Abstract
Endothelial-to-mesenchymal transition (EndMT) involves the phenotypic conversion of endothelial-to-mesenchymal cells, and was first discovered in association with embryonic heart development. EndMT can regulate various processes, such as tissue fibrosis and cancer. Recent findings have shown that EndMT is related to resistance to cancer therapy, such as chemotherapy, antiangiogenic therapy, and radiation therapy. Based on the known effects of EndMT on the cardiac toxicity of anticancer therapy and tissue damage of radiation therapy, we propose that EndMT can be targeted as a strategy for overcoming tumor resistance while reducing complications, such as tissue damage. In this review, we discuss EndMT and its roles in damaging cardiac and lung tissues, as well as EndMT-related effects on tumor vasculature and resistance in anticancer therapy. Modulating EndMT in radioresistant tumors and radiation-induced tissue fibrosis can especially increase the efficacy of radiation therapy. In addition, we review the role of hypoxia and reactive oxygen species as the main stimulating factors of tissue damage due to vascular damage and EndMT. We consider drugs that may be clinically useful for regulating EndMT in various diseases. Finally, we argue the importance of EndMT as a therapeutic target in anticancer therapy for reducing tissue damage.
Subject terms: Cancer microenvironment, Cancer microenvironment
Cell biology: effect of changes in cell type on disease
A process of cellular conversion known as endothelial-to-mesenchymal transition (EndMT) may offer a valuable target for treating cancer and other diseases. In EndMT, the cells lining blood vessels undergo a striking change in shape and physiology, acquiring features of cells called fibroblasts. Fibroblasts form the body’s connective tissue, but also produce scar tissue that impairs organ function. Researchers led by Yoon-Jin Lee of the Korea Institute of Radiological & Medical Sciences in Seoul, South Korea, have reviewed the impact of this transformation on human disease. EndMT is seen as a prelude to heart failure, in lung tissue affected by pulmonary fibrosis, and within tumors, where the process recruits cells that further stimulate cancer progression. The authors highlight the potential of using drugs that target EndMT to bolster the efficacy and safety of tumor therapy.
Introduction
Endothelial-to-mesenchymal transition (EndMT) was initially observed by electron microscopy in 1975 during a detailed analysis of endocardium differentiation during the formation of heart valves in vertebrate embryogenesis1. This process has also been observed in pathological contexts, such as vascular calcification2, atherosclerosis3, and cardiac and pulmonary fibrosis4. Endothelial cells (ECs) display high plasticity, particularly under pathological conditions and in EndMT. During EndMT, endothelial cells lose their characteristic markers, such as CD31, VE cadherin, Tie2, and vWF, while gaining increased expression of mesenchymal markers, such as fibroblast-specific protein-1 (FSP1), alpha 2 smooth muscle actin (α-SMA), and type I/III collagen. Transforming growth factor (TGF)-β1 may induce a phenotypic transformation of proliferating endothelial cells into fibroblast-like cells. TGF-β1, TGF-β2, and bone morphogenetic proteins are also well-known to cause EndMT5,6. Several groups have reported on signaling systems and molecules that induce EndMT, including Wnt/β-catenin7 and Notch signaling8, as well as hypoxia9 and oxidative stress10. These signaling pathways are related to transcription factors involved in mesenchymal transition, such as Snail, Slug, ZEB1, and ZEB211,12. EndMT exhibits phenotypic changes similar to the epithelial–mesenchymal transition (EMT), which is considered the main process driving phenotypic changes of cells into mesenchymal cells. TGF-β also plays important roles in the EMT process in the tumor microenvironment during tumorigenesis and metastases, and in fibroblastic changes of epithelial cells that occur during the development of fibrotic diseases. EndMT has been further studied in atherosclerosis and tumor angiogenesis13,14. Recently, increasing evidence has suggested that EndMT-related processes are important contributors to the microenvironmental plasticity of cancerous tumors4. EndMT leads to the production of cancer-associated fibroblasts (CAFs)15, which affect tumor growth and metastases. ECs can differentiate via EndMT into adipocytes and mural cells, such as pericytes and smooth muscle cells16. It has also been reported that endothelial progenitor cells differentiate into smooth muscle-like progenitor cells via TGF-β1-driven EndMT17. Moreover, tumor EndMT induces the formation of both a CAF-associated tumor microenvironment and aberrant tumor vessels18,19. Here, we review the role of EndMT in tissue fibrosis and cancer. We focus on EndMT in lung and cardiac tissue, which are often injured during anticancer therapy. In addition, we discuss the therapeutic advantages of targeting EndMT in anticancer therapy.
EndMT effects on tissue damage
EndMT in cardiac tissue damage
Cardiac fibrosis is a common feature in patients with advanced heart failure. Anti-fibrotic therapy may be useful for improving the function of a diseased heart, but the development of therapies has been limited by the incomplete understanding of the origin of fibroblasts in the heart. Cardiac fibrosis occurs because of excessive deposition of the extracellular matrix, which is mediated by the reduction of microcirculation and destruction of the normal myocardial structure. Cardiac fibrosis is associated with the appearance of EndMT20. TGF-β1 caused EndMT in Tie1Cre;R26RstoplacZ mice (in which the endothelial origin is marked by lacZ expression) and FSP1-GFP mice (expressing green flourescent protein (GFP) under the promoter activity of FSP1), whereas bone morphogenetic protein-7 preserved the endothelial phenotype and inhibited cardiac fibrosis in mice with pressure overload and chronic allograft rejection (Table 1)20. EndMT also occurs during the neohypertensive reaction following grafting of veins into the arterial circulation in both mice and humans. An intervening vein graft was evaluated in different mouse models, including a constitutive EndotrackYFP model and an EndotrackLacZ and tamoxifen-induced EndotrackYFP transgenic mouse model. Staining for endothelial-specific markers revealed that in neointimal (but not luminal) YFP+ cells, the expression of endothelial-specific markers was lost after grafting. In addition, the expression of the vascular smooth muscle cell markers, SMA and smooth muscle protein 22-alpha (SM22a), progressively increased in YFP+ cells (Table 1). Previous data revealed TGF-β–Smad2/3–Slug signaling as a principal pathway regulating EndMT during vein graft remodeling21. A study using ET-1f/f;Tie2-Cre transgenic mice with specific ET-1 inhibition showed that diabetes mellitus-induced cardiac fibrosis was associated with the emergence of fibroblasts, and that ET-1 further promoted cardiac fibrosis and heart failure through the accumulation of fibroblasts via EndMT (Table 1)22. It has been reported that hypoxic conditions can induce EndMT in cardiac damage. Hypoxia induced EndMT in human coronary endothelial cells, an effect mediated by hypoxia-inducible factor (HIF)-1α-induced activation of SNAIL, an EndMT master-regulatory transcriptional factor23.
Table 1.
The effects of miRNAs on EndMT.
| miRNA | Target | Function | Research model | Refs. |
|---|---|---|---|---|
| miR-200b | TGF-β-dependent Smad2/Snail1 | Inhibit EndMT | Retina in diabetic animals | 108 |
| miR-18a-5p | Notch2 | Attenuate EndMT | Diabetic cardiomyopathy | 110 |
| miR-126a-5p | TGF-β signaling | Increase EndMT | Neonatal pulmonary hypertension | 49 |
| miR-302c | Metadherin | Inhibit EndMT | Hepatocellular carcinoma | 53 |
| miR-199a-5p | Snail/miR-199a-5p axis | Promote EndMT via the Snail/miR-199a-5p axis | Irradiated human umbilical vein endothelial cells | 95 |
EndMT in lung injury
Recently, EndMT was reported to play a role in pulmonary fibrosis. Chronic hypoxia is an important contributing factor to pulmonary hypertension. EndMT was observed in the pulmonary arteries of rat models of pulmonary hypertension. In pulmonary microvascular ECs, hypoxia-induced HIF‑1α activity modulates the transdifferentiation of ECs via Twist24. It has been reported that vildagliptin (a dipeptidyl peptidase 4 [DPP-4] inhibitor that serves as an antidiabetic drug) improves vascular dysfunction. In a model of lipopolysaccharide-induced septic lung injury, vildagliptin reduced pulmonary fibrosis by attenuating EndMT (Table 1)25. Recent data demonstrated that the mesenchymal transition of pulmonary capillary ECs served as the origin of fibroblasts found during bleomycin-induced pulmonary fibrosis using the Tie2-Cre/CAG-CAT-LacZ double-transgenic mouse system, which enabled tracing of LacZ expression in Tie2-positive ECs26. Cotreatment with TGF-β and activated Ras caused a persistent EndMT phenotype, inducing α-SMA expression, which was not induced by TGF-β or activated Ras alone. The data showed that, via TGF-β signaling, Ras/MAPK regulated the completion of EndMT in bleomycin-induced pulmonary fibrosis27. Nintedanib is a tyrosine kinase inhibitor that targets platelet-derived growth factor (PDGF) and the fibroblast growth factor receptor, and reduces vascular remodeling-related neointimal lesions and the medial wall thickness of pulmonary arteries. Moreover, Twist expression and related EndMT were reduced by treatment with nintedanib28.
Role of oxidative stress and hypoxia in EndMT
Reactive oxygen species (ROS) have been reported to induce EndMT during oxidative stress10,29. Oxidative stress promotes TGF-β1 secretion, a key inducer of EndMT30. Oxidants can directly activate latent TGF-β31. In addition, many oxidants can activate the nuclear factor-kappa B (NF-κB) pathway32, and subsequently induce the production of inflammatory cytokines, which directly stimulate EndMT33,34.
Recent reports have shown that EndMT contributes to fibroblast accumulation during heart and kidney fibrosis and cancer6,13,35. Although ROS, TGF-β, inflammatory cytokines, and hypoxia are known to be involved in the development of chronic fibrosis in the lungs, the precise mechanism underlying the progression of initial radiation injuries to chronic fibrosis remains unclear36,37. It has also been demonstrated that radiation-induced hypoxia can trigger EndMT through HIF-1α-mediated activation of TGF-β1/Smad signaling in human pulmonary artery ECs. Moreover, regulating EndMT has been suggested as an effective strategy for stopping radiation-induced pulmonary fibrosis at an early stage38. Cells derived from EndMT were previously found during atherosclerotic development in intimal plaques, and this process was guided by TGF-β signals, oxidative stress, and hypoxia in vitro and in vivo in mice39.
Salvianolic acid A (which has been widely used for the clinical therapy of various diseases involving vascular disturbance) upregulates CD31 and downregulates α-SMA, and can attenuate EndMT, suppresses oxidative stress, and alleviates pulmonary vascular remodeling. The Nrf2/HO-1 signaling pathway may be involved in these processes40.
The inhibition of ROS production in ECs reduces oxidative stress-induced EndMT. Brain and muscle ARNT-like protein-1 (BMAL1), an essential clock transcription activator, can prevent the accumulation of intracellular ROS caused by lipoproteins, and subsequent EndMT in human endothelial aortic cells41–43 in vitro. In addition, BMAL1 deficiency aggravated intracellular ROS accumulation and EndMT progression via bone morphogenetic protein-mediated signaling. These results provide a mechanism to explain the central role of BMAL1 in atherosclerosis progression, and the phenotype switch of plaque cells43. Low-intensity pulsed ultrasound protects endothelial human aortic cells from oxidative stress-induced EndMT, which has been associated with PI3K/AKT signaling44. As previously reported, EndMT occurs when oxidative stress and NF-κB activity are increased in ECs by TGF-β1 and TGF-β245,46. Taken together, these findings suggest that inflammation, hypoxia, and endothelial oxidative stress exacerbate EndMT by inducing canonical TGF-β signaling30. NADPH oxidases and ROS play key roles in mediating fibrotic responses induced by TGF-β via Smad2/Smad3 activation30. Oxidative stress can induce EndMT conversion via TGF-β1- and TGF-β2-dependent pathways and the intracellular ALK5/Smad3/NF-κB pathway30,47.
Recent findings have also suggested that EndMT can be regulated using nanoparticles or natural compounds. Polyglucose sorbitol carboxymethyether-modified Fe2O3 (PSC-Fe2O3) iron oxide nanoparticles decreased the expression of the endothelial markers CD31 and VE cadherin at an acutely noncytotoxic concentration, and increased the expression of the mesenchymal marker α-SMA. FSP, via its peroxidase-like activity, enhanced EC migration and inhibited angiogenic function, which clearly indicated the occurrence of EndMT in ECs30. EndMT leads to vascular damage during angiotensin-II-treated chronic inflammation, which can be prevented by treatment with schisandrin B. Schisandrin B is a natural product derived from Schisandra chinensis that can inhibit the activation of NF-κB. Schisandrin B was also found to suppress inflammation/ROS-mediated EndMT by inhibiting NF-κB48. Hypoxia is the main factor promoting the occurrence of EndMT. The relationship between hypoxia and TGF-β signaling is regulated by the expression of microRNAs (miRNAs). miR-126a-5p, which inhibits TGF-β signaling, was upregulated in hypoxia-induced persistent pulmonary hypertension of newborns as a cardiac syndrome (Table 2)49. Chronic hypoxia increased oxygen consumption and activated fibroblasts in cardiac fibrosis, resulting in aberrant ventricular remodeling50. Under hypoxic conditions, the EndMT of human cardiac microvascular ECs promoted tube formation. Autophagy provides protective effects against the EndMT of human cardiac microvascular ECs by degrading Snail under hypoxic conditions51. In addition, it has been suggested that hypoxia induces EndMT in human coronary ECs via Hif1a-activated Snail, indicating that endocardium-derived ECs undergo EndMT23.
Table 2.
Genetically engineered mouse models (GEMMs) used to study EndMT.
| GEMM(s) | Genetic change(s) | Biological effects | Research animal model | Refs. |
|---|---|---|---|---|
| Tie1Cre;R26RstoplacZ and FSP1-GFP mice | EC-specific LacZ expression and fibroblast-specific GFP expression | The phenotypic change in ECs was traced in cardiac fibrosis | Cardiac fibrosis | 20 |
| Tamoxifen-induced EndotrackYFP | EC-specific YFP expression | YFP+ cells increased SMA and SM22a expression after vein grafting | Vein-grafting models | 21 |
| ET-1f/f;Tie2-Cre | EC-specific ET-1 deletion | ET-1 deletion enhanced fibroblast accumulation | Diabetes mellitus-induced cardiac fibrosis | 22 |
| Tie2-cre;R26Rosa-lox-Stop-lox-LacZ | EC-specific LacZ expression | Fibroblastic tumor cells were derived from EndMT | B16F10 tumor | 52 |
| Tie2-Cre Metfl/fl | EC-specific Met deletion | Met inhibited GBM-associated fibroblast-like cells with an EC origin | GBM | 19 |
| RIP1-Tag2;Eng+/− | Endoglin-deficient tumor | Endoglin-deficient tumors exhibited the hallmark of EndMT | Pancreatic neuroendocrine tumor | 77 |
| Tie2-p53fl/fl | EC-specific p53 deletion | p53 deletion inhibited EndMT in the tumor vasculature | Radiation therapy for lung cancer | 18 |
| Tie2-TGFbR2fl/fl | EC-specific TGFbR2 deletion | TGFbR2 deletion increased radiation-induced tumor EndMT | Radiation therapy for lung cancer | 18 |
| Hey2flx/flx/Ve-CadCre−/− | EC-specific Hey2 deletion | Hey2 deletion decreased the EndMT frequency | Acute radiation proctitis | 94 |
Effects of EndMT on cancer
EndMT is a specific mechanism resulting in CAF accumulation; antiangiogenic tumor therapy may directly decrease the abundance of activated fibroblasts that are likely to promote cancer progression52.
Tumor EndMT and CAFs
ECs contribute to the abundance of CAFs through EndMT in the tumor microenvironment52. Using Tie2-cre;R26Rosa-lox-Stop-lox-LacZ transgenic mice, it was discovered that approximately 30% of fibroblastic cells (FSP+ cells) and 12% of α-SMA+ cells in the B16F10 tumor stroma were derived from EndMT (Table 1)52. Cancer cells can induce EndMT via TGF-β through several mechanisms. In hepatocellular carcinoma, miR-302c inhibits tumor growth through metadherin, a factor that contributes to cell motility (Table 2)4,53. The levels of miR-302c expressed by ECs isolated from tumor tissues were significantly lower than the corresponding levels in normal liver tissues53. The levels of miR-302c in ECs correlated negatively with the proliferation rate of the hepatocellular carcinoma cell line HCCLM353.
Tumor-induced EndMT is mediated by factors secreted from tumor cells, such as TGF-β2 and interleukin (IL)-1β. Tumor-driven EndMT is accompanied by the activation of proinflammatory pathways in ECs54. The expression of cyclooxygenase-2, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 is increased, and NF-κB is activated in EndMT-transformed ECs3. ECs showed phenotypic changes consistent with EndMT when cocultured with OE33 esophageal adenocarcinoma cells expressing high levels of IL-1β and TGF-β2. CAFs, which were likely a result of EndMT, were found at the invasive front of esophageal adenocarcinoma, indicating the significance of EndMT in tumor progression54. Notably, a remarkably large number of these EndMT-derived CAFs were located close to the invasive tumor front3. ECs undergoing tumor-induced EndMT express higher levels of the vascular endothelial growth factor (VEGF) gene, whereas VEGF receptor 2 (VEGFR2) was downregulated in ECs3. EndMT-transformed esophageal ECs may be an important source of VEGF in the tumor microenvironment, and function more in a paracrine than in an autocrine manner54.
Loss of Tie-1, an EC-specific receptor essential for the vascular system, induces EndMT in human ECs and pancreatic tumors. Downregulation of Tie-1 triggers EndMT by activating the Slug promoter55. EndMT plays an important role in cancer progression and metastasis. ECs that undergo EndMT are more invasive, as they lose expression of their endothelial markers (CD31, von Willebrand factor VIII, and VE cadherin) and acquire a mesenchymal phenotype and an increased migration ability. The tumor promotes a mesenchymal shift in ECs that is regulated by Smad signaling through the synergistic stimulation of TGF-β and Notch pathways in breast cancer cells. Tumor cells increase the mesenchymal phenotypes of ECs, but maintain their endothelial phenotypes. It was shown that tumor-stimulated processes that increase extracellular matrix formation are also regulated by activation of the Notch pathway via phosphorylation of TGF-β/Smad1/556–60. HSPB1 has been described as a key regulator of EndMT in lung cancer. Endothelial HSPB1 deficiency in the mesenchymal transition of vascular ECs contributes to lung fibrosis and tumorigenesis61.
Osteopontin is a multifunctional phospho-glycoprotein that stimulates angiogenesis in ECs. In colorectal cancer, the presence of osteopontin–integrin αVβ3 induces HIF-1α expression via a PI3K/Akt/tuberous sclerosis complex 2-mediated and mTORC1-dependent protein synthesis pathway, which transactivates TCF12 gene expression. These findings indicate that HIF-1α promotes EndMT by inducing TCF1262. EndMT reversal contributes to the control of chemoresistance, irrespective of the level of soluble TGF-β that is present. In a xenograft mouse model of multicellular tumor spheroids containing lung cancer cells and human umbilical vein endothelial cells (HUVECs), GSK-3β inhibition reduced the lung cancer volume by inhibiting EndMT, and had a synergistic anticancer effect on non-small-cell lung cancer cells in combination with gefitinib63.
The PLEK2–SHIP2 axis promotes both lung cancer cell migration (via TGF-β/PI3K/AKT signaling) and vascular invasion. This suggests that PLEK2 can serve as a valuable prognostic marker and a promising target in non-small-cell lung cancer therapy64. Researching functional genes and regulatory mechanisms associated with EndMT is important for revealing the mechanisms driving lung cancer metastasis and improving targeted diagnoses or treatments.
EndMT and the tumor vasculature
EndMT also plays a major role in the tumor vasculature. ECs undergoing tumor-mediated EndMT show functional alterations, such as greater migratory capacity, higher proliferation rates, and a gain of contractile capacity, whereas the cells lose their angiogenic ability to form capillary-like tubes54. In glioblastoma, c-Met-mediated EC plasticity induces mesenchymal transformation to promote EC proliferation and migration, resulting in aberrant vasculature formation and chemoresistance to multiple therapies (Table 1)19. EndMT contributes to metastatic extravasation and intravasation. Primary tumor vasculature showing EndMT exhibits the loss of endothelial cell–cell junctions, which causes the transendothelial migration of metastatic cells65. For example, Rock was shown to reduce microvascular–endothelial hyperpermeability via the Rho/ROCK/MLC pathway of actin stress-fiber formation66. The Rho/RACK pathway rescued tight-junction integrity in brain ECs67. ROCK inhibition prevented tumor endothelial migration68. Downregulated ERG and FL1 expression caused EndMT in intratumoral ECs in B16F10 melanoma tumors, indicating the aberrant behavior of ECs in pathological environments69.
EndMT in anticancer therapy
EndMT and CAF chemoresistance
After chemotherapy, the activated stromal compartment contributes to the survival of residual cancer cells and tumor resistance70. CAFs promote tumor-supportive functions through cytokine and metabolite release. The CD10- and GPR77-positive CAF subset exhibits chemoresistance and poor survival in patients with breast or lung cancer71. CAFs may originate from ECs via EndMT, bone marrow-derived cells, adipocytes, and stellate cells72. Various reports have shown that CAFs mediate tumor progression and resistance, as well as relapse after chemotherapy14.
EndMT and anti-VEGF therapy
Aberrant vascularization is a characteristic of resistance to chemotherapy. Human glioblastoma multiforme (GBM) tissues show an aberrant vasculature altered by EndMT. In GBM, chemoresistance and cancer progression are related to c-Met-mediated EndMT. EC-specific Met deletion resulted in EndMT, inhibited aberrant vascularization and tumor growth, and prolonged the survival of GBM-bearing mice after temozolomide treatment19. In GBM mouse models, PDGF-mediated EndMT decreased VEGFR2 expression via the PDGF/NF-κB/Snail axis in ECs, and thus induced resistance to anti-VEGF/VEGFR therapy. EC-specific PDGFR-B deletion sensitized tumors to anti-VEGF therapy73. SMA-positive perivascular cells were evaluated for their resistance to antiangiogenic therapy in vivo in malignant melanoma74 and pancreatic neuroendocrine tumor mouse models using RIP1-Tag2 mice75. Aberrant α-SMA+ perivascular cells were related to increased metastasis in RIP1-Tag2 mice deficient in neural cell adhesion molecule76. Endoglin-deficient tumors showed increased α-SMA- and NG2-positive cells in the blood vessels, and endoglin-deficient tumor ECs in RIP1-Tag2 mice caused EndMT, which induced Twist expression. Endoglin-deficient tumors exhibited more hepatic metastases than wild-type tumors, but maintained their sensitivity to anti-VEGF therapy, suggesting that the synergistic interaction of endoglin deficiency and VEGF inhibition can be used as a combined therapeutic strategy (Table 1)77. EC-specific miR-302c inhibited metadherin expression, reduced EndMT, and suppressed tumor growth in hepatocellular carcinoma. Metadherin and Mir-302c may be useful as antiangiogenic therapies in hepatocellular carcinoma (Table 2)53.
Cardiotoxicity of anticancer drugs
Chemotherapeutic agents, including anthracycline and doxorubicin (Dox), and targeted therapies in anticancer therapy are well known to cause cardiac toxicity. Recently, data from several studies demonstrated that EndMT occurs during the development of cardiac toxicity due to anticancer chemotherapies. A recent study showed that Dox and Herceptin increased drug permeability by affecting tight-junction formation by human cardiac microvascular–endothelial cells, showing decreased expression of ZO-1 and tight-junction protein-1, which resulted in cardiotoxicity23,78. Cardiotoxicity is also caused by monotherapy with Herceptin (trastuzumab), a humanized monoclonal antibody used to treat breast cancers.
Calcitriol, an active form of vitamin D3 that inhibits the growth of cancer cells, has been shown to attenuate Dox-induced myocardial fibrosis and fibrotic proteins, and improve diastolic function by reducing TGF-β–Smad2-mediated EndMT and fibroblast-to-myofibroblast transition. The hearts of mice with EC-specific GFP expression showed increased levels of vimentin+ GFP+ ECs (indicative of EndMT), whereas calcitriol treatment attenuated these effects79. Administration of arsenic trioxide induced cardiac fibrosis in rats. In human aortic ECs, the AKT/GSK-3β/cochlear pathway was activated in arsenic trioxide-mediated EndMT, and EndMT was inhibited by the AKT inhibitor LY29400280.
EndMT in radiation therapy
Approximately half of all patients with cancer are treated with radiation therapy. Despite recent technological advances, tumor radioresistance and normal tissue damage remain challenges to improving cancer-cure rates. Normal tissue damage in radiation therapy remains a dose-limiting factor81.
In previous studies, we reported the occurrence of EndMT in irradiated tumor and normal tissues after radiation therapy18,61,82,83. Radiation-induced EndMT causes fibrotic changes in both tumors and the normal tissue microenvironment, such as in the lungs and heart vessels, which contribute to tumor radioresistance and normal tissue damage, respectively. Therefore, strategies targeting EndMT may enhance the efficacy of radiotherapy to overcome normal tissue damage and tumor radioresistance.
Radiation-induced tumor EndMT
There is increasing interest in combining radiotherapy with antiangiogenic therapy or immunotherapy to further improve the effectiveness of radiotherapy (Fig. 1)83.
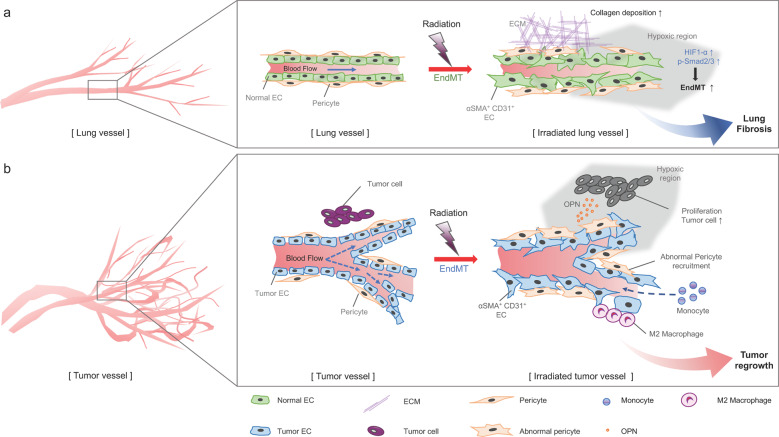
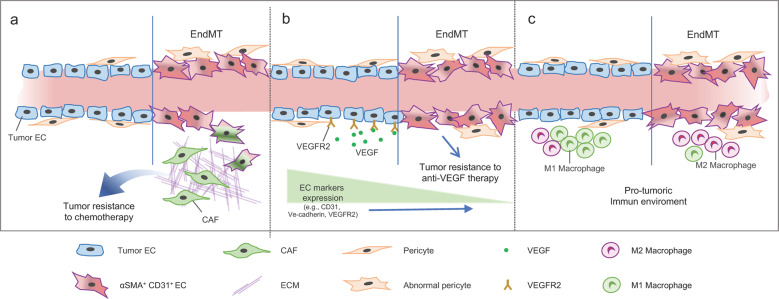
Fig. 1. Schematic illustration of the effects of tumor EndMT on the tumor microenvironment.
Mesenchymal transition of tumor ECs results in CAF formation and an abnormal tumor vasculature. a CAFs can affect tumor resistance to chemotherapy. b Fibrotic changes in ECs cause the loss of EC-specific characteristics with downregulated VEGF receptor expression, which can cause resistance to anti-VEGF therapy. c Immune cell infiltration into the tumor microenvironment after chemotherapy and radiation therapy can promote the generation of more protumor-immune cells via EndMT, resulting in a fibrotic vascular environment.
Combining radiation therapy with antiangiogenic therapy has been reported to be effective, and VEGFR2 blockade may normalize the abnormal tumor vasculature with increased pericyte and basement membrane coverage, resulting in tumor oxygenation and an increased response to radiation therapy84. Therefore, understanding the tumor endothelial transition in radiation therapy may lead to more efficient combined radiotherapies. Radiation-induced EndMT results in the formation of a tumor vasculature with irregular SMA+NG2+ pericyte recruitment in adenocarcinoma lung tumors with p53 deletion and KRAS mutation (Fig. 2). EndMT has also been observed in human non-small-cell lung cancer tissues following irradiation. EC-specific p53 deletion inhibits tumor EndMT and subsequent irregular SMA+NG2+ pericyte recruitment, resulting in decreased tumor regrowth after radiation therapy (Table 1). In addition, EC-specific TGFbR2 deletion in the tumor is associated with increased tumor regrowth after radiation therapy because of enhanced TGFβR1 signaling (Table 1). Tumor EndMT occurring after radiotherapy results in osteopontin secretion, which stimulates CD44V6+ cancer stem cell proliferation, and contributes to the M2 polarization of macrophages. Coculture of monocytic cells and irradiated tumor ECs facilitated the conversion of monocytic cells into M2 macrophages18. In a neuroblastoma xenograft model, high-dose radiation therapy (HDRT) combined with Notch inhibition decreased tumor EC numbers more than HDRT alone. Notch inhibition reduced HDRT-induced EndMT, as demonstrated by decreased SMA-stained ECs. HDR increased Notch1 signaling, which was not observed at doses less than 2 Gy. These results suggest that Notch1 activation can protect tumor vessels and control the EndMT process in HDRT85.
Fig. 2. Schematic illustration of radiation-induced EndMT during radiation therapy in patients with lung cancer.
a Radiation-induced lung vascular damage causes the mesenchymal transition of lung ECs, and thus, hypoxic regions formed by damaged vessels enhance the process of tissue fibrosis. Increased HIF-α expression on vascular ECs enhances EndMT via Smad2/3 signaling. b In lung cancer, radioresistant tumors can regrow after radiation therapy. ECs remaining following irradiation undergo EndMT, which increases the tumor burden, and leads to the recruitment of abnormal pericytes. Tumor EndMT promotes tumor regrowth via EndMT-related secreted molecules (such as OPN), and tumor EndMT promotes M2 macrophage polarization of monocytes recruited into the tumor microenvironment after radiation therapy.
Radiation-induced vascular fibrosis
During radiation therapy, radiation-induced tissue damage can cause morbidity, which is considered a treatment-limiting factor. In radiation-induced tissue damage, vascular fibrosis is well known as a prominent occurrence86. Radiation directly induces DNA damage, and ionizing radiation causes ROS production and the activation of inflammatory processes, which result in fibrosis by increasing collagen deposition and reducing vascularity87. Blood vascular damage-induced tissue hypoxia and ischemia contribute to severe tissue injury, such as fibrosis and necrosis, and vascular fibrosis can greatly worsen the prognosis of patients undergoing radiotherapy81,88.
Irradiated ECs acquire a proinflammatory, procoagulant, and prothrombic phenotype, and increase the proliferation and migration of vascular smooth muscle cells88.
Radiation-induced vascular endothelium damage can lead to the burst release of ROS, and change the balance of angiogenesis, lipid-metabolism pathways, and immune homeostasis89. These acute effects can cause long-term vascular dysfunction89.
Radiation-induced heart disease
Cardiovascular complications limit the use of thoracic radiation therapy for treating Hodgkin lymphoma, lung cancer, and breast cancer90. A meta-analysis revealed excess mortality due to cardiac disease, including cardiovascular damage, in women undergoing radiation therapy for left-sided breast cancer91. Atherosclerosis occurs as a delayed side effect of radiation-induced heart disease92.
Previously, we reported that radiation-induced EndMT occurs in human aortic ECs. Oxidized low-density lipoprotein increased radiation-induced EndMT, and irradiated ApoE–/– mice showed increased oxidized low-density lipoprotein levels and a more fibrotic phenotype of ECs82.
Radiation-induced intestinal damage
Gastrointestinal toxicity after radiotherapy is a major treatment-related complication. Radiation proctitis commonly occurs following inflammation of the rectal lining after treatment for cervical, prostate, and colon cancer. Recently, Mintet et al. showed that EndMT occurred during radiation proctitis development in Tie2-GFP mice, and that tissue inflammation caused the phenotypic conversion of endothelial-to-mesenchymal cells93. In conditional endothelial Hey2-deleted mice, EndMT occurrence and rectal tissue damage were reduced after irradiation. Microvascular protection reduced stem/clonogenic EC loss. These results suggest that EndMT can be targeted to mitigate radiation-induced intestinal damage (Table 1)94.
Radiation-induced pulmonary fibrosis (RIPF)
Thoracic radiotherapy can cause RIPF as a late side effect. In particular, RIPF is one of the most frequent complications after radiotherapy for lung cancer. RIPF is characterized by fibroblast proliferation and leukocyte recruitment, as well as excessive extracellular matrix deposition. Previously, we reported that the presence of EndMT significantly increased during the early phase of RIPF development. In that study, it was demonstrated that initial vascular hypoxic damage induced EndMT at various irradiation doses in different tissue volumes. Vascular EndMT prominently appeared prior to EndMT of alveolar epithelial II cells38.
In irradiated human pulmonary artery ECs, EndMT was dependent on HIF-1α expression via TGF-βR1/Smad signaling. Data generated in that study revealed HIF-1α-related EndMT in human RIPF tissues95. A recent study provided evidence that in a coculture system of human fetal lung fibroblasts (MRC-5) and irradiated HUVECs, Snail and vimentin expression was upregulated, and CD31 expression was downregulated. Radiation-induced EndMT enhances the differentiation of fibroblasts to myofibroblasts via the Snail/miR-199a-5p axis (Table 2)95.
Drugs with EndMT-inhibiting effects
Recently, several drugs in clinical testing have been reported to inhibit EndMT in various animal disease models (Table 1). These drugs inhibit EndMT by targeting various signaling molecules, such as DPP-425,96, Smad97, TGF-β97–99, AMPK100, and other proteins28,101–106.
Nintedanib, which has been approved for treating pulmonary fibrosis, showed anti-vascular remodeling effects in pulmonary hypertension28. Macitentan was approved for treating pulmonary fibrosis and inhibited TGF-β- and ET-1-mediated EndMT in ECs isolated from patients with systemic sclerosis98. Recent data demonstrated that diabetic complications were related to EndMT. High glucose levels can lead to EndMT, which is regulated by miRNAs (miR-200b and miR-18a-5p) (Table 2)107–110.
Vildagliptin, a drug used to treat diabetes, regulates DPP-4-dependent EndMT and showed anti-fibrotic effects in a mouse model of sepsis25. Liraglutide, linagliptin, and losartan, which have been approved for treating diabetes and its complications, inhibited EndMT in diabetic mice and diabetic complications in mouse models96,97,100. In addition, the GSK inhibitor, CHIR99021, was shown to inhibit radiation-induced EndMT in HUVECs101. Imatinib, a PDGF receptor antagonist, regulated EndMT in pulmonary artery remodeling of pulmonary artery hypertension in rats111. Table 3 shows various drugs that are used clinically to inhibit EndMT.
Table 3.
Drugs regulating EndMT in various animal disease models.
| Drug | Target(s)a | Mechanism(s)b | Biological effects in EndMTc | Research animal model/cellsd | Biological effect(s) (clinical phase)e | Clinical trial status (disease) | Refs. |
|---|---|---|---|---|---|---|---|
| Nintedanib | PDGFR, FGFR, and VEGFR | Inhibits kinase signaling pathways and EndMT | Anti-vascular remodeling effects | Pulmonary arterial hypertension | Inhibits kinases and cell proliferation | Phase 3 completed (fibrosis) | 28 |
| Vildagliptin | Dipeptidyl peptidase 4 (DPP-4) | Inhibits DPP-4 signaling and EndMT | Anti-fibrotic effect | Sepsis models | Blood glucose regulation | Phase 4 completed (diabetes) | 25 |
| Liraglutide | Glucagon-like peptide 1 (GLP-1) receptor | Inhibits high glucose-induced EndMT via the AMPK pathway | Inhibits neointima formation to increase reendothelialization | Endovascular injury in diabetic mice | Blood glucose regulation | Phase 2 completed (type-1 and -2 diabetes mellitus) | 100 |
| Cinacalcet | Extracellular calcium-sensing receptor | Inhibits the expression of extracellular matrix elements (type I collage and fibronectin) and EndMT | Ameliorates cardiac fibrosis | Uremic hearts | Acts as a calcium mimetic | Phase 4 completed (chronic kidney disease) | 103 |
| Linagliptin | DPP-4 | Suppresses DPP-4 and inhibits EndMT via microRNA29 induction | Ameliorates kidney fibrosis | Streptozotocin-induced diabetic mice | Blood glucose regulation | Phase 2 completed (type-2 diabetes mellitus) | 96 |
| Macitentan | Endothelin-1 receptor, and endothelin B receptor | Inhibits the TGF-β- and ET-1-mediated EndMT | Inhibits fibroblast accumulation | ECs isolated from patients with systemic sclerosis (in vitro) | Antagonist of endothelin receptors on blood vessels and smooth muscle | Phase 2 completed (pulmonary hypertension) | 98 |
| Rapamycin (sirolimus) | Inhibited cell migration and extracellular matrix degradation | Inhibits EndMT and MMP-2/9 secretion | Inhibits EC angiogenesis in vitro | EA.hy926 cell line, a permanent endothelial cell line derived from HUVECs (in vitro) | Immunosuppressive macrolide | Phase 4 completed (transplantation, kidney) | 104 |
| Rapamycin | Inhibits VEGF, TGF-β, and TNF-α levels | Decreases PD-induced angiogenesis and EndMT | Protective effects, i.e., preservation of the peritoneal membrane | In vivo mouse model of peritoneal dialysis | Immunosuppressive macrolide | Phase 4 completed (transplantation, kidney) | 105 |
| Spironolactone | Abrogates TGF-β-induced fibrosis in EndMT | Inhibits EndMT by blocking Notch signaling and TGF-β | - | HUVECs (in vitro) | Aldosterone receptor blocker | Phase 2 completed (congenital heart disease, endomyocardial fibrosis, and heart failure) | 99 |
| CHIR‐99021 | Reduces FSP1 and α-SMA expression | Inhibits radiation- induced EndMT | - | HUVECs (in vitro) | GSK-3 inhibitor | N/A | 101 |
| Losartan | Inhibits ERK phosphorylation | Inhibits EndMT | - | Hypertension, cardiac valve endothelial cells (in vitro) | Type-1 angiotensin-II receptor antagonist | Phase 2 completed (fibrosis, inflammatory reaction) | 102 |
| Losartan | TGF-β1/Smad2/3 pathway | Inhibits EndMT, oxidative stress damage, and the TGF-b1/Smad signaling pathway | Reduces high-fat diet-induced hyperglycemia | Diabetic nephropathy (DN)-induced renal fibrosis (in vivo) | Type-1 angiotensin-II receptor antagonist | Hypertension in the left ventricle, DN | 97 |
| Hydrocortisone | EPAC–RAP1 pathway | Inhibits various signaling pathways, including RAP1 activity | Enhances the barrier properties of human brain microvascular endothelial cells | Blood–brain barrier models (in vitro) | Blocks the immunosuppressive hormone cortisol | Phase 4 completed (cardiovascular insufficiency, leukemia) | 106 |
a–cTargets, mechanism, and biological effects of drugs that were found to inhibit EndMT in vivo or in vitro.
dIn vivo disease model used to study EndMT.
eBiological effects of drugs in clinical trials.
- Blank: not described.
N/A not applicable.
Concluding remarks
Taken together, the results of various studies have demonstrated that EndMT contributes to tissue fibrosis and cancer. EndMT can affect tumor progression via the mesenchymal transition of cells to CAFs, or effects on the abnormal tumor vasculature. Moreover, fibrotic changes and specific gene loss in tumor ECs may cause tumor resistance to and relapse after anti-VEGF therapy. Considering these tumor vasculature EndMT-related phenotypic changes, antiangiogenic therapies can be developed, particularly anti-VEGF therapy combined with chemo- and radiation therapy. The role of EndMT in regulating normal tissue damage, such as tissue fibrosis, should also be evaluated to reduce the side effects of anticancer therapy, such as cardiovascular damage, cardiotoxicity, and kidney and lung fibrosis. In particular, EndMT modulation may provide a new strategy for radiation therapy. Regulating EndMT in irradiated normal tissues around the tumor can reduce vascular damage and tissue fibrosis. Simultaneously, regulating tumor EndMT after radiation therapy may prevent tumor regrowth and tumor microenvironmental changes, such as those mediating the protumorigenic activities of immune cells in the fibrotic phase. Further studies are necessary to understand the exact mechanism behind tumor EndMT in human cancer.
Understanding EndMT may provide new therapeutic strategies against cancer, and reduce the related side effects, such as tissue damage.
Acknowledgements
This work was supported by grants from the National Research Foundation (NRF-2017M2A2A7A02019482, 2020R1A2B5B020027, and NRF-2017R1A2B2004156) and Korea Institute of Radiological & Medical Sciences (KIRAMS, 50531-2020) funded by the Ministry of Science and ICT (MSIT), Republic of Korea.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kyu Jin Choi, Jae-Kyung Nam
References
- 1.Cui X, et al. Venous endothelial marker COUP-TFII regulates the distinct pathologic potentials of adult arteries and veins. Sci. Rep. 2015;5:16193. doi: 10.1038/srep16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krenning G, Barauna VG, Krieger JE, Harmsen MC, Moonen JR. Endothelial plasticity: shifting phenotypes through force feedback. Stem Cells Int. 2016;2016:9762959. doi: 10.1155/2016/9762959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong L, et al. EndMT: a promising and controversial field. Eur. J. Cell Biol. 2018;97:493–500. doi: 10.1016/j.ejcb.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Platel V, Faure S, Corre I, Clere N. Endothelial-to-mesenchymal transition (EndoMT): roles in tumorigenesis, metastatic extravasation and therapy resistance. J. Oncol. 2019;2019:8361945. doi: 10.1155/2019/8361945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tinkelman DG. Theophylline-use and misuse in pediatric asthma. Hosp. Pract. 1988;23:179–184. doi: 10.1080/21548331.1988.11703423. [DOI] [PubMed] [Google Scholar]
- 6.van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res. 2012;347:177–186. doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong A, Mirzaei Z, Simmons CA. The roles of matrix stiffness and ss-catenin signaling in endothelial-to-mesenchymal transition of aortic valve endothelial. Cells Cardiovasc. Eng. Technol. 2018;9:158–167. doi: 10.1007/s13239-018-0363-0. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Dong F, Jeong J, Masuda T, Lobe CG. Constitutively active Notch1 signaling promotes endothelialmesenchymal transition in a conditional transgenic mouse model. Int J. Mol. Med. 2014;34:669–676. doi: 10.3892/ijmm.2014.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, et al. Bone morphogenetic protein-7 inhibits endothelial-mesenchymal transition in pulmonary artery endothelial cell under hypoxia. J. Cell Physiol. 2018;233:4077–4090. doi: 10.1002/jcp.26195. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, et al. Kallistatin inhibits TGF-beta-induced endothelial-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression. Exp. Cell Res. 2015;337:103–110. doi: 10.1016/j.yexcr.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song S, et al. Foxm1 is a critical driver of TGF-beta-induced EndMT in endothelial cells through Smad2/3 and binds to the Snail promoter. J. Cell Physiol. 2019;234:9052–9064. doi: 10.1002/jcp.27583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoud MM, et al. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci. Rep. 2017;7:3375. doi: 10.1038/s41598-017-03532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito A. EMT and EndMT: regulated in similar ways? J. Biochem. 2013;153:493–495. doi: 10.1093/jb/mvt032. [DOI] [PubMed] [Google Scholar]
- 14.Fiori ME, et al. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol. Cancer. 2019;18:70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Nakayama H, Klagsbrun M, Mulliken JB, Bischoff J. Glucose transporter 1-positive endothelial cells in infantile hemangioma exhibit features of facultative stem cells. Stem Cells. 2015;33:133–145. doi: 10.1002/stem.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moonen JR, et al. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res. 2010;86:506–515. doi: 10.1093/cvr/cvq012. [DOI] [PubMed] [Google Scholar]
- 18.Choi SH, et al. Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nat. Commun. 2018;9:5108. doi: 10.1038/s41467-018-07470-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang M, et al. c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J. Clin. Invest. 2016;126:1801–1814. doi: 10.1172/JCI84876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 21.Cooley BC, et al. TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med. 2014;6:227ra234. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widyantoro B, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–2418. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, et al. Snail is a direct target of hypoxia-inducible factor 1alpha (HIF1alpha) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J. Biol. Chem. 2015;290:16653–16664. doi: 10.1074/jbc.M115.636944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, et al. Hypoxia induces endothelialmesenchymal transition in pulmonary vascular remodeling. Int. J. Mol. Med. 2018;42:270–278. doi: 10.3892/ijmm.2018.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T, et al. Vildagliptin ameliorates pulmonary fibrosis in lipopolysaccharide-induced lung injury by inhibiting endothelial-to-mesenchymal transition. Respir. Res. 2017;18:177. doi: 10.1186/s12931-017-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataraj D, Ernst A, Kalluri R. Idiopathic pulmonary fibrosis is associated with endothelial to mesenchymal transition. Am. J. Respir. Cell Mol. Biol. 2010;43:129–130. doi: 10.1165/rcmb.2010-0044ED. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto N, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutsumi T, et al. Nintedanib ameliorates experimental pulmonary arterial hypertension via inhibition of endothelial mesenchymal transition and smooth muscle cell proliferation. PLoS ONE. 2019;14:e0214697. doi: 10.1371/journal.pone.0214697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan F, et al. Glucagon-Like peptide 1 protects against hyperglycemic-induced endothelial-to-mesenchymal transition and improves myocardial dysfunction by suppressing poly(ADP-Ribose) polymerase 1 activity. Mol. Med. 2015;21:15–25. doi: 10.2119/molmed.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montorfano I, et al. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-beta1 and TGF-beta2-dependent pathway. Lab. Invest. 2014;94:1068–1082. doi: 10.1038/labinvest.2014.100. [DOI] [PubMed] [Google Scholar]
- 31.Pociask DA, Sime PJ, Brody AR. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab. Invest. 2004;84:1013–1023. doi: 10.1038/labinvest.3700109. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Good RB, et al. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am. J. Pathol. 2015;185:1850–1858. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Rieder F, et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011;179:2660–2673. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brush J, et al. Molecular mechanisms of late normal tissue injury. Semin. Radiat. Oncol. 2007;17:121–130. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother. Oncol. 1995;35:83–90. doi: 10.1016/0167-8140(95)01540-w. [DOI] [PubMed] [Google Scholar]
- 38.Choi SH, et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis. Clin. Cancer Res. 2015;21:3716–3726. doi: 10.1158/1078-0432.CCR-14-3193. [DOI] [PubMed] [Google Scholar]
- 39.Evrard SM, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016;7:11853. doi: 10.1038/ncomms11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, et al. Activation of Nrf2 attenuates pulmonary vascular remodeling via inhibiting endothelial-to-mesenchymal transition: an insight from a plant polyphenol. Int. J. Biol. Sci. 2017;13:1067–1081. doi: 10.7150/ijbs.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anea CB, et al. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ. Res. 2012;111:1157–1165. doi: 10.1161/CIRCRESAHA.111.261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khapre RV, Kondratova AA, Susova O, Kondratov RV. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle. 2011;10:4162–4169. doi: 10.4161/cc.10.23.18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu M, et al. BMAL1 suppresses ROS-induced endothelial-to-mesenchymal transition and atherosclerosis plaque progression via BMP signaling. Am. J. Transl. Res. 2018;10:3150–3161. [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, et al. Low-intensity pulsed ultrasound prevents the oxidative stress induced endothelial-mesenchymal transition in human aortic endothelial cells. Cell Physiol. Biochem. 2018;45:1350–1365. doi: 10.1159/000487561. [DOI] [PubMed] [Google Scholar]
- 45.Lee ES, Boldo LS, Fernandez BO, Feelisch M, Harmsen MC. Suppression of TAK1 pathway by shear stress counteracts the inflammatory endothelial cell phenotype induced by oxidative stress and TGF-beta1. Sci. Rep. 2017;7:42487. doi: 10.1038/srep42487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maleszewska M, et al. IL-1beta and TGFbeta2 synergistically induce endothelial to mesenchymal transition in an NFkappaB-dependent manner. Immunobiology. 2013;218:443–454. doi: 10.1016/j.imbio.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Jiang F, Liu GS, Dusting GJ, Chan EC. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol. 2014;2:267–272. doi: 10.1016/j.redox.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You S, et al. Schizandrin B attenuates angiotensin II induced endothelial to mesenchymal transition in vascular endothelium by suppressing NF-kappaB activation. Phytomedicine. 2019;62:152955. doi: 10.1016/j.phymed.2019.152955. [DOI] [PubMed] [Google Scholar]
- 49.Xu YP, et al. MiR-126a-5p is involved in the hypoxia-induced endothelial-to-mesenchymal transition of neonatal pulmonary hypertension. Hypertens. Res. 2017;40:552–561. doi: 10.1038/hr.2017.2. [DOI] [PubMed] [Google Scholar]
- 50.Watson CJ, et al. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum. Mol. Genet. 2014;23:2176–2188. doi: 10.1093/hmg/ddt614. [DOI] [PubMed] [Google Scholar]
- 51.Zou, J. et al. Autophagy attenuates endothelial-to-mesenchymal transition by promoting Snail degradation in human cardiac microvascular endothelial cells. Biosci. Rep. 37, BSR20171049 (2017). [DOI] [PMC free article] [PubMed]
- 52.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 53.Zhu K, et al. MiR-302c inhibits tumor growth of hepatocellular carcinoma by suppressing the endothelial-mesenchymal transition of endothelial cells. Sci. Rep. 2014;4:5524. doi: 10.1038/srep05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie L, et al. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: role of IL-1beta and TGF-beta2. Am. J. Physiol. Cell Physiol. 2014;307:C859–C877. doi: 10.1152/ajpcell.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia J, et al. Tie1 deficiency induces endothelial-mesenchymal transition. EMBO Rep. 2012;13:431–439. doi: 10.1038/embor.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noseda M, et al. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ. Res. 2004;94:910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- 57.Timmerman LA, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blokzijl A, et al. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell Biol. 2003;163:723–728. doi: 10.1083/jcb.200305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghiabi P, et al. Breast cancer cells promote a notch-dependent mesenchymal phenotype in endothelial cells participating to a pro-tumoral niche. J. Transl. Med. 2015;13:27. doi: 10.1186/s12967-015-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi SH, et al. HSPB1 inhibits the endothelial-to-mesenchymal transition to suppress pulmonary fibrosis and lung tumorigenesis. Cancer Res. 2016;76:1019–1030. doi: 10.1158/0008-5472.CAN-15-0952. [DOI] [PubMed] [Google Scholar]
- 62.Fan CS, et al. Osteopontin-integrin engagement induces HIF-1alpha-TCF12-mediated endothelial-mesenchymal transition to exacerbate colorectal cancer. Oncotarget. 2018;9:4998–5015. doi: 10.18632/oncotarget.23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SH, Song Y, Seo HR. GSK-3beta regulates the endothelial-to-mesenchymal transition via reciprocal crosstalk between NSCLC cells and HUVECs in multicellular tumor spheroid models. J. Exp. Clin. Cancer Res. 2019;38:46. doi: 10.1186/s13046-019-1050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu DM, et al. PLEK2 mediates metastasis and vascular invasion via the ubiquitin-dependent degradation of SHIP2 in non-small cell lung cancer. Int J. Cancer. 2019;146:2563–2575. doi: 10.1002/ijc.32675. [DOI] [PubMed] [Google Scholar]
- 65.Dudley AC. Tumor endothelial cells. Cold Spring Harb. Perspect. Med. 2012;2:a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13:237–247. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 67.Fujii M, et al. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem. Int. 2012;60:327–333. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li B, et al. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 2006;580:4252–4260. doi: 10.1016/j.febslet.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 69.Nagai N, et al. Downregulation of ERG and FLI1 expression in endothelial cells triggers endothelial-to-mesenchymal transition. PLoS Genet. 2018;14:e1007826. doi: 10.1371/journal.pgen.1007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan TS, et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. J. Exp. Med. 2016;213:2967–2988. doi: 10.1084/jem.20151665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su S, et al. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856 e816. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Prakash J. Cancer-associated fibroblasts: perspectives in cancer therapy. Trends Cancer. 2016;2:277–279. doi: 10.1016/j.trecan.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Liu T, et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun. 2018;9:3439. doi: 10.1038/s41467-018-05982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Helfrich I, et al. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franco M, Paez-Ribes M, Cortez E, Casanovas O, Pietras K. Use of a mouse model of pancreatic neuroendocrine tumors to find pericyte biomarkers of resistance to anti-angiogenic therapy. Horm. Metab. Res. 2011;43:884–889. doi: 10.1055/s-0031-1284381. [DOI] [PubMed] [Google Scholar]
- 76.Xian X, et al. Pericytes limit tumor cell metastasis. J. Clin. Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderberg C, et al. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. J. Exp. Med. 2013;210:563–579. doi: 10.1084/jem.20120662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilkinson EL, Sidaway JE, Cross MJ. Cardiotoxic drugs Herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol. Open. 2016;5:1362–1370. doi: 10.1242/bio.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsai TH, Lin CJ, Hang CL, Chen WY. Calcitriol attenuates doxorubicin-induced cardiac dysfunction and inhibits endothelial-to-mesenchymal transition in mice. Cells. 2019;8:865. doi: 10.3390/cells8080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, et al. Endothelial to mesenchymal transition contributes to arsenic-trioxide-induced cardiac fibrosis. Sci. Rep. 2016;6:33787. doi: 10.1038/srep33787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 82.Kim M, et al. The effect of oxidized low-density lipoprotein (ox-LDL) on radiation-induced endothelial-to-mesenchymal transition. Int J. Radiat. Biol. 2013;89:356–363. doi: 10.3109/09553002.2013.763193. [DOI] [PubMed] [Google Scholar]
- 83.Goedegebuure RSA, de Klerk LK, Bass AJ, Derks S, Thijssen V. Combining radiotherapy with anti-angiogenic therapy and immunotherapy; a therapeutic triad for cancer? Front. Immunol. 2018;9:3107. doi: 10.3389/fimmu.2018.03107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 85.Banerjee D, et al. High-dose radiation increases Notch1 in tumor vasculature. Int J. Radiat. Oncol. Biol. Phys. 2019;106:857–866. doi: 10.1016/j.ijrobp.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J. Am. Coll. Cardiol. 2010;55:1237–1239. doi: 10.1016/j.jacc.2009.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Straub JM, et al. Radiation-induced fibrosis: mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015;141:1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dorresteijn LD, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J. Clin. Oncol. 2002;20:282–288. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 89.Venkatesulu BP, et al. Radiation-induced endothelial vascular injury: a review of possible mechanisms. JACC Basic Transl. Sci. 2018;3:563–572. doi: 10.1016/j.jacbts.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 91.Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 92.Boerma M, Hauer-Jensen M. Potential targets for intervention in radiation-induced heart disease. Curr. Drug Targets. 2010;11:1405–1412. doi: 10.2174/1389450111009011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mintet E, et al. Identification of endothelial-to-mesenchymal transition as a potential participant in radiation proctitis. Am. J. Pathol. 2015;185:2550–2562. doi: 10.1016/j.ajpath.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 94.Mintet E, et al. Endothelial Hey2 deletion reduces endothelial-to-mesenchymal transition and mitigates radiation proctitis in mice. Sci. Rep. 2017;7:4933. doi: 10.1038/s41598-017-05389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yi M, et al. Irradiated human umbilical vein endothelial cells undergo endothelial-mesenchymal transition via the Snail/miR-199a-5p axis to promote the differentiation of fibroblasts into myofibroblasts. Biomed. Res. Int. 2018;2018:4135806. doi: 10.1155/2018/4135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanasaki K, et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63:2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 97.Yao Y, et al. Losartan alleviates renal fibrosis and inhibits endothelial-to-mesenchymal transition (EMT) under high-fat diet-induced hyperglycemia. Front. Pharm. 2018;9:1213. doi: 10.3389/fphar.2018.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cipriani P, et al. The endothelial-mesenchymal transition in systemic sclerosis is induced by endothelin-1 and transforming growth factor-beta and may be blocked by macitentan, a dual endothelin-1 receptor antagonist. J. Rheumatol. 2015;42:1808–1816. doi: 10.3899/jrheum.150088. [DOI] [PubMed] [Google Scholar]
- 99.Chen X, et al. Protective effect of spironolactone on endothelial-to-mesenchymal transition in HUVECs via Notch pathway. Cell Physiol. Biochem. 2015;36:191–200. doi: 10.1159/000374063. [DOI] [PubMed] [Google Scholar]
- 100.Tsai TH, et al. Liraglutide inhibits endothelial-to-mesenchymal transition and attenuates neointima formation after endovascular injury in streptozotocin-induced diabetic mice. Cells. 2019;8:589. doi: 10.3390/cells8060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song Y, et al. Identification of radiation-induced EndMT inhibitors through cell-based phenomic screening. FEBS Open Bio. 2019;9:82–91. doi: 10.1002/2211-5463.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wylie-Sears J, Levine RA, Bischoff J. Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-beta-induced phosphorylation of ERK. Biochem. Biophys. Res. Commun. 2014;446:870–875. doi: 10.1016/j.bbrc.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu M, et al. Cinacalcet ameliorates cardiac fibrosis in uremic hearts through suppression of endothelial-to-mesenchymal transition. Int. J. Cardiol. 2014;171:e65–e69. doi: 10.1016/j.ijcard.2013.11.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao H, Zhang J, Liu T, Shi W. Rapamycin prevents endothelial cell migration by inhibiting the endothelial-to-mesenchymal transition and matrix metalloproteinase-2 and -9: an in vitro study. Mol. Vis. 2011;17:3406–3414. [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez-Mateo GT, et al. Rapamycin protects from type-i peritoneal membrane failure inhibiting the angiogenesis, lymphangiogenesis, and endo-MT. Biomed. Res. Int. 2015;2015:989560. doi: 10.1155/2015/989560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Furihata T, et al. Hydrocortisone enhances the barrier properties of HBMEC/cibeta, a brain microvascular endothelial cell line, through mesenchymal-to-endothelial transition-like effects. Fluids Barriers CNS. 2015;12:7. doi: 10.1186/s12987-015-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Invest. Ophthalmol. Vis. Sci. 2014;55:7321–7331. doi: 10.1167/iovs.14-15167. [DOI] [PubMed] [Google Scholar]
- 108.Feng B, et al. miR-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes. 2016;65:768–779. doi: 10.2337/db15-1033. [DOI] [PubMed] [Google Scholar]
- 109.Kumarswamy R, et al. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler. Thromb. Vasc. Biol. 2012;32:361–369. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- 110.Geng H, Guan J. MiR-18a-5p inhibits endothelial-mesenchymal transition and cardiac fibrosis through the Notch2 pathway. Biochem. Biophys. Res. Commun. 2017;491:329–336. doi: 10.1016/j.bbrc.2017.07.101. [DOI] [PubMed] [Google Scholar]
- 111.Song S, et al. The role of PDGF-B/TGF-beta1/neprilysin network in regulating endothelial-to-mesenchymal transition in pulmonary artery remodeling. Cell Signal. 2016;28:1489–1501. doi: 10.1016/j.cellsig.2016.06.022. [DOI] [PubMed] [Google Scholar]