Abstract
Diabetic retinopathy (DR) is a major cause of acquired blindness among working adults. The retinal pigment epithelium (RPE), constitutes an outer blood-retinal barrier, is vastly affected in diabetic humans and animals. Lower levels of lutein in the serum and retina of diabetic population, and beneficial effects of carotenoids supplementation in diabetic retinopathy patients created an interest to examine the protective effect of lutein on hyperglycemia-mediated changes in oxidative stress and antioxidant defense system in ARPE-19 cells. The WST-1 assay was performed to analyze the impact of glucose, and lutein on the viability of ARPE-19. The intracellular oxidative stress was measured by a DCF (dichlorofluorescein) assay, mitochondrial membrane potential (MMP) was monitored using a JC-10 MMP assay kit and GSH level was examined using GSH/GSSG ratio detection kit. The oxidative stress markers, protein carbonyl and malondialdehyde were spectrophotometrically measured using 2,4-dinitrophenylhydrazine and 2-thiobarbituric acid, respectively. The expression of endogenous antioxidant enzymes and regulatory proteins in ARPE-19 was quantified by western blotting. The localization of Nrf2 protein was examined by immunofluorescent staining. The results show that lutein (up to 1.0 μM) did not affect the viability of ARPE-19 grown in both normal and high-glucose conditions. Lutein treatment blocked high glucose-mediated elevation of intracellular ROS, protein carbonyl and malondialdehyde content in ARPE-19 cells. The decreased MMP and GSH levels observed in ARPE-19 grown under high-glucose condition were rescued by lutein treatment. Further, lutein protected high glucose-mediated down-regulation of a redox-sensitive transcription factor, Nrf2, and antioxidant enzymes, SOD2, HO-1, and catalase. This protective effect of lutein was linked with activated nuclear translocation of Nrf2, which was associated with increased activation of regulatory proteins such as Erk and AKT. Our study indicates that improving the concentration of lutein in the retina could protect RPE from diabetes-associated damage.
Keywords: Lutein, Hyperglycemia, ARPE-19, ROS, Redox signaling
Introduction
The Retinal Pigment Epithelium (RPE) is a tightly aligned array of monolayered pigmented epithelial cells which form the outer blood-retinal barrier (BRB). RPE is situated between photoreceptor’s outer segments and Bruch’s membrane that separates RPE from the fenestrated endothelium of choriocapillaris. It has several vital functions in retina, which include transportation of water, ions and metabolites (Hamann 2002; Sparrow et al. 2003), maintenance of photoreceptor health (Young and Bok 1969), metabolism and recycling of retinal (Bernstein et al. 1987), absorption of light and protection against photooxidation (Boulton and Dayhaw-Barker 2001), and secretion and synthesis of immunoregulatory molecules and growth factors (Sugasawa et al. 1994; Ishida et al. 2003). This ensures that the functional coordination between RPE and neighboring retinal cells is essential for the integrity of the retina. Therefore, it is plausible that any cellular or metabolic disruption in RPE can cause retinal dysfunction, which leads to loss of visual function. In diabetic retina, cellular and biochemical alterations in the RPE occur before vision loss or before diabetic retinopathy (DR) is clinically identified (Decanini et al. 2008; Xu and Le 2011). Also, recent report highlights that early RPE dysfunction is concomitant with hyperglycemia in mouse models of type 1 and type 2 diabetes (Desjardins et al. 2016).
Hyperglycemia is a well-known major cause of DR and other microvascular complications associated with diabetes. Thus, it is apparent that hyperglycemia-induced oxidative stress in RPE plays a significant role in diabetic retinopathy which in turn leads to subsequent development of vision impairment. Under diabetic condition, retinal cells including RPE cells experience increased oxidative stress due to sustained hyperglycemia, which disrupts the normal cellular functions leading to the development of retinopathy (Nita and Grzybowski 2016). The elevated reactive oxygen species (ROS) levels are considered as a potential contributor to increased oxidative stress in RPE (Cai et al. 2000). The mitochondrial over-production of superoxide followed by activation of at least four crucial pathological changes such as increased flux of the polyol pathway, PKC activation, increased production of AGEs and over-activation of the hexosamine pathway are proven to increase intracellular ROS production in hyperglycemic retina (Kowluru and Mishra 2015). On the other hand, diabetic retina experiences compromised antioxidant defense system; as a result, the condition of imbalance between intracellular ROS and antioxidants occurs, which leads to domination of the state of oxidative stress.
RPE possesses an effective defense system against oxidative damage, particularly rich in antioxidants such as vitamin C, α-tocopherol, β-carotene, macular pigments, and antioxidant enzymes. A previous study reported that endogenous defense system against oxidative damage in RPE cells protects well against damage to mitochondria and endoplasmic reticulum (Lu et al. 2006). There are substantial evidence from previous experimental studies indicate that Nrf2 (Nuclear factor-erythroid 2-related factor-2) is a crucial stress-response transcription factor which acts by triggering anti-oxidant response element (ARE) bearing genes that are critical for the intracellular redox homeostasis in many cell types (Nguyen et al. 2003; Wang et al. 2007; Dieter 2015). Several other studies have shown that this adaptive response by Nrf2 is significantly reduced in the later stage of diabetic mice as well as human subjects (Siewert et al. 2013; Bai et al. 2013). This emphasizes an important role of Nrf2 system as the body’s natural defense against hyperglycemia-induced cell damage. Thus, it is plausible that activation of Nrf2 signaling pathway with small molecule Nrf2 activators in hyperglycemic RPE could prevent the development of diabetic retinopathy.
In the retina, lutein, zeaxanthin, and meso-zeaxanthin, so-called macular pigments, have been found to show important photoprotective and antioxidant activities (Whitehead et al. 2006). Epidemiological studies showed an inverse association between the plasma levels of macular carotenoids and age-related macular degeneration, and cataract (Coyne et al. 2005; Zhong and Kowluru 2011). A few recent in vivo studies have reported that these macular carotenoids down-regulate the expression of VEGF which is the major contributor of vascular complication in the retina of diabetic mice (Madsen-Bouterse and Kowluru 2008; Santos et al. 2014). Recently, lutein and zeaxanthin were found to induce Nrf2-mediated phase II enzymes in RPE cells cultured in DMEM/F12 medium with recommended glucose level (Zou et al. 2014; Frede et al. 2017), which emphasize their protective efficacy towards conditions like age-related macular degeneration (AMD). However, the degree of Nrf2 activation was found to vary from one carotenoid to the other (Koushan et al. 2013; Zou et al. 2014; Frede et al. 2017). We have set an experimental protocol that aids the researchers to test any drug molecule on hyperglycemia-mediated changes in redox signaling in ARPE-19 cells (Shivarudrappa et al. 2019). Despite numerous in vivo and in vitro studies, our understanding of the mechanisms underlying the benefits of lutein concerning hyperglycemia-induced oxidative stress in RPE is still limited. In this context, this study aimed to investigate whether lutein protects RPE cells from hyperglycemia-mediated oxidative stress, which would, in turn, due to the activation of Nrf2 signaling or not. We chose to use human adult retinal pigment epithelial (ARPE-19) cell line as in vitro model of RPE. ARPE-19 has been widely used as an alternative to primary cultures, which exhibit similar epithelial cell morphology and express several genes specific for the RPE, such as RPE65, CRALBP, and ZO-1 (Maminishkis et al. 2006; Kannan et al. 2006).
Materials and methods
Chemicals and reagents
Bovine serum albumin (BSA), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), mounting medium, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) and 2-thiobarbituric acid (TBA) were purchased from Sigma-Aldrich Co., USA. The mitochondrial membrane potential (MMP) assay kit and GSH/GSSG ratio detection kit were purchased from Abcam Inc., UK. The WST-1 reagent was procured from Roche Life Science, USA. Syringe filters (Acrodisc) were purchased from Pall Corporation Inc., USA, and the cell culture media and supplements were from Life Technologies, USA. ARPE-19 cells were obtained from ATCC, USA. The DC protein assay kit, polyvinylidene difluoride (PVDF) membrane, and Clarity™ Western ECL substrate solution were procured from Bio-Rad Laboratories, USA. Primary antibodies to Nrf2, HO-1, AKT, pAKT, Erk1/2, pErk1/2, p38, p-p38 and β-actin were purchased from Cell Signaling Technology Inc., USA, and antibody to catalase was from Cloud-Clone Corp., USA. The antibodies to SOD2 and lamin were procured from Abcam Inc., UK. The secondary antibody, enzyme-conjugated anti-rabbit IgG was purchased from Cell Signaling Technology Inc., USA, and Alexa Fluor 594 goat anti-rabbit IgG was procured from Thermofisher Scientific Inc., USA. The carotenoid, lutein used in this study was isolated and purified from Chenopodium album. Trichloroacetic acid (TCA) and 2, 4-dinitrophenylhydrazine (DNPH) were purchased from Sisco Research Laboratories Pvt. Ltd. India. Skimmed milk powder was procured from Hi-Media Laboratories Pvt. Ltd., India. All other reagents and chemicals were of the analytical grade commercially available. In all experiments, Milli-Q (Merck Millipore Corporation, USA) water was used.
Preparation of lutein
The carotenoids were extracted from the lyophilized powder of Chenopodium album using acetone as described in Sowmya Shree et al. (2017). Briefly, the acetone extract was subjected to saponification using methanolic-KOH. The unsaponified fraction was phase-separated using hexane. Then the carotenoid-rich hexane fraction was washed with distilled water to remove the potassium salts. The resulted extract was condensed under reduced pressure using rotary flash evaporator (Heidolph, Germany). From the total extract, lutein-rich fraction was separated by open column chromatography, and was subjected to preparative HPLC for purification of lutein. Lutein was identified based on the absorption spectrum of lutein peak measured using a photodiode array detector (SPD-M10A, Shimadzu, Japan) attached to the Shimadzu HPLC system. Purified carotenoid (purity ≥95%) was stored at −80 °C for further analysis.
Cell culture
ARPE-19, a human retinal pigment epithelial cell line was cultured in DMEM/F12 medium (1:1 mixture of Dulbecco’s modified Eagles medium and Ham’s F12 containing glucose concentration of 17.5 mM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were cultured at 37 °C in a humidified atmosphere with 5% CO2. The sub-culturing was performed by trypsinizing the cells with 0.05% trypsin-EDTA solution. For cell viability assay, lutein treatment was done along with hyperglycemic condition for 24 h. For all other experiments, the cells were pre-treated with lutein at noted concentrations for 3 h and then the hyperglycemic condition was established for 24 h to examine the protective effect of lutein on hyperglycemia-mediated oxidative stress in ARPE-19.
Cell viability and morphology
The viability of ARPE-19 was analyzed by the water-soluble tetrazolium-1 (WST-1) assay. In brief, cells at a density of 5.0 × 104 cells/ml were seeded (100 μl/well) in a 96-well plate for 18 h, and the effect of lutein treatment on viability of ARPE-19 cultured in both normal and high-glucose condition (25 mM) was analyzed after 24 h. To examine the impact of hyperglycemia on the viability of ARPE-19, glucose at two different concentrations (25 and 30 mM) were analyzed. DMSO was used as a vehicle for lutein with the final level of 0.05% (v/v) in the culture medium. After 24 h of treatment, a tetrazolium salt, WST-1 (2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) reagent was added (10 μl/well) to each well and incubated at 37 °C for 1 h. The cleavage of WST-1 to formazan by metabolically active cells was quantified spectrophotometrically at 450 nm using a multimode plate reader (Infinite-M200 PRO, Tecan, Switzerland). The morphological changes in control and lutein-treated ARPE-19 cells were observed under Phase-contrast microscope (Model 1X73, Olympus Corporation, Japan). The images captured were analyzed and compared with respective control.
ROS assay
Intracellular ROS level was measured spectrofluorimetrically by using 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Within the cells, DCFH-DA, a fluorogenic dye, reacts with ROS, which results in the formation of a fluorescent product, DCF that reflects the level of ROS. Briefly, ARPE-19 cells seeded in a clear bottom 96-well black plate (Eppendorf Ltd., Germany) for overnight were pre-treated with lutein (0.5 μM and 1 μM) for 3 h followed by high-glucose (25 mM) media for 24 h. After the treatment, cells were subjected with 10 μM DCFH-DA solution and incubated for 30 min. The fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a multimode plate reader (Infinite-M200 Pro, Tecan, Switzerland). Finally, the cells were washed with PBS and examined under an inverted fluorescent microscope (Model 1X73, Olympus Corp., Japan).
Spectrophotometric determination of MDA-TBA
One of the predominant byproducts of lipid peroxidation is malondialdehyde (MDA). The MDA content in ARPE-19 was measured spectrophotometrically according to the method described by Prabhakar et al. (2012). Briefly, 50 μL aliquot of cell lysate from each experimental group was mixed with 200 μL of TBA-TCA reagent [0.375% thiobarbituric acid (w/v) and 15% trichloroacetic acid (w/v)], and the volume was made up to 500 μL with distilled water and incubated at 95 °C for 20 min. The reaction mixture was allowed to cool at room temperature. The TBA–MDA complex was then extracted by adding 500 μL of n-butanol. The pink-colored extract in n-butanol was measured at 532 nm using a spectrophotometer (Epoch-2, Biotek, US). The amount of MDA was expressed as % of control of μmol MDA per mg wet weight of cell lysate.
Protein carbonyl assay
As described by Dalle-Donne et al. (2003), 150 μg of pelleted protein from each experimental group was incubated with 150 μL of 10 mM DNPH (2,4-dinitrophenylhydrazine) at room temperature under dark condition for an hour. The mixture was vortexed every 10 min. After incubation, the solution was precipitated using 10% TCA, and the protein pellet was washed with ethanol: ethyl acetate at the ration of 1:1 ratio (v/v) to remove any free DNPH. The resulting pellet was re-suspended with 6.0 M guanidine hydrochloride and incubated for 15 min at 37 °C. Protein carbonyl content was measured spectrophotometrically (Epoch-2, Biotek, US) at 366 nm and calculated using the molar extinction coefficient of 22,000 Mˉ1cm ˉ1. Protein carbonyl was expressed as % of control.
Mitochondrial membrane potential (MMP) assay
The MMP assay was performed as described in the manufacturer’s protocol of the JC-10 MMP assay kit. Briefly, ARPE-19 cells (5 × 104 cells/ml) seeded in a 96-well clear bottom black plate (Eppendorf Ltd., Germany) were pre-treated with lutein (0.5 μM and 1 μM) for 3 h followed by high-glucose media (25 mM) for 24 h. Following the incubation, JC-10 dye solution (JC-10 and assay buffer A 1:100 v/v) was added (50 μl/well) to the control and treated cells. The plate was kept under dark condition for 30 min. Then, assay buffer B (50 μl/well) was added, and the fluorescent intensity was measured at 490/525 nm (Green) and 540/590 nm (Red) using a multimode plate reader (Infinite-M200 Pro, Tecan, Switzerland). The ratio of red/green fluorescent intensity was used to determine the MMP. To visualize the protective effect of lutein on high glucose-mediated loss of MMP, the plate was analyzed under inverted fluorescent microscope (Model 1X73, Olympus Corp., Japan). Carbonyl cyanide m-chlorophenylhydrazone (CCCP) was used as a positive control.
Total GSH assay
The GSH assay was performed by following the manufacturer’s protocol of the GSH/GSSG ratio detection assay kit. Briefly, ARPE-19 cells (2.5 × 105 cells/ml) seeded in a 6-well plate (Eppendorf Ltd., Germany) were treated with DMSO (control) or lutein (1 μM) for 3 h followed by glucose treatment (25 mM) for 24 h. Then, the cells were extracted using lysis buffer, deproteinized with TCA, and neutralized using sodium bicarbonate. Prepared samples with an equal volume of reagents were loaded on to a 96-well black plate (Eppendorf Ltd., Germany) along with standards as prescribed in the manufacturer’s protocol. Fluorescent intensity was measured at an excitation wavelength of 490 nm and an emission wavelength of 520 nm using a multimode plate reader (Infinite-M200 Pro, Tecan, Switzerland). The levels of GSH were detected using a standard curve, as mentioned in the manufacturer’s manual.
Protein expression
Cellular proteins of control and treated cells were extracted by using lysis buffer (20 mM Tris-buffered saline, 1% Triton-X100, protease inhibitor cocktail, 50 mM sodium fluoride, and 1 mM orthovanadate). The extract was centrifuged at 14000 rpm for 30 min at 4 °C and supernatant was collected. Nuclear proteins were extracted using a standard method described by Johnson et al. (2009). The concentration of protein in the supernatant was quantified using a DC protein assay kit. Protein concentration of 30 μg/lane was subjected to electrophoretic separation on 8% (Nrf2), 10% (catalase, p38, Erk and AKT) and 12.5% (HO-1 and SOD2) SDS-polyacrylamide gels. The protein bands were then transferred on to polyvinylidene difluoride membranes and were blocked using 3% skimmed milk powder for 2 h. Next, the membranes were probed with specific primary antibodies for 1 h and subsequently with secondary horseradish peroxidase-conjugated anti-rabbit IgG antibody for 1 h. Finally, the bands were visualized with the substrate, Clarity™ western ECL using a Bio-Rad visualizer (Bio-Rad Laboratories, USA). The beta-actin and lamin were used as a loading control for cytoplasmic and nuclear protein, respectively.
Immunofluorescent staining
ARPE-19 cells were seeded on 8-chambered cell imaging slide (Eppendorf Ltd., Germany) for 18 h. Cells were then pre-treated with lutein for 3 h, followed by exposure to high-glucose condition for 24 h. H2O2 treated for 24 h at a concentration of 100 μM was used as positive control. After the treatment, cells were washed with PBS and fixed with 2% paraformaldehyde. The fixed cells were permeabilized using 0.1% triton X100 and then blocked with 3% BSA for 1 h at 4 °C. To examine the localization of Nrf2, cells were incubated with a monoclonal antibody against Nrf2 (1/200 dilution) and with Alexa Fluor 594-labeled secondary goat anti-rabbit IgG (1/2000). After washing the cells with PBS, the nuclei were counter stained with DAPI. Finally, the slide was mounted using mounting medium, and was subjected to microscopic observation. The images for Nrf2 and nucleus were taken separately using a fluorescent microscope (Model 1X73, Olympus Corporation, Japan) and were processed using an Olympus Cell Sens Entry 1.16 software. The overall experiment was done at 4 °C under dark condition.
Statistical analysis
Values are presented as means ± SD. Data were analyzed by one-way (ANOVA) analysis of variance with the Tukey-Kramer post-hoc test to identify significant differences (p < 0.05).
Results
Effect of lutein on the viability and morphology of ARPE-19
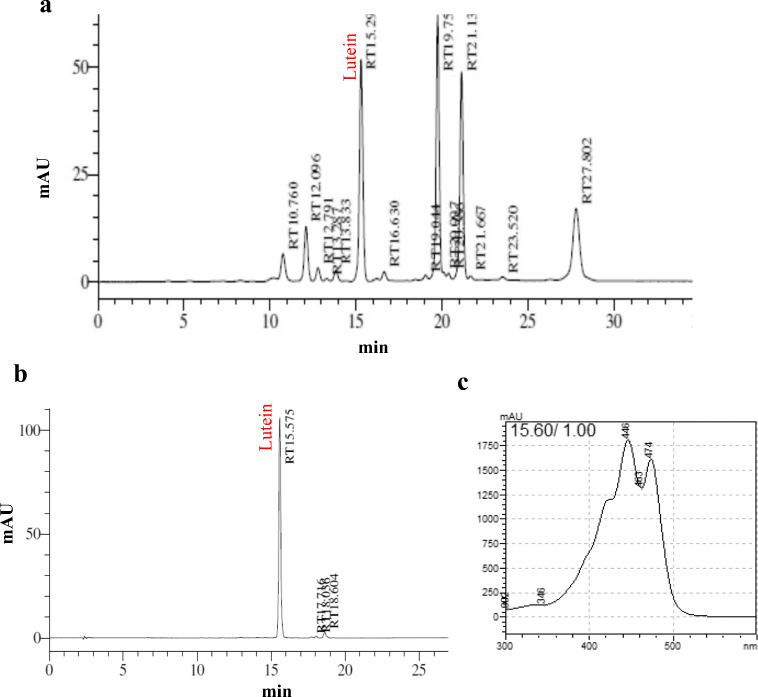
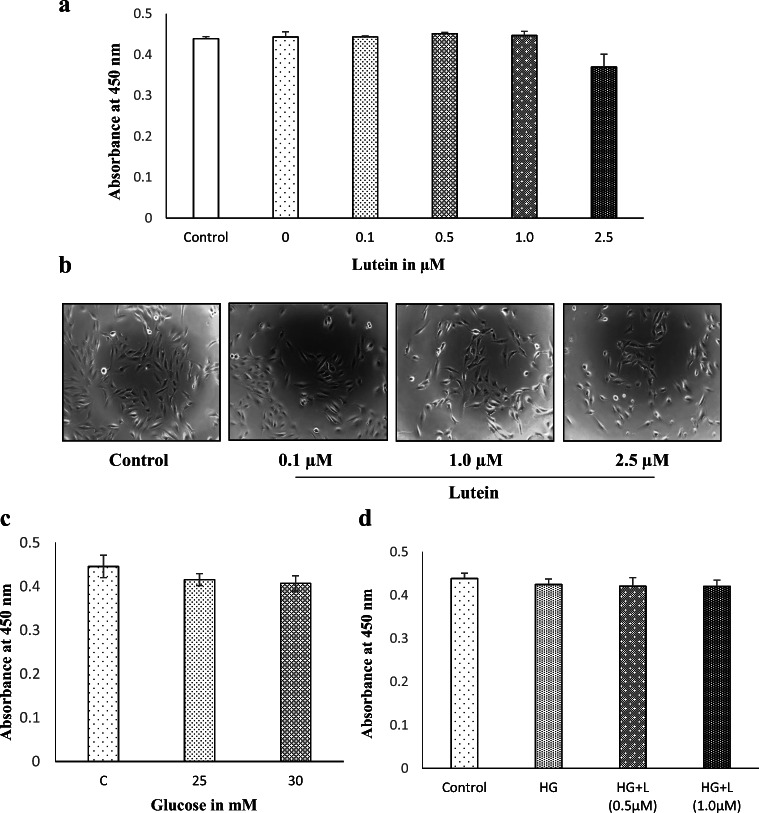
Lutein was found to be a major carotenoid in Chenopodium album and was identified based on the retention time (Fig. 1a and b) and specific absorption spectrum (Fig. 1c) on the HPLC chromatogram as described in our previous paper (Kavalappa et al. 2019). To examine the effect of purified lutein on the viability of ARPE-19 cells grown in both standard and high-glucose media, the treated cells were incubated for 24 h. Treatment with lutein at concentrations ranging from 0.1 to 1 μM neither inhibited the viability nor affected the morphology of ARPE-19 cells grown in standard media (Fig. 2a and b). But, lutein concentration at 2.5 μM was found to exert slightly reduced viability, though the reduction was not significant (p > 0.05). The microscopic observation also displayed cell shrinkage and reduced cell density in experimental group treated with 2.5 μM of lutein. Increased glucose concentrations (25 mM and 30 mM) did not show any significant effect on the viability of ARPE-19 (Fig. 2c). Thus, 25 mM was chosen to generate a hyperglycemic condition. Also, the concentrations of lutein (0.5 and 1 μM) tested for cell viability on ARPE-19 grown in high-glucose (25 mM) media did not show any significant effect (Fig. 2d). Based on these results, non-cytotoxic concentrations of lutein (0.5 and 1 μM) were used further to examine its protective effects against hyperglycemia-mediated changes in oxidative and redox status of ARPE-19 cells.
Fig. 1.
Isolation and identification of lutein. a Carotenoids profile of Chenopodium album.b HPLC chromatogram of purified lutein from the total carotenoid extract of C. album. c UV-VIS-spectrum of the respective peak of lutein
Fig. 2.
Effect of lutein and glucose on the viability and morphology of ARPE-19 cells. a The cell viability of ARPE-19 treated with lutein at the noted concentrations (0, 0.1, 0.5, 1.0 & 2.5 μM) for 24 h was analyzed by WST-1 assay. Values are mean ± SD (n = 3). b The cell morphology of ARPE-19 treated with lutein for 24 h was observed under a phase-contrast microscope, and the representative picture is presented. c The cell viability of ARPE-19 treated with glucose at the different concentrations (25 & 30 mM) for 24 h was analyzed by WST-1 assay. Values are mean ± SD (n = 3). d The effect of lutein (L-1 μM) on the viability of ARPE-19 cells treated with high-glucose (HG-25 mM) was analyzed by WST-1 assay. Values are mean ± SD (n = 3)
Lutein counteracts oxidative stress generated by high-glucose
To determine the protective role of lutein against oxidative stress caused by high-glucose condition in ARPE-19, we compared the intracellular reactive oxygen species (ROS) levels and mitochondrial membrane potential (MMP) of the high-glucose group with lutein pre-treated group. As predicted, we found a raised fluorescent intensity when the cells were subjected to high-glucose condition. The increase was found to be around 18% compared to control (Fig. 3a). An increased green fluorescence observed under fluorescent microscope further confirms this elevated intracellular oxidative stress (Fig. 3b). Interestingly, pre-treatment with lutein (1 μM) reversed this high glucose-mediated elevation of ROS in ARPE-19. Similarly, two significant markers of oxidative stress, MDA and protein carbonyl levels were significantly increased in hyperglycemic ARPE-19, whereas lutein pre-treatment brought those levels down as close to that of control (Fig. 3c and d). From these data, it is noticed that lutein prevents high glucose-mediated oxidative stress in ARPE-19.
Fig. 3.
Effect of lutein on oxidative status of ARPE-19 cells upon glucose induction. a The intracellular ROS levels were measured as indicated in materials and methods section after pre-treating ARPE-19 with lutein (0.5 μM & 1 μM) for 3 h and with glucose (25 mM) for 24 h. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group. b The microscopic images of the intensity of DCF fluorescence of respective experimental group. c ARPE-19 cells were exposed to lutein (1 μM) for 3 h followed by glucose (25 mM) for 24 h. MDA content was assessed using Thiobarbituric Acid-Reactive Substances (TBARS) assay as described in materials and methods. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group. d The carbonyl content in ARPE-19 cells treated with lutein (1 μM) for 3 h followed by glucose (25 mM) for 24 h was measured by spectrophotometric DNPH assay as detailed in materials and methods. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group
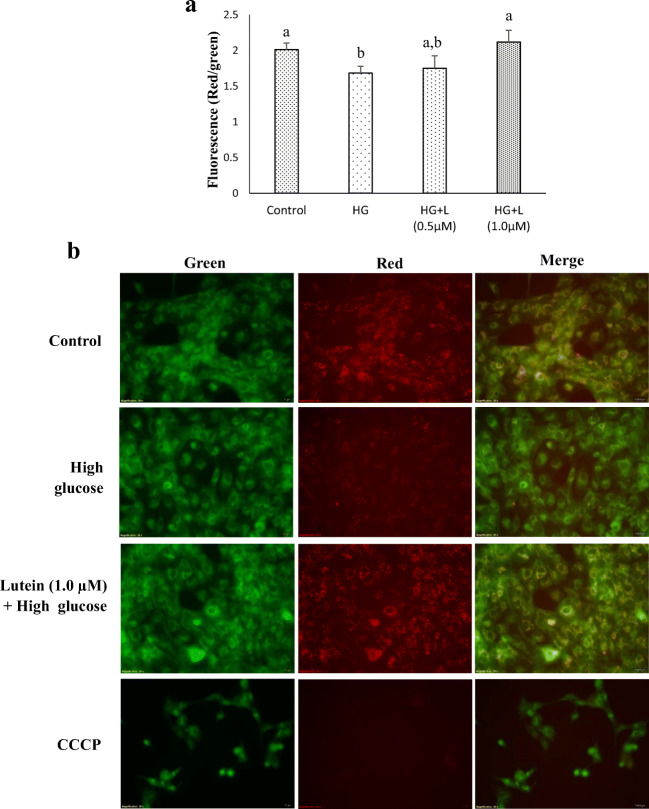
High-glucose treatment also caused significant decrease in MMP, with an observed reduction of 16%. Incubation of ARPE-19 with high-glucose, but prior pre-treatment with lutein at 1 μM for 3 h showed an increase in MMP (Fig. 4a). To corroborate the data, fluorescent microscopic images of ARPE-19 stained with JC-10 were analyzed. As shown in merged images of Fig. 4b, control cells exhibited green cytosolic fluorescence with orange colored thread-like appearance of mitochondria, whereas high glucose-treated cells showed distinctly less red fluorescence, demonstrating mitochondria with lesser MMP. Carotenoid treatment ameliorated this high glucose-mediated loss of MMP, with an increased red fluorescent aggregates. The cells treated with CCCP (positive control) exhibited a complete loss of MMP with almost no red fluorescence. Thus, it appears that excess glucose disrupts MMP in ARPE-19, which may be due to elevated generation of ROS, and lutein effectively protects this glucose-mediated disruption.
Fig. 4.
Effect of lutein on mitochondrial membrane potential (MMP) in ARPE-19 cells. The MMP was analyzed in ARPE-19 cells treated with lutein (0.5 μM & 1 μM) for 3 h followed by glucose (25 mM) for 24 h using a JC-10 assay kit as indicated in materials and methods. a The quantitative analysis of MMP in the respective treatment group. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group. b The fluorescence images of MMP in the respective treatment group
Protective role of lutein against high glucose-mediated blockage of antioxidant markers
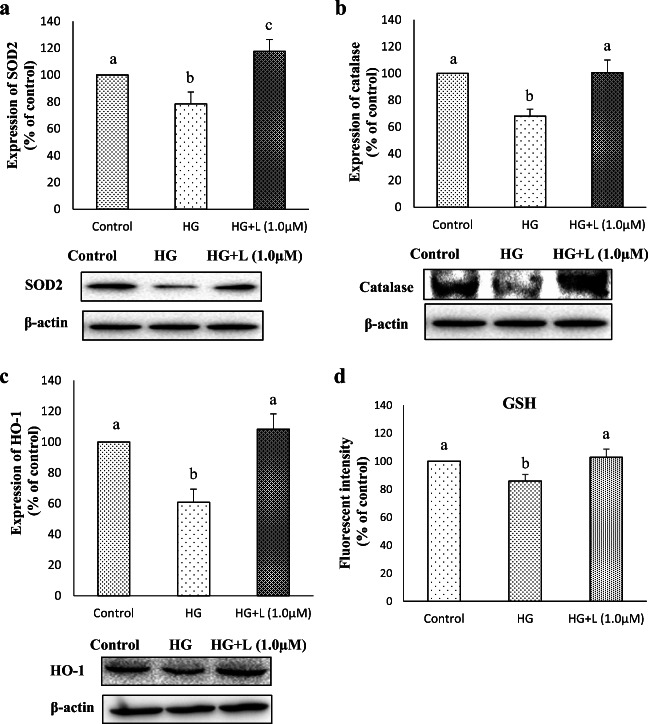
Since an impaired antioxidant defense is evident in diabetic and pre-diabetic condition alongside with elevated oxidative stress (Siewert et al. 2013; Bai et al. 2013), we next interested to study the effect of lutein on high glucose-mediated changes of antioxidant markers in ARPE-19 cells. When there is a threat to the cells due to pro-oxidant state, antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GP) play a critical role in maintaining the normal cellular homeostasis. In the current study, SOD2 (MnSOD) and catalase were down-regulated by glucose treatment compared to control, whereas lutein pre-treatment reversed this glucose-mediated suppression of those antioxidant enzymes. The reduction in the expression of SOD2 and catalase upon glucose treatment was 22% and 32% respectively compared to control, which was reversed by 39% and 32% respectively upon lutein pre-treatment (Fig. 5a and b). Similarly, a long-known stress response enzyme, heme oxygenase-1 (HO-1) was repressed (39%) by glucose treatment, while lutein pre-treatment ameliorated this effect (Fig. 5c). The intracellular concentration of GSH also followed a similar trend as that of antioxidant enzymes studied (Fig. 5d). Transcriptional regulators such as Nrf2 and nuclear factor kappa-B (NF-kB) play pivotal roles in the events of cellular defense against oxidative stress through activating the genes mentioned above. The results indicate that hyperglycemic condition for 24 h suppresses the expression of primary antioxidant enzymes that are necessary for the cellular defense against glucose-mediated oxidative stress in ARPE-19 cells, and lutein restores this glucose-mediated effect, which might be through activation of transcriptional regulators, Nrf2 or NF-kB.
Fig. 5.
Effect of lutein on the expression of redox markers in ARPE-19 cells upon glucose induction. Protein expression of SOD2 (a), catalase (b) and HO-1 (c), and GSH levels (d) in cellular extract of ARPE-19 pre-treated with lutein (1 μM) for 3 h followed by glucose (25 mM) for 24 h was detected by western blotting as described in materials and methods. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group. d GSH levels were assessed using GSH assay kit after pre-treatment with lutein (1 μM) followed by glucose (25 mM) treatment. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group
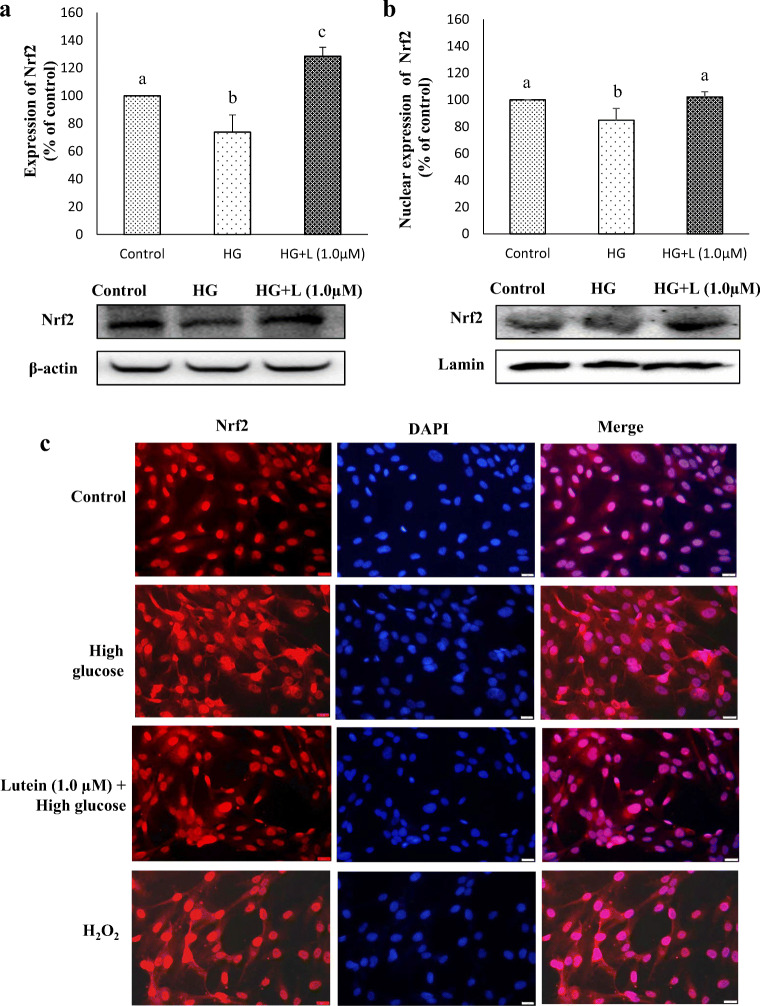
Lutein induces translocation of Nrf2
Based on the above hypothesis, we evaluated the effect of lutein on translocation of transcription factor, Nrf2 from the cytosol to the nucleus in ARPE-19 grown under hyperglycemic condition (Fig. 6). From Fig. 6a and b, it is evident that lutein pre-treatment induced cytoplasmic and nuclear expression of Nrf2 in ARPE-19, which was found to hinder by hyperglycemia. To confirm this result, an immunofluorescence assay was performed with a fluorescent-tagged (Alexa Fluor 594) secondary antibody. Our observations showed that Nrf2 is predominantly localized in the cytoplasm of hyperglycemic cells, nevertheless lutein pre-treatment stimulated translocation of Nrf2 with an increased localization in the nucleus (Fig. 6c). A widely known stress inducer, H2O2 used in this study as positive control exhibited similar result as that of hyperglycemic cells indicate that sustained oxidative stress blocks Nrf2 activation, which is in accordance with our findings that hyperglycemia increased the intracellular levels of ROS in ARPE-19. From these data, it is demonstrated that lutein pre-treatment alleviates hyperglycemia-mediated obstruction in the translocation of Nrf2 in ARPE-19.
Fig. 6.
Effect of lutein on the expression and translocation of Nrf2. a Protein expression of Nrf2 in the cellular extract of ARPE-19 cells pre-treated with lutein (1 μM) for 3 h followed by glucose (25 mM) for 24 h was detected by western blotting as described in materials and methods. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group. b Nuclear translocation of Nrf2 in ARPE-19 cells was analyzed by western blotting using the nuclear extract as depicted in materials and methods. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group. c Nuclear translocation was confirmed by immunofluorescent assay
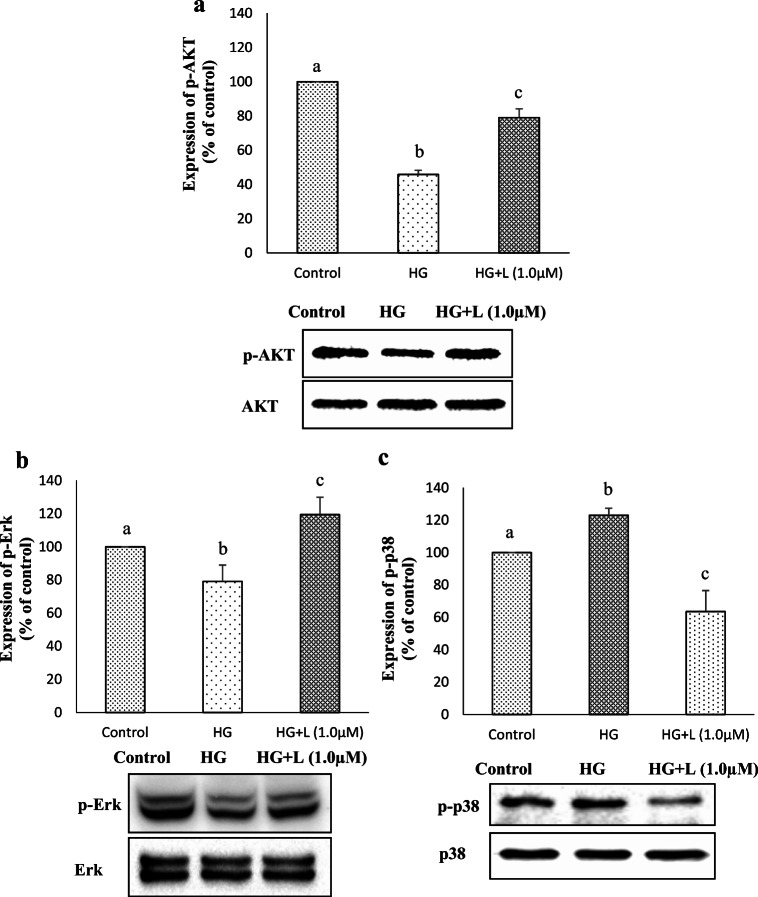
Regulatory mechanism of lutein
The PI3K/AKT, and mitogen-activated protein kinases (MAPKs) such as Erk1/2, JNK, and p38 have been shown to regulate Nrf2 in different types of cells (Yu et al. 2000; Varì et al. 2011; Filomeni et al. 2012). To determine whether Nrf2 activation in hyperglycemic ARPE-19 cells by lutein is associated with those regulators or not, we measured the activation of AKT, Erk and p-38 (Fig. 7). The results showed that hyperglycemia suppressed the activation of both AKT (Fig. 7a) and Erk (Fig. 7b), whereas lutein pre-treatment rescued this hyperglycemia-mediated suppression. In contrast, phosphorylated p-38 expression was increased under hyperglycemic condition, which was again diminished by lutein pre-treatment (Fig. 7c). Together our data indicate that lutein protects ARPE-19 cells from hyperglycemia-mediated oxidative stress through activation of Nrf2 by regulating the expression of pAKT and pErk positively, and negatively the phosphorylation of p38.
Fig. 7.
Effect of lutein on the regulatory markers of Nrf2. Relative activation of AKT (a), Erk (b) and p-38 (c) was analyzed by western blotting as described in materials and methods. Values are mean ± SD (n = 3); Bars with different letter indicate significant difference (p < 0.05) between the group
Discussion
Even though biochemical changes related to several cellular and molecular signaling pathways occur in the human RPE in diabetic patients (Vinores et al. 2000; Decanini et al. 2008; Wang et al. 2010), the impact of RPE dysfunction in diabetic retinopathy is poorly defined. A vast number of studies highlighted that diabetic retinopathy is majorly a microvascular (inner blood-retinal barrier) complication of diabetes mellitus but, a few recent investigations identified the breakdown of outer blood-retinal barrier, RPE in diabetic subjects and animal models (Johnson et al. 2009; Wang et al. 2010; Samuels et al. 2014). Elevated levels of ROS and impaired antioxidant defense found in the retina of diabetic animals and human subjects indicate that there is a strong correlation between hyperglycemia-associated oxidative stress and RPE dysfunction. In our previous study, we found a sustained increase in ROS levels in ARPE-19 cells cultured under hyperglycemic condition from 24 h to till 96 h (Shivarudrappa et al. 2019). We also found that a major component of antioxidant defense and regulator of endogenous antioxidant enzymes, Nrf2 is down-regulated in hyperglycemic ARPE-19. Lutein is one of the macular carotenoids present predominantly in the peripheral macula, which is known for its antioxidant, anti-inflammatory, and anti-apoptotic properties (Thomas and Harrison 2016). In general, presence of conjugated double-bonds and hydroxy group make these carotenoids as potent scavengers of singlet oxygen (1O2) and peroxyl radicals (Choe and Min 2006). In addition to their role as a scavenger of reactive oxygen species (ROS), carotenoids including lutein act by making interaction with cellular signaling cascades, such as NF-kB, MAPK, or Nrf2 (Shi and Zhou 2010; Min et al. 2011). A recent dietary supplementation study reported that carotenoids dietary supplementation [lutein (10 mg), zeaxanthin (2 mg) and meso-zeaxanthin (10 mg)] improves the visual function of type 2 diabetes patients (Moschos et al. 2017). In our current study, we demonstrate that lutein effectively protects ARPE-19 from damage generated by hyperglycemia by activating Nrf2 through its regulators, suggesting the preventive role of lutein against diabetic retinopathy.
Reports highlight that serum concentration of lutein in normal subjects varies from 0.08–0.35 μg/ml and this level is significantly reduced in patients with diabetic retinopathy (Bone et al. 2003; Hu et al. 2011). However, supplementation of lutein at 10 mg/day could achieve a maximum serum concentration of 1.4 μg/ml after 18 days in healthy subjects, and by supplementation of lutein, the serum concentration increases significantly in diabetic retinopathy patients (Hu et al. 2011). Thus, it is essential to note that hyperglycemia may prevent absorption of lutein, which subsequently reflects its low levels in the retina of diabetic patients. Since carotenoids are not synthesized by humans, intake of lutein-rich foods may improve the serum levels and accumulation in the retina. In this study, lutein up to the concentration of 1 μM did not show any cytotoxic effect both in normal and hyperglycemic ARPE-19; instead, it showed protection against hyperglycemia-mediated oxidative stress. The concentration of 1 μM lutein is equal to 0.568 μg/ml, which is physiologically achievable through intake of lutein-rich foods or supplementation.
The two major events of cellular damage due to oxidative stress in the diabetic retina are lipid peroxidation and oxidation of glycated proteins (van Reyk et al. 2003). Our initial findings on the functional role of lutein on hyperglycemia-mediated oxidative damage in ARPE-19 demonstrate that it prevents hyperglycemia-mediated intracellular ROS generation, and reduces the levels of ROS-specific markers such as protein carbonyl and malondialdehyde. We hypothesize that conjugated double bonds in lutein and activation of antioxidant defense system by Nrf2 could have involved in scavenging intracellular ROS, which in turn contributed to reduced levels of the markers of oxidative damage. Oxidative stress caused by ROS was reported to induce depolarization of mitochondrial membrane potential (MMP) (Garg and Chang 2004). We also noticed a significant loss of MMP in hyperglycemic ARPE-19, and lutein brings back this loss. Further, disrupted MMP observed with the addition of a relatively stable and membrane permeable ROS, H2O2 indicate that hyperglycemic condition generated adequate ROS which is sufficient to affect the MMP in ARPE-19. In earlier studies, lutein showed its potency as a powerful antioxidant in ARPE-19 under different oxidative stress conditions. To demonstrate a few, in one study, lutein suppressed ROS levels in photo-stressed RPE-choroid (Kamoshita et al. 2016). Another study observed that lutein at 5 and 10 μM concentrations reduce H2O2-mediated elevation of ROS (Liu et al. 2017).
To determine whether or not lutein protects hyperglycemia-mediated down-regulation of antioxidant defense enzymes, we measured the expression levels of three major proteins, SOD2, catalase and HO-1 in hyperglycemic ARPE-19 pre-treated with lutein. In antioxidant defense process, as the first line of defense, SOD catalyzes the dismutation of superoxide (O2• –) to hydrogen peroxide (H2O2), which consequently converted into a water molecule and molecular oxygen by the action of catalase. Another antioxidant enzyme in this line, HO-1 is involved in the conversion of heme into bilirubin, a potent antioxidant (Ndisang et al. 2014). Interestingly, lutein exhibited significant protection over hyperglycemia-mediated down-regulation of SOD2, catalase, and HO-1. Recent studies reported that lutein and zeaxanthin significantly up-regulated mRNA and protein expression of HO-1 in ARPE-19 cells (Zou et al. 2014; Frede et al. 2017). Further, increased levels of GSH observed in lutein-treated ARPE-19 in this study might be due to the activation of enzymes that catalyze GSH synthesis such as GCLc, and GCLm. Carotenoids, astaxanthin, zeaxanthin, and lutein were found to activate expression of GCLc and GCLm in different cells including ARPE-19 (Li et al. 2013; Zou et al. 2014; Frede et al. 2017; Nishimoto et al. 2017). These observations indicate that the protection showed by lutein against hyperglycemia-mediated oxidative stress in ARPE-19 is probably through the enhancement of phase II antioxidant enzyme system.
Next, we underwent to decipher the potential mechanism by which lutein protects ARPE-19 from hyperglycemia-mediated oxidative stress. The Nrf2 signaling pathway plays a vital role in regulating the transcription of several antioxidant genes such as enzymes related to GSH synthesis, and SOD, NQO1, HO-1 and catalase (Li et al. 2013). Studies enunciate that Nrf2 is decreased in diabetic mice and in patients with type 2 diabetes mellitus (T2DM) which contributes to increased oxidative stress. But, overexpression and small molecule activation of Nrf2 prevents the onset of T2DM and reduces oxidative stress, respectively (David et al. 2017; Matzinger et al. 2018). As cellular levels of Nrf2 is critical in maintaining the redox status, it was reported that the nuclear fractions of peripheral blood mononuclear cells (PBMC) from pre-diabetic and diabetic patients contain diminished level of Nrf2 (Jiménez-Osorio et al. 2014). As expected, the expression of Nrf2 in both cytosolic and nuclear fraction is decreased in hyperglycemic ARPE-19, and lutein treatment successfully restored these changes. Therefore, we assume that activation of Nrf2 pathway might be accounting for the benefits of lutein against diabetic retinopathy. Further to confirm this mechanism, we tested the localization of Nrf2 as it release from Keap1 in the cytoplasm and translocate into the nucleus to activate transcription. The results indicate that lutein promotes Nrf2 nuclear translocation, which was blocked when the cells were grown only with high-glucose. Previous studies reported that few carotenoids including lutein activate Nrf2 in ARPE-19 cells under normal growth condition, suggesting its potency in the context of age-related macular degeneration (AMD) (Ben-Dor et al. 2005; Frede et al. 2017). The present study for the first time demonstrates the activation of Nrf2 signaling by lutein in hyperglycemic ARPE-19, which mimic the condition of diabetic retinopathy.
Several studies have proved the involvement of the PI3K/AKT and the MAP kinases pathways, including Erk, p38, and JNK, in the regulation of Nrf2 (Yu et al. 2000; Li et al. 2013; Zou et al. 2014). It was demonstrated that p38 negatively regulates the induction of antioxidant response element (ARE)-dependent phase II gene expression, but in contrast, Erk and AKT regulate the expression of ARE-dependent enzymes (Goodwin 1980; Yu et al. 1999, 2000; Wang et al. 2008; Pitha-Rowe et al. 2009; Das et al. 2013) positively. Consistent with the earlier studies, in the current study, phosphorylation of AKT and Erk1/2 was significantly blocked in hyperglycemic ARPE-19 cells, whereas the phosphorylation of p38 was promoted. These changes were retrieved when the cells were pre-treated with lutein. In support of our study, astaxanthin in a previous study showed a protective effect against H2O2-mediated oxidative stress in ARPE-19 via up-regulation of Nrf2 and its regulatory antioxidant enzymes through activation of AKT (Li et al. 2013). Similarly, zeaxanthin in an earlier study exhibited increased Nrf2 expression in ARPE-19 cells cultured in normal growth condition that was associated with elevated phosphorylation of AKT and Erk1/2, but the phosphorylation of p38 was unaffected (Zou et al. 2014). These data demonstrate that activation of Nrf2 in hyperglycemic ARPE-19 by lutein is through an upregulated expression of its positive regulators, pAKT and pErk1/2, and down-regulated expression of a negative regulator, phospho-p38. Though lutein reverses hyperglycemia-mediated elevation of oxidative stress and associated dysregulation of Nrf2 and its intracellular signal transducers, the upstream signaling molecule(s) from which the signal is transmitted needs to be studied.
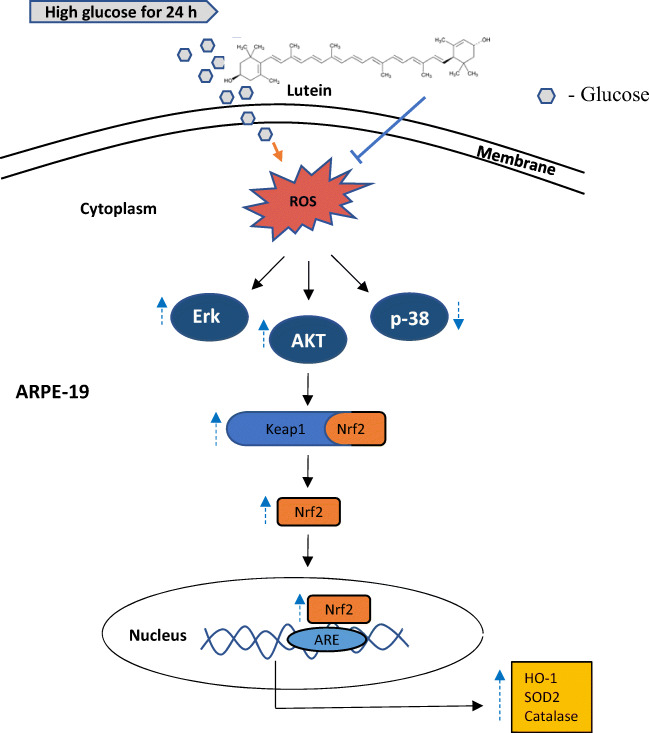
n conclusion, as illustrated in Fig. 8, lutein potently activates Nrf2 nuclear translocation in hyperglycemic ARPE-19 by activating the PI3K/AKT and Erk1/2 pathways, thereby protecting the cells from hyperglycemia-mediated oxidative damage. An account with recent studies, the present study here specifies that lutein not only activates Nrf2 signaling in conditions like age-related macular disorders but also in diabetic retinopathy. We consider our study imparts an additional benefit to lutein for its effects related to the treatment of diabetes-related retinal disorders.
Fig. 8.
A putative model for therapeutic targets of lutein on hyperglycemia-mediated oxidative damage in ARPE-19. Lutein activates Nrf2 translocation through activating its upstream regulators, PI3K-AKT and Erk1/2, and thereby upregulates protective antioxidant enzymes in hyperglycemic ARPE-19 cells
Acknowledgments
The author, Arpitha H S, acknowledges the University Grants Commission (UGC), New Delhi, India for granting Research Fellowship. The authors thank the Director, CSIR-CFTRI for the constant support.
Funding information
We would like to thank the Council for Scientific and Industrial Research (CSIR), New Delhi for granting financial support under the 12th Five Year Plan Project (BSC-0404).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–186. [PubMed] [Google Scholar]
- Bernstein PS, Law WC, Rando RR. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci U S A. 1987;84:1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133:992–998. doi: 10.1093/jn/133.4.992. [DOI] [PubMed] [Google Scholar]
- Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye. 2001;15:384–389. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/S1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Choe E, Min DB. Chemistry and reactions of reactive oxygen species in foods. Crit Rev Food Sci Nutr. 2006;46:1–22. doi: 10.1080/10408390500455474. [DOI] [PubMed] [Google Scholar]
- Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S, Leonard D, Shaw J. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–693. doi: 10.1093/ajcn/82.3.685. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Das BN, Kim YW, Keum YS (2013, 2013) Mechanisms of Nrf2/keap1-dependent phase II cytoprotective and detoxifying gene expression and potential cellular targets of chemopreventive isothiocyanates. Oxidative Med Cell Longev. 10.1155/2013/839409 [DOI] [PMC free article] [PubMed]
- David JA, Rifkin WJ, Rabbani PS, Ceradini DJ. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res. 2017;2017:1–15. doi: 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia. 2008;51:1051–1061. doi: 10.1007/s00125-008-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins DM, Yates PW, Dahrouj M, Liu Y, Crosson CE, Ablonczy Z. Progressive early breakdown of retinal pigment epithelium function in hyperglycemic rats. Investig Opthalmol Vis Sci. 2016;57:2706–2713. doi: 10.1167/iovs.15-18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter BP. Dysregulation of Nrf2 signaling in diabetes: an opportunity for a multitarget approach. J Diabetes Metab. 2015;06:1–12. doi: 10.4172/2155-6156.1000475. [DOI] [Google Scholar]
- Filomeni G, Piccirillo S, Rotilio G, Ciriolo MR. P38 MAPK and ERK1/2 dictate cell death/survival response to different pro-oxidant stimuli via p53 and Nrf2 in neuroblastoma cells SH-SY5Y. Biochem Pharmacol. 2012;83:1349–1357. doi: 10.1016/j.bcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Frede K, Ebert F, Kipp AP, Schwerdtle T, Baldermann S. Lutein activates the transcription factor Nrf2 in human retinal pigment epithelial cells. J Agric Food Chem. 2017;65:5944–5952. doi: 10.1021/acs.jafc.7b01929. [DOI] [PubMed] [Google Scholar]
- Garg TK, Chang JY. 15-deoxy-delta 12,14-prostaglandin J2 prevents reactive oxygen species generation and mitochondrial membrane depolarization induced by oxidative stress. BMC Pharmacol. 2004;4:6. doi: 10.1186/1471-2210-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. Functions of carotenoids. Biochem Carotenoids. 1980;1:77–95. doi: 10.1007/978-94-009-5860-9_3. [DOI] [Google Scholar]
- Hamann S. Molecular mechanisms of water transport in the eye. Int Rev Cytol. 2002;215:395–431. doi: 10.1016/S0074-7696(02)15016-9. [DOI] [PubMed] [Google Scholar]
- Hu B-J, Hu Y-N, Lin S, Ma W-J, Li X-R. Application of lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011;4:303–306. doi: 10.3980/j.issn.2222-3959.2011.03.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K, Panjwani N, Cao Z, Streilein JW. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol Inflamm. 2003;11:91–105. doi: 10.1076/ocii.11.2.91.15914. [DOI] [PubMed] [Google Scholar]
- Jiménez-Osorio AS, Picazo A, González-Reyes S, Barrera-Oviedo D, Rodríguez-Arellano ME, Pedraza-Chaverri J. Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci. 2014;15:20290–20305. doi: 10.3390/ijms151120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Maher P, Hanneken A. The flavonoid, eriodictyol, induces long-term protection in arpe-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Investig Ophthalmol Vis Sci. 2009;50:2398–2406. doi: 10.1167/iovs.08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshita M, Toda E, Osada H, Narimatsu T, Kobayashi S, Tsubota K, Ozawa Y (2016) Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci Rep 6:–10. 10.1038/srep30226 [DOI] [PMC free article] [PubMed]
- Kannan R, Zhang N, Sreekumar PG, Spee CK, Rodriguez A, Barron E, Hinton DR. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006;12:1649–1659. [PubMed] [Google Scholar]
- Kavalappa YP, Udayawara Rudresh D, Gopal SS, Haranahalli Shivarudrappa A, Stephen NM, Rangiah K, Ponesakki G. β-Carotene isolated from the marine red alga, Gracillaria sp. potently attenuates the growth of human hepatocellular carcinoma (HepG2) cells by modulating multiple molecular pathways. J Funct Foods. 2019;52:165–176. doi: 10.1016/j.jff.2018.11.015. [DOI] [Google Scholar]
- Koushan K, Rusovici R, Li W, Ferguson LR, Chalam KV. The role of lutein in eye-related disease. Nutrients. 2013;5:1823–1839. doi: 10.3390/nu5051823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta Mol basis Dis. 2015;1852:2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y, Hou D, Zhang X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/AKT. Mol Vis. 2013;19:1656–1666. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu W, Zhou X, Long C, Kuang X, Hu J, Tang Y, Liu L, He J, Huang Z, Fan Y, Jin G, Zhang Q, Shen H. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol Med Rep. 2017;16:2069–2074. doi: 10.3892/mmr.2017.6838. [DOI] [PubMed] [Google Scholar]
- Lu L, Hackett SF, Mincey A, Lai H, Campochiaro PA. Effects of different types of oxidative stress in RPE cells. J Cell Physiol. 2006;206:119–125. doi: 10.1002/jcp.20439. [DOI] [PubMed] [Google Scholar]
- Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Investig Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger M, Fischhuber K, Heiss EH. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol Adv. 2018;36:1738–1767. doi: 10.1016/j.biotechadv.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Lee JT, Hye JE, Kwon TK. An IκBα phosphorylation inhibitor induces heme oxygenase-1(HO-1) expression through the activation of reactive oxygen species (ROS)-Nrf2-ARE signaling and ROS-PI3K/AKT signaling in an NF-κB-independent mechanism. Cell Signal. 2011;23:1505–1513. doi: 10.1016/j.cellsig.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Moschos MM, Dettoraki M, Tsatsos M, Kitsos G, Kalogeropoulos C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. 2017;4:23. doi: 10.1186/s40662-017-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndisang JF, Jadhav A, Mishra M. The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in zucker diabetic fatty rats. PLoS One. 2014;9:e87936. doi: 10.1371/journal.pone.0087936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Huang H-C, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- Nishimoto S, Koike S, Inoue N, Suzuki T, Ogasawara Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: increase in GSH levels is a critical event for the detoxification mechanism. Biochem Biophys Res Commun. 2017;483:874–879. doi: 10.1016/j.bbrc.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the Pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med Cell Longev. 2016;2016:1–23. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha-Rowe I, Liby K, Royce D, Sporn M. Synthetic Triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Investig Opthalmology Vis Sci. 2009;50:5339. doi: 10.1167/iovs.09-3648. [DOI] [PubMed] [Google Scholar]
- Prabhakar PV, Reddy UA, Singh SP, Balasubramanyam A, Rahman MF, Indu Kumari S, Agawane SB, Murty USN, Grover P, Mahboob M. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J Appl Toxicol. 2012;32:436–445. doi: 10.1002/jat.1775. [DOI] [PubMed] [Google Scholar]
- Samuels IS, Bell BA, Pereira A, Saxon J, Peachey NS. Early retinal pigment epithelium dysfunction is concomitant with hyperglycemia in mouse models of type 1 and type 2 diabetes. J Neurophysiol. 2014;113:1085–1099. doi: 10.1152/jn.00761.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JM, Mishra M, Kowluru RA. Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: implications in diabetic retinopathy and metabolic memory phenomenon. Exp Eye Res. 2014;121:168–177. doi: 10.1016/J.EXER.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zhou B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci. 2010;115:391–400. doi: 10.1093/toxsci/kfq066. [DOI] [PubMed] [Google Scholar]
- Shivarudrappa A, Gopal SS, Ponesakki G. An in vitro protocol to study the effect of hyperglycemia on intracellular redox signaling in human retinal pigment epithelial (ARPE-19) cells. Mol Biol Rep. 2019;46:1263–1274. doi: 10.1007/s11033-019-04597-x. [DOI] [PubMed] [Google Scholar]
- Siewert S, González I, Santillán L, Lucero R, Ojeda MS, Gimenez MS. Downregulation of Nrf2 and HO-1 expression contributes to oxidative stress in type 2 diabetes mellitus: a study in Juana Koslay City, San Luis, Argentina. J Diabetes Mellit. 2013;03:71–78. doi: 10.4236/jdm.2013.32011. [DOI] [Google Scholar]
- Sowmya Shree G, Yogendra Prasad K, Arpitha HS, Deepika UR, Nawneet Kumar K, Mondal P, Ganesan P. β-Carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol Cell Biochem. 2017;436:1–12. doi: 10.1007/s11010-017-3071-4. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Fishkin N, Zhou J, Cai B, Jang YP, Krane S, Itagaki Y, Nakanishi K. A2E, a byproduct of the visual cycle. Vis Res. 2003;43:2983–2990. doi: 10.1016/S0042-6989(03)00475-9. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Deguchi J, Okami T, Yamamoto A, Omori K, Uyama M, Tashiro Y. Immunocytochemical analyses of distributions of Na, K-ATPase and GLUT1, insulin and transferrin receptors in the developing retinal pigment epithelial cells. Cell Struct Funct. 1994;19:21–28. doi: 10.1247/csf.19.21. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Harrison EH. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J Lipid Res. 2016;57:1865–1878. doi: 10.1194/jlr.M070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reyk DM, Gillies MC, Davies MJ. The retina: oxidative stress and diabetes. Redox Rep. 2003;8:187–192. doi: 10.1179/135100003225002673. [DOI] [PubMed] [Google Scholar]
- Varì R, D’Archivio M, Filesi C, Carotenuto S, Scazzocchio B, Santangelo C, Giovannini C, Masella R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J Nutr Biochem. 2011;22:409–417. doi: 10.1016/j.jnutbio.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Macular Edema. Dordrecht: Springer Netherlands; 2000. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema; pp. 13–24. [DOI] [PubMed] [Google Scholar]
- Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43:408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen Y, Sternberg P, Cai J. Essential roles of the PI3 kinase/AKT pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Investig Opthalmology Vis Sci. 2008;49:1671–1678. doi: 10.1167/iovs.07-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xu E, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol. 2006;124:1038–1045. doi: 10.1001/archopht.124.7.1038. [DOI] [PubMed] [Google Scholar]
- Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Investig Ophthalmol Vis Sci. 2011;52:2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Simmons-Menchaca M, Gapor A, Sanders BG, Kline K. Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr Cancer. 1999;33:26–32. doi: 10.1080/01635589909514744. [DOI] [PubMed] [Google Scholar]
- Yu R, Mandlekar S, Lei W, Fahl WE, Tan TH, Kong AN. p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J Biol Chem. 2000;275:2322–2327. doi: 10.1074/JBC.275.4.2322. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Kowluru RA. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–1313. doi: 10.2337/db10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Gao J, Zheng Y, Wang X, Chen C, Cao K, Xu J, Li Y, Lu W, Liu J, Feng Z. Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death. Cell Death Dis. 2014;5:e1218–e1218. doi: 10.1038/cddis.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]