Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors with poor prognosis. Aryl hydrocarbon receptor (AHR) is a ligand-dependent transcription factor and emerging evidence shows it is associated with tumor initiation and promotion. However, the relationship between AHR and ESCC is not clear and it is meaningful to explore whether AHR could be a therapeutic target. In the present study, immunohistochemistry was performed to determine AHR expression levels in ESCC tissues. Knockdown of AHR expression in ESCC cell lines genetically and modulation of AHR by 3, 3′-diindolylmethane (DIM) pharmacologically both in vitro and in vivo were utilized to examine the corresponding alterations in cell growth, migration and invasion. Our study indicated that AHR expression levels were elevated in ESCC and associated with poor prognosis. Both knockdown and modulation of AHR inhibited tumor progression through down-regulating expression levels of PCNA, Bcl-2, Cyclin D1, MMP1, MMP2, MMP9 and up-regulating expression levels of Bax, Cleaved-Caspase 3. Our findings also indicated that repressing COX2/PGE2/STAT3 axis exerted inhibitory effects on ESCC both in vitro and in vivo assays. Taken together, AHR plays the key role in ESCC progression and targeting AHR as a therapeutic strategy with DIM is deserved for further exploration.
Keywords: AHR, COX2, ESCC, PGE2, STAT3
Introduction
Esophageal carcinoma is the sixth main cause of cancer-related mortality and the eighth most common cancer worldwide. Meanwhile, it ranks the forth in terms of cancer diagnosis and death in China. Esophageal squamous cell carcinoma (ESCC) is the predominant histological type, especially in Asian countries. Generally speaking, the most well-known risk factors for ESCC are tobacco use and alcohol consumption (Lin et al. 2013; Pennathur et al. 2013). Meanwhile, environmental and dietary factors play a pivotal role in ESCC initiation.
Aryl hydrocarbon receptor (AHR) has been found in the study process of environmental pollutants, such as polycyclic aromatic hydrocarbon compounds. AHR is a ligand-dependent transcription factor diffusely located in the cytoplasm. Once being ligand-activated, the AHR-ligand complex then translocates into the nuclei, binding with the aryl hydrocarbon receptor nuclear translocator (ARNT). With that, the AHR-ligand-ARNT complex is recruited to the xenobiotic response elements (XREs) in the regulatory regions to further participate in transcription of target genes (Rothhammer and Quintana 2019). Among these target genes, the most predominant ones are the microsomal cytochrome P450-dependent monooxygenases CYP1A1 and CYP1A2 (Cella and Colonna 2015). Hence, the increasing production of CYP1A1 or CYP1A2 is cited as the exact symbol of AHR translocation for detecting the interaction between the ligand and receptor in bulks of researches on AHR (Wang et al. 2009; Callero et al. 2012; Yin et al. 2012). Apart from its capacity of mediating the toxicity, AHR has been gradually deemed as a collaborator to take part in tumorigenesis, promotion, progression and metastasis. Emerging evidence has shown that AHR expression levels are elevated in tumors and can be found chronically activated to facilitate the tumor malignant biological properties (Murray et al. 2014). According to recent studies, AHR is involved in various malignant tumors including lung cancer, gastric cancer, breast cancer, oral squamous cell carcinoma and so on (Hanieh 2015; Stanford et al. 2016; Wei et al. 2016; Ye et al. 2018). However, researches on AHR in esophageal carcinoma are so limited that it is meaningful to set particular focus on ESCC. As a ligand-dependent receptor, AHR exerts its specific function on cell growth, apoptosis and metastasis in line with the certain ligand. The well-accepted classification of AHR ligands is comprised of exogenous and endogenous ligands. However, the interest in exploring the different transcriptional activity of different ligands binding with AHR leads to a more appropriate classification, including the complete agonist, the selective modulators and the pure antagonist (Smith et al. 2011). 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) is the most ever known potent prototypic exogenous complete agonist for AHR along with the property of high toxicity (Boffetta et al. 2011). Meanwhile, 3,3′-diindolylmethane (DIM), as a selective modulator for AHR, is a small molecule compound and a major active metabolite of indole-3-carbinol (I3C) which is mainly present in cruciferous vegetables, such as broccoli, cabbage, and cauliflower (Aggarwal and Ichikawa 2005). DIM is deemed as a selective modulator of AHR with its non-toxic property in cell lines study. As one of indole derivatives from cruciferous vegetables, DIM could exert huge anti-tumor effects with evidence that I3C mainly targets the aryl hydrocarbon receptor pathway to suppress proliferation of different tumor cells. Meanwhile, I3C has been demonstrated that it can promote AHR translocated into the nuclei to bind to the P450 promoter and inhibit the binding of AHR to the COX2 promoter. As a matter of course, DIM may repress the COX2 signaling (Popolo et al. 2017).
Cyclooxygenase (COX) is a key rate-limiting enzyme that catalyzes the conversion of arachidonic acid to prostaglandins to finally induce the production of prostaglandin E2 (PGE2). COX2 is an inducible enzyme activated by pro-inflammation cytokines and growth factors, and a large body of researches has demonstrated the high level of COX2 expression rather than COX1 in malignant tumors, such as non-small cell lung cancer, prostate cancer, breast cancer, colorectal cancer and ESCC (Miyashita et al. 2006; Akutsu et al. 2011; Garg et al. 2018; Peng et al. 2018; Shimizu et al. 2018; Zhou et al. 2018). The COX2-PGE2 pathway has emphasized its importance of tumorigenesis in colorectal cancer on aspects of tumor proliferation, angiogenesis and metastasis (Greenhough et al. 2009). Meanwhile, Signal transducer and activator of transcription 3 (STAT3) stresses its crucial involvement in various cellular processes such as cell proliferation, apoptosis and drug resistance, and has recently been broadcast to be aberrantly activated in many cancers (Zhao et al. 2016). A recent study has elucidated the role of COX2/PGE2/STAT3 axis in initiation of epithelial-mesenchymal transition (EMT) induced by castration in prostate cancer (Tong et al. 2017). The far-reaching mysterious function of the axis in tumorigenesis and progression is deserved to be lucubrated to seek for new targeted therapy. In this study, we focus on AHR high expression levels in ESCC so as to explore the gene function change by knockdown of AHR and pharmacologically modulation of AHR by DIM both in vitro and in vivo experiments to investigate the corresponding reflection on cell progression. Evidence indicates that DIM is an efficient anti-cancer drug for ESCC treatment through modulation of AHR by repressing the COX2/PGE2/STAT3 axis.
Materials and methods
Patients and clinical specimens
54 patients who underwent esophageal carcinoma resection in Department of Thoracic Surgery of the First Hospital of China Medical University between 2011 and 2013 were enrolled in this study. All patients were diagnosed with ESCC confirmed by pathologists with postoperative pathological biopsy and had never ever experienced any radiotherapy or chemotherapy before surgery. Paired tumoral and non-tumoral (at least 5 cm distance from the tumor margin) tissues were obtained. The clinical pathological data and follow-up information were collected for further analysis. The Eighth Edition of American Joint Committee on Cancer (AJCC) Esophageal Cancer Staging System was used for tumor staging. The overall survival (OS) time was calculated from the date of surgery to the date mortality from ESCC. If the patient was alive till end, then the OS time was calculated from the surgery time to the last date of follow-up. A letter of consent was signed by each patient.
Cell culture and reagents
Human ESCC cell lines TE1 and KYSE150 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS, Biological Industries, Israel) and 1% penicillin and streptomycin (Biological Industries, Israel), then cultured in incubators with a humidified atmosphere of 5% CO2 at 37 °C. 3, 3′-diindolylmethane (DIM), Resveratrol (RSV), Celecoxib and Prostaglandin E2 (PGE2) were purchased from MCE, USA.
Lentivirus transfection
The shRNA coding sequence of human AHR (sh-AHR sequence: 5’-CCGGCGGCATAGAGACCGACTTAATCTCGAGATTAAGTCGGTCTCTATGCCGTTTTTG-3′) and negative control sequence (sh-NC sequence: 5′- CCGGGCAAGCTGACCCTGAAGTTCATCTCGAGATGAACTTCAGGGTCAGCTTGCTTTTTT-3′) were designed and constructed with the GV248 lentivirus vector (Genechem, Shanghai, China). TE1 and KYSE150 cell lines were transfected with AHR (sh-AHR) and negative control (sh-NC) lentivirus respectively. After transfection, the cells were reseeded in medium with 2 μg/ml puromycin (Sigma Aldrich, USA) to finally wipe out the untransfected cells. Western blot was performed to identify the transfection efficiency.
Immunohistochemistry (IHC)
The Ultra™Sensitive SP (Mouse/Rabbit) IHC Kit (Maixin, China) was used for IHC. Primary antibodies against AHR (1:200, Santa Cruz, USA), proliferating cell nuclear antigen (PCNA, 1:4000, CST, USA), Bax, Bcl-2, Cleaved-Caspase 3, Cyclin D1, MMP1, MMP9 (1:200, CST, USA), MMP2 (1:400, OriGene, USA), COX2, STAT3, p-STAT3 (1:600, CST, USA) were applied to the slices followed by secondary antibody conjugated with streptavidin-biotin-horseradish peroxidase complex. Staining was performed with DAB (3, 3′-Diaminobenzidine) kit (Maixin, China) to produce the brown reactions. Then the slices were counterstained with hematoxylin, dehydrated, transparentized and covered with coverslips using neutral resin. IHC scores for clinical samples were performed independently by two experienced pathologists who were blind to any relevant information. Scores were calculated by evaluating the average staining intensity and the staining ratio at 5 high-power fields of slices at random. The staining intensity was scored as: 0 (no staining), 1 (weak staining), 2 (moderate staining), 3 (strong staining). The staining ratio of positive reaction cells was scored as: 0 (<5%), 1 (5–25%), 2 (26–50%), 3 (51–75%), 4 (>75%). Staining intensity and ratio were then multiplied to form the final IHC score with the range from 0 to 12. Final score ≥ 4 was deemed as high expression of AHR and final score < 4 was deemed as low expression of AHR.
Cell viability assay
Cell viability assay was evaluated by the Cell Counting Kit-8 (CCK8, MCE, USA). Cell counting was performed by a Scepter™ Handheld Automated Cell Counter (Merck Millipore, Germany). In brief, 3 × 103 cells were seeded in 96-well plate with 100 μl medium with or without different concentrations of DIM (0-60 μmol/L) or RSV (1, 5, 10, 20 μmol/L) to incubate for 24, 48, 72 and 96 h. 4 h before the time point, 10 μl CCK-8 reagent was added into each corresponding well. Cell viability was measured by detecting the absorbance at 450 nm to obtain the optical density (OD) value. All assays were repeated at least three times.
Colony formation assay
For transfected cells (sh-NC and sh-AHR), approximately 100 cells were seeded in 24-well plate with 1 ml medium for 10 days. For TE1, 500 cells were seeded with 1 ml medium containing various concentrations of DIM (0-60 μmol/L). 10 days later, the medium was removed and the colonies were washed gently with PBS, fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for 15 min.
DNA synthesis assay
For transfected cells, Cell-Light Apollo488 EdU Stain Kit (Ribobio, Guangzhou, China) was used for detecting DNA synthesis. EdU (5-Ethynyl-2′- deoxyuridine) of 50 μmol/L dissolved in medium was added into each culture dish and cultured for 2 h. Then, medium was removed and each culture dish was washed with PBS for three times and fixed with 4% paraformaldehyde for 15 min and 0.5% Triton-X100 was used to permeate for 10 min. Apollo Stain Kit was used according to the protocol and DAPI (Solarbio, Beijing, China) was used to stain the nuclei. After that, we used the inverted fluorescence microscope (Leica, Germany) to observe the results of EdU positive staining.
Cell cycle assay
Cell cycle detection was performed by flow cytometry. For transfected cells, cells were prepared at logarithmic phase and washed with cold PBS, then fixed with 70% cold ethanol overnight at 4 °C. The second day, cells were centrifuged at 1000 rpm for 3 min and washed to eliminate the ethanol. 490 μl PBS was added to resuspend the cells and RNase A (Sigma Aldrich, USA) was utilized to break the interference of RNA. After incubation at 37 °C in water bath for 30 min, the cells were stained with 10 μl propidium Iodide (PI, 50 μg/ml, BD Biosciences, USA) for another 30 min placed in cold ice away from light. Cell cycle distribution was detected by a BD LSRFortessa instrument (BD Bioscience, USA) and the results were analyzed with the ModFit software (Becton Dickinson, USA).
For TE1, cells were previously cultured in serum-free medium for 24 h in order to synchronize initial cycles. Next, medium were replaced with complete medium with different concentrations of DIM (0-60 μmol/L). After 48 h’ incubation, the cells were treated with aforementioned steps.
Apoptosis assay
Cell apoptosis was detected using the Annexin V-FITC/PI Apoptosis Detection Kit (BD Bioscience, USA) by flow cytometry. Transfected cells and TE1 cells were treated with different medium aforementioned. Next, cells were trypsinized, washed and resuspended in 100 μl binding buffer, then counterstained with the Annexin V and PI for 15 min in dark place. After that, cell apoptosis were detected and analyzed with the use of BD LSRFortessa instrument.
Wound scratch assay
Cell migration was monitored by wound scratch assay and the extent of wound healing was regarded as the cell mobility. All cells were seeded in 6-well plate and when the cell confluence reached almost 100%, at least 3 scratches were made per well using a 10 μl pipette. After scratching, cells were washed with PBS for three times to swill out the dissociative cells and the medium was replaced with serum-free medium. Various concentrations of DIM (0, 20, 40, 60 μmol/L) were dissolved in serum-free medium to incubate for 0, 24, 48 and 72 h. At each time point, images of scratches were captured with the use of an inverted fluorescence microscope (Leica, Germany).
Transwell assay
Cell invasion assay was carried out using an 8 μm pore size 24-well Transwell Chambers (Costar, USA). The upper chamber was coated with diluted Matrigel (BD Bioscience, USA) and ready for use when the matrigel was the form of solidification. Totally 2 × 104 cells were seeded onto the upper chamber in 1% FBS medium containing 40 μmol/L DIM or not. 600 μl medium with 20% FBS was filled into the lower chamber. After incubation for 48 h, the upper chamber was moved out and washed gently with PBS. 4% paraformaldehyde was used to fix the cells traversing the membrane at the outside bottom of chamber. The invaded cells were stained with 0.1% crystal violet and at least 3 randomly selected fields were captured and counted averagely by the inverted microscope.
Western blot
Cells were harvested after certain treatment, washed with PBS and then lysed with RIPA lysis buffer (Beyotime, China) supplemented with 1 mM of PMSF (Beyotime, China) and phosphatase inhibitor (MCE, USA). The concentration of cell lysates was measured using the Enhanced BCA Protein Assay Kit (Beyotime, China). Proteins with SDS loading buffer boiled at 95 °C were separated by 10% SDS-PAGE and then transferred to the polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Germany). The PVDF membranes were blocked by 5% non-fat milk (Solarbio, China) and primary antibodies against AHR, CYP1A1, PCNA, Bcl-2, Bax, Caspase3, Cleaved-Caspase3, Cyclin D1, COX2, STAT3, p-STAT3, MMP1, MMP2 and MMP9 were used overnight at 4 °C with gentle shaking. Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies and ECL detection reagents (Beyotime, China) were used to visualize the proteins. All experimental results were repeated in triplicate. Densitometry analysis for Western blots was conducted by Image J software.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used for quantification of PGE2. Approximately 1 × 105 TE1 cells per well were seeded in 12-well plate with 2 ml serum-free medium containing varying concentrations of DIM (0, 10, 20, 30, 40, 50, 60 μmol/L) for 48 h. The medium were collected and centrifuged to obtain the supernates. The PGE2 released in supernates was measured by the Parameter™ PGE2 Assay Kit (R&D Systems, USA) according to the manufacturer’s instructions.
Tumor xenograft assay
The animal study was approved by the Animal Ethics and Experimental Committee of China Medical University (Approved number: 2018146). 4--6-week-old male BALB/c nude mice were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. and raised under specific pathogen-free conditions in Animal Center of China Medical University. Totally 2 × 106 TE1 cells suspended in 200 μl PBS were injected subcutaneously into the flanks of each mouse. After one week for visible tumor generation, all 16 mice were randomly allocated into two groups (8 mice each group). Mice in control group were treated by intragastrical gavage with 200 μl PBS daily, whereas the mice in DIM group were treated with 10 mg/kg/day of DIM suspended in 200 μl PBS. Mice weight and tumor size were monitored twice a week throughout the study. The tumor volume was calculated by the formula V = 1/2 × length × width2 (mm3). After gavage for 28 days, the mice were sacrificed and the tumor tissues were stripped, weighed and subsequently processed for IHC staining.
Statistical analysis
All statistical analyses were performed with the use of SPSS software (version 20, USA). GraphPad Prism version 8.0 was used for creating the artwork. Experimental data were presented as means±standard deviation (SD). To compare the statistical significance of difference between two groups, data were analyzed by paired or unpaired two-tailed Student’s t test and χ2-test or corrected χ2-test or Fisher’s Exact Test. For more than two groups’ comparison, one-way ANOVA was utilized. The survival analysis was performed with the Kaplan-Meier method. A p value <0.05 was considered statistically significant.
Results
AHR expression levels are elevated in tumor tissues and correlate with poor prognosis of ESCC
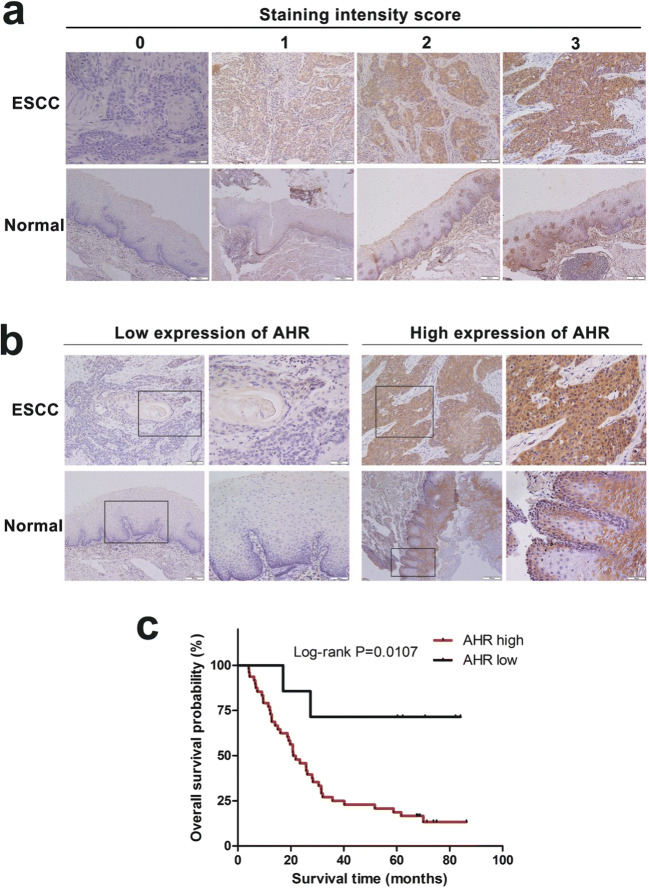
To investigate whether AHR expression levels in ESCC were different from that in normal esophageal tissues, we collected 54 ESCC patients’ surgical samples (aged from 40 to 81, average 59.46 years old) including paired tumor and normal tissues from 2011 to 2013 for IHC. IHC staining intensity scores were assessed individually according to slices’ gradation of reaction color (Fig.1a). Results showed that AHR expression levels were elevated in tumors compared with normal tissues and positive staining was mainly located in cytoplasm and nucleus. Whereas in paired normal esophageal tissues, staining was stressed mainly in epithelial basal layer (Fig. 1b). To explore whether AHR expression in tumors had any correlation with ESCC progression, we analyzed its relationship with clinical pathological parameters (Table 1). Among 54 patients, AHR was extremely overexpressed in 47 patients and expression of AHR was significantly related with lymph node metastasis and clinical stage. It showed no significant relationship with patients’ age, gender, T stage and differentiation. The Kaplan-Meier survival analysis was conducted to determine whether AHR expression was correlated with prognosis. As expected, ESCC patients with high AHR expression had significantly shorter overall survival time than those with low AHR expression (Fig. 1c). Evidence showed that AHR expression levels may be a potential biomarker in diagnosis.
Fig. 1.
High expression of AHR in ESCC correlates with poor prognosis. a Representative images of IHC staining intensity level, 0(no staining), 1(weak staining), 2(moderate staining), 3(strong staining). Magnification: 200×. b Representative IHC images of low or high AHR expression in ESCC and normal tissues. Magnification: 200×, left panel; 400×, right panel. c The Kaplan-Meier survival analysis of AHR expression in 54 patients
Table 1.
Expression levels of AHR in ESCC and their correlation with clinicopathological parameters
| Parameters | Number of cases | Expression of AHR | P value | |
|---|---|---|---|---|
| Low | High | |||
| Paired normal tissues | ||||
| Low | 49 | 4 | 45 | 0.010* |
| High | 5 | 3 | 2 | |
| Age (years) | ||||
| ≤ 60 | 32 | 5 | 27 | 0.772 |
| > 60 | 22 | 2 | 20 | |
| Gender | ||||
| Male | 46 | 4 | 42 | 0.095 |
| Female | 8 | 3 | 5 | |
| T stage | ||||
| T1-T2 | 28 | 5 | 23 | 0.480 |
| T3-T4 | 26 | 2 | 24 | |
| Lymph node metastasis | ||||
| Negative | 32 | 7 | 25 | 0.033* |
| Positive | 22 | 0 | 22 | |
| Clinical stage | ||||
| I-II | 32 | 7 | 25 | 0.033* |
| III-IV | 22 | 0 | 22 | |
| Differentiation | ||||
| Well | 12 | 4 | 8 | 0.058 |
| Moderate / Poor | 42 | 3 | 39 | |
Statistical analyses were performed by χ2-test or corrected χ2-test or Fisher’s Exact Test. * P < 0.05
Knockdown of AHR inhibits cell growth and promotes cell cycle arrest
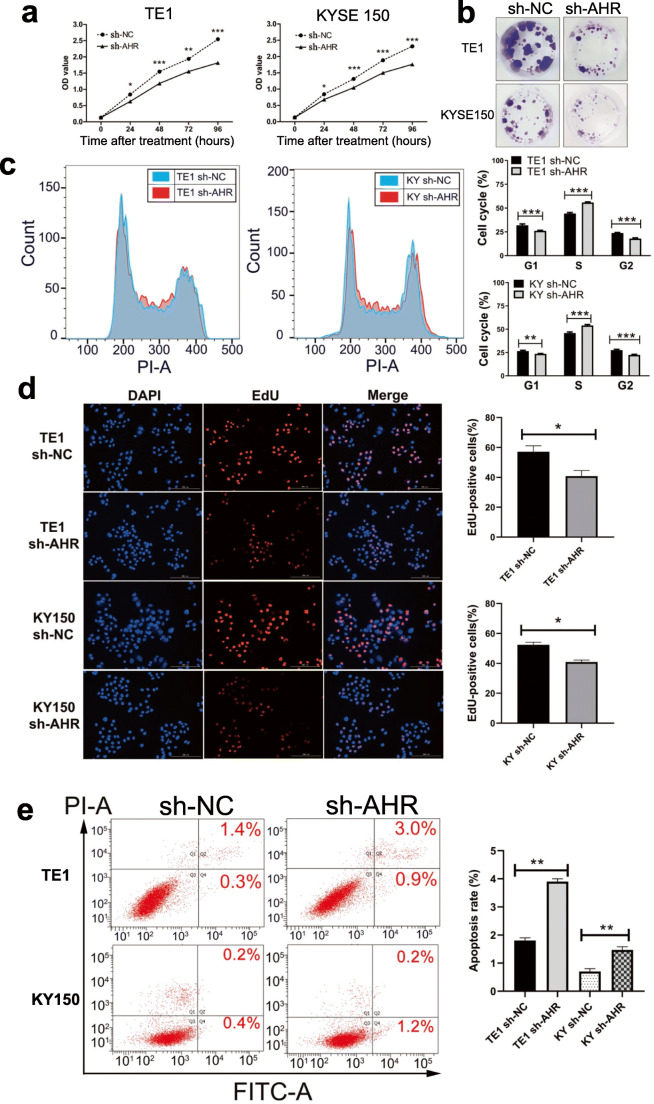
Since AHR expression was high in ESCC, we had tried to establish the knockdown of AHR cell lines via transfection with lentivirus. We performed the CCK8 assay to investigate cell viability after knockdown of AHR. For both two cell lines, sh-AHR cells proliferated more slowly than sh-NC cells (Fig. 2a). Colony formation assay indicated that after a long certain time for incubation, sh-AHR cells formed fewer colonies (Fig. 2b). Flow cytometry was used to confirm the cell cycle arrest since cell cycle was vital for cell growth. Results indicated that compared with sh-NC cells, sh-AHR cells were arrested in S phase accounting for approximate a more 10% part and compensatorily decreased in G1 and G2 phase (Fig. 2c). Therefore, we performed the EdU staining assay to show DNA synthesis change caused by knockdown of AHR and results (Fig. 2d) significantly indicated S phase was blocked when depleting AHR. Since cell growth was mediated by AHR, we further examined whether AHR was involved in apoptosis. Not very much, two cell lines after transfection exhibited different sensitivity in AHR-silence-related apoptosis since TE1 sh-AHR cells apoptosis rate rose significantly from 1.7% to 3.9% and KYSE150 sh-AHR cells rose from 0.6% to 1.4% (Fig. 2e). Although the apoptotic rate was minimal, especially of KYSE150 cells, data statistics indicated results were significant. All in all, knockdown of AHR exactly suppressed ESCC cell growth and promoted cell cycle arrest.
Fig. 2.
Knockdown of AHR inhibits proliferation and promotes apoptosis. a CCK8 assay was performed to evaluate cell viability post-transfection. b Knockdown of AHR reduced the colony formation. c Cell cycle was detected by flow cytometry and more cells were arrested in S phase after knockdown. d
EdU staining of sh-NC and sh-AHR cells was performed to show DNA synthesis change. e Cell apoptosis assay was done to indicate that knockdown of AHR promotes apoptosis. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
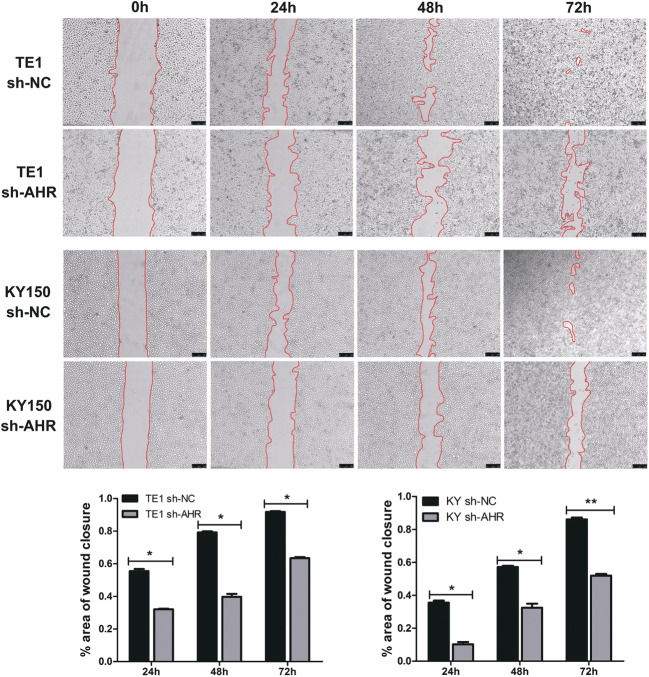
Knockdown of AHR inhibits cell migration and invasion
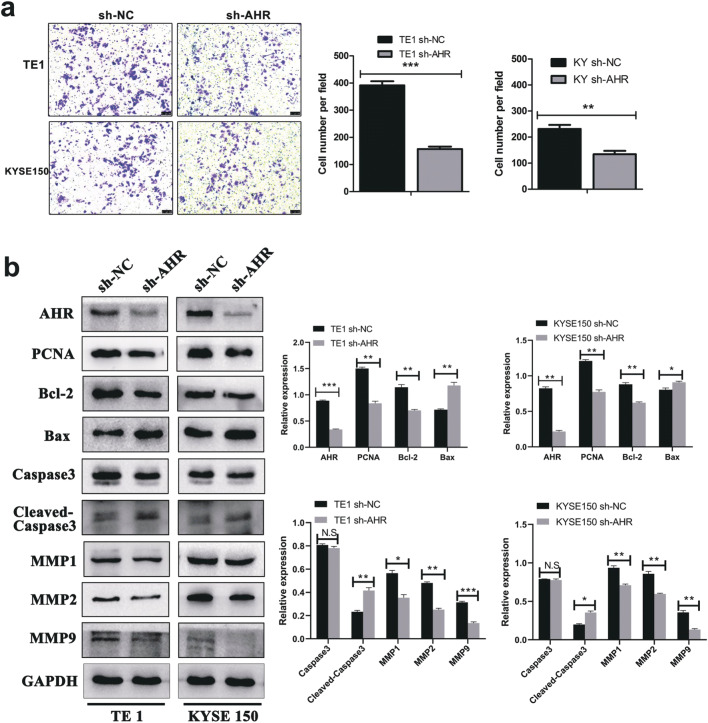
ESCC is outstanding in metastasis so that examining cell migration and invasion is of significance. The wound scratch assay was performed and results showed that silence of AHR correspondingly repressed ESCC cells migratory ability (Fig. 3). Transwell assay was conducted to confirm if AHR mediated tumor invasion and from the cell number traversing the membrane, we could see that knockdown of AHR indeed limited the ESCC cell invasive ability (Fig. 4a). Western blot showed the underlying mechanism that PCNA and Bcl-2 were significantly down-regulated in sh-AHR cells and to the contrary, apoptosis-related proteins such as Bax and Cleaved-Caspase 3 expression were correspondingly elevated. Absolutely, knockdown of AHR reduced the MMP1, MMP2 and MMP9 metastasis-related proteins expression (Fig. 4b).
Fig. 3.
Knockdown of AHR inhibits ESCC migration. *, P < 0.05; **, P < 0.01
Fig. 4.
Knockdown of AHR inhibits ESCC invasion and results in relative protein expression levels change. a Transwell assay indicated that after transfection, ESCC cell lines lost the capacity of invasion compared with high AHR expression cells. Magnification: 100×. b Western blot showed the down-regulation of relative protein expression regarding cell growth, apoptosis, migration and invasion. *, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S, no significance
Modulation of AHR by DIM inhibits cell growth, induces cell cycle arrest and promotes cell apoptosis
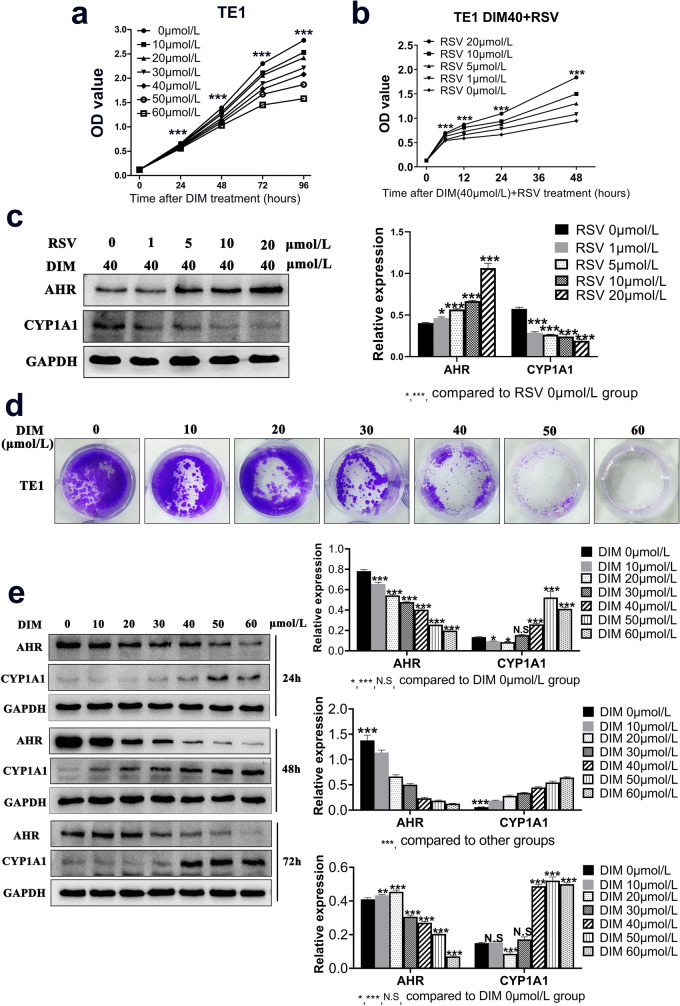
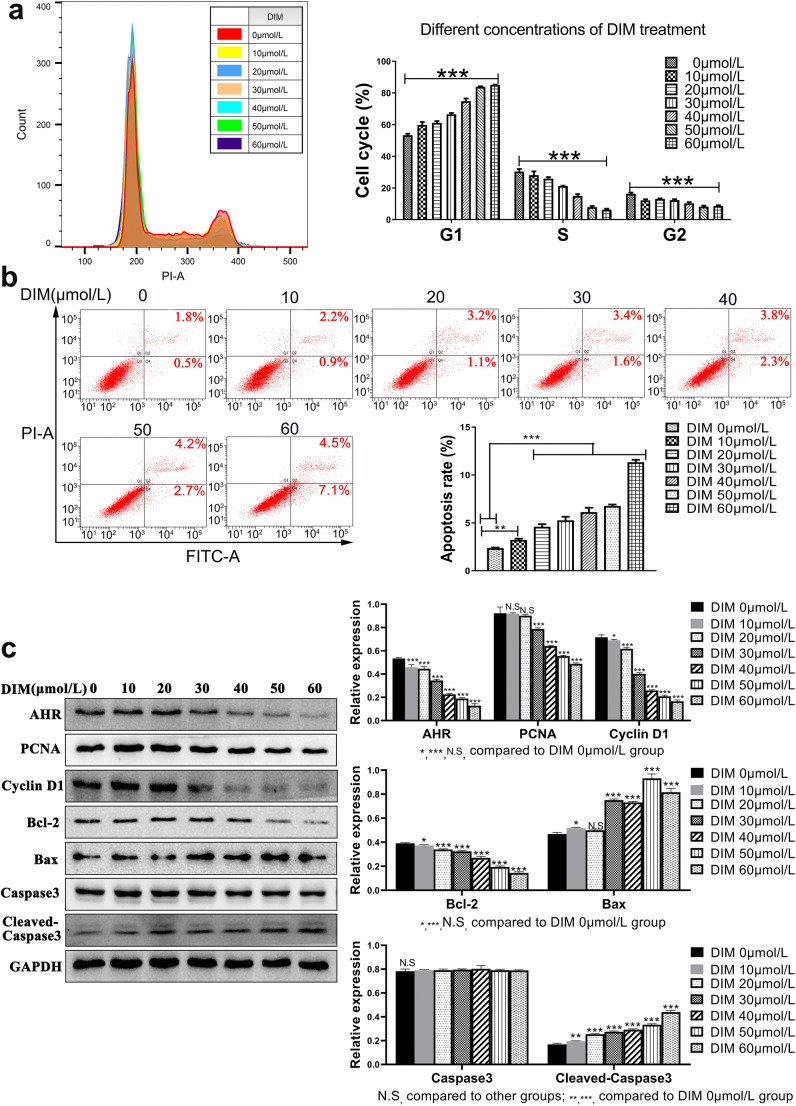
DIM was an indole derivative from cruciferous vegetables digested by small intestine and a natural compound without toxicity. Results indicated that cell viability was prohibited in a dose- and time-dependent manner (Fig. 5a). To verify whether the process of AHR modulation by DIM could be reversed, another AHR selective antagonist RSV was applied. As expected, RSV partly reversed the inhibitory effects on cell viability in a dose-dependent manner (Fig. 5b). Western blot demonstrated that RSV indeed reversed the translocation of AHR by DIM and DIM-induced CYP1A1 production, the classic target gene of AHR transcription activity (Fig. 5c). Therefore, DIM modulated ESCC progression in an AHR-dependent manner. Colony formation assay further visualized that DIM could inhibit cell growth for quite a long time incubation (Fig. 5d). In order to determine the exact DIM effective time point for regulating cell function, we treated cells with DIM for 24, 48, 72 h, respectively. Western blot showed the results that at the 48 h time point, cells exerted the potent reactivity when exposing DIM treatment in that the expression of AHR reduced gradually on the basis of increased DIM concentrations and the production of CYP1A1 increased in the same way (Fig. 5e). In light of this, we confirmed the incubation time for DIM treatment in the following experiments. Since modulation of AHR inhibited cell viability, we then examined the cell cycle change to find out the underlying inhibitory mechanism. Evidence obviously exhibited that after DIM treatment, cells were starkly arrested in G1 phase accompanied with the compensation by a decrease of cells in S and G2 phase (Fig. 6a). Meanwhile, to test whether DIM could induce cell apoptosis in a dose-dependent manner, we examined the early and later apoptosis rate for each DIM concentration treatment. Results showed the apoptosis rate increased ranging from 2.3% to 11.6% in total (Fig. 6b). That might partly explain how DIM inhibited ESCC progression except for mediating cell cycle.
Fig. 5.
DIM inhibits ESCC growth in an AHR-dependent manner. a CCK8 assay indicated that DIM inhibited cell viability through the time-dependent and dose-dependent manner. b An antagonist of AHR, Resveratrol (RSV, 0, 1, 5, 10, 20 μmol/L), was added in medium with DIM 40 μmol/L for 6 h to reverse cell viability partially. c Western blot visualized the corresponding protein expression change after RSV treatment with DIM. d Colony formation visualized that DIM inhibited cell growth. e Proper incubation time for DIM treatment was selected by western blot. The 48-h time point was applied in next assays. *, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S, no significance
Fig. 6.
Modulation of AHR by DIM induces ESCC cell cycle arrested in G1 phase and ESCC apoptosis with underlying related protein changes. a Cell cycle using flow cytometry indicated after DIM treatment, more cells were significantly arrested in G1 phase. b Apoptosis assay showed both early and later apoptosis rate increased in a dose-dependent manner. c Western blot indicated the down-regulation of growth and apoptosis-related proteins. *, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S, no significance
To elucidate mechanism of modulating AHR by DIM, western blot was utilized for detecting related proteins expression. Along with the increased DIM concentration, PCNA expression was down-regulated and so did the G1-phase-related protein Cyclin D1. Meanwhile, Bcl-2, as an anti-apoptosis protein, was also down-regulated whereas pro-apoptosis proteins like Bax and Cleaved-Caspase 3 were up-regulated, contributing to a higher apoptosis rate which resulted in cell growth restriction (Fig. 6c).
DIM inhibits cell migratory and invasive capacity by down-regulating MMPs expression
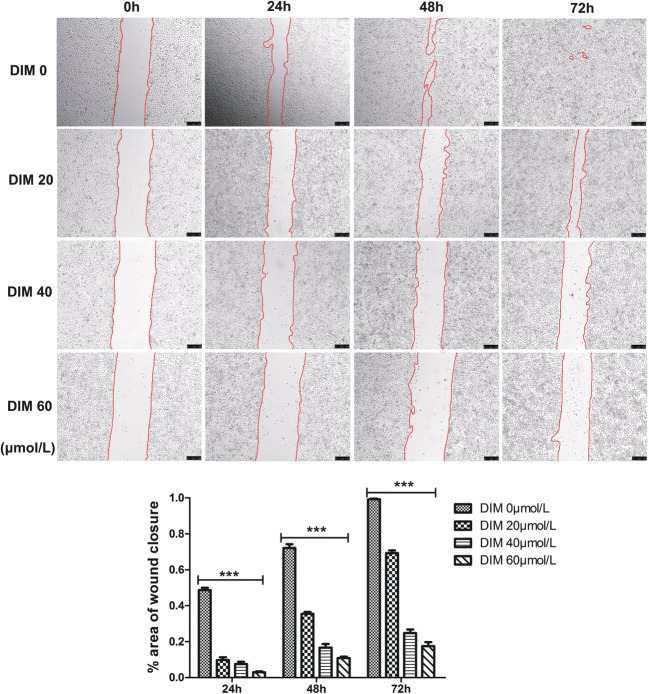
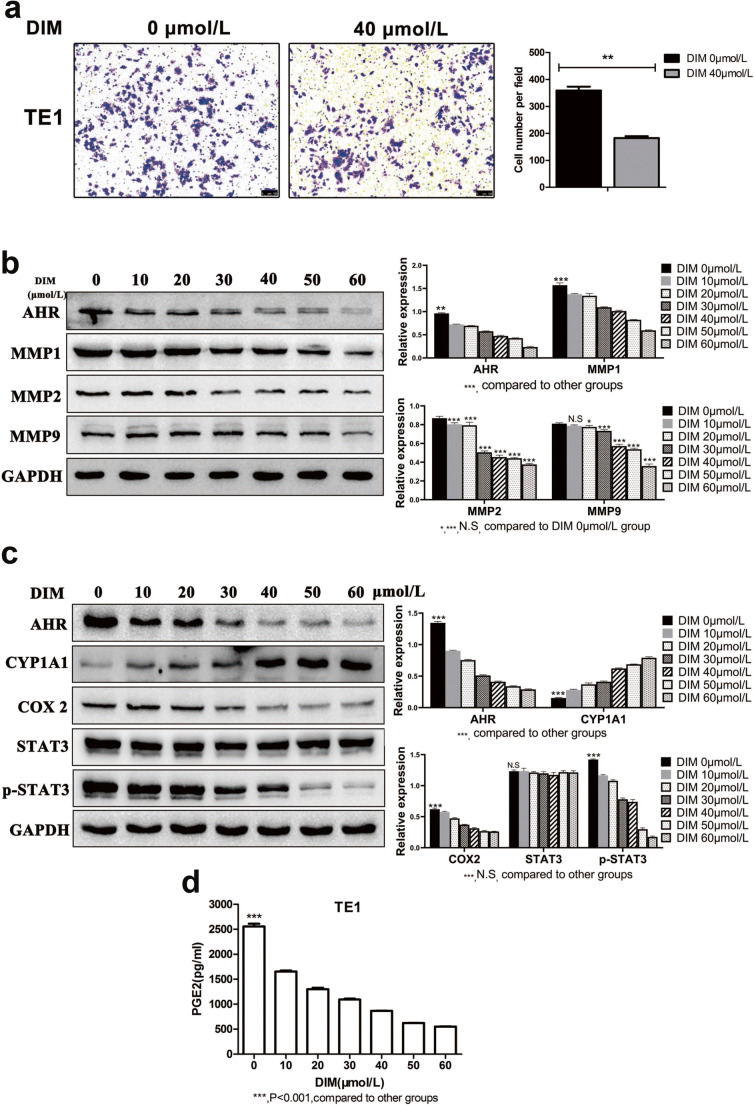
The same as the aforementioned results, DIM of 20, 40 and 60 μmol/L significantly reduced cell migratory capacity compared with negative control group (Fig. 7). Transwell assay obtained the same result that DIM of 40 μmol/L significantly decreased the cell number traversing the membrane which in other way meant cell invasive capacity was weakened (Fig. 8a). It was a truism that matrix metalloproteinases (MMPs) were associated with tumor metastasis and we examined MMPs expression levels after DIM treatment by western blot. Results were in accordance with expectation that MMP1, MMP2 and MMP9 expression levels were down-regulated (Fig. 8b). Therefore, we concluded that DIM inhibited cell migration and invasion via down-regulating MMPs expression.
Fig. 7.
Modulation of AHR by DIM weakens ESCC migratory capacity in a dose-dependent manner. ***, P < 0.001
Fig. 8.
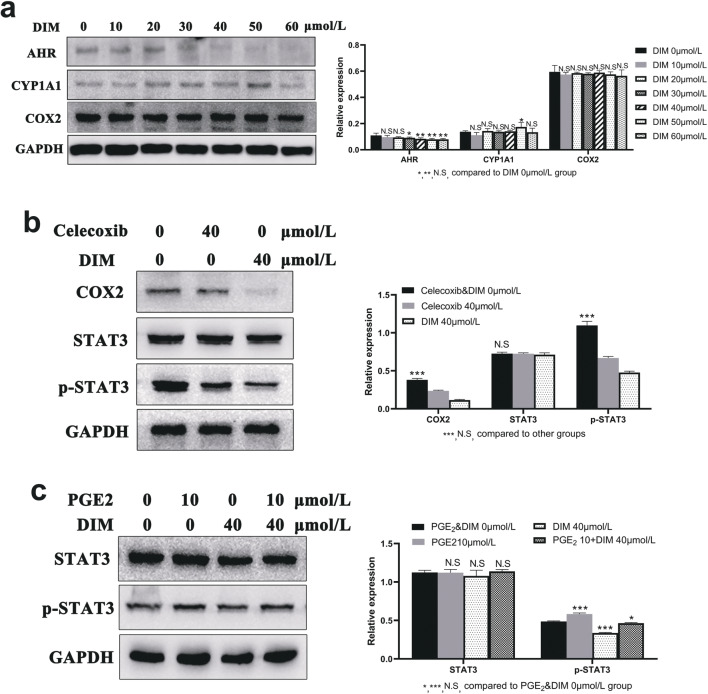
DIM inhibits ESCC invasion with relative MMPs changes and induces the COX2/PGE2/STAT3 axis repression. a DIM of 40 μmol/L significantly weakened ESCC invasive ability. Magnification: 100×. b Western blot showed the decreased MMP1, MMP2, MMP9 expression after DIM treatment. c DIM treatment induced the COX2/PGE2/STAT3 axis repression. d ELISA was conducted to quantify the production of PGE2 in medium. A significant decrease in PGE2 was in agreement with decreased COX2 expression. *, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S, no significance
Effects of DIM-induced AHR modulation on expression levels of COX2, PGE2 and STAT3 phosphorylation
Western blot showed the results that along with the CYP1A1 expression levels being up-regulated, COX2 expression levels were down-regulated in a dose-dependent manner. What is more, DIM exerted no obvious effect on total STAT3 protein expression, whereas the phosphorylated STAT3 (p-STAT3) expression levels were gradually down-regulated (Fig. 8c). ELISA was applied to quantify the release of PGE2 generated by COX2 catalyzation in medium. Consistent with DIM inhibitory effects on COX2 expression levels, the PGE2 levels were significantly lower in DIM treated cells compared with control cells (Fig. 8d). In order to verify whether DIM induced down-regulation of COX2 in an AHR-dependent manner, we utilized TE1 sh-AHR cells for another 48 h’ incubation with DIM. Results showed that COX2 expression levels had not changed obviously with increasing concentrations of DIM (Fig. 9a). All above evidence demonstrated that DIM induced down-regulation of COX2 expression levels in an AHR-dependent manner. Meanwhile, to verify whether COX2 or PGE2 exactly had effects on tumor progression by targeting STAT3, we used the selective COX2 inhibitor Celecoxib and COX2 catalysate PGE2 for validation. As shown in Fig. 9b and c, p-STAT3 expression levels were down-regulated by Celecoxib while when treated with PGE2, the result opposed. What is more, when co-incubated with DIM and PGE2, the latter could reverse the DIM inhibitory effects on p-STAT3. Therefore, COX2 and PGE2 did have effects on tumor progression.
Fig. 9.
DIM inhibits ESCC progression by modulating AHR to repress the COX2/PGE2/STAT3 axis. a To certify if DIM reduced the COX2 expression level through the upstream modulation of AHR, established AHR knockdown cells were incubated with DIM for 48 h. b Celecoxib, as a selective COX2 inhibitor, down-regulated p-STAT3 expression levels. c PGE2 and DIM alone or together had different effects on p-STAT3 regulation. *, P < 0.05; **, P < 0.01; ***, P < 0.001; N.S, no significance
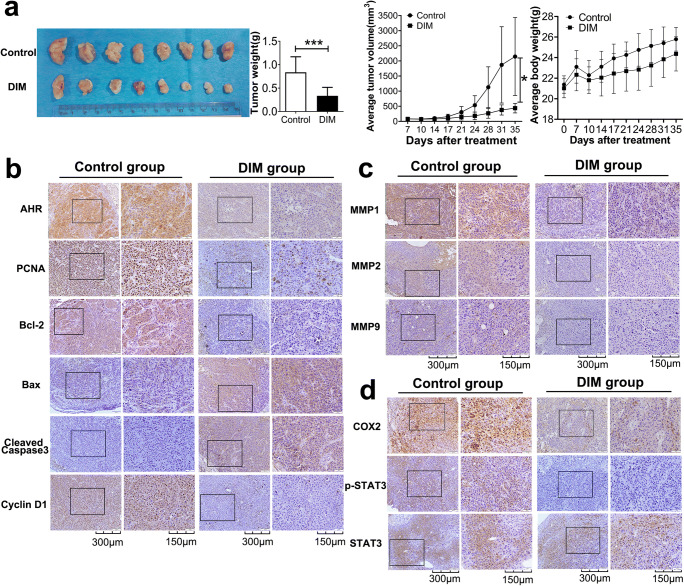
DIM inhibits progression of TE1 cell xenografts in vivo
Since all previous experiments were conducted in vitro, we constructed the mouse xenograft model for examining DIM effect on cell growth in vivo. After gavage with only PBS for Control group and DIM dissolved in PBS for DIM group for up to 28 days, mice were humanely sacrificed and xenografts were stripped and processed for IHC. As we could see, DIM group had formed obviously smaller tumors and the average tumor volume and weight were also significantly less than Control group even though average mouse body weight showed no significance between Control and DIM group (Fig. 10a). To better elucidate the inhibitory effect on tumor growth in vivo, tumors were cut into slices for IHC staining. In agreement with previous results, DIM gavage still activated the AHR which caused the growth suppression by down-regulating PCNA, Bcl-2 and Cyclin D1 proteins expression levels and up-regulating pro-apoptosis-related proteins (Fig. 10b). Meanwhile, MMPs expression levels were also down-regulated in order to limit metastasis capacity (Fig. 10c). Activation of AHR in vivo could actually reduce the COX2 and p-STAT3 production without affecting total STAT3 (Fig. 10d).
Fig. 10.
DIM inhibits tumor growth, invasion and promotes apoptosis in vivo by performing tumor xenograft assay. a Imgaes of ESCC tissues collected from male BALB/c nude mice. DIM group had tendency to obtain smaller volume tumor formation compared to Control group. b IHC staining results showed the decreased growth-related and increased apoptosis-related proteins expression. c IHC staining for invasion-related proteins expression. MMPs were down-regulated. d IHC staining for COX2, p-STAT3, STAT3. Results were in agreement with previous evidences obtained in vitro. *, P < 0.05; ***, P < 0.001
Discussion
Aryl hydrocarbon receptor, as a ligand-activated receptor, behaves as a key transcription factor participating in cell proliferation, migration, invasion and differentiation (Roman et al. 2018). In recent years, numerous studies have set focus on AHR for its mysterious function when treated with specific tumor types and specific ligand-binding. For example, melanoma B16F10 cells lacking AHR expression were injected in wild type mice and exacerbated tumorigenesis (Contador-Troca et al. 2013). Whereas silence of AHR suppressed three NRAS-mutant cell lines growth (Barretina et al. 2012). On the other hand, leflunomide-induced melanoma growth inhibition was mediated by AHR (O'Donnell et al. 2012). Thus, it is full of interest to explore why and how AHR regulates tumorigenesis and progression since no comprehensive researches about AHR were related with esophageal squamous cell carcinoma. Therefore, we first collected 54 pairs of surgical tumor and normal esophageal tissue samples for IHC to investigate whether AHR was high expressed in ESCC. Results were in line with previous only one research about ESCC that AHR expression levels were elevated (Zhang et al. 2012). Thereafter, we established the AHR knockdown cell lines genetically and using DIM as a selective AHR modulator to examine the AHR effects on ESCC malignant biological properties. In the end, tumor xenograft assay was performed to further verify the hypothesis that modulation of AHR by DIM could inhibit tumor progression. From experimental results, knockdown of AHR actually suppressed cell growth, migration and invasion as well as promoted cell cycle arrest and apoptosis with modest effect. Regarding apoptosis, shAHR cells showed no apparent evidence even the data statistics were significant in terms of flow cytometry and Western blot for detecting cleaved caspase-3. However, we think it is necessary to show the current results because knockdown of AHR actually did not translocate AHR into nuclei and triggered the transcription of AHR downstream genes. After DIM treatment, DIM had modulated AHR translocated into the nuclei with the evidence of increasing CYP1A1 production and triggered the transcription to finally induce the apoptosis with the apoptosis rate increased ranging from 2.3% to 11.6% in total. We agree that AHR has more of a cytostatic effect rather than apoptotic induction since the range of apoptotic rates is not apparent in terms of AHR transfection and DIM treatment. Therefore, we suppose AHR alone may not be the key gene for regulating apoptosis. Modulation of AHR by DIM pharmacologically impacted cell function aforementioned through repressing COX2/PGE2/STAT3 axis. In vivo experiment showed DIM gavage indeed reduced tumor formation and made the same effects on ESCC in vitro.
COX2/PGE2 pathway plays key roles in hallmarks of cancer and adaptation to the tumor microenvironment since the pathway is characterized by evasion of apoptosis, self-sufficiency in growth signals, promotion of tumor invasion and metastasis (Greenhough et al. 2009). Several researches had demonstrated that COX2 was over-expressed in ESCC and correlated with poor prognosis and chemoresistance (Xi et al. 2005; Akutsu et al. 2011; Yoon et al. 2011). Meanwhile, AHR-mediated lymphoma was associated with increased expression of COX2 (Vogel et al. 2007) and TCDD activated AHR with up-regulation of COX2 in breast cancer (Degner et al. 2009). Apart from malignant tumors, TCDD-induced AHR activation also mediated lung fibroblast migration and differentiation with increased COX2 expression and arachidonic acid metabolism (Su et al. 2016). And PGE2, as the metabolite of arachidonic acid, was reported to mediate acute myeloid leukemia (AML) drug resistance and promote colorectal cancer cell proliferation induced by IL-33 (Li et al. 2018b; Carter et al. 2019). STAT3 belongs to signal transducer and activator of transcription (STAT) family and is the most frequently implicated one involved in tumor cell differentiation, proliferation, angiogenesis and immune evasion through phosphorylation (Huynh et al. 2019). Thus, targeting COX2/PGE2/STAT3 axis as a therapeutic strategy is of significance. Our results showed that DIM effectively reduced the expression levels of COX2/PGE2 and p-STAT3 but without affecting the expression level of total STAT3 and in turn weakened a series of ESCC cell malignant properties through down-regulating growth-related PCNA, Bcl-2, CyclinD1 and up-regulating Bax, Cleaved-Caspase3 proteins expression as well as inhibiting metastasis with reduced expression of MMP1, MMP2 and MMP9. As mentioned before, AHR knockdown induced an S phase arrest while DIM treatment induced a G1 phase arrest. It seemed inconsistent but as a ligand-dependent transcription factor diffusely located in the cytoplasm, AHR knockdown did not translocate AHR into nuclei to trigger downstream transcription and conversely, DIM as an AHR modulator could do this with the symbol of increasing CYP1A1 production to exert more extensive effect on cell cycle and apoptosis. Therefore, this may explain the inconsistence of cell cycle arrest and more apparent apoptosis after DIM treatment compared with AHR knockdown.
In addition, when we used the AHR knockdown TE1 cells treated with the same DIM concentration gradient for examining the COX2 output, no clear change in COX2 expression was detected. This outcome demonstrated that DIM induced COX2 inhibition in an AHR-dependent manner. It is a highlight for us to apply DIM for modulating AHR state along with finding that COX2 was also down-regulated since COX2-PGE2 pathway participated in anti-apoptosis and increasing Bcl-2 expression through activation of the Ras-MAPK/ERK pathway and PI3K-AKT pathway (Logsdon and Lu 2016; Li et al. 2018a; Tu et al. 2019). We used COX2 selective inhibitor Celecoxib and COX2 catalysate PGE2 to test whether p-STAT3 expression had alterations and results were consistent with DIM inhibitory effect on COX2/PGE2/STAT3 axis. Meanwhile, down-regulation of STAT3 activation was associated with ESCC therapy in several recent researches (Liu et al. 2018; Wang et al. 2018). Modulation of AHR by DIM via repressing the COX2/PGE2/STAT3 axis both in vitro and in vivo synergistically inhibited the ESCC growth, progression and metastasis. Nevertheless, our experimental design still has some limitations that clinical samples for IHC and analysis with pathological parameters are incomprehensive in distribution of clinical stage, especially in Low expression of AHR group, and more ESCC cell lines should be applied to fully verify the exact progression inhibitory mechanism induced by DIM.
Since aryl hydrocarbon receptor plays essential role in regulating tumor initiation and progression, we should target AHR with certain ligand such as agonist or antagonist or selective modulator as a new therapeutic strategy. It is essential for us to design and select appropriate AHR ligand based on distinct mechanism of certain cancer-related promotion in order to discover new molecules with therapeutic value (Kolluri et al. 2017). DIM is a health promoting phytochemical and noteless in the field of cancer therapy even if it exerts huge impacts on cancer cell growth inhibition through modulating AHR or androgen receptor (Goldberg et al. 2014). Therefore, more researches are in dire need of evidence to exploit the potential of AHR and DIM as a novel cancer chemotherapy.
In conclusion, this is the first study to systematacially investigate the correlation between AHR and ESCC. AHR expression levels are elevated in ESCC and correlated with poor prognosis. Knockdown of AHR and modulation of AHR by DIM pharmacologically both inhibit cell growth, migration and invasion, and the latter exerts its function through repressing COX2/PGE2/STAT3 axis in vitro and in vivo. AHR, as a potential therapeutic target, is deserved to be further exploited.
Acknowledgments
This work was supported in part by a grant from Department of Education of Liaoning Province (LK201614). We thank the NHC Key Laboratory of Immunodermatology (China Medical University) for experiments carried out. We also thank Professor Ruiqun Qi for experimental instructions.
Abbreviations
- AHR
Aryl hydrocarbon receptor
- COX2
Cyclooxygenase 2
- CYP1A1
Cytochrome P450, family 1, member A1
- DIM
3, 3′-diindolylmethane
- ESCC
Esophageal squamous cell carcinoma
- OD
Optical density
- OS
Overall survival
- PCNA
Proliferating cell nuclear antigen
- PGE2
Prostaglandin E2
- RSV
Resveratrol
- STAT3
Signal transducer and activator of transcription 3
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Ethics and Experimental Committee of China Medical University (Approved number: 2018146).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruiqun Qi, Email: xiaoqiliumin@163.com.
Shuguang Zhang, Email: shgzhang@cmu.edu.cn.
References
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4(9):1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- Akutsu Y, Hanari N, Yusup G, Komatsu-Akimoto A, Ikeda N, Mori M, Yoneyama Y, Endo S, Miyazawa Y, Matsubara H. COX2 expression predicts resistance to chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18(10):2946–2951. doi: 10.1245/s10434-011-1645-z. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jane-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P, Jr, de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, Garraway LA. The Cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Mundt KA, Adami HO, Cole P, Mandel JS. TCDD and cancer: a critical review of epidemiologic studies. Crit Rev Toxicol. 2011;41(7):622–636. doi: 10.3109/10408444.2011.560141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callero MA, Suarez GV, Luzzani G, Itkin B, Nguyen B, Loaiza-Perez AI. Aryl hydrocarbon receptor activation by aminoflavone: new molecular target for renal cancer treatment. Int J Oncol. 2012;41(1):125–134. doi: 10.3892/ijo.2012.1427. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Mak PY, Wang X, Tao W, Ruvolo V, Mak D, Mu H, Burks JK, Andreeff M. An ARC-regulated IL1beta/cox-2/PGE2/beta-catenin/ARC circuit controls leukemia-microenvironment interactions and confers drug resistance in AML. Cancer Res. 2019;79(6):1165–1177. doi: 10.1158/0008-5472.CAN-18-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Colonna M. Aryl hydrocarbon receptor: linking environment to immunity. Semin Immunol. 2015;27(5):310–314. doi: 10.1016/j.smim.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contador-Troca M, Alvarez-Barrientos A, Barrasa E, Rico-Leo EM, Catalina-Fernandez I, Menacho-Marquez M, Bustelo XR, Garcia-Borron JC, Gomez-Duran A, Saenz-Santamaria J, Fernandez-Salguero PM. The dioxin receptor has tumor suppressor activity in melanoma growth and metastasis. Carcinogenesis. 2013;34(12):2683–2693. doi: 10.1093/carcin/bgt248. [DOI] [PubMed] [Google Scholar]
- Degner SC, Papoutsis AJ, Selmin O, Romagnolo DF. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3′-diindolylmethane in breast cancer cells. J Nutr. 2009;139(1):26–32. doi: 10.3945/jn.108.099259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Blando JM, Perez CJ, Lal P, Feldman MD, Smyth EM, Ricciotti E, Grosser T, Benavides F, Kazanietz MG. COX-2 mediates pro-tumorigenic effects of PKCepsilon in prostate cancer. Oncogene. 2018;37(34):4735–4749. doi: 10.1038/s41388-018-0318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AA, Titorenko VI, Beach A, Abdelbaqi K, Safe S, Sanderson JT. Ring-substituted analogs of 3,3′-diindolylmethane (DIM) induce apoptosis and necrosis in androgen-dependent and -independent prostate cancer cells. Investig New Drugs. 2014;32(1):25–36. doi: 10.1007/s10637-013-9979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- Hanieh H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol Cancer. 2015;14:172. doi: 10.1186/s12943-015-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J, Chand A, Gough D, Ernst M. Therapeutically exploiting STAT3 activity in cancer - using tissue repair as a road map. Nat Rev Cancer. 2019;19(2):82–96. doi: 10.1038/s41568-018-0090-8. [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Jin UH, Safe S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch Toxicol. 2017;91(7):2497–2513. doi: 10.1007/s00204-017-1981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Gao D, Du M, Cheng X, Mao X. Casein glycomacropeptide hydrolysates inhibit PGE2 production and COX2 expression in LPS-stimulated RAW 264.7 macrophage cells via Akt mediated NF-kappaB and MAPK pathways. Food Funct. 2018;9(4):2524–2532. doi: 10.1039/c7fo01989k. [DOI] [PubMed] [Google Scholar]
- Li Y, Shi J, Qi S, Zhang J, Peng D, Chen Z, Wang G, Wang Z, Wang L. IL-33 facilitates proliferation of colorectal cancer dependent on COX2/PGE2. J Exp Clin Cancer Res. 2018;37(1):196. doi: 10.1186/s13046-018-0839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23(4):233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Zeng S, Zhang X, Zhao J, Zhang X, Chen X, Yang W, Yang Y, Dong Z, Zhu J, Xu X, Tian F. The natural polyphenol curcumin induces apoptosis by suppressing STAT3 signaling in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2018;37(1):303. doi: 10.1186/s13046-018-0959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon CD, Lu W. The significance of Ras activity in pancreatic Cancer initiation. Int J Biol Sci. 2016;12(3):338–346. doi: 10.7150/ijbs.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita M, Makino H, Katsuta M, Nomura T, Shinji S, Kashiwabara M, Takahashi K, Kudo M, Ishiwata T, Naito Z, Tajiri T. Cyclo-oxygenase-2 over-expression is associated with human esophageal squamous cell carcinoma. J Nippon Med Sch. 2006;73(6):308–313. doi: 10.1272/jnms.73.308. [DOI] [PubMed] [Google Scholar]
- Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14(12):801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell EF, Kopparapu PR, Koch DC, Jang HS, Phillips JL, Tanguay RL, Kerkvliet NI, Kolluri SK. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS One. 2012;7(7):e40926. doi: 10.1371/journal.pone.0040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Wang Y, Tang N, Sun D, Lan Y, Yu Z, Zhao X, Feng L, Zhang B, Jin L, Yu F, Ma X, Lv C. Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J Exp Clin Cancer Res. 2018;37(1):248. doi: 10.1186/s13046-018-0926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- Popolo A, Pinto A, Daglia M, Nabavi SF, Farooqi AA, Rastrelli L. Two likely targets for the anti-cancer effect of indole derivatives from cruciferous vegetables: PI3K/Akt/mTOR signalling pathway and the aryl hydrocarbon receptor. Semin Cancer Biol. 2017;46:132–137. doi: 10.1016/j.semcancer.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Roman AC, Carvajal-Gonzalez JM, Merino JM, Mulero-Navarro S, Fernandez-Salguero PM. The aryl hydrocarbon receptor in the crossroad of signalling networks with therapeutic value. Pharmacol Ther. 2018;185:50–63. doi: 10.1016/j.pharmthera.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, Quintana FJ (2019) The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol [DOI] [PubMed]
- Shimizu K, Okita R, Saisho S, Maeda AI, Nojima Y, Nakata M. Impact of COX2 inhibitor for regulation of PD-L1 expression in non-small cell lung Cancer. Anticancer Res. 2018;38(8):4637–4644. doi: 10.21873/anticanres.12768. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Murray IA, Tanos R, Tellew J, Boitano AE, Bisson WH, Kolluri SK, Cooke MP, Perdew GH. Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. J Pharmacol Exp Ther. 2011;338(1):318–327. doi: 10.1124/jpet.110.178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford EA, Ramirez-Cardenas A, Wang Z, Novikov O, Alamoud K, Koutrakis P, Mizgerd JP, Genco CA, Kukuruzinska M, Monti S, Bais MV, Sherr DH. Role for the aryl hydrocarbon receptor and diverse ligands in Oral squamous cell carcinoma migration and tumorigenesis. Mol Cancer Res. 2016;14(8):696–706. doi: 10.1158/1541-7786.MCR-16-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HH, Lin HT, Suen JL, Sheu CC, Yokoyama KK, Huang SK, Cheng CM. Aryl hydrocarbon receptor-ligand axis mediates pulmonary fibroblast migration and differentiation through increased arachidonic acid metabolism. Toxicology. 2016;370:116–126. doi: 10.1016/j.tox.2016.09.019. [DOI] [PubMed] [Google Scholar]
- Tong D, Liu Q, Liu G, Xu J, Lan W, Jiang Y, Xiao H, Zhang D, Jiang J. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett. 2017;389:23–32. doi: 10.1016/j.canlet.2016.12.031. [DOI] [PubMed] [Google Scholar]
- Tu C, Huang X, Xiao Y, Song M, Ma Y, Yan J, You H, Wu H. Schisandrin a inhibits the IL-1beta-induced inflammation and cartilage degradation via suppression of MAPK and NF-kappaB signal pathways in rat chondrocytes. Front Pharmacol. 2019;10:41. doi: 10.3389/fphar.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Li W, Sciullo E, Newman J, Hammock B, Reader JR, Tuscano J, Matsumura F. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am J Pathol. 2007;171(5):1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CK, Chang H, Chen PH, Chang JT, Kuo YC, Ko JL, Lin P. Aryl hydrocarbon receptor activation and overexpression upregulated fibroblast growth factor-9 in human lung adenocarcinomas. Int J Cancer. 2009;125(4):807–815. doi: 10.1002/ijc.24348. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou P, Qin S, Xu D, Liu Y, Fu W, Ruan B, Zhang L, Zhang Y, Wang X, Pan Y, Wang S, Yan H, Qin J, Wang X, Liu Q, Du Z, Liu Z, Wang Y. The Curcumin analogs 2-Pyridyl Cyclohexanone induce apoptosis via inhibition of the JAK2-STAT3 pathway in human esophageal squamous cell carcinoma cells. Front Pharmacol. 2018;9:820. doi: 10.3389/fphar.2018.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhao L, He W, Yang J, Geng C, Chen Y, Liu T, Chen H, Li Y. Benzo[a]pyrene promotes gastric cancer cell proliferation and metastasis likely through the aryl hydrocarbon receptor and ERK-dependent induction of MMP9 and c-myc. Int J Oncol. 2016;49(5):2055–2063. doi: 10.3892/ijo.2016.3674. [DOI] [PubMed] [Google Scholar]
- Xi H, Baldus SE, Warnecke-Eberz U, Brabender J, Neiss S, Metzger R, Ling FC, Dienes HP, Bollschweiler E, Moenig S, Mueller RP, Hoelscher AH, Schneider PM. High cyclooxygenase-2 expression following neoadjuvant radiochemotherapy is associated with minor histopathologic response and poor prognosis in esophageal cancer. Clin Cancer Res. 2005;11(23):8341–8347. doi: 10.1158/1078-0432.CCR-04-2373. [DOI] [PubMed] [Google Scholar]
- Ye M, Zhang Y, Gao H, Xu Y, Jing P, Wu J, Zhang X, Xiong J, Dong C, Yao L, Zhang J, Zhang J. Activation of the aryl hydrocarbon receptor leads to resistance to EGFR TKIs in non-small cell lung Cancer by activating Src-mediated bypass signaling. Clin Cancer Res. 2018;24(5):1227–1239. doi: 10.1158/1078-0432.CCR-17-0396. [DOI] [PubMed] [Google Scholar]
- Yin XF, Chen J, Mao W, Wang YH, Chen MH. A selective aryl hydrocarbon receptor modulator 3,3′-Diindolylmethane inhibits gastric cancer cell growth. J Exp Clin Cancer Res. 2012;31:46. doi: 10.1186/1756-9966-31-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MS, Nam TK, Lee JS, Cho SH, Song JY, Ahn SJ, Chung IJ, Jeong JU, Chung WK, Nah BS. VEGF as a predictor for response to definitive chemoradiotherapy and COX-2 as a prognosticator for survival in esophageal squamous cell carcinoma. J Korean Med Sci. 2011;26(4):513–520. doi: 10.3346/jkms.2011.26.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zong H, Li S, Zhang D, Zhang L, Xia Q. Activation of aryl hydrocarbon receptor suppresses invasion of esophageal squamous cell carcinoma cell lines. Tumori. 2012;98(1):152–157. doi: 10.1177/030089161209800121. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li H, Lin HJ, Yang S, Lin J, Liang G. Feedback activation of STAT3 as a Cancer drug-resistance mechanism. Trends Pharmacol Sci. 2016;37(1):47–61. doi: 10.1016/j.tips.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Zhou FF, Huang R, Jiang J, Zeng XH, Zou SQ. Correlated non-nuclear COX2 and low HER2 expression confers a good prognosis in colorectal cancer. Saudi J Gastroenterol. 2018;24(5):301–306. doi: 10.4103/sjg.SJG_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]