Abstract
Purpose
The exocyst complex is a conserved protein complex that mediates fusion of intracellular vesicles to the plasma membrane and is implicated in processes including cell polarity, cell migration, ciliogenesis, cytokinesis, autophagy, and fusion of secretory vesicles. The essential role of these genes in human genetic disorders, however, is unknown.
Methods
We performed homozygosity mapping and exome sequencing of consanguineous families with recessively inherited brain development disorders. We modeled an EXOC7 splice variant in vitro and examined EXOC7 messenger RNA (mRNA) expression in developing mouse and human cortex. We modeled exoc7 loss-of-function in a zebrafish knockout.
Results
We report variants in exocyst complex members, EXOC7 and EXOC8, in a novel disorder of cerebral cortex development. In EXOC7, we identified four independent partial loss-of-function (LOF) variants in a recessively inherited disorder characterized by brain atrophy, seizures, and developmental delay, and in severe cases, microcephaly and infantile death. In EXOC8, we found a homozygous truncating variant in a family with a similar clinical disorder. We modeled exoc7 deficiency in zebrafish and found the absence of exoc7 causes microcephaly.
Conclusion
Our results highlight the essential role of the exocyst pathway in normal cortical development and how its perturbation causes complex brain disorders.
Keywords: exocyst, EXOC7, EXOC8, microcephaly, developmental delay
INTRODUCTION
Eight genes in the human genome, EXOC1–EXOC8, encode the exocyst complex, a multimeric, evolutionarily conserved complex that traffics vesicles within the cell to the plasma membrane for fusion. The exocyst complex has been shown to play a role in several cellular processes, including cell polarity, cell migration, ciliogenesis, cytokinesis, autophagy, and fusion of secretory vesicles,1 but human disorders associated with definitive loss-of-function variants in any of these components have not yet been reported. Although a missense variant in EXOC82 was reported in a single case of Joubert syndrome (MIM 213300), and a missense variant in EXOC43 was reported in a case of Meckel–Gruber syndrome (MIM 249000), the pathogenicity of these two variants has not yet been confirmed. As such, the essential role of individual proteins of the exocyst complex remains unclear.
Several of the reported functions of the exocyst complex, cell polarity and migration, cytokinesis, and ciliogenesis,1,4–6 are integral processes during cerebral cortical development and so motivated us to test the hypothesis that variants in exocyst encoding genes cause brain development disorders. First, establishing cell polarity and cell migration are essential for cortical development. Variants in radial glial cell progenitors (RGC) polarity genes, such as Pals1 and Par3, disrupt cortical development through massive cell death or premature cell cycle exit, respectively.7,8 In addition, variants in genes required for neuronal migration can cause one of several cortical malformations, including lissencephaly (MIM 607432, PAFAH1B1/LIS19), double cortex syndrome (MIM 300067, DCX10,11), and cortical dysplasia (MIM 610031, TUBB3,12 TUBB5,13 KIF5C,14 KIF2A14). Second, robust and rapid cell division of cortical progenitors is essential for cortical development and several genetic causes of microcephaly (MIM 251200) exhibit disrupted cytokinesis as a result of supernumerary (CDK5RAP2,15,16 KATNB117,18), or missing spindle poles (ASPM,19 WDR6220) stemming from dysfunctional centrosomes.21 Finally, defects in cilia formation lead to ciliopathy syndromes (MIM 209900), complex syndromes with disrupted brain development (INPP5E,22 C2CD3,23 BBS124).
Here we provide a systematic analysis of variants in two exocyst components, by defining several variants in EXOC7 and EXOC8. We identify four independent, partial loss-of-function variants in EXOC7, associated with developmental brain disorders of variable severity characterized by developmental delay, seizures, brain atrophy, microcephaly, and infantile death. We also describe one loss-of-function variant in EXOC8 similarly associated with severe developmental delay, seizures, brain atrophy, microcephaly, and premature death. We further provide a zebrafish genetic model of EXOC7 loss-of-function and offer genetic evidence that EXOC7 is required for neuron survival.
MATERIALS AND METHODS
Human subjects
This study was conducted with the approval of institutional review boards and according to the ethical standards of the participating institutions: Boston Children’s Hospital; University of California–San Diego; the Faculty of Medicine, United Arab Emirates University; and AP-HP Sorbonne Université. Informed consent was received from all participants. Permission was received to publish patient photographs.
IACUC approval of zebrafish housing and experiments
A complete description of the husbandry and environmental conditions in housing for the fish used in these experiments is available as a collection in protocols.io (10.17504/protocols.io.mrjc54n). All animals were cared for humanely and all experiments were approved by Boston Children’s Hospital Institutional Animal Care and Use Committee (IACUC).
Exome sequencing
DNA was extracted from whole blood and exome sequencing was performed (See Supplement). We filtered out variants with allele frequency >10% in the Middle Eastern population.25
Sanger sequencing
Primers surrounding the reported variant in each family were used for polymerase chain reaction (PCR) and subsequent Sanger sequencing to confirm genotype from exome sequencing and determine segregation within the family.
Minigene cloning and expression
A ~5-kb section of human EXOC7 locus was amplified with primers (F: AAGGACTGAAGGAGCATTTC, R: CAGGGAGTCGAAGGTCTTCT) from a BAC and cloned into pCAG expression vector. The splice acceptor variant from family I was introduced with site-directed mutagenesis. Wild-type (WT) or splice variant containing vector was transfected into mouse N2A cells and after 48 hours RNA was isolated and retrotranscribed into complementary DNA (cDNA). N2A cells were cultured at 37 °C and 5% CO2 in high-glucose DMEM (GIBCO) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin.
HAP1 mutant cell line
HAP1 human cell line was cultured at 37 °C and 5% CO2 in high-glucose DMEM (GIBCO) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. The splice acceptor variant from family I was introduced into a HAP1 cell line as a hemizygous variant using CRISPR/Cas9 mutagenesis (Supplemental Methods)26 (Horizon Discovery). Immunoblot was performed using human EXOC7 antibody (Abcam, ab118792).
Exoc7 alternative splicing in developing cortex
Alternative splicing analysis of EXOC7 in developing human (GW13–16) and mouse (E14.5) cortex was performed as described previously.27 Aligned BAM files from RNA sequencing data sets were analyzed with the MISO pipeline (version 0.4.6) to determine the inclusion frequency of alternatively spliced exons.
Generation of exoc7 mutant zebrafish lines
Exoc7 mutant zebrafish were generated by CRISPR/Cas9 mutagenesis. Cas9 messenger RNA (mRNA) (250 ng/μl) and exoc7 targeting guide RNA (target: CCGTCCTCATCCTGGACGCC, 80 ng/μl) were injected into 1-cell embryos. Embryos developed to adulthood and then Sanger sequencing was used to identify potential heterozygous exoc7 mutant carriers in F1 progeny. A 1-bp frameshift deletion in exon 5 was identified and this fish was backcrossed to WT to generate heterozygous carriers. This allele is mh111.
Toluidine blue staining of zebrafish
Five days postfertilization (dpf) embryos were fixed in 4% PFA overnight at 4 °C and then embedded in JB-4 resin according to manufacturer’s protocol (Polysciences Inc). Fish were sectioned at 2 μm, and then matching sections were stained with toluidine blue and imaged with a bright-field microscope.
Immunostaining of zebrafish progenitor cells
Five dpf embryos were fixed in 4% PFA overnight at 4 °C, embedded in OCT, and sectioned coronally at 20 μm on a cryostat. Matched sections were stained with a primary antibody against Sox2 (Abcam, ab97959). Tissue was permeabilized and blocked in 3% BSA, 0.3% Triton X-100, 0.3% sodium azide in PBS. Primary antibodies were incubated overnight at 4 °C. Sections were stained with Alexa secondary antibodies and Hoechst. Imaging was done on Zeiss 510 confocal microscope. Sox2-positive nuclei in telencephalon were counted.
TUNEL staining in developing zebrafish
Five dpf embryos were fixed in 4% PFA overnight at 4 °C, embedded in OCT, and cryosectioned coronally. Apoptotic cells in matched sections were labeled with TUNEL staining using the Apoptag kit (Millipore) according to the manufacturer’s instructions. Imaging was done on Zeiss 510 confocal microscope. TUNEL positive cells in telencephalon were counted.
RNAscope
RNAscope on human fetal brain tissue was performed according to manufacturer’s protocol (ACDBio). Tissue was fixed in 4% PFA, frozen, and sectioned at 20 μm on a cryostat.
Quantification and statistical analysis
In all analyses, mean values are presented for pooled data and errors bars are SEM. For all quantifications, statistical significance was determined using a two-tailed, unpaired t test (GraphPad Prism).
RESULTS
EXOC7 and EXOC8 variants in recessive developmental disorders
In mapping developmental disorders affecting the cerebral cortex, we identified variants in EXOC7 and EXOC8 associated with recessive brain development syndromes with a range of symptom severity including developmental delay, seizures, brain atrophy, microcephaly, and infantile death (Table 1).
Table 1.
Variant summary for each family.
| Family I | Family II | Family III | Family IV | Family V | |
|---|---|---|---|---|---|
| Maximum LOD score | 1.93 | Singleton | Nonconsanguineous | 2.9 | 2.5 |
| Gene | EXOC7 | EXOC7 | EXOC7 | EXOC7 | EXOC8 |
| Variant type | Splice variant | In-frame deletion | Splice variant & in-frame deletion | Missense | Frameshift deletion |
| Variant | Exon 7 splice acceptor (c.809–2A>G) | Ser48del (GGAT>G) | Exon 7 splice acceptor (c.809–2A>G) & exon 10: c.1212_1226 delTGGGCTG ATGCTTGA | Ala523Thr (C>T) | Asp607Ter (CCT>C) |
| Segregates in family | Yes | Yes | Yes | Yes | Yes |
| gnomAD frequency | 2/251,414 alleles, heterozygous | 2/276,426 alleles, heterozygous | Splice: 2/251,414 alleles, heterozygous & deletion: absent | 2/277,066 alleles, heterozygous | Absent |
LOD logarithm of the odds.
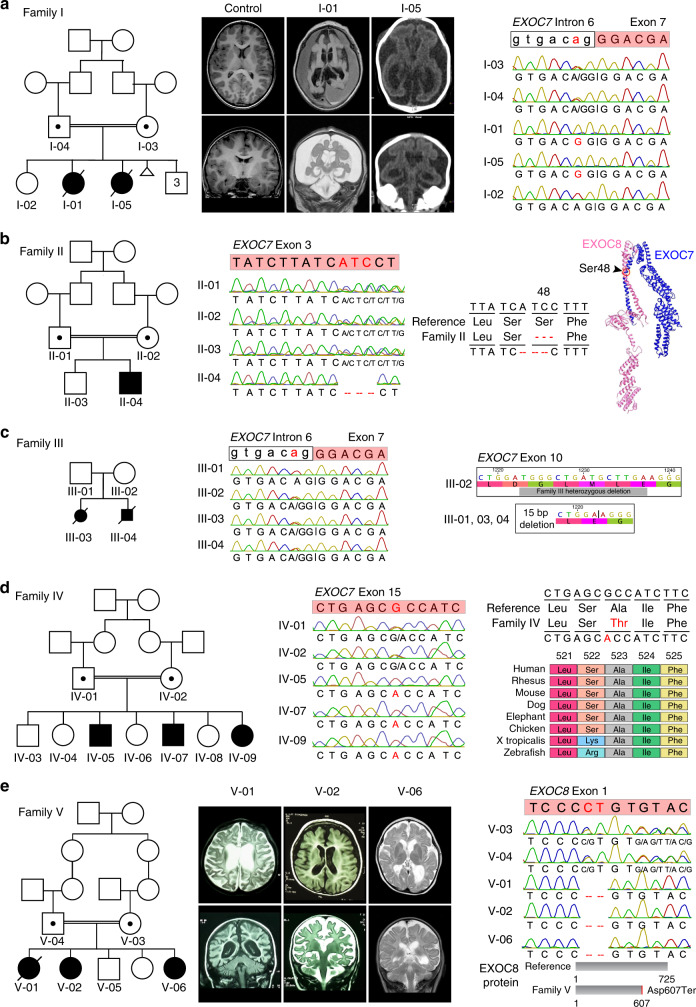
Family I is a consanguineous family with the most severely affected children, who have infantile lethality with neonatal microencephaly, seizures, and arthrogryposis (Fig. 1a, S1A). The family had two daughters who were born with myoclonic seizures and arthrogryposis multiplex. One had documented microcephaly (−2.7 SD), and both died within the first months of life. Imaging of both siblings showed a cerebrum smaller than the skull cavity with a thin cortex and extremely simplified gyri, enlarged ventricles, reduced white matter, and a very small cerebellum and brainstem (Table S1). These findings are consistent with global cerebral cortical maldevelopment, and likely brain atrophy, reflecting neuronal loss (Fig. 1a). Homozygosity mapping in this family identified a region on chromosome 17 linked to disease with a statistically suggestive maximum logarithm of the odds (LOD) score of 1.93 (Fig. S2A). Exome sequencing identified a homozygous splice variant in EXOC7 within this region (exon 7 splice acceptor, NC_000017.10:g.74087318T>C [hg19], c.809–2A>G, Fig. 1a); this variant is heterozygous in 2/251,414 alleles from normal controls (frequency = 7.96 × 10−6) and never homozygous (gnomAD28). This variant mutates a highly conserved base, disrupts the canonical splice acceptor for exon 7 (ag|G>gg|G), and segregates perfectly with disease in this family. One additional rare homozygous variant was found in the same linkage region that caused a missense variant in CYB5D2 (NC_000017.10:g.4057982C>T [hg19], p.Arg136Trp), a gene that encodes a heme binding protein29 with low expression in the developing cerebral cortex (Fig. S4). In the gnomAD database, this CYB5D2 variant is heterozygous in 3/276,426 alleles from normal controls (frequency = 1.22 × 10−5, 0 homozygous alleles), and there are three additional homozygous missense and one homozygous stop-gain variants in CYB5D2 in gnomAD. Although little is known about CYB5D2 function, its expression is much lower than EXOC7 in developing cortex and the greater relative severity of the EXOC7 splice acceptor variant favors it as causative in this family.
Fig. 1. EXOC7 variants cause a recessive brain development disorder.
(a) Left, pedigree of family I showing consanguineous parents and recessive inheritance of lethal microcephaly in I-01 and I-05. Middle, coronal and axial brain magnetic resonance image (MRI) (I-01) or computed tomography (CT) (I-05) show extremely simplified gyral pattern, small cortex, and fluid accumulation with age-matched normal MRI for comparison. Right, Sanger sequencing of EXOC7 intron 6/exon 7 boundary shows intronic A>G variant that mutates the canonical splice acceptor (ag|G to gg|G). This variant is homozygous in affected individuals and segregates with disease. (b) Left, pedigree of family II showing consanguineous parents and recessive inheritance of brain atrophy, microcephaly, and seizures in II-04. Middle, Sanger sequencing of EXOC7 exon 3 reveals a homozygous 3-base-pair ATC deletion in the affected individual that segregates with disease. Right, this deletion removes amino acid Serine 48, which is located in the EXOC7 N-terminal region responsible for binding to EXOC8.30 (c) Left, pedigree of family III showing recessive inheritance of fetal microcephaly and cerebellar hypoplasia in III-03, III-04. Middle, Sanger sequencing showing EXOC7 exon 7 heterozygous splice acceptor variant. Right, diagram of 15-bp heterozygous deletion in EXOC7 exon 10. (d) Left, pedigree of family IV showing consanguineous parents and recessive inheritance of brain atrophy and seizures in IV-05, IV-07, and IV-09. Middle, Sanger sequencing of EXOC7 exon 15 reveals a homozygous G>A variant in affected individuals that segregates with disease. Right, this variant changes amino acid 523, a highly conserved amino acid from humans to zebrafish, from alanine to threonine. (e) Family V has recessive inheritance of a syndrome of developmental regression and delay, seizures, brain atrophy, and early death. Homozygosity mapping and exome sequencing reveals a homozygous 2-base-pair deletion in EXOC8 that causes early protein truncation. aa amino acid.
Family II has one affected male child, of consanguineous parents, with severe developmental delay, seizures, and mild microcephaly (−2.6 SD) (Fig. 1b). Brain imaging showed central and cortical atrophy prominent in the temporal lobes (Table S1). Exome sequencing identified a homozygous 3-base-pair deletion in exon 3 of EXOC7 that removes a serine at position 48 (NC_000017.10:g.74097928_74097930delGAT (hg19), Ser48del, Fig. 1b). This variant is heterozygous in 2/276,426 alleles from normal controls (frequency = 7.24 × 10−6) and never homozygous (gnomAD), and segregates perfectly with disease. Recent work presents two important roles for EXOC7’s N-terminal domain, (1) binding to EXOC8 to promote exocyst complex assembly30 and (2) binding to Wave regulatory complex for cell migration.31 As Ser48 is located in this region, its deletion could disrupt these functions, consistent with it being a pathogenic variant.
Family III is nonconsanguineous with two affected fetuses with mild microcephaly and cerebellar hypoplasia, confirmed by autopsy following termination of pregnancy (Fig. 1c, S1F–H, Table S1). Exome and Sanger sequencing identified two compound heterozygous variants in EXOC7 in the affected fetuses that segregated with disease. One variant is the same exon 7 splice variant found in family I (NC_000017.10:g.74087318T>C [hg19], c.809–2A>G, Fig. 1c). The second variant is an in-frame deletion of 15 bp that removes five amino acids in exon 10 (NM_001145297.2: c.1212_1226delTGGGCTGATGCTTGA, Fig. 1c, S3). This variant is absent from normal controls (gnomAD).
Family IV is consanguineous with three siblings affected with recessively inherited seizures, intellectual disability, and developmental delay (Fig. 1d). Brain imaging showed atrophy in two of the three affected siblings (Table S1). Homozygosity mapping identified a single linkage region on chromosome 17 with the maximum possible LOD score of 2.9 (Fig. S2B). Exome sequencing identified a homozygous missense variant in exon 15 of EXOC7 within the linkage region (NC_000017.10:g.74081807C>T [hg19], Ala523Thr, Fig. 1d). This variant is heterozygous in 2/277,066 alleles from normal controls (frequency = 7.22 × 10−6) and never homozygous (gnomAD), and segregates perfectly with disease. Alanine 523 is a highly conserved amino acid that is conserved from humans to zebrafish.
Family V exhibits recessively inherited global developmental delay with regression, seizures, and microcephaly in three daughters of consanguineous parents (Fig. 1e, S1B–E). Brain imaging showed atrophy in all three affected individuals (Table S1). Homozygosity mapping identified multiple linkage regions with a maximum possible LOD score of 2.5, including a large region on chromosome 1 (Fig. S2C). Exome sequencing identified a homozygous 2-base-pair deletion in exon 1 of EXOC8 in the linkage region (NC_000001.10:g.231471676_231471677delCT (hg19), Asp607Ter, Fig. 1e), which is absent from normal controls (gnomAD). This frameshifting deletion creates a premature stop codon at amino acid 607, short of the full-length protein (725 aa), and segregates perfectly with disease in this family. One additional homozygous variant was detected in the family (stop-gain in RP1L1). In gnomAD, this variant is present as a homozygote in one individual and RP1L1 is associated with adult-onset retinitis pigmentosum (OMIM 613587), so this variant is unlikely to contribute to microcephaly in this family.
In total, we report four novel missense and splice site variants in EXOC7 and one novel truncating variant in EXOC8 in families with a recessive syndrome of brain atrophy, seizures, and developmental delay, and in more severe cases microcephaly and infantile death (Table 1). The presence of cerebral atrophy in all families indicates neurodegeneration and suggests EXOC7 and EXOC8 are required for neuronal survival. Loss-of-function variants in EXOC7 have not been previously linked to human disease, and we have recently reported one homozygous missense variant in EXOC8 in a single case of Joubert syndrome.2
EXOC7 splice variant disrupts mRNA splicing patterns and reduces protein expression
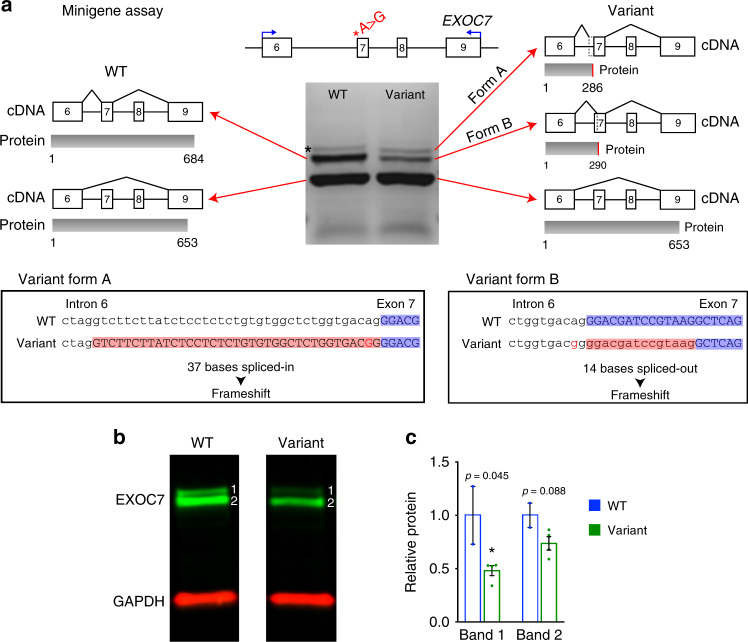
EXOC7 is an alternatively spliced gene with 5 verified transcripts;32 three of which include exon 7. We generated a minigene assay to model the exon 7 splice acceptor variant found in both families I and III and found this variant disrupts splicing and decreases EXOC7 protein level. The minigene was constructed using 5 kb of genomic DNA from the human EXOC7 locus spanning exon 6 to exon 9 (Fig. 2a). Reverse transcription PCR (RT-PCR) of mRNA transcribed from the minigene plasmid expressed in mouse N2A cells revealed three splicing disruptions caused by the human variant (Fig. 2a). First, cDNA encoding a high-abundance transcript (including exons 6, 7, and 9) was isolated from wild-type minigene but completely absent from the variant (Fig. 2a). A low-abundance larger product that could not be subcloned for sequencing was found in wild-type and likely encodes a transcript including exons 6, 7, 8, and 9 (*, Fig. 2a). Second, two novel out-of-frame splice forms that are predicted to encode early truncations were found exclusively in variant minigene cDNA (Fig. 2a). Form A splices in the last 37 bases of intron 6 causing a frameshift that is predicted to create a premature stop codon at amino acid 286, well short of the full-length protein of 684 amino acids. Form B splices out the first 14 bases of exon 7 causing a frameshift, predicted to create a premature stop codon at amino acid 290. An isoform that skips exon 7 and includes exons 6 and 9 was observed in both wild-type and variant minigenes. These minigene results show that the variant disrupts EXOC7 mRNA splicing. To assess the variant’s effect on EXOC7 protein we mutated a HAP1 cell line to encode the patient variant (hemizygous). Immunoblot of EXOC7 protein showed reduced expression in variant HAP1 cells (Fig. 2b, c). We detected two isoforms: (1) a larger isoform was significantly reduced by 50% (two-tailed t test, p = 0.045) and (2) a smaller isoform showed a trend towards reduction (two-tailed t test, p = 0.088). Together, these results support the pathogenicity of this variant.
Fig. 2. EXOC7 exon 7 splice acceptor variant disrupts splicing.
(a) Top, diagram of EXOC7 human minigene construct with splice acceptor variant. Blue arrows mark reverse transcription polymerase chain reaction (RT-PCR) primers used to generate complementary DNA (cDNA) products shown in gel image. Arrows from each band in gel point to splicing diagram determined by Sanger sequencing of the cDNA. Wild-type (WT) minigene generated two in-frame isoforms (left), whereas variant minigene generated one in-frame isoform and two novel out-of-frame isoforms (right). Two variant isoforms encode novel stop codons that lead to premature protein truncation. Asterisk (*) indicates low-abundance product that could not be subcloned for sequencing. (b) Immunoblot of EXOC7 protein in WT and variant HAP1 cells showing reduction of two EXOC7 isoforms. GAPDH is a protein loading control. (c) Quantification of (b) showed significant 50% reduction of larger EXOC7 band (band 1, p = 0.045), while the lower weight band (band 2) was not significantly reduced. P values calculated by two-tailed t test. Error bars represent SEM.
EXOC7 is highly expressed in developing cerebral cortex
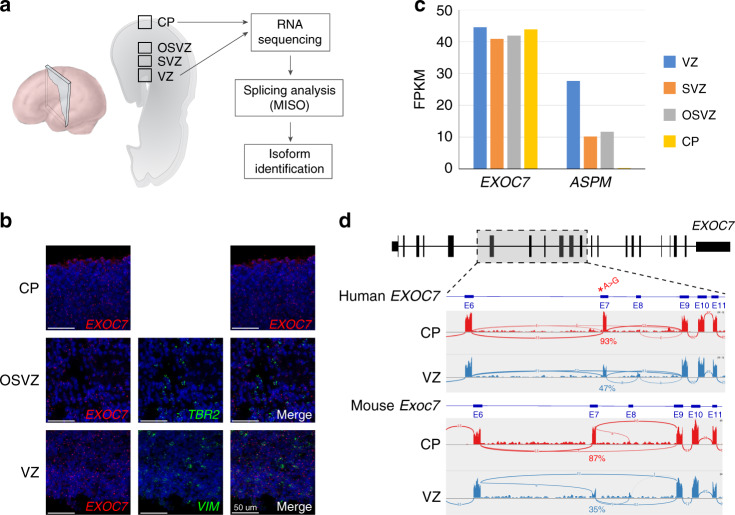
EXOC7 is highly expressed in the developing human cortex, consistent with the affected individuals’ phenotypes. In situ hybridization using RNAscope in human fetal cortex showed abundant EXOC7 expression in the ventricular zone (adjacent to the ventricle and coexpressing VIM), the outer subventricular zone (coexpressing TBR2) and in the cortical plate (adjacent to the pial surface) (Fig. 3a, b). Similarly, Exoc7 is expressed in developing mouse cortex (MGI). RNA sequencing analysis of human fetal cortex33 confirms this expression pattern with expression in the ventricular zone and the inner and outer subventricular zones at a similar level as ASPM, a canonical microcephaly gene, and in the cortical plate (Fig. 3c). EXOC7 expression in both progenitors and postmitotic neurons suggests it has important roles in both cell types during cortical development. Notably, exon 7 of EXOC7, which contains a splice acceptor variant in families I and III, is differentially spliced in cortical progenitors compared with postmitotic cortical neurons. Using a previously published method,27 we identified differentially spliced exons in the developing cortex with RNA sequencing of separated cortical progenitors and postmitotic neurons isolated from both developing mouse and human brain tissue. During fetal human cortical development (GW15), exon 7 is included in 93% of transcripts from the cortical plate and 47% of transcripts from ventricular zone (Fig. 3d). Similarly, during cortical development in mice (E14.5), exon 7 is included in 87% of transcripts isolated from the cortical plate and 35% of transcripts from the ventricular zone (Fig. 3d). The role of this differential splicing in the regulation of EXOC7 function in development is unknown, but our evidence that the splicing variant identified in families I and III alters this differential expression, by eliminating expression of the exon 7 included isoform (Fig. 2), suggests this variant is pathogenic by disrupting cortical development.
Fig. 3. EXOC7 is highly expressed in developing cortex.
(a) Diagram of cortical section of human fetal cortex indicating locations of RNAscope imaging in (b) and RNA sequencing in (c). (b) RNAscope imaging of fetal human cortex shows EXOC7 expression in VZ and OSVZ, two progenitor zones, and in CP, the location of postmitotic neurons. (c) EXOC7 is highly expressed in developing human cortex and shown in comparison with ASPM. Expression levels measured based on RNA sequencing.33 (d) RNA sequencing data from developing human fetal cortex (GW15) and mouse cortex (E14.5) showing differential inclusion of exon 7 in CP vs. VZ. FPKM fragments per kilobase of transcript per million mapped reads.
Exoc7 is essential for vertebrate embryonic development
EXOC7 protein is highly conserved among vertebrates (Fig. 4a). To further characterize the function of EXOC7 in brain development, we examined Exoc7 loss in both zebrafish and mouse. Mice deficient for Exoc7 have been created by Lexicon Pharmaceuticals and are reported to be homozygous lethal (personal communication). This is consistent with the phenotype for loss-of-function variants in other exocyst components also reported to be early embryonic lethal,34,35 and suggests that the three human alleles identified retain some function and are partial loss-of-function.
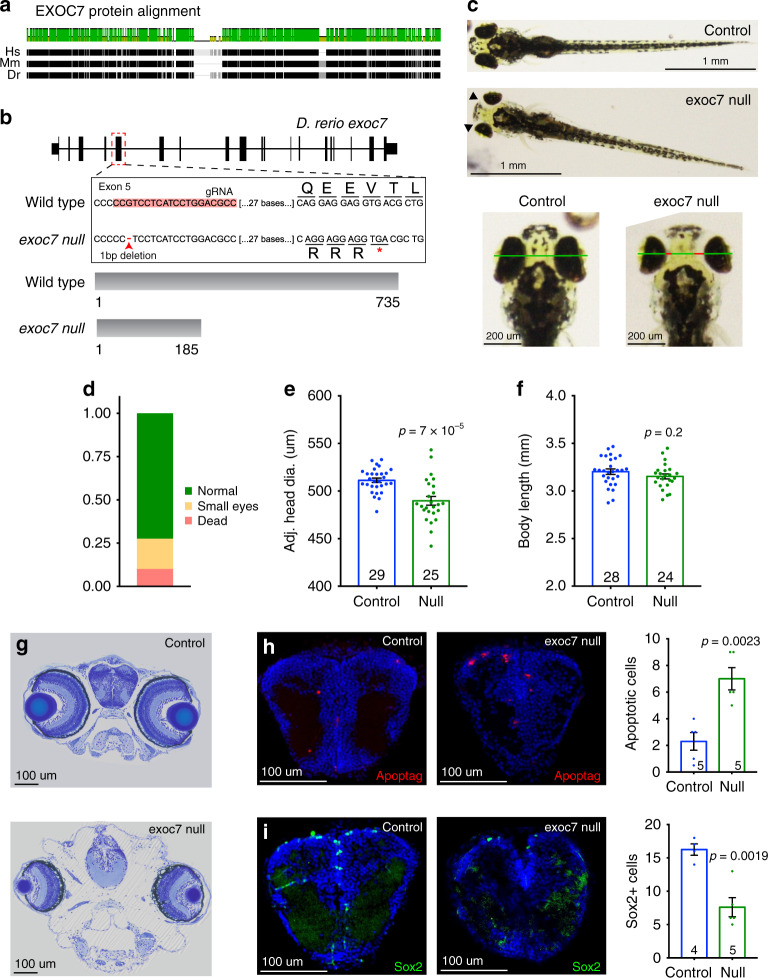
Fig. 4. Exoc7 is essential for zebrafish telencephalon development.
(a) Exoc7 amino acid sequence is highly conserved between human, mice, and zebrafish. (b) exoc7 1-bp frameshift deletion variant in exon 5 is confirmed by DNA sequencing and predicted to cause a frameshift and subsequently a protein truncation through a premature stop codon at amino acid 186. gRNA guide RNA. (c) exoc7 homozygous mutant fish have gross developmental abnormalities by 5 dpf, notably small eyes and head edema. Green line shows measurement of adjusted head diameter calculated by subtracting edema (red lines). (d) Heterozygous exoc7 zebrafish crosses generated mutant fish (small eye/edema or dead) at expected Mendelian ratio. Genotyping confirmed that phenotypically mutant larvae were homozygous for the exoc7 variant. (e) Quantification of adjusted head diameter, which is significantly reduced in homozygous mutant fish. (f) Body length is not significantly changed in homozygous mutant fish. (g) Toluidine blue stain of 5-dpf wild-type and exoc7 mutant zebrafish. (h) Apoptag staining shows a significant increase of apoptotic cells in the exoc7 mutant telencephalon. (i) Immunohistochemical staining of neuronal progenitors using Sox2. The number of Sox2+ progenitors is significantly decreased in the exoc7 mutant telencephalon. P values calculated with two-tailed t test. Error bars represent SEM.
To facilitate analysis of Exoc7 function during development, we took advantage of the ease of genome editing in the zebrafish. We created an exoc7 mutant zebrafish with predicted loss of function of the encoded protein that allowed us to examine the function of exoc7 in development. We used CRISPR/Cas9 mutagenesis to create a 1-bp deletion in exon 5 of exoc7 that generates a predicted frameshift and subsequent nonsense variant. This variant is predicted to lead to a prematurely truncated protein 185 amino acids long, well short of the full-length peptide at 735 (Fig. 4b).
We found that exoc7 is essential for zebrafish development. At 5 dpf, approximately 25% of progeny from a heterozygous incross showed head edema and small eyes, consistent with Mendelian inheritance (Fig. 4c, d, g). Genotyping confirmed that these phenotypes were associated with loss of exoc7 and demonstrated that the phenotype was highly penetrant (Fig. S5A). The mutant fish from the incross (edema and small eyes) die shortly after day 5, showing exoc7 is essential for early zebrafish survival.
Quantification of the small eye phenotype revealed that the eye area was reduced by 26% in mutant fish (two-tailed t test, 3.6 × 10−24, Fig. S5B). Broader characterization of the phenotype in exoc7 mutant fish revealed general defects in head size although this was partially masked with the present edema. To account for the changes caused by the edema, we measured the distance from the outside of one eye to the other and then subtracted the regions of edema (Fig. 4c). Even with this conservative measure, head diameter was 4% smaller in exoc7 null fish compared with clutch controls (two-tailed t test, p = 6.8 × 10−5, Fig. 4e). We detected no difference in body length in mutant fish, suggesting that these defects are specifically caused by the loss of exoc7 and not simply by developmental delay or allometric changes (two-tailed t test, p = 0.21, Fig. 4f).
Cellular defects in exoc7 null developing telencephalon
To identify cellular mechanisms underlying microphthalmia and microcephaly in exoc7 mutant zebrafish (Fig. 4g), we measured apoptosis and counted progenitor cells in the developing telencephalon. At 5 dpf, we found a threefold increase in the number of apoptotic cells (TUNEL stain) in the telencephalon of exoc7 null zebrafish (control n = 5, exoc7 knockout [KO] n = 5, two-tailed t test, p = 0.0023, Fig. 4h). At the same age, we also found a 53% decrease in the number of Sox2-positive telencephalon progenitor cells (control n = 4, exoc7 KO n = 5, two-tailed t test, p = 0.0019, Fig. 4i). The number of Sox2-positive neuroprogenitors was also decreased in the retina of exoc7 null fish, with a 76% decrease compared with controls (control n = 11, exoc7 KO n = 10, two-tailed t test, p = 3.0 × 10−6, Fig. S5C). We examined Hoechst-stained mitotic figures in developing telencephalon and did not find a detectable increase in abnormal mitoses in exoc7 null fish, suggesting normal cytokinesis (Fig. S5E). Together, these results highlight specific cellular defects that drive microcephaly in zebrafish in the absence of exoc7, and further suggest that the atrophy and microcephaly observed in humans with EXOC7 variants reflect loss of proliferating progenitor cells and postmitotic neurons.
DISCUSSION
We identified four independent presumably hypomorphic variants in EXOC7 and one predicted loss-of-function variant in EXOC8 causing a recessive human brain development disorder characterized by brain atrophy, seizures, and developmental delay and in more severe cases, microcephaly and infantile death. We show that EXOC7, a member of the mammalian exocyst complex, is highly expressed in developing human cortex. In addition, a zebrafish model of Exoc7 deficiency recapitulates the human disorder with increased apoptosis and decreased progenitor cells during telencephalon development, suggesting that the brain atrophy in human cases reflects neuronal degeneration. These findings provide key inroads into understanding the role of the exocyst complex in normal cortical development and complex neurodegenerative disorders.
Exocyst variants cause a range of brain development disorders
Our work represents the first systematic genetic analysis of the role of exocyst components in human genetic disease. The four distinct alleles that we describe in EXOC7 show a range of severity consistent with the degree to which they likely damage the protein, with the splicing variant being most severe, a 5–amino acid deletion having similar severity, a 1–amino acid deletion being less severe, and an amino acid substitution being the mildest. However, all families share central nervous system (CNS) disease and specifically cortical atrophy.
We also report a null variant in EXOC8 associated with severe phenotypes within this spectrum. Interestingly, we previously reported that a single affected individual with Joubert syndrome had a homozygous missense variant (E265G) in EXOC8.2 This variant occurred at a highly conserved amino acid and was predicted to be damaging to protein function. Careful clinical review of the affected individuals here confirms that they do not have classic signs of Joubert syndrome. It is possible that these two alleles, E265G and Asp607Ter, lead to different clinical syndromes based on differing variant severity, where a hypomorphic missense variant causes Joubert syndrome and a null variant causes cortical atrophy and microcephaly. Consistent with this idea, homozygous null Exoc8 mice are reported to have early embryonic lethality (MGI, Mouse Phenotyping Consortium). Here we report that loss-of-function variants in either EXOC7 or EXOC8 lead to highly overlapping clinical features, suggesting perhaps that disruption of the exocyst complex broadly impairs normal cortical development. This is supported by previous work reporting an individual with Meckel–Gruber syndrome and microcephaly had a homozygous missense variant (Gln578Arg) in another exocyst component, EXOC4.3 The exact mechanism for exocyst dysfunction causing microcephaly and cortical atrophy is unknown, but previous work and our current results suggest the exocyst may be essential for multiple molecular processes during cortical development. Joubert syndrome and Meckel–Gruber syndrome both have features of ciliopathies, and the exocyst is reported to localize to the primary cilium where some members are required for normal ciliogenesis (EXOC536). In developing zebrafish, a ciliopathy phenotype of abdominal or cardiac edema, upward tail curvature, and small eyes has been observed with loss of exoc5,37 and knockdown of Joubert syndrome gene arl13b38 (OMIM 612291). We find that exoc7 mutant zebrafish have a phenotype with some ciliopathy features including small eyes with edema but missing other features such as abdominal edema or upward tail curvature (Fig. S5D). Further investigation will be required to determine if loss of exoc7 causes mild cilia dysfunction. Interestingly, the patients we report with EXOC7 hypomorphic variants do not have classic ciliopathy features. We find that loss of EXOC7 leads to apoptosis, cell loss, and atrophy in the developing brain and further studies will determine which cellular processes (or combination thereof) are disrupted including RGC polarity and cilia function.
Exocyst complex in cortical development
Our results agree with previous studies of Exoc7 function in neurons and add new details for its role in brain development. Previous work reported that expression of dominant negative Exoc7 in developing mouse cortex impaired neuron migration39 and in vitro Exoc7 knockdown in cultured neurons disrupted polarization and prevented process outgrowth.4,5 A conditional mouse knockout of Arp2/3, an actin binding protein that interacts with Exoc7, in neuroprogenitors showed cortical disorganization characterized by radial glia process truncation, impaired neuron migration, and abundant apoptosis.40 Here we find exoc7 deficiency in the zebrafish developing telencephalon is also associated with abundant apoptosis.
We report the first identification of human variants in an exocyst member, EXOC7, and show these variants and a null variant in another exocyst member, EXOC8, cause a neurodevelopmental syndrome of brain atrophy, seizures, and developmental delay with microcephaly and infantile death. This study exposes key, shared properties of two exocyst components in brain development. Our exoc7 loss-of-function zebrafish model provides a new tool that can shed light on the mechanisms of the exocyst complex in the developing brain supporting the role of EXOC7 as the cause of this complex neurological disorder.
Supplementary information
Acknowledgements
We thank the families for their invaluable participation in our study. M.E.C. was supported by F30 MH102909, Howard Hughes Medical Institute Medical Student Fellowship, and Nancy Lurie Marks Family Foundation Medical Student Fellowship. C.A.W. was supported by R01 NS35129 and R01NS032457 from the National Institute of Neurological Disorders and Stroke (NINDS), U01MH106883 from the National Institute of Mental Health (NIMH), and the Allen Discovery Center program through The Paul G. Allen Frontiers Group. C.A.W. and J.G.G. are Investigators of the Howard Hughes Medical Institute. X.Z. was supported by K01MH109747 from the NIMH. K.H. and M.P.H. were supported in part through funding from Children’s Hospital Orthopaedic Surgery Foundation. This work was also supported by the Broad Center for Mendelian Genomics (UM1 HG008900) funded by the National Human Genome Research Institute (NHGRI). E.M.D. was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) under award 5T32EB1680. R.S.S. was supported by NINDS (F32NS100033801, K99NS112604). J.A., N.C., and L.B. were supported by the French Health Ministry (PNMR2-PNMR3).
Disclosure
The authors declare no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joseph G. Gleeson, Email: jogleeson@ucsd.edu.
Christopher A. Walsh, Email: christopher.walsh@childrens.harvard.edu.
Supplementary information
The online version of this article (10.1038/s41436-020-0758-9) contains supplementary material, which is available to authorized users.
References
- 1.Polgar N, Fogelgren B. Regulation of cell polarity by exocyst-mediated trafficking. Cold Spring Harb Perspect Biol. 2018;10:a031401.. doi: 10.1101/cshperspect.a031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon-Salazar TJ, Silhavy JL, Udpa N, et al. Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med. 2012;4:138ra178. doi: 10.1126/scitranslmed.3003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaheen R, Faqeih E, Alshammari MJ, et al. Genomic analysis of Meckel–Gruber syndrome in Arabs reveals marked genetic heterogeneity and novel candidate genes. Eur J Hum Genet. 2013;21:762–768. doi: 10.1038/ejhg.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupraz S, Grassi D, Bernis ME, et al. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci. 2009;29:13292–13301. doi: 10.1523/JNEUROSCI.3907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita A, Koinuma S, Yasuda S, et al. GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PLoS ONE. 2013;8:e79689. doi: 10.1371/journal.pone.0079689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Zhao Y, Sun Y, et al. Exo70 stimulates the Arp2/3 complex for lamellipodia formation and directional cell migration. Curr Biol. 2012;22:1510–1515. doi: 10.1016/j.cub.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Lehtinen MK, Sessa A, et al. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron. 2010;66:69–84. doi: 10.1016/j.neuron.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa MR, Wen G, Lepier A, Schroeder T, Gotz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- 9.Reiner O, Carrozzo R, Shen Y, et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 10.Gleeson JG, Allen KM, Fox JW, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/S0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 11.des Portes V, Pinard JM, Billuart P, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/S0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 12.Poirier K, Saillour Y, Bahi-Buisson N, et al. Mutations in the neuronal ss-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum Mol Genet. 2010;19:4462–4473. doi: 10.1093/hmg/ddq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breuss M, Heng JI, Poirier K, et al. Mutations in the beta-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep. 2012;2:1554–1562. doi: 10.1016/j.celrep.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirier K, Lebrun N, Broix L, et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lizarraga SB, Margossian SP, Harris MH, et al. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. 2010;137:1907–1917. doi: 10.1242/dev.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagnamenta AT, Murray JE, Yoon G, et al. A novel nonsense CDK5RAP2 mutation in a Somali child with primary microcephaly and sensorineural hearing loss. Am J Med Genet A. 2012;158A:2577–2582. doi: 10.1002/ajmg.a.35558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu WF, Pomp O, Ben-Omran T, et al. Katanin p80 regulates human cortical development by limiting centriole and cilia number. Neuron. 2014;84:1240–1257. doi: 10.1016/j.neuron.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra-Gorur K, Caglayan AO, Schaffer AE, et al. Mutations in KATNB1 cause complex cerebral malformations by disrupting asymmetrically dividing neural progenitors. Neuron. 2014;84:1226–1239. doi: 10.1016/j.neuron.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bond J, Roberts E, Mochida GH, et al. ASPM is a major determinant of cerebral cortical size. Nat Genet. 2002;32:316–320. doi: 10.1038/ng995. [DOI] [PubMed] [Google Scholar]
- 20.Yu TW, Mochida GH, Tischfield DJ, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet. 2010;42:1015–1020. doi: 10.1038/ng.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill RS, Schoborg TA, Rusan NM. Same but different: pleiotropy in centrosome-related microcephaly. Mol Biol Cell. 2018;29:241–246. doi: 10.1091/mbc.E17-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielas SL, Silhavy JL, Brancati F, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thauvin-Robinet C, Lee JS, Lopez E, et al. The oral-facial-digital syndrome gene C2CD3 encodes a positive regulator of centriole elongation. Nat Genet. 2014;46:905–911. doi: 10.1038/ng.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller J, Stoetzel C, Vincent MC, et al. Identification of 28 novel mutations in the Bardet-Biedl syndrome genes: the burden of private mutations in an extensively heterogeneous disease. Hum Genet. 2010;127:583–593. doi: 10.1007/s00439-010-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott EM, Halees A, Itan Y, et al. Characterization of Greater Middle Eastern genetic variation for enhanced disease gene discovery. Nat Genet. 2016;48:1071–1076. doi: 10.1038/ng.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Essletzbichler P, Konopka T, Santoro F, et al. Megabase-scale deletion using CRISPR/Cas9 to generate a fully haploid human cell line. Genome Res. 2014;24:2059–2065. doi: 10.1101/gr.177220.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Chen MH, Wu X, et al. Cell-type-specific alternative splicing governs cell fate in the developing cerebral cortex. Cell. 2016;166:1147–62 e1115. doi: 10.1016/j.cell.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruce A, Rybak AP. CYB5D2 requires heme-binding to regulate HeLa cell growth and confer survival from chemotherapeutic agents. PLoS ONE. 2014;9:e86435. doi: 10.1371/journal.pone.0086435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mei K, Li Y, Wang S, et al. Cryo-EM structure of the exocyst complex. Nat Struct Mol Biol. 2018;25:139–146. doi: 10.1038/s41594-017-0016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biondini M, Sadou-Dubourgnoux A, Paul-Gilloteaux P, et al. Direct interaction between exocyst and Wave complexes promotes cell protrusions and motility. J Cell Sci. 2016;129:3756–3769. doi: 10.1242/jcs.187336. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Liu J, Liu S, et al. Exo70 isoform switching upon epithelial-mesenchymal transition mediates cancer cell invasion. Dev Cell. 2013;27:560–573. doi: 10.1016/j.devcel.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fietz SA, Lachmann R, Brandl H, et al. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci USA. 2012;109:11836–11841. doi: 10.1073/pnas.1209647109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno S, Takami K, Daitoku Y, et al. Peri-implantation lethality in mice carrying megabase-scale deletion on 5qc3.3 is caused by Exoc1 null mutation. Sci Rep. 2015;5:13632. doi: 10.1038/srep13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich GA, Hildebrand JD, Soriano P. The secretory protein Sec8 is required for paraxial mesoderm formation in the mouse. Dev Biol. 1997;192:364–374. doi: 10.1006/dbio.1997.8727. [DOI] [PubMed] [Google Scholar]
- 36.Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 2009;20:2522–2529. doi: 10.1091/mbc.e08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobo GP, Fulmer D, Guo L, et al. The exocyst is required for photoreceptor ciliogenesis and retinal development. J Biol Chem. 2017;292:14814–14826. doi: 10.1074/jbc.M117.795674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seixas C, Choi SY, Polgar N, et al. Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell. 2016;27:308–320. doi: 10.1091/mbc.e15-02-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letinic K, Sebastian R, Toomre D, Rakic P. Exocyst is involved in polarized cell migration and cerebral cortical development. Proc Natl Acad Sci USA. 2009;106:11342–11347. doi: 10.1073/pnas.0904244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang PS, Chou FS, Ramachandran S, et al. Crucial roles of the Arp2/3 complex during mammalian corticogenesis. Development. 2016;143:2741–2752. doi: 10.1242/dev.130542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.