal membranes [15]. We recently identified phosphatidic acid (PA) and phosphatidylinositolphosphates (PIPs) as lipid interaction partners of HsAFP1 [16]. Upon accumulation at the cell surface, HsAFP1 is internalized in S. cerevisiae cells, resulting in membrane permeabilization [16]. Although HsAFP1’s internalization is at least partially endocytosis-mediated [16], the exact mechanisms remain unclear. In addition, HsAFP1 also induces the production of reactive oxygen species (ROS) and apoptosis in C. albicans [17]. By screening a S. cerevisiae deletion mutant library, yeast tolerance mechanisms towards HsAFP1 treatment were identified, and involve mitogen-activated protein kinase (MAPK) cascades and general stress response pathways [17].
In this study, the antifungal activity and yeast tolerance mechanisms of HsAFP1 were further unraveled via transcriptome analysis and additional genetic and biochemical tests. Based on these data, we demonstrated that GPI-anchored proteins are involved in HsAFP1’s internalization, and that HsAFP1 induces autophagy, vacuolar dysfunction and impairment of the cell cycle.
2. Materials and methods
2.1. Strains and reagents:
The strains used in this study are listed in Table 1. Yeast cells were cultured in the following liquid media: YPD (yeast extract (10 g/L; LabM, UK), peptone (20 g/L; LabM, UK) and glucose (20 g/L; Sigma-Aldrich, USA), YNB (yeast nitrogen base without amino acids; MP Biomedicals, USA) (6.7 g/L), PDB/YPD (potato dextrose broth (19.2 g/L; BD, USA), yeast extract (2 g/L), peptone (4 g/L) and glucose (4 g/L)) adjusted to pH 7 with 50 mM HEPES (Sigma-Aldrich, USA), synthetic complete (SC) medium (CSM (complete amino acid supplement mixture; MP Biomedicals, USA) (0.77 g/L), YNB (6.7 g/L) and glucose (20 g/L)) adjusted to pH 7 with 50 mM HEPES or 1/5th PDB/YNB; at 30°C and 37°C for S. cerevisiae and C. albicans, respectively. Yeast cells were grown on following solid media: YPD agar plates containing 2% or 0.05% glucose (YPD + 15 g/L agar (Invitrogen, USA)) for all FC50 and replicative lifespan experiments. HsAFP1 and HsAFP1[H32A][R52A] were produced recombinantly, as described previously [4, 11].
Table 1:
Yeast strains used in this study.
2.2. Antifungal activity:
Exponentially growing S. cerevisiae BY4741 cells in YPD (grown for 5 h at 30°C starting from OD600 nm = 0.3) were washed. The pellet was resuspended in PDB/YPD with 50 mM HEPES pH 7 and incubated (OD600 nm = 1) with HsAFP1 for 150 minutes at 30°C. Both at the start and at the end of the treatment, surviving yeast cells were determined via plating assays. In a plating assay, 10-fold dilution series of yeast cells in PBS were prepared, after which 100 μL was plated on YPD plates. After 2 days of incubation at 30°C, the number of colony forming units (CFUs) was counted. FC50 values, being 25 μg/mL HsAFP1 for 107 S. cerevisiae cells/mL and 5 μg/mL HsAFP1 for 107 C. albicans cells/mL, indicate the fungicidal concentration resulting in 50% cell death.
2.3. Cell treatment for transcriptome analysis:
Exponentially growing C. albicans SC5314 cells in YPD (grown for 5 h at 37°C starting from OD600 nm = 0.1) were washed. The pellet was resuspended in PDB/YPD with 50 mM HEPES pH 7 and incubated (OD600 nm = 1) with HsAFP1 (2x FC50; 10 μg/mL) or MQ water (control) for 150 minutes at 37°C. After treatment, cells were washed with PBS and, subsequently, cell pellets were flash-frozen in liquid nitrogen before storage at −80°C until RNA isolation. Samples of 3 independent experiments were collected. In parallel to transcriptome analysis, plating assays were performed to determine CFU counts at FC50, as described above.
2.4. RNA isolation, library preparation, RNA sequencing and data-analysis:
RNA was isolated from treated cell pellets, as described above, using the Yeast RiboPure RNA Purification kit (AmBion) according to manufacturer’s instructions. Next, organic solvents were removed by ethanol precipitation, followed by RNA dissolving in 0.1% (v/v%) diethylpyrocarbonate (DEPC)-MQ water (Sigma-Aldrich, USA). The quality and integrity of RNA was determined via NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA), Experion Automated Electrophoresis System (Biorad, USA) and BioAnalyser (Agilent, USA). Library preparation for RNA sequencing was performed using an Illumina (USA) TruSeq kit. Next, sequencing was performed in one lane of an Illumina NextSeq sequencer using sequencer kit H75. On average 4,497,979 reads per sample (75 bp) were generated. A first step in the preprocessing of the raw reads was quality trimming, in which low quality ends (Q < 20) were trimmed and adapters were trimmed at the end if there was ≥ 10 bp overlap and 90% match. Secondly, reads < 35 bp after trimming, poly-A reads, ambiguous reads, low quality reads and artefacts were discarded. After preprocessing, 99% of all reads remained. These preprocessed reads were subsequently subjected to mapping analysis to the reference genome sequence of C. albicans SC5314_A21 using Tophat v2.0.13. Reads with non-primary mappings or with mapping quality of < 20 were discarded. On average 91% of the preprocessed reads could be mapped to the reference genome. Using htseq0.6.1p1, the number of reads that are overlapping with gene features was determined. The last step in the data processing was the removal of genes that do not have > 1 count per million reads for all three replicates for at least one treatment (MQ water or HsAFP1). Differential expression of genes was determined after a within-sample [22] and between-sample [23] normalization, using EdgeR packages of Bioconductor. Genes with a False Discovery Rate [24] (FDR) < 0.05 and a |log2 (Fold Change)| > 1 (calculated by EdgeR) were considered as differentially expressed. To identify important processes in HsAFP1 treatment compared to the control treatment, the differentially expressed genes were subjected to Gene Ontology (GO) analysis using the Candida Genome Database GO Term Finder Tool [25].
2.5. Vacuolar pH measurements:
The vacuolar pH was measured using the acetoxymethyl ester of 2’,7’-bis-(2-carboxyethyl)-5-,6-carboxyfluorescein (BCECF-AM; Thermo Fisher Scientific, USA), adapted from the protocol described in [26]. Exponentially growing S. cerevisiae BY4741 cells (grown for 5 h at 30°C starting from OD600 nm = 0.3) were washed. The pellet was resuspended in PDB/YPD with 50 mM HEPES pH 7 and incubated (OD600 nm = 1) with 1/2 FC50, FC50, 2x FC50 HsAFP1 (12.5 μg/mL; 25 μg/mL; 50 μg/mL), 2x FC50 HsAFP1[H32A][R52A] (50 μg/mL), 10 μM concanamycin A (ConA; positive control; Sigma-Aldrich, USA) or MQ water (negative control) for 150 minutes at 30°C. After 2 h of incubation, 50 μM BCECF-AM was added to all samples and subsequently incubated for another 30 minutes at 30°C. Next, samples were centrifuged, washed and resuspended in PBS, followed by fluorescence measurements with the ratio of fluorescence intensity from excitation at 490 nm (I490 nm) to the intensity from excitation at 450 nm (I450 nm) (both measured at an emission wavelength of 535 nm) used as a measure of the vacuolar pH [26] and OD490 nm used as a measure of cell biomass. The fluorescence signal (I490 nm/I450 nm) was normalized for the number of cells present in the sample (OD490 nm).
2.6. Replicative lifespan experiments:
Replicative lifespan experiments were performed on S. cerevisiae BY4742 cells as described previously [27, 28]. All lifespan experiments were performed on YPD plates buffered to pH 7 with 50 mM HEPES containing HsAFP1 and 2% glucose, except for the low glucose experiments. The latter experiments were carried out on YPD plates with only 0.05% of glucose. The HsAFP1 dose used is the maximum dose that does not affect replicative lifespan in 2% glucose.
2.7. Autophagy and mitophagy assay:
The monitoring of autophagy and mitophagy was performed according to the protocol described by Mendl and co-workers [20] and Noda and Klionsky [29]. Briefly, strains deleted for PHO8 were transformed with cytPHO8- or mtPHO8-expressing plasmids. Exponentially growing S. cerevisiae BY4741 cells containing plasmid pYX242-cytPho8/pYX242-mtPho8, in YNB supplemented with 2% glucose, histidine (50 μg/mL), methionine (50 μg/mL) and uracil (100 μg/mL) (grown for 5 h at 30°C starting from OD600 nm = 0.3) were washed. The pellet was resuspended in 1/5th PDB/YNB (supplemented with 2% glucose, histidine (50 μg/mL), methionine (50 μg/mL) and uracil (100 μg/mL)) with 50 mM HEPES pH 7 and incubated (OD600 nm = 1) with HsAFP1 (FC50; 25 μg/mL and 1.33x FC50; 33.25 μg/mL), HsAFP1[H32A][R52A] (1.33x FC50; 33.25 μg/mL) or MQ water (control) for 180 minutes at room temperature. Next cells were collected, harvested, washed in 2 mL of ice-cold water containing 0.85% NaCl, 1 mM PMSF, and resuspended in 300 μL lysis buffer (20 mM PIPES, 0.5% Triton X-100, 50 mM KCl, 100 mM potassium acetate, 10 mM MgSO4, 10 μM ZnSO4, and 1 mM PMSF). An equal volume of acid-washed glass beads was added and the cells were lysed by vortexing for 7 minutes. To start the assay, 100 μL of extract was added to 400 μL reaction buffer (250 mM Tris-HCl, pH 8.5, 0.4% Triton X-100, 10 mM MgSO4, 4 mM nitrophenyl phosphate (Sigma-Aldrich, USA)), and samples were incubated 15 minutes at 37°C before terminating the reaction by adding 500 μL of stop buffer (2 M glycine, pH 11). Evolution of nitrophenol was monitored by measuring absorbance at 405 nm using a microplate reader Model680 (Bio-Rad, USA). Protein concentration in the extracts was measured with the RC DC kit (Bio-Rad, USA) according to the manufacturer’s instructions. Monitoring autophagy was also achieved through the GFP-Atg8 processing assay by immunoblotting analysis. In this assay, delivery of Atg8 to the vacuole was followed by a GFP N-terminally tagged to Atg8 [30, 31]. It is known that GFP is more resistant to the vacuolar degradation than Atg8, therefore bulk autophagy results in the accumulation of free GFP in the vacuole. GFP-Atg8 and free GFP are detectable by western blotting using a GFP-specific antibody. To perform this assay, cells were transformed with the plasmid pRS416-GFPAtg8 with fusion gene under the control of the ATG8 endogenous promoter. Exponentially growing S. cerevisiae BY4741 cells containing plasmid pRS416GFP-ATG8, in YNB supplemented with 2% glucose, histidine (50 μg/mL), leucine (300 μg/mL) and methionine (50 μg/mL) (grown for 5 h at 30°C starting from OD600 nm = 0.3) were washed. The pellet was resuspended in 1/5th PDB/YNB (supplemented with 2% glucose, histidine (50 μg/mL), leucine (300 μg/mL) and methionine (50 μg/mL)) with 50 mM HEPES pH 7 and incubated (OD600 nm = 1) with HsAFP1 (FC50; 25 μg/mL and 1.33x FC50; 33.25 μg/mL) HsAFP1[H32A][R52A] (1.33x FC50; 33.25 μg/mL) or MQ water (control) for 180 minutes at room temperature. For detection of protein levels by western blot, the total cellular extracts were collected and extracted as previously described [32]. Briefly, cells were pre-treated with 2 M lithium acetate for 5 minutes at room temperature. After lithium acetate removal, 0.4 M NaOH was added and cells were incubated for 5 minutes on ice. Next, the cells were resuspended in SDS-PAGE sample buffer and boiled for 5 minutes. Twenty μg of proteins was resolved on a 12% SDS gel and transferred to a nitrocellulose membrane during 7 minutes at 25V. Phosphoglycerate kinase 1 (Pgk1) was used as a control protein in this assay. The membranes were blocked with Tris buffered saline (TBS) with 0.1% Tween 20 (TBST) containing 5% skim milk, followed by incubation with anti-GFP (1:5000; Abcam, UK) and anti-PGK (1:5000; Invitrogen, USA) primary antibodies in TBST containing 1% skim milk. After washing with TBS, the membranes were incubated with the secondary antibody, being HRP-conjugated anti-goat IgG and anti-mouse IgG at a dilution of 1:5000 in 1% skim milk, respectively. Protein levels were detected after incubation with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, USA) or Clarity Western ECL Substrate (Bio-Rad, USA). Digital images of the western blotting were obtained in a ChemiDoc XRS System with Quantity One software (Bio-Rad, USA).).
2.8. HsAFP1 internalization confocal microscopy and flow cytometry:
Exponentially growing S. cerevisiae BY4741 cells in YPD (grown for 5 h at 30°C starting from OD600 nm = 0.3) were washed. The pellet was resuspended in PDB/YPD with 50 mM HEPES pH 7 and incubated (OD600 nm = 1) with HsAFP1 for 150 minutes at 30°C. Internalization of HsAFP1 (via fluorescently labelled HsAFP1; BODIPY-HsAFP1) as well as membrane permeabilization (via the fluorescent dye propidium iodide; 3 μM) were studied in S cerevisiae WT and Δbst1 mutant yeasts both via confocal microscopy [16] and via flow cytometry. For the latter, cells were subjected on a BD InfluxTM cell sorter. Per treatment, 100,000 cells were monitored for fluorescence at 530/40 nm (FL2_λex = 488 nm) and 610/20 nm (FL11_ λex = 561 nm) for the detection of HsAFP1 internalization (BODIPY+) and membrane permeabilization (PI+), respectively. PDB/YPD with 50 mM HEPES pH 7 was supplemented with 2 mM CaSO4, 2 mM CuSO4, 2 mM MgSO4 or 2 mM FeSO4 to evaluate the effect of divalent cations on the mode of action of HsAFP1.
2.9. Cell cycle analysis:
Exponentially growing S. cerevisiae BY4741 cells, in YNB supplemented with 2% glucose, histidine (50 μg/mL), leucine (300 μg/mL), methionine (50 μg/mL) and uracil (100 μg/mL) (grown for 5 h at 30°C starting from OD600 nm = 0.3) were washed. The pellet was resuspended in 1/5th PDB/YNB (supplemented with 2% glucose, histidine (50 μg/mL), leucine (300 μg/mL), methionine (50 μg/mL) and uracil (100 μg/mL)) with 50 mM HEPES pH 7 and incubated (OD600 nm = 1) with HsAFP1 (FC50; 25 μg/mL and 1.33x FC50; 33.25 μg/mL), HsAFP1[H32A][R52A] (1.33x FC50; 33.25 μg/mL) or MQ water (control) for 180 minutes at room temperature. Next, the cell cycle phase of all treated cells was determined as described by Vriens and colleagues [33]. Briefly, cells were fixed with ethanol, after which they were treated with RNAse and proteinase K. The cell cycle phase was determined via flow cytometric analysis on cells stained with the DNA dye SYBR Green 10,000 x and ModFit LT software.
2.10. Data analysis:
Data were analyzed with GraphPad Prism 6. Significant differences were determined on means ± SD with P < 0.05 as considered statistically significant (represented by * in the figures). We assumed that all data are normally distributed. For the flow cytometry experiments, significant differences in the population size between the control treatment and all other treatments were determined via two-way ANOVA followed by Tukey multiple comparison. Immunoblot bands were quantified by densitometric analysis using the Quantity One software. Then, the ratio of free GFP and total GFP was used as a readout to evaluate the autophagic flux. This ratio is normalized using the control protein Pgk1. For the autophagy/mitophagy (Immunoblot and alkaline phosphatase assay) and the vacuolar pH experiments, significant differences between the negative control and all other treatments were determined via one-way ANOVA followed by Dunnett multiple comparison. Kaplan-Meier survival curves [34] were plotted using GraphPad Prism 6. For the cell cycle experiments, significant differences in the population size of each phase between the control treatment and all other treatments were determined via two-way ANOVA followed by Tukey multiple comparison, while significant differences in the CFU counts were determined via one-way ANOVA followed by Dunnett multiple comparison.
3. Results and discussion
3.1. Transcriptome analysis of HsAFP1-treated C.albicans cells
To identify fungal pathways that are induced or repressed upon HsAFP1 treatment, a transcriptome analysis was performed on C. albicans cells treated with MQ water (control) or HsAFP1 at 2x FC50 (10 μg/mL; i.e. fungicidal concentration resulting in 50% cell death for 107 cells/mL; nomenclature according to recent guidelines [35]), which is a moderate HsAFP1 dose. Upon HsAFP1 treatment, 570 genes were differentially expressed (FDR < 0.05 and |log(Fold Change)| > 1), with 370 and 200 being up- and down-regulated, respectively.
The top50-induced and -repressed genes (red and green in Supplementary Table S1, respectively) were selected, and Gene Ontology (GO) analysis was performed on all differentially expressed genes (Supplementary Table S2). Multiple top-induced genes encode for proteins located at the cell surface, including mannosyltransferases (MNN15, MNN4–4 and BMT7) and other enzymes (PGA13 and KRE1) that play a role in cell wall biosynthesis. Other induced genes coding for glycosylphosphatidylinositol (GPI)-anchored cell surface proteins are DAG7, PCL1, orf19.6840, ECM331, RBR1, PGA23, PGA39, PGA49 and orf19.675.1. Genes involved in lipid regulation and transport were also up-regulated by HsAFP1 treatment, including orf19.2726 (orthologue in S. cerevisiae regulates phosphatidylinositol(PI)-4-phosphate levels), RTA2 and RTA4 (sphingolipid base transport). A shift in lipid metabolism is not surprising as we recently discovered that HsAFP1 specifically interacts with phosphate-containing phospholipids such as phosphatidic acid and phosphatidylinositol phosphates [16]. Enhanced expression of two proteins involved in cell cycle progression and polarized growth, cyclins Pcl1p and Pcl2p, was induced by HsAFP1. Genes coding for proteins that are linked to cation homeostasis (calcium-induced: orf19.851 and iron-regulated: orf19.675) and multidrug efflux pumps (MDR1) were up-regulated as well as genes from the oxidative stress-response (OYE23 and SOD5). Lastly, CEK1, coding for a MAP kinase that plays a role in mating and invasive growth, and YIP3, of which the orthologue in S. cerevisiae is involved in vesicle transport from the ER to the Golgi complex, were also up-regulated by HsAFP1.
Genes that were repressed by HsAFP1 also included genes encoding cell surface proteins, such as those coding for GPI-anchored proteins (PGA45, PGA26, PGA7, RBT5, ALS4 and PLB4.5), adhesin-like proteins orf19.3988 and orf19.5267, chitinase CHT3 and proteins related to (phospho)lipid degradation (PLB1, PHO100, PHO112 and PLB4.5). In addition, phosphate metabolism (PHO112 and GIT1) and its transport protein (FGR2) were also down-regulated. Various virulence factors, such as host cell penetration (PLB1), adhesion (ALS2, ALS3 and ALS4), biofilm formation/dispersion (YWP1) and production of cytotoxic peptides (ECE1) were down-regulated by HsAFP1, which is not surprising as HsAFP1 can inhibit C. albicans biofilm formation [11]. The transcription of various hyphal proteins (ECE1, PGA7 and HWP1) was inhibited as well. Another group of HsAFP1-repressed genes are related to iron (ALS3, CFL2, FET34, FET99, FRE10, FRP1, FTR1, PGA10, RBT5 and RNR3) or copper homeostasis (CRP1 and FET34). In addition, one gene coding for ABC (multidrug) transporter CDR4 and three genes coding for oligopeptide transporters OPT4, OPT7 and IFC3, were also repressed by HsAFP1.
To obtain a broader view on the pathways involved in HsAFP1’s mode of action in C. albicans, all differentially expressed genes (FDR < 0.05 and |log(Fold Change)| > 1) were subjected to Gene Ontology (GO) analysis (Supplementary Table S2). Groups of genes, clustered according to their cellular component, molecular function or biological process that were overrepresented by HsAFP1 treatment, were identified (Figure 1). Similar pathways were identified as in the analysis of the top50-induced, except for the significantly up-regulated GO groups of genes involved in carbohydrate and the sulphur metabolic processes. In line, similar pathways were identified in the GO and the top50-repressed genes analysis, except for the significantly down-regulated GO group of genes involved in ribosome biosynthesis and rRNA processing, as well as nucleolar genes (Figure 1), which might point to reduced transcription and translation.
Figure 1: GO groups that were significantly overrepresented in either the up-regulated (A) or down-regulated (B) genes in Candida albicans SC5314 by HsAFP1, as determined via the GO Term Finder at the Candida Genome Database website (FDR < 0.05, Supplementary Table S2).

Relative enrichment of GO groups (%) is based on the amount of annotated differentially expressed genes from the corresponding group compared to the total amount of differentially expressed genes upon HsAFP1 treatment.
In conclusion, various gene clusters were differentially expressed upon HsAFP1 treatment, from which four (namely GPI-anchored proteins, cation homeostasis, autophagy and cell cycle) were further investigated in this study (Figure 2) for several reasons. Firstly, 19 and 17 genes encoding for GPI-anchored proteins were up- or down-regulated, respectively, by HsAFP1 treatment. Altered expression of genes coding for biosynthesis of GPI-anchor building blocks, such as phosphatidylinositol (PI) (e.g. CDS1, CHO1, INO1 and PDR16) and phosphatidylethanolamine (PE) (orf19.6951) could be observed, as well as up-regulation of genes involved in GPI-anchor biosynthesis (e.g. GPI13 and GPI15). Secondly, 10 out of the top50 down-regulated genes by HsAFP1 treatment were involved in iron/copper assimilation and homeostasis, while various calcium transporter genes were up-regulated, indicating that cation homeostasis may be affected by HsAFP1 treatment. Thirdly, 5 genes (CLG1, IPK2, IRS4, PCL1 and PCL5) that are involved in macroautophagy were up-regulated and 1 gene (TRS33) that is involved in macroautophagy was down-regulated upon HsAFP1 treatment. According to GO term finder, CLG1, PCL1 and TRS33 are directly annotated to the term ‘positive regulation of macroautophagy’. IPK2, IRS4 and PCL5 are directly annotated to the term ‘macroautophagy’, ‘autophagy’ and ‘negative regulation of macroautophagy’, respectively. Finally, genes coding for proteins that are located in the cellular bud and/or that are involved in cell wall biosynthesis and/or cell cycle were significantly up-regulated, indicating that cell growth and/or reproduction may be affected by HsAFP1 treatment (Figure 2).
Figure 2: Expression profile of Candida albicans SC5314 cultures determined via RNA-seq analysis.
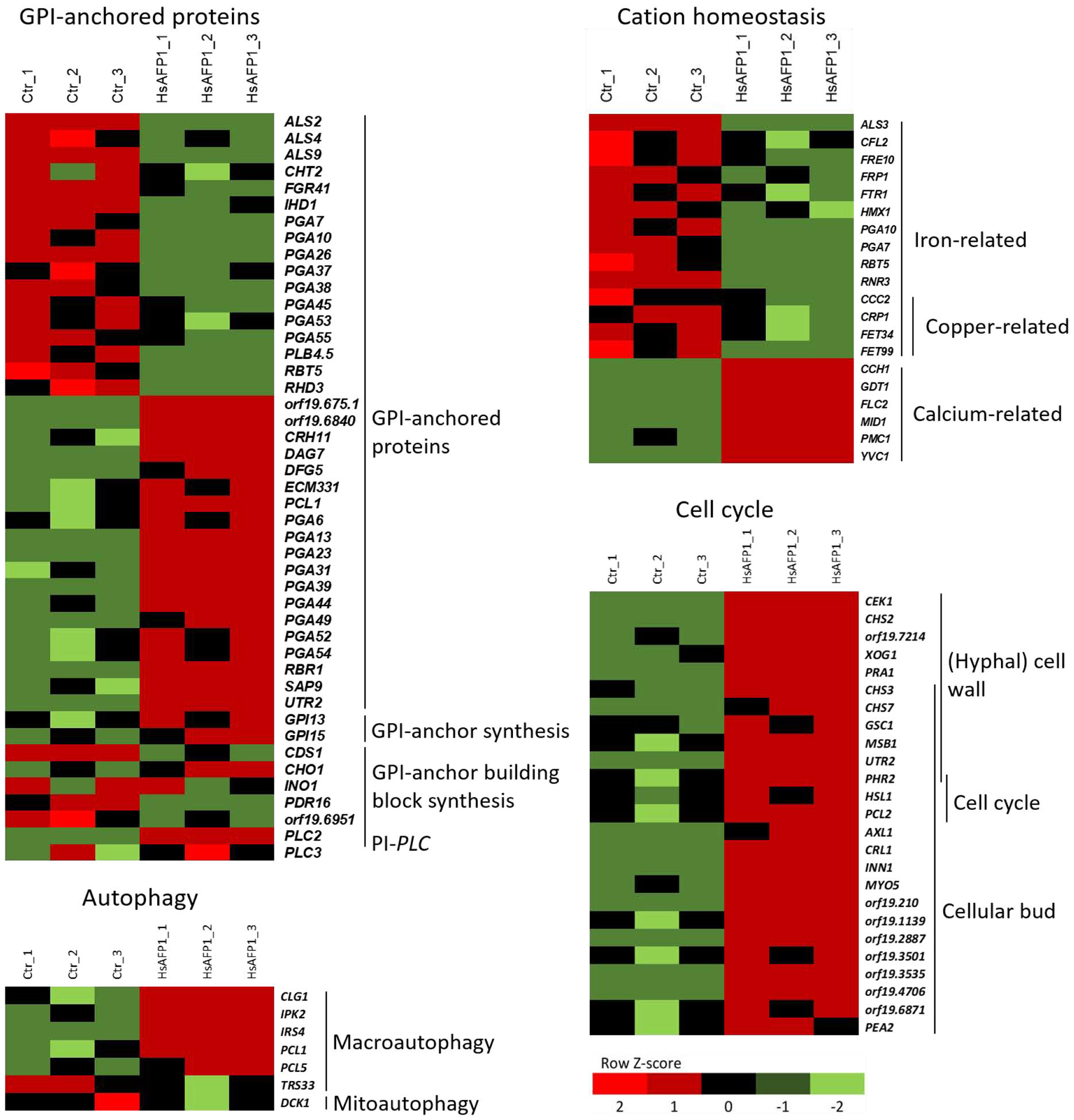
Ctr_1, Ctr_2, Ctr_3 and HsAFP1_1, HsAFP1_2, HsAFP1_3 represent the control and HsAFP1 treatment of 3 independent experiments, respectively. C. albicans SC5314 cells were treated for 150 minutes with HsAFP1 (2x FC50; 10 μg/mL) in PDB/YPD with 50 mM HEPES pH 7. Colored bars indicate the Z-score, representing the relative expression of a gene compared to the mean of expression for the corresponding gene over all six samples. Up- and down-regulated genes coding for: GPI-anchored proteins, GPI-anchor synthesis, GPI-anchor building block biosynthesis and phosphatidylinositol-specific phospholipase C (PI-specific PLC). Down-regulated iron- and copper-related genes and up-regulation of calcium-related genes. Up- and down-regulated genes involved in macro- or mitophagy, respectively. Up-regulated genes coding for: cell wall biosynthesis and cell cycle enzymes as well as cellular bud located proteins.
Previously at our laboratory we screened a S. cerevisiae deletion mutant library for both hypersensitivity and resistance to HsAFP1 [17]. To link this study with our current study, and given the plethora of (genetic) tools that are available for S. cerevisiae, we validated the C. albicans transcriptome data further in S. cerevisiae. An important tool we used throughout the validation experiments was HsAFP1[H32A][R52A], a mutant HsAFP1 peptide that is devoid of antifungal activity [16]. In this way, we could discriminate between HsAFP1-induced cellular read-outs that are linked to its antifungal activity and actions that do not, the latter assumed to be also induced by the inactive HsAFP1 mutant.
3.2. GPI-anchored proteins mediate HsAFP1’s internalization
As the transcriptomic data pointed to effects of HsAFP1 on the expression of GPI-anchored proteins and GPI-anchor (building block) biosynthesis (Figure 2 and Supplementary Table S2), we first focused on a potential role of GPI-anchored proteins in HsAFP1’s antifungal mode of action. We previously showed that deletion of the GPI-anchor remodeling gene BST1 resulted in 32-fold increased resistance of S. cerevisiae to HsAFP1 treatment, while deletion of particular GPI-anchor biosynthesis genes of yeast did not result in altered sensitivity to HsAFP1 [17]. Hence, it seems that GPI-anchor remodeling is most important for HsAFP1’s antifungal activity.
Bst1p is an inositol deacylase that removes the acyl-chain from GPI-anchors in the endoplasmic reticulum immediately after GPI attachment to proteins [36]. In human cells it has been shown that the mutation of Pga1, the Bst1’s human functional orthologue, results in cell surface GPI-anchored proteins with abnormal GPI-anchors [37]. We earlier demonstrated that upon accumulation at the cell surface, HsAFP1 is internalized in S. cerevisiae cells, resulting in membrane permeabilization [16]. In the current study we further investigated whether GPI-anchored proteins might be important for HsAFP1’s internalization in S. cerevisiae cells. To this end, we assessed HsAFP1’s internalization via microscopy and flow cytometry analysis using green fluorescently labelled HsAFP1 (BODIPY-HsAFP1). In parallel, the red fluorescent dye propidium iodide (PI) was used to monitor membrane permeabilization. Firstly, to maximally visualize the specific interaction of BODIPY-HsAFP1 with S. cerevisiae cells, the highest HsAFP1 concentration possible was used, to which the bst1 deletion mutant was still fully resistant (10x FC50; 250 μg/mL, Figure 3A). Previously, we demonstrated that HsAFP1 accumulates at the cell surface of S. cerevisiae WT cells with intact membranes, most notably at the buds and septa. Subsequently HsAFP1 is internalized, followed by membrane permeabilization [16]. In contrast to S. cerevisiae WT cells, neither internalization nor membrane permeabilization was observed in Δbst1 cells upon treatment with this very high HsAFP1 dose (Figure 3A). Secondly, to further investigate the importance of the Bst1p for the interaction of HsAFP1 with S. cerevisiae cells, flow cytometry was used. Here as well as in the rest of the manuscript, we used HsAFP1 doses of up to 2x FC50 (50 μg/mL), which is still sufficient to fully investigate the mode of action of HsAFP1. Note that treatment of HsAFP1 (2x FC50; 50 μg/mL) resulted in 52% BODIPY positive WT cells of which 46% were PI positive, thereby corroborating our previous findings that HsAFP1 is internalized in S. cerevisiae cells, immediately followed by membrane permeabilization [16]. In contrast, < 1% Δbst1 cells were PI positive (Figure 3B–C). All of this indicates that Δbst1 cells are resistant to HsAFP1 treatment due to a compromised HsAFP1 internalization pathway. Hence, GPI-anchor remodeling enzymes, such as Bst1, play an important role in HsAFP1’s internalization.
Figure 3: Glycosylphosphatidylinositol (GPI)-anchor remodeling by Bst1p is important for HsAFP1 internalization and HsAFP1-induced cell death.
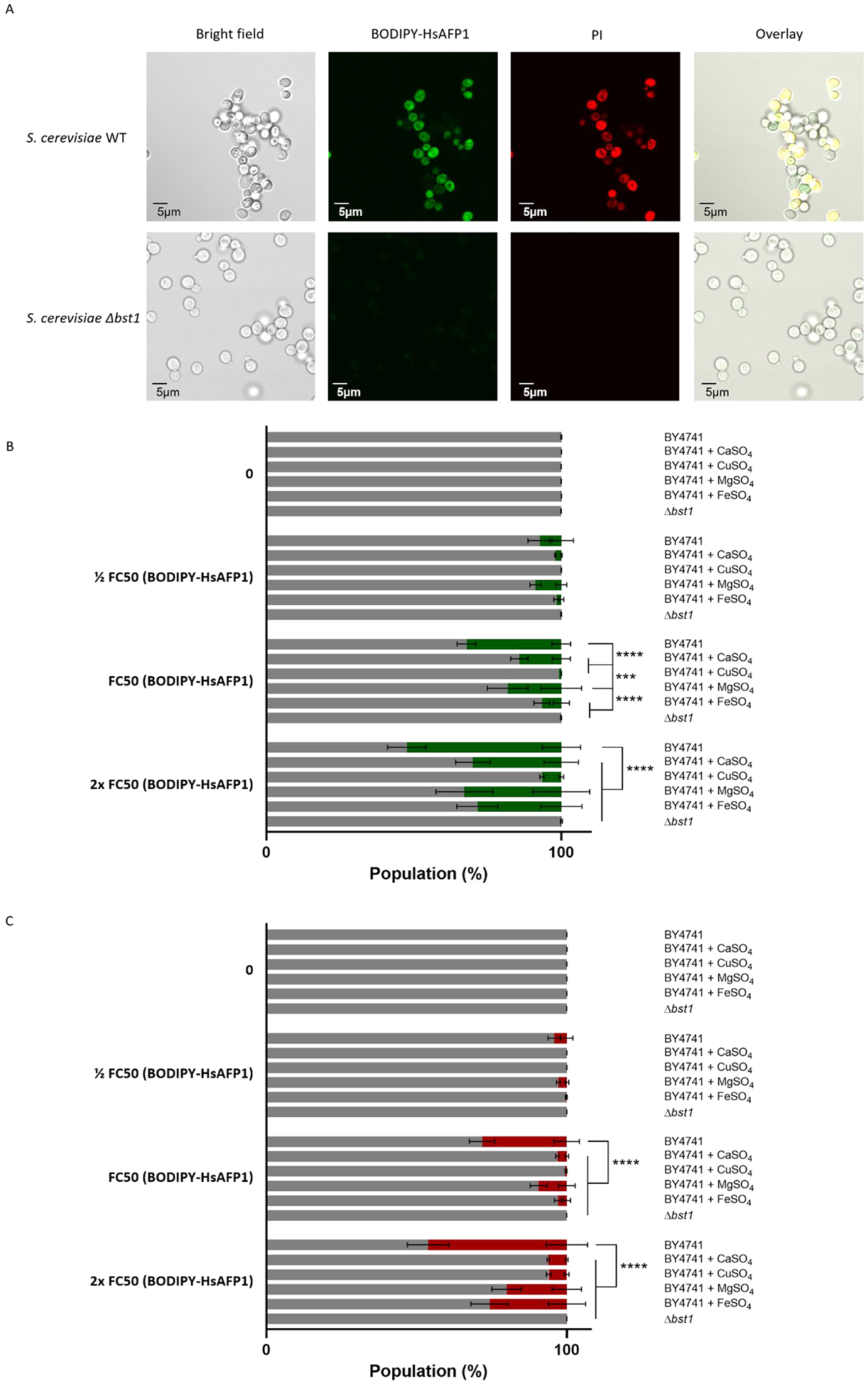
S. cerevisiae WT and Δbst1 mutant cells were treated for 150 minutes in PDB/YPD with 50 mM HEPES pH 7 at 30°C. BODIPY-HsAFP1 and propidium Iodide (PI; 3 μM) were used as markers for peptide internalization and cell death, respectively. (A) Confocal microscope images of S. cerevisiae cells treated with high HsAFP1 doses (10x FC50; 250 μg/mL). Scale bar: 5 μm. (B) Flow cytometry of WT and Δbst1 mutant cells. WT cells are co-administrated with BODIPY-HsAFP1 (1/2 FC50; 12.5 μg/mL, FC50; 25 μg/mL and 2x FC50; 50 μg/mL)and 2 mM CaSO4, 2 mM CuSO4, 2 mM MgSO4 or 2 mM FeSO4. The % of cells that do have BODIPY-HsAFP1 associated with their surface or internalized (green) and the % of cells that do not have BODIPY-HsAFP1 associated with their surface or internalized (grey) is presented. (C) Flow cytometry of WT and Δbst1 mutant cells. WT cells are co-administrated with BODIPY-HsAFP1 (1/2 FC50; 12.5 μg/mL, FC50; 25 μg/mL and 2x FC50; 50 μg/mL) and 2 mM CaSO4, 2 mM CuSO4, 2 mM MgSO4 or 2 mM FeSO4. The % of cells that both have BODIPY-HsAFP1 associated with their surface/internalized and have compromised membranes is presented in red. All other subpopulations are presented in grey, including the % of cells that do not have BODIPY-HsAFP1 internalized or associated with their surface and do not have compromised membranes, the % of cells that do not have BODIPY-HsAFP1 internalized or associated with their surface and do have compromised membranes, and the % of cells that do have BODIPY-HsAFP1 internalized or associated with their surface and do not have compromised membranes. Data are means ± SD for n ≥ 3 independent experiments. To analyze significant differences in the size of the subpopulations, two-way ANOVA followed by Tukey multiple comparison was performed, with *** and **** representing P < 0.001 and P < 0.0001, respectively.
As the transcriptomic data revealed down-regulation of various iron/copper homeostasis genes and up-regulation of various calcium transporter genes upon HsAFP1 treatment (Figure 2 and Supplementary Table S2), we further investigated the role of ions in HsAFP1’s mode of action. In general, divalent cations including Mg2+ are known to reduce the antifungal activity of plant defensins [12]. Hence, we hypothesized that positively charged compounds might interfere with HsAFP1 for its binding to negatively charged cell surfaces and/or internalization [16]. Using flow cytometry, we indeed observed that co-administrating HsAFP1 and cations, such as Ca2+, Cu2+, Mg2+ and Fe2+, inhibited HsAFP1’s interaction with the fungal cell surface/internalization and associated fungal cell killing (Figure 3B–C). Cu2+ was the most effective in reducing the association of BODIPY-HsAFP1 with the cell surface and/or internalization (Figure 3B), whereas the presence of both Ca2+ or Cu2+ resulted in the most effective reduction of cells with permeabilized membranes within the population of BODIPY-HsAFP1 positive cells (Figure 3C).
3.3. HsAFP1 induces autophagy and impairs vacuolar function
In addition, the transcriptomic data also revealed up-regulation of genes involved in macroautophagy, the best characterized type of autophagy (Figure 2 and Supplementary Table S2).
Hence, we measured the autophagy flux in S. cerevisiae cells treated with moderate HsAFP1 doses using the GFP-Atg8 processing assay and an alkaline phosphatase assay. Through the GFP-Atg8 assay, we were able to monitor the vacuolar delivery and subsequent breakdown of GFP-Atg8. As a result, the free GFP levels were detected and quantified by western blot analysis [38]. Data revealed that HsAFP1 treatment led to an accumulation of free GFP, demonstrating enhanced degradation of GFP-Atg8 pointing to increased autophagy (Figure 4A). We also evaluated the effect of equimolar concentrations of HsAFP1 [H32A][R52A], for which no increased free GFP levels were found (Figure 4A). In the second assay, the activity of the vacuolar alkaline phosphatase, Pho8, directly proportional to the cytosolic (cyPho8) cargo delivered to the vacuoles for degradation and hence, indicative for autophagy levels, was determined [38]. In accordance with the GFP-Atg8 assay, we found increased activity of cyPho8 upon HsAFP1 treatment, indicating that autophagy is induced by these HsAFP1 doses (orange bars in Figure 4B). In addition, to test whether HsAFP1-induced autophagy is linked to HsAFP1’s antifungal activity, we evaluated cyPho8 activity in S. cerevisiae cells treated with equimolar concentrations of HsAFP1[H32A][R52A] [16]. In contrast to HsAFP1, no increased cyPho8 activity upon HsAFP1[H32A][R52A] treatment was observed, indicating that autophagy induction is linked to HsAFP1’s antifungal activity (Figure 4B).
Figure 4: Macroautophagy is induced by moderate HsAFP1 doses.
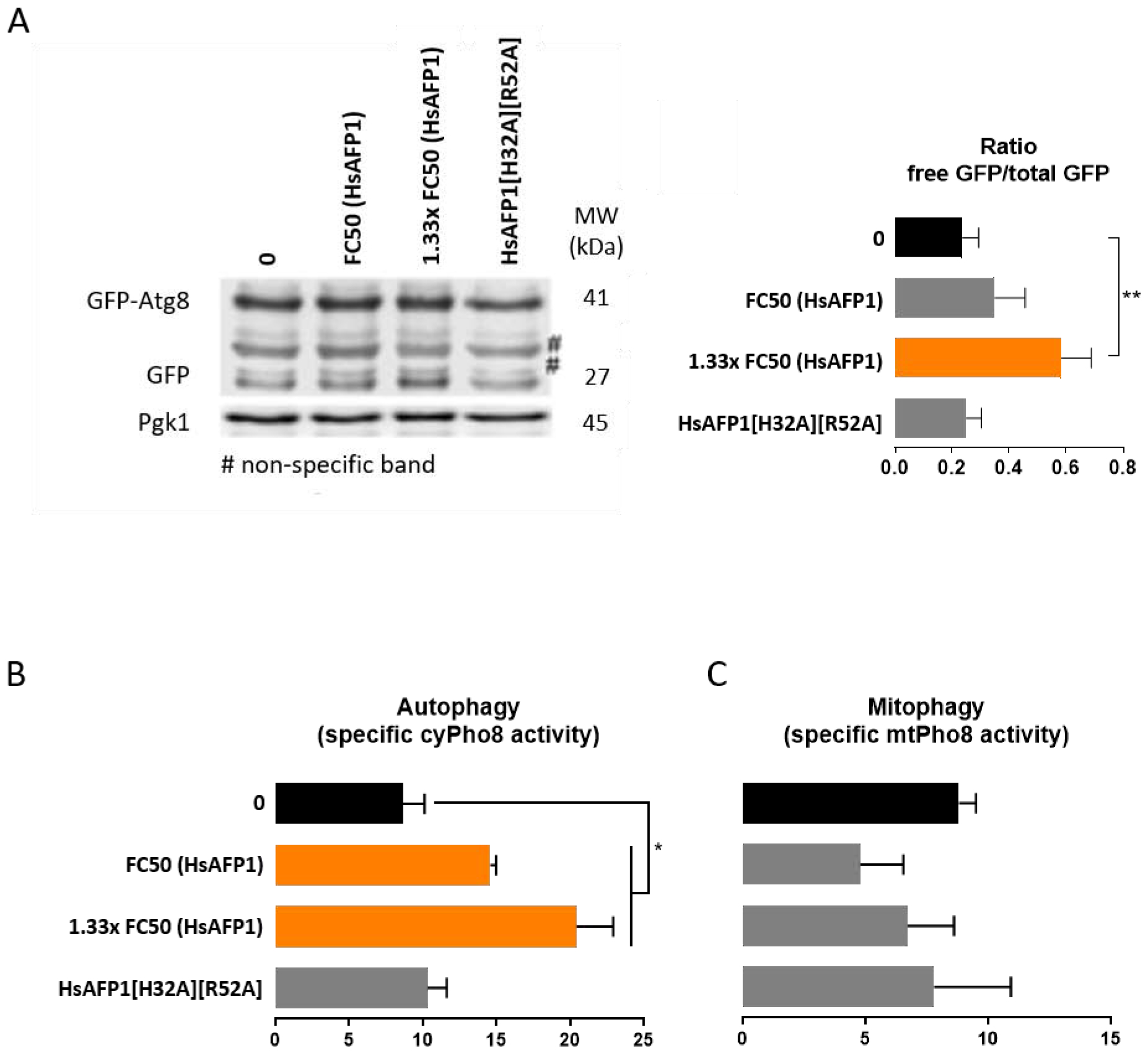
S. cerevisiae WT cells treated 180 min with moderate HsAFP1 (FC50; 25 μg/mL and 1.33x FC50; 33.25 μg/mL) or HsAFP1[H32A][R52A] doses (equivalent to the highest tested HsAFP1 dose) in 1/5th PDB/YNB (supplemented with 2% glucose, histidine (50 μg/mL), methionine (50 μg/mL) and either leucine (300 μg/mL; A) or uracil (100 μg/mL; B-C)) with 50 mM HEPES pH 7 at room temperature. (A) Vacuolar degradation of Atg8 was quantified using western blotting. Increase of free GFP levels upon HsAFP1 treatment demonstrate an increased degradation of GFP-Atg8, pointing to autophagy upon HsAFP1 treatment. Equimolar concentrations of HsAFP1[H32A][R52A] did not result in increased free GFP levels. One western blot of a representative experiment is shown. The ratio of free GFP / total GFP (mean ± SD, for n = 3 independent experiments) is normalized using the control protein Pgk1. (B) Dose-dependent increase of the specific cyPho8 activity of HsAFP1-treated S. cerevisiae cells (mean ± SD, for n = 3 independent experiments) as compared to the control (MQ water; black bar), determined via an alkaline phosphatase assay. Equimolar HsAFP1[H32A][R52A] doses (as the highest tested HsAFP1 dose) did not affect the specific cyPho8 activity. (C) Specific mtPho8 activity of HsAFP1-treated S. cerevisiae cells (mean ± SD, for n = 3 independent experiments) as compared to the control (MQ water; black bar), determined via an alkaline phosphatase assay. No increase of the specific mtPho8 activity could be demonstrated. Equimolar HsAFP1[H32A][R52A] doses (as the highest tested HsAFP1 dose) did not affect the specific mtPho8 activity. (A-C) Significant differences between the control treatment (black bars) and all other treatments were determined via one-way ANOVA followed by Dunnett multiple comparison, with * and ** representing P < 0.05 and P < 0.01, respectively (presented by orange bars).
The transcriptomic data also revealed down-regulation of one gene involved in mitophagy, as DCK1 is directly annotated to the term ‘autophagy of mitochondrion’ according to GO term finder (Figure 2 and Supplementary Table S2), a process that includes the selective degradation of mitochondria (mt) by autophagy [39]. To investigate whether mitophagy is involved in yeast tolerance to HsAFP1, we evaluated mtPho8 activity in treated S. cerevisiae cells (Figure 4C). No increased expression of mtPho8 upon treatment with moderate HsAFP1 doses could be observed, indicating that HsAFP1-induced autophagy does not include degradation of mitochondria.
Functional vacuoles are indispensable for the induction of autophagy [40, 41]. We previously reported that S. cerevisiae deletion mutants defective for vacuolar H+-ATPase function (Δvma mutants) have increased sensitivity to HsAFP1 [17], pointing to a role for functional vacuoles in governing tolerance to HsAFP1. Hence, we further assessed vacuolar functioning in the presence of various doses of HsAFP1. The vacuolar pH in functional vacuoles is typically between 5.6 and 6.3, while elevated pH has been linked to dysfunctional vacuoles [26, 42]. Here, we assessed the vacuolar pH in HsAFP1-treated S. cerevisiae cells using the vacuolar dye BCECF-AM and the vacuolar H+-ATPase inhibitor concanamycin A (ConA) as a positive control [27]. ConA-treated cells were characterized by significantly increased vacuolar pH, while their survival was not affected (Figure 5A). In the case of HsAFP1 treatment, the vacuolar pH of treated cells was increased in a dose-dependent manner and was significantly different from control cells at high HsAFP1 doses (2x FC50; 50 μg/mL, orange bars Figure 5A). In contrast to HsAFP1, the vacuolar pH was not affected by equimolar doses of HsAFP1[H32A][R52A] [16] (Figure 5A).
Figure 5: Vacuolar dysfunction is induced by high HsAFP1 doses.
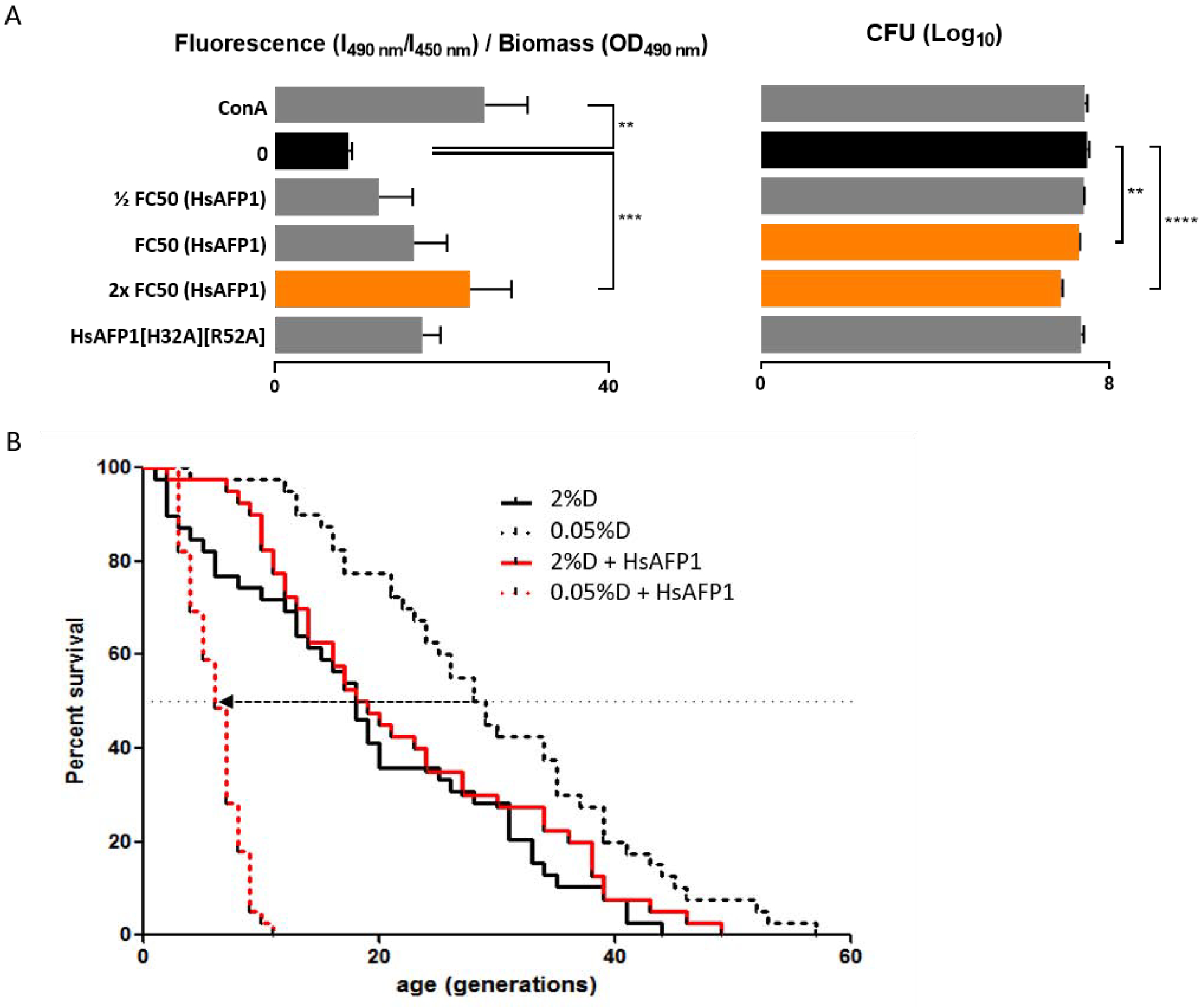
(A) S. cerevisiae WT cells treated 150 min with HsAFP1 (1/2 FC50; 12.5 μg/mL, FC50; 25 μg/mL and 2x FC50; 50 μg/mL) or HsAFP1[H32A][R52A] doses (equivalent to the highest tested HsAFP1 dose) in PDB/YPD with 50 mM HEPES pH 7 at 30°C. Dose-dependent increase of the vacuolar pH and decrease of survival of HsAFP1-treated S. cerevisiae cells as compared to the control (MQ water; black bars), determined via the vacuolar dye BCECF-AM and CFU counting, respectively. Concanamycin A (ConA) was used as positive control for increased vacuolar pH, whereas cell survival was not affected. Equimolar HsAFP1[H32A][R52A] doses (as the highest tested HsAFP1 dose) did not affect vacuolar pH or survival. Means ± SD are presented for n ≥ 3 independent experiments. Significant differences in vacuolar pH or Log10 numbers of CFU between the negative control (MQ water; black bars) and all other treatments were determined via one-way ANOVA followed by Dunnett multiple comparison, with **, *** and **** representing, P < 0.01, P < 0.001 and P < 0.0001, respectively (presented by orange bars). (B) Decrease in replicative lifespan of S. cerevisiae BY4742 cells by HsAFP1 treatment under dietary restriction (DR) conditions, as represented by the dotted arrow. Replicative lifespan of cells on agar plates in the presence or absence of HsAFP1 and containing 2% or 0.05% glucose (= dextrose (D)), with 0.05% glucose representing DR conditions. The HsAFP1 dose used is the maximum dose that does not affect replicative lifespan in 2% glucose. Replicative lifespan results are presented from an experiment containing n = 40 cells tested per condition.
Together, our results point to autophagy as an important S. cerevisiae tolerance mechanism to cope with moderate HsAFP1 doses (≤ FC50; 25 μg/mL). However, at high HsAFP1 doses (≥ 2x FC50; 50 μg/mL), vacuoles are affected, possibly resulting in impaired autophagy. These data are consistent with the model that vacuolar dysfunction induced by high HsAFP1 doses results in effective killing of S. cerevisiae cells.
Functional vacuoles are also essential for replicative lifespan (RLS) under dietary restriction (DR) conditions, as mutants defective for vacuolar ATPase activity show a dramatic shortening of RLS by DR [21]. As HsAFP1 impairs vacuolar function, we investigated whether HsAFP1 also affects RLS under DR (0.05% glucose). Indeed, moderate HsAFP1 doses reduced RLS under DR by > 3-fold, while these doses had no effect on RLS under non-DR conditions (2% glucose) (Figure 5B). These results corroborate the above findings on HsAFP1-induced vacuolar dysfunction, potentially affecting RLS in yeast. Note however, that RLS is the result of a multifactorial process and that apart from vacuolar dysfunction, other factors may contribute as well.
3.4. HsAFP1 affects S. cerevisiae cell cycle in the G2/M phase
The transcriptomic data further revealed that the GO groups cellular bud and polarized growth, including genes involved in the cell cycle, were significantly up-regulated upon HsAFP1 treatment (Figure 2 and Supplementary Table S2). Moreover, two genes coding for cyclins, PCL1 and PCL2, were identified as top18- and top47-induced upon HsAFP1 treatment, respectively (Supplementary Table S1). Therefore, we investigated whether up-regulation of these genes resulted from HsAFP1-induced cell cycle impairment. For that, we analyzed the DNA content using flow cytometry and found that moderate HsAFP1 doses did not have any impact on the cell cycle profile. Although, a cell cycle impairment at G2/M phase was detected (Figure 6A), this impairment was also present upon treatment with the HsAFP1 mutant [16], HsAFP1[H32A][R52A], when tested at equimolar concentrations, indicating that HsAFP1-induced cell cycle impairment is not linked to its antifungal activity (Figure 6A–B). Therefore, we hypothesized that cell cycle impairment is a rather non-specific secondary effect than the primary cause of HsAFP1’s killing activity. Whether the HsAFP1-induced effects on G2/M cell cycle result in increased hyphal branching, as documented before in case of HsAFP1 treatment of the filamentous fungus Fusarium culmorum [12], remains unclear.
Figure 6: Cell cycle impairment in the G2/M phase is induced by moderate HsAFP1 doses.
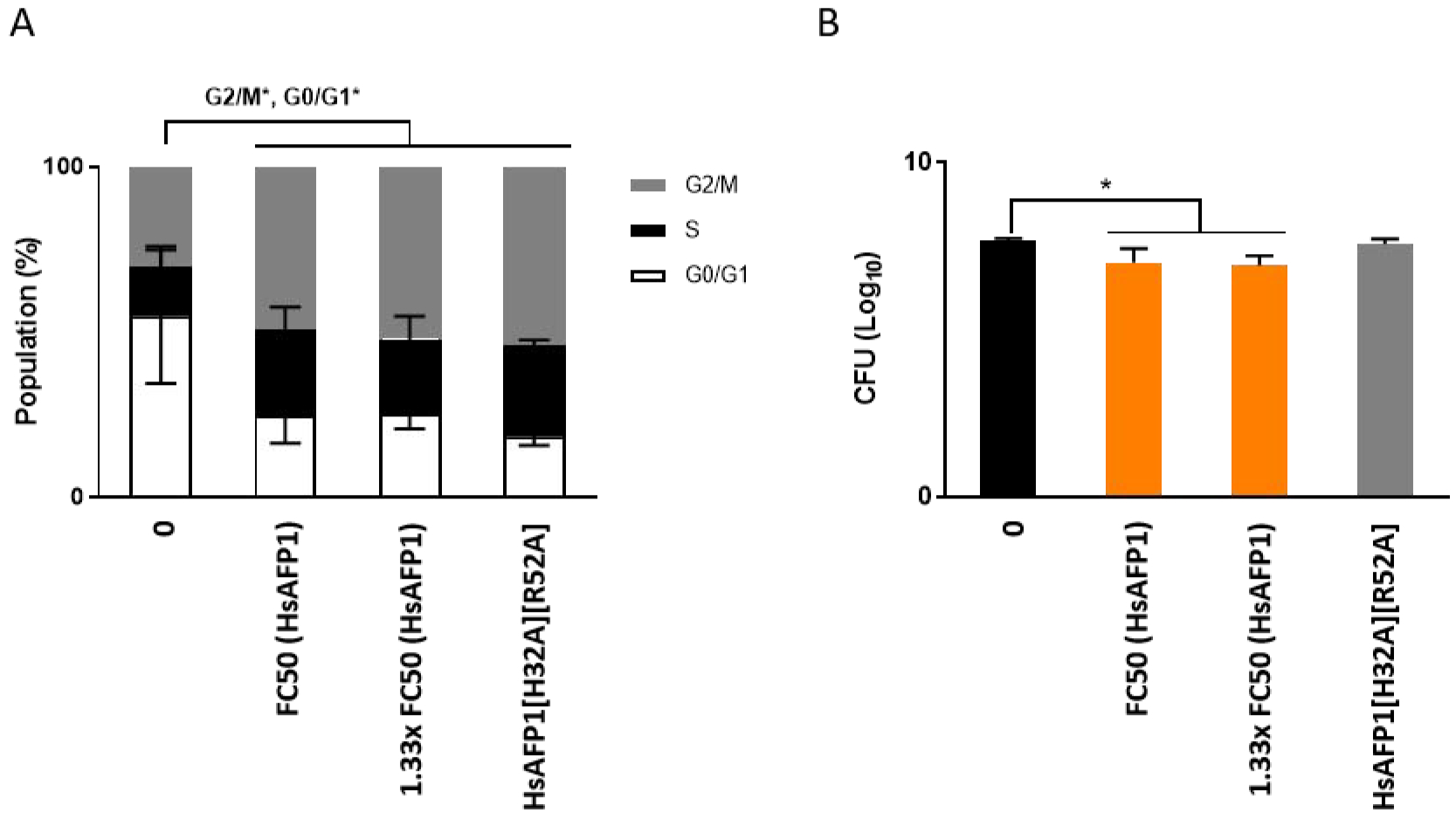
S. cerevisiae WT cells treated 180 min with moderate HsAFP1 (FC50; 25 μg/mL and 1.33x FC50; 33.25 μg/mL) or HsAFP1[H32A][R52A] doses (equivalent to the highest tested HsAFP1 dose) in 1/5th PDB/YNB (supplemented with 2% glucose, histidine (50 μg/mL), leucine (300 μg/mL), methionine (50 μg/mL) and uracil (100 μg/mL)) with 50 mM HEPES pH 7 at room temperature. (A) Grey, black and white bars represent the percentage of cells (mean ± SD, for n = 3 independent experiments) in the G2/M-, S-, and G0/G1-phase, respectively. Significant differences in the population size of each phase between the control treatment and all other treatments were determined via two-way ANOVA followed by Tukey multiple comparison, with * representing P < 0.05. (B) The amount of viable cells after treatment with HsAFP1 or HsAFP1[H32A][R52A] was determined via CFU counting. Log10 numbers of CFU ± SD, for n = 3 independent experiments. Significant differences between the control treatment (black bar) and all other treatments were determined via one-way ANOVA followed by Dunnett multiple comparison, with * representing P < 0.05 (presented by orange bars in (B)).
4. Conclusion
In this study, we further investigated the mode of action of HsAFP1 via a transcriptome analysis on C. albicans cells. Various genes and/or pathways, important for HsAFP1’s antifungal activity or tolerance to HsAFP1’s action were identified based on differentially expressed gene clusters upon HsAFP1 treatment. The main affected pathways were linked to GPI-anchored proteins, cation homeostasis, autophagy and the cell cycle, as numerous genes involved in these pathways were up- or down-regulated in C. albicans upon HsAFP1 treatment. In a previous genome-wide study on HsAFP1’s mode of action, we screened a S. cerevisiae deletion mutant library for both hypersensitivity and resistance to HsAFP1 [17]. The main affected pathways identified in that study were related to cell wall organization, stress responses and vacuolar acidification [17].
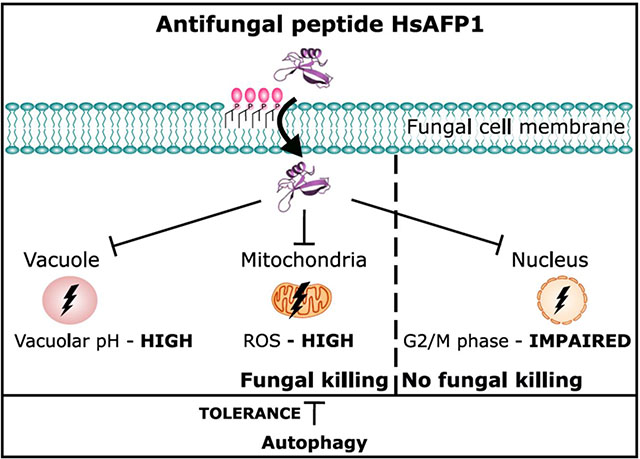
We propose the following scheme (Figure 7) for the mode of action of HsAFP1 (right panel), either resulting in fungal killing or not, and the effect of HsAFP1 on replicative life span (RLS) (left panel). The scheme integrates all findings from our 3 previous reports [12, 16, 17] (blue boxes) and the findings of our current study (red boxes). Firstly, HsAFP1 is internalized in S. cerevisiae cells, by targeting cell surface GPI-anchored proteins and/or their GPI-anchor. This internalization is at least partially endocytosis-mediated, as it was significantly reduced in a S. cerevisiae mutant that is affected in endocytosis (Δend3) [16]. Due to their positive charge, divalent cations inhibit the uptake of HsAFP1. Upon interaction and/or internalization, HsAFP1 induces different cellular pathways resulting in killing of S. cerevisiae cells. These include (i) mitochondrial dysfunction, resulting in the production of endogenous reactive oxygen species (ROS) and induction of apoptosis [17], (ii) vacuolar dysfunction, and (iii) cell cycle impairment (the latter is not linked to HsAFP1’s antifungal action). In contrast to high HsAFP1 doses (≥ 2x FC50; 50 μg/mL), moderate HsAFP1 doses (≤ FC50; 25 μg/mL) induce autophagy, probably as a fungal tolerance/pro-survival mechanism against the HsAFP1 action. Dietary restriction (DR) extends RLS, thereby maintaining an acidic vacuolar pH [21]. The disruption of vacuolar pH homeostasis, via e.g. HsAFP1 administration, results in a shortened RLS under DR (left panel).
Figure 7: Model for the mode of action of HsAFP1 on yeast cells.
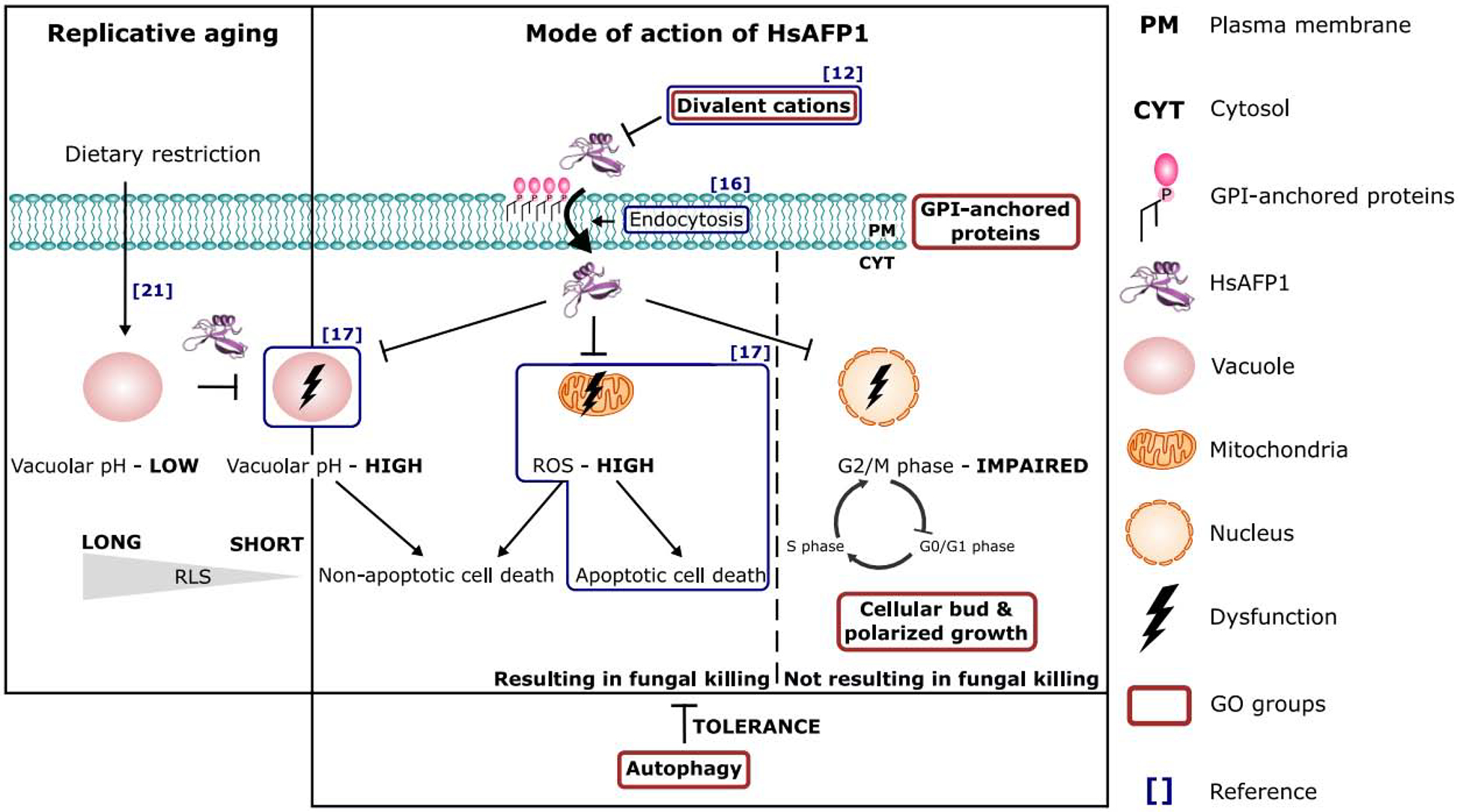
The model is based on a compilation of previous reports [12, 16, 17, 21] and results from the current study. We refer to the conclusion section for detailed description.
Supplementary Material
Supplementary Table S1: Genes and expression profile of Candida albicans SC5314 cultures treated with HsAFP1 (2x FC50; 10 μg/mL) or control (MQ water) in PDB/YPD with 50 mM HEPES pH 7, determined via RNA-seq analysis.
Supplementary Table S2: GO analysis of the differentially expressed genes (FDR < 0.05 and |log(Fold Change)| > 1) between the control (MQ water) and HsAFP1 treatment, as determined via the Candida Genome Database GO Term Finder Tool on www.candidagenome.org.Gene clusters (based on cellular component, biological process or molecular function) that were enriched in the HsAFP1 up-regulated or down-regulated genes are presented, with the cluster frequency being the relative amount of genes in the pool that corresponds to a specific gene cluster and the FDR (False Discovery Rate) corresponding to the significance by which the gene cluster was selected. FDR < 0.05 was considered as significantly enriched.
Highlights.
GPI-anchored proteins mediate HsAFP1’s internalization
HsAFP1 induces autophagy and impairs vacuolar function
HsAFP1 affects S. cerevisiae cell cycle in the G2/M phase Graphical abstract
7. Acknowledgements
Special thanks to the lab of Prof. Patrick Van Dijck (VIB-KU Leuven) for the use of their confocal microscope.
8. Funding
This work was supported by Fonds Wetenschappelijk Onderzoek (FWO) - Vlaanderen (FWO Bilateral Project (G.0D51.13N), research projects (G0080016N and G0C6418N), travel grant (K218717N), SB fellowship (1S38618N)), NIH grant P30AG013280 to MK and the Industrial Research Fund - KU Leuven (IOFm/05/022 to KT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Fisher MC, Henk DA, Briggs CJ et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484(7393), 186–194 (2012). DOI: 10.1038/nature10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauthier GM, Keller NP. Crossover fungal pathogens: the biology and pathogenesis of fungi capable of crossing kingdoms to infect plants and humans. Fungal Genetics and Biology 61, 146–157 (2013). DOI: 10.1016/j.fgb.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 3.Maubon D, Garnaud C, Calandra T et al. Resistance of Candida spp. to antifungal drugs in the ICU: where are we now? Intensive Care Medicine 40(9), 1241–1255 (2014). DOI: 10.1007/s00134-014-3404-7 [DOI] [PubMed] [Google Scholar]
- 4.Cools TL, Struyfs C, Cammue BP et al. Antifungal plant defensins: increased insight in their mode of action as a basis for their use to combat fungal infections. Future Microbiology 12, 441–454 (2017). DOI: 10.2217/fmb-2016-0181 [DOI] [PubMed] [Google Scholar]
- 5.De Souza Candido E, E Silva Cardoso MH, Sousa DA et al. The use of versatile plant antimicrobial peptides in agribusiness and human health. Peptides 55, 65–78 (2014). DOI: 10.1016/j.peptides.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Shafee TM, Lay FT, Hulett MD et al. The defensins consist of two independent, convergent protein superfamilies. Molecular Biology and Evolution (2016). DOI: 10.1093/molbev/msw106 [DOI] [PubMed] [Google Scholar]
- 7.Carvalho AO, Gomes VM. Plant defensins - prospects for the biological functions and biotechnological properties. Peptides 30(5), 1007–1020 (2009). DOI: 10.1016/j.peptides.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 8.De Coninck B, Cammue BP, Thevissen K. Modes of antifungal action and in planta functions of plant defensins and defensin-like peptides. Fungal Biology Reviews 26, 10–120 (2013). DOI: 10.1016/j.fbr.2012.10.002 [DOI] [Google Scholar]
- 9.Tavares PM, Thevissen K, Cammue BP et al. In vitro activity of the antifungal plant defensin RsAFP2 against Candida isolates and its in vivo efficacy in prophylactic murine models of candidiasis. Antimicrobial Agents & Chemotherapy 52(12), 4522–4525 (2008). DOI: 10.1128/AAC.00448-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thevissen K, Kristensen HH, Thomma BP et al. Therapeutic potential of antifungal plant and insect defensins. Drug Discovery Today 12(21), 966–971 (2007). DOI: 10.1016/j.drudis.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 11.Vriens K, Cools TL, Harvey PJ et al. Synergistic activity of the plant defensin HsAFP1 and caspofungin against Candida albicans biofilms and planktonic cultures. PLoS One 10(8), e0132701 (2015). DOI: 10.1371/journal.pone.0132701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborn RW, De Samblanx GW, Thevissen K et al. Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Letters 368(2), 257–262 (1995). DOI: 10.1016/0014-5793(95)00666-w [DOI] [PubMed] [Google Scholar]
- 13.Gao AG, Hakimi SM, Mittanck CA et al. Fungal pathogen protection in potato by expression of a plant defensin peptide. Nature Biotechnology 18(12), 1307–1310 (2000). DOI: 10.1038/82436 [DOI] [PubMed] [Google Scholar]
- 14.Lacerda AF, Vasconcelos EA, Pelegrini PB et al. Antifungal defensins and their role in plant defense. Frontiers in Microbiology 5, 116 (2014). DOI: 10.3389/fmicb.2014.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thevissen K, Osborn RW, Acland DP et al. Specific, high affinity binding sites for an antifungal plant defensin on Neurospora crassa hyphae and microsomal membranes. Journal of Biological Chemistry 272(51), 32176–32181 (1997). DOI: 10.1074/jbc.272.51.32176 [DOI] [PubMed] [Google Scholar]
- 16.Cools TL, Vriens K, Struyfs C et al. The antifungal plant defensin HsAFP1 is a phosphatidic acid-interacting peptide inducing membrane permeabilization. Frontiers in Microbiology 21(8), 2295 (2017). DOI: 10.3389/fmicb.2017.02295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aerts AM, Bammens L, Govaert G et al. The antifungal plant defensin HsAFP1 from Heuchera sanguinea induces apoptosis in Candida albicans. Frontiers in Microbiology 2, 47 (2011). DOI: 10.3389/fmicb.2011.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134(3), 717–728 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guedes A, Ludovico P, Sampaio-Marques B Caloric restriction alleviates alpha-synuclein toxicity in aged yeast cells by controlling the opposite roles of Tor1 and Sir2 on autophagy Mechanisms of Ageing and Development. 161, 270–276 (2017). DOI: 10.1016/j.mad.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Mendl N, Occhipinti A, Müller M et al. Mitophagy in yeast is independent of mitochondrial fission and requires the stress response gene WHI2. Journal of Cell Science, 124, 1339–1350, (2011). DOI: 10.1242/jcs.076406 [DOI] [PubMed] [Google Scholar]
- 21.Schleit J, Johnson SC, Bennett CF et al. Molecular mechanisms underlying genotype-dependent responses to dietary restriction. Aging Cell 12(6), 1050–1061 (2013). DOI: 10.1111/acel.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risso D, Schwartz K, Sherlock G et al. GC-content normalization for RNA-seq data. BMC Bioinformatics 12, 480 (2011). DOI: 10.1186/1471-2105-12-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology 11(3), R25 (2010). DOI: 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of Royal Statistical Society 57, 289–300 (1995). DOI: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 25.Skrzypek MS, Arnaud MB, Costanzo MC et al. New tools at the Candida Genome Database: biochemical pathways and full-text literature search. Nucleic Acids Research 38(Database issue), D428–432 (2010). DOI: 10.1093/nar/gkp836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diakov TT, Tarsio M, Kane PM. Measurement of vacuolar and cytosolic pH in vivo in yeast cell suspensions. Journal of Visualized Experiments 74 (2013). DOI: 10.3791/50261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaeberlein M, Kirkland KT, Fields S et al. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biology 2(9), E296 (2004). DOI: org/10.1371/journal.pbio.0020296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steffen KK, Kennedy BK, Kaeberlein M. Measuring replicative life span in the budding yeast. Journal of Visualized Experiments 28 (2009). DOI: 10.3791/1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noda T, Klionsky DJ. The quantitative Pho8Delta60 assay of nonspecific autophagy. Methods Enzymology 451, 33–42 (2008). DOI: 10.1016/S0076-6879(08)03203-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T, Lei J, Yang H et al. An improved method for whole protein extraction from yeast Saccharomyces cerevisiae Yeast 28, 795–798, (2011). DOI: 10.1002/yea.1905 [DOI] [PubMed] [Google Scholar]
- 31.Cheong H, Nair U, Geng J et al. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Molecular Biology of the Cell 19(2), 668–681 (2008). DOI: 10.1091/mbc.e07-08-0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Abdelmohsen K, Abe A et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) [published correction appears in Autophagy. 2016;12(2):443. [DOI] [PMC free article] [PubMed] [Google Scholar]; Selliez Iban [corrected to Seiliez, Iban]]. Autophagy 12(1), 1–222 (2016). DOI: 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vriens K, Tewari Kumar P, Struyfs C et al. Increasing the fungicidal action of amphotericin B by inhibiting the nitric oxide-dependent tolerance pathway. Oxidative Medicine and Cellular Longevity 2017, 4064628 (2017). DOI: 10.1155/2017/4064628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan E, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 53(282), 457–481 (1958). DOI: 10.2307/2281868 [DOI] [Google Scholar]
- 35.Van Dijck P, Sjollema J, Cammue BPA et al. Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microbial Cell 5(7), 300–326 (2018). DOI: 10.15698/mic2018.07.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka S, Maeda Y, Tashima Y et al. Inositol deacylation of glycosylphosphatidylinositol-anchored proteins is mediated by mammalian PGAP1 and yeast Bst1p. Journal of Biological Chemistry 279(14), 14256–14263 (2004). DOI: 10.1074/jbc.M313755200 [DOI] [PubMed] [Google Scholar]
- 37.Murakami Y, Tawamie H, Maeda Y et al. Null mutation in PGAP1 impairing Gpi-anchor maturation in patients with intellectual disability and encephalopathy. PLoS Genetics 10(5), e1004320 (2014). DOI: 10.1371/journal.pgen.1004320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kainz K, Tadic J, Zimmermann A et al. Methods to Assess Autophagy and Chronological Aging in Yeast. Methods Enzymology 588, 367–394 (2017). DOI: 10.1016/bs.mie.2016.09.086 [DOI] [PubMed] [Google Scholar]
- 39.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Research 8(1), 3–5 (2005). DOI: 10.1089/rej.2005.8.3 [DOI] [PubMed] [Google Scholar]
- 40.Deprez MA, Eskes E, Wilms T et al. pH homeostasis links the nutrient sensing PKA/TORC1/Sch9 ménage-à-trois to stress tolerance and longevity. Microbial Cell 5(3), 119–136 (2018). DOI: 10.15698/mic2018.03.618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampaio-Marques B, Burhans WC, Ludovico P Yeast at the Forefront of Research on Ageing and Age-Related Diseases In: Sá-Correia I. (eds) Yeasts in Biotechnology and Human Health. Progress in Molecular and Subcellular Biology, 58, Springer, (2019). DOI: 10.1007/978-3-030-13035-0_9 [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Munoz GA, Kane P. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. Journal of Biological Chemistry 283(29), 20309–20319 (2008). DOI: 10.1074/jbc.M710470200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Genes and expression profile of Candida albicans SC5314 cultures treated with HsAFP1 (2x FC50; 10 μg/mL) or control (MQ water) in PDB/YPD with 50 mM HEPES pH 7, determined via RNA-seq analysis.
Supplementary Table S2: GO analysis of the differentially expressed genes (FDR < 0.05 and |log(Fold Change)| > 1) between the control (MQ water) and HsAFP1 treatment, as determined via the Candida Genome Database GO Term Finder Tool on www.candidagenome.org.Gene clusters (based on cellular component, biological process or molecular function) that were enriched in the HsAFP1 up-regulated or down-regulated genes are presented, with the cluster frequency being the relative amount of genes in the pool that corresponds to a specific gene cluster and the FDR (False Discovery Rate) corresponding to the significance by which the gene cluster was selected. FDR < 0.05 was considered as significantly enriched.


