Abstract
The mitochondrial calcium uniporter (MCU) plays essential roles in mitochondrial calcium homeostasis and regulates cellular functions, such as energy synthesis, cell growth, and development. Thus, MCU activity is tightly controlled by its regulators as well as post-translational modification, including phosphorylation by protein kinases such as proline-rich tyrosine kinase 2 (Pyk2) and AMP-activated protein kinase (AMPK). In our in vitro kinase assay, the MCU N-terminal domain (NTD) was phosphorylated by protein kinase C isoforms (PKCβII, PKCδ, and PKCε) localized in the mitochondrial matrix. In addition, we found the conserved S92 was phosphorylated by the PKC isoforms. To reveal the structural effect of MCU S92 phosphorylation (S92p), we determined crystal structures of the MCU NTD of S92E and D119A mutants and analysed the molecular dynamics simulation of WT and S92p. We observed conformational changes of the conserved loop2-loop4 (L2-L4 loops) in MCU NTDS92E, NTDD119A, and NTDS92p due to the breakage of the S92-D119 hydrogen bond. The results suggest that the phosphorylation of S92 induces conformational changes as well as enhancements of the negative charges at the L2-L4 loops, which may affect the dimerization of two MCU-EMRE tetramers.
Subject terms: X-ray crystallography, Biochemistry
Introduction
Under physiological conditions, mitochondria, which uptake and sequester Ca2+ into the matrix, play essential roles in the regulation of ATP synthesis through the tricarboxylic acid cycle (TCA), buffering of cytosolic Ca2+, and cell growth and development1. However, prolonged overload of mitochondrial Ca2+ uptake can trigger the production of large amounts of reactive oxygen species (ROS), induce opening of the mitochondrial permeability transition pore, cause disruption of mitochondrial membrane potential, and eventually lead to apoptotic and necrotic cell death1. The malfunction of mitochondrial Ca2+ homeostasis causes pathological diseases, including ischemia reperfusion, myocardial infarction, and epilepsy2–4.
A key pathway for mitochondrial Ca2+ uptake across the inner mitochondrial membrane (IMM) is through the mitochondrial calcium uniporter (MCU) complex, which facilitates Ca2+ entry into the IMM through the electrochemical potential gradient driven by the mitochondrial membrane potential (Δψ = approximately −180 mV)5,6. The MCU is the pore-forming subunit of the MCU complex and acts as a selective Ca2+ channel. The MCU complex consists of MCU, along with its regulatory proteins, MCU paralog (MCUb), mitochondrial calcium uptake 1, 2 & 3 (MICU1, MICU2, and MICU3), essential MCU regulator (EMRE), and mitochondrial calcium uniporter regulator 1 (MCUR1). The interaction of these regulatory proteins with MCU controls MCU Ca2+ uptake activity under different mitochondrial conditions and in different cell types2,7,8.
Mitochondrial calcium uptake greatly varies depending on the cell types and tissues due to the differential expression levels of MCU complex components9,10. The mitochondrial Ca2+ conductance measurements using whole-mitoplast current recording suggest that the level of Ca2+ entry mediated by MCU differs between mouse tissues, including skeletal muscle, heart, brown fat, kidney, and liver9. In addition, uncoupling proteins 2 and 3 (UCP2 and UCP3) interact with methylated MICU1 and can differentially modulate the mitochondrial calcium uptake in different cells, including HeLa, Ea.hy926, HUVEC, and PAEC cells10.
The role of the MCU as an essential Ca2+ channel for mitochondrial Ca2+ uptake is supported by functional studies of the MCU in human cells and mouse models7,11–16. Inhibition of Ca2+ uptake in the matrix has been previously demonstrated by blocking the MCU pore with ruthenium red (Ru360)16, and by genetic ablation of MCU7,11. Previous research has also shown that the microRNA miR-25 can reduce the mRNA level of MCU and directly down-regulate MCU expression, thus inhibiting the mitochondrial Ca2+ uptake12. In the MCU pore-forming region, mutations of negatively charged acidic residues (E257, D261, E264) in human MCU, have also been shown to inhibit the MCU activity16. Although the MCU knock-out mouse was reported as the mild phenotype, unexpected compensatory changes that affect cytosolic Ca2+ homeostasis or modulate mitochondrial Ca2+-dependent metabolism impair the short-term mitochondrial Ca2+ uptake at a “fight-or-flight” response13–15.
The N-terminal domain (NTD) of the MCU (MCU NTD) plays essential roles in the dimerization of two MCU-EMRE tetramers, MCUR1 interaction, MCUb NTD interaction, Mg2+ binding selectivity, redox sensor, and regulation of MCU Ca2+ uptake activity6,7,17–19. In addition, the MCU NTD can be altered by post-translational modifications in the mitochondrial matrix space7,20,21. Under inflammatory and hypoxic conditions, MCU undergoes S-glutathionylation in a highly conserved C97 residue and functions as a mitochondrial ROS sensor in the mitochondrial matrix7. Phosphorylation of the MCU (predicted Y158 in the NTD and Y289, Y317 in the C-terminal domain) by proline-rich tyrosine kinase 2 (Pyk2) induces an increase in the mitochondrial Ca2+ uptake by facilitating formation of the MCU channel via MCU oligomerization20. S57 phosphorylation in the MCU by AMP-activated protein kinase (AMPK) facilitates mitochondrial Ca2+ entry during mitosis and boosts mitochondrial respiration to maintain energy homeostasis21. Regulatory functions of MCU Ca2+ uptake by Ca2+/calmodulin-dependent protein kinase II (CaMKII) still remain controversial22–26, although Nguyen et al. suggest S92 phosphorylation (S92p) of MCU by CaMKII in vivo25. Since our study focuses on the S92p of MCU by protein kinase C (PKC), we exclude an argument about the functional role of MCU driven by CaMKII.
PKC isoforms, a heterogeneous family of serine/threonine (Ser/Thr) kinases, are encoded by nine genes (α, β, γ, δ, ε, η, θ, ι, ζ) in human27, and PKCβII (one of the splice variants of PKCβ), PKCδ, and PKCε are localized in the mitochondrial matrix in response to ROS27–29. PKC directly phosphorylates a wide range of cellular substrates and regulates various cellular functions, such as cell migration, differentiation, proliferation, senescence, and apoptosis27–29.
In this study, we observed that the MCU S92 was phosphorylated by mitochondrial PKC isoforms, including PKCβII, PKCδ, and PKCε, via in vitro kinase assay. To uncover the structural effects of phosphorylated S92 (S92p), we determined two crystal structures of MCU NTDS92E, an S92p mimic, and NTDD119A mutants at a resolution of 2.50 Å and 2.85 Å, respectively, and analysed the molecular dynamics simulation for NTDWT and NTDS92p. We propose that phosphorylation at S92 induces conformational and electrostatic changes in the L2-L4 loops of the MCU NTDWT due to the breakage of S92-D119 hydrogen bonds. As a result, it may affect the dimerization of the two MCU-EMRE tetramers.
Results
The MCU NTD S92 is phosphorylated by PKCβII, PKCδ, and PKCε
The MCU NTD sequence, which is encoded by exon 3 and 4 (residues 75−165) of the MCU gene, was highly conserved based on 230 MCU NTD homologous protein sequences in the ConSurf server (Fig. 1A–C, Supplementary Fig. S1)30. The MCU NTD has six serines (S87, S92, S105, S107, S129, and S138) and four threonines (T76, T100, T139, and T157). Among these, the highly conserved S92 in the 89-RLPS-92 sequences (the RxxS motif, where x is any residue) was determined to be a putative recognition site for phosphorylation by Ser/Thr kinases containing CaMKII, cAMP-dependent protein kinases (PKA), and PKC, using the KinasePhos 2.0 server and Group-based Prediction System (GPS) 2.0 softwares (Fig. 1C, Supplementary Fig. S1)22,31–35. Previously, Nguyen et al. isolated mitochondria from vascular smooth muscle cells (VSMC) and detected the S92p of MCU by CaMKII using specific MCU S92p antibodies in an immunoblotting25. To further investigate whether S92 is phosphorylated by other Ser/Thr kinases such as PKA and PKC localized in the mitochondrial matrix28,36,37, we performed in vitro kinase assays with myelin basic protein (MBP; positive control), MCU NTDWT, MCU NTDAAS92 (all alanine mutations of the nine Ser/Thr residues in the NTD except S92), and [γ-32P]ATP. In control experiments, MBP, a multiple phosphorylation target by Ser/Thr kinases38–40, was phosphorylated by PKA and PKC isoforms (α, β, γ mixtures, βII, δ, and ε) (Supplementary Fig. S2). Under the same conditions, MCU NTDWT was phosphorylated by PKC, but not by PKA (Fig. 1D) and MCU NTDAAS92 was also phosphorylated by PKC (Fig. 1F). In all nine PKC isoforms, three PKC isoforms, including PKCβII, PKCδ, and PKCε are localized in the mitochondrial matrix and regulate the reactive oxygen species (ROS) formation in the matrix27–29. In additional in vitro kinase assays, we observed that PKCβII, PKCδ, and PKCε phosphorylated S92, and that S92 phosphorylation activities by PKCβII and PKCδ were stronger than that of PKCε (Fig. 1E,F). Thus, we suggest that PKCβII, PKCδ, and PKCε localized in mitochondrial matrix can phosphorylate the S92 in the MCU NTD.
Figure 1.
Conserved S92 is phosphorylated by protein kinase C isoforms. (A) Schematic diagram of the MCU. The MCU consists of a mitochondrial targeting sequence (MTS), N-terminal domain (NTD), linker helix domain (LHD), two transmembrane domains (TM1 and TM2), a TM linker (L), and two coiled-coils (CC). (B) Surface and ribbon diagrams of the MCU NTD coloured by scoring the residue conservation from 230 MCU NTD homologues using the ConSurf server. Highly conserved and variable residues are shown in red and green, respectively. The β-strands (β1 − β6), α-helices (α1, α2), and loops (L1 − L8) are shown in arrows, cylinders, and lines, respectively. (C) Detailed view of the highly conserved L2-L4 loop regions in the MCU NTD (PDB ID, 4XTB). The residues and hydrogen bonds are denoted in stick and dashed lines (red). (D–F) In vitro kinase assays of MCU NTDWT (residues 75–165) (D,E) and MCU NTDAAS92 (F). Autoradiography analysis of MCU NTDWT (residues 75–165) and MCU NTDAAS92 proteins that were incubated with protein kinase A (PKA), protein kinase C (PKC) isoforms (PKC mixture of α, β, and γ isoforms with lesser δ and ζ; PKCβII; PKCδ; PKCε), and [γ-32P]ATP (P-32). We designed all Ser/Thr (T76, S87, S92, T100, S105, S107, S129, S138, T139, and T157) mutants of the MCU NTD except the S92 (MCU NTDAAS92). Full autoradiography results in Supplementary Fig S4. The reaction samples were resolved by SDS-PAGE, and visualized by autoradiography. Data are representative of three independent experiments.
In details of conformational and electrostatic changes of MCU NTD by S92 phosphorylation
To reveal the structural effect of S92 phosphorylation in the MCU NTD, we generated the S92E mutant, an S92p mimic, of MCU NTD fused with the bacteriophage T4 lysozyme at the N-terminal end of MCU NTD (T4-MCU NTDS92E) to improve protein solubility for crystallographic studies6. We determined the structure of T4-MCU NTDS92E at a resolution of 2.50 Å by molecular replacement using the MCU NTDWT (PDB ID: 4XSJ) and T4 lysozyme (PDB ID: 2LZM) structures as templates (Fig. 2A; Table 1).
Figure 2.
Structural comparison between the MCU NTDWT and the NTDS92E or NTDD119A mutants. (A) Overall structure of MCU NTDS92E mutant (green) fused with bacteriophage T4 lysozyme (gray) at the N-terminus end of the MCU NTD. (B) Ribbon diagrams of superimposed MCU NTDWT (PDB ID: 4XSJ) and mutant structures of MCU NTDS92E and MCU NTDD119A. The ribbon diagrams are represented in different colours: magenta (WT), green (S92E), and blue (D119A). The side chains of residues are shown in stick, the red dashed lines denote hydrogen bonds, and the L2 loop of conformational changes are represented by black arrows. (C,D) Detailed view of superimposed L2-L4 loops of MCU NTDWT (magenta) and MCU NTDS92E (green) (C) or MCU NTDWT (magenta) and MCU NTDD119A (blue) (D). The backbone and side chains of residues are represented in ribbon and stick, respectively. The arrows represent movement of the residues participating in hydrophobic interaction (black) or hydrogen bonds (red). Dashed-lines (green in S92E and magenta in WT) denote hydrogen bonds.
Table 1.
Data collection and refinement statistics.
| Proteins | T4 lysozyme-MCU NTD S92E | T4 lysozyme-MCU NTD D119A |
|---|---|---|
| PDB ID: | 6JG0 | 6KVX |
| Data Collection | ||
| Space group | P65 | P65 |
| X-ray sourcea | PAL-5C | PAL-5C |
| Detector | ADSC Q315 | ADSC Q315 |
| Wavelength (Å) | 0.9795 | 0.9795 |
| Unit cell: a, b, c (Å) | 97.9, 97.9, 61.5 | 97.9, 97.9, 61.6 |
| Resolution range (Å)b | 50.0‒2.50 (2.54‒2.50) | 50.0‒2.85 (2.90‒2.85) |
| Rmergec | 8.3 (61.9) | 9.3 (59.1) |
| CC1/2d in outer shell (%) | 60.7 | 70.8 |
| I/σ(I) | 14.3 (3.0) | 12.3 (3.0) |
| Completeness (%) | 99.7 (99.8) | 98.7 (99.8) |
| Redundancy | 4.8 (3.5) | 4.2 (4.1) |
| Refinement | ||
| Resolution range (Å) | 42.5‒2.50 | 29.0‒2.85 |
| No. reflections | 11122 | 7489 |
| Rworke (%)/Rfree (%) | 19.0/26.0 | 18.0/26.2 |
| No. atoms/residues | ||
| Protein | 2011/253 | 2027/253 |
| SO42− | 10/2 | 20/4 |
| Water | 77 | − |
| B-factors (Å2) | ||
| Protein | 52.1 | 55.0 |
| SO42− | 88.6 | 81.9 |
| Water | 46.4 | − |
| Model statistics | ||
| rmsd bond length (Å) | 0.012 | 0.014 |
| rmsd bond angles (°) | 1.40 | 1.20 |
| Ramachandran plot (%) favoured/allowed/disallowed | 97.6/2.4/0.0 | 96.0/4.0/0.0 |
aBeamline 5 C at Pohang Acceleratory Laboratory (PAL) in South Korea.
bValues in parentheses are for highest-resolution shell.
cRmerge = ∑h ∑i │I(h)i − ‹I(h)›│/∑h ∑iI(h)i, where I(h) is the intensity of reflection of h, ∑h is the sum overall reflections and ∑i is the sum over i measurements of reflection h.
dCC1/2 in outer shell were calculated from HKL2000.
eRwork = Σhkl ||Fo | -|Fc ||/Σhkl | Fo | ; 5% of the reflections were excluded for the Rfree calculation.
The overall structure of MCU NTDS92E was similar to the structure of MCU NTDWT (PDB ID: 4XSJ) with root-mean-square deviation (RMSD) of 0.57 Å for 87 Cα atoms, and consisted of two helices (α1 and α2) and six β-strands (Fig. 2A,B). The S92-D119 in the L2-L4 loops of MCU NTDWT formed a hydrogen bond at a distance of 2.5 Å; the R93 interacted with the E118 to form a salt bridge and stabilized the closed form of L2 loop (Fig. 2B,C). The mutation of S92 to E92 results in atomic clashes of the side chains between E92 and D119, broke the S92-D119 hydrogen bond, and induced conformational changes from the closed form of the L2 loop in MCU NTDWT to the open form (Fig. 2B,C). The peptide backbone of the L2 loop in the MCU NTDS92E moved away from L2 loop of MCU NTDWT (Cα atom distance of 4.6 Å), and the side chain of R93 moved up to the position of S92 and formed a new hydrogen bond with E92 and D119. The MCU NTDS92E L90 in the hydrophobic interior, which also contained V88, L115, I122, V125, and I153 in MCU NTDS92E moved away from that of MCU NTDWT at a distance of 2.2 Å (Fig. 2B,C). Based on the MCU NTDS92E structure, we can suggest that the additional phosphate group by the S92 phosphorylation break the S92-D119 hydrogen bond due to atomic clashes between the phosphate group of S92p and the carboxyl group of D119, and induces a conformational change similar to that of the MCU NTDS92E.
In our previous studies, we unintentionally observed that the S92A mutation abolish the S92-D119 hydrogen bond in the structure of the MCU NTDS92A6 (Supplementary Fig. S3A). Intriguingly, the conformational changes of the L2-L4 loops in MCU NTDS92A were similar to that of MCU NTDS92E (Supplementary Fig. S3B) and were maintained in the open form of L2 loop in comparison with the closed form of MCU NTDWT (Fig. 2C, Supplementary Fig. S3A), hypothesizing that S92 phosphorylation might modulate the open or closed conformation of the NTD L2-L4 loops.
To further investigate whether the S92-D119 hydrogen bond is important for maintaining the closed conformation of the L2-L4 loops, we prepared the D119A mutant to break the S92-D119 hydrogen bond. We determined the structure of the MCU NTDD119A mutant fused with N-terminus T4-lysozyme fusion (T4-MCU NTDD119A) at 2.85 Å resolution. Overall, the structures of MCU NTDWT and the MCU NTDD119A mutant were similar, with an RMSD of 0.61 Å for 86 Cα atoms (Fig. 2B). As expected, the MCU NTDD119A also broke the S92-D119 hydrogen bond from the L2-L4 loops of MCU NTDWT and caused structural changes in the L2-L4 loops, similar to that observed in the structure of MCU NTDS92E (Fig. 2B,D). The L2 loop conformation of the MCU NTDD119A moved away at a Cα atom distance of 5.1 Å from that of MCU NTDWT, while the side chain of R93 residue, which moved up to the position of S92, did not form a new hydrogen bond because of lack of a hydrogen bonding counterpart by D119A mutation (Fig. 2B,D).
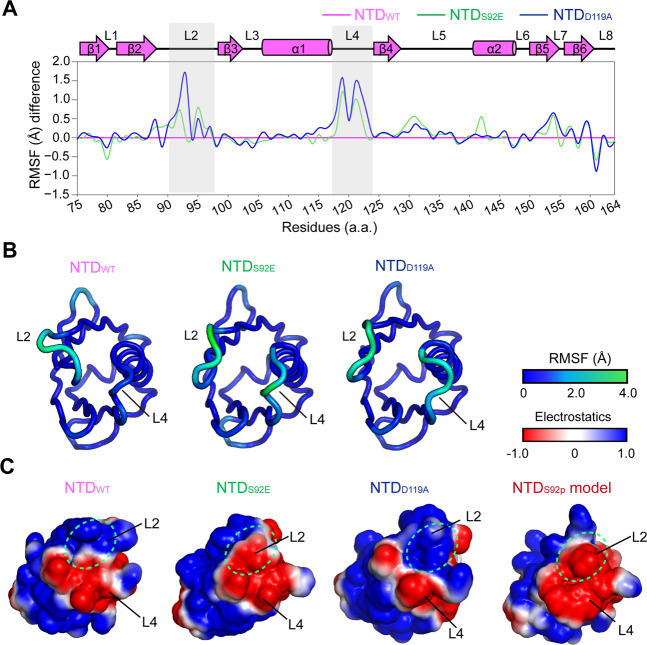
In addition, to understand whether the S92-D119 hydrogen bond disruption by S92p might contribute to flexibility of L2-L4 loops in the MCU NTD, we performed the ensemble refinement using PHENIX and calculated the root-mean-square fluctuation (RMSF) (Å) from the ensemble refinement results of the MCU NTDWT and the mutants (S92E and D119A)41,42. Overall structures of two mutants showed similar RMSF scores in dynamics to that of the MCU NTDWT, while dramatic RMSF changes were observed in the L2-L4 loops of the MCU NTD mutants compared to the MCU NTDWT (Fig. 3A,B).
Figure 3.
Structural dynamics and electrostatic surface charges in MCU NTDWT and mutants. (A) Ensemble refinements of MCU NTDWT (magenta; PDB ID: 4XSJ), MCU NTDS92E (green), and MCU NTDD119A (blue). Plots of the root-means-square fluctuation (RMSF) (Å) difference. The RMSF of each mutant, S92E (green) and D119A (blue), were relatively calculated by subtracting RMSF of the WT (magenta). Dramatic RMSF difference of the L2-L4 loops are highlighted in gray boxes. The β-strands (β1 − β6), α-helices (α1, α2), and loops (L1 − L8) are shown in arrows, cylinders, and lines, respectively. (B) Cartoon diagrams of ensemble refinements of MCU NTDWT, MCU NTDS92E, and MCU NTDD119A in the RMSF (Å) coloured from blue (rigid) to green (flexible), depended on scoring of the residue dynamics. (C) Electrostatic surface charges of MCU NTDWT and the mutants, S92E and D119A, and MD simulated MCU NTDS92p model were calculated using PDB2PQR server with the CHARMM force field at the mitochondrial matrix pH of approximately 7.8, and the surface diagrams were generated using PyMOL software. The different surface charge regions are highlighted in green circles.
To investigate whether phosphorylation of S92 in the MCU NTD affected electrostatic charges, we calculated and compared the side chain charges of the residues S92, S92p, S92E, and D119A, at the mitochondrial matrix pH of approximately 7.8 using the Henderson–Hasselbalch equation43,44. Negative charges in the mutant S92E (pKa ~4.3) and S92p (pKa1 ~1.5, pKa2 ~6.3) by deprotonation of the hydroxyl group were increased by −1.0 and −2.0, respectively, whereas the negative charge of −1.0 in the D119A mutant (pKa ~3.9) was reduced in comparison with the MCU NTDWT. In agreement with the changes of the negative charge, electrostatic surface charge was enhanced in the L2-L4 loops of the MCU NTDS92E and the S92p model structures, whereas the positive surface charge of MCU NTDD119A was increased compared to the MCU NTDWT (Fig. 3C).
Collectively, these findings suggest that the S92-D119 hydrogen bond formation or disruption, which depends on S92 phosphorylation, regulates the conformation of L2-L4 loops and additional negative charges in the phosphate group of S92p in the MCU complex.
Molecular dynamics simulation analysis of NTDWT and NTDS92p monomers
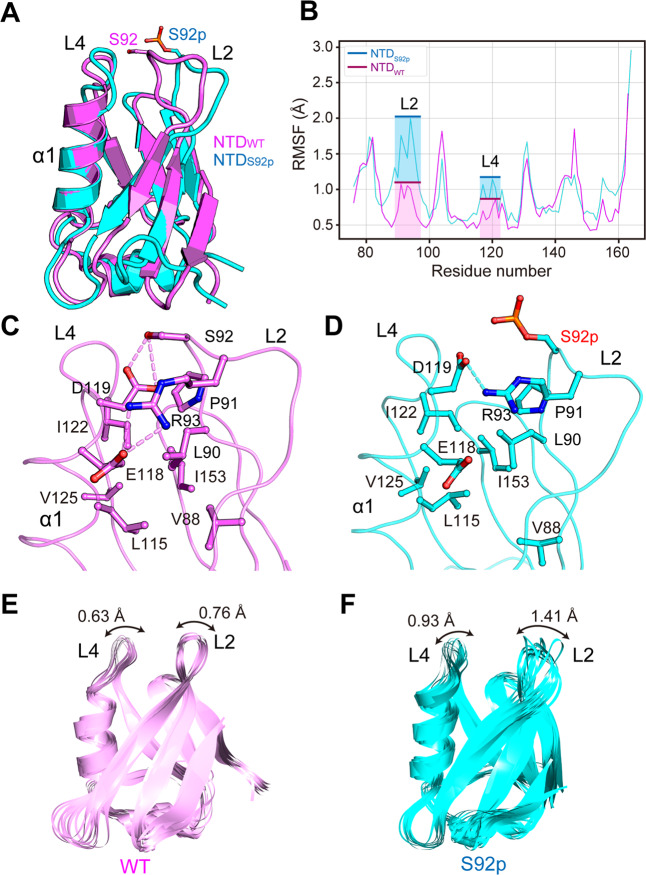
Molecular dynamics (MD) simulations were performed on the NTDWT and NTDS92p monomer structures to identify the intra structural changes caused by phosphorylation of S92 in the NTD. The MD simulations clearly showed the flexibility change of the L2-L4 loop region (Fig. 4A,E,F). The fluctuations of all amino acid residues in NTDWT and NTDS92p monomer structures were measured by plotting of the RMSF. The RMSF values of the L2 and L4 loops of the NTDS92p structure were significantly higher than the values of the NTDWT (Fig. 4B). The average RMSF values of the L2 loop for the NTDWT and NTDS92p were 0.76 Å and 1.41 Å, respectively; the values of the L4 loop were 0.63 Å and 0.93 Å, respectively.
Figure 4.
L2-L4 structure comparison between NTDWT and NTDS92p monomers during 10 ns molecular dynamics (MD) simulation. (A-D) MD snapshot structures of NTDWT and NTDS92p structures at 10 ns. (A) NTDWT and NTDS92p structures are superimposed and colored in pink and cyan, respectively. The S92 and S92p residues are displayed in a stick model with the same color. (B) The root-means-square fluctuation (RMSF) plots for Cα atoms of the NTDWT and the NTDS92p are shown as pink and cyan lines, respectively. The RMSF values of the L2 and L4 loops are highlighted by the boxes in the same color. (C,D) Atomic interaction analysis between residues of L2 and L4 loops on NTDWT (pink) and NTDS92p (cyan). Hydrogen bond interactions are shown as dashed lines (pink in NTDWT and cyan in NTDS92p). (E,F) MD snapshot structure superimposition. The 20 trajectories are extracted every 0.5 ns during 10 ns simulation time and aligned for NTDWT (pink) (E) and NTDSS92p. (cyan) (F).
To investigate details of the atomic interaction between the residues near the L2 and L4 loops, the final MD trajectory structure was extracted. In NTDWT, the S92 and R93 in the L2 loop were hydrogen-bonded with D119 in the L4 loop (Fig. 4C). Conversely, in NTDS92p, only R93 participated in the hydrogen bond interaction, as the interaction of S92 with D119 was broken (Fig. 4D). Therefore, it can be inferred that phosphorylation on S92 can break the interaction between the S92 and D119.
Effects of S92 phosphorylation on the dimerization of the MCU-EMRE tetramer
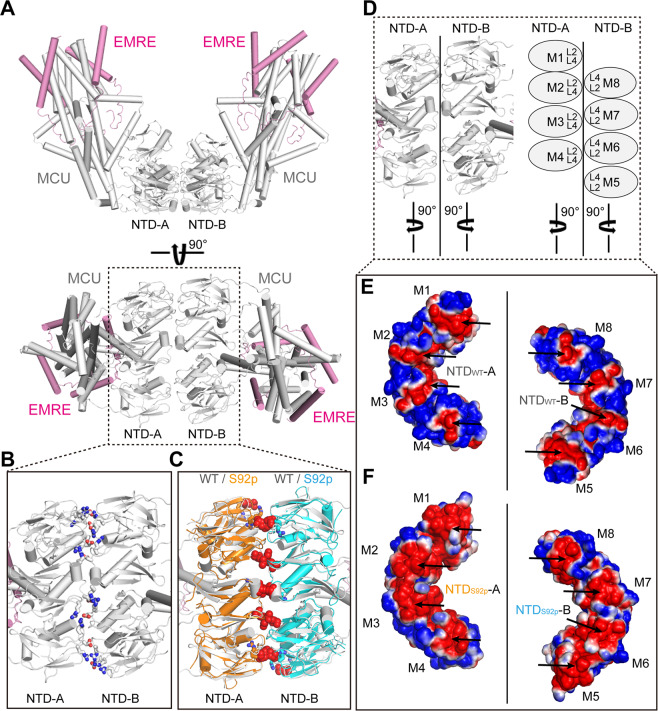
Upon examination of the structures of the MCU NTDS92E, MCU NTDD119A, and MD simulated S92p models, we expected that S92 phosphorylation in the MCU induces conformational changes as well as enhancements in the negative charges in the local L2-L4 loops (Figs. 2 and 4). Wang et al. recently reported that the tetrameric MCU-EMRE channels underwent extensive interactions with each other resulting in the formation of dimers at the MCU NTDs, including the L2-L4 loops (Fig. 5A,B,D). Additionally, the MCU-EMRE channel interactions formed a V-shaped tetrameric MCU-EMRE dimer. Moreover, a single mutation, D123R, in the L4 loop of the MCU NTD abolished the dimerization of the two MCU-EMRE channels, possibly by disrupting the electrostatic interactions with the neighboring arginine residues, R93 and R12419. To elucidate the effect of S92p on the dimerization of tetrameric MCU-EMRE, we compared the binding energy difference for the dimerization of the tetramer between WT and S92p NTDs using the PRODIGY web server45. The binding free energy of NTD S92p (−7.4 kcal/mol) was higher than that of WT (−10.5 kcal/mol), suggesting that the conformational changes and enhancement of negative charges by S92 phosphorylation may affect the dimerization of two MCU-EMRE channels (Fig. 5C–F).
Figure 5.
Structural comparison between the NTDWT and the NTD S92-phosphorylated (NTDS92p) model in the dimerization of two MCU-EMRE channels. (A) The overall structure of two MCU-EMRE channels (PDB ID: 6O58). MCU and EMRE are shown in grey and magenta colored ribbons, respectively. (B) Detailed view of the interacting surfaces of NTD-A and NTD-B in dimer of the two MCU-EMRE channels. The residues (R93, D123, and R124) forming salt bridges and hydrogen bonds are shown in sticks. (C) Detailed view of the superimposed MCU NTDS92p MD simulated model structures (10-nsec snapshot) onto NTDWT-A or -B of the two MCU-EMRE channel complexes. The MCU NTDS92p residues that are expected to disrupt salt bridges and hydrogen bonds (R93, D123, and R124) are depicted as orange sticks and cyan sticks, respectively. Atomic clashes between NTD-A and NTD-B of the MCU NTDS92p are denoted by red spheres. (D‒F) Differences between the electrostatic surface charges of the interacting interfaces of the MCU NTDWT -A or -B (M1‒M8) superimposed on the MCU NTDS92p -A or -B (M1‒M8). Enhancement of negative charges in the MCU NTDS92p are highlighted with black arrows. Blue surfaces and red surfaces in the NTDWT and in the MD simulated NTDS92p indicate positive and negative charges, respectively.
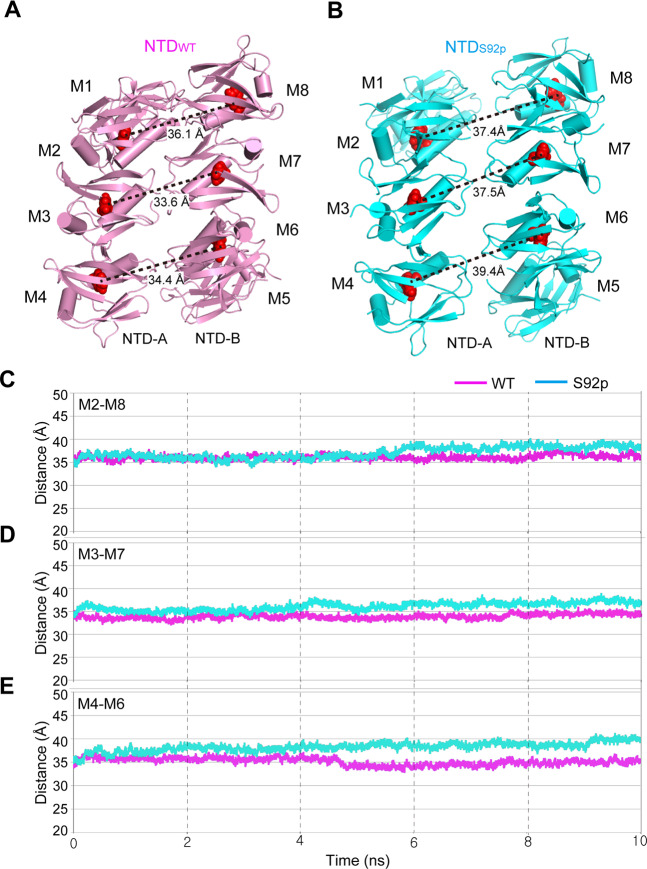
To confirm this hypothesis, we performed MD simulations for the NTDWT and NTDS92p octamer structures. To compare the distance between the two tetramers (NTD-A and NTD-B), the three key monomer pair distances (M2-M8, M3-M7, and M4-M6) were monitored during 10 ns simulations times (Fig. 6). The snapshot structures at 10 ns (Fig. 6A,B) and the distance trajectory during the MD simulation (Fig. 6C–E) show that the distances between the paired monomers in NTDS92p were significantly increased compared to that of the NTDWT by approximately 1.5 to 5 Å. It suggests that the additional negative charges from the phosphate group might contribute to push each tetramers (NTD-A and NTD-B) away. Overall, our MD simulation studies suggest that the S92 phosphorylation can weaken dimerization of the MCU-EMRE tetramer.
Figure 6.
Distance comparison between NTDWT and NTDS92p octamers using MD simulation structures. (A-B) The 10 ns-snapshot structures of NTDWT (A) and NTDS92p octamers (B) are shown in pink and cyan, respectively. Each of the eight monomers are designated as M1 − M8. The distances between the center of monomer were calculated. (C-E) The three key monomer pair distances in the central region of the octamer were measured during 10 ns simulations times: M2-M8 (C), M3-M7 (D), and M4-M6 (E). The Cα atom of F111 (red sphere) located at the nearest position of the center of monomer was selected for the distance measurement.
Discussion
MCU activity is modulated by its regulatory proteins, including MICU1, MICU2, MCUb, EMRE, and MCUR1, as well as post-translational modifications such as phosphorylation2,7,8,20,21. In addition, the MCU NTD plays pivotal roles in MCUR1 interaction, MCUb NTD interaction, Mg2+ binding selectivity, phosphorylation, redox sensor, oligomerization of MCU-EMRE channel complexes, and regulation of MCU Ca2+ uptake activity6,7,17–19. Thus, we believe functional roles of MCU NTD for its Ca2+ uptake activity warrants further investigation, although recent studies of NTD deletion of MCU appears to be functionally dispensable in mitochondrial Ca2+ uptake19,46
Protein kinases can be localized in the sarcoplasmic reticulum (SR) and mitochondria, and modulate function of Ca2+ channels by phosphorylation. Phosphorylation of Ca2+ channels containing ryanodine receptor 2 and inositol 1,4,5-trisphosphate receptors regulates Ca2+ release in the SR through PKA and CaMKII20,47–51. The MCU is directly phosphorylated by Pyk2 and AMPK and phosphorylated MCU facilitates Ca2+ entry into the mitochondria20,21. The conserved S92 in the MCU is a putative recognition site (89-RLPS-92; RxxS motif) for phosphorylation by Ser/Thr kinases such as CaMKII, PKA, and PKC on the basis of prediction of KinasePhos 2.0 server and GPS 2.0 software31–35. Nguyen et al. observed S92 phosphorylation of MCU by CaMKII using MCU S92 phospho-specific antibody in vivo25, although the regulatory functions of MCU activity by CaMKII still remain controversial22–26. Instead, our in vitro kinase assay results indicated MCU S92 was phosphorylated by PKC isoforms (PKCβII, PKCδ, and PKCε) localized in the mitochondrial matrix, but was not phosphorylated by PKA (Fig. 1D,F)27,28. Further studies are needed to understand the functional roles of MCU NTD phosphorylation by PKC isoforms.
Free radicals, such as ROS and reactive nitrogen species (RNS), generate in a well-modulated manner to maintain cellular homeostasis as signalling second messengers, and play critical roles in the activation of enzymes and alteration of lipids, protein, and DNA52,53. Under physiological conditions, the MCU uptakes Ca2+ ions into the matrix; Ca2+ ions play an essential role modulating ATP synthesis through TCA cycle and the electron transfer chain, and finally induce ROS production as by-products in the mitochondria54. However, continuous overload of mitochondrial Ca2+ entry can produce large amounts of ROS and eventually lead to apoptotic or necrotic cell death54. Upon production of ROS, PKCβII, PKCδ, and PKCε translocate to the mitochondria and modulate functions of enzymes and Ca2+ channels by Ser/Thr phosphorylation, as well as ROS production27–29. In our studies, we observed that PKCβII, PKCδ, and PKCε phosphorylated the MCUS92 in vitro. We speculate that regulation of the S92 phosphorylation by the PKC isoforms under physiological conditions play important roles in ROS homeostasis or programmed cell death by excessive ROS, driven by the MCU Ca2+ uptake. Additional experimental evidence will be required to clarify the functional roles of PKC isoforms in the MCU29–31.
In conclusion, we identified that the PKC isoforms, PKCβII, PKCδ, and PKCε, are capable of phosphorylating S92 in the MCU NTD. We also characterized local conformational changes in our structural determination of MCU NTDS92E and NTDD119A as well as in MD simulation analysis of the WT and S92p. The conformational changes and enhancement of negative charge of the L2-L4 loops in the MCU NTD by S92 phosphorylation may be essential for regulating MCU activity, despite there lacks of functional data for the MCU activity modulation by S92 phosphorylation. Further studies are required to reveal the functional effects of MCU S92 phosphorylation by the PKC. The results provide a framework for further studies investigating the functional and structural roles of MCU phosphorylation by PKC.
Materials and Methods
DNA constructs
For structural studies, human MCU NTD (residues 75 − 165), including N-terminal His6-bacteriophage T4 lysozyme (residues 2 − 161; triple mutants of D20N/C54T/C97A)55,56 in the modified pET21a vector (Novagen), was constructed as previously described6. Point mutagenesis using polymerase chain reaction (PCR) was performed to construct the S92E or D119A mutants. For the in vitro kinase assay of the MCU NTD S92, we designed all Ser/Thr to Ala mutants (T76, S87, S92, T100, S105, S107, S129, S138, T139, and T157; MCU NTDAA). PCR was used to synthesize the MCU NTDAA construct using 12 oligonucleotides; then, MCU NTDAAS92 was generated using the A92S mutation from the MCU NTDAA.
Purification of MCU constructs
The T4 lysozyme-MCU NTDS92E or T4 lysozyme-MCU NTDD119A was purified using the same method for T4 lysozyme-MCU NTDWT as previously described6. The proteins were expressed in the Escherichia coli strain BL21-CodonPlus (DE3), followed by purification using Ni-NTA affinity and size exclusion chromatography (SEC) on a HiLoad 16/60 Superdex 75 column (GE Healthcare Life Science). The samples were concentrated by centrifugation using Amicon Ultra-15 10 K filter units (Millipore) to 5 mg mL−1 in final buffer containing 20 mM Tris-HCl (pH 8.0), 50 mM NaCl, 5% (v/v) glycerol, and 1 mM DTT.
For in vitro kinase assays, MCU NTDWT and MCU NTDAAS92 were purified by a similar procedure using the following buffers: lysis buffer [50 mM Tris-HCl (pH 8.0), 500 mM NaCl, 10 mM imidazole, 5% (v/v) glycerol, 1 mM PMSF, 1 mM β-mercaptoethanol], wash buffer [50 mM Tris-HCl (pH 8.0), 500 mM NaCl, 40 mM imidazole, 5% (v/v) glycerol], and elution buffer [50 mM Tris-HCl (pH 8.0), 500 mM NaCl, 500 mM imidazole, 5% (v/v) glycerol]. The samples were then purified using SEC on a HiLoad 16/60 Superdex 75 column (GE Healthcare Life Science) pre-equilibrated with a buffer [50 mM Tris-HCl (pH 7.5) and 150 mM NaCl]. Then, the fractions containing human MCU NTDWT and MCU NTDAAS92 were collected. The protein was concentrated using an Amicon Ultra-15 10 K filter unit (Millipore) at a concentration of 0.3 mg mL−1. Final human MCU NTD proteins were stored at −80 °C.
In vitro kinase assays
Six micrograms (6 μg) of purified MCU NTDWT, 20 μg of purified MCU NTDAAS92, and 32 μg of commercially obtained MBP (Enzo, ALX-202-075) were phosphorylated by PKA (Promega, V5161), PKC mixtures (α, β, and γ isoforms with lesser δ and ζ; Promega, V5261), PKCβII (Promega, V3741), PKCδ (Promega, V3401), and PKCε (Promega, V4036). In vitro phosphorylation of PKA was performed in 25 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 2 mM DTT, 5 mM β-glycerophosphate, 0.1 mM Na3VO4, 0.2 mM Mg-ATP, and 3 pmol of [γ-32P]ATP (3000 Ci/mmol) with 20 ng PKA for MBP and 100 ng PKA for MCU NTDWT and MCU NTDAAS92. In vitro phosphorylation assays of PKC mixtures, PKCβII, PKCδ, and PKCε were performed in 1 × reaction buffer A (SignalChem, K03-09) [20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 0.02% (v/v) Tween-20], 2 mM DTT (SignalChem, D86-09B), 1 × PKC lipid activator (SignalChem, L51-39), 0.2 mM Mg-ATP, and 3 pmol of [γ-32P]ATP (3000 Ci/mmol) with 100 ng PKC mixtures, PKCβII, PKCδ, and PKCε for MBP and 100 ng PKC, 50 ng PKCβII, 200 ng PKCδ, and 200 ng PKCε for MCU NTDWT, and MCU NTDAAS92. All in vitro kinase assays were performed at 30 °C for 60 min. The reaction was halted by the addition of SDS-PAGE sample buffer. Then, reaction samples were resolved by SDS-PAGE and visualized by autoradiography.
Crystallization
The T4 lysozyme-MCU NTDS92E or T4 lysozyme-MCU NTDD119A was crystalized using the same method for T4 lysozyme-MCU NTDS92A as previously described6. Crystals of T4 lysozyme-MCU NTDS92E or T4 lysozyme-MCU NTDD119A were produced using the hanging drop vapour diffusion and microseeding method, using T4 lysozyme-MCU NTDWT crystals as seeds in the reservoir solution containing 20% (w/v) polyethylene glycol (PEG) 3350, 5% (v/v) glycerol, 0.3 M (NH4)2SO4, and 0.1 M Bis-Tris-HCl (pH 5.5). Once the microcrystals (<0.01 − 0.02 mm) of the T4 lysozyme-MCU NTDWT grew at 20 °C, 2 μL of T4 lysozyme-MCU NTDS92E or T4 lysozyme-MCU NTDD119A proteins and 2 μL of the reservoir solution were added directly to the 1 μL drop containing T4 lysozyme-MCU NTDWT seed crystals. The final T4 lysozyme-MCU NTDS92E or NTDD119A crystals were grown at 20 °C in 5 μL mixtures containing the WT and the S92E or D119A mutant at a 1:4 molar ratio. The crystals were directly flash-frozen in liquid nitrogen.
Data collection, structure determination, and refinement
Diffraction data of T4 lysozyme-MCU NTDS92E or T4 lysozyme-MCU NTDD119A crystals were collected at 100 K using synchrotron X-ray sources on beamlines 5 C at the Pohang Acceleratory Laboratory (PAL) (Pohang, South Korea). We finally collected diffraction data for T4 lysozyme-MCU NTDS92E at a resolution of 2.50 Å and for T4 lysozyme-MCU NTDD119A at 2.85 Å using a single wavelength, 0.9795 Å. The diffraction data were processed using the HKL2000 suite57. Molecular replacement was carried out using Phaser in the CCP4 suite58, using the structures of the bacteriophage T4 lysozyme (PDB ID: 2LZM) and MCU NTD (PDB ID: 4XTB) as templates. The obtained models were subjected to iterative rounds of model building and refinement using programs Coot59 and REFMAC5 in CCP4 suite58. The details of data collection and refinement statistics are provided in Table 1.
Structural analysis
All structural figures were generated using PyMOL version 1.5.0.4 (Schrödinger LLC). The amino acid sequence and protein surface conservation of the MCU NTD were calculated using the ConSurf server30. The CCP4 program LSQKAB was used to superimpose the structures of MCU NTDWT (PDB ID: 4XTB), MCU NTDS92E, and MCU NTDD119A and to estimate RMSD (Å) scores of Cα atoms58. The electrostatic surface charges of MCU NTDs (WT, S92E, S92p, and D119A) were analysed using the PDB2PQR server60 and visualized using PyMOL version 1.5.0.4 (Schrödinger LLC).
Ensemble refinement
Ensemble refinement for T4 lysozyme-MCU NTDWT (PDB ID: 4XSJ), T4 lysozyme-MCU NTDS92E, and T4 lysozyme-MCU NTDD119A was performed using structures and structural factors by phenix.ensemble_refinement41. Default parameters were used in the phenix.ensemble_refinement, including pTLS = 0.8 and Tbath = 5 K, and solvent updated every 25 cycles. The simulations have an equilibration phase (10τx) in which the temperature, X-ray weight and averaged structure factors stabilize, followed by an acquisition phase (10τx). The output structures by ensemble refinement were visualized using PyMOL version 1.5.0.4 (Schrödinger LLC) with a script ‘ens_tool.py’. The RMSF difference histogram for the MCU NTDWT and mutants (S92E and D119A) was plotted using SigmaPlot 12.
Molecular dynamic simulations
Four molecular dynamics (MD) simulations of NTDWT and NTDS92p monomer and octamer structures were carried out using GROMACS (GROningen Machine for Chemical Simulations) 2018.4 package61–63 with amber99sb-star-ILDNP force field64. Molecular topologies for phosphorylated S92 were generated by AnteChamber Python Parser interface (ACPYPE) with generalized AMBER force field 2 (GAFF2)65,66. All four systems were solvated with TIP3P water molecules67 in a dodecahedron box and Na+ counter ions were added to neutralize the net changes of the systems by replacing water molecules. In all cases, bond lengths were constrained with LINCS68 and long-range electrostatics were calculated using the smooth particle mesh Ewald (PME) method with a cut-off of 1.0 nm69,70. A cut-off of short-range non-bonded interactions, van der Waals (vdW), were truncated at 1.0 nm. All MD simulations were conducted energy minimization using the steepest descent method. Equilibration was then performed in two phases, during which position restraints applied to all heavy atoms of the protein. First, the simulations were run under NVT conditions at 300 K, using Berendsen’s coupling algorithm71 for 100 ps. The second phase of equilibration was carried out an NPT ensemble for 100 ps, using the Nose-Hoover thermostat72,73 and the Parrinello-Rahman barostat74,75 with coupling time constants of 2.0 ps and 5.0 ps to maintain 300 K and 1 bar, respectively. Production MD was then conducted for 10 ns without any restraint and under the same conditions as the NPT ensemble. All analyses of MD simulation results were performed using the analysis tools in the GROMACS package.
Accession numbers
Atomic coordinates and structure factors of T4 lysozyme-MCU NTDS92E and T4 lysozyme-MCU NTDD119A have been deposited in the PDB with the accession numbers, 6JG0 and 6KVX, respectively.
Supplementary information
Acknowledgements
We thank the staff at the beamline BL-5C and 7 A of the Pohang Accelerator Laboratory (PAL; Pohang, South Korea) for their kind help with data collection. This work was supported by the GIST Research Institute (GRI) ARI grant funded by the GIST in 2020 and by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) & funded by the Korean government (MSIT) (No. NRF-2018M3A9A7057263).
Author contributions
S.H.E. and Y.L. planned and organized the experiments. Y.L. performed purification, crystallization, collection of X-ray diffraction data, structure determination and analysis, in vitro kinase assays, ensemble refinement, and data analysis. P.J. performed purification, collection of X-ray diffraction data, and data analysis. G.L., S.Y., and K.W.L. performed the molecular dynamics simulation analyses. Y.L. and P.J. carried out gene cloning and expression. T.G.K., T.Y., C.K.M., and D.H.K. contributed to experimental design and data interpretation. S.H.E., Y.L., and K.W.L. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Youngjin Lee and Jongseo Park.
Contributor Information
Youngjin Lee, Email: yjlee86@kribb.re.kr.
Soo Hyun Eom, Email: eom@gist.ac.kr.
Supplementary information
is available for this paper at 10.1038/s41598-020-65994-y.
References
- 1.Chaudhuri D, Artiga DJ, Abiria SA, Clapham DE. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proc Natl Acad Sci USA. 2016;113:E1872–1880. doi: 10.1073/pnas.1602264113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallilankaraman K, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomar D, et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016;15:1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren T, et al. MCUR1-Mediated Mitochondrial Calcium Signaling Facilitates Cell Survival of Hepatocellular Carcinoma via Reactive Oxygen Species-Dependent P53 Degradation. Antioxid Redox Signal. 2018;28:1120–1136. doi: 10.1089/ars.2017.6990. [DOI] [PubMed] [Google Scholar]
- 5.Jin M, et al. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. Journal of experimental & clinical cancer research: CR. 2019;38:136. doi: 10.1186/s13046-019-1135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, et al. Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter. EMBO Rep. 2015;16:1318–1333. doi: 10.15252/embr.201540436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Z, et al. Mitochondrial Ca(2+) Uniporter Is a Mitochondrial Luminal Redox Sensor that Augments MCU Channel Activity. Mol Cell. 2017;65:1014–1028 e1017. doi: 10.1016/j.molcel.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patron M, Granatiero V, Espino J, Rizzuto R, De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26:179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun. 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madreiter-Sokolowski CT, et al. PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake in immortalized cells. Nat Commun. 2016;7:12897. doi: 10.1038/ncomms12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchi S, et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan X, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong JQ, et al. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luongo TS, et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, et al. Molecular characterization of the N-terminal domains of the human mitochondrial calcium uniporter (MCU) and its paralog, MCUb. Biodesign. 2015;3:175–184. [Google Scholar]
- 18.Lee SK, et al. Structural Insights into Mitochondrial Calcium Uniporter Regulation by Divalent Cations. Cell Chem Biol. 2016;23:1157–1169. doi: 10.1016/j.chembiol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Structural Mechanism of EMRE-Dependent Gating of the Human Mitochondrial Calcium Uniporter. Cell. 2019;177:1252–1261 e1213. doi: 10.1016/j.cell.2019.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O-Uchi Jin, Jhun Bong Sook, Xu Shangcheng, Hurst Stephen, Raffaello Anna, Liu Xiaoyun, Yi Bing, Zhang Huiliang, Gross Polina, Mishra Jyotsna, Ainbinder Alina, Kettlewell Sarah, Smith Godfrey L., Dirksen Robert T., Wang Wang, Rizzuto Rosario, Sheu Shey-Shing. Adrenergic Signaling Regulates Mitochondrial Ca2+ Uptake Through Pyk2-Dependent Tyrosine Phosphorylation of the Mitochondrial Ca2+ Uniporter. Antioxidants & Redox Signaling. 2014;21(6):863–879. doi: 10.1089/ars.2013.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, et al. AMPK-mediated activation of MCU stimulates mitochondrial Ca(2+) entry to promote mitotic progression. Nat Cell Biol. 2019;21:476–486. doi: 10.1038/s41556-019-0296-3. [DOI] [PubMed] [Google Scholar]
- 22.Joiner ML, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correll RN, Molkentin JD. CaMKII does it again: even the mitochondria cannot escape its influence. Circ Res. 2013;112:1208–1211. doi: 10.1161/CIRCRESAHA.113.301263. [DOI] [PubMed] [Google Scholar]
- 24.Fieni F, Johnson DE, Hudmon A, Kirichok Y. Mitochondrial Ca2+ uniporter and CaMKII in heart. Nature. 2014;513:E1–2. doi: 10.1038/nature13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen EK, et al. CaMKII (Ca(2+)/Calmodulin-Dependent Kinase II) in Mitochondria of Smooth Muscle Cells Controls Mitochondrial Mobility, Migration, and Neointima Formation. Arterioscler Thromb Vasc Biol. 2018;38:1333–1345. doi: 10.1161/ATVBAHA.118.310951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickel Alexander G., Kohlhaas Michael, Bertero Edoardo, Wilhelm Daniel, Wagner Michael, Sequeira Vasco, Kreusser Michael M., Dewenter Matthias, Kappl Reinhard, Hoth Markus, Dudek Jan, Backs Johannes, Maack Christoph. CaMKII does not control mitochondrial Ca 2+ uptake in cardiac myocytes. The Journal of Physiology. 2020;598(7):1361–1376. doi: 10.1113/JP276766. [DOI] [PubMed] [Google Scholar]
- 27.Voris JP, et al. Functional alterations in protein kinase C beta II expression in melanoma. Pigment Cell Melanoma Res. 2010;23:216–224. doi: 10.1111/j.1755-148X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 28.Kornfeld OS, et al. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res. 2015;116:1783–1799. doi: 10.1161/CIRCRESAHA.116.305432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim S, et al. Regulation of mitochondrial functions by protein phosphorylation and dephosphorylation. Cell Biosci. 2016;6:25. doi: 10.1186/s13578-016-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landau M, et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005;33:W299–302. doi: 10.1093/nar/gki370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo MG, et al. Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J Cell Physiol. 2011;226:236–247. doi: 10.1002/jcp.22335. [DOI] [PubMed] [Google Scholar]
- 32.Mah AS, et al. Substrate specificity analysis of protein kinase complex Dbf2-Mob1 by peptide library and proteome array screening. BMC Biochem. 2005;6:22. doi: 10.1186/1471-2091-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher TL, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaffe MB, Elia AE. Phosphoserine/threonine-binding domains. Curr Opin Cell Biol. 2001;13:131–138. doi: 10.1016/s0955-0674(00)00189-7. [DOI] [PubMed] [Google Scholar]
- 35.Yaffe MB, Smerdon SJ. The use of in vitro peptide-library screens in the analysis of phosphoserine/threonine-binding domain structure and function. Annu Rev Biophys Biomol Struct. 2004;33:225–244. doi: 10.1146/annurev.biophys.33.110502.133346. [DOI] [PubMed] [Google Scholar]
- 36.Sardanelli AM, et al. Topology of the mitochondrial cAMP-dependent protein kinase and its substrates. FEBS Lett. 1996;396:276–278. doi: 10.1016/0014-5793(96)01112-x. [DOI] [PubMed] [Google Scholar]
- 37.Mishra S, Gray CB, Miyamoto S, Bers DM, Brown JH. Location matters: clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ Res. 2011;109:1354–1362. doi: 10.1161/CIRCRESAHA.111.248401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martenson RE, Law MJ, Deibler GE. Identification of multiple in vivo phosphorylation sites in rabbit myelin basic protein. J Biol Chem. 1983;258:930–937. [PubMed] [Google Scholar]
- 39.Ulmer JB, Edwards AM, McMorris FA, Braun PE. Cyclic AMP decreases the phosphorylation state of myelin basic proteins in rat brain cell cultures. J Biol Chem. 1987;262:1748–1755. [PubMed] [Google Scholar]
- 40.Cicirelli MF, Pelech SL, Krebs EG. Activation of multiple protein kinases during the burst in protein phosphorylation that precedes the first meiotic cell division in Xenopus oocytes. J Biol Chem. 1988;263:2009–2019. [PubMed] [Google Scholar]
- 41.Burnley BT, Afonine PV, Adams PD, Gros P. Modelling dynamics in protein crystal structures by ensemble refinement. Elife. 2012;1:e00311. doi: 10.7554/eLife.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Po HN, SenoZan NM. Henderson-Hasselbalch Equation: Its History and Limitations. Journal of Chemical Education. 2001;78:1499–1503. [Google Scholar]
- 44.Porcelli AM, et al. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- 45.Xue LC, Rodrigues JP, Kastritis PL, Bonvin AM, Vangone A. PRODIGY: a web server for predicting the binding affinity of protein-protein complexes. Bioinformatics. 2016;32:3676–3678. doi: 10.1093/bioinformatics/btw514. [DOI] [PubMed] [Google Scholar]
- 46.Oxenoid K, et al. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533:269–273. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 48.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 49.Wojcikiewicz RJ, Luo SG. Phosphorylation of inositol 1,4,5-trisphosphate receptors by cAMP-dependent protein kinase. Type I, II, and III receptors are differentially susceptible to phosphorylation and are phosphorylated in intact cells. J Biol Chem. 1998;273:5670–5677. doi: 10.1074/jbc.273.10.5670. [DOI] [PubMed] [Google Scholar]
- 50.Vanderheyden V, et al. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 52.Devasagayam TP, et al. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 53.Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 55.Pjura PE, Matsumura M, Wozniak JA, Matthews BW. Structure of a thermostable disulfide-bridge mutant of phage T4 lysozyme shows that an engineered cross-link in a flexible region does not increase the rigidity of the folded protein. Biochemistry. 1990;29:2592–2598. doi: 10.1021/bi00462a023. [DOI] [PubMed] [Google Scholar]
- 56.Tong J, et al. Crystallization and preliminary X-ray crystallographic analysis of the oxysterol-binding protein Osh3 from Saccharomyces cerevisiae. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68:1498–1502. doi: 10.1107/S1744309112042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 58.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 60.Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Der Spoel D, et al. GROMACS: fast, flexible, and free. J Comput Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 62.Bekker H, et al. Gromacs: A parallel computer for molecular dynamics simulations. Phys Comput. 1993;29:252–256. [Google Scholar]
- 63.Abraham MJ, et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1:19–25. [Google Scholar]
- 64.Aliev AE, et al. Motional timescale predictions by molecular dynamics simulations: case study using proline and hydroxyproline sidechain dynamics. Proteins. 2014;82:195–215. doi: 10.1002/prot.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sousa da Silva AW, Vranken WF. ACPYPE - AnteChamber PYthon Parser interfacE. BMC Res Notes. 2012;5:367. doi: 10.1186/1756-0500-5-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J Comput Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 67.Jorgensen WL, Chandrasekhar J, Madura JD. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 68.Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: A linear constraint solver for molecular simulations. Journal of Computational Chemistry 18, 1463-1472, doi:10.1002/(sici)1096-987x(199709)18:12<1463::Aid-jcc4>3.0.Co;2-h (1997).
- 69.Darden T, York DM, Pedersen LG. Particle mesh Ewald: AnN⋅log(N) method for Ewald sums in large systems. The. Journal of Chemical Physics. 1993;98:10089–10092. [Google Scholar]
- 70.Essmann Ulrich, Perera Lalith, Berkowitz Max L., Darden Tom, Lee Hsing, Pedersen Lee G. A smooth particle mesh Ewald method. The Journal of Chemical Physics. 1995;103(19):8577–8593. [Google Scholar]
- 71.Berendsen HJ, Postma JV, van Gunsteren WF, DiNola ARHJ, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 72.Nosé S. A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys. 1984;81:511–519. [Google Scholar]
- 73.Hoover WG. Canonical dynamics: Equilibrium phase-space distributions. Phys Rev A Gen Phys. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 74.Parrinello M, Rahman A. Polymorphic transitions in single crystals: A new molecular dynamics method. J Appl Phys. 1981;52:7182–7190. [Google Scholar]
- 75.Nosé S, Klein ML. Constant pressure molecular dynamics for molecular systems. Mol Phys. 1983;50:1055–1076. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.