Highlights
-
•
Disseminated M. szulgai infections invade bone, skin and lymph node more common instead of pulmonary involvement.
-
•
The disseminated infection is associated with immunocompromised status or hematologic malignancies.
-
•
Neutralizing anti–interferon-γ autoantibodies are commonly detected in Asian adults with disseminated NTM infections.
-
•
Most patients respond to rifampin and ethambutol-based combination regimens.
Keywords: Disseminated mycobacterial infection, Mycobacterium szulgai, Anti-interferon-gamma autoantibodies
Abstract
Incidence of nontuberculous mycobacterial infections has increased during the past decades. Disseminated infections are relatively rare and associated with immunocompromised status. We report a case of disseminated Mycobacterium szulgai infection of cervical lymphadenitis and pulmonary involvement with positive anti-interferon-gamma autoantibodies. The patient was successfully treated with rifampin, ethambutol, and clarithromycin. The case reports and series through search engines of Pubmed and Google with the keyword of disseminated infection of M. szulgai were reviewed. Fifteen patients of disseminated M. szulgai infection were reviewed and included. DisseminatedM. szulgaiinfection involves bone, skin and lymph node more common instead of pulmonary involvement, and most are associated with immunocompromised status with neoplastic hematologic disorders. In patients with disseminated M. szulgai infection, long term anti-mycobacterial agents are necessary. Most patients will respond to rifampin and ethambutol combination regimens.
Introduction
Incidence of nontuberculous mycobacterial (NTM) infections has significantly increased during the past decades [[1], [2], [3], [4], [5]]. Male patients older than 50 years with alcohol abuse, COPD, smoking or previous pulmonary tuberculosis are associated with high risks of infection [[6], [7], [8]]. Mycobacterium szulgai is a scotochromogen of slow-growing NTM. It was first isolated in 1972 [1] and to be a rare pathogen in human beings [6,9,10], usually causing pulmonary infection similar to that caused by M. tuberculosis [11,12]. Due to increasing reports of clinical disease related to M. szulgai, isolation of M. szulgai should be considered a significant pathogen.
The diagnosis of M. szulgai infection should be considered when it is isolated [12]. Disseminated M. szulgai infection is rarely reported. In the article, we summarize the literature on M. szulgai infection with disseminated presentations.
Case report
A 71-year-old man with left nasal alar melanoma post wide excision with a nasolabial flap was admitted due to progressive left side costal pain associated with dyspnea. Physical examination revealed crackle breath sounds over the left lower lung field. A chest X-ray revealed accumulated left pleural effusion and a chest CT scan showed left loculated pleural effusion with pleural thickening. Video-assisted thoracic surgery (VATS) with left side decortication was performed due to poor response to piperacillin/tazobactam and imipenem/cilastatin. The pathological findings showed acute suppurative inflammation composed of dense neutrophilic and eosinophilic infiltration with extensive necrosis which indicated empyema and tissue culture did not yield bacterial or mycobacterial pathogens. Sputum and pleural effusion acid-fast stain also failed to identify specific pathogen. The patient received ciprofloxacin for pneumonia complicated with empyema. The fever episode subsided except dry cough and persistent pneumonia consolidation in the right upper lung field.
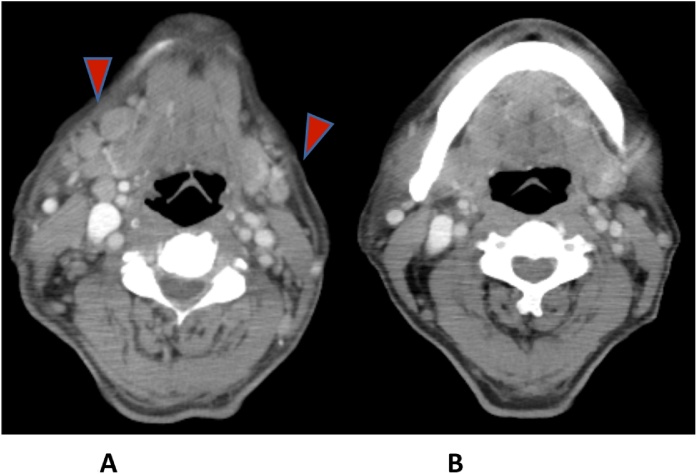
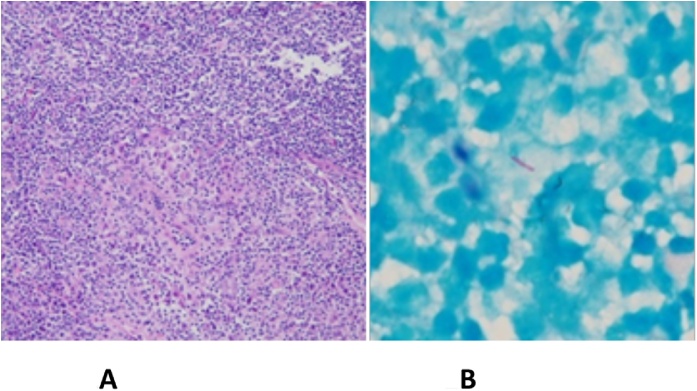
Progressive bilateral cervical lymphadenopathy was noticed by the patient one month after discharge (Fig. 1A). Additionally, abnormal granular nodules over nasopharynx were found by otolaryngologist during follow up for melanoma. Excisional biopsy of the left-side cervical lymph node was performed. The pathological report showed ill-defined granulomatous inflammation (Fig. 2A), and acid-fast positive bacilli was identified (Fig. 2B). M. szulgai was also grown from the cervical lymph node. M. szulgai was isolated from the following three sets of sputum. HIV screening was negative whereas anti-interferon-γ autoantibodies were positive.
Fig. 1.
(A) Computed tomography (CT) of head and neck revealed multiple lymphadenopathy over bilateral cervical areas (arrowheads); (B) Resolved cervical lymphadenopathy after 16-month anti-tuberculous therapies.
Fig. 2.
(A) Granulomatous formation with infiltrations of neutrophils and eosinophils in the left cervical lymph node biopsy (hematoxylin and eosin stain); (B) Acid-fast positive bacilli was found in the cervical lymph node.
Anti-mycobacterial combination therapies of clarithromycin, ethambutol, and rifampin were administered. Clinical improvement of cervical lymphadenitis was noted during follow up. Sputum culture for mycobacteria turned negative after five months of combination therapies. Follow up chest X-ray showed gradual resolution of consolidation over the right upper lung field. The patient is regularly followed up at the outpatient clinic on the therapy planned for 12 months after the sputum culture turns negative.
Literature review
M. szulgai infection is less common among NTM species. It presents with pulmonary involvement in most patients, indistinguishable with Mycobacterium tuberculosis [11,12]. Extrapulmonary infection of M. szulgai mainly included cutaneous infection, peripheral lymphadenitis, tenosynovitis, olecranon bursitis and osteomyelitis [7]. The disseminated infection had been rarely reported in immunocompromised patients. To identify the reported cases, we reviewed all case reports and case series through search engines of Pubmed and Google with the keyword of disseminated infection of M. szulgai. Disseminated infection was defined as involvements more than one site or organ of M. szulgai infection. The patients with a single site or organ involvement or absence of cultural evidence were excluded.
Results
Patient characteristics
Of fifteen cases with disseminated M. szulgai infection have been reported from 1984 to 2013. Twelve patients were male and three patients were female. The average age was 46 year old. There was a four year old boy who suffered from cutaneous infection and lymphadenitis. Among the ten male patients, the average age was 42 years old (Table 1). All three female patients were over 60 years old of age.
Table 1.
Characteristics of the fifteen patients with disseminated Mycobacterium szulgai infection.
| First author/ publish year |
Age/Sex | Comorbidity | Site of infection | Treatment/duration | Outcome | Reference | |
|---|---|---|---|---|---|---|---|
| 1 | Gur et al., 1984 | 18/M | Lymphocyte dysfunction | Bone Skin, LNs |
INH, RIF, EMB/> 2 Years | Persistent infection | [23] |
| 2 | Cross et al., 1985 | 51/M | Steroid use | Skin, bone | INH, RIF, EMB/ 24 months | Cure | [25] |
| 3 | Roig et al., 1993 | 67/M | HIV | Bone, kidney | Streptomycin, INH, Ethionamide/ unknown duration | ND | [30] |
| 4. | Luque et al., 1998 | 37/M | AIDS, HBV, HCV | Bone, lung, blood | INH, EMB, Clofazimine/ 5 months | Death due to cryptococcal meningitis, liver failure | [9] |
| 5 | Hurr and Sorg, 1998 | 68/F | Steroid use | Bone, LNs | Surgery INH, RIF / 1 month |
Cure | [8] |
| 6 | Fang et al., 1999 | 59/M | Chemotherapy | Bone, LNs | RIF, EMB, Ciprofloxacin/ 1 year; INH/ 6months | Cure | [26] |
| 7 | Nakada et al., 2001 | 64/F | MDS | Bone marrow, lung | Clarithromycin / unknown duration | Death due to myocardial infarction | [14] |
| 8 | Frisk et al., 2003 | 4/M | Leukemia status post bone marrow transplantation | Skin, LNs | RIF, EMB / 9 months | Cure | [27] |
| 9 | Tappe et al., 2004 | 36/M | AIDS | Skin, bone | Surgery Clarithromycin, EMB, Ciprofloxacin /1 year |
Cure | [32] |
| 10 | Kapur et al., 2004 | 27/M | Unknown immunosuppression | Skin, bone marrow | INH, EMB, Clarithromycin/ unknown duration |
Cure | [28] |
| 11 | Manalac et al., 2007 | 65/M | CLL, lymphoma | Multiple joints | I&D RIF, Levofloxacin, Clarithromycin/unknown duration |
Death due to respiratory failure | [15] |
| 12 | Meyer et al., 2008 | 66/F | CLL | Skin, bone | RIF, INH, EMB/ 2 years | Death due to SDH | [16] |
| 13 | Ohta et al., 2011 | 59/M | HBV carrier | Skin, lung | RIF, INH, PZA, Streptomycin/unknown duration | Cure | [29] |
| 14 | Riedel et al., 2012 | 59/M | AML | Bone marrow, LNs | No target treatment | Death | [13] |
| 15 | Shamriz et al., 2013 | 17/M | Partial STAT1 deficiency | Bone, LNs | INH, RIF, EMB, PZA/ 2 months; RIF, EMB, Azithromycin/ > 20 months | Cure | [31] |
HIV = human immunodeficiency virus; AIDS = acquired Immunodeficiency syndrome; MDS = myelodysplastic syndrome; HBV = hepatitis B virus; HCV = hepatitis C virus; CLL = chronic lymphocytic leukemia; AML=acute myeloid leukemia; STAT1=signal transducer and activator of transcription 1; LNs = lymph nodes; INH = isoniazid; RIF = rifampin; EMB = ethambutol; PZA = pyrazinamide; MI = myocardial infarction; SDH= subdural hematoma.
Site of infection
Among fifteen patients with disseminated M. szulgai infection, the most commonly involved sites of infection were bone with skin (four patients) and bone with lymph nodes (four patients), followed by bone and pulmonary involvement (two patients). For classification of involved sites, the bony involvement (12) was the most common, followed by skin (7) and lymph nodes (6) involvements. The results were significantly in contrast to single organ involvement in the general infected population with mainly pulmonary infection.
Among twelve male patients with disseminated M. szulgai infection, the most commonly involved sites or organs were bone (9), skin (5), and lymph nodes (5). Relatively rare infection sites included pulmonary involvement (2), joint (1), urinary tract (1) and bloodstream (1) involvement. Among three female patients, the most common infected sites were bone (3), respiratory tract (2), and skin (2). Besides, there is one case of solitary lymph node involvement.
Patient comorbidities
The comorbidities were commonly associated with immunocompromised status including malignancy, HIV infection or AIDS, HBV or HCV carrier, usage of immunosuppressive agents or uncategorized immunosuppression. Interestingly, the underlying malignancy were all associated with neoplastic hematologic disorders. There were two patients undertaken long term steroids and one patient had received a 7-day-course of chemotherapy with cyclophosphamide, prednisolone, and vincristine.
Treatment and prognosis
The optimal regimens for disseminated M. szulgai infection are uncertain during the past three decades [12]. Most patients received rifampin and ethambutol-base combination therapy in our literature review, but the treatment duration was uncertain. Surgical intervention or incision and drainage was done in some patients with bony and joint involvement. Most patients reached clinical improvement or even complete remission. One patient died 20 days after admission due to a postmortem diagnosis of M. szulgai infection without target treatment [13]. Another patient died of complications related to the co-infection of cryptococcal meningitis and liver failure without adequate antimicrobial therapy [9].
There were three cases of fatality during follow up, which were not caused by M. szulgai infection itself or disease activity. A 64 year old woman with pulmonary and bone marrow involvement died of myocardial infarction three months after resolution of M. szulgai infection [14]. A 65 year old man with disseminated joint involvement died of respiratory failure during the rehabilitation process [15]. Another 66 year old woman with skin and bone involvement of M. szulgai died from unrelated subdural hematoma two months after discontinuation of anti-mycobacterial therapy [16].
Discussion
M. szulgai infection is recognized as a significant pathogen causing clinical disease, although the disseminated infection is rare. The clinical, radiological and pathological presentations of M. szulgai infection are similar to M. tuberculosis infection [11,12], but there is no evidence proved for person-to-person transmission [17]. The diagnosis of M. szulgai infection is based on clinical presentation, radiological and cultural specimens for mycobacteria. In contrast to other NTM viewed as an environmental contaminant, identification of M. szulgai should be considered a significant pathogen [12]. However, the classic cultural system for mycobacteria species identification is time-consuming. Highly accurate nucleic acid probes are commercially available for isolation of M. szulgai and other NTM such as Mycobacterium avium complex (MAC) and M. kansasii within one day of identification [18].
Due to its rare prevalence, isolation of M. szulgai provides a pathological significance [12]. Pulmonary infection is the most common manifestation in a single site or organ involvement, and lymphadenitis, cutaneous or disseminated disease has been rarely reported [7]. However, among populations with disseminated M. szulgai in our study, the most commonly involved sites and organs included osteomyelitis, cutaneous involvement, and lymphadenopathy.
Browne et al. demonstrated that neutralizing anti–interferon-γ autoantibodies were detected in Asian adults with NTM infections [19]. Patients with anti–interferon-γ autoantibodies with impaired interferon-γ signaling were vulnerable to disseminated infections with intracellular pathogens, especially non-tuberculosis mycobacterium [[19], [20], [21]].
Our case had positive anti–interferon-γ autoantibodies which were considered to be associated with the adult-onset immunodeficiency syndrome, similar to that of advanced HIV infection. Treatment with EE-IFN-g might be worth investigating in patients producing anti–interferon-γ. Rituximab was under investigation for patients with relapsed anti–interferon-γ autoantibody-associated NTM infections [22].
Most patients reviewed in our study with disseminated M. szulgai infection responded well to rifampin and ethambutol based combination therapies [13]. Only one patient suffered from a persistent infection after at least a 24-month period of isoniazid, rifampin, and ethambutol [23].
Although M. szulgai is susceptible to most anti-mycobacterial agents, no optimal agents and duration of treatment were well established. Current guidance recommends the combination therapy with rifampin, ethambutol and a macrolide such as a clarithromycin for at least one year after sputum cultures turn negative [11,12,24]. Nevertheless, in vitro resistance to clarithromycin and ciprofloxacin have been reported [7,23,24].
Conclusion
For increasing reports of clinical diseases, the isolation of M. szulgai should be considered as a pathogen [12]. The patients with disseminated M. szulgai disease were relatively rare, and it was considered to be associated with adult-onset immunodeficiency syndrome. The combination therapies of anti-tuberculous agents should be administered for at least twelve months after the sputum culture turns negative.
Author contributions
N.Y.L and L.S.S conceived the study. N.Y.L and L.S.S provided data collection, N.Y.L and Z.P.W analyzed the data. N.Y.L and Z.P.W prepared the manuscript. All authors reviewed and edited the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
Financial support. This study was supported by the grants from National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH-10902060).
References
- 1.Marks J., Jenkins P.A., Tsukamura M. Mycobacterium szulgai--a new pathogen. Tubercle. 1972;53(3):210–214. doi: 10.1016/0041-3879(72)90018-9. [DOI] [PubMed] [Google Scholar]
- 2.Henkle E., Hedberg K., Schafer S. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc. 2015;12(5):642–647. doi: 10.1513/AnnalsATS.201412-559OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namkoong H., Kurashima A., Morimoto K. Epidemiology of pulmonary nontuberculous mycobacterial disease. Japan. Emerg Infec Dis. 2016;22(6):1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henkle E., Hedberg K., Schafer S.D. Surveillance of extrapulmonary nontuberculous mycobacteria infections, Oregon, USA, 2007-2012. Emerg Infect Dis. 2017;23(10):1627–1630. doi: 10.3201/eid2310.170845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y.C., Wu C.J., Ko P.S. Mycobacterial infections in adult recipients of allogeneic hematopoietic stem cell transplantation: a cohort study in a high endemic area. J Microbiol Immunol Infect. 2020;53(2):274–282. doi: 10.1016/j.jmii.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Benator D.A., Kan V., Gordin F.M. Mycobacterium szulgai infection of the lung: case report and review of an unusual pathogen. Am J Med Sci. 1997;313(6):346–351. doi: 10.1097/00000441-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Maloney J.M., Gregg C.R., Stephens D.S. Infections caused by Mycobacterium szulgai in humans. Rev Infect Dis. 1987;9(6):1120–1126. doi: 10.1093/clinids/9.6.1120. [DOI] [PubMed] [Google Scholar]
- 8.Hurr H., Sorg T. Mycobacterium szulgai osteomyelitis. J Infect. 1998;37(2):191–192. doi: 10.1016/s0163-4453(98)80178-3. [DOI] [PubMed] [Google Scholar]
- 9.Luque A.E., Kaminski D., Reichman R. Mycobacterium szulgai osteomyelitis in an AIDS patient. Scand J Infect Dis. 1998;30(1):88–91. doi: 10.1080/003655498750002376. [DOI] [PubMed] [Google Scholar]
- 10.Pozniak A., Bull T. Recently recognized mycobacteria of clinical significance. J Infect. 1999;38(3):157–161. doi: 10.1016/s0163-4453(99)90243-8. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Alarcos J.M., De Miguel-Díez J., Bonilla I. Pulmonary infection due to Mycobacterium szulgai. Respiration. 2003;70(5):533–536. doi: 10.1159/000074214. [DOI] [PubMed] [Google Scholar]
- 12.Griffith D.E., Aksamit T., Brown-Elliott B.A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 13.Riedel S., Dionne K., Ellis C. Mycobacterium szulgai: an unusual cause of disseminated mycobacterial infections. Infection. 2012;40(4):463–468. doi: 10.1007/s15010-011-0213-6. [DOI] [PubMed] [Google Scholar]
- 14.Nakada S., Sekikawa T., Takahara S. Nontuberculous atypical mycobacterial infection with progressive pancytopenia in a patient with myelodysplastic syndrome. Rinsho Ketsueki. 2001;42(7):543–548. [PubMed] [Google Scholar]
- 15.Christopher Manalac Tyrone, Hector Bonilla. Disseminated Mycobacterium szulgai infection: case report and review of literature. Infect Dis Clin Pract. 2007;15(5):341–344. [Google Scholar]
- 16.Meyer Jj, Gelman Ss. Multifocal osteomyelitis due to Mycobacterium szulgai in a patient with chronic lymphocytic leukemia. J Infect. 2008;56(2):151–154. doi: 10.1016/j.jinf.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Falkinham J.O., 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9(2):177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telenti A., Marchesi F., Balz M. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbio. 1993;31(2):175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browne S.K., Burbelo P.D., Chetchotisakd P. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valour F., Perpoint T., Sénéchal A. Interferon-gamma autoantibodies as predisposing factor for nontuberculous mycobacterial infection. Emerg Infect Dis. 2016;22(6):1124–1126. doi: 10.3201/eid2206.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki A., Sakagami T., Yoshizawa K. Clinical significance of interferon-γ neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis. 2018;66(8):1239–1245. doi: 10.1093/cid/cix996. [DOI] [PubMed] [Google Scholar]
- 22.Browne S.K., Zaman R., Sampaio E.P. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated. Blood. 2012;119(17):3933–3939. doi: 10.1182/blood-2011-12-395707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gur H., Porat S., Haas H. Disseminated mycobacterial disease caused by Mycobacterium szulgai. Arch Intern Med. 1984;144(9):1861–1863. [PubMed] [Google Scholar]
- 24.Newshan G., Torres Ra. Pulmonary infection due to multidrug-resistant Mycobacterium szulgai in a patient with AIDS. Clin Infect Dis. 1994;18(6):1022–1023. doi: 10.1093/clinids/18.6.1022-a. [DOI] [PubMed] [Google Scholar]
- 25.Cross G.M., Guill M.A., Aton J.K. Cutaneous Mycobacterium szulgai infection. Arch Dermatol. 1985;121(2):247–249. [PubMed] [Google Scholar]
- 26.Fang C.T., Chang S.C., Luh K.T. Successful treatment of disseminated Mycobacterium szulgai infection with ciprofloxacin, rifampicin, and ethambutol. J Infect. 1999;38(3):195–197. doi: 10.1016/s0163-4453(99)90252-9. [DOI] [PubMed] [Google Scholar]
- 27.Frisk P., Boman G., Pauksen K. Skin infection caused by Mycobacterium szulgai after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2003;31(6):511–513. doi: 10.1038/sj.bmt.1703861. [DOI] [PubMed] [Google Scholar]
- 28.Kapur N., Schuster H., Parker N. Severe sporotrichoid infection with Mycobacterium szulgai. Clin Exp Dermatol. 2004;29(4):377–379. doi: 10.1111/j.1365-2230.2004.01542.x. [DOI] [PubMed] [Google Scholar]
- 29.Ohta H., Miyauchi E., Ebina M. A case of cutaneous infection caused by Mycobacterium szulgai with progression to acute respiratory distress syndrome. Clin Med Insights Case Rep. 2011;4:29–33. doi: 10.4137/CCRep.S7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roig P., Nieto A., Navarro V. Mycobacteriosis from Mycobacterium szulgai in a patient with human immunodeficiency virus infection. An Med Interna. 1993;10(4):182–184. [PubMed] [Google Scholar]
- 31.Shamriz O., Engelhard D., Rajs A.P. Mycobacterium szulgai chronic multifocal osteomyelitis in an adolescent with inherited STAT1 deficiency. Pediatr Infect Dis J. 2013;32(12):1345–1347. doi: 10.1097/01.inf.0000437067.43859.4c. [DOI] [PubMed] [Google Scholar]
- 32.Tappe D., Langmann P., Zilly M. Osteomyelitis and skin ulcers caused by Mycobacterium szulgai in an AIDS patient. Scand J Infect Dis. 2004;36(11-12):883–885. doi: 10.1080/00365540410024736. [DOI] [PubMed] [Google Scholar]