ABSTRACT
Genomic imprinting, a phenomenon in which the two parental alleles are regulated differently, is observed in mammals, marsupials and a few other species, including seed-bearing plants. Dysregulation of genomic imprinting can cause developmental disorders such as Beckwith-Wiedemann syndrome (BWS) and Silver-Russell syndrome (SRS). In this Review, we discuss (1) how various (epi)genetic lesions lead to the dysregulation of clinically relevant imprinted loci, and (2) how such perturbations may contribute to the developmental defects in BWS and SRS. Given that the regulatory mechanisms of most imprinted clusters are well conserved between mice and humans, numerous mouse models of BWS and SRS have been generated. These mouse models are key to understanding how mutations at imprinted loci result in pathological phenotypes in humans, although there are some limitations. This Review focuses on how the biological findings obtained from innovative mouse models explain the clinical features of BWS and SRS.
KEY WORDS: Imprinting disorders, Beckwith-Wiedemann syndrome, Silver-Russell syndrome, H19, Igf2, Cdkn1c, Kcnq1ot1, Imprinting control regions
Summary: Beckwith-Wiedemann syndrome (BWS) and Silver-Russell syndrome (SRS) can be caused by various (epi)genetic lesions leading to the dysregulation of genomic imprinting. This Review focuses on the mouse models used to understand how such perturbations contribute to the human BWS/SRS phenotypes.
Introduction
A small number of mammalian genes are expressed in a parent-of-origin-specific manner via an epigenetic phenomenon known as genomic imprinting (Barlow and Bartolomei, 2014). Imprinted genes are typically found clustered in the genome and have important developmental roles, including the control of fetal growth and placental function (Plasschaert and Bartolomei, 2014). Genomic imprinting is largely regulated by the differential DNA methylation of discrete genomic elements located within imprinted clusters, which are called imprinting control regions (ICRs) (Li et al., 1993; Edwards and Ferguson-Smith, 2007). ICRs are methylated on a single parental allele during gametogenesis. Normally, this methylation state is maintained throughout development, despite the genome-wide epigenetic reprogramming that occurs after fertilization in the early embryo (Barlow and Bartolomei, 2014). Offspring with improper DNA methylation, inherited or somatic mutations and partial deletions of ICRs may exhibit abnormal genomic imprinting. Perturbations of imprinted genes and their regulation often lead to abnormal pre- and postnatal growth, as exemplified by the human imprinting disorders Beckwith-Wiedemann syndrome (BWS), Silver-Russell syndrome (SRS), Prader-Willi syndrome and Angelman syndrome. In this Review, we discuss how mouse models have contributed to our understanding of two of these imprinting disorders: BWS and SRS.
Why the mouse model?
Since the discovery of genomic imprinting (McGrath and Solter, 1984; Surani et al., 1984), mouse models have been a powerful tool to study genetic disorders associated with abnormal imprints. The main advantage of the mouse lies in the largely evolutionarily conserved mechanisms that govern genomic imprinting (Edwards and Ferguson-Smith, 2007; Lee and Bartolomei, 2013). For each imprinted cluster, the genes, ICRs and epigenetic modifications responsible for parent-of-origin-specific expression are largely conserved between mice and humans. Additionally, the prevalence of single-nucleotide polymorphisms in numerous mouse strains allows for elegant mating strategies, and tracking of the maternal and paternal alleles in offspring. Furthermore, the marks that designate the parental identity of imprinted genes are established in the germline and can readily be evaluated in murine gametes. Importantly, loss of imprinting often occurs early in development in cells and tissues that are not easily accessible in humans but are accessible in the mouse. Nearly 40 years after its discovery, mouse models continue to serve as a vital tool to understand the physiological consequences of genomic imprinting.
BWS
BWS [Online Mendelian Inheritance in Man (OMIM) catalog #130650] is one of the most common fetal overgrowth syndromes, with an incidence of 1 in 10,000 to 13,700 births (Vora and Bianchi, 2009; Mussa et al., 2013). Clinical features include pre- and postnatal overgrowth, hemihypertrophy, macroglossia, neonatal hypoglycemia, hyperinsulinism and hypoglycemia, organomegaly, renal abnormalities, anterior abdominal wall defects, umbilical hernia, ear creases, increased susceptibility to congenital/childhood tumors such as Wilms' tumor, adrenocortical carcinoma, hepatoblastoma and neuroblastoma, placentomegaly and placental mesenchymal dysplasia (DeBaun et al., 2000; Bliek, 2001; Weksberg et al., 2010; Brioude et al., 2018a,b). Notably, these symptoms can vary substantially among individuals. Although the molecular etiology of BWS is multifold, the vast majority of cases involve genetic and epigenetic perturbations of two clusters of imprinted genes on chromosome 11p15: the H19/IGF2 cluster and CDKN1C/KCNQ1OT1 cluster.
Imprinting mechanisms of the H19/IGF2 cluster and CDKN1C/KCNQ1OT1 cluster
The H19/IGF2 locus includes two imprinted genes located at chromosome 11p15.5 in human and at distal chromosome 7 in mouse (Fig. 1). In humans, telomeric to the ICR (designated as IC1) resides H19, which encodes a cytoplasmic long noncoding RNA (lncRNA). H19 is associated with growth suppression, delayed placental growth, cell cycle regulation, cardiac remodeling, tumorigenesis and metastasis (Yoshimizu et al., 2008; Lecerf et al., 2019; Zhou et al., 2019). H19 lncRNA may be processed into a microRNA (MIR675/Mir675), although its exact role remains unclear (Mineno et al., 2006; Keniry et al., 2012).
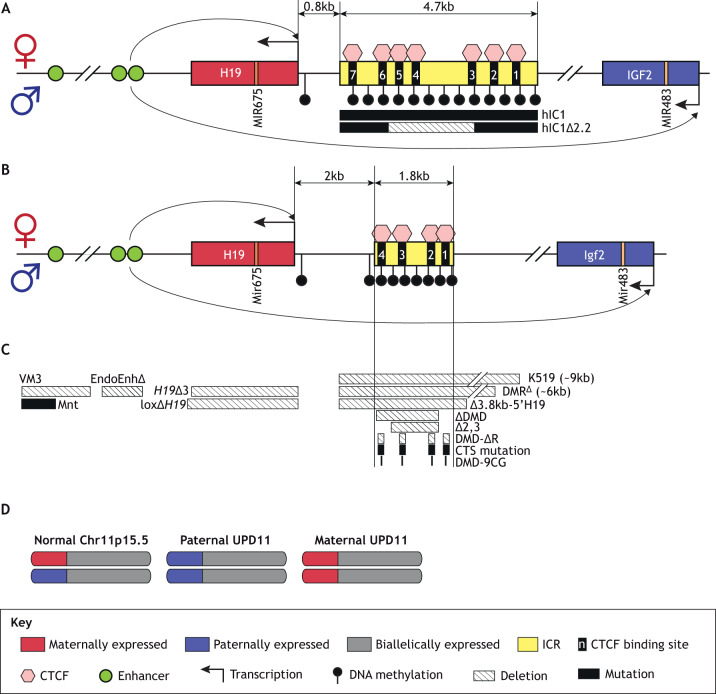
Fig. 1.
H19/IGF2 imprinted locus. (A) Human H19/IGF2 cluster. On the maternal allele, CTCF binds to the unmethylated IC1 and insulates the IGF2 promoter from the downstream enhancers, enabling H19 expression. The methylated paternal IC1 silences the paternal H19 allele and allows IGF2 expression. IGF2 is expressed from multiple promoters. MIR675 is located within the first exon of H19, whereas MIR483 is encoded within the second-to-last intron of IGF2. The BWS-related mutation (hIC1Δ2.2), which was introduced in the humanized IC1 allele in mice, is shown at the bottom of the panel. (B) Mouse H19/Igf2 cluster. The H19/Igf2 ICR is smaller in size and has fewer CTCF binding sites compared to human IC1, but the imprinting mechanism, detailed in A, is conserved between two species. (C) Summary of the mouse models described in this Review. Striped boxes indicate deletions and solid-filled boxes indicate mutations. (D) Simplified depiction of uniparental disomy (UPD). Sizes in kb are approximate and figures are not drawn to scale.
Centromeric to IC1 resides IGF2, which encodes insulin-like growth factor 2, a fetal growth-promoting protein essential for proper organ development, and an intronic microRNA (MIR483/miR483) (Fig. 1). In various tissues, IGF2 promotes cell proliferation and differentiation (Borensztein et al., 2013; Ferrón et al., 2015). In both mouse and human, H19 and Igf2 are regulated by the ICR and share tissue-specific enhancers located downstream of H19 (Fig. 1A,B) (Leighton et al., 1995; Kaffer et al., 2001). The maternal ICR is unmethylated and is bound by the architectural protein CTCF (Bell et al., 1999; Bell and Felsenfeld, 2000; Lee and Bartolomei, 2013). CTCF maintains the unmethylated state of the maternal ICR and functions as an insulator to prevent the downstream enhancers from interacting with the maternal Igf2 promoter (Yoon et al., 2007). Consequently, Igf2 is silenced and H19 is expressed on the maternal allele. Conversely, the paternal H19/Igf2 ICR is methylated during spermatogenesis. Following fertilization, this methylation spreads to the paternal H19 promoter and silences the paternal H19 (Thorvaldsen et al., 1998). Additionally, the methylation of the paternal ICR interferes with CTCF binding and formation of the insulator. As a result, Igf2 is expressed and H19 is silenced on the paternal allele. The ICR is critical for allele-specific regulation of Igf2 and H19, as shown in a mouse model with the ICR deletion [Fig. 1C, Δ3.8kb-5′H19 (Thorvaldsen et al., 2006) and K519 (Kaffer et al., 2000)]. When the H19/Igf2 ICR was deleted on the maternal allele, the normally silent maternal Igf2 was activated, increasing the fetal weight.
Centromeric to the human H19/IGF2 cluster (Fig. 2A) and telomeric to the mouse cluster (Fig. 2B) is the CDKN1C/KCNQ1OT1 cluster. The ICR of this cluster (designated as IC2 in humans and as KvDMR1 in mice) and its regulatory mechanisms are conserved. KvDMR1 regulates allelic expression of multiple imprinted genes in the cluster, including Kcnq1ot1, cyclin-dependent kinase inhibitor (Cdkn1c or p57Kip2), Slc22a18, Phlda2, Ascl2 and Kcnq1. Each of these imprinted genes plays important roles in development. For example, Cdkn1c regulates cell proliferation and promotes cell cycle arrest (Hatada and Mukai, 1995; Matsuoka et al., 1996); Kcnq1 encodes a potassium channel and is imprinted at specific developmental stages and tissues (Wang et al., 1996; Lee et al., 1997); Ascl2 encodes a placenta-specific transcription factor (Guillemot et al., 1995; McLaughlin et al., 1996); and Phlda2 regulates placental growth and hormone synthesis (Jensen et al., 2014).
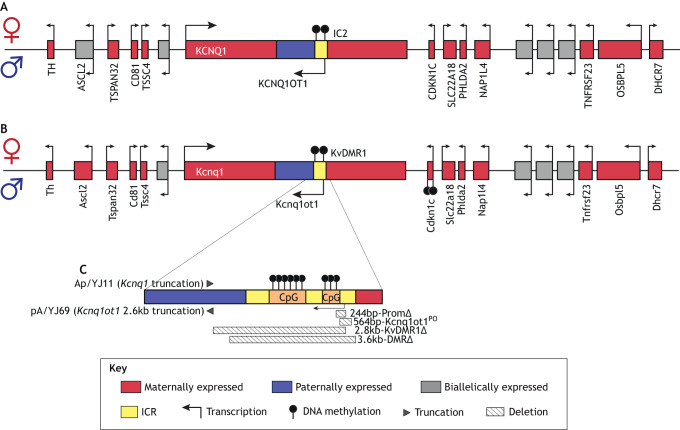
Fig. 2.
CDKN1C/KCNQ1OT1 imprinted locus. (A) Human CDKN1C/KCNQ1OT1 cluster. On the maternal allele, IC2, which includes the transcription start site of KCNQ1OT1, is methylated. Thus, the maternal KCNQ1OT1 is repressed, allowing the expression of maternal-allele-specific linked genes such as KCNQ1 and CDKN1C. On the paternal allele, IC2 is unmethylated and KCNQ1OT1 is transcribed, silencing the linked imprinted genes on the paternal allele. (B) Mouse Cdkn1c/Kcnq1ot1 cluster. Note that IC2 is designated as KvDMR1 in mouse. The methylation on the Cdkn1c promoter and gene body is acquired postfertilization, and is absent in human (Bhogal et al., 2004). (C) Mouse models described in this Review. Striped boxes indicate deletions and filled triangles indicate insertions of truncation cassettes. Figures are not drawn to scale.
The Cdkn1c/Kcnq1ot1 locus is primarily regulated by the lncRNA Kcnq1ot1. The Kcnq1ot1 promoter resides within KvDMR1, which is methylated on the maternal allele and unmethylated on the paternal allele. Kcnq1ot1 transcription is essential for proper imprinting of the Cdkn1c/Kcnq1ot1 locus. The unmethylated paternal KvDMR1 promotes the transcription of Kcnq1ot1 to silence the linked imprinted genes, likely via the recruitment of repressive epigenetic modulators including histone modifiers and DNA methyltransferases (Pandey et al., 2008; Mohammad et al., 2010). Conversely, the maternal KvDMR1 methylation silences Kcnq1ot1, enabling the expression of the linked imprinted genes. This mechanism is supported by numerous studies in mouse. For example, deleting KvDMR1 on the paternal allele disrupted allele-specific regulation of Cdkn1c and other linked genes [Fig. 2C, 2.8kb-KvDMR1Δ (Fitzpatrick et al., 2002) and 3.6kb-DMRΔ (Mancini-DiNardo et al., 2006)]. Deletion of the paternal Kcnq1ot1 promoter in mice led to abnormal methylation and expression of genes in the cluster [Fig. 2C, 244bp-PromΔ (Mancini-DiNardo et al., 2006) and 564bp-Kcnq1ot1PO (Schultz et al., 2015)]. Indeed, the transcriptional activity of the Kcnq1ot1 promoter ensures KvDMR1 hypomethylation and repression of Cdkn1c. Interestingly, others have shown that Kcnq1ot1 may control the locus in most, but not all, tissues. Truncation of the paternal Kcnq1ot1 transcript de-repressed paternal Cdkn1c expression in brain, heart, gut and placenta, but not in liver, kidney and lung [Fig. 2C, pA/YJ69 (Shin et al., 2008)]. Thus, although the full Kcnq1ot1 transcript is required to silence Cdkn1c and other genes in the cluster in most tissues, an alternative mechanism, independent of Kcnq1ot1, may mediate Cdkn1c silencing in some tissues.
Genetic and epigenetic errors of BWS
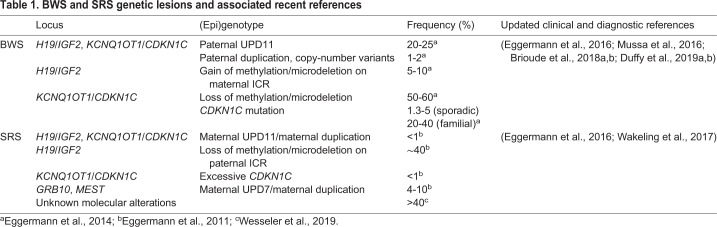
Most BWS patients have genetic or epigenetic errors at the H19/IGF2 and/or the CDKN1C/KCNQ1OT1 clusters, such as DNA methylation perturbations, copy-number variants and loss-of-function mutations of imprinted genes (Table 1). Indeed, the various epigenotypes of BWS patients correlate with different phenotypes. For example, hemihypertrophy and hypoglycemia are associated with paternal uniparental disomy (pUPD) of 11p15 (DeBaun et al., 2002). Individuals with abnormal IC1 methylation are reported to have increased tumor risk (Bliek, 2001). H19/IGF2 imprinting is consistently dysregulated in BWS patients presenting with Wilms' tumors (Dao et al., 1999; Frevel et al., 1999). Gain of methylation (GoM) on IC1 is strongly correlated with neonatal macrosomia and disproportionate overgrowth (Mussa et al., 2016). Premature birth is associated with loss of methylation (LoM) on IC2 or CDKN1C mutations (Mussa et al., 2016). Finally, patients with abdominal wall defects exhibit a higher frequency of abnormal methylation on the CDKN1C/KCNQ1OT1 cluster (DeBaun et al., 2002). Thus, elucidating the epigenetic mechanisms for the BWS imprinted clusters would increase our understanding of how certain mutations cause defined pathological phenotypes, ultimately leading to better treatment plans for patients. Given the conserved biology and regulation of IC1/IC2, the investigation of dysregulated human imprinting using mouse models has been particularly informative.
Table 1.
BWS and SRS genetic lesions and associated recent references
pUPD11 in BWS
Twenty to 25% of BWS patients have pUPD of chromosome 11p15.5, where both copies of 11p15.5 are inherited from the father (Fig. 1D and Table 1). Modeling pUPD11 in mice has been difficult because pUPD of the corresponding region in mice (distal chromosome 7) is lethal at midgestation (McLaughlin et al., 1996; Rentsendorj et al., 2010; Singh et al., 2011). Mouse embryos with pUPD7 were developmentally delayed at 10.5 days post coitum (dpc), lacked placental spongiotrophoblasts and were resorbed before 13.5 dpc. Although these embryos lacked H19 and exhibited increased Igf2 expression, deleting paternal Igf2 failed to rescue their viability (Rentsendorj et al., 2010). Similarly, when only the paternal Cdkn1c/Kcnq1ot1 cluster is left functional due to the truncation of maternal distal chromosome 7, lethality was observed by 10.5 dpc in mice [DelTel7 (Oh et al., 2008)]. The difference in UPD lethality between human and mouse may arise from the typical mosaic presentation of UPD and non-UPD cells in human patients, whereas all cells in pUPD7 mice are constitutively UPD. Nevertheless, and perhaps more importantly, midgestation lethality in mouse may be caused by loss of expression of the maternally expressed imprinted gene Ascl2, which is not imprinted in humans (Fig. 2A) (Westerman et al., 2001; Miyamoto et al., 2002; Rentsendorj et al., 2010). Consistent with this hypothesis, the embryonic lethality of the aforementioned DelTel7 mouse model was partially rescued by restoring Ascl2 expression (Tunster et al., 2018).
The H19/IGF2 cluster in BWS
Five to 10% of BWS patients exhibit GoM or IC1 hypermethylation at the H19/IGF2 locus (Table 1). DNA methylation antagonizes CTCF binding (Kanduri et al., 2000; Wang et al., 2012). Therefore, hypermethylation of IC1 on the normally unmethylated maternal allele can prevent CTCF binding, which disfavors H19 expression and increases IGF2 expression. CTCF is indispensable for both insulating IGF2 and maintaining the unmethylated state of maternal IC1. In mice, targeted deletion of all four CTCF binding sites (CTSs) within the maternal H19/Igf2 ICR resulted in hypermethylation of mutant maternal ICR, loss of CTCF-mediated insulator function and therefore biallelic Igf2 expression [Fig. 1C, DMD-ΔR (Engel et al., 2006)]. Similar results were obtained when the four CTSs were mutated on the maternal H19/Igf2 ICR, where the mice exhibited variable acquisition of DNA methylation and maternal Igf2 activation [Fig. 1C, CTS mutation (Schoenherr et al., 2003; Szabo et al., 2004)]. These models underscore the importance of proper CTCF binding to the ICR for allele-specific regulation of H19/Igf2 expression.
Given that H19 and IGF2 are key regulators in development, it is expected that perturbations in gene dose promote abnormal growth. For example, overexpression of Igf2 caused by introducing an Igf2 transgene in mice causes fetal overgrowth similar to the human BWS phenotype (Sun et al., 1997). A mouse carrying an H19 deletion on the maternal allele exhibited fetal overgrowth and tissue-specific activation of maternal Igf2 [Fig. 1C, H19Δ3 (Ripoche et al., 1997) and loxΔH19 (Schmidt et al., 1999)]. Additionally, H19 and IGF2 can work together to control growth. Early work suggested that H19 is a trans-acting repressor of Igf2 (Li et al., 1998). Transgenic overexpression of H19 in H19Δ3 mice restored H19 and Igf2 expression to wild-type levels and rescued the overgrowth phenotype (Gabory et al., 2009; Martinet et al., 2016), suggesting that H19 may function as a repressor of Igf2. In conclusion, absence of H19 and/or increased Igf2 can lead to overgrowth in early development.
The causes of IC1 hypermethylation remain unclear. The relationship between IC1 mutations, IC1 hypermethylation and BWS phenotypes is complex (Table 1) (Brioude et al., 2018a,b; Duffy et al., 2019b). IC1 hypermethylation is often associated with microdeletions (Sparago et al., 2004), which may change the spacing of CTSs and affect CTCF binding, insulator activity and DNA methylation of IC1. Therefore, it is possible that the variable BWS phenotypes may stem from the spatial arrangement of CTCF binding sites specific to each microdeletion (Beygo et al., 2013). For example, in one representative BWS family, transmission of a specific IC1 mutation over generations was accompanied by a gradual increase in DNA methylation (Berland et al., 2013). Conversely, another BWS family with an IC1 microdeletion had no IC1 methylation abnormalities (Prawitt et al., 2005). In this study, a family member carrying a maternally inherited IC1 microdeletion and a duplication of chromosome 11p15 presented with BWS symptoms, whereas other relatives with the same maternal IC1 deletion did not. Additional studies document incomplete penetrance of BWS features despite all relatives carrying the same IC1 microdeletion (Cerrato et al., 2008; Berland et al., 2013). One factor that may contribute to this clinical heterogeneity is the developmental stage at which the IC1 deletion occurs. For example, one BWS study identified a point mutation within IC1 that originated from the paternal allele of a patient's grandmother (Berland et al., 2013). During oogenesis in this grandmother, IC1 reprogramming (i.e. removal of paternally methylated epigenetic marks and establishment of maternally unmethylated marks) could have been disrupted by the mutation, resulting in incomplete demethylation of the mutant IC1. Once this hypermethylated IC1 was maternally inherited, methylation gradually accumulated on the mutant IC1 over generations. Consistently, mouse studies showed imprinted H19 expression was not affected when the paternal H19/Igf2 ICR was deleted after methylation of the H19 promoter was established [Fig. 1C, DMRΔ (Srivastava et al., 2000)].
It is important to note that genetic lesions at IC1 are not always accompanied by hypermethylation, and the aberrant methylation patterns are not necessarily caused by IC1 mutations. For example, a subset of BWS patients exhibited IC1 hypermethylation but did not carry any identified genetic lesions (Cerrato et al., 2008). Another factor that can explain the heterogeneity in BWS is epigenetic mosaicism (Abi Habib et al., 2017). IC1 hypermethylation is often mosaic in BWS patients and is independent of the mutations (Cerrato et al., 2008). Mosaic expression was also observed at a single-cell level in mice harboring ICR mutations (Ginart et al., 2016). The fact that hypermethylation exhibits a mosaic pattern suggests that the hypermethylation is acquired after fertilization instead of in the germline.
Two important questions remain regarding hypermethylation and IC1 microdeletions associated with BWS: (1) do both maternally derived genetic lesions and hypermethylation cause BWS, or (2) is GoM the consequence of the microdeletion? To understand the relationship between microdeletions and IC1 hypermethylation, researchers have utilized mouse models with partial deletions in the H19/Igf2 ICR. Although the regulatory mechanisms of the locus are well conserved, the mouse H19/Igf2 ICR differs from the human IC1 in the number of CTSs, genomic size and nucleotide sequence (Fig. 1A,B). Consequently, the optimal way to understand BWS lesions using a mouse model is through ICR deletions that remove CTCF binding sites. Deletion of two of the four CTSs on the maternal H19/Igf2 ICR resulted in tissue-specific activation of maternal Igf2 and increased birth weight, despite the normal methylation of the mutant ICR [Fig. 1C, Δ2,3 (Ideraabdullah et al., 2014)]. This model demonstrates that partial deletion of the H19/Igf2 ICR is not always accompanied by ICR hypermethylation and that ICR hypermethylation is not strictly required for abnormal H19/Igf2 expression and growth. In contrast, when a larger ICR deletion containing three CTSs was maternally transmitted, the mutant maternal ICR gained methylation and maternal Igf2 was activated [Fig. 1C, ΔDMD (Thorvaldsen et al., 1998, 2002, 2006)]. Similarly, when all four CTSs were deleted or mutated in the maternal H19/Igf2 ICR, the mutant ICR acquired methylation and H19/Igf2 imprinting was disrupted ubiquitously [Fig. 1C, CTS mutation (Schoenherr et al., 2003; Szabo et al., 2004; Engel et al., 2006)]. These models suggest that the nature of ICR deletions determines whether there will be accompanying hypermethylation, although it is unclear whether the deletion size, position or sequence is critical to determine whether the ICR is protected from ectopic hypermethylation. Therefore, a subset of IC1 microdeletions that disrupt the key aspects of IC1 function may result in its hypermethylation and, ultimately, BWS.
As stated above, due to a lack of ICR sequence conservation, mice are limited in modeling the exact BWS mutations observed in humans. To recapitulate human mutations more precisely, Hur and colleagues generated a mouse strain with human IC1 (hIC1) sequence substituting the endogenous murine H19/Igf2 ICR [Fig. 1A, hIC1 (Hur et al., 2016)]. The paternally transmitted hIC1 allele failed to acquire and maintain the normal hypermethylation (see further discussion below). However, when maternally inherited, the hIC1 allele exhibited normal hypomethylation and CTCF insulator function, suggesting a possible system to model human BWS mutations endogenously in mice. For example, Freschi et al. designed a mouse strain with hIC1 including a 2.2-kb deletion found in BWS patients [Fig. 1A, hIC1Δ2.2 (Freschi et al., 2018)]. Maternal transmission of this mutant hIC1 resulted in tissue-specific overexpression of Igf2, successfully mimicking clinical BWS phenotypes including pre-/postnatal overgrowth, nephromegaly, macroglossia and kidney asymmetry. Notably, mutant hIC1 gained methylation when maternally transmitted, possibly because the removal of three CTSs reduced CTCF binding to the IC1. Thus, humanized mouse models offer a powerful tool to mimic the epigenetic and genetic errors found in human BWS patients and examine their physiological consequences.
The CDKN1C/KCNQ1OT1 cluster in BWS
Fifty percent of BWS patients exhibit LoM at IC2 (Table 1). In these cases, the lncRNA KCNQ1OT1 is predicted to be ectopically expressed from the maternal allele, which silences the growth suppressor CDKN1C and other maternally expressed genes. Importantly, loss of CDKN1C expression is a major cause of the overgrowth phenotype in BWS patients, and knockout of maternal Cdkn1c in mice causes similar fetal overgrowth, as well as cleft palate, abdominal wall defects and placental defects such as placentomegaly that approximate the BWS phenotype (Yan et al., 1997; Zhang et al., 1997; Tunster et al., 2011).
Dysregulation of other IC2-controlled genes in addition to CDKN1C can contribute to BWS-like phenotypes. One such pathway involves PHLDA2, a maternally expressed gene within the IC2 cluster (Fig. 2). In mice, maternal inheritance of a Phlda2 deletion resulted in placentomegaly (Frank et al., 2002; Salas et al., 2004), which suggests that the dysregulation of Phlda2 expression caused by disrupted imprinting of the IC2 cluster can lead to adverse placental phenotypes, one of the hallmark BWS symptoms.
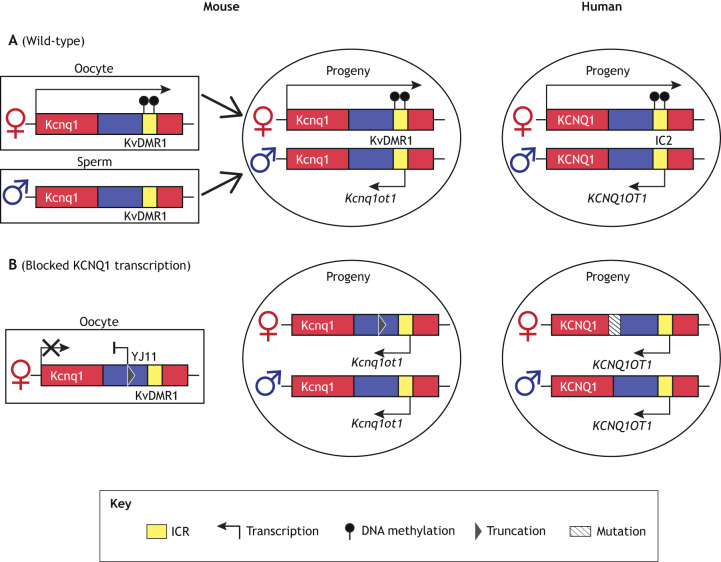
In a subset of BWS patients, LoM on IC2 may result from maternally transmitted mutations, particularly those that affect transcription. Notably, work in mice has shown that establishment of maternal-allele-specific methylation on KvDMR1 requires active transcription of Kcnq1 through KvDMR1 during oocyte maturation (Fig. 3A) (Chotalia et al., 2009; Singh et al., 2017). Thus, a defect in transcription may result in the failure to establish proper methylation on maternal KvDMR1 (Fig. 3B). Human BWS cases support this model. Valente et al. described a BWS patient with a maternally inherited mutation affecting KCNQ1 transcription and complete LoM at IC2 (Valente et al., 2019). In a second BWS family, disruption of KCNQ1 transcription by translocation of KCNQ1 exons to a different chromosome was associated with abnormal IC2 methylation (Beygo et al., 2019). In a third report, a maternally inherited microdeletion in KCNQ1OT1 coincided with IC2 LoM, CDKN1C silencing and BWS features (Niemitz et al., 2004). To genetically dissect the role of transcription in IC2 imprinting in more detail, mouse models harboring a truncated maternal Kcnq1 transcript just prior to KvDMR1 have been generated. These mice exhibited LoM on the maternal KvDMR1, biallelic expression of Kcnq1ot1 and repression of normally maternally expressed genes, including Cdkn1c [Figs 2C and 3B, Ap/YJ11 (Singh et al., 2017)]. Together, these human and mouse studies suggest that a mutation disrupting Kcnq1 transcription leads to the loss of imprinting of the entire cluster.
Fig. 3.
Establishment of DNA methylation at the CDKN1C/KCNQ1OT1 imprinted locus. (A) The transcription of Kcnq1 in mouse oocytes is postulated to establish maternal-allele-specific methylation on KvDMR1, which silences maternal Kcnq1ot1 expression in the progeny. The allele-specific regulation of this cluster is conserved in humans. (B) Truncating the maternal Kcnq1 transcript in mice [Ap/YJ11 (Singh et al., 2017)] resulted in hypomethylation on the maternal KvDMR1. In humans, maternally inherited mutations, which disrupt KCNQ1 transcription, are hypothesized to disrupt the establishment of methylation on maternal IC2, which can lead to LoM on IC2 in the progeny (Niemitz et al., 2004; Beygo et al., 2019; Valente et al., 2019). See text for details.
Despite solid evidence for this maternally based transcription model for LoM, the origin of IC2 hypomethylation for many BWS patients remains unclear. Given the typical mosaic pattern of LoM in different tissues, it is likely that the methylation defect occurs after fertilization. This is particularly relevant for patients conceived using assisted reproductive technologies (ART) and, more specifically, in vitro fertilization (IVF). IVF is associated with a higher-than-expected frequency of BWS and SRS cases that involve DNA methylation defects (Johnson et al., 2018). One possible explanation for this observation is that IVF involves manipulations of gametes and embryos at a time of epigenetic reprogramming. The genome undergoes an extensive DNA methylation reprogramming after fertilization, but DNA methylation at ICRs must survive this reprogramming (Morgan et al., 2005). The environmental perturbations inherent to ART, i.e. hormone treatments and ex vivo manipulations, may affect the fidelity of DNA methylation at imprinted genes in the early embryo (Vrooman and Bartolomei, 2017). Consistent with this hypothesis, mouse models of IVF have shown that simply culturing embryos ex vivo can result in significant changes in DNA methylation (Doherty et al., 2000; Mann et al., 2004). Therefore, environmental insults during early pregnancy may cause BWS in children without inherited genetic mutations.
CDKN1C mutations and loss of expression
Five to 10% of all BWS patients carry deficiency of or loss-of-function mutations in the growth suppressor CDKN1C (Table 1) (Choufani et al., 2013; Mussa et al., 2016). Early clinical work determined that maternal CDKN1C expression was absent in Wilms' tumors (Matsuoka et al., 1996), suggesting that maternally inherited loss-of-function mutations in CDKN1C can cause some of the classic symptoms of BWS. This human mutation inspired the generation of mouse models with Cdkn1c mutations to study its role in BWS (Yan et al., 1997; Zhang et al., 1997; Tunster et al., 2011). Yan et al. reported an increase in the number of apoptotic cells in affected organs, underscoring the role of Cdkn1c as a cell cycle regulator (Yan et al., 1997). Cdkn1c mutant embryos were oversized at midgestation (Tunster et al., 2011) and showed abnormalities in muscles covering the abdominal wall, recapitulating the abdominal wall defects seen in BWS patients (Zhang et al., 1997; Tunster et al., 2011). To note, the fetal overgrowth of Cdkn1c mutant mice was reversed rapidly in late gestation. Tunster et al. have suggested that intrauterine competition in mice may have masked the fetal overgrowth caused by the lack of Cdkn1c, and singleton pregnancy in human allows presentation of the overgrowth phenotype in BWS offspring (Tunster et al., 2011).
SRS
SRS (OMIM #180860) is a fetal undergrowth genetic disorder with an incidence range of 1 in 30,000 to 100,000 (Wakeling et al., 2017). Representative SRS symptoms include lower birth weight, growth restriction lasting to adulthood, skeletal/limb asymmetry, cognitive impairment together with delayed language development, macrocephaly, fifth-finger clinodactyly and characteristic triangular face (Wakeling et al., 2017). SRS is a ‘sister’ epigenetic disorder of BWS. The dysregulation of the same gene cluster can cause either the overgrowth observed in BWS or undergrowth in SRS. Consistently, depending on the parental origin, the same genetic defect in imprinted clusters can result in increased or decreased expression of these imprinted growth regulators. Here, we discuss how both human and mouse studies have informed our understanding of the abnormal imprinting patterns of IC1 and SRS.
The H19/IGF2 cluster in SRS
Loss of IC1 methylation, independent of a genetic mutation in cis, is found in 50% of SRS patients (Table 1). Normally, methylation on paternal IC1 prevents CTCF binding and silences paternal H19. In SRS, the hypomethylated paternal IC1 is proposed to enable the formation of an ectopic CTCF insulator, as demonstrated in an SRS mouse model (Engel et al., 2004; Gicquel et al., 2005). As a result, the paternal H19 is activated and IGF2 expression is decreased. Altered H19 and/or IGF2 expression can lead to abnormal growth because H19 and IGF2 are growth regulators. In mice, paternal-specific deletion of Igf2 resulted in total loss of Igf2 expression and pre- and postnatal growth restriction (DeChiara et al., 1991; Haley et al., 2012). Moreover, transgenic H19 overexpression caused embryonic growth restriction (Gabory et al., 2009). Therefore, improper H19/Igf2 expression caused by IC1 LoM can cause abnormal development in SRS.
The consequences of IC1 LoM found in humans are explored further in the aforementioned humanized mouse model [Fig. 1A, hIC1 (Hur et al., 2016)]. In these mice, the paternally transmitted hIC1 failed to establish proper methylation during spermatogenesis, enabling CTCF to function as an insulator on the paternal allele. Therefore, the offspring of these sires exhibit highly elevated H19 expression, undetectable Igf2 expression, severe growth restriction and perinatal lethality. In a different mouse model, mutating CpGs within the CTSs of the paternal H19/Igf2 ICR caused LoM [Fig. 1C, DMD-9CG (Engel et al., 2004)]. This mutant ICR was unable to maintain methylation after fertilization, thereby promoting CTCF binding and ectopic insulator function on the paternal allele. These offspring also display activated paternal H19 and reduced Igf2 expression, culminating in restricted embryonic growth.
What can cause the IC1 LoM in SRS patients? As with BWS methylation abnormalities, SRS patients typically present with a mosaic pattern of methylation (Table 1) (Eggermann et al., 2016). This mosaic pattern suggests that LoM may occur postfertilization and may be linked to an unfavorable embryonic/fetal environment. ART/IVF strategies represent an example of an environmental insult on the methylome, and, similar to BWS, SRS appears in IVF conceptuses more often than predicted by random chance (Hattori et al., 2019). Additionally, ART-conceived SRS patients have higher variability in methylation compared to spontaneously conceived SRS individuals (Hattori et al., 2019). Because ART procedures occur in the developmental window when the epigenome is reprogrammed, the epigenetic regulators responsible for maintaining imprints at IC1 may be perturbed, predisposing ART babies to a higher risk for SRS.
IC1 LoM can occasionally be caused by genetic lesions. SRS patients with IC1 hypomethylation may also present with paternally inherited IC1 deletions (Abi Habib et al., 2017). A BWS-related IC1 deletion that caused GoM on maternally inherited IC1 in a different family member caused LoM on IC1 when paternally inherited (Kraft et al., 2019). As described in the hIC1Δ2.2 mouse model, paternal transmission of the mutant hIC1 allele resulted in incomplete methylation of the paternal IC1, recapitulating LoM in SRS [Fig. 1A, hIC1Δ2.2 (Freschi et al., 2018)]. The incomplete establishment of methylation on the mutant IC1 may be due to the inability of the mutated allele to be recognized by DNA methyltransferases during spermatogenesis and/or the subsequent failure to maintain the previously established methyl marks. Animals with a hypomethylated paternal ICR showed high H19 and low Igf2 expression, and were pre- and postnatally growth restricted. The liver and kidneys were more severely affected than other organs in this model, closely resembling the organ-specific growth restriction in SRS.
The KRAB zinc finger protein ZFP57 is another factor that can affect DNA methylation of the H19/IGF2 locus. ZFP57, which interacts with ICRs in a methylation-dependent manner, binds to methylated CpGs in ICRs and protects them from genome-wide demethylation during the postfertilization reprogramming window (Li et al., 2008; Strogantsev et al., 2015). This binding enables the preservation of parental allele-specific methylation of imprinted clusters. Previous work suggested that ZFP57 was involved in the etiology of IC1 hypomethylation in SRS (Hirasawa and Feil, 2008), in that loss-of-function mutation of ZFP57 resulted in mosaic hypomethylation of many imprinted loci (Li et al., 2008; Mackay et al., 2008). Consistently, Sparago et al. suggested that, in the hIC1 transgenic mouse model (Hur et al., 2016), the paternally transmitted hIC1 could not be properly methylated possibly because the inserted hIC1 sequence lacked a ZFP57 binding site. They suggested that insertion of an additional ZFP57 binding site in the hIC1 construct would enable ZFP57 to bind and protect paternally established methylation (Sparago et al., 2018). However, patients with transient neonatal diabetes mellitus 1 caused by loss-of-function mutations of ZFP57 exhibited normal methylation of IC1 (Mackay et al., 2008; Boonen et al., 2013). Moreover, SRS patients with IC1 hypomethylation were found to have no functional mutation of ZFP57 (Spengler et al., 2009). Although the role of ZFP57 at IC1 remains to be determined, it is likely that other zinc finger proteins, such as the recently described ZFP445, preserve DNA methylation and may be mutated in SRS patients (Takahashi et al., 2019).
Mutations in the H19/IGF2 cluster other than IC1 LoM are also found in SRS patients. Chromosomal structural variations in the H19/IGF2 enhancer region were reported in a group of SRS patients (Grønskov et al., 2011). Paternally inherited genetic lesions that disrupt the interaction between the mesodermal enhancer and the IGF2 promoter resulted in delayed growth in patients. In mice, deletion of the endodermal H19/Igf2 enhancers on the paternal allele led to growth-restricted offspring (to 70% of that of wild type at birth) and reduced Igf2 expression in endodermal tissues such as liver and kidney [Fig. 1C, EndoEnhΔ (Leighton et al., 1995)]. Additionally, paternally transmitted disruption of mesodermal H19/Igf2 enhancers resulted in growth retardation (50% of wild type at birth), repressing Igf2 expression in mesodermal tissues including tongue and kidney [Fig. 1C, Mnt (Davies and Reik, 2002)]. The deletion of mesodermal H19/Igf2 enhancers on the paternal allele also resulted in reduced Igf2 expression in mesodermal tissues [Fig. 1C, VM3 (Kaffer et al., 2001)].
mUPD11 and duplication of maternal chromosome 11p15
Rarely, SRS patients have maternal UPD (mUPD) or maternal duplication of chromosome 11p15 [Table 1, Fig. 1D (Luk et al., 2016)]. In mice, mUPD of mouse distal chromosome 7, which includes the H19/Igf2 and Cdkn1c/Kcnq1ot1 clusters, resulted in growth deficiency and perinatal lethality (Han et al., 2010). In the presence of two maternal distal chromosome 7 alleles, the maternally expressed genes are overexpressed and the paternally expressed genes are silent. Accordingly, mUPD7 mice had loss of Igf2 and increased H19 and Cdkn1c expression, which would result in severe undergrowth. To understand the source of the perinatal lethality, the H19/Igf2 ICR and the Cdkn1c gene were deleted in mUPD7 mice. When H19/Igf2 ICR was deleted on one allele, the mUPD7 pups were viable, but the growth restriction persisted, albeit to a smaller extent. Here, Igf2 expression was restored to wild-type levels, but Cdkn1c expression remained high. Deletion of Cdkn1c from the paternal allele of mUPD7 mice restored embryonic weight to the wild-type level but lethality persisted. This demonstrates that excessive Cdkn1c expression was not responsible for mUPD7 lethality. Indeed, IC1 and IC2 clusters may contribute uniquely to the growth dysregulation in mUPD11 SRS patients.
mUPD7 and duplication of chromosome 7
Five to 10% of SRS patients are reported to have duplication (Monk et al., 2000; 2002; Eggermann et al., 2012b) or mUPD on chromosome 7 (Yuan et al., 2016), where growth factor receptor-bound protein 10 [GRB10; also known as maternally expressed gene 1 (MEG1)] resides. SRS patients with mUPD7 are more likely to have verbal dyspraxia, learning difficulties, myoclonus dystonia and autistic spectrum disorder compared to other SRS subgroups (Wakeling et al., 2017). GRB10 functions as a growth suppressor, binding to insulin and IGF1 receptors to inhibit the growth-promoting activity of IGF. Therefore, increased GRB10 in SRS patients can result in an undergrowth phenotype. Grb10 is located on chromosome 11 in mouse, and the somatic isoform is maternally expressed. Maternal duplication of mouse proximal chromosome 11, which includes the Grb10 locus, resulted in prenatal growth retardation (Miyoshi et al., 1998). In contrast, paternal duplication of the same region resulted in growth enhancement, proving that the allele-specific regulation of Grb10 expression is crucial for normal development. To note, Grb10 isoforms are expressed from different parental alleles in somatic and neuronal tissues in mice. Absence of the paternal, brain-specific isoform of GRB10 might thus cause the brain-specific phenotypes in mUPD7 patients.
Another imprinted gene implicated in SRS is located on chromosome 7q: mesoderm-specific transcript [MEST; also known as paternally expressed gene 1 (PEG1)]. The MEST promoter is methylated on the maternal allele, and MEST is expressed from the paternal allele (Riesewijk et al., 1997). Several SRS patients with mild symptoms were reported to have mUPD for chromosome 7q (Hannula et al., 2001; Reboul et al., 2006; Eggermann et al., 2008) or a deletion on paternal 7q spanning MEST (Eggermann et al., 2012a). In mice, loss of Mest expression from the paternal allele resulted in pre- and postnatal growth restriction (Lefebvre et al., 1998), suggesting a growth regulatory role for MEST.
Mutations related to the CDKN1C/KCNQ1OT1 cluster
A small number of SRS patients have mutations in the CDKN1C/KCNQ1OT1 cluster. Maternally inherited activating mutations of CDKN1C were found in SRS patients (Table 1; Brioude et al., 2013). A microduplication encompassing the CDKN1C/KCNQ1OT1 cluster, but not the H19/IGF2 cluster, was found on the maternal allele of an SRS patient (Schönherr et al., 2006). In a different SRS family, a microduplication of the CDKN1C/KCNQ1OT1 cluster resulted in SRS only when maternally inherited (Bonaldi et al., 2011). Transgene modeling this duplicated region led to increased Cdkn1c expression in mice, together with growth reduction, which was dependent on Cdkn1c dosage and lasted to adulthood (Andrews et al., 2007). This mouse model also showed neonatal hypoglycemia, decreased body fat in adults (Van De Pette et al., 2016) and behavioral abnormalities (McNamara et al., 2016), phenotypes that recapitulate representative SRS features. Mice with deletion of paternal KvDMR1 exhibited biallelic expression of Cdkn1c and fetal growth retardation, further demonstrating the growth repressor functions of Cdkn1c [Fig. 2C, 2.8kb-KvDMR1Δ (Fitzpatrick et al., 2002) and 3.6kb-DMRΔ (Mancini-DiNardo et al., 2006)]. When the paternal Cdkn1c was activated due to the truncation of the paternal Kcnq1ot1 transcript, mice exhibited growth deficiency [Fig. 2C, pA/YJ69 (Shin et al., 2008)]. Transgenic overexpression of Phlda2 and Slc22a18 resulted in placental growth retardation without involving a change in Cdkn1c expression (Salas et al., 2004; Tunster et al., 2010). Further research showed that the increased Phlda2 expression contributed more to placental insufficiency and asymmetric growth restriction (Tunster et al., 2014). These results suggest that other genes in the cluster can contribute the growth restriction phenotype of SRS.
MLID: lesions on multiple loci
Multi-locus imprinting disturbances (MLID) exhibit imprinting defects at multiple loci, in addition to the locus primarily relevant to the patient's symptoms (Sanchez-Delgado et al., 2016). Up to 25% of BWS patients with IC2 LoM and 10% of SRS patients with IC1 LoM were reported to have MLID (Azzi et al., 2009; Eggermann et al., 2011). Although the exact cause of MLID is unknown, some MLID patients carry defects in trans regulators such as ZFP57 (Mackay et al., 2008), NLRP2 (Meyer et al., 2009), NLRP5 (Docherty et al., 2015) and PADI6 (Begemann et al., 2018) that may contribute to the variable phenotypes. Among these, deletion of the oocyte-specific gene Nlrp5 (also known as Mater) caused sterility in female mice, producing embryos that developmentally arrest at the two-cell stage (Tong et al., 2000). Nlrp2-deficient females were subfertile and produced developmentally delayed and smaller offspring (Mahadevan et al., 2017), with more rapidly decreasing reproductive rates, as one would expect with accelerated aging (Kuchmiy et al., 2016). Mice do not have an ortholog of NLRP7 and, therefore, defects in NLRP7 cannot be modeled. Overall, it would be important to understand the roles of these trans regulators that may be crucial for establishing and maintaining DNA methylation on multiple imprinted loci.
Conclusions and future directions
Mouse models have been widely utilized to understand how genomic imprinting is established, maintained and inherited in humans. To mimic the pathologic genotypes of imprinting disorders such as BWS and SRS in humans, researchers have generated numerous mouse models. These mouse models revealed (1) the main regulatory mechanisms of imprinted clusters such as H19/Igf2 and Cdkn1c/Kcnq1ot1, (2) how the lack of key components of imprinted clusters lead to dysregulation, and (3) the roles of linked genes in the imprinted clusters.
Although manipulating mouse genomes has provided important knowledge, as discussed in this Review, modeling human epigenetic mutations in mice has several limitations. For example, pUPD11 is a significant cause of BWS, but modeling pUPD11 in mice has been challenging due to embryonic lethality. This prenatal death is caused by loss of expression of the imprinted Ascl2 gene, which is not imprinted in humans (Westerman et al., 2001; Miyamoto et al., 2002; Rentsendorj et al., 2010). Additionally, the mosaicism observed in human pUPD11 patients is not evident in mice. Together, these factors complicate the modeling of human pUPD11.
To overcome such limitations, several other methods have been developed to study imprinting disorders, including human induced pluripotent cells (hiPSCs). Derived from BWS patients' fibroblasts, the strength of this system is the preserved pathological genetic environment. This eliminates the need to manipulate the genome to achieve the desired mutations and therefore avoids the possible off-target effects of gene editing. Another advantage of the system is that hiPSCs can be differentiated into clinically relevant tissues that are difficult to study in human BWS patients. Combining information gleaned from hiPSCs and traditional mouse models will enhance our understanding of the genetic mechanisms underlying imprinting disorders such as BWS and SRS.
Acknowledgements
The authors thank Aimee Juan and Dr Joanne Thorvaldsen for their critical reading of this Review.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health (GM051279).
References
- Abi Habib W., Brioude F., Azzi S., Salem J., Das Neves C., Personnier C., Chantot-Bastaraud S., Keren B., Le Bouc Y., Harbison M. D. et al. (2017). 11p15 ICR1 Partial Deletions Associated with IGF2/H19 DMR Hypomethylation and Silver–Russell Syndrome. Hum. Mutat. 38, 105-111. 10.1002/humu.23131 [DOI] [PubMed] [Google Scholar]
- Andrews S. C., Wood M. D., Tunster S. J., Barton S. C., Surani A. M. and John R. M. (2007). Cdkn1c (p57Kip2) is the major regulator of embryonic growth within its imprinted domain on mouse distal chromosome 7. BMC Dev. Biol. 7, 53 10.1186/1471-213X-7-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi S., Rossignol S., Steunou V., Sas T., Thibaud N., Danton F., Le Jule M., Heinrichs C., Cabrol S., Gicquel C. et al. (2009). Multilocus methylation analysis in a large cohort of 11p15-related foetal growth disorders (Russell Silver and Beckwith Wiedemann syndromes) reveals simultaneous loss of methylation at paternal and maternal imprinted loci. Hum. Mol. Genet. 18, 4724-4733. 10.1093/hmg/ddp435 [DOI] [PubMed] [Google Scholar]
- Barlow D. P. and Bartolomei M. S. (2014). Genomic imprinting in mammals. Cold Spring Harbor Perspect. Biol. 6, a018382-a018382 10.1101/cshperspect.a018382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann M., Rezwan F. I., Beygo J., Docherty L. E., Kolarova J., Schroeder C., Buiting K., Chokkalingam K., Degenhardt F., Wakeling E. L. et al. (2018). Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J. Med. Genet. 55, 497-504. 10.1136/jmedgenet-2017-105190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. C. and Felsenfeld G. (2000). Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405, 482-485. 10.1038/35013100 [DOI] [PubMed] [Google Scholar]
- Bell A. C., West A. G. and Felsenfeld G. (1999). The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98, 387-396. 10.1016/S0092-8674(00)81967-4 [DOI] [PubMed] [Google Scholar]
- Berland S., Appelbäck M., Bruland O., Beygo J., Buiting K., Mackay D. J. G., Karen Temple I. and Houge G. (2013). Evidence for anticipation in Beckwith-Wiedemann syndrome. Eur. J. Hum. Genet. 21, 1344-1348. 10.1038/ejhg.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beygo J., Citro V., Sparago A., De Crescenzo A., Cerrato F., Heitmann M., Rademacher K., Guala A., Enklaar T., Anichini C. et al. (2013). The molecular function and clinical phenotype of partial deletions of the IGF2/H19 imprinting control region depends on the spatial arrangement of the remaining CTCF-binding sites. Hum. Mol. Genet. 22, 544-557. 10.1093/hmg/dds465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beygo J., Bürger J., Strom T. M., Kaya S. and Buiting K. (2019). Disruption of KCNQ1 prevents methylation of the ICR2 and supports the hypothesis that its transcription is necessary for imprint establishment. Eur. J. Hum. Genet. 27, 903-908. 10.1038/s41431-019-0365-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal B., Arnaudo A., Dymkowski A., Best A. and Davis T. L. (2004). Methylation at mouse Cdkn1c is acquired during postimplantation development and functions to maintain imprinted expression. Genomics 84, 961-970. 10.1016/j.ygeno.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Bliek J. (2001). Increased tumour risk for BWS patients correlates with aberrant H19 and not KCNQ1OT1 methylation: occurrence of KCNQ1OT1 hypomethylation in familial cases of BWS. Hum. Mol. Genet. 10, 467-476. 10.1093/hmg/10.5.467 [DOI] [PubMed] [Google Scholar]
- Bonaldi A., Mazzeu J. F., Costa S. S., Honjo R. S., Bertola D. R., Albano L. M. J., Furquim I. M., Kim C. A. and Vianna-Morgante A. M. (2011). Microduplication of the ICR2 domain at chromosome 11p15 and familial Silver-Russell syndrome. American Journal of Medical Genetics, Part A 155, 2479-2483. 10.1002/ajmg.a.34023 [DOI] [PubMed] [Google Scholar]
- Boonen S. E., Mackay D. J. G., Hahnemann J. M. D., Docherty L., Gronskov K., Lehmann A., Larsen L. G., Haemers A. P., Kockaerts Y., Dooms L. et al. (2013). Transient neonatal diabetes, ZFP57, and hypomethylation of multiple imprinted loci. Diabetes Care 36, 505-512. 10.2337/dc12-0700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borensztein M., Monnier P. and Dandolo L. (2013). Dandolo_Borensztein et al., Development, 2013_MyoD and Igf2 on mesodermal enhancer. Development 140, 1231-1239. 10.1242/dev.084665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioude F., Oliver-Petit I., Blaise A., Praz F., Rossignol S., Jule M. L., Thibaud N., Faussat A.-M., Tauber M., Bouc Y. L. et al. (2013). CDKN1C mutation affecting the PCNA-binding domain as a cause of familial Russell Silver syndrome. J. Med. Genet. 50, 823-830. 10.1136/jmedgenet-2013-101691 [DOI] [PubMed] [Google Scholar]
- Brioude F., Kalish J. M., Mussa A., Foster A. C., Bliek J., Ferrero G. B., Boonen S. E., Cole T., Baker R., Bertoletti M. et al. (2018a). Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nature Reviews Endocrinology 14, 229-249. 10.1038/nrendo.2017.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioude F., Hennekam R., Bliek J., Coze C., Eggermann T., Ferrero G. B., Kratz C., Bouc Y. L., Maas S. M., Mackay D. J. G. et al. (2018b). Revisiting Wilms tumour surveillance in Beckwith-Wiedemann syndrome with IC2 methylation loss, reply. Eur. J. Hum. Genet. 26, 471-472. 10.1038/s41431-017-0074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrato F., Sparago A., Verde G., De Crescenzo A., Citro V., Cubellis M. V., Rinaldi M. M., Boccuto L., Neri G., Magnani C. et al. (2008). Different mechanisms cause imprinting defects at the IGF2/H19 locus in Beckwith - Wiedemann syndrome and Wilms’ tumour. Hum. Mol. Genet. 17, 1427-1435. 10.1093/hmg/ddn031 [DOI] [PubMed] [Google Scholar]
- Chotalia M., Smallwood S. A., Ruf N., Dawson C., Lucifero D., Frontera M., James K., Dean W. and Kelsey G. (2009). Transcription is required for establishment of germline methylation marks at imprinted genes. Genes Dev. 23, 105-117. 10.1101/gad.495809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choufani S., Shuman C. and Weksberg R. (2013). Molecular findings in beckwith-wiedemann syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 163, 131-140. 10.1002/ajmg.c.31363 [DOI] [PubMed] [Google Scholar]
- Dao D., Walsh C. P., Yuan L., Gorelov D., Feng L., Hensle T., Nisen P., Yamashiro D. J., Bestor T. H. and Tycko B. (1999). Multipoint analysis of human chromosome 11p15/mouse distal chromosome 7: inclusion of H19/IGF2 in the minimal WT2 region, gene specificity of H19 silencing in Wilms’ tumorigenesis and methylation hyper-dependence of H19 imprinting. Hum. Mol. Genet. 8, 1337-1352. 10.1093/hmg/8.7.1337 [DOI] [PubMed] [Google Scholar]
- Davies K. and Reik W. (2002). Disruption of mesodermal enhancers for Igf2 in the minute mutant. Development 129, 1657-1668. [DOI] [PubMed] [Google Scholar]
- Debaun M. R., King A. A. and White N. (2000). Hypoglycemia in Beckwith-Wiedemann syndrome. Semin. Perinatol. 24, 164-171. 10.1053/sp.2000.6366 [DOI] [PubMed] [Google Scholar]
- Debaun M. R., Niemitz E. L., Mcneil D. E., Brandenburg S. A., Lee M. P. and Feinberg A. P. (2002). Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am. J. Hum. Genet. 70, 604-611. 10.1086/338934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechiara T. M., Robertson E. J. and Efstratiadis A. (1991). Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64, 849-859. 10.1016/0092-8674(91)90513-X [DOI] [PubMed] [Google Scholar]
- Docherty L. E., Rezwan F. I., Poole R. L., Turner C. L. S., Kivuva E., Maher E. R., Smithson S. F., Hamilton-Shield J. P., Patalan M., Gizewska M. et al. (2015). Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat. Commun. 6 10.1038/ncomms9086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty A. S., Mann M. R. W., Tremblay K. D., Bartolomei M. S. and Schultz R. M. (2000). Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo1. Biol. Reprod. 62, 1526-1535. 10.1095/biolreprod62.6.1526 [DOI] [PubMed] [Google Scholar]
- Duffy K. A., Sajorda B. J., Yu A. C., Hathaway E. R., Grand K. L., Deardorff M. A. and Kalish J. M. (2019a). Beckwith–Wiedemann syndrome in diverse populations. Am. J. Med. Genet. A 179, 525-533. 10.1002/ajmg.a.61109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy K. A., Cielo C. M., Cohen J. L., Gonzalez-Gandolfi C. X., Griff J. R., Hathaway E. R., Kupa J., Taylor J. A., Wang K. H., Ganguly A. et al. (2019b). Characterization of the Beckwith-Wiedemann spectrum: diagnosis and management. Am. J. Med. Genet. C Semin. Med. Genet. 181, 693-708. 10.1002/ajmg.c.31740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A. and Ferguson-Smith A. C. (2007). Mechanisms regulating imprinted genes in clusters. Curr. Opin. Cell Biol. 19, 281-289. 10.1016/j.ceb.2007.04.013 [DOI] [PubMed] [Google Scholar]
- Eggermann T., Schönherr N., Jäger S., Spaich C., Ranke M. B., Wollmann H. A. and Binder G. (2008). Segmental maternal UPD(7q) in Silver-Russell syndrome. Clin. Genet. 74, 486-489. 10.1111/j.1399-0004.2008.01057.x [DOI] [PubMed] [Google Scholar]
- Eggermann T., Buiting K. and Temple I. K. (2011). Clinical utility gene card for: Silver-Russell syndrome. Eur. J. Hum. Genet. 19, 3-3 10.1038/ejhg.2010.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann T., Spengler S., Begemann M., Binder G., Buiting K., Albrecht B. and Spranger S. (2012a). Deletion of the paternal allele of the imprinted MEST/PEG1 region in a patient with Silver-Russell syndrome features. Clin. Genet. 81, 298-300. 10.1111/j.1399-0004.2011.01719.x [DOI] [PubMed] [Google Scholar]
- Eggermann T., Begemann M., Gogiel M., Palomares M., Vallespín E., Fernández L., Cazorla R., Spengler S. and García-Miñaúr S. (2012b). Heterogeneous growth patterns in carriers of chromosome 7p12.2 imbalances affecting GRB10. Am. J. Med. Genet. A 158A, 2815-2819. 10.1002/ajmg.a.35612 [DOI] [PubMed] [Google Scholar]
- Eggermann T., Algar E., Lapunzina P., Mackay D., Maher E. R., Mannens M., Netchine I., Prawitt D., Riccio A., Temple I. K. et al. (2014). Clinical utility gene card for: Beckwith-Wiedemann Syndrome. Eur. J. Hum. Genet. 22, 435-435 10.1038/ejhg.2013.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann T., Brioude F., Russo S., Lombardi M. P., Bliek J., Maher E. R., Larizza L., Prawitt D., Netchine I., Gonzales M. et al. (2016). Prenatal molecular testing for Beckwith-Wiedemann and Silver-Russell syndromes: a challenge for molecular analysis and genetic counseling. Eur. J. Hum. Genet. 24, 784-793. 10.1038/ejhg.2015.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel N., West A. G., Felsenfeld G. and Bartolomei M. S. (2004). Antagonism between DNA hypermethylation and enhancer-blocking activity at the H19 DMD is uncovered by CpG mutations. Nat. Genet. 36, 883-888. 10.1038/ng1399 [DOI] [PubMed] [Google Scholar]
- Engel N., Thorvaldsen J. L. and Bartolomei M. S. (2006). CTCF binding sites promote transcription initiation and prevent DNA methylation on the maternal allele at the imprinted H19/Igf2 locus. Hum. Mol. Genet. 15, 2945-2954. 10.1093/hmg/ddl237 [DOI] [PubMed] [Google Scholar]
- Ferrón S. R., Radford E. J., Domingo-Muelas A., Kleine I., Ramme A., Gray D., Sandovici I., Constancia M., Ward A., Menheniott T. R. et al. (2015). Differential genomic imprinting regulates paracrine and autocrine roles of IGF2 in mouse adult neurogenesis. Nat. Commun. 6, 8265 10.1038/ncomms9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick G. V., Soloway P. D. and Higgins M. J. (2002). Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat. Genet. 32, 426-431. 10.1038/ng988 [DOI] [PubMed] [Google Scholar]
- Frank D., Fortino W., Clark L., Musalo R., Wang W., Saxena A., Li C.-M., Reik W., Ludwig T. and Tycko B. (2002). Placental overgrowth in mice lacking the imprinted gene lpl. Proc. Natl. Acad. Sci. USA 99, 7490-7495. 10.1073/pnas.122039999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi A., Hur S. K., Valente F. M., Ideraabdullah F. Y., Sparago A., Gentile M. T., Oneglia A., Di Nucci D., Colucci-D'Amato L., Thorvaldsen J. L. et al. (2018). Tissue-specific and mosaic imprinting defects underlie opposite congenital growth disorders in mice. PLoS Genet. 14, e1007243 10.1371/journal.pgen.1007243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevel M. A. E., Sowerby S. J., Petersen G. B. and Reeve A. E. (1999). Methylation sequencing analysis refines the region of H19 epimutation in Wilms tumor. J. Biol. Chem. 274, 29331-29340. 10.1074/jbc.274.41.29331 [DOI] [PubMed] [Google Scholar]
- Gabory A., Ripoche M.-A., Le Digarcher A., Watrin F., Ziyyat A., Forne T., Jammes H., Ainscough J. F. X., Surani M. A., Journot L. et al. (2009). H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development 136, 3413-3421. 10.1242/dev.036061 [DOI] [PubMed] [Google Scholar]
- Gicquel C., Rossignol S., Cabrol S., Houang M., Steunou V., Barbu V., Danton F., Thibaud N., Merrer M. L., Burglen L. et al. (2005). Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat. Genet. 37, 1003-1007. 10.1038/ng1629 [DOI] [PubMed] [Google Scholar]
- Ginart P., Kalish J. M., Jiang C. L., Yu A. C., Bartolomei M. S. and Raj A. (2016). Visualizing allele-specific expression in single cells reveals epigenetic mosaicism in an H19 loss-of-imprinting mutant. Genes Dev. 30, 567-578. 10.1101/gad.275958.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønskov K., Poole R. L., Hahnemann J. M. D., Thomson J., Tumer Z., Brondum-Nielsen K., Murphy R., Ravn K., Melchior L., Dedic A. et al. (2011). Deletions and rearrangements of the H19/IGF2 enhancer region in patients with Silver-Russell syndrome and growth retardation. J. Med. Genet. 48, 308-311. 10.1136/jmg.2010.086504 [DOI] [PubMed] [Google Scholar]
- Guillemot F., Caspary T., Tilghman S. M., Copeland N. G., Gilbert D. J., Jenkins N. A., Anderson D. J., Joyner A. L., Rossant J. and Nagy A. (1995). Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat. Genet. 9, 235-242. 10.1038/ng0395-235 [DOI] [PubMed] [Google Scholar]
- Haley V. L., Barnes D. J., Sandovici I., Constancia M., Graham C. F., Pezzella F., Bühnemann C., Carter E. J. and Hassan A. B. (2012). Igf2 pathway dependency of the Trp53 developmental and tumour phenotypes. EMBO Mol. Med. 4, 705-718. 10.1002/emmm.201101105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Szabó P. E. and Mann J. R. (2010). Postnatal survival of mice with maternal duplication of distal chromosome 7 induced by a Igf2/H19 imprinting control region lacking insulator function. PLoS Genet. 6, e1000803 10.1371/journal.pgen.1000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula K., Lipsanen-Nyman M., Kontiokari T. and Kere J. (2001). A narrow segment of maternal uniparental disomy of chromosome 7q31-qter in Silver-Russell syndrome delimits a candidate gene region. Am. J. Hum. Genet. 68, 247-253. 10.1086/316937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada I. and Mukai T. (1995). Genomic imprinting of p57KIP2, a cyclin–dependent kinase inhibitor, in mouse. Nat. Genet. 11, 204-206. 10.1038/ng1095-204 [DOI] [PubMed] [Google Scholar]
- Hattori H., Hiura H., Kitamura A., Miyauchi N., Kobayashi N., Takahashi S., Okae H., Kyono K., Kagami M., Ogata T. et al. (2019). Association of four imprinting disorders and ART. Clin. Epigenetics 11, 21 10.1186/s13148-019-0623-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa R. and Feil R. (2008). A KRAB domain zinc finger protein in imprinting and disease. Dev. Cell 15, 487-488. 10.1016/j.devcel.2008.09.006 [DOI] [PubMed] [Google Scholar]
- Hur S. K., Freschi A., Ideraabdullah F., Thorvaldsen J. L., Luense L. J., Weller A. H., Berger S. L., Cerrato F., Riccio A. and Bartolomei M. S. (2016). Humanized H19/Igf2 locus reveals diverged imprinting mechanism between mouse and human and reflects Silver-Russell syndrome phenotypes. Proc. Natl. Acad. Sci. USA 113, 10938-10943. 10.1073/pnas.1603066113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideraabdullah F. Y., Thorvaldsen J. L., Myers J. A. and Bartolomei M. S. (2014). Tissue-specific insulator function at H19/Igf2 revealed by deletions at the imprinting control region. Hum. Mol. Genet. 23, 6246-6259. 10.1093/hmg/ddu344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. B., Tunster S. J. and John R. M. (2014). The significance of elevated placental PHLDA2 in human growth restricted pregnancies. Placenta 35, 528-532. 10.1016/j.placenta.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Beischel L., Schwanke C., Styren K., Crunk A., Schoof J. and Elias A. F. (2018). Overrepresentation of pregnancies conceived by artificial reproductive technology in prenatally identified fetuses with Beckwith-Wiedemann syndrome. J. Assist. Reprod. Genet. 35, 985-992. 10.1007/s10815-018-1228-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffer C. R., Srivastava M., Park K. Y., Ives E., Hsieh S., Batlle J., Grinberg A., Huang S. P. and Pfeifer K. (2000). A transcriptional insulator at the imprinted H19/Igf2 locus. Genes Dev. 14, 1908-1919. [PMC free article] [PubMed] [Google Scholar]
- Kaffer C. R., Grinberg A. and Pfeifer K. (2001). Regulatory mechanisms at the mouse Igf2/H19 locus. Mol. Cell. Biol. 21, 8189-8196. 10.1128/MCB.21.23.8189-8196.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanduri C., Pant V., Loukinov D., Pugacheva E., Qi C.-F., Wolffe A., Ohlsson R. and Lobanenkov V. V. (2000). Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr. Biol. 10, 853-856. 10.1016/S0960-9822(00)00597-2 [DOI] [PubMed] [Google Scholar]
- Keniry A., Oxley D., Monnier P., Kyba M., Dandolo L., Smits G. and Reik W. (2012). The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 14, 659-665. 10.1038/ncb2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft F., Wesseler K., Begemann M., Kurth I., Elbracht M. and Eggermann T. (2019). Novel familial distal imprinting centre 1 (11p15.5) deletion provides further insights in imprinting regulation. Clin. Epigenetics 11 10.1186/s13148-019-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchmiy A. A., D'Hont J., Hochepied T. and Lamkanfi M. (2016). NLRP2 controls age-associated maternal fertility. J. Exp. Med. 213, 2851-2860. 10.1084/jem.20160900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecerf C., Le Bourhis X. and Adriaenssens E. (2019). The long non-coding RNA H19: an active player with multiple facets to sustain the hallmarks of cancer. Cell. Mol. Life Sci. 76, 4673-4687. 10.1007/s00018-019-03240-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. T. and Bartolomei M. S. (2013). X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 152, 1308-1323. 10.1016/j.cell.2013.02.016 [DOI] [PubMed] [Google Scholar]
- Lee M. P., Hu R.-J. H., Johnson L. A. and Feinberg A. P. (1997). Human KVLQT1 gene shows tissue-specific imprinting and encompasses Beckwith-Wiedemann syndrome chromosomal rearrangements. Nat. Genet. 15, 181-185. 10.1038/ng0297-181 [DOI] [PubMed] [Google Scholar]
- Lefebvre L., Viville S., Barton S. C., Ishino F., Keverne E. B. and Surani M. A. (1998). Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat. Genet. 20, 163-169. 10.1038/2464 [DOI] [PubMed] [Google Scholar]
- Leighton P. A., Saam J. R., Ingram R. S., Stewart C. L. and Tilghman S. M. (1995). An enhancer deletion affects both H19 and Igf2 expression. Genes Dev. 9, 2079-2089. 10.1101/gad.9.17.2079 [DOI] [PubMed] [Google Scholar]
- Li E., Beard C. and Jaenisch R. (1993). Role for DNA methylation in genomic imprinting. Nature 366, 362-365. 10.1038/366362a0 [DOI] [PubMed] [Google Scholar]
- Li Y. M., Franklin G., Cui H.-M., Svensson K., He X.-B., Adam G., Ohlsson R. and Pfeifer S. (1998). The H19 transcript is associated with polysomes and may regulate IGF2 expression in trans. J. Biol. Chem. 273, 28247-28252. 10.1074/jbc.273.43.28247 [DOI] [PubMed] [Google Scholar]
- Li X., Ito M., Zhou F., Youngson N., Zuo X., Leder P. and Ferguson-Smith A. C. (2008). A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev. Cell 15, 547-557. 10.1016/j.devcel.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk H. M., Ivan Lo F.-M., Sano S., Matsubara K., Nakamura A., Ogata T. and Kagami M. (2016). Silver–Russell syndrome in a patient with somatic mosaicism for upd(11)mat identified by buccal cell analysis. Am. J. Med. Genet. A 170, 1938-1941. 10.1002/ajmg.a.37679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D. J. G., Callaway J. L. A., Marks S. M., White H. E., Acerini C. L., Boonen S. E., Dayanikli P., Firth H. V., Goodship J. A., Haemers A. P. et al. (2008). Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat. Genet. 40, 949-951. 10.1038/ng.187 [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Sathappan V., Utama B., Lorenzo I., Kaskar K. and Van den Veyver I. B. (2017). Maternally expressed NLRP2 links the subcortical maternal complex (SCMC) to fertility, embryogenesis and epigenetic reprogramming. Sci. Rep. 7, 44667 10.1038/srep44667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini-Dinardo D., Steele S. J., Levorse J. M., Ingram R. S. and Tilghman S. M. (2006). Elongation of the Kcnq1ot1 transcript is required for genomic imprinting of neighboring genes. Genes Dev. 20, 1268-1282. 10.1101/gad.1416906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. R. W., Lee S. S., Doherty A. S., Verona R. I., Nolen L. D., Schultz R. M. and Bartolomei M. S. (2004). Selective loss of imprinting in the placenta following preimplantation development in culture. Development 131, 3727-3735. 10.1242/dev.01241 [DOI] [PubMed] [Google Scholar]
- Martinet C., Monnier P., Louault Y., Benard M., Gabory A. and Dandolo L. (2016). H19 controls reactivation of the imprinted gene network during muscle regeneration. Development (Camb.) 143, 962-971. 10.1242/dev.131771 [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Thompson J. S., Edwards M. C., Bartletta J. M., Grundy P., Kalikin L. M., Harper J. W., Elledge S. J. and Feinberg A. P. (1996). Imprinting of the gene encoding a human cyclin-dependent kinase inhibitor, p57KIP2, on chromosome 11p15. Proc. Natl. Acad. Sci. USA 93, 3026-3030. 10.1073/pnas.93.7.3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgrath J. and Solter D. (1984). Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37, 179-183. 10.1016/0092-8674(84)90313-1 [DOI] [PubMed] [Google Scholar]
- Mclaughlin K. J., Szabó P., Haegel H. and Mann J. R. (1996). Mouse embryos with paternal duplication of an imprinted chromosome 7 region die at midgestation and lack placental spongiotrophoblast. Development 122, 265-270. [DOI] [PubMed] [Google Scholar]
- Mcnamara G. I., Davis B. A., Dwyer D. M., John R. M. and Isles A. R. (2016). Behavioural abnormalities in a novel mouse model for Silver Russell Syndrome. Hum. Mol. Genet. 25, 5407-5417. 10.1093/hmg/ddw357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E., Lim D., Pasha S., Tee L. J., Rahman F., Yates J. R. W., Woods C. G., Reik W. and Maher E. R. (2009). Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (beckwith-wiedemann syndrome). PLoS Genet. 5, e1000423 10.1371/journal.pgen.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineno J., Okamoto S., Ando T., Sato M., Chono H., Izu H., Takayama M., Asada K., Mirochnitchenko O., Inouye M. et al. (2006). The expression profile of microRNAs in mouse embryos. Nucleic Acids Res. 34, 1765-1771. 10.1093/nar/gkl096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Hasuike S., Jinno Y., Soejima H., Yun K., Miura K., Ishikawa M. and Niikawa N. (2002). The human ASCL2 gene escaping genomic imprinting and its expression pattern. J. Assist. Reprod. Genet. 19, 240-244. 10.1023/A:1015362903486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N., Kuroiwa Y., Kohda T., Shitara H., Yonekawa H., Kawabe T., Hasegawa H., Barton S. C., Surani M. A., Kaneko-Ishino T. et al. (1998). Identification of the Meg1/Grb10 imprinted gene on mouse proximal chromosome 11, a candidate for the Silver-Russell syndrome gene. Proc. Natl. Acad. Sci. USA 95, 1102-1107. 10.1073/pnas.95.3.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad F., Mondal T., Guseva N., Pandey G. K. and Kanduri C. (2010). Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development 137, 2493-2499. 10.1242/dev.048181 [DOI] [PubMed] [Google Scholar]
- Monk D., Wakeling E. L., Proud V., Hitchins M., Abu-Amero S. N., Stanier P., Preece M. A. and Moore G. E. (2000). Duplication of 7p11.2-p13, including GRB10, in Silver-Russell syndrome. Am. J. Hum. Genet. 66, 36-46. 10.1086/302717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk D., Bentley L., Hitchins M., Myler R., Clayton-Smith J., Ismail S., Price S., Preece M., Stanier P. and Moore G. (2002). Chromosome 7p disruptions in Silver Russell syndrome: Delineating an imprinted candidate gene region. Hum. Genet. 111, 376-387. 10.1007/s00439-002-0777-4 [DOI] [PubMed] [Google Scholar]
- Morgan H. D., Santos F., Green K., Dean W. and Reik W. (2005). Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14, R47-R58. 10.1093/hmg/ddi114 [DOI] [PubMed] [Google Scholar]
- Mussa A., Russo S., De Crescenzo A., Chiesa N., Molinatto C., Selicorni A., Richiardi L., Larizza L., Silengo M. C., Riccio A. et al. (2013). Prevalence of beckwith-wiedemann syndrome in North West of Italy. American Journal of Medical Genetics, Part A 161A, 2481-2486. 10.1002/ajmg.a.36080 [DOI] [PubMed] [Google Scholar]
- Mussa A., Russo S., De Crescenzo A., Freschi A., Calzari L., Maitz S., Macchiaiolo M., Molinatto C., Baldassarre G., Mariani M. et al. (2016). Fetal growth patterns in Beckwith–Wiedemann syndrome. Clin. Genet. 90, 21-27. 10.1111/cge.12759 [DOI] [PubMed] [Google Scholar]
- Niemitz E. L., Debaun M. R., Fallon J., Murakami K., Kugoh H., Oshimura M. and Feinberg A. P. (2004). Microdeletion of LIT1 in familial Beckwith-Wiedemann syndrome. Am. J. Hum. Genet. 75, 844-849. 10.1086/425343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh R., Ho R., Mar L., Gertsenstein M., Paderova J., Hsien J., Squire J. A., Higgins M. J., Nagy A. and Lefebvre L. (2008). Epigenetic and phenotypic consequences of a truncation disrupting the imprinted domain on distal mouse chromosome 7. Mol. Cell. Biol. 28, 1092-1103. 10.1128/MCB.01019-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R. R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-Dinardo D. and Kanduri C. (2008). Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 32, 232-246. 10.1016/j.molcel.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Plasschaert R. N. and Bartolomei M. S. (2014). Genomic imprinting in development, growth, behavior and stem cells. Development (Camb.) 141, 1805-1813. 10.1242/dev.101428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawitt D., Enklaar T., Gartner-Rupprecht B., Spangenberg C., Oswald M., Lausch E., Schmidtke P., Reutzel D., Fees S., Lucito R. et al. (2005). Microdeletion of target sites for insulator protein CTCF in a chromosome 11p15 imprinting center in Beckwith-Wiedemann syndrome and Wilms’ tumor. Proc. Natl. Acad. Sci. USA 102, 4085-4090. 10.1073/pnas.0500037102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul M. P., Tandonnet O., Biteau N., Belet-De Putter C., Rebouissoux L., Moradkhani K., Vu P. Y., Saura R., Arveiler B., Lacombe D. et al. (2006). Mosaic maternal uniparental isodisomy for chromosome 7q21-qter. Clin. Genet. 70, 207-213. 10.1111/j.1399-0004.2006.00664.x [DOI] [PubMed] [Google Scholar]
- Rentsendorj A., Mohan S., Szab P. and Mann J. R. (2010). A genomic imprinting defect in mice traced to a single gene. Genetics 186, 917-927. 10.1534/genetics.110.118802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesewijk A. M., Hu L., Schulz U., Tariverdian G., Höglund P., Kere J., Ropers H.-H. and Kalscheuer V. M. (1997). Monoallelic expression of human PEG1/MEST is paralleled by parent- specific methylation in fetuses. Genomics 42, 236-244. 10.1006/geno.1997.4731 [DOI] [PubMed] [Google Scholar]
- Ripoche M. A., Kress C., Poirier F. and Dandolo L. (1997). Deletion of the H19 transcription unit reveals the existence of a putative imprinting control element. Genes Dev. 11, 1596-1604. 10.1101/gad.11.12.1596 [DOI] [PubMed] [Google Scholar]
- Salas M., John R., Saxena A., Barton S., Frank D., Fitzpatrick G., Higgins M. J. and Tycko B. (2004). Placental growth retardation due to loss of imprinting of Phlda2. Mech. Dev. 121, 1199-1210. 10.1016/j.mod.2004.05.017 [DOI] [PubMed] [Google Scholar]
- Sanchez-Delgado M., Riccio A., Eggermann T., Maher E. R., Lapunzina P., Mackay D. and Monk D. (2016). Causes and Consequences of Multi-Locus Imprinting Disturbances in Humans. Trends Genet. 32, 444-455. 10.1016/j.tig.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Schmidt J. V., Levorse J. M. and Tilghman S. M. (1999). Enhancer competition between H19 and Igf2 does not mediate their imprinting. Proc. Natl. Acad. Sci. USA 96, 9733-9738. 10.1073/pnas.96.17.9733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenherr C. J., Levorse J. M. and Tilghman S. M. (2003). CTCF maintains differential methylation at the Igf2/H19 locus. Nat. Genet. 33, 66-69. 10.1038/ng1057 [DOI] [PubMed] [Google Scholar]
- Schönherr N., Meyer E., Roos A., Schmidt A., Wollmann H. A. and Eggermann T. (2006). The centromeric 11p15 imprinting centre is also involved in Silver-Russell syndrome. J. Med. Genet. 44, 59-63. 10.1136/jmg.2006.044370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz B. M., Gallicio G. A., Cesaroni M., Lupey L. N. and Engel N. (2015). Enhancers compete with a long non-coding RNA for regulation of the Kcnq1 domain. Nucleic Acids Res. 43, 745-759. 10.1093/nar/gku1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. Y., Fitzpatrick G. V. and Higgins M. J. (2008). Two distinct mechanisms of silencing by the KvDMR1 imprinting control region. EMBO J. 27, 168-178. 10.1038/sj.emboj.7601960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Wu X., Lee D.-H., Li A. X., Rauch T. A., Pfeifer G. P., Mann J. R. and Szabo P. E. (2011). Chromosome-wide analysis of parental allele-specific chromatin and DNA methylation. Mol. Cell. Biol. 31, 1757-1770. 10.1128/MCB.00961-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. B., Sribenja S., Wilson K. E., Attwood K. M., Hillman J. C., Pathak S. and Higgins M. J. (2017). Blocked transcription through KvDMR1 results in absence of methylation and gene silencing resembling Beckwith-Wiedemann syndrome. Development (Camb.) 144, 1820-1830. 10.1242/dev.145136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparago A., Cerrato F., Vernucci M., Ferrero G. B., Silengo M. C. and Riccio A. (2004). Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat. Genet. 36, 958-960. 10.1038/ng1410 [DOI] [PubMed] [Google Scholar]
- Sparago A., Cerrato F. and Riccio A. (2018). Is ZFP57 binding to H19/IGF2: IG-DMR affected in Silver-Russell syndrome? Clin. Epigenetics 10 10.1186/s13148-018-0454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler S., Gogiel M., Schönherr N., Binder G. and Eggermann T. (2009). Screening for genomic variants in ZFP57 in Silver-Russell syndrome patients with 11p15 epimutations. Eur. J. Med. Genet. 52, 415-416. 10.1016/j.ejmg.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Srivastava M., Hsieh S., Grinberg A., Williams-Simons L., Huang S. P. and Pfeifer K. (2000). H19 and Igf2 monoallelic expression is regulated in two distinct ways by a shared cis acting regulatory region upstream of H19. Genes Dev. 14, 1186-1195. [PMC free article] [PubMed] [Google Scholar]
- Strogantsev R., Krueger F., Yamazawa K., Shi H., Gould P., Goldman-Roberts M., Mcewen K., Sun B., Pedersen R. and Ferguson-Smith A. C. (2015). Allele-specific binding of ZFP57 in the epigenetic regulation of imprinted and non-imprinted monoallelic expression. Genome Biol. 16 10.1186/s13059-015-0672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F. L., Dean W. L., Kelsey G., Allen N. D. and Reik W. (1997). Transactivation of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature 389, 809-815. 10.1038/39797 [DOI] [PubMed] [Google Scholar]
- Surani M. A. H., Barton S. C. and Norris M. L. (1984). Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308, 548-550. 10.1038/308548a0 [DOI] [PubMed] [Google Scholar]
- Szabo P. E., Tang S.-H. E., Silva F. J., Tsark W. M. K. and Mann J. R. (2004). Role of CTCF binding sites in the Igf2/H19 imprinting control region. Mol. Cell. Biol. 24, 4791-4800. 10.1128/MCB.24.11.4791-4800.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Coluccio A., Thorball C. W., Planet E., Shi H., Offner S., Turelli P., Imbeault M., Ferguson-Smith A. C. and Trono D. (2019). ZNF445 is a primary regulator of genomic imprinting. Genes Dev. 33, 49-54. 10.1101/gad.320069.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsen J. L., Duran K. L. and Bartolomei M. S. (1998). Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 12, 3693-3702. 10.1101/gad.12.23.3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsen J. L., Mann M. R. W., Nwoko O., Duran K. L. and Bartolomei M. S. (2002). Analysis of sequence upstream of the endogenous H19 gene reveals elements both essential and dispensable for imprinting. Mol. Cell. Biol. 22, 2450-2462. 10.1128/MCB.22.8.2450-2462.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsen J. L., Fedoriw A. M., Nguyen S. and Bartolomei M. S. (2006). Developmental profile of H19 differentially methylated domain (DMD) deletion alleles reveals multiple roles of the DMD in regulating allelic expression and DNA methylation at the imprinted H19/Igf2 locus. Mol. Cell. Biol. 26, 1245-1258. 10.1128/MCB.26.4.1245-1258.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z. B., Gold L., Pfeifer K. E., Dorward H., Lee E., Bondy C. A., Dean J. and Nelson L. M. (2000). Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 26, 267-268. 10.1038/81547 [DOI] [PubMed] [Google Scholar]
- Tunster S. J., Tycko B. and John R. M. (2010). The imprinted Phlda2 gene regulates extraembryonic energy stores. Mol. Cell. Biol. 30, 295-306. 10.1128/MCB.00662-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunster S. J., Van De Pette M. and John R. M. (2011). Fetal overgrowth in the Cdkn1c mouse model of Beckwith-Wiedemann syndrome. DMM Dis. Model. Mech. 4, 814-821. 10.1242/dmm.007328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunster S. J., Van De Pette M. and John R. M. (2014). Isolating the role of elevated Phlda2 in asymmetric late fetal growth restriction in mice. DMM Dis. Model. Mech. 7, 1185-1191. 10.1242/dmm.017079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunster S. J., Van de Pette M., Creeth H. D. J., Lefebvre L., John R. M. (2018). Fetal growth restriction in a genetic model of sporadic Beckwith-Wiedemann syndrome. DMM Dis. Model. Mech. 11, dmm035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente F. M., Sparago A., Freschi A., Hill-Harfe K., Maas S. M., Frints S. G. M., Alders M., Pignata L., Franzese M., Angelini C. et al. (2019). Transcription alterations of KCNQ1 associated with imprinted methylation defects in the Beckwith–Wiedemann locus. Genet. Med. 21, 1808-1820. 10.1038/s41436-018-0416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Pette M., Tunster S. J., Mcnamara G. I., Shelkovnikova T., Millership S., Benson L., Peirson S., Christian M., Vidal-Puig A. and John R. M. (2016). Cdkn1c boosts the development of brown adipose tissue in a murine model of silver russell syndrome. PLoS Genet. 12, e1005916 10.1371/journal.pgen.1005916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora N. and Bianchi D. W. (2009). Genetic considerations in the prenatal diagnosis of overgrowth syndromes. Prenat. Diagn. 29, 923-929. 10.1002/pd.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrooman L. A. and Bartolomei M. S. (2017). Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 68, 72-84. 10.1016/j.reprotox.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling E. L., Brioude F., Lokulo-Sodipe O., O'connell S. M., Salem J., Bliek J., Canton A. P. M., Chrzanowska K. H., Davies J. H., Dias R. P. et al. (2017). Diagnosis and management of Silver-Russell syndrome: first international consensus statement. Nat. Rev. Endocrinol. 13, 105-124. 10.1038/nrendo.2016.138 [DOI] [PubMed] [Google Scholar]
- Wang Q., Curran M. E., Splawski I., Burn T. C., Millholland J. M., Vanraay T. J., Shen J., Timothy K. W., Vincent G. M., De Jager T. et al. (1996). Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat. Genet. 12, 17-23. 10.1038/ng0196-17 [DOI] [PubMed] [Google Scholar]
- Wang H., Maurano M. T., Qu H., Varley K. E., Gertz J., Pauli F., Lee K., Canfield T., Weaver M., Sandstrom R. et al. (2012). Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Res. 22, 1680-1688. 10.1101/gr.136101.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R., Shuman C. and Beckwith J. B. (2010). Beckwith-Wiedemann syndrome. Eur. J. Hum. Genet. 18, 8-14. 10.1038/ejhg.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseler K., Kraft F. and Eggermann T. (2019). Molecular and clinical opposite findings in 11p15.5 associated imprinting disorders: Characterization of basic mechanisms to improve clinical management. Int. J. Mol. Sci. 20, 4219 10.3390/ijms20174219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman B. A., Poutsma A., Looijenga L. H. J., Wouters D., Van Wijk I. J. and Oudejans C. B. M. (2001). The human achaete scute homolog 2 gene contains two promotors, generating overlapping transcripts and encoding two proteins with different nuclear localization. Placenta 22, 511-518. 10.1053/plac.2001.0695 [DOI] [PubMed] [Google Scholar]
- Yan Y., Frisen J., Lee M. H., Massague J. and Barbacid M. (1997). Ablation of the CDK inhibitor p57(Kip2) results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 11, 973-983. 10.1101/gad.11.8.973 [DOI] [PubMed] [Google Scholar]
- Yoon Y. S., Jeong S., Rong Q., Park K.-Y., Chung J. H. and Pfeifer K. (2007). Analysis of the H19ICR Insulator. Mol. Cell. Biol. 27, 3499-3510. 10.1128/MCB.02170-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T., Miroglio A., Ripoche M.-A., Gabory A., Vernucci M., Riccio A., Colnot S., Godard C., Terris B., Jammes H. et al. (2008). The H19 locus acts in vivo as a tumor suppressor. Proc. Natl. Acad. Sci. USA 105, 12417-12422. 10.1073/pnas.0801540105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Huang L., Hu X., Li Q., Sun X., Xie Y., Kong S. and Wang X. (2016). FGFR3 gene mutation plus GRB10 gene duplication in a patient with achondroplasia plus growth delay with prenatal onset. Orphanet J. Rare Dis. 11, 89 10.1186/s13023-016-0465-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Liégeois N. J., Wong C., Finegold M., Hou H., Thompson J. C., Silverman A., Harper J. W., Depinho R. A. and Elledge S. J. (1997). Altered cell differentiation and proliferation in mice lacking p57(KIP2) indicates a role in Beckwith-Wiedemann syndrome. Nature 387, 151-158. 10.1038/387151a0 [DOI] [PubMed] [Google Scholar]
- Zhou H., Wang B., Yang Y. X., Jia Q. J., Zhang A., Qi Z. W. and Zhang J. P. (2019). Long noncoding RNAs in pathological cardiac remodeling: a review of the update literature. BioMed Res. Int. 2019, 1-11. 10.1155/2019/7159592 [DOI] [PMC free article] [PubMed] [Google Scholar]