Abstract
It is widely believed that extracellular vesicles (EVs) mediate intercellular communications by functioning as messengers. EVs contain various biomolecules, including nucleic acids and proteins, as cargo in the internal space. Thus, it has been postulated that this cargo can be transferred from donor cells to recipient cells, leading to phenotypic changes in the recipient cells. However, there is a lack of experimental evidence for the aforementioned hypothesis, that EVs function as messengers. This is presumably because of a lack of rigorous methodologies for EV research. Although cells usually incorporate nanoparticles (NPs) from the extracellular space via endocytosis, these NPs are processed through the endo/lysosomal system and do not escape to the cytoplasm unless they disrupt or fuse with the endo/lysosomal membrane. Whether EVs actually are capable of escaping endo/lysosomes is still debatable. In contrast, viruses have evolved to efficiently deliver their cargo (viral proteins and genetic material) into the cytoplasm of host (recipient) cells by circumventing endo/lysosomal degradation. Thus, it may be helpful to compare EVs to viruses in terms of cargo delivery. The present technological issues that hinder obtaining support for the “EV cargo transfer hypothesis” are summarized and potential solutions for EV research are proposed.
Keywords: Exosome, Extracellular vesicle, Cargo, Delivery, Intercellular communication
Introduction
Extracellular vesicles (EVs) are nanoparticles (NPs) that are secreted from virtually all cell types that range in size from 20 to 1000 nm. Several EV nomenclatures have been proposed, including exosomes, microvesicles, and apoptotic bodies, depending on their size, site of biogenesis, and function (Raposo and Stoorvogel 2013; Théry et al. 2018). Certain molecules are enriched in EVs, thus cells likely employ a sorting mechanism to package specific molecules into EVs (Hagiwara et al. 2015; Shurtleff et al. 2016; Ageta et al. 2018). Notably, Valadi et al. reported that small EVs secreted from human and mouse cells contain RNA species such as microRNAs (miRNAs) and messenger RNAs (mRNAs) (Valadi et al. 2007).
Numerous studies have explored the physiological and pathological roles of EVs and their potential as intercellular delivery tools for cargo, mainly in mammalian systems. Nevertheless, despite considerable research over the past few decades, many details regarding the functions of EVs remain unclear (Margolis and Sadovsky 2019). Although the “EV cargo transfer hypothesis” has attracted many scientists from broad fields of biology and numerous studies have argued that EVs can deliver cargo from donor to recipient cells based on the findings of in vitro experiments, rigorous confirmational in vivo studies have not been reported. This is presumably because the true nature of EVs is difficult to assess, due to difficulties in purification, no standardization of materials and methods, and a lack of reliable bioassays for determining the functionality of EVs and obtaining solid evidence of intracellular trafficking. In addition to these technological problems, a fixed bias in support of the “EV cargo transfer hypothesis” has probably hampered the interpretation of EV research results.
In contrast to EVs, there is strong evidence that natural viruses are capable of delivering their cargo (i.e., genetic materials) into host cells. This is because viruses employ a sophisticated mechanism that overcomes the cellular barriers to delivering their genetic materials and establishing an infection. Viruses utilize viral proteins that enable specific receptor binding, cellular uptake, and membrane fusion with the host cell membrane and thus function as delivery vesicles for viral material cargo. Thus, it would be useful to compare the cellular uptake and delivery mechanisms of viruses with those of EVs. Therefore, the cargo delivery mechanism of viruses is discussed in this review.
Based on these considerations, the “EV cargo transfer hypothesis” in mammalian systems (derived mainly from human and mouse studies) is carefully reviewed and the present methodological issues are summarized. In 2018, the International Society for Extracellular Vesicles (ISEV) published “MISEV2018” as a general guideline for EV research (Théry et al. 2018). Certain issues discussed in the MISEV2018 somewhat overlap with those discussed in this review. Although the MISEV2018 and this review both highlight the importance of rigorous research, this review specifically focuses on the “EV cargo transfer hypothesis.”
EV-mediated cargo delivery
RNA cargo in EVs
EVs contain various molecules in their inner space, and RNA is the most widely studied EV cargo. This RNA cargo is thought to be transferred from donor cells to recipient cells and involved in intercellular communications in mammalian systems (Valadi et al. 2007; Kosaka et al. 2010; Pegtel et al. 2010; Zhang et al. 2010). The RNA species detected inside EVs include miRNAs (Mittelbrunn et al. 2011; Chevillet et al. 2014), mRNAs (Ratajczak et al. 2006; Xiao et al. 2012; Yokoi et al. 2017), and long-noncoding RNAs (Liu et al. 2016), as well as other RNA species (Baglio et al. 2015). Numerous studies have reported that specific RNA species are enriched in EVs, and it was shown that small RNAs are predominant (Valadi et al. 2007), presumably because smaller RNA species are easier to encapsulate into EVs than larger RNAs, such as rRNAs and mRNAs. Among the small RNA species found in EVs, tRNAs might be one of the most abundant, and tRNAs comprise >50% and ~30% of total small RNAs in human adipose-derived and bone marrow-derived mesenchymal stem cells, respectively (Baglio et al. 2015). In contrast, the fraction of regulatory RNAs, such as miRNAs and small nucleolar RNAs, in EVs is relatively small, at 2–5% of the total small RNAs. However, it is important to note that the RNA species present in EVs vary between studies, depending on the raw materials (body fluid or cell type), sample processing protocol, and analytical methods used (Schageman et al. 2013; Shurtleff et al. 2017). In addition, the cellular total RNA composition generally differs from the EV-enriched RNA composition, indicating that cells may utilize specific mechanisms for sorting RNAs into EVs (Ferguson and Nguyen 2016).
A previous study reported that the average copy number per particle for a single miRNA of interest is less than 0.01 (Chevillet et al. 2014). This estimation is in agreement with other reports stating that the amount of miRNA is much less than one copy per EV (He et al. 2019). These studies suggested that the majority of EVs do not possess small regulatory RNAs, such as miRNAs. Whether abundant miRNAs can be encapsulated in EVs or if only a specific population of EVs contains substantial functional RNAs remains unclear.
Protein cargo in EVs
In addition to RNA cargo, several proteins have been reported to be present inside EVs. EV-enriched proteins, such as Alix (Baietti et al. 2012), glyceraldehyde-3-phosphate dehydrogenase, and heat shock proteins (Théry et al. 1999), are found in the ExoCarta (http://www.exocarta.org) (Keerthikumar et al. 2016) and Vesiclepedia (http://microvesicles.org) databases (Kalra et al. 2012). Certain proteins, including Alix and TSG101(Théry et al. 2001), in EVs are thought to be involved in the biogenesis of EVs. Some RNA-binding proteins have also been detected in EVs. For example, YBX1 is present in EVs and may control global RNA sorting into EVs (Shurtleff et al. 2017). In addition, cytoplasmic proteins, such as actin and tubulin are loaded into EVs (Théry et al. 2001), presumably by passive loading machinery, during the biogenesis of EVs.
The protein cargo composition inside EVs largely depends on the parental cells and the subpopulation of EVs. The protein concentration in EVs is presumed to be equivalent to that of the parental cells. Considering that a single HeLa cell, with a diameter of 10–20 μm (BNID 115568 and 100432 (Milo et al. 2010)), contains ~150 pg of protein mass (BNID 109385 (Milo et al. 2010)), a single vesicle, at 100 nm in diameter, representing about one-millionth of the volume compared to that of the cell, would contain ~1.5 × 10−16 g of protein mass. This assumption is in accordance with a previous review (Sverdlov 2012). If the average molecular weight of a protein is 50 kDa, the number of proteins in a single EV is on the order of thousands, and may be ~2000/vesicle. Smaller vesicles, such as those that are only 30 nm in diameter, theoretically have hundreds of protein molecules.
EV cargo delivery mechanism
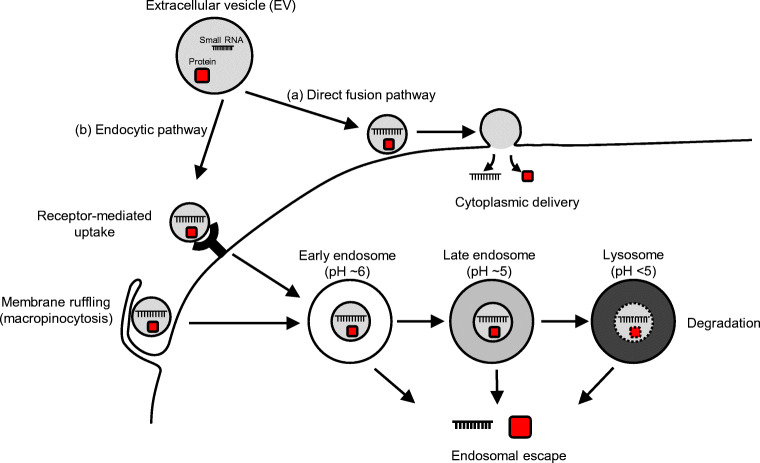
EVs are generated, with their cargo, secreted from parental cells, and taken up by other cells. During EV uptake, it is presumed that certain molecules on the recipient cell bind to EVs. Candidate EV-binding molecules on recipient cells have been described in the literature (Mulcahy et al. 2014). A few studies have suggested that EVs can directly fuse with the cellular membrane (Fig. 1a) (Parolini et al. 2009; Montecalvo et al. 2012). Although direct fusion between EVs and the plasma membrane cannot be ruled out (Prada and Meldolesi 2016), experimental evidence for direct fusion is lacking. Most studies have hypothesized that EVs are taken up by cells via the endocytosis pathway (Fig. 1b). Notably, cellular uptake of EVs depends on not only the biomolecules present on the EVs but also their physicochemical properties, such as their size (Lu et al. 2009) and surface potential (He et al. 2010). The correlation between the physicochemical properties of NPs and their cellular uptake has been extensively investigated in the field of nanotechnology. These studies will likely be helpful for understanding how EVs are internalized in recipient cells.
Fig. 1.
Schematic representation of the proposed cargo delivery pathways of EVs. EVs are taken up by recipient cells via either (a) a direct fusion pathway or (b) the endocytic pathway. a In the direct fusion pathway, EVs directly fuse with the plasma membrane and release their cargo into the cytoplasm. b In the endocytic pathway, after endocytosis (i.e., receptor-mediated endocytosis or micropinocytosis), EVs are transported to early endosomes, late endosomes, and eventually lysosomes. During endocytic transport, the pH of the endosomal compartment decreases, and EVs are ultimately processed and degraded in the lysosome. EVs may only achieve cargo delivery if the cargo leaves the endosomal compartment and enters the cytoplasm (endosomal escape)
Numerous studies have implicated endocytosis pathways in the uptake of EVs, such as phagocytosis (Feng et al. 2010), clathrin-mediated endocytosis (Tian et al. 2014), micropinocytosis (Tian et al. 2014; Costa Verdera et al. 2017), and caveolae-mediated endocytosis (Nanbo et al. 2013). The uptake pathway depends on several factors, such as the parental cell type, recipient cell type, and even the culture conditions of the recipient cell.
Once EVs are taken up, they are transported by the vesicular system via membrane trafficking. When the endosomes mature, the EVs are processed from early endosomes to late endosomes, and eventually to lysosomes. In the lysosomes, which are acidic degradation compartments, components of EVs may be enzymatically degraded. To complete cargo delivery, EVs need to escape the endo/lysosomal membrane (Fig. 1); in a process is known as “endosomal escape.” This may be the rate-limiting step in cargo delivery by EVs.
Endosomal escape: The rate-limiting step of cargo delivery
In the delivery of EV cargo to recipient cells, endosomal escape is the rate-limiting step. Hung and Leonard demonstrated that the mRNA and protein cargo within EVs from HEK293FT cells can be delivered to the endo/lysosomes of recipient PC-3 cells. However, the cargo was unable to escape from the endosomal compartment into the cytoplasm (Hung and Leonard 2016). They carefully designed their experiments and included appropriate controls, and further concluded that despite cellular uptake of the EVs by the recipient cells, the low efficiency of endosomal escape limits the delivery of cargo into the cytoplasm. Sutaria et al. reported that EVs from 293 T cells were unable to deliver an miRNA to recipient cells to silence its target gene (Sutaria et al. 2017), indicating that EVs are generally incapable of delivering cargo into the cytoplasm of recipient cells. Other studies demonstrated that RNA transfer by EVs is not available because of the lack of efficient endosomal escape (Kanada et al. 2015).
If the “EV cargo transfer hypothesis” holds true, then EVs must escape from the endosome or lysosome. Numerous studies have claimed that EVs can functionally deliver cargo to recipient cells and induce substantial phenotypic changes (Alvarez-Erviti et al. 2011; Ohno et al. 2013; Kamerkar et al. 2017). However, there is no direct evidence that EVs can escape the endo/lysosome. Thus, there is a knowledge gap in the “EV cargo transfer hypothesis,” as it is in fact still unclear whether EVs are capable of escaping the endo/lysosome or not.
EV-mediated RNA delivery
Problems in RNA/EV research
In addition to the lack of direct evidence for cargo delivery, there are several other issues and difficulties in EV research; for example, the lack of stoichiometric experiments and appropriate methodologies. Typically, the transfer of small RNAs via EVs has been studied as follows: EVs containing a cargo RNA are isolated from donor cells and added to a recipient cell culture, and then the cells are observed for some phenotypic change (gene expression, cellular function, etc.). The EV cargo is analyzed, and the small RNA that is responsible for the phonotypic change observed in the recipient cells is identified. These aforementioned studies were conducted in a well-organized manner. However, alteration of target gene expression in the recipient cells does not prove that the RNA cargo of the EVs was functionally delivered, as there are several potential unexpected causes, such as non-cargo EV components, contaminants in the EV sample, non-RNA cargo, etc. Indeed, our group has reported that both empty liposomes and EVs from bovine milk can alter lipid metabolism, suggesting that uptake of lipid NPs, without any specific cargo, can induce phenotypic changes in recipient cells (Fujita et al. 2019). Thus, various unexpected and cargo-independent factors can affect recipient cells and induce phenotypic changes.
Stoichiometric speculation: miRNA delivery by EVs
In experimental biology, it is generally essential to interpret the data and draw conclusions in a stoichiometric manner. Unfortunately, in some EV studies, a stoichiometric analysis was out of question. Here, the experimental design of EV studies was validated in a stoichiometric manner. To simplify the evaluation, only the bulk and averaged values were used.
The materials included EVs of interest and recipient cells. In Table 1, a typical experimental design is presented. About 10 μg of EVs from donor cells was mixed with recipient cells, and the results were observed. Herein, 1 μg of EVs corresponds to ~109–1010 particles in some studies (Sverdlov 2012; Webber and Clayton 2013). Therefore, 10 μg of EVs, equivalent to 1010–1011 particles, are present in the culture supernatant. The miRNA present in EVs is not a single species; in fact, sometimes thousands of miRNA species can be detected in EVs. Based on stoichiometric studies (Chevillet et al. 2014; He et al. 2019), if the EVs contain 0.01 copy of miRNA per particle, a maximum of 108–109 copies of an miRNA are present in the medium. If a phenotypic change is observed in the recipient cells, one might expect that an miRNA in the EVs caused the change.
Table 1.
In vitro experimental design for the evaluation of EVs and their cargo (miRNA or protein)
| Culture | EVs with miRNA/protein cargo | miRNA transfection | |||
|---|---|---|---|---|---|
| EVs | miRNA* | Protein | miRNA mimic | ||
| Experimental setting | 105 cells in 1 mL of medium | 10 μg of protein | 0.01 copies/EV | 10 μg (50 kDa) | 1–10 nM |
|
Number [mol] |
– | 1010–1011 particles |
108–109 [0.2–1.7 × 10−15 mol] |
1.2 × 1014 [2.0 × 10−10 mol] |
6 × 1011–1012 [1–10 pmol] |
| Molecules/cell | – | 103–104 | 1.2 × 109 | 106–107 | |
In contrast, when the function of a given miRNA is studied, cells are transfected with miRNA mimics or miRNA-expression vectors. Transfection with an miRNA mimic using conventional transfection reagents typically requires 1–10 nM miRNA (as shown in Table 1), and for some miRNAs, a higher concentration is required to obtain a silencing effect. This corresponds to 1–10 pmol or 1011–1012 copies of a specific miRNA in the medium. Comparison of the cargo miRNAs present in EVs to the miRNA mimics used for transfection reveals a large difference in miRNA copy number, on the order of 102–104. Moreover, experimental evidence has shown inefficient endosomal escape (1–3.5% of the total intracellular siRNA) of siRNAs delivered using synthetic NPs, which enable functional delivery of the siRNA and potent silencing in vivo (Gilleron et al. 2013; Wittrup et al. 2015). Although it is possible that the transfer efficiency of EVs is far superior to that of conventional transfection reagents and delivery tools in some situation, it seems to be unlikely. One study indicated that EVs are far less efficient to deliver miRNA than conventional transfection reagent (Stremersch et al. 2016).
Similar stoichiometric speculation has already been described in the literature (Sverdlov 2012); however, only a few studies have provided quantitative analyses. Stoichiometric and quantitative interpretations are notably neglected in most EV research. Furthermore, because the EV fraction contains heterologous EVs with numerous diverse miRNAs, it is nearly impossible to identify a single species of miRNA as the molecule that is responsible for any phenotypic change in the recipient cells, especially since a combination of miRNAs (and even other molecules) can induce the same phenotypic change.
A physiologically relevant amount of EVs must be used in cargo transfer experiments, as an excessive amount of EVs may lead to inappropriate interpretations. It is still a matter of debate whether the 10 μg of EVs (equivalent to 1010–1011 particles; Table 1) used in many in vitro experiments is a physiologically relevant amount. The yield of EVs from raw materials (culture supernatant or body fluid) is often quite low (0.5 μg of protein/mL of supernatant, and even lower in many EV studies); thus, extensive enrichment of EVs is typically performed. Considering an yield of 0.5 μg of protein/mL of supernatant, 10 μg of EVs can be obtained from 20 mL of medium. In this experiment, EVs were enriched 20 fold.
Delivery of RNA by EVs: A lack of evidence for functional delivery
Similar to miRNA, mRNA transfer by EVs has also been reported. However, even in the historical paper published in 2007 (Valadi et al. 2007), only a limited evidence of EV-mediated functional mRNA transfer was provided. The study proved that exosomes contain mRNA that is potentially translated to full-length proteins by in vitro translation technique. Although transfer of mRNA into recipient cells was confirmed by using 3H uracil-labelled RNA, functional cargo delivery (translation of mRNA into proteins inside recipient cells) was not verified. They detected mouse proteins in the recipient human cells treated with mouse cell-derived exosomes by mass spectrometry. However, it is still possible that direct transfer of mouse proteins in mouse cell-derived exosome fraction was detected, not from the mRNA-mediated production in recipient human cells.
To obtain evidence for EV-mediated transfer of mRNA cargo, a reporter assay using a bacteriophage P1-derived Cre recombinase was developed (Zomer et al. 2015). Utilizing this Cre reporter assay, Zomer et al. demonstrated that a Cre-encoding mRNA can be transferred from donor cancer cells to recipient cells via an in vivo EV model. Despite their excellent study, a small amount of Cre protein, even undetectable by western blotting, may exist inside the EVs and contribute to the observed recombination, as tiny amounts of Cre, theoretically a single molecule, can induce recombination in the recipient reporter cells. Furthermore, the recombination efficiency of EV-mediated mRNA transfer was low in in vivo studies. Thus, it remains unclear whether EV-mediated mRNA transfer is a physiologically significant event. Another problem is that the Cre reporter assay is a qualitative assay, as the reporter cell can only be off or on.
In another study, it was reported that 5′-triphosphate RNA can be transferred to recipient cells via EVs (Boelens et al. 2014). 5′-triphosphate RNA from stromal cells is recognized by the RNA sensor RIG-I in the cytoplasm of cancer cells, which activates antiviral immunity. In the study, the effect of EVs in the recipient cells was completely abrogated by RNase treatment of the EVs, suggesting that a non-EV RNA may be responsible for the phenotypic change since the RNAs within EVs should be protected from the RNase. Based on these findings, although cargo RNA transfer by EVs is still possible, it remains unclear whether EV-mediated mRNA transfer is a physiologically relevant phenomenon.
EV-mediated protein cargo delivery
Protein cargo delivery by EVs
Similar to RNA, proteins may be transferred from donor cells to recipient cells by EVs. A Cre reporter assay was used evaluate the transfer of Cre proteins from donor cells to recipient cells via EVs as only cytoplasmically delivered Cre can induce recombination and the recombination-dependent readout in the reporter cells. It is obvious that some fraction of Cre-containing EVs was taken up by the recipient cells and that recombination was induced; however, Cre-containing EVs generally yield a limited fraction of the recombination-positive cells in vitro (~1% of the total reporter cells (Sterzenbach et al. 2017)). This is presumably owing to the inefficient endosomal escape of Cre after endocytosis of the EVs.
In contrast, Yim et al. reported the efficient transfer of Cre protein via HEK293T-derived EVs using a light-activated protein engineering system called EXPLORs (Yim et al. 2016). They confirmed the presence of up to one protein molecule per vesicle in their system. The engineered EVs delivered Cre protein and achieved nearly 100% recombination in vitro. They claimed that EVs were delivered by direct fusion with the plasma membrane of the recipient cells, although relevant evidence was missing from the study. While the study did not include proper controls, such as non-EV Cre, for the Cre-containing EVs, it is plausible that EVs enriched with a specific cargo can achieve functional delivery.
Stoichiometric speculation: Protein delivery by EVs
EVs contain several proteins. Stoichiometric speculation was used to validate whether EV-mediated cargo protein delivery is possible in a model experiment (Table 1). If we assume (for simplicity) that all the protein mass in the 10 μg of EVs is cargo protein, and the protein of interest (50-kDa) comprises 1% of the total EV protein, a maximum of 0.1 μg (1.2 × 107 molecules/cell) of protein may potentially be delivered to the recipient cells. However, due to the low efficiency of endosomal escape, only a limited amount of protein would be delivered. Assuming that the efficiency of endosomal escape delivers 1% of the total protein, 1.2 × 105 molecules/cell of the protein of interest would reach the cytoplasm. Since the median protein copy number in HeLa cells is 18,000 (BNID: 108425 (Milo et al. 2010)), it is likely that 1.2 × 105 protein molecules/cell may be enough to exert its function in the recipient cells. Nevertheless, the amount of each cargo protein in the EVs varies. Furthermore, the efficiency of endosomal escape of EVs remains unclear. Thus, the hypothesis needs to be carefully interpreted in a stoichiometric manner.
Is the EV-mediated intercellular communication hypothesis true or false?
Is the cargo in EVs substantially transferred?
In the aforementioned sections, it was shown that EV-mediated cargo transfer is likely possible in few circumstances. However, experimental artifacts and a lack of rigorous methodology, which may lead to in a misunderstanding or misinterpretation of study results, remain a concern. The potential risk factors that create confusion regarding the “EV cargo transfer hypothesis” include EV fraction contaminants and the non-cargo molecules in EVs.
Contaminants in the EV fraction
EV research is intrinsically complex because EVs are multicomponent materials. Because researchers cannot uniformly purify EVs to a similar extent as recombinant proteins, EVs are often used as a crude fraction that presumably contains abundant non-EV components. The potential non-EV contaminants depend on the raw materials, i.e., culture supernatant or body fluid, and include cytokines, hormones, lipoprotein particles, protein aggregates, and RNA-protein complexes (Arroyo et al. 2011), as well as others that can potentially affect the function of recipient cells. Furthermore, in experiments using cell culture supernatant, materials from fetal bovine serum may be contaminants. Tosar et al. reported that the RNAs detected in the EV fraction can be contaminants (Tosar et al. 2017). In addition, some serum-free defined media also contain biological materials, such as growth factors and proteins. These materials can bring non-EV RNAs to the EV fraction. Similar criticisms have been published recently (Auber et al. 2019), indicating that despite using serum-free medium, non-EV RNAs can contaminate the EV fraction. These studies suggested that substantial non-EV materials can contaminate the EV fraction, and thus interfere with the experiments.
Non-cargo components of EVs
In addition to the cargo, non-cargo molecules in EVs can also function in intercellular communication. Recent reports suggest that functional proteins on the surface of EVs can stimulate recipient cells. For example, it was discovered that TGFβ-1 on the surface of EVs derived from mast cells can stimulate mesenchymal stem cells and induce a migrating phenotype (Shelke et al. 2019). Moreover, it was reported that cancer-derived EVs carry EV-bound proteins that induce phenotypic changes in recipient cells. A shorter form of vascular endothelial growth factor (VEGF) can bind to EVs via its affinity to heparin and can evade neutralizing antibodies (Ko et al. 2019). Based on these observations, it is clear that the surface molecules on EVs might be involved in EV-mediated intercellular communications. Stremerschs et al. attempted to deliver endogenous miRNA with B16F10 cell-derived EVs, which failed (Stremersch et al. 2016). They concluded that the non-specific effects of a non-cargo component (lipid or protein) of the EVs can impede the interpretation of cargo delivery studies.
What is the true role of EVs?
Although we believe that EVs play a role in intercellular communication by delivering cargo, they may have other biological functions. One possibility is that secretion of EVs with unnecessary materials is a mechanism of cellular homeostasis. In fact, it was demonstrated that EVs can exclude fragmented DNA from the cytoplasm to the extracellular space in senescence (Takahashi et al. 2017). Although cells can degrade intracellular materials through various mechanisms, such as autophagy (Mizushima 2007), is seems to be reasonable that EV secretion is a cellular disposal mechanism for maintaining homeostasis.
Comparison of EVs and viruses: Two vehicles for cargo delivery
Delivery mechanism of virus
Viruses are natural delivery vehicles that contain genetic materials. There are two major groups of viruses, enveloped and capsid. In this review, only enveloped viruses are considered in the comparison of EVs with viruses as delivery vehicles.
Infection, essentially the delivery mechanism of viruses, varies according to virus, but the mechanisms are generally classified into two types: direct fusion and endosomal escape, which is thought to be similar to that of EVs (Fig. 1) (Cohen 2016). The former mechanism is utilized by paramyxovirus (Aguilar et al. 2016) and human immunodeficiency virus-1 (HIV-1) (Stein et al. 1987). In this mechanism, interaction of a viral protein with a host receptor on the cell surface leads to a conformational change in the viral fusion protein, resulting in the fusion of the viral envelope and plasma membrane. In the latter mechanism, which is used by vesicular stomatitis virus (VSV) (Kim et al. 2017) and influenza virus (Skehel et al. 1982), after endocytosis, the decreased pH of the endosomal compartment induces a conformational change in the viral fusion protein, leading to membrane fusion. These two mechanisms share an important characteristic: viral fusogenic proteins are involved in the membrane fusion process; thus, viral cargo delivery requires efficient fusion. The essential part of membrane fusion is that specific receptors, a pH change, or other stimuli, lead to the fusogenic activity of the envelope proteins and the induction of membrane fusion.
Comparison of the fusion capability of EVs to that of viruses
In contrast to viruses, the membrane fusion capability of EVs is unknown. This concern was also described in the position paper of ISEV published recently (Russell et al. 2019). If the “EV cargo transfer hypothesis” is true, like viruses, EVs must achieve either direct fusion or endosomal escape. Moreover, if EVs are capable of fusing with the cellular membrane, specific molecules that are responsible for membrane fusion must exist. Some EV-specific proteins, such as CD9 and CD81, are known to be involved in membrane fusion (Stein et al. 2004). It would be interesting to determine whether these EV-specific molecules contribute to the membrane fusion of EVs during cargo delivery.
As mentioned above, experimental proof of the fusogenicity of EVs has been reported in few studies (Parolini et al. 2009; Montecalvo et al. 2012). In these pioneering studies, a lipophilic fluorescence dye, octadecyl rhodamine B chloride (R18), was used as a probe to monitor the membrane fusion of EVs with a model membrane or cellular membrane. However, it is generally accepted that assays using R18 often yield false-positive results, as R18 inserted into the membrane can diffuse via non-specific probe transfer between membranes (Stegmann et al. 1993; Trikash et al. 2010). Therefore, results from membrane fusion assays utilizing R18 must be appropriately controlled and carefully interpreted.
For some viruses, membrane fusion with host cells was studied using an imaging technique. In one study, Blanc et al. demonstrated that membrane fusion of VSV in endo/lysosomes can be visualized by using a lipophilic fluorescence dye (Le Blanc et al. 2005). The membrane fusion process of EVs could be visualized with a similar method. Although direct evidence demonstrating that EVs fuse with the cellular membrane of recipient cells in physiologically-relevant experiments is lacking, these imaging techniques may help to answer the question of whether EVs are capable of fusing with the cellular membrane of recipient cells.
Possible cargo transfer mechanism of EVs using viral infection machinery
During viral infection, cells express viral components, such as fusogenic proteins. Therefore, it is possible that EVs secreted from virus-infected cells can acquire fusogenic activity. Indeed, certain studies have suggested that EVs from virally infected cells deliver viral cargo molecules to recipient non-infected cells (Rodrigues et al. 2018). Additionally, a recent report demonstrated that hepatitis D virus (HDV), a satellite virus that usually requires the envelope protein of hepatitis B virus (HBV) for infectivity, can become infectious in the absence of HBV envelope proteins by using the envelope proteins from other viruses, such as VSV or hepatitis C virus (Perez-Vargas et al. 2019). This study suggested that viral envelope proteins may promote the delivery of nonspecific cellular components from virus-infected cells to the surrounding cells.
Mammalian cells possess endogenous virus-derived genes in their genome, such as endogenous retrovirus (ERV) sequences. Sometimes these genes are activated and express virus-like fusion proteins at certain developmental stages or in specific organs. If an endogenous fusion protein, such as syncytin, is present on the surface of the EVs, they may become fusogenic and efficiently deliver the cargo molecules to recipient cells. However, such endogenous fusion proteins are only expressed in limited organs (reproductive organs)(Mi et al. 2000) or at early developmental stages. In some diseases, such as various cancers (Larsson et al. 2007) and systemic lupus erythematosus (Tokuyama et al. 2019), the expression levels of endogenous retroviral fusion proteins are significantly upregulated. Under these circumstances, EVs can bear endogenous retroviral proteins and become fusogenic.
Potential solutions for rigorous EV studies
As previously mentioned, although the “EV cargo transfer hypothesis” was validated, a few methodological problems still exist in the field of EV research. In this section, potential solutions that may facilitate more reliable and rigorous EV research are summarized (Table 2).
Table 2.
Present challenges and potential solutions for EV cargo transfer studies
| Challenges | Solutions |
|---|---|
| Stoichiometry |
Stoichiometric experiments a) Absolute quantification of cargo molecules b) Stoichiometrically appropriate control |
| Lack of reliable bioassays |
Development of a feasible reporter assay a) Quantitative reporter assay b) Fusion assay c) Imaging to track intracellular trafficking |
| Interference by non-EV contaminants |
Removal of non-EV components by a combination of affinity purification methods Removal of EVs by immunoprecipitation to confirm that EVs are essential for the phenotypic changes in recipient cells |
| Cargo-specificity |
Using specific inhibitors that diminish the function of cargo a) miRNA inhibitors for miRNA cargo b) siRNAs for mRNA cargo |
Stoichiometric experiments
In general, it is important to design experiment in a stoichiometrically appropriate and physiologically relevant manner. “Always include appropriate controls” is an aphorism in experimental biology. The inclusion of appropriate controls may help to provide support for the RNA cargo transfer hypothesis. In EV cargo transfer experiments, a conventional transfection reagent might be an appropriate positive control. In experiments assessing EV-mediated small RNA transfer in which recipient cells are treated with EVs, as a control, “recipient” cells can be transfected with a small RNA mimic at the same dose as predicted to be inside the EVs. If the recipient cells in both experiments show the same phenotype, such as target gene silencing, the small RNA in the EVs might be functional in the recipient cells. Conventional transfection reagents are generally good enough to functionally deliver small RNAs into cells. Since the transfer efficiencies of EVs and conventional transfection reagents may differ, a stoichiometrically adequate control may also be necessary to support the possibility of small RNA transfer by EVs.
The same applies to mRNA and protein transfer experiments. For example, in mRNA transfer experiments, the recipient cells are treated with mRNA-containing EVs, whereas in the control, the cells can be transfected with in vitro-synthesized mRNA using a transfection reagent. The mRNA dose should be equivalent in both assays. If the two different mRNA transfers lead to a similar change in gene expression or phenotype, then it can be concluded that mRNA transfer and expression by EVs are possible.
For RNA studies, stoichiometrically designed experiments require absolute quantification of target RNA. Methods and instruments that enable absolute quantification of cargo RNA are available, including digital PCR (Bellingham et al. 2017) and other specific devices (He et al. 2019; Ramshani et al. 2019). These methods should help improve stoichiometric analysis and experiments in EV research.
Reliable, physiologically relevant assays
As discussed above, the Cre reporter system is useful for EV transfer experiments, but it is also highly sensitive. Thus, a more physiologically relevant reporter assay is required to validate cargo transfer by EVs. One approach is to use a component of the widely available Tet-OFF system (Gossen and Bujard 1992). Using this system, it is possible to monitor the transfer of tTA protein from donor cells to recipient cells by measuring the expression of a reporter gene under the control of the tetracycline response element (TRE) promoter (Mangeot et al. 2011). Since the expression level of the reporter gene under the TRE promoter reflects the quantity of functional cargo delivered into the recipient cells, use of this assay would enable quantitative evaluation of EV-mediated transfer of tTA protein or mRNA.
Rigorous membrane fusion assays are another key for validating the fusogenicity and cargo transfer ability of EVs. As described in the literature, the R18-based fusion assay often leads to false-positive results. Therefore, the development of a more reliable and rigorous membrane fusion assay may help to understand whether EVs are capable of fusing with the cellular membrane. Identification of the membrane fusion proteins on EVs is the key to understanding how EVs escape the endosome and deliver cargo into the cytoplasm. The siRNA cargo delivery mechanism of lipid NPs was extensively studied using imaging techniques (Wittrup et al. 2015). Using this imaging technology, the bursting of the NP cage and the release of the siRNA cargo into the cytoplasm can be quantitatively evaluated. A systematic and integrated approach is required to evaluate the cargo delivery mechanism of EVs.
EV-specific purification
As described above, the presence of non-EV components in the EV fraction may lead to false-positive results, as phenotypic changes in the recipient cells can be induced by non-EV components in the EV fraction. Thus, there is an urgent need to develop a specific purification method to isolate pure EVs from crude materials, such as culture supernatant and body fluids. Purification by ultracentrifugation is still considered the gold standard, and it is a popular, reliable method for purifying EV from any sample. However, pure EVs cannot be isolated by pelleting using ultracentrifugation, as the EV pellet always contains non-EV components.
Affinity-based methods would be more specific and may help remove non-EV components from crude materials. Recently, a purification method using the phosphatidylserine-binding protein TIM4 was reported (Nakai et al. 2016). In addition, the heparin-binding affinity of EVs may also be useful for purifying EVs from raw materials (Balaj et al. 2015). Presumably, a combination of these methods could improve the purity of EVs. Shurtleff et al. carefully isolated EVs by combining serial ultracentrifugation with immunoisolation using an anti-CD63 antibody to analyze the RNA cargo of EVs (Shurtleff et al. 2017). These methods can exclude the possibility of interference by non-EV components.
Conversely, the EV-cargo transfer hypothesis could be validated by the removal of EVs from an EV sample. If the EVs are required to induce the phenotypic changes in the recipient cells, depletion of the EVs would negate the effect. Immunoprecipitation using an EV-specific antibody can deplete specific EV components from the EV fraction, leaving non-EV components.
Inhibitors
Pretreatment of recipient cells with specific inhibitors can be a good control measure to verify the cargo transfer activity of EVs. For example, in experiments evaluating miRNA transfer by EVs, pretreatment of the recipient cells with an anti-miRNA should prevent the phenotypic change in recipient cells induced by the EVs and its cargo. Similarly, for mRNA transfer experiments, pretreatment of recipient cells with a specific siRNA against the target mRNA should diminish the functionality of the EV cargo mRNA. It is also recommended to use a translational inhibitor, such as cycloheximide, to distinguish whether the phenotypic change is induced by mRNA or protein cargo (Hung and Leonard 2016). Thus, cargo-specific inhibitors could help support the “EV cargo transfer hypothesis.”
Conclusions
As reported in the MISEV2018, various factors should be considered in EV research. In particular, the functional aspects of EVs must be carefully investigated. Interpretation of many EV studies has been biased by the assumption that EVs deliver cargo to recipient cells and induce phenotypic changes. The development of rigorous methodologies will improve our understanding of the true functions of EVs. The mystery of the tiny particles present in EVs also needs to be solved. Since EVs have been considered to be potential delivery vesicles for therapeutic cargo, such as synthetic siRNAs (Alvarez-Erviti et al. 2011), deciphering the delivery mechanism of EVs may lead to future clinical applications.
Acknowledgments
This work was supported by JSPS KAKENHI (Grant-in-Aid for Early-career Scientists no. 18 K18386) and a Research Grant from the JGC-S Scholarship Foundation.
Abbreviations
- EV
Extracellular vesicle
- miRNA
MicroRNA
- NP
Nanoparticle
- R18
Octadecyl rhodamine B chloride
- VSV
Vesicular stomatitis virus
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ageta H, Ageta-Ishihara N, Hitachi K, Karayel O, Onouchi T, Yamaguchi H, Kahyo T, Hatanaka K, Ikegami K, Yoshioka Y, Nakamura K, Kosaka N, Nakatani M, Uezumi A, Ide T, Tsutsumi Y, Sugimura H, Kinoshita M, Ochiya T, Mann M, Setou M, Tsuchida K. UBL3 modification influences protein sorting to small extracellular vesicles. Nat Commun. 2018;9:1–12. doi: 10.1038/s41467-018-06197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar HC, Henderson BA, Zamora JL, Johnston GP. Paramyxovirus glycoproteins and the membrane fusion process. Curr Clin Microbiol Rep. 2016;3:142–154. doi: 10.1007/s40588-016-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auber M, Fröhlich D, Drechsel O, Karaulanov E, Krämer-Albers EM. Serum-free media supplements carry miRNAs that co-purify with extracellular vesicles. J Extracell Vesicles. 2019;8:1656042. doi: 10.1080/20013078.2019.1656042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Balaj L, Atai NA, Chen W, Mu D, Tannous BA, Breakefield XO, Skog J, Maguire CA. Heparin affinity purification of extracellular vesicles. Sci Rep. 2015;5:10266. doi: 10.1038/srep10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Shambrook M, Hill AF. Quantitative analysis of Exosomal miRNA via qPCR and digital PCR. In: Hill AF, editor. Exosomes and microvesicles: methods and protocols. New York: Springer New York; 2017. pp. 55–70. [DOI] [PubMed] [Google Scholar]
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, ter Brugge P, Jonkers J, Slingerland J, Minn AJ. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111:14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen FS. How viruses invade cells. Biophys J. 2016;110:1028–1032. doi: 10.1016/j.bpj.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100–108. doi: 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Fujita K, Somiya M, Kuroda S, Hinuma S (2019) Induction of lipid droplets in non-macrophage cells as well as macrophages by liposomes and exosomes. Biochem Biophys Res Commun. 10.1016/j.bbrc.2019.01.078 [DOI] [PubMed]
- Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stöter M, Epstein-Barash H, Zhang L, Koteliansky V, Fitzgerald K, Fava E, Bickle M, Kalaidzidis Y, Akinc A, Maier M, Zerial M. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Katsuda T, Gailhouste L, Kosaka N, Ochiya T. Commitment of Annexin A2 in recruitment of microRNAs into extracellular vesicles. FEBS Lett. 2015;589:4071–4078. doi: 10.1016/j.febslet.2015.11.036. [DOI] [PubMed] [Google Scholar]
- He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- He D, Wang H, Ho S-L, Chan HN, Hai L, He X, Wang K, Li HW. Total internal reflection-based single-vesicle in situ quantitative and stoichiometric analysis of tumor-derived exosomal microRNAs for diagnosis and treatment monitoring. Theranostics. 2019;9:4494–4507. doi: 10.7150/thno.33683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung ME, Leonard JN. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J Extracell Vesicles. 2016;1:1–13. doi: 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers EM, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-'t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom B, Vázquez J, Vidal M, Wauben MH, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar S, LeBleu VS, Sugimoto H et al (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 10.1038/nature22341 [DOI] [PMC free article] [PubMed]
- Kanada M, Bachmann MH, Hardy JW et al (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci:201418401. 10.1073/pnas.1418401112 [DOI] [PMC free article] [PubMed]
- Keerthikumar S, Chisanga D, Ariyaratne D, al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: a web-based compendium of Exosomal cargo. J Mol Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Jenni S, Stanifer ML, Roth E, Whelan SP, van Oijen A, Harrison SC. Mechanism of membrane fusion induced by vesicular stomatitis virus G protein. Proc Natl Acad Sci. 2017;114:E28–E36. doi: 10.1073/pnas.1618883114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SY, Lee W, Kenny HA, Dang LH, Ellis LM, Jonasch E, Lengyel E, Naora H. Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake. Commun Biol. 2019;2:386. doi: 10.1038/s42003-019-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L-I, Holck S, Christensen IJ. Prognostic role of syncytin expression in breast cancer. Hum Pathol. 2007;38:726–731. doi: 10.1016/j.humpath.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Le Blanc I, Luyet P-P, Pons V, et al. Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang X, Gao S et al (2016) Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget 7. 10.18632/oncotarget.13465 [DOI] [PMC free article] [PubMed]
- Lu F, Wu S-H, Hung Y, Mou C-Y. Size effect on cell uptake in well-suspended, uniform Mesoporous silica nanoparticles. Small. 2009;5:1408–1413. doi: 10.1002/smll.200900005. [DOI] [PubMed] [Google Scholar]
- Mangeot P-E, Dollet S, Girard M, Ciancia C, Joly S, Peschanski M, Lotteau V. Protein transfer into human cells by VSV-G-induced Nanovesicles. Mol Ther. 2011;19:1656–1666. doi: 10.1038/mt.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis L, Sadovsky Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol. 2019;17:e3000363. doi: 10.1371/journal.pbio.3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC Jr, McCoy J. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- Milo R, Jorgensen P, Moran U, Weber G, Springer M. BioNumbers—the database of key numbers in molecular and cell biology. Nucleic Acids Res. 2010;38:D750–D753. doi: 10.1093/nar/gkp889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:1–14. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N, Naruse K, Sadamura Y, Hanayama R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6:33935. doi: 10.1038/srep33935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via Caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87:10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microrna to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven M, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vargas J, Amirache F, Boson B, Mialon C, Freitas N, Sureau C, Fusil F, Cosset FL. Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo. Nat Commun. 2019;10:2098. doi: 10.1038/s41467-019-10117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada I, Meldolesi J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int J Mol Sci. 2016;17:1296. doi: 10.3390/ijms17081296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshani Z, Zhang C, Richards K, Chen L, Xu G, Stiles BL, Hill R, Senapati S, Go DB, Chang HC. Extracellular vesicle microRNA quantification from plasma using an integrated microfluidic device. Commun Biol. 2019;2:189. doi: 10.1038/s42003-019-0435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Fan J, Lyon C, Wan M, Hu Y. Role of extracellular vesicles in viral and bacterial infections: pathogenesis, diagnostics, and therapeutics. Theranostics. 2018;8:2709–2721. doi: 10.7150/thno.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AE, Sneider A, Witwer KW, et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J Extracell Vesicles. 2019;8:1684862. doi: 10.1080/20013078.2019.1684862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schageman J, Zeringer E, Li M, et al. The complete exosome workflow solution: from isolation to characterization of RNA cargo. Biomed Res Int. 2013;2013:1–15. doi: 10.1155/2013/253957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelke GV, Yin Y, Jang SC, Lässer C, Wennmalm S, Hoffmann HJ, Li L, Gho YS, Nilsson JA, Lötvall J. Endosomal signalling via exosome surface TGFβ-1. J Extracell Vesicles. 2019;8:1650458. doi: 10.1080/20013078.2019.1650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, et al. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife. 2016;5:1–23. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R, Lambowitz AM. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci. 2017;114:E8987–E8995. doi: 10.1073/pnas.1712108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Bayley PM, Brown EB, Martin SR, Waterfield MD, White JM, Wilson IA, Wiley DC. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci. 1982;79:968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T, Schoen P, Bron R, Wey J, Bartoldus I, Ortiz A, Nieva JL, Wilschut J. Evaluation of viral membrane fusion assays. Comparison of the octadecylrhodamine dequenching assay with the pyrene excimer assay. Biochemistry. 1993;32:11330–11337. doi: 10.1021/bi00093a009. [DOI] [PubMed] [Google Scholar]
- Stein BS, Lifson D, Bensch KG, Engleman’t EG. pH-independent HIV entry into CD4-positive T cells iia virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Stein KK, Primakoff P, Myles D. Sperm-egg fusion: events at the plasma membrane. J Cell Sci. 2004;117:6269–6274. doi: 10.1242/jcs.01598. [DOI] [PubMed] [Google Scholar]
- Sterzenbach U, Putz U, Low L-H, et al. Engineered exosomes as vehicles for biologically active proteins. Mol Ther. 2017;25:1–10. doi: 10.1016/j.ymthe.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremersch S, Vandenbroucke RE, Van Wonterghem E, et al. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Control Release. 2016;232:51–61. doi: 10.1016/j.jconrel.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Sutaria DS, Jiang J, Elgamal OA, Pomeroy SM, Badawi M, Zhu X, Pavlovicz R, Azevedo-Pouly ACP, Chalmers J, Li C, Phelps MA, Schmittgen TD. Low active loading of cargo into engineered extracellular vesicles results in inefficient miRNA mimic delivery. J Extracell Vesicles. 2017;6:1333882. doi: 10.1080/20013078.2017.1333882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverdlov ED. Amedeo Avogadro’s cry: what is 1μg of exosomes? BioEssays. 2012;34:873–875. doi: 10.1002/bies.201200045. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, Hara E. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Boussac M, Véron P, et al. Proteomic analysis of dendritic cell-derived Exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;8:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Zhu Y-L, Zhou Y-Y, Liang GF, Wang YY, Hu FH, Xiao ZD. Exosome uptake through Clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama M, Gunn BM, Venkataraman A et al (2019) Antibody against envelope protein from human endogenous retrovirus activates neutrophils in systemic lupus erythematosus. bioRxiv. 10.1101/776468
- Tosar JP, Cayota A, Eitan E, et al. Ribonucleic artefacts : are some extracellular RNA discoveries driven by cell culture medium components ? J Extracell Vesicles. 2017;6:1–10. doi: 10.1080/20013078.2016.1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikash I, Gumenyuk V, Lishko V. The fusion of synaptic vesicle membranes studied by lipid mixing: the R18 fluorescence assay validity. Chem Phys Lipids. 2010;163:778–786. doi: 10.1016/j.chemphyslip.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2:1–6. doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup A, Ai A, Liu X, Hamar P, Trifonova R, Charisse K, Manoharan M, Kirchhausen T, Lieberman J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat Biotechnol. 2015;33:870–876. doi: 10.1038/nbt.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, Zacharias W, Hao H, McMasters K. Identifying mRNA, MicroRNA and protein profiles of melanoma Exosomes. PLoS One. 2012;7:e46874. doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim N, Ryu S-W, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park JH, Kim D, Heo WD, Choi C. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda SI, Kato T, Kiyono T, Takeshita F, Kajiyama H, Kikkawa F, Ochiya T. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470. doi: 10.1038/ncomms14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu D, Chen X, et al. Secreted Monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ, Wurdinger T, Pegtel DM, van Rheenen J. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]