Glycyrrhizic acid (GA, 1) is the main triterpene glycoside from roots of Glycyrrhiza glabra L. and G. uralensis Fisch. and a leading natural glycoside with potential as a medicine for developing new immunomodulators and antiviral agents [1, 2]. Several conjugates of GA with amino acids and dipeptides displayed pronounced antiviral activity against human immunodeficiency (HIV) [3, 4], atypical pneumonia of SARS-CoV [5], Epstein–Barr [6], and flu A/H1N1 viruses [7]. Previously, we proposed methods for preparing conjugates of GA with alkyl and benzyl esters of L- and D-amino acids via activation of the GA carboxylic acids using N-hydroxybenzotriazole (HOBt) and N,N′-dicyclohexylcarbodiimide (DCC) [8], N-hydroxyphthalimide and DCC [9], or N-hydroxysuccinimide (HOSu) and DCC [3]. The main drawback of these methods for preparing amino-acid conjugates of GA is their two steps (preparation of GA activated ester and subsequent reaction of it with an amine). Furthermore, the dicyclohexylurea formed if DCC is used contaminates the target conjugates. Therefore, water-soluble carbodiimides, in particular 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (DEC), which does not form dicyclohexylurea, are more attractive as condensing reagents.
The present communication reports the selective synthesis of amino-acid conjugates of GA with free amino acids in the glycoside carbohydrate part using DEC and L-amino-acid t-butyl ester hydrochlorides.
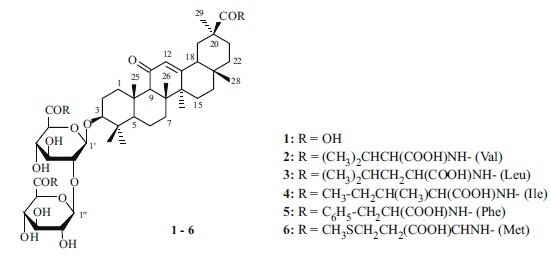
Condensation of GA with amino-acid t-butyl ester hydrochlorides (amino-component, AC) was carried out at room temperature in DMF in the presence of DEC with GA–AC/DEC mole ratios 1:2.5–3.0:3.0–3.2 in the presence of an excess of Et3N. The ACs were L-amino-acid t-butyl esters (valine, leucine, isoleucine, phenylalanine, methionine). The obtained carboxy-substituted conjugates were deblocked by CF3COOH in CH2Cl2. The target GA conjugates (2–6) were isolated by column chromatography in 55–60% yields. Compounds 2 and 3 were prepared first. The physicochemical properties of 4–6 agreed with those reported earlier [3]. The structures of 3–5 were confirmed by high-resolution PMR (500 MHz) and 13C NMR spectra (125 MHz). 13C NMR spectra of the obtained GA conjugates showed additional resonances for the amino-acid COOH atoms at weak field (172–175 ppm) and sets of amino-acid resonances. The resonance of the aglycon free COOH group appeared at δ 178.6–180.2 ppm. The PMR spectrum of GA conjugate 5 displayed strong multiplets for aromatic protons at weak field (7.35–7.19 ppm). The aromatic C atoms of phenylalanine were observed in the 13C NMR spectrum at weak field (136.39–126.80 ppm). The purity of the compounds (95–99%) was monitored by TLC and HPLC.
Thus, a simple and selective method for preparing amino-acid conjugates of GA containing free amino-acids in the glycoside carbohydrate part was proposed.
General Comments.13C NMR spectra were recorded in CDCl3 on a Bruker Avance-III pulsed spectrometer at operating frequency 500.13 (1H) and 125.47 MHz (13C) or a Bruker AMX-300 spectrometer at operating frequency 300 for 1H and 75.5 MHz for 13C. Chemical shifts were given in ppm vs. TMS internal standard. IR spectra were recorded from mineral-oil mulls on an IR Prestige-21 spectrophotometer (Shimadzu). Optical activity was measured on a PerkinElmer 341 polarimeter in a 1-dm tube at 20–22°C (λNa 546 nm).
TLC used Sorbfil plates (Sorbpolimer, Russia). Spots of compounds were detected using H2SO4 (5%) in EtOH followed by heating at 110–120°C for 2–3 min. Column chromatography used KSK silica gel (50–160 μm fraction) (Sorbpolimer, Russia). HPLC used an LC-20 liquid chromatograph (Shimadzu) equipped with a spectrophotometric diode array detector at 25 ± 2°C and Zorbax RX C18 reversed-phase (250 . 4.6 mm, 5 μm) (Agilent, USA) or Atlantis C18 columns (250 . 4.6 mm, 5 μm) (Waters, USA). UV detection was made at 254 nm. The mobile phase was MeOH at flow rate 1 mL/min.
GA from G. uralensis roots (Altai) was used in the work [10]. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma-Aldrich, Germany) and L-amino acid t-butyl esters (Chemapol, Hungary) were used in the work.
General Method for Preparing Amino-acid Conjugates of GA (2–6). A solution of 1 (0.82 g, 1 mmol) in DMF (20 mL) was treated with 1-ethyl-3(3-dimethylaminopropyl)carbodiimide hydrochloride (3.1–3.2 mmol), amino-acid t-butyl ester hydrochloride (2.8–3.0 mmol), and Et3N (0.8–1.0 mL), stirred at 20–22°C for 24 h, diluted with cold H2O, and acidified with citric acid (pH 3–4). The precipitate was filtered off, rinsed with H2O, dried, and worked up with CF3COOH in CH2Cl2 (2 mL/10 mL). The solvent and CF3COOH were evaporated. The solid was chromatographed over a column of silica gel with elution by CHCl3–MeOH–H2O (400:10:1, 200:10:1, 100:10:1, 50:10:1) with TLC monitoring.
3-O-{2-O-[N-(β-D-Glucopyranosyluronoyl)-L-valine]-N-(β-D-glucopyranosyluronoyl)-L-valine}-(3β,20β)-11-oxo-30-norolean-12-ene (2R) (C55) (2). Yield 55% (amorphous compound), Rf 0.42 (CHCl3–EtOH, 5:1); [α]D20 +55° (c 0.06, EtOH). IR spectrum (ν, cm–1): 3500–3200 (OH, NH), 1725 (COOH), 1660 (C=O), 1552 (CONH). 13C NMR spectrum (75.5 MHz, CD3OD, δ, ppm): 17.0 (C-25), 17.3 (C-24), 18.0 (C-6), 19.8 (C-26), 23.8 (C-27), 26.1 (C-2), 27.5 (C-15), 27.7 (C-16), 28.5 (C-28), 29.1 (C-23), 29.4 (C-29), 31.8 (C-21), 33.0 (C-17), 33.9 (C-7), 38.2 (C-10), 38.8 (C-22), 40.4 (C-1), 40.7 (C-4), 42.8 (C-19), 44.6 (C-14), 45.0 (C-8), 46.8 (C-20), 48.2 (C-18), 56.5 (C-5), 63.2 (C-9), 73.8, 73.7 (C-4′, 4′′), 75.2 (C-2′′), 76.1 (C-3′′), 76.4 (C-3′), 77.4 (C-5′), 77.8 (C-5′′), 81.7 (C-2′), 90.3 (C-3), 104.8 (C-1′), 105.1 (C-1′′), 129.2 (C-12), 171.5, 171.6 (C-6′′, 6′), 172.4 (C-13), 178.6 (C-30), 202.7 (C-11); 2Val: 13.9, 18.5, 18.7, 19.5, 19.6, 31.8, 32.1, 32.2, 58.9, 58.4, 174.2 (COOH), 175.0 (COOH). Found, %: N 2.65. C52H80N2O18. Calcd, %: N 2.74. H = 1021.17.
3-O-{2-O-[N-(β-D-Glucopyranosyluronoyl)-L-leucine]-N-(β-D-glucopyranosyluronoyl)-L-leucine}-(3β,20β)-11-oxo-30-norolean-12-ene (3). Yield 60% (amorphous compound); Rf 0.49 (CHCl3–MeOH–H2O, 45:10:1); HPLC (95.8 ± 1.5%), τ 2.34 min (Zorbax RX-C18); [α]D20 +54° (c 0.04, EtOH). IR spectrum (ν, cm–1): 3500–3200 (OH, NH), 1727 (COOH), 1662 (C=O), 1540 (CONH). 13C NMR spectrum (125 MHz, CD3OD, δ, ppm): 15.6 (C-25), 16.0 (C-24), 17.1 (C-6), 18.0 (C-26), 22.6 (C-27), 26.0 (C-2), 26.2 (C-15), 26.3 (C-16), 27.0 (C-28), 27.4 (C-23), 27.9 (C-29), 30.6 (C-21), 31.6 (C-17), 32.4 (C-7), 36.7 (C-10), 37.6 (C-22), 39.0 (C-1), 39.3 (C-4), 41.0 (C-19), 43.2 (C-14), 43.5 (C-8), 45.4 (C-20), 47.4 (C-18), 55.0 (C-5), 61.7 (C-9), 71.8 (C-4′′), 72.2 (C-4′), 74.3 (C-2′′), 74.6 (C-3′′), 74.8 (C-3′), 75.7 (C-5′), 76.1 (C-5′′), 80.5 (C-2′), 89.0 (C-3), 103.7 (C-1′), 103.8 (C-1′′), 127.5 (C-12), 169.6, 170.2 (C-6′′, 6′), 171.6 (C-13), 179.0 (C-30), 201.1 (C-11); 2Leu: 20.7, 20.8, 22.1, 22.2, 24.5, 24.6, 40.4, 40.5, 51.2, 51.7, 174.4 (COOH), 174.5 (COOH). Found, %: N 2.54. C54H84N2O18. Calcd, %: N 2.67. M = 1049.2.
3-O-{2-O-[N-(β-D-Glucopyranosyluronoyl)-L-isoleucine]-N-(β-D-glucopyranosylyronoyl)-L-isoleucine}-(3β,20β)-11-oxo-30-norolean-12-ene (4). Yield 57% (amorphous compound), Rf 0.42 (CHCl3–EtOH, 5:1), HPLC (98.8 ± 1.5%), τ 3.35 min (Atlantis C18); [α]D20 +58° (c 0.06, MeOH). Lit. [3]: [α]D20 +60°C (c 0.04, MeOH). IR spectrum (ν, cm–1): 3500–3200 (OH, NH), 1730 (COOH), 1662 (C=O), 1539 (CONH). The 13C NMR spectrum (125 MHz, CD3OD, δ, ppm) agreed with that published [3]. Found, %: N 2.54. C54H84N2O18. Calcd, %: N 2.67. M = 1049.2.
3-O-{2-O-[N-(β-D-Glucopyranosyluronoyl)-L-phenylalanine]-N-(β-D-glucopyranosylyronoyl)-Lphenylalanine}-(3β,20β)-11-oxo-30-norolean-12-ene (5). Yield 60% (amorphous compound), Rf 0.40 (CHCl3–EtOH, 5:1); [α]D20 +59° (c 0.04, MeOH). Lit. [3]: [α]D20 +60° (c 0.02, MeOH). The 13C NMR spectrum (125 MHz, CD3OD, δ, ppm) agreed with that published [3]. Found, %: N 2.40. C60H80N2O18. Calcd, %: N 2.50. M = 1117.24.
3-O-{2-O-[N-(β-D-Glucopyranosyluronoyl)-L-methionine]-N-(β-D-glucopyranosylyronoyl)-L-methionine}-(3β,20β)-11-oxo-30-norolean-12-ene (6). Yield 56% (amorphous compound), Rf 0.42 (CHCl3–EtOH, 5:1), HPLC (99.4 ± 1.5%): τ 2.16 min (Zorbax RX C18); [α]D20 +54° (c 0.05, MeOH). Lit. [3]: [α]D20 +56° (c 0.05, MeOH). IR spectrum (ν, cm–1): 3450–3200 (OH, NH), 1734 (COOH), 1659 (C=O), 1558 (CONH). The 13C NMR spectrum (125 MHz, CD3OD, δ, ppm) agreed with that published [3]. Found, %: N 2.48, S 5.70. C52H80N2O18S2. Calcd, %: N 2.58, S 5.92. M = 1083.27.
Acknowledgment
The work was financially supported by the RFBR (Grant 18-53-52004_MNT_a) and State Task Topic No. AAAA-A17-117011910025-6 using equipment at the Khimiya CUC, UfIC, UFRC and Agidel2 RCUC, UFRC, RAS.
Footnotes
Translated from Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2020, pp. 488–490.
References
- 1.Pompei R, Laconi S, Ingianni A. Mini-Rev. Med. Chem. 2009;9:996. doi: 10.2174/138955709788681636. [DOI] [PubMed] [Google Scholar]
- 2.Baltina LA, Kondratenko RM, Baltina LA, Jr, Plyasunova OA, Pokrovskii AG, Tolstikov GA. Pharm. Chem. J. 2009;43(10):539. doi: 10.1007/s11094-010-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltina LA, Jr, Kondratenko RM, Baltina LA, Baschenko NZ, Plyasunova OA. Russ. J. Bioorg. Chem. 2009;35:510. doi: 10.1134/S1068162009040141. [DOI] [Google Scholar]
- 4.Baltina LA, Jr, Chistoedova ES, Baltina LA, Kondratenko RM, Plyasunova OA. Chem. Nat. Compd. 2012;48:262. doi: 10.1007/s10600-012-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoever G, Baltina L, Michaelis M, Kondratenko R, Baltina L, Tolstikov GA, et al. J. Med. Chem. 2005;48:1256. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 6.Lin J-C, Cherng J-M, Hung M-S, Baltina LA, Baltina L, Kondratenko R. Antiviral Res. 2008;79:6. doi: 10.1016/j.antiviral.2008.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baltina LA, Zarubaev VV, Baltina LA, Orshanskaya IA, Fairushina AI, Kiselev OI, Yunusov MS. Bioorg. Med. Chem. Lett. 2015;25:1742. doi: 10.1016/j.bmcl.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baltina LA, Ryzhova SA, Vasil’eva EV, Kapina AP, Tolstikov GA. Zh. Obshch. Khim. 1993;63:2140. [Google Scholar]
- 9.Baltina LA, Fairushina AI, Baltina LA. Russ. J. Gen. Chem. 2015;85:2735. doi: 10.1134/S1070363215120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stolyarova OV, Baltina LA, Jr, Mikhlailova LR, Gabbasov TM, Baltina LA, Tolstikov GA. Chem. Sustainable Develop. 2008;16:563. [Google Scholar]


