Abstract
Introduction
Pain is considered an unpleasant sensory and emotional experience, being considered as one of the most important causes of human suffering. Computational chemistry associated with bioinformatics has stood out in the process of developing new drugs, through natural products, to manage this condition.
Objective
To analyze, through literature data, recent molecular coupling studies on the antinociceptive activity of essential oils and monoterpenes.
Data source
Systematic search of the literature considering the years of publications between 2005 and December 2019, in the electronic databases PubMed and Science Direct.
Eligibility Criteria
Were considered as criteria of 1) Biological activity: non-clinical effects of an OE and/or monoterpenes on antinociceptive activity based on animal models and in silico analysis, 2) studies with plant material: chemically characterized essential oils and/or their constituents isolated, 3) clinical and non-clinical studies with in silico analysis to assess antinociceptive activity, 4) articles published in English. Exclusion criteria were literature review, report or case series, meta-analysis, theses, dissertations, and book chapter.
Results
Of 16,006 articles, 16 articles fulfilled all the criteria. All selected studies were non-clinical. The most prominent plant families used were Asteraceae, Euphorbiaceae, Verbenaceae, Lamiaceae, and Lauraceae. Among the phytochemicals studied were α-Terpineol, 3-(5-substituted-1,3,4-oxadiazol-2-yl)-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene] propane hydrazide, β-cyclodextrin complexed with citronellal, (−)-α-bisabolol, β-cyclodextrin complexed with farnesol, and p-Cymene. The softwares used for docking studies were Molegro Virtual Docker, Sybyl®X, Vlife MDS, AutoDock Vina, Hex Protein Docking, and AutoDock 4.2 in PyRx 0.9. The molecular targets/complexes used were Nitric Oxide Synthase, COX-2, GluR2-S1S2, TRPV1, β-CD complex, CaV1, CaV2.1, CaV2.2, and CaV2.3, 5-HT receptor, delta receptor, kappa receptor, and MU (μ) receptor, alpha adrenergic, opioid, and serotonergic receptors, muscarinic receptors and GABAA opioid and serotonin receptors, 5-HT3 and M2 receptors. Many of the covered studies used molecular coupling to investigate the mechanism of action of various compounds, as well as molecular dynamics to investigate the stability of protein-ligand complexes.
Conclusions
The studies revealed that through the advancement of more robust computational techniques that complement the experimental studies, they may allow some notes on the identification of a new candidate molecule for therapeutic use.
Keywords: nociception, essential oils, docking, in silico, molecular target
Introduction
Pain was conceptualized for the first time in 1986 by the International Association for the Study of Pain (IASP); being defined as an response of the Central Nervous System to a tissue injury or an emotional change and classifiable as acute or chronic (McKune et al., 2015), adaptive or non-adaptive (Hellyer et al., 2007; de Oliveira Alves et al., 2017), and as physiological or pathological (Klaumann et al., 2008). The neural process of coding and processing noxious stimuli is called nociception (Naidu and Pham, 2015).
Various animal models have been developed with the purpose of understanding the mechanisms involved in nociception, including hot-plate, Hargreaves, von Frey, Randall-Selitto, capsaicin, glutamate, and formalin methods among others. Most of the non-clinical experimental models involve the use of stimuli; of a chemical, thermal, or mechanical nature where characteristic behaviors reflecting the nociceptive response are recorded. Animal models should be able to predict the effects of new drugs in humans, as well as clinical analgesic sensitivity. For this purpose, the animals are usually rodents (mice and rats), but alternative species have been used (Barrot, 2012; Barrett, 2015).
Computational chemistry associated with bioinformatics has led the process of developing new drugs with various activities, especially analgesia. Molecular docking is a computational technique that predicts the positioning (orientation and conformation) of a ligand (drug or molecule of therapeutic interest) in a target interaction site, and aids in the understanding of biological activity, by both explaining and predicting possible interactions, and helping to evaluate pharmacological properties and relations between chemical structure and biological activity (Chen and Zhi, 2001; Kirchmair et al., 2012). Thus, for molecules of therapeutic interest, molecular docking serves as a predictive model that can contribute to in vivo evaluations of pharmacological activity.
Since natural products derived from plants have a wide variety of bioactive chemical compounds, they present an important alternative in the search for therapeutic agents. Essential oils and their constituents, monoterpenes and sesquiterpenes, have several pharmacological properties, among them the potential analgesic effect. (Bahmani et al., 2014; Gomathi and Manian, 2015; de Oliveira Júnior et al., 2018). However, these products are often unexplored (Leonardão et al., 2016), and this fact collaborates to promote the scientific hypothesis related to the antinociceptive effect, including performing the molecular anchorage studies.
The objective of this study was to conduct a survey of recent molecular docking studies involving the antinociceptive activity of essential oils and monoterpenes.
Materials and Methods
The Question Under Study
This systematic review was carried out to address the specific question: “What are the scientific findings associating non-clinical animal studies and in silico analysis when evaluating the antinociceptive activity of essential oils?”
Search Strategy and Selection of Studies
The guidelines of the PRISMA guide of Systematic Reviews and Meta-Analyses) (Liberati et al., 2009) were followed. Two databases were systematically searched for experimental antinociceptive in vivo studies and in silico analysis of essential oil activity—as published through December 20, 2019 (Table 1).
Table 1.
Search mechanism and bibliographic databases used to choose the articles for this review.
| Primary bibliographic sources | Search strategy (descriptors/combinations with Boolean operators) |
|---|---|
|
Science Direct (2005–2019) |
|
|
Pubmed (2005–2019) |
|
Eligibility Criteria
Systematic screening of the articles was performed by two independent examiners according to the following inclusion criteria:
-
Biological activity: non-clinical effect of essential oil (EO) on nociceptive activity based on animal models and in silico analysis.
Primary outcomes of interest: acetic acid-induced abdominal writhing, formalin-induced nociception, orofacial formalin-induced nociception test, chronic muscle pain test, tail-flick test, hot plate test, tail immersion test, and von Frey test.
Secondary outcomes of interest—Studies of the antinociceptive mechanisms of action: Involvement of opioid receptors, Involvement of ATP-sensitive KATPchannels, in silico (molecular docking) analyses.
Plant material and chemical elucidation: chemically characterized essential oils and/or their isolated constituents from aromatic plants.
Study design: non-clinical animal studies, clinical studies, and in silico analysis to evaluate the antinociceptive activity of essential oils.
Methodological quality: accuracy of methods and outcomes; internal and external validity.
Language: for articles written in English, in cases of inconsistency, the examiners would give the final verdict on which articles should be included in this review would be reached by consensus.
Study Selection
To compose the sample of this review, a database was initially searched according to the strategies mentioned in Table 1. In this phase of the search, the results were compared, and duplicated articles found between the databases were excluded, and studies that were explicitly different from the criteria and objective of this review were excluded through the evaluation of titles and abstracts. Thus, 16 articles were included in this review, which deals with evaluations of monoterpene and sesquiterpene antinociceptive activity via in silico docking studies from 2011 to December 2019.
Data Collection and Analysis
The following variables were collected: plant family, plant species, source, phytochemical, molecular target, route of administration, animal species, antinociceptive test, software, results, country, and reference.
This information is detailed in Tables 2 and 3. The research data were analyzed based on the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) published by the Animal Center for the Replacement, Refinement & Reduction of Animals in Research (Kilkenny et al., 2010).
Table 2.
Information ethnobotanical, molecular, pharmacological, and docking programs used in vivo studies involving the antinociceptive activity of essential oils.
| Plant Family | Plant Species | Phytochemical | Molecular target | Source | Route of administration | Animal(s) species | Antinociceptive test | Software | Results | Country | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Verbenaceae | Lippia grata Schauer | Bicyclogermacrene | Alpha Adrenergic, Opioid and serotoninergic receptors. | Northeastern Brazil | Gavage | Swiss | Chronic muscle pain model | Molegro Virtual Docker v. 6.0.1. | Anti-hyperalgesic activity of LG-β-CD seems to involve opioid and serotoninergic receptors | Brazil | Siqueira-Lima et al., 2017. |
| Lauraceae |
Cinnamomum sintoc bl. |
Eugenol | COX-2. | Yogyakarta district | Ip | Swiss albino mice | Acetic acid induced writhing method | – | Eugeunol presented better molecular prediction for naproxen (control ligand-blue carbon) at binding site of COX-2. | Indonesia | Sumiwi et al., 2015. |
| Euphorbiaceae |
Croton conduplicatus Kunth |
Spathulenol Caryophyllene oxide |
Muscarinic receptors and GABAA. | Northeastern Brazil | Ip | Male Swiss mice | Acetic-acid-writhing-induced nociception, Formalin-induced nociception, Hot plate test. | Molegro Virtual Docker, v. 6.0.1. | Majority EO compounds (1,8-cineole, spathulenol, caryophyllene oxide and p-cymene) were dose-dependent and appear to involve muscarinic, opioid, and GABAA receptors. |
Brazil | de Oliveira Júnior et al., 2018. |
| Euphorbiaceae |
Croton conduplicatus Kunth |
(E)-caryophyllene Caryophyllene oxide |
Muscarinic receptors. | Northeastern Brazil | Ip | Male Swiss mice | Acetic-acid-writhing-induced nociception, Formalin-induced nociception, Hot plate test. | Molegro Virtual Docker. | Majority compound structures of camphor, caryophyllene oxide, and (E)-caryophyllene submitted to molecular docking—EO acts through central and peripheral mechanisms, possibly involving KATP channels and muscarinic receptors |
Brazil | De Oliveira Junior et al., 2017. |
| Lamiaceae | Hyptis pectinata |
Caryophyllene oxide Germacrene D |
Opioid and serotonin receptors. | Malhada dos Bois (Sergipe State), in northeastern Brazil |
Subcutaneous | Male Swiss mice | Acid Saline-Induced Chronic Muscle Pain, Mechanical Sensitivity of the Muscle (Primary Hyperalgesia), Mechanical Sensitivity of the Paw (secondary hyperalgesia) |
Molegro Virtual Docker v. 6.0.1. |
Main components of EOH were β- caryophyllene, caryophyllene oxide, germacrene D, and linalool. Central analgesic activity seems to be evoked by the action of NE-EOH on the opioid and serotonin systems. |
Brazil | Quintans-Junior et al., 2018. |
| Asteraceae | Ageratina glabrata | Chromene derivative Meloxicam |
COX-2. | Mexico | Ip | Sprague Dawley rats | Hot plate | Sybyl®X software suite. |
Antinociceptive effect (COX-2 inhibition) Not affected by hormonal changes |
Mexico | García et al. (2011) |
| Asteraceae | Achillea falcata L. | trans-Sabinol | – | Syria | Ip | BALB/c mice | Ach writhing Hot plate Tail immersion |
– | Antinociceptive effect Toxicity of some derivatives not ruled out |
Serbia | Radulović et al. (2015) |
| Asteraceae | Vanillosmopsis arborea Baker | (−)-α-bisabolol | 5-HT3 and M2 receptors. | Brazil | Gavage | Swiss mice Wistar rats |
Formalin Capsaicin Acidic saline Glutamate |
Molegro Virtual Docker. | Antinociceptive effect | Brazil | Leite et al. (2019) |
This table summarizes the main results obtained in research where essential oils from natural products were used to test for possible antinociceptive activity.
Table 3.
Information ethnobotanical, phytochemical, molecular, pharmacological, and docking programs used in silico studies involving the antinociceptive activity isolated from essential oils.
| Phytochemical | Molecular target | Chemical marker | Route of administration | Animal(s) species | Antinociceptive test | Software | Results | Country | Ref |
|---|---|---|---|---|---|---|---|---|---|
| α-Terpineol | Nitric Oxide Synthase enzyme | TP, amino guanidine, dexamethasone, Nitro- L-arginine methyl ester (L-NAME) |
Subcutaneous | Male Swiss | Mechanical hyperalgesia was assessed by means of digital von Frey | Molegro Virtual Docker v. 6.0.1. |
Antinociceptive effect of TP probably occurs via mechanisms related to modulation of oxidative stress, with maintenance of endogenous antioxidant substances and reduction of iNOS levels. |
Brazil | Gouveia et al., 2018. |
| 3-(5-substituted-1,3,4-oxadiazol-2-yl)-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]propane hydrazides | COX-2 | indomethacin | Po | Albino Wistar mice | Hot plate | Vlife MDS. | Antinociceptive effect | India | Kerzare et al., 2016 |
| β-cyclodextrin (CT-βCD) complexed with citronellal (CT) |
GluR2-S1S2 | 1FTJ protein complexed with glutamate | Po | Swiss mice | Digital von Frey Grip strength meter |
AutoDock Vina. | CT-βCD has a greater analgesic effect than the free form (CT alone) | Brazil | Santos et al., 2016 |
| (−)-α-bisabolol | TRPV1 | – | Intraocular | Swiss mice | Hypertonic saline-induced corneal nociception | Hex Protein Docking (HEX) | Nanoencapsulated BISA is topically active—attenuates 5 M NaCl-induced corneal nociception | Brazil | Teixeira et al., 2017 |
| (−)-α-bisabolol | TRPV1 | – | Po and topical | Adult male Swiss albino mice and adult male Wistar rats | Orofacial formalin test Orofacial cinnamaldehyde test Temporomandibular joint formalin test |
Hex Protein Docking (HEX) | The study confirmed the anti-nociceptive effect of BISA on orofacial pain. The effect may in part be due to TRPA1 antagonism | Brazil | Melo et al., 2017 |
| B-cyclodextrin complexed with farnesol | β-CD complex | – | Ip | Male Swiss mice | Formalin, Orofacial capsaicin, glutamate | AutoDock 4.2 software in the PyRx 0.9. | Farnesol complexed with β-CD presented best antinociceptive activity, probably via 5-HT3 receptor | Brazil | (Silva et al., 2017). |
| p-Cymene | CaV1, CaV2.1, CaV2.2 and CaV2.3 | p-cymene, nicardipine, ω-agatoxin IVA, ω-conotoxin GVIA, and N-Triazole Oxindole | Subcutaneous | Male Albino Wistar mice | Digital von Frey Grip strength meter |
Molegro Virtual Docker | p-Cymene was able to reduce calcium current density | Brazil | Santos et al., 2019 |
| α-terpineol | 5-HT receptor Delta receptor Kappa receptor MU receptor |
– | Ip | Male Swiss mice | Mechanical hyperalgesia induced by acid saline Formalin-induced nociception test |
Molegro Virtual Docker 6.0. | β-CD improves the anti-hyperalgesic effect of α-TPN; α-TPN-βCD enhances analgesic profile producing a longer-lasting analgesic profile when compared to α-TPN alone; Docking study demonstrated that anti-hyperalgesic effect produced by α-TPN-βCD implies opioid and serotoninergic receptors | Brazil | Oliveira et al., 2016 |
The table summarizes the main results obtained in research where isolated molecules of essential oils from natural products were used to test for possible antinociceptive activity.
Results
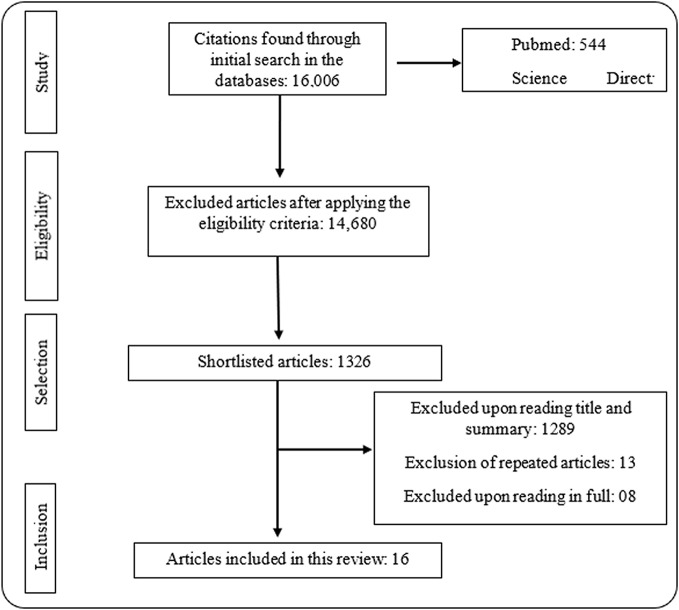
The initial search of the databases (with the strategies presented in Table 1) allowed the identification of 16,006 citations. After filtering the remaining texts included English language articles and various complete free articles; review studies were excluded, leaving 1326 articles, from which a selection based on titles and abstracts for the inclusion criteria mentioned above was performed. At this stage, 1289 articles were excluded, leaving only 37. Upon removal of 13 repeated articles, 24 remained. These studies were subsequently completely read, and finally, 16 articles were selected; 8 articles did not meet all inclusion criteria and were excluded. The selection process can be better visualized in Figure 1 below, the search flowchart.
Figure 1.
Flowchart of article selection for systematic review. The bibliographic study started with 16,006 articles, which after applying the eligibility criteria, 14,680 remained. Among these, 1326 were selected. A total of 1,289 was excluded after reading the title, 13 were excluded by repetition and 8 were excluded after full reading. In total, 16 articles fit the purpose and were selected for this review.
The studies identified were concentrated between 2011 and 2019 and are considered current.
There was variability in the study regions for the selected manuscripts, with 75% of the papers coming from the American continents, with 6.25% from North America and 68.75% from South America, 12.5% of the articles originated from the European continent, and 12.5% from the Asian continent.
Of the countries in North America, Mexico represented 6.25% of the publications, in South America, Brazil represented 68.75%, in the European continent, Italy represented 6.25% and Serbia represented 6.25%. In Asia, India and Indonesia stood out, both represented 6.25% of publications.
The most prominent plant families used in the studies identified were Asteraceae, (García et al., 2011; Radulović et al., 2015; Leite et al., 2019); Euphorbiaceae, (De Oliveira Junior et al., 2017; de Oliveira junior et al., 2018); Verbenaceae, (Siqueira-Lima et al., 2017); Lamiaceae, (Quintans-Junior et al., 2018), and Lauraceae (Sumiwi et al., 2015).
Of the species, Croton conduplicatus Kunth prevailed (De Oliveira Junior et al., 2017; de Oliveira junior et al., 2018); with Vanillosmopsis arborea Baker (Leite et al., 2019); then Achillea falcata L.; (Radulović et al., 2015); Ageratin glabrata; (García et al., 2011); Hyptis pectinata; (Quintans-Junior et al., 2018); Cinnamomum; sintoc bl.; (Sumiwi et al., 2015); and Grateful Lippia Schauer (Siqueira-Lima et al., 2017).
Among the phytochemicals studied were α-Terpineol (Oliveira et al., 2016; Gouveia et al., 2018); 3-(5-substituted-1,3,4-oxadiazol-2-yl)-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene] propane hydrazide (Kerzare et al., 2016); β-cyclodextrin (CT-βCD) complexed with citronellal (CT) (Santos et al., 2016); (−)-α-bisabolol (Melo et al., 2017; Teixeira, et al., 2017); β-cyclodextrin complexed with farnesol (Silva et al., 2017), and p-Cymene (Santos et al., 2019).
All of the selected studies were non-clinical, the animals used in the studies were Swiss Mice, BALB/c, Wistar, and Sprague Dawley Rats. The tests for evaluation of antinociceptive activity included chemical nociception induction tests: Formalin Test (De Oliveira Junior et al., 2017; de Oliveira Júnior et al., 2018; Leite et al., 2019); Capsaicin (Silva et al., 2017; Leite et al., 2019); Acetic acid (Sumiwi et al., 2015); Glutamate (Silva et al., 2017; Leite et al., 2019); Orofacial formalin (Silva et al., 2017); Saline-induced chronic muscle pain (Siqueira-Lima et al., 2017; Quintans-Junior et al., 2018); Thermal induction tests: Hot Plate Test (Radulović et al., 2015; García et al., 2011; Kerzare et al., 2016; De Oliveira Junior et al., 2017; de Oliveira Júnior et al., 2018); Tail immersion (Radulović et al., 2015); and Mechanical nociceptive induction testing: Von Frey (Santos et al., 2016; Gouveia et al., 2018 Santos et al., 2019).
The in silico programs used for docking studies were; Molegro Virtual Docker (Oliveira et al., 2016; De Oliveira Junior et al., 2017; Siqueira-Lima et al., 2017; de Oliveira Júnior et al., 2018; Gouveia et al., 2018; Leite et al., 2019; Santos et al., 2019); Sybyl®X software suite (García et al., 2011), Vlife MDS (Kerzare et al., 2016); AutoDock Vina (Santos et al., 2016); Hex Protein Docking (HEX) (Teixeira et al., 2017; Melo et al., 2017); and AutoDock 4.2 software in PyRx 0.9 (Silva et al., 2017).
The molecular targets/complexes used in the docking studies were Nitric Oxide Synthase (Gouveia et al., 2018), COX-2 (García et al., 2011; Sumiwi et al., 2015; Kerzare et al., 2016); GluR2-S1S2 (Santos et al., 2016); TRPV1 (Teixeira et al., 2017; Melo et al., 2017); β-CD complex (Silva et al., 2017); CaV1, CaV2.1, CaV2.2, and CaV2.3 (Santos et al., 2019); 5-HT receptor, Delta receptor, Kappa receptor, and MU (µ) receptor (Oliveira et al., 2016); Alpha Adrenergic, Opioid, and Serotonergic receptors (Siqueira-Lima et al., 2017); Muscarinic receptors and GABAA (De Oliveira Junior et al., 2017; de Oliveira Júnior et al., 2018); Opioid and Serotonin receptors (Quintans-Junior et al., 2018); 5-HT 3 and M2 receptors (Leite et al., 2019).
The study results were analyzed separately and can be seen in Table 2, along with other information from the articles.
Discussion
With technological development and advances in understanding the pathophysiological bases of pain/nociception, alternative screening methods for naturally occurring compounds have gained specified target approaches. Various models have been developed and tested against natural compounds. Previous studies, including a systematic review, showed the potential antinociceptive effect of essential oils and their phytoconstituents, especially monoterpenes. However, most of the reports of these investigations present obtained by experimental animal assays (Guimarães et al., 2012; Sá et al., 2017). Through this review, although scarce, studies coupling molecular anchoring with EO antinociceptive activity and their constituents (monoterpene - sesquiterpene) (Table 4) allow observations concerning the obtained molecular hits. This increases the chances of finding candidate molecules for therapeutic use. Certain species were studied for possible antinociceptive activity with the aid of molecular coupling. For these, it was possible to predict the preferential orientation (when linked together to form a stable complex) of one molecule to another, and further elucidated molecular interactions.
Table 4.
Interactions observed in docking studies involving antinociceptive activity.
| Ligand | Molecular target | Interacting amino acids | Reference |
|---|---|---|---|
| Citronellal | GluR2-S1S2 | Arg96, Ser142 e Thr143. | Santos et al., 2016 |
| α-Terpineol | Nitric Oxide Synthase | Thr324, Trp325 e Ile327. | Gouveia et al., 2018. |
| (−)-α-bisabolol | TRPV1 | Ala680, Gly683, Asn687. | Teixeira et al., 2017. |
| (−)-α-bisabolol | TRPV1 | Ile695, Ser972, Leu973 and Lys969. | Melo et al., 2017 |
| p-Cymene | CaV1, CaV2.1, CaV2.2 and CaV2.3 | Glu84, Glu87, Ala88, Val91, Met144. | Santos et al., 2019 |
| α-Terpineol | 5-HT receptor Delta receptor Kappa receptor MU receptor |
Asp129 and Cys133. Asp128. Leu67 and Val63. Asp147. |
Oliveira et al., 2016 |
| (−)-α-bisabolol | 5-HT3 and M2 receptors. |
Tyr64, Arg65, Thr154, Trp156 and Glu209. Asp103, Tyr104, Ala194 and Tyr403. |
Leite et al., 2019. |
| Camphor, transcarophyllene and bicyclogermacrene | Alpha adrenergic, µ Opioid, and 5-HT. | Arg14, Tyr15, Ile18 and Thr19. Asp135, Val136, Thr140, Phe340 and Phe341. Gln124, Tyr148, Val236, His297, Trp318. |
Siqueira-Lima et al., 2017. |
| Eugenol | COX-2 | Val116, Arg120, Val349, Leu352, Tyr355, Phe518, Met522. | Sumiwi et al., 2015. |
| b-FNA, Germacrene D, Caryophyllene oxide, Linalool and β-caryophyllene |
Opioid and serotonin receptors. | Thr134 and Val201. | Quintans-Junior et al., 2018. |
| 3-(5-substituted-1,3,4-oxadiazol-2-yl)-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]propane hydrazides derivatives | COX-2 | Pro127, Tyr373, Gly536, Gln374, Arg376 and Ser541. | Kerzare et al., 2016. |
| 1,8-Cineole, Caryophyllene oxide, p-Cymene, Spathulenol, | Muscarinic receptors and GABAA. | Not described. | de Oliveira Júnior et al., 2018. |
| 10-benzoiloxi-6,8,9-isobutirato de tri-hidroxi-timol | COX-2 | Not described. | García et al., 2011. |
| trans-sabinol and trans-sabinyl acetate | AChE | Ser200, Glu327, His440, Phe330 and Trp 84. | Radulović et al, 2015. |
| B-cyclodextrin complexed with Farnesol | β-CD complex | Not described. | Silva et al., 2017. |
The table summarizes the ligands and molecular targets used in molecular docking research, as well as the residues that showed interaction with the compounds.
Vanillosmopsis arborea Baker
Vanillosmopsis arborea Baker (Asteraceae) is a native plant to northeastern Brazil, especially the state of Ceará (Matos et al., 1988). There are insufficient studies reporting the biological effects of the essential oil extracted from this plant, but its leishmanicidal (Colares et al., 2013), gastroprotective (De Oliveira et al., 2009), and antimalarial (Mota et al., 2012) activities have been described. According to De Oliveira et al. (2009), an analysis by gas chromatography coupled to mass spectrometry (GC/MS) of V. arborea stem bark essential oil (EOVA) evidenced the existence of α-bisabolol (70%), and also α-cadinol (8.4%), elemicin (6.21%), β-bisabolene (4.46%), δ-guaiene (2.31%), β-cubebene (1.76%), and estragole (1.08%).
To increase the bioavailability and pharmacological properties of essential oils, complexation with β-cyclodextrin is very useful (Santos et al., 2016). Cyclodextrins are cyclic oligosaccharides presenting a hydrophobic center that complexes with molecules yet improves water solubility and reduces toxic effects (Oliveira et al., 2009; Abril-Sánchez et al., 2019). EOVA and its form as complexed with β-cyclodextrin (EOVA-pCD) at a dose of 50 mg/kg, i.p. reduced orofacial nociception induced by various stimuli, as well as in a model of temporomandibular joint dysfunction caused by the administration of formalin. The study also suggested that EOVA modulates type 1 vanilloid transient potential receptors, yet without interacting with the glutamatergic inhibitory pathway. Fos protein expression in the dorsal horn of the spinal cord was decreased by pCDEVA, inferring a reduction in pain-sensitive neuronal activation. In the same study, (through molecular docking) the researchers observed favorable interactions between bisabolol, the major constituent of EOVA, the 5-HT3 receptor, and type 3 muscarinic receptors (Leite et al., 2019).
Citronellal
Citronellal (Table 5, ID 01) is a monoterpene isolated from aromatic plants of the genus Cymbopogon (Quintans-Junior et al., 2008). Several pharmacological activities have been described for this compound, which acts as an anti-atherosclerotic (Lu et al., 2019), antifungal (Wu et al., 2016), anti-inflammatory (De Santana et al., 2013), and anticonvulsant (Melo et al., 2011).
Table 5.
Main docking compounds used in the articles included in this review.
| ID | Name | Structure | Reference | ID | Name | Structure | Reference |
|---|---|---|---|---|---|---|---|
| 01 | Citronellal |
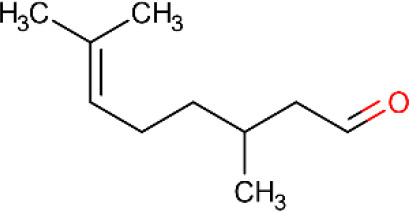
|
Santos et al., 2016 | 09 | Linalool |
|
Quintans-Junior et al., 2018. |
| 02 | α-Terpineol |
|
Gouveia et al., 2018. Oliveira et al., 2016 |
10 | β-caryophyllene |
|
Quintans-Junior et al., 2018. |
| 03 | (−)-α-bisabolol |
|
Teixeira et al., 2017 Melo et al., 2017. Leite et al., 2019. |
11 | 1,8-Cineole |
|
de Oliveira Júnior et al., 2018. |
| 04 | p-Cymene |
|
de Oliveira Júnior et al., 2018. | 12 | Spathulenol |
|
de Oliveira Júnior et al., 2018. |
| 05 | Camphor |
|
Siqueira-Lima et al., 2017. | 13 | trans-sabinol |
|
Radulović et al, 2015. |
| 06 | Eugenol |
|
Sumiwi et al., 2015. | 14 | sabinyl acetate |
|
Radulović et al, 2015. |
| 07 | Germacrene D |
|
Quintans-Junior et al., 2018. | 15 | Farnesol |
|
Silva et al., 2017. |
| 08 | Caryophyllene oxide |
|
Quintans-Junior et al., 2018. |
This table summarizes the structure of compounds that interact with protein targets in docking studies.
Despite the known effects of isolated monoterpenes, the complexation of monoterpenes with β-cyclodextrin has produced more promising results for treating pain and inflammation (Quintans-Junior et al., 2013; Nascimento et al., 2014; Silva et al., 2016). In a chronic non-inflammatory muscle pain model, chronic treatment using a citronellal/β-cyclodextrin complex at a dose of 50 mg/kg, p.o. presented longer-lasting antihyperalgesic effects than citronellal alone. The results can be explained by an increase in c-Fos protein expression in the ventrolateral periaqueductal gray and rostroventral medullary areas of the brain and reductions in this activity in the superficial dorsal horn, suggesting inhibitory modulation of descending pain pathways (Santos et al., 2018). In a molecular docking study, a favorable energy bond between citronellal and the structure GluR2-S1S2J, an ionotropic glutamate receptor responsible for painful stimulus propagation was observed (Santos et al., 2016).
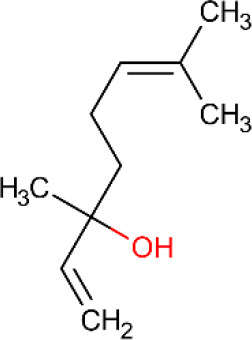
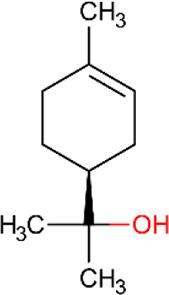
α-Terpineol
α-Terpineol (Table 5, ID 02) is a monoterpene present in the essential oils of Ravensara aromatica, Melaleuca qinquenervia, and mainly, species of the genus Eucalyptus (Elaissi et al., 2010). Studies show the clinical potential of this compound to treat various types of pain (Quintans-Júnior et al., 2011; De Oliveira et al., 2012; Oliveira et al., 2016; Safaripour et al., 2018).
In an experimental model of cancer pain consisting of subcutaneous implantation (in the plantar region) of tumor cells in mice, subcutaneous treatment with α-terpineol (12.5, 25, and 50 mg/kg, sc) promotes an anti-hyperalgesic effect and seems to reduce allodynia in these painful conditions, without promoting myorelaxant effects. The effect may be related to antioxidant capacity and reduced inducible nitric oxide synthase (iNOS) levels in the tumor microenvironment. Molecular docking corroborates these results, since it was observed that the monoterpene binds to iNOS in the same regions as N-Nitroarginine methyl ester (an iNOS inhibitor) (Gouveia et al., 2018).
A complex containing α-terpineol and cyclodextrin (αTPN-pCD) at doses of 25, 50, and 100 mg/kg, p.o. reduced mechanical hyperalgesia caused in an animal model of fibromyalgia. In the same study, the involvement of the opioid and serotonergic system in the analgesic activity was observed both through the use of pharmacological antagonists in animals, and when employing molecular docking (Oliveira et al., 2016).
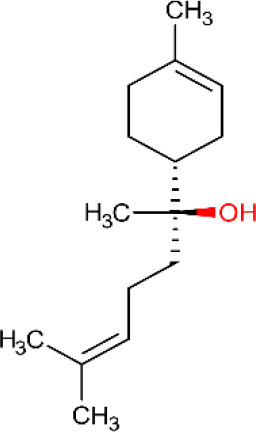
α-Bisabolol
α-Bisabolol (Table 5, ID 03) is a monocyclic sesquiterpene extracted from the essential oils of various plants, mainly the flowers of Matricaria chamomilla and the bark of Vanillosmopsis arborea Baker (Kamatou and Viljoen, 2010; Inocencio Leite et al., 2014). Several biological effects have been described for α-bisabolol including neuroprotective (Fernandes et al., 2019), antiparasitic (de Menezes et al., 2019), anticancer (Wu et al., 2018), and antibacterial activity (de Sousa Oliveira et al., 2017).
In an orofacial antinociceptive evaluation of α-bisabolol, the non-clinical efficacy of this compound when administered systemically or topically was confirmed. In a temporomandibular joint dysfunction model, α-bisabolol reduced nociceptive orofacial rubbing behavior in rats. The pharmacological effects occur independently of ATP-sensitive nitrergic systems, opioids, and potassium channels. Molecular docking revealed a strong interaction and the absence of significant electrostatic repulsion between α-bisabolol and the TRPA1 receptor, suggesting possible antagonism (Melo et al., 2017). Texeira et al. (2017), observing TRPV1 antagonism in molecular docking studies, revealed that nanocapsules containing α-bisabolol present antinociceptive activity in an orofacial pain model caused by administration of hypertonic saline into the mouse cornea.
Still talking about terpenes and TRPV1 receptors, Jansen et al. (2019), found that myrcene has a significant participation in calcium inflows mediated by the TRPV1 receptor. Myrcene has been identified as an analgesic in previous studies, demonstrating its anti-nociceptive potential in mice (Rao et al., 1990). Responses to myrcene showed total dependence on the presence of TRPV1 and were effectively blocked by capsaicin, a TRPV1 antagonist. Patch Clamp studies showed that myrcene is indeed an effective TRPV1 ligand and in cells that contain high TRPV1 receptor densities, leading to intracellular calcium release. While for many pain applications there is a focus on TRPV1 antagonists, other applications depend on the chronic agonism of TRPV1, leading to the desensitization of TRPV1 at the cellular level and the induction of neuronal cell death by large inflows of calcium and sodium through the channel (Derry et al., 2017). Preliminary molecular coupling studies suggest that myrcene is interacting hydrophobically, non-covalently, and in a way that does not depend heavily on reactive cysteines. Tyr 554 is involved in the binding site, which has also been implicated in capsaicin binding (Elokely et al., 2016) and forms part of the S4-S5 loop between the fourth and fifth transmembrane domains of TRPV1 (Boukalova et al., 2010).
Like myrcene, camphor also desensitizes TRPV1 receptors, but in a more rapidly and completely way than capsaicin, it activates TRPV3 and inhibits TRPA1, correlating to its analgesic properties (Xu et al., 2005).
The endocannabinoid system involves the central and peripheral nervous systems. It is involved in inflammatory and pain processes (Rodriguez de Fonseca et al., 2005). The endocannabinoid system appears to work both independently and synergistically (Mallat et al., 2011; Baron, 2018). Cannabidiol (CBD) is the second major cannabinoid and has much lower affinity for CB1 and CB2 receptors as compared to Δ9-tetrahydrocannabinol (THC), and it acts as a non-competitive CB1 and CB2 receptor antagonist (Thomas et al., 2007). CBD has additional actions that may account for its anti-inflammatory and analgesic effects including TRPA1 agonist, TRPV1 agonist (similar to capsaicin, although without the noxious side effects), TRPM8 antagonist (De Petrocellis et al., 2011; Baron, 2018).
Jansen et al. (2019) show that myrcene and CBD share elements of a binding site and can influence one another physiologically. Their data suggest that several minor cannabinoids discriminate between TRPV1, TRPA1, and TRPM8 (Starkus et al., 2019). In CBD docking studies, a binding pocket partially over-lapping with that of Myrcene was identified and the best scoring pose showed a docking score of −26.5 kcal/mol. In this site, Thyr 554 and Arg 491 are important, as for Myrcene. The remaining residues implicated in CBD binding show both similarities and differences to the Myrcene site (Jansen et al., 2019).
Croton conduplicatus Kunth
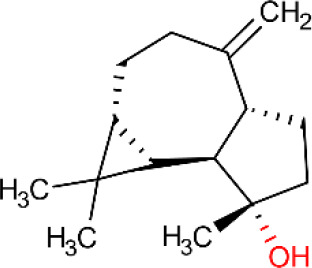
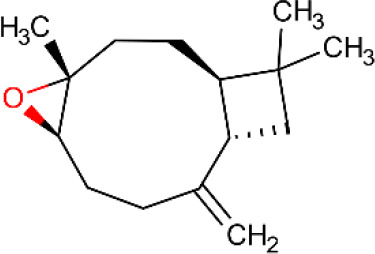
Croton conduplicatus Kunth is a Brazilian medicinal plant, belonging to the Euphorbiaceae family, called as “quebra-faca” by the locals; it is found in South America. In the Brazilian northeast, folk medicine uses its leaves to treat stomach pain (Cartaxo et al., 2010). GC/MS analysis of Croton conduplicatus Kunth stem bark (essential oil) revealed a majority presence of the terpenoids (E)-caryophyllene (13.72%), caryophyllene oxide (Table 5, ID 08) (13.15%) and camphor (8.25%). The essential oil presents central and peripheral antinociceptive activity via possible involvement of KATP channels and muscarinic receptors, yet without participation of opioid receptors. In the same study, a satisfactory link was observed in molecular docking between the constituent terpenoids and muscarinic receptors (M2, M3, and M4) (De Oliveira Júnior et al., 2017).
Chemical analysis of Croton conduplicatus Kunth leaf essential oil revealed 1,8-cineole (Table 5, ID 11) (21.42%), p-cymene (12.41%), spathulenol (Table 5, ID 12) (15.47%), and caryophyllene oxide (12.15%) as the main constituents. The oil presents antinociceptive activity equal to that of the leaves, yet involvement of the opioid system is observed. In molecular docking, the interactivity of spathulenol and caryophyllene oxide with opioid and muscarinic receptors was verified (de Oliveira Júnior et al., 2018).
Complexed β-cyclodextrin (βCD)—Lippia grata essential oil
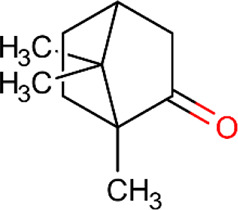
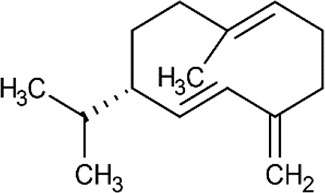
Lippia grata Schauer is a shrub widely distributed in northeastern Brazil. Folk medicine uses it to treat pain conditions (Viana et al., 1981). Terpene-rich L. grata (LG) leaf essential oil is known to promote an orofacial analgesic profile. Since it is known that the therapeutic analgesic effects of certain EO and its constituents can be enhanced by forming a β-cyclodextrin (βCD) inclusion complex (Oliveira et al., 2016), Siqueira-Lima et al., 2017 evaluated the antinociceptive effect of a β-cyclodextrin (βCD) complex with Lippia grata essential oil in an animal model of chronic musculoskeletal pain. The model mimicked painful muscle diseases such as fibromyalgia (FM), which is a rheumatic disease characterized by widespread chronic pain and is difficult to treat. The low clinical efficacy and side effects of current treatments make therapeutic adherence more difficult, and the lack of knowledge about the pathophysiology of FM complicates the development of new drugs. Animal models are an alternative for reproducing FM symptoms. In a model of chronic non inflammatory muscle pain, caused by saline acid injection in the mouse gastrocnemius, oral administration of LG-βCD produced excellent antihyperalgesic activity. LG-βCD also did not alter muscle strength, discarding the chance of reduced motor performance, common in some CNS drugs. An assay to evaluate possible antagonist involvement of opioid, serotoninergic, and noradrenergic pathways revealed that the antihyperalgesic effect of LG-βCD may involve serotonergic and opioid receptors, indicating probable participation of the inhibitory modulation system of descending pain. This was partially supported by the in silico study. The receptor structures were obtained from Protein Data Bank (PDB). The investigated receptors, by comparing binding energy with ligands; camphor (Table 5, ID 05), trans-caryophyllene, bicyclogermacrene (Table 5, ID 07) (major LG compounds) were alpha-adrenergic, μOpioid, and 5HT. In silico target validation analysis favored the understanding of the drug-receptor interaction, and confirmed the observed result within in vivo tests, demonstrating that camphor and E-caryophyllene bind to the alpha-adrenergic receptor, while bicyclogermacrene binds with moderate energy to 5-HT and μOpioid receptors (Siqueira-Lima et al., 2017).
Hyptis pectinata Leaf Essential Oil
Hyptis pectinata belongs to the Lamiaceae family, which is characterized by the presence of strongly aromatic plants, present in North and South America, mainly in tropical areas. In traditional medicine, H. pectinata extracts have been used as medicinal teas in pain treatment. The anti-inflammatory and analgesic profile of H. pectinata has been reported in many studies in the literature. The analgesic effects of H. pectinata EO occur due to the terpenoid compounds, such as β-caryophyllene (Table 5, ID 10), caryophyllene oxide, linalool (Table 5, ID 09), and limonene (McNeil et al., 2011). The antinociceptive effect of β-caryophyllene occurs due to its cannabimimetic effects (Gertsch et al., 2008). The complex containing β-caryophyllene and β-cyclodextrin has anti-hyperalgesic properties in the model of chronic muscle pain via inhibition of c-Fos expression in the lumbar spinal cord (Quintans-Júnior et al., 2016).
The EO of H. pectinata complexed with β-cyclodextrin is able to increase the analgesic effect, as well as extend its duration (Menezes et al., 2015). Quintans-Junior et al. (2018) evaluated the capacity of a nanostructured thermoreversible subcutaneous hydrogel aggregated with H. pectinata essential oil (NE-EOH) to promote long-term antihyperalgesic effect in a fibromyalgia (FM) animal model. This formulation (containing H. pectinata essential oil) produced a lasting antihyperalgesic effect in a non-inflammatory muscle pain model in mice. NE-EOH produces analgesia through CNS inhibitory mechanisms. Through a molecular anchoring study, it was possible to predict that this antihyperalgesic response involves central pain inhibitory pathways and endogenous serotonin and opioid neurotransmitters.
To evaluate the binding ability of NE-EOH to the tested targets (serotonin and opioid receptors), coupling analysis using MolDock was performed, taking into account the binding energy of the major H. pectinata EO compounds (β-caryophyllene, caryophyllene oxide, germacrene D, and linalool) with μ-OR β-FNA receptors and 5-HT1B dihydroergotamine. A hydrogen bond was observed between the epoxy portion of caryophyllene oxide and 5-HT1B (Thr 134), an equal interaction could be seen for dihydroergotamine. Caryophyllene oxide and germacrene D presented the lowest binding energies of the studied secondary metabolites, suggesting that germacrene D and caryophyllene oxide possibly contributes in the antihyperalgesic activity of NE-EOH, via the μ-OR pathway (Quintans-Junior et al., 2018).
Cinnamomum sintoc bl Bark Essential Oil (sintoc)
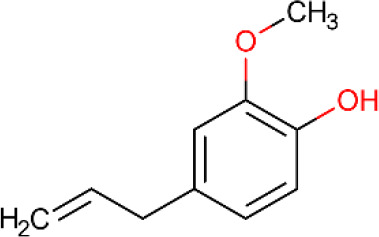
Sintoc (C. sintoc bl) is a plant grown in Indonesia, Malaysia, and Thailand and used in folk medicine to treat swelling (inflammation). Sumiwi et al., 2015 investigated the anti-inflammatory and analgesic activity of Cinnamomum sintoc bl (sintoc) bark essential oil, (inhibiting the enzyme COX-2), using animal models, together with molecular coupling to predict the interaction of sintoc compounds with COX-2. Eugenol (a constituent of sintoc (Table 5, ID 06)) presented a good visual interaction with COX-2. The phenol part of eugenol forms hydrogen bonds with Gly 526 and Met522 of COX-2, as well as the naproxen phenol. However, eugenol presents no electrostatic interaction between carboxyl and ammonium ions, such as naproxen carboxyl groups with Arg120 ammonium ions.
In the in vivo tests, the essential oil of sintoc bark presented analgesic activity in the acetic acid-induced abdominal writhing test and anti-inflammatory activity in the carrageenan-induced paw edema test. In a previous molecular anchorage study, the results revealed that isoeugenol can effectively inhibit the enzymatic activity of cyclooxygenase and lipoxygenase. Isoeugenol anchored at the active site with an orientation similar to that of indomethacin (Zarlaha et al., 2014).
Ageratina glabrata
Species of the genus Ageratina belong to the Asteraceae family. In general, this genus is known to have therapeutic activities, among which its analgesic effects stand out (Mandal et al, 2005; Chakravarty et al., 2011).
Ageratina glabrata (Kunth) is commonly known in Mexico as “chamizo blanco” or “hierba del coup”. Folk medicine reveals the use of this plant for pain relief. The literature describes the presence of flavones, thymol derivatives, and other phenolic terpenoids in its chemical composition (Vivar et al., 1971; Bohlmann et al., 1977; Guerrero et al., 1978).
Using the hot plate test, García et al. (2011), verified the presence of analgesic effect in a group of animals treated at 100mg/kg with A. glabrata leaf extract. The results, because of the duration and pain suppression characteristics, and is similar to those observed in the positive control treated with meloxicam, suggest that the molecular mechanism involved may be via cyclooxygenase (COX).
After isolation and purification, Garcića et al. (2011) identified the presence of trihydroxy thymol 10-benzoyloxy-6,8,9-isobutyrate, which became the molecule from A. glabrata extract chosen to conduct a docking study using the Sybyl® software. The protein was downloaded from the Protein Data Bank with code 3LN0. Both meloxicam and the derivative was successfully docked in the same position and orientation at the PDB ligand, at the active site of the COX-2 enzyme. The binding and formation of the ligand-enzyme complex were in agreement with the crystallographic structure, showing the potential of this derivative to interact with COX-2 and promote the analgesic effect evidenced in the in vivo thermal nociception model.
Achillea falcata L.
Achillea falcata L. is an endemic Mediterranean species widely considered for its pharmacological effects, and traditionally used to treat fever, stomach pain, and hemorrhoids (Aburjai et al., 2007; Alzweiri et al., 2011).
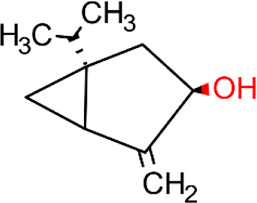
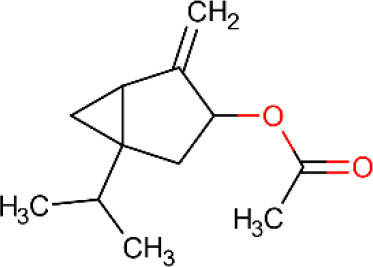
Radulović et al. (2015) have been able to provide clear evidence that A. falcata is capable to produce trans-sabinol (Table 5, ID 13) and some of its esters. Its constituents are biosynthesized and accumulate in the aerial parts and roots of the plant (Kürkçüoğlu et al., 2003; AburjaiHudaib, 2006). The compounds formate and tiglate have been recently discovered (Radulović et al., 2015).
Achillea falcata essential oil characterization (aerial parts and roots) was performed near the city of Ma’loula, Syria, revealing the presence of two principal constituents, trans-sabinol (19.1%) and trans-sabinyl acetate (Table 5, ID 14) (11.4%), as well as their rare esters. Using different models such as abdominal contortions and hot plate, Radulović et al. (2015) screened to investigate the analgesic potential of trans-sabinol and its esters.
Due to the structural similarity of these compounds with rivastigmine, an acetylcholinesterase (AChE) inhibitor, there was an interest in verifying in silico (molecular docking), the ability of these esters to interact with AChE. The active site of AChE is known to be deep within the enzyme. The residues of Ser200, His440, and Glu327, located at the bottom depth, form the catalytic triad and participates directly in hydrolysis of acetylcholine (Sussman et al., 1991; Millard et al., 1999).
All of the esters found energetically favorable coupling poses, placing the ligands at the catalytic site, and suggesting that the compounds may indeed approach the amino acid residue triad. The most favorable anchorage position was achieved by trans-sabinyl. This compound was placed with the electrophilic ester/bond group near the residues of Ser200, Glu327, His440, Phe330, and Trp 84, which are relevant for AChE function (Sussman et al., 1991; Millard et al., 1999). The ligand disposal indicates that it might interact with the enzyme allowing covalent modification. The other esters came with a similar outcome, and the calculated binding energies suggests that trans-sabinyl tiglate and senecioate initially bind more strongly to the enzyme (8.5 kcal/mol), while trans-sabinyl formate likely has the lowest affinity for AChE (Radulović et al., 2015).
The mentioned compounds also presented antinociceptive activity in two different animal models. Trans-sabinol promoted a reduction in the animal’s response to thermal stimuli in the hot plate test, and a reduction in the abdominal writhing test response as well. When subjected to the hot plate test at the dose of 50 mg/kg, trans-sabinol was able to reach its maximum effect after 15 min. In the same test, time and dose, trans-sabinyl tiglate increased the residence time by 140% (Radulović et al., 2015). These results, associated with molecular anchoring tests, indicate that trans-sabinol and its esters interact with different targets and influence both the periphery and the central nervous system, been capable to promote a considerable antinociceptive effect.
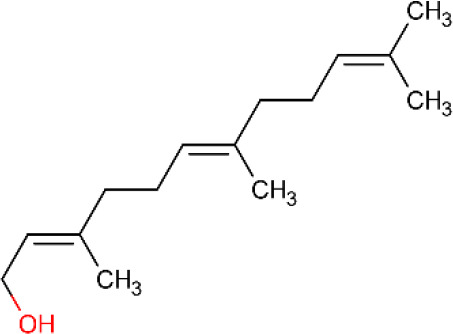
β-Cyclodextrin Complexed With Farnesol
Farnesol (FAR) (Table 5, ID 15) is a naturally occurring sesquiterpene alcohol known to exhibit multiple functions including Ca2+ channel inhibition (Endo et al., 2011; Khan and Sultana, 2011; Santhanasabapathy et al., 2015), an important target for drugs used in chronic pain (Clark et al., 2016). This sesquiterpene has been shown to possess anti-inflammatory and analgesic properties but without considerable neurotoxicity in the brain of adult mice (De Oliveira Junior et al., 2013).
A molecular docking study was performed to predict the likely interaction between β-cyclodextrin (β-CD) and FAR. It was observed that of the ten conformations generated by FAR with β-CD there were stable fittings (forming complexes) with the lowest energy value being - 3.45 kcal/mol. The presence of hydrogen bonds between farnesol and β-CD was also observed.
In the formalin test, administration of FAR (50 mg/kg) was able to reduce face rubbing time (p < 0.05); FAR at 100 mg/kg, and FAR + β-CD in 50 and 100 mg/kg doses reduced this behavior in both phases of the formalin test (p < 0.001). From the results, FAR + β-CD was significantly more effective in reducing pain behavior than FAR alone (Silva et al., 2017).
Experiments using a capsaicin-induced orofacial pain model showed that administration of FAR (50 mg/kg) also reduced face rubbing time (p < 0.05), yet FAR at 100 mg/kg, and the FAR + β-CD complex at doses of 50 and 100 mg/kg reduced this behavior more effectively (p < 0.001). Two targets act in this pain pathway, TRP receptors, and Ca2+ channels, thus suggesting their inhibition by the compounds (Silva et al., 2017).
Glutamate tests revealed that FAR, and FAR + β-CD at doses of 50 and 100 mg/kg significantly inhibited nociception (p < 0.0001). Statistical differences between FAR + β-CD at 100 mg/kg dose, and doses of isolated FAR at 50 and 100 mg/kg (p < 0.0001) indicated once again that the complex is more effective (Silva et al., 2017).
In a study on the mechanism of action, both isolated FAR and FAR + ondansetron presented statistical differences (p < 0.0001), demonstrating potential interactions with serotonergic receptors. The 5-HT3 receptor plays a pro-nociceptive role, mediating descending excitatory pathways in the spinal cord dorsal horn (Azimaraghi et al., 2014).
Overall, FAR + β-CD demonstrated a better pharmacological effect than the active compound alone. FAR + β-CD reduced orofacial pain behavior, which according to the investigation of the mechanism of action, was potentially mediated by interaction with 5-HT3 receptors. The inclusion complex that contains FAR + β-CD, therefore, suggest having therapeutic potential in the treatment of some types of dysfunctional pain, such as orofacial pain.
2-Oxoindolin-3-ylidene-3-(5-substituted phenyl-1,3,4-oxadiazol-2-yl) Propanehydrazide Derivatives
The hybrid approach involves the development more effective synergistic molecules by hybrid mixing of two or more already active biomolecules to produce new derivatives that have better pharmacological activity (Gediya and Njar, 2009).
The target portions selected for the formation of 2-oxoindolin-3-ylidene-3-(5-substituted phenyl-1,3,4-oxadiazol-2-yl)-propanehydrazide hybrids were based on studies revealing the analgesic and anti-inflammatory properties of indole and oxadiazole nuclei (Pandeya et al., 1998; Wagle et al., 2008; Chikhale et al., 2009; Jayashankar et al., 2009; Singh et al., 2010; Chaluvaraju et al., 2011).
Fifteen different hybrids of indole and oxadiazole were synthesized. In silico determinations of potentials compound–receptor interactions were performed through molecular coupling studies of the ligands at the cyclooxygenase (COX) site (Chikhale et al., 2015). Docking of these derivatives with COX-2 (PDB code 4Z0L) was performed using Vlife MDS Molecular Modeling 4.3.1 software. One of the reasons for choosing this enzyme was that its crystallographic structure already provides complexing with an indole derivative, and it can act as a reference molecule for coupling. As well, the animal model used also favored the choice. Anchoring was performed for all of the synthesized compounds. Three compounds, 50 (p-OH), 51 (p-CH3), and 53 (o-OCH3, m′-OCH3) exhibited the best activity, with 50 and 51 the most active. As energias de 50 (−4,44) e 51 (−4,37) foram as mais altas da série de derivados sintetizados, comparáveis à indometacina, o medicamento padrão, com uma pontuação de 4,47. Compound 50 was shown to bind at the active site of COX-2, forming hydrogen bonds at GLY536 and TYR373. Hydrophobic interactions were found mainly at GLN374 and ARG376. Compound 51 also stood out for interacting at the COX-2 active site, forming two hydrogen bonds at ASN375 (Kerzare et al., 2016). The presence of methyl or hydroxyl groups, as well as a 1,3,4-oxadiazole nitro substitution, increased the activity. The nesting of these engineered molecules demonstrated their entry into a deep region of the enzyme. Thus, the presence of an indole ring and oxadiazole in the molecule are considered beneficial for activity, but the presence of halogens such as chlorine or fluorine reduces activity.
Was evaluated the analgesic activity of the synthesized compounds using the hot plate test. They were tested at an oral dose of 100 mg·kg−1, and compared to indomethacin at a dose of 100 mg·kg−1 (v.o.), the tested compound series showed analgesic activity after 90 min ranging from 25.13% to 84.11%. The results revealed compounds 50, 51, and 52 (m-NO2) as presenting good analgesic activity, while compounds 49 and 61 (o-F) presented intermediate activity, and compounds 56 (p-COCH3) and 57 presented lesser activity as compared to the medicinal standard. The results indicate that compounds possessing electron-withdrawing groups with para and meta substituents can increase analgesic activity, while electron donor groups decrease activity (Kerzare et al., 2016).
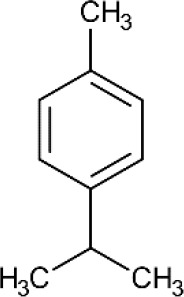
p-Cymene
P-cymene (Table 5, ID 04) is a monoterpene found in the essential oil of approximately 100 herb species, and also present in over 200 types of food (Selvaraj et al., 2002). Its many biological effects, such as anti-inflammatory and analgesic activity, have been studied and demonstrated in various parts of the world (Bonjardim et al., 2012; De Souza Siqueira Quintans et al., 2013; Quintans-Junior et al., 2013; De Santana et al., 2015).
As a potential alternative to cancer-associated pain, Santos et al. (2019) evaluated the effects of p-cymene on animal models of Sarcoma 180 (S180) induced nociception and investigated how it may act to promote such effect.
The animals submitted to the sarcoma-causing agent received the treatment with p-cymene at doses of 12.5, 25 or 50 mg/kg subcutaneously for 15 days in a row. The rats were evaluated for sensitivity to mechanical stimulation using the von Frey test on the tumor-bearing paw. Four measurements were taken between 3-min intervals to verify the stimulus intensity. It was seen that p-cymene at a dose of 50 mg/kg was able to reverse the hyperalgesic graph, starting from the 11th to the 15th day, with 60.4% of inhibition, equivalent to morphine, which similarly caused a decrease in animal perception (Santos et al., 2019).
The molecular interactions between p-cymene and the various voltage-dependent calcium channel subtypes were analyzed by docking studies, using p-cymene, nicardipine, ω-conotoxin GVIA, ω-agatoxin IVA, and N-triazole oxindole as ligands. The Protein Data Bank provided the macromolecules for this study, which were CaV1 calcium channel (type L) (PDB ID 5GJV), CaV2.1 calcium channel (type P/Q) (PDB ID 3BXK), CaV2.2 calcium channel (type N) (PDB ID 3DVE) and CaV2.3 calcium channel (type R) (PDB ID 3BXL). All ligands were subjected to molecular anchoring using the MolDock algorithm (Thomsen and Christensen, 2006; Santos et al., 2019).
When p-cymene interacted with CaV1, CaV2.1, CaV2.2, and CaV2.3 calcium channels, the respective negative energy values of −60.118, −59.60, −49.55, and −59.95 kcal/mol suggested that binding between the targets is both favorable and likely occurs since such negative values suggest lower energy expenditure to assume a more stable interaction (Du et al., 2016). These voltage-dependent calcium channels can be found at presynaptic terminals and participate in the release of neurotransmitters, such as substance P, glutamate, and CGRP (Lee, 2013). The density of the calcium stream was significantly reduced by p-cymene. It is known that direct inhibition of calcium channels alone by exogenous ligands may cause antinociception (Freitas et al., 2018). In addition, this mechanism of action is equivalent to certain existing drugs that are currently used to treat chronic pain, such as gabapentin (Santos et al., 2019; Catterall and Swanson, 2015).
We observed that many studies covered in this review used molecular coupling to investigate the mechanism of action of several compounds, one of the objectives of molecular docking studies. Thus, the studies described in this review use docking in the second stage, that is, after the experimental tests. However, there are other docking approaches that are used in a first stage, that is, before biological assessment to avoid spending on reagents and the irrational use of animal models. Therefore, instead of attempts to find potential compounds, other methodologies can be used to select the most promising compounds with the potential therapeutic effect even before biological assays.
With the advancement of more robust computational techniques, in silico studies guarantee greater reliability of results and rational drug planning.
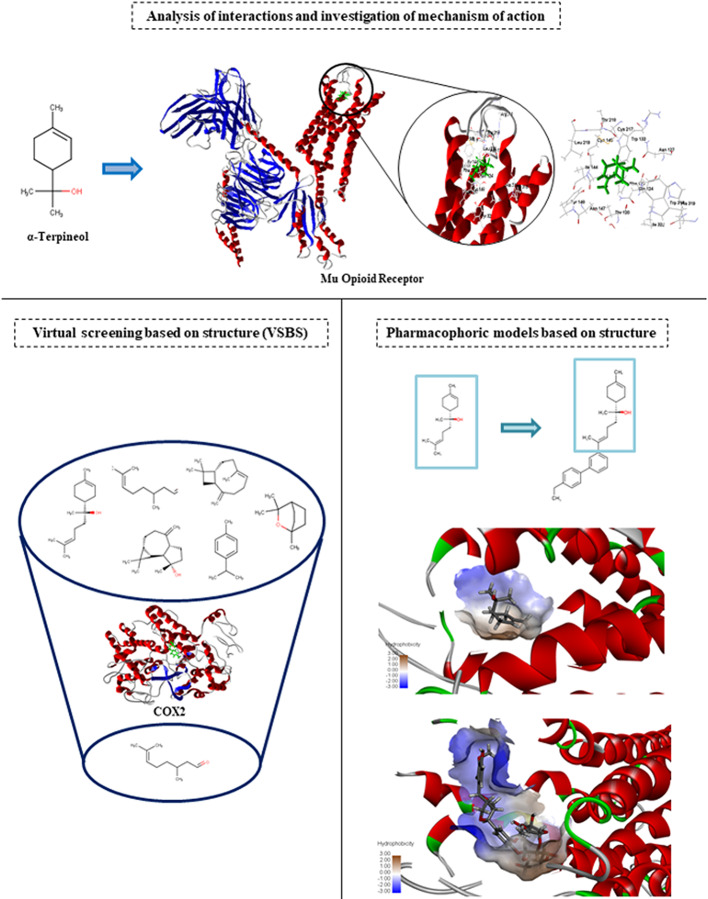
Virtual screening is one of the methods that can be used in the investigation of compounds with antinociceptive activity. This method consists of screening chemical compound libraries using computational models or molecular docking in order to evaluate and/or select compounds with desired properties. Virtual screening is a fast and low-cost alternative for screening and selecting potential compounds for experimental evaluation (Alves et al., 2017). Docking is the main technique used in virtual screening based on structure. In this case, the molecules are coupled to the binding site and classified based on their predicted binding affinity or complementarity.
Pharmacophoric models based on structure are also a good alternative for the investigation of compounds with therapeutic potential. A pharmacophore model consists of a molecular recognition of a biological target shared by a group of compounds. Structure-based pharmacophores (SBPs) can work with either a free structure (apo) or a structure of the macromolecule-ligand complex (holo) (Pirhadi et al., 2013). These methods use the potential interactions observed between the ligand and the protein, while the SBP method, which aims to derive the pharmacophore from the free protein of the ligand, uses only information from the active site of the protein. This type of method also reduces costs and is considered a valuable tool for optimizing hits for leads, virtual screening, scaffolding jumping and design of drugs with multiple targets (Pirhadi et al., 2013).
Consensus docking uses various docking programs or various types of scoring functions to increase docking accuracy. The method helps to increase the classification power and, therefore, the hit rates, but combines information about the predicted connection modes instead of predicted connection affinities (Houston and Walkinshaw, 2013). Ao usar mais um programa de encaixe para visualizar uma pose de ligação, as poses podem atingir um índice de 82% (Houston and Walkinshaw, 2013).
Different Molecular Docking Approaches Applied to Antinociceptive Studies
Most of the studies reported in this review describe the use of docking to investigate the mechanism of action, characterize interactions between targets and ligands, and assess antinociceptive activity at the molecular level. However, there are some Docking approaches that can be used to assess antinociceptive activity for different purposes.
An interesting study addressed by Poli et al. (2019), used virtual screening as a method to filter the compounds with the greatest potential to be tested experimentally. Considered one of the first examples of virtual screening studies focused on the identification of new peptide ligands as opioid modulators, the authors used parallel virtual screening from an internal library. The library containing 198,000 tetrapeptides was filtrated using a pharmacophore coupled to the X-ray structure of the µ-opioid receptor linked to the β-FNA morphine antagonist (PDB 4DKL code) using the LigandScout software. With this first filter, 28,070 compounds were selected and submitted to Docking using the software Glide. The software was able to select 146 compounds that were subjected to a second coupling using the Autodock Vina software. Vina was able to select 15 best compounds with probability of antinociceptive activity in opioid targets that were subjected to molecular dynamics studies to investigate the affinity of interactions in the presence of factors, such as solvent and ions. The three most promising peptide compounds were synthesized and subjected to biological evaluation. The results showed that peptide 1 showed selectivity for MOR and demonstrated an appreciable inverse agonist effect in MOR. The authors concluded that this peptide may represent a promisingly successful new compound to be used as a starting point for the optimization of structure-based ligands, with the aim of discovering potent opioid modulators.
Another study by Khanna et al. (2019) also used virtual screening to identify small molecules that disrupt the CaVα–CaVβ interaction. A commercially available library at ChemBridge was used to couple 50,000 small commercially available drug-like molecules. Of these compounds, 49 compounds were screened for their ability to inhibit calcium influx induced by depolarization in rat DRG neurons. These compounds were purchased, 13 were found to be insoluble or killed neurons, and 11 compounds inhibited the influx of Ca by 50%. The anchored compound, 2-(3,5-dimethylisoxazol-4-yl)-N-((4-((3-phenylpropyl)amino)quinazolin-2-yl)methyl)acetamide (IPPQ) was capable to interrupt the CaVα interaction CaVβ and considered as a non-opioid therapy for chronic pain.
An approach based on the design of compounds from docking and virtual screening was used by Lee et al. (2014). The researchers observed through bibliographic research that some compounds with a piperidine portion, such as haloperidol, penfluridol, pimozide, flunazirine, and TTA-P2, were well known as type T calcium channel inhibitors. Thus, inspired by these compounds and other types second generation, new compounds with greater potential for the treatment of neuropathic pain were designed. To predict a binding affinity of the projected compounds to the T-type calcium channel before synthesizing them, the researchers mapped the 3D ligand-based pharmacophore model generated by a common resource generation approach (HipHop) implemented in the CATALYST program (Doddareddy et al., 2007). The results showed that 4-phenyl compounds and 4-(3,4-dichloro) phenyl tetrahydropyridine compounds 7a and 7b showed bonds and interactions with the target similar to that of their tetrahydropyridine analogs 7. These compounds were evaluated for effect antinociceptive in rat models for neuropathic pain and were significantly effective in decreasing pain responses to mechanical mechanisms.
Daga et al. (2014), presented a combined method based on structure and ligand. They used a manual structure-based pharmacophore, which has the advantage of finding the binding conformation of the ligand in the bioactive state of the receptor. The structure of the nociceptin receptor (NOP), of the family of opioid receptors was used to fit with agonist ligands. Given the structure-activity relationships for known NOP ligands, the researchers developed a hybrid method that combines a structure-based and ligand-based approach, using the active state NOP receptor, as well as the pharmacophoric resources of ligands showed greater effectiveness than methods individual. The results showed that the NOP receptor binding affinity of a selected set of high-scoring hits resulted in the identification of several compounds with measurable binding affinity at the NOP receptor, one of which had a new chemotype for binding to the NOP receptor.
A summary of all docking approaches observed in this review is shown in Figure 2.
Figure 2.
Different molecular docking approaches that can be applied in studies of antinociceptive activity. The interaction analysis allows to evaluate the main connections and interactions observed between compounds and targets before and after experimental tests. The virtual screening based on the structure consists of selecting selective compounds according to the binding affinity with the target protein. Pharmacophoric models based on structure consist of the molecular recognition of a target shared by a group of compounds with a similar structural base.
Conclusion
The data presented in this review demonstrate the importance of studies with natural products, more specifically involving pharmacology with the help of bioinformatics/cheminformatics techniques, which is currently complementing and facilitating the discovery of new compounds, guiding and orienting studies towards specific molecular targets.
The most cited compounds in docking studies in this review are monoterpenes, especially α-Terpineol, (−)-α-bisabolol, Camphor, and p-Cymene. Many of the research addressed in this review used molecular docking to investigate the mechanism of action of various compounds and molecular dynamics to elucidate the stability of protein–ligand complexes in systems containing water, ions, temperature, and pressure. For new investigations, future perspectives include the advancement of more robust computational techniques and the increase in silico studies that complement the experimental studies, the tendency is to use computational resources in the first stage in the investigation of molecules with potential biological activity for certain diseases. Computational methods contribute to the selection of chemical structures with the highest probability of biological activity and the rationalization of these compounds. Several studies use QSAR (Quantitative Structure-Activity Relationship) methods to identify potential molecules with antinociceptive activity.
Author Contributions
All authors contributed to the development of the article. DA, HA, DD, and HA held the discussion of the articles. RC, RB, and NB performed the methodology, bibliographic search, and selection of articles. MM and LS were in charge of generating the tables and discussing the content of chemoinformatics. MS and RA responsible for the general review of the content.
Funding
This study was supported by funds from the Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq) and the Federal University of Paraíba (UFPB) for funding the article invoice.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abril-Sánchez C., Matencio A., Navarro-Orcajada S., García-Carmona F., López-Nicolás J. M. (2019). Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids 219, 72–78. 10.1016/j.chemphyslip.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Aburjai T., Hudaib M. (2006). Antiplatelet, antibacterial and antifungal activities of Achillea falcata extracts and evaluation of volatile oil composition. Pharmacogn. Mag. 2 (7), 191–198. [Google Scholar]
- Aburjai T., Hudaib M., Tayyem R., Yousef M., Qishawi M. (2007). Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 110 (2), 294–304. 10.1016/j.jep.2006.09.031 [DOI] [PubMed] [Google Scholar]
- Alves V., Braga R. C., Muratov E. N., Andrade C. H. (2017). Quimioinformática: uma introdução. Química Nova 41, 202. 10.21577/0100-4042.20170145 [DOI] [Google Scholar]
- Alzweiri M., Sarhan A., Mansi K., Hudaib M., Aburjai T. (2011). Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 137 (1), 27–35. 10.1016/j.jep.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Azimaraghi O., Aghajani Y., Molaghadimi M., Khosravi M., Eslami K., Ghadimi F., et al. (2014). Ondansetron reducing pain on injection of etomidate: a controlled randomized study. Braz. J. Anesthesiol. (English Edition) 64 (3), 169–172. 10.1016/j.bjane.2013.06.013 [DOI] [PubMed] [Google Scholar]
- Bahmani M., Shirzad H., Majlesi M., Shahinfard N., Rafieian-Kopaei M. (2014). A review study on analgesic applications of Iranian medicinal plants. Asian Pacific J. Trop. Med. 7 (S1), S43–S53. 10.1016/S1995-7645(14)60202-9 [DOI] [PubMed] [Google Scholar]
- Baron E. P. (2018). Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 58 (7), 1139–1186. 10.1111/head.13345 [DOI] [PubMed] [Google Scholar]
- Barrett J. E. (2015). The pain of pain: challenges of animal behavior models. Eur. J. Pharmacol. 753, 183–190. 10.1016/j.ejphar.2014.11.046 [DOI] [PubMed] [Google Scholar]
- Barrot M. (2012). Tests and models of nociception and pain in rodents. Neuroscience 211, 39–50. 10.1016/j.neuroscience.2011.12.041 [DOI] [PubMed] [Google Scholar]
- Bohlmann F., Jakupovic J., Lonitz M. (1977). Naturlich vorkommende Terpen Derivate. 76. Uber Inhaltsstoffe der Eupatorium Gruppe Chemische Berichte 110 (1), 301–314. 10.1002/cber.19771100132 [DOI] [Google Scholar]
- Bonjardim L. R., Cunha E. S., Guimarães A. G., Santana M. F., Oliveira M. G., Serafini M. R., et al. (2012). Evaluation of the anti-inflammatory and antinociceptive properties of p-cymene in mice. Z. für Naturforschung C. 67 (1-2), 15–21. [DOI] [PubMed] [Google Scholar]
- Boukalova S., Marsakova L., Teisinger J., Vlachova V. (2010). Conserved residues within the putative S4-S5 region serve distinct functions among thermosensitive vanilloid transient receptor potential (TRPV) channels. J. Biol. Chem. 285 (53), 41455–41462. 10.1074/jbc.M110.145466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartaxo S. L., de Almeida Souza M. M., de Albuquerque U. P. (2010). Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 131 (2), 326–342. 10.1016/j.jep.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Swanson T. M. (2015). Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol. Pharmacol.. 88 (1), 141–150. 10.1124/mol.114.097659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty A. K., Mazumder T., Chatterjee S. N. (2011). Anti-inflammatory potential of ethanolic leaf extract of eupatorium adenophorum spreng. Through alteration in production of TNF-α, ROS and expression of certain genes. Evidence-Based Complementary Altern. Med. 2011. 10.1093/ecam/neq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaluvaraju K. C., Zaranappa X. (2011). Synthesis and biological evaluation of some isatin derivatives for antimicrobial properties. Res. J. Pharm. Biol. Chem. Sci. 2, 541–546. [Google Scholar]
- Chen Y. Z., Zhi D. G. (2001). Ligand-protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins: Struct. Funct. Genet. 43 (2), 217–226. [DOI] [PubMed] [Google Scholar]
- Chikhale R. V., Bhole R. P., Khedekar P. B., Bhusari K. P. (2009). Synthesis and pharmacological investigation of 3-(substituted 1-phenylethanone)-4-(substituted phenyl)-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylates. Eur. J. Med. Chem. 44 (9), 3645–3653. 10.1016/j.ejmech.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Chikhale R., Thorat S., Pant A., Jadhav A., Thatipamula K. C., Bansode R., et al. (2015). Design, synthesis and pharmacological evaluation of pyrimidobenzothiazole-3-carboxylate derivatives as selective L-type calcium channel blockers. Bioorg. Med. Chem. 23 (20), 6689–6713. 10.1016/j.bmc.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Clark G. T., Padilla M., Dionne R. (2016). Medication Treatment Efficacy and Chronic Orofacial Pain. Oral. Maxillofacial Surg. Clinics 28 (3), 409–421. 10.1016/j.coms.2016.03.011. W.B. Saunders. [DOI] [PubMed] [Google Scholar]
- Colares A. V., Almeida-Souza F., Taniwaki N. N., Souza C.da S.F., da Costa J. G. M., Calabrese K.da S., et al. (2013). In Vitro Antileishmanial Activity of Essential Oil of Vanillosmopsis arborea (Asteraceae) Baker. Evidence-Based Complementary Altern. Med.: ECAM 2013 (727042), 1–7. 10.1155/2013/727042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga P. R., Polgar W. E., Zaveri N. T. (2014). Structure-Based Virtual Screening of the Nociceptin Receptor: Hybrid Docking and Shape-Based Approaches for Improved Hit Identification. J. Chem. Inf. Model. 54, 2732–2743. 10.1021/ci500291a [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Menezes R. R. P. P. B., Sampaio T. L., Lima D. B., Sousa P. L., de Azevedo I. E. P., Magalhaes E. P., et al. (2019). Antiparasitic effect of (-)-alpha-bisabolol against Trypanosoma cruzi Y strain forms. Diagn. Microbiol. Infect. Dis. 95 (3), 114860. 10.1016/j.diagmicrobio.2019.06.012 [DOI] [PubMed] [Google Scholar]
- De Oiveira L G., da Penha A. R., Fernandes C. N., Souza H. H. F., da Costa J. G. M., Campos A. R. (2009). Gastroprotective mechanism of Vanillosmopsis arborea bark essential oil. Fitoterapia 80 (1), 77–80. 10.1016/j.fitote.2008.10.008 [DOI] [PubMed] [Google Scholar]
- De Oliveira M. G. B., Marques R. B., de Santana M. F., Santos A. B. D., Brito F. A., Barreto E. O., et al. (2012). alpha-terpineol reduces mechanical hypernociception and inflammatory response. Basic Clin. Pharmacol. Toxicol. 111 (2), 120–125. 10.1111/j.1742-7843.2012.00875.x [DOI] [PubMed] [Google Scholar]
- de Oliveira Alves J. E., Silveira M. D., Vieira E. M. P., de Moura Vidal L. W. (2017). Mecanismos fisiopatológicos da nocicepção e bases da analgesia perioperatória em pequenos animais. Acta Biomed. Brasiliensia 8 (1), 56–68. 10.18571/acbm.122 [DOI] [Google Scholar]
- De Oliveira Júnior W. M., Benedito R. B., Pereira W. B., de Arruda Torres P., Ramos C. A. F., Costa J. P., et al. (2013). Farnesol: antinociceptive effect and histopathological analysis of the striatum and hippocampus of mice. Fundam. Clin. Pharmacol. 27 (4), 419–426. 10.1111/j.1472-8206.2012.01030.x [DOI] [PubMed] [Google Scholar]
- de Oliveira Júnior R. G., Ferraz C. A. A., Silva J. C., de Andrade Teles R. B., Silva M. G., Diniz T. C., et al. (2018). Neuropharmacological effects of essential oil from the leaves of Croton conduplicatus Kunth and possible mechanisms of action involved. J. Ethnopharmacol. 221, 65–76. 10.1016/j.jep.2018.04.009 [DOI] [PubMed] [Google Scholar]
- De Oliveira Junior R. G., Ferraz C. A. A., Silva J. C., de Oliveira A. P., Diniz T. C., E Silva M. G., et al. (2017). Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules 22 (6), 1–14. 10.3390/molecules22060900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L., Ligresti A., Moriello A. S., Allarà M., Bisogno T., Petrosino S., et al. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494. 10.1111/j.1476-5381.2010.01166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santana M. T., de Oliveira M. G. B., Santana M. F., De Sousa D. P., Santana D. G., Camargo E. A., et al. (2013). Citronellal, a monoterpene present in Java citronella oil, attenuates mechanical nociception response in mice. Pharm. Biol. 51 (9), 1144–1149. 10.3109/13880209.2013.781656 [DOI] [PubMed] [Google Scholar]
- De Santana M. F., Guimarães A. G., Chaves D. O., Silva J. C., Bonjardim L. R., de Lucca Júnior W., et al. (2015). The anti-hyperalgesic and anti-inflammatory profiles of p -cymene: Evidence for the involvement of opioid system and cytokines. Pharm. Biol. 53 (11), 1583–1590. 10.3109/13880209.2014.993040 [DOI] [PubMed] [Google Scholar]
- de Sousa Oliveira F., de Freitas T. S., da Cruz R. P., do Socorro Costa M., Pereira R. L. S., Quintans-Junior L. J., et al. (2017). Evaluation of the antibacterial and modulatory potential of alpha-bisabolol, beta-cyclodextrin and alpha-bisabolol/beta-cyclodextrin complex. Biomed. Pharmacother. = Biomedecine Pharmacotherapie 92, 1111–1118. 10.1016/j.biopha.2017.06.020 [DOI] [PubMed] [Google Scholar]
- De Souza Siqueira Quintans J., Menezes P. P., Santos M. R. V., Bonjardim L. R., Almeida J. R. G. S., Gelain D. P., et al. (2013). Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine 20 (5), 436–440. 10.1016/j.phymed.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Derry S., Rice A. S., Cole P., Tan T., Moore R. A., et al. (2017). Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 1, 1–67. 10.1002/14651858.CD007393.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddareddy M. R., Choo H., Cho Y. S., Rhim H., Koh H. Y., Lee J.-H., et al. (2007). 3D pharmacophore based virtual screening of T-type calcium channel blockers. Bioorg. Med. Chem. 15, 1091e1105. 10.1016/j.bmc.2006.10.013 [DOI] [PubMed] [Google Scholar]
- Du X., Li Y., Xia Y. L., Ai S. M., Liang J., Sang P., et al. (2016). Insights into protein–ligand interactions: mechanisms, models, and methods. Int. J. Mol. Sci. 17 (2), 144. 10.3390/ijms17020144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaissi A., Medini H., Marzouki H., Larbi Khouja M., Lynene F., Chemli R., et al. (2010). Variation in volatile leaf oils of twelve eucalyptus species harvested from Hajeb Layoun arboreta (Tunisia). Chem. Biodivers. 7 (3), 705–716. 10.1002/cbdv.200900169 [DOI] [PubMed] [Google Scholar]
- Elokely K., Velisetty P., Delemotte L., Palovcak E., Klein M. L., Rohacs T., et al. (2016). Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin. Proc. Natl. Acad. Sci. U S A 113, 137–145. 10.1073/pnas.1517288113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S., Matsunaga T., Ohta C., Soda M., Kanamori A., Kitade Y., et al. (2011). Roles of rat and human aldo-keto reductases in metabolism of farnesol and geranylgeraniol. Chemico-Biol. Interact. 191, 261–268. 10.1016/j.cbi.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M. Y. D., Carmo M. R. S., do Fonteles A. A., de Sousa Neves J. C., da Silva A. T. A., Pereira J. F., et al. (2019). (-)-alpha-bisabolol prevents neuronal damage and memory deficits through reduction of proinflammatory markers induced by permanent focal cerebral ischemia in mice. Eur. J. Pharmacol. 842, 270–280. 10.1016/j.ejphar.2018.09.036 [DOI] [PubMed] [Google Scholar]
- Freitas A. C., Peigneur S., Macedo F. H., Menezes-Filho J. E., Millns P., Medeiros L. F., et al. (2018). O peptídeo PnPP-19, um derivado da toxina da aranha, ativa os receptores μ-opioides e modula os canais de cálcio. Toxinas 10 (1), 43. 10.3390/toxins10010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García G. P., García E. P., Martínez I. G., Scior T. R. F., Salvador J. L., Martínez P M. M., et al. (2011). Analgesic effect of leaf extract from Ageratina glabrata in the hot plate test. Braz. J. Pharmacogn. 21 (5), 928–935. 10.1590/S0102-695X2011005000158 [DOI] [Google Scholar]
- Gediya L. K., Njar V. C. (2009). Promise and challenges in drug discovery and development of hybrid anticancer drugs. Expert Opin. Drug Dis. 4 (11), 1099–1111. 10.1517/17460440903341705 [DOI] [PubMed] [Google Scholar]
- Gertsch J., Leonti M., Raduner S., Racz I., Chen J. Z., Xie X. Q., et al. (2008). Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U.S.A. 105 (26), 9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomathi R., Manian S. (2015). Analgesic and acetylcholinesterase inhibition potential of polyphenols from Scolopia crenata (Flacourtiaceae): An endemic medicinal plant of India. Ind. Crops Prod. 73, 134–143. 10.1016/j.indcrop.2015.03.090 [DOI] [Google Scholar]
- Gouveia D. N., Costa J. S., Oliveira M. A., Rabelo T. K., Silva A. M., de O. E., et al. (2018). alpha-Terpineol reduces cancer pain via modulation of oxidative stress and inhibition of iNOS. Biomed. Pharmacother. = Biomedecine Pharmacotherapie 105, 652–661. 10.1016/j.biopha.2018.06.027 [DOI] [PubMed] [Google Scholar]
- Guerrero C., Silva M., Maldonado E., Martínez y M. (1978). Eupaglabric acid a new compound isolated from Eupatorium glabratum HBK. Rev. Latinoamer. Quím. 9, 71–75. [Google Scholar]
- Guimarães A. G., Quintans J. S. S., Quintans-Júnior L. J. (2012). Monoterpenes with analgesic activity – a systematic review. Phytother. Res. 27 (1), 1–15. 10.1002/ptr.4686 [DOI] [PubMed] [Google Scholar]
- Hellyer P., Rodan I., Brunt J., Downing R., Hagedorn J. E., Robertson S. A. (2007). AAHA/AAFP pain management guidelines for dogs & cats. J. Am. Anim. Hosp. Assoc. 43 (5), 235–248. 10.5326/0430235 [DOI] [PubMed] [Google Scholar]
- Houston D. R., Walkinshaw M. D. (2013). Consensus docking: improving the reliability of docking in a virtual screening context. J. Chem. Inf. model. 53 (2), 384–390. 10.1021/ci300399w [DOI] [PubMed] [Google Scholar]
- Inocencio Leite L. H., Leite G., de O., Silva Coutinho T., de Sousa S. D. G., Sampaio R. S., et al. (2014). Topical Antinociceptive Effect of Vanillosmopsis arborea Baker on Acute Corneal Pain in Mice. Evidence-Based Complementary Altern. Med.: ECAM 2014, 708636. 10.1155/2014/708636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C., Shimoda L. M. N., Kawakami J. K., Ang L., Bacani A. J., Baker J. D., et al. (2019). Myrcene and terpene regulation of TRPV. Channels 13 (1), 344–366. 10.1080/19336950.2019.1654347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayashankar B., Lokanath Rai K. M., Baskaran N., Sathish H. S. (2009). Synthesis and pharmacological evaluation of 1,3,4-oxadiazole bearing bis(heterocycle) derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 44 (10), 3898–3902. 10.1016/j.ejmech.2009.04.006 [DOI] [PubMed] [Google Scholar]
- Kamatou G. P., Viljoen A. M. (2010). ). A review of the application and pharmacological properties of α-Bisabolol and α-Bisabolol-rich oils. J. A.m Oil Chem. Soc 87 (1), 1–7. 10.1007/s11746-009-1483-3 [DOI] [Google Scholar]
- Kürkçüoğlu M., Demirci B., Tabanca N., Özek T., Başer K. H. C. (2003). The essential oil of Achillea falcata L. Flavour Fragrance J. 18 (3), 192–194. 10.1002/ffj.1176 [DOI] [Google Scholar]
- Kerzare D., Chikhale R., Bansode R., Amnerkar N., Karodia N., Paradkar A., et al. (2016). Design, synthesis, pharmacological evaluation and molecular docking studies of substituted oxadiazolyl-2-oxoindolinylidene propane hydrazide derivatives. J. Braz. Chem. Soc. 27 (11), 1998–2010. 10.5935/0103-5053.20160090 [DOI] [Google Scholar]
- Khan R., Sultana S. (2011). Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of Wistar rats. Chemico-Biol. Interact. 192 (3), 193–200. 10.1016/j.cbi.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Khanna R., Yu J., Yang X., Moutal A., Chefdeville A., Gokhale V., et al. (2019). Targeting the CaVα–CaVβ interaction yields an antagonist of the N-type CaV2.2 channel with broad antinociceptive efficacy. Pain 160 (7), 1644–1661. 10.1097/j.dor.0000000000001524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C., Browne W. J., Cuthill I. C., Emerson M., Altman D. G. (2010). Improving Bioscience Research Reporting: The ARRIVE Guidelines Checklist Animal Research: Reporting In Vivo Experiments. PloS Biol. 8 (6), 1–5. 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchmair J., Williamson M. J., Tyzack J. D., Tan L., Bond P. J., Bender A., et al. (2012). Computational prediction of metabolism: Sites, products, SAR, P450 enzyme dynamics, and mechanisms. J. Chem. Inf. Model. 52 (3), 617–648. 10.1021/ci200542m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaumann P. R., Wouk A. F. P. F., Sillas T. (2008). Patofisiologia da dor. Arch. Vet. Sci. 13 (1), 1–12. 10.5380/avs.v13i1.11532 [DOI] [Google Scholar]
- Lee J.-H., Seo S. H., Lim E. J., Cho N.-C., Nama G., Kang S. B., et al. (2014). Synthesis and biological evaluation of 1-(isoxazol-5- ylmethylaminoethyl)-4-phenyl tetrahydropyridine and piperidine derivatives as potent T-type calcium channel blockers with antinociceptive effect in a neuropathic pain model. Eur. J. Med. Chem. 74, 246e257. 10.1016/j.ejmech.2013.12.056 [DOI] [PubMed] [Google Scholar]
- Lee S. (2013). Pharmacological Inhibition of Voltage-gated Ca2+ Channels for Chronic Pain Relief. Curr. Neuropharmacol. 1 (6), 606–620 . 10.2174/1570159X11311060005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite L. H. I., Leite G. O., da Silva B. A. F., Santos S. A. A. R., Magalhaes F. E. A., Menezes P. P., et al. (2019). Molecular mechanism underlying orofacial antinociceptive activity of Vanillosmopsis arborea Baker (Asteraceae) essential oil complexed with beta-cyclodextrin. Phytomedicine: Int. J. Phytother. Phytopharmacology 55, 293–301. 10.1016/j.phymed.2018.09.173 [DOI] [PubMed] [Google Scholar]
- Leonardão E. J., Savegnago L., Jacob R. G., Victoria F. N., Martinez D. M. (2016). Antinociceptive effect of essential oils and their constituints: na update review. J. Braz. Chem. Soc. 27 (3), 435–474. 10.5935/0103-5053.20150332 [DOI] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P. A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. 6 (7), e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.-X., Guo C., Ou W.-S., Jing Y., Niu H.-F., Song P., et al. (2019). Citronellal prevents endothelial dysfunction and atherosclerosis in rats. J. Cell. Biochem. 120 (3), 3790–3800. 10.1002/jcb.27660 [DOI] [PubMed] [Google Scholar]
- Mallat A., Teixeira-Clerc F., Deveaux V., Manin S., Lotersztajn S. (2011). The endocannabinoid system as a key mediator during liver dis-eases: new insights and therapeutic openings. Br. J. Pharmacol. 163, 1432–1440. 10.1111/j.1476-5381.2011.01397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S. K., Boominathan R., Parimaladevi B., Dewanjee S., Mandal S. C. (2005). Analgesic activity of methanol extract of Eupatorium adenophorum Spreng. leaves. Indian J. Exp. Biol. 43 (7), 662–663. [PubMed] [Google Scholar]
- Matos M. E. O., De Sousa M. P., Matos F. J. A., Craveiro A. A. (1988). Sesquiterpenes from Vanillosmopsis arborea. J. Natural Prod. 51 (4), 780–782. 10.1021/np50058a023 [DOI] [PubMed] [Google Scholar]
- McKune M. C., Murrell J. C., Nolan A. M., White K. L., Wright B. D. (2015). “Nociception and pain,” in Veterinary anesthesia and analgesia - The fifth edition of Lumb and Jones. Eds. Grimm K. A., Lamont L. A., Tranquilli W. J., Greene S. A., Robertson S. A. (Hoboken: Wiley-Blackwell; ), 584–616. [Google Scholar]
- McNeil M., Facey P., Porter R. (2011). Essential oils from the Hyptis genus-a review (1909-2009). Nat. Prod. Commun. 6 (11), 1934578X1100601149. [PubMed] [Google Scholar]
- Melo MônicaS., de Santana M. T., Guimarães A. G., Siqueira R. S., De Sousa DamiãoP., Santos R. V., et al. (2011). Bioassay-guided evaluation of central nervous system effects of citronellal in rodents. Rev. Bras. Farmacognosia 21 (4), 697–703. 10.1590/S0102-695X2011005000124 [DOI] [Google Scholar]
- Melo L. T., Duailibe M. A. B., Pessoa L. M., da Costa F. N., Vieira-Neto A. E., de Vasconcellos Abdon A. P., et al. (2017). (-)-alpha-Bisabolol reduces orofacial nociceptive behavior in rodents. Naunyn-Schmiedeberg’s Arch. Pharmacol. 390 (2), 187–195. 10.1007/s00210-016-1319-2 [DOI] [PubMed] [Google Scholar]
- Menezes P., A de S Araujo A., Doria A. A., J Quintans-Junior L., GB de Oliveira M., RV dos Santos M., et al. (2015). Physicochemical characterization and analgesic effect of inclusion complexes of essential oil from Hyptis pectinata L. Poit leaves β-cyclodextrin. Curr. Pharm. Biotechno. 16 (5), 440–450. [DOI] [PubMed] [Google Scholar]
- Millard C. B., Kryger G., Ordentlich A., Greenblatt H. M., Harel M., Raves M. L., et al. (1999). Crystal Structures of Aged Phosphonylated Acetylcholinesterase: Nerve Agent Reaction Products at the Atomic Level † , ‡. Biochemistry 38 (22), 7032–7039. 10.1021/bi982678l [DOI] [PubMed] [Google Scholar]
- Mota M. L., Lobo L. T. C., da Costa J. M. G., Costa L. S., Rocha H. A. O., Rocha e Silva L. F., et al. (2012). In vitro and in vivo antimalarial activity of essential oils and chemical components from three medicinal plants found in northeastern Brazil. Planta Med. 78 (7), 658–664. 10.1055/s-0031-1298333 [DOI] [PubMed] [Google Scholar]
- Naidu R. K., Pham T. M. (2015). “Pain management,” in Basic Clinical Anesthesia. Eds. Sikka P. K., Beaman S. T., Street J. A. (New York: Springer; ), 265–296. [Google Scholar]
- Nascimento S. S., Camargo E. A., DeSantana J. M., Araujo A. A. S., Menezes P. P., Lucca-Junior W., et al. (2014). Linalool and linalool complexed in beta-cyclodextrin produce anti-hyperalgesic activity and increase Fos protein expression in animal model for fibromyalgia. Naunyn-Schmiedeberg’s Arch. Pharmacol. 387 (10), 935–942. 10.1007/s00210-014-1007-z [DOI] [PubMed] [Google Scholar]
- Oliveira R., Santos Delfim, Coelho P. (2009). “Ciclodextrinas: formação de complexos e sua aplicação farmacêutica,” in Revista da Faculdade de Ciências da Saúde, vol. 6 (Porto: Edições Universidade Fernando Pessoa; ), 70–83. ISSN 1646-0480. [Google Scholar]
- Oliveira M. G. B., Brito R. G., Santos P. L., Araujo-Filho H. G., Quintans J. S. S., Menezes P. P., et al. (2016). alpha-Terpineol, a monoterpene alcohol, complexed with beta-cyclodextrin exerts antihyperalgesic effect in animal model for fibromyalgia aided with docking study. Chemico-Biol. Interact. 254, 54–62. 10.1016/j.cbi.2016.05.029 [DOI] [PubMed] [Google Scholar]
- Pandeya S. N., Sriram D. (1998). Synthesis and screening for antibacterial activity of schiff’s and mannich bases of isatin and its derivatives. Acta. Pharm. Turc. 40, 33–38. [Google Scholar]
- Pirhadi S., Shiri F., Ghasemi J. B. (2013). Methods and applications of structure based pharmacophores in drug discovery. Curr. topics med. Chem. 13 (9), 1036–1047. 10.2174/1568026611313090006 [DOI] [PubMed] [Google Scholar]
- Poli G., Dimmito M. P., Mollica A., Zengin G., Benyhe S., Zador F., et al. (2019). Discovery of Novel µ-Opioid Receptor Inverse Agonist from a Combinatorial Library of Tetrapeptides through Structure-Based Virtual Screening. Molecules 24 (21), 3872. 10.3390/molecules24213872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintans-Junior L. J., Souza T. T., Leite B. S., Lessa N. M. N., Bonjardim L. R., Santos M. R. V., et al. (2008). Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine: Int. J. Phytother. Phytopharmacology 15 (8), 619–624. 10.1016/j.phymed.2007.09.018 [DOI] [PubMed] [Google Scholar]
- Quintans-Junior L. J., Oliveira M. G. B., Santana M. F., Santana M. T., Guimaraes A. G., Siqueira J. S., et al. (2011). alpha-Terpineol reduces nociceptive behavior in mice. Pharm. Biol. 49 (6), 583–586. 10.3109/13880209.2010.529616 [DOI] [PubMed] [Google Scholar]
- Quintans-Junior L. J., Barreto R. S. S., Menezes P. P., Almeida J. R. G. S., Viana A. F. S. C., Oliveira R. C. M., et al. (2013). beta-Cyclodextrin-complexed (-)-linalool produces antinociceptive effect superior to that of (-)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 113 (3), 167–172. 10.1111/bcpt.12087 [DOI] [PubMed] [Google Scholar]
- Quintans-Júnior L. J., Araújo A. A., Brito R. G., Santos P. L., Quintans J. S. S., Menezes P. P., et al. (2016). β-caryophyllene, a dietary cannabinoid, complexed with β-cyclodextrin produced anti-hyperalgesic effect involving the inhibition of Fos expression in superficial dorsal horn. Life Sci. 149, 34–41. 10.1016/j.lfs.2016.02.049 [DOI] [PubMed] [Google Scholar]
- Quintans-Junior L. J., Brito R. G., Quintans J. S. S., Santos P. L., Camargo Z. T., Barreto P. A., et al. (2018). Nanoemulsion Thermoreversible Pluronic F127-Based Hydrogel Containing Hyptis pectinata (Lamiaceae) Leaf Essential Oil Produced a Lasting Anti-hyperalgesic Effect in Chronic Noninflammatory Widespread Pain in Mice. Mol. Neurobiol. 55 (2), 1665–1675. 10.1007/s12035-017-0438-1 [DOI] [PubMed] [Google Scholar]
- Radulović N. S., Mladenović M. Z., Randjelovic P. J., Stojanović N. M., Dekić M. S., Blagojević P. D. (2015). Toxic essential oils. Part IV: The essential oil of Achillea falcata L. as a source of biologically/pharmacologically active trans-sabinyl esters. Food Chem. Toxicol. 80, 114–129. 10.1016/j.fct.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Rao V. S., Menezes A. M., Viana G. S. (1990). Effect of myrcene on nociception in mice. J. Pharm. Pharmacol. 42, 877–878. 10.1111/j.2042-7158.1990.tb07046.x [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F., Del Arco I., Bermudez-Silva F. J., Bilbao A., Cippitelli A., Navarro M. (2005). The endocannabinoid system: phys-iolo gy and pharmacology. Alcohol Alcohol. 40, 2–14. 10.1093/alcalc/agh110 [DOI] [PubMed] [Google Scholar]
- Sá R. C. S., Lima T. C., Nóbrega F. R., Brito A. E. M., Sousa D. P. (2017). Analgesic-Like Activity of Essential Oil Constituents: An Update. Int. J. Mol. Sci. 18 (12), e2392. 10.3390/ijms18122392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaripour S., Nemati Y., Parvardeh S., Ghafghazi S., Fouladzadeh A., Moghimi M. (2018). Role of l-arginine/SNAP/NO/cGMP/KATP channel signalling pathway in antinociceptive effect of alpha-terpineol in mice. J. Pharm. Pharmacol. 70 (4), 507–515. 10.1111/jphp.12864 [DOI] [PubMed] [Google Scholar]
- Santhanasabapathy R., Vasudevan S., Anupriya K., Pabitha R., Sudhandiran G. (2015). Farnesol quells oxidative stress, reactive gliosis and inflammation during acrylamide-induced neurotoxicity: Behavioral and biochemical evidence. Neuroscience 308, 212–227. 10.1016/j.neuroscience.2015.08.067 [DOI] [PubMed] [Google Scholar]
- Santos P. L., Brito R. G., Oliveira M. A., Quintans J. S. S., Guimaraes A. G., Santos M. R. V., et al. (2016). Docking, characterization and investigation of beta-cyclodextrin complexed with citronellal, a monoterpene present in the essential oil of Cymbopogon species, as an anti-hyperalgesic agent in chronic muscle pain model. Phytomedicine: Int. J. Phytother. Phytopharmacology 23 (9), 948–957. 10.1016/j.phymed.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Santos P. L., Brito R. G., Matos J. P. S. C. F., Quintans J. S. S., Quintans-Junior L. J. (2018). Fos Protein as a Marker of Neuronal Activity: a Useful Tool in the Study of the Mechanism of Action of Natural Products with Analgesic Activity. Mol. Neurobiol. 55 (6), 4560–4579. 10.1007/s12035-017-0658-4 [DOI] [PubMed] [Google Scholar]
- Santos W. B. R., Melo M. A. O., Alves R. S., de Brito R. G., Rabelo T. K., Prado L., et al. (2019). p-Cymene attenuates cancer pain via inhibitory pathways and modulation of calcium currents. Phytomedicine. 10.1016/j.phymed.2019.152836 [DOI] [PubMed]
- Selvaraj M., Pandurangan A., Seshadri K. S., Sinha P. K., Krishnasamy V., Lal K. B. (2002). Comparison of mesoporous Al-MCM-41 molecular sieves in the production of p-cymene for isopropylation of toluene. J. Mol. Catal. A: Chem. 186 (1–2), 173–186. 10.1016/S1381-1169(02)00134-6 [DOI] [Google Scholar]
- Silva J. C., Almeida J. R. G. S., Quintans J. S. S., Gopalsamy R. G., Shanmugam S., Serafini M. R., et al. (2016). Enhancement of orofacial antinociceptive effect of carvacrol, a monoterpene present in oregano and thyme oils, by β-cyclodextrin inclusion complex in mice. Biomed. Pharmacother. 84, 454–461. 10.1016/j.biopha.2016.09.065 [DOI] [PubMed] [Google Scholar]
- Silva J. C., de Moraes Alcantara L. F., Dias Soares J. M., e Silva M. G., de Lavor É.M., Andrade V. M., et al. (2017). Docking, characterization and investigation of β-cyclodextrin complexed with farnesol, an acyclic sesquiterpene alcohol, produces orofacial antinociceptive profile in experimental protocols. Process. Biochem. 62, 193–204. 10.1016/j.procbio.2017.07.022 [DOI] [Google Scholar]
- Singh U. K., Pandeya S. N., Singh A. K., Srivastava B. K., Pandey M. (2010). Synthesis And Antimicrobial Activity Of Schiff's And n-Mannich Bases Of Isatin And Its Derivatives With 4-Amino-n-Carbamimidoyl Benzene Sulfonamide. Int. J. Pharm. Sci. Drug Res. 2 (2), 151–154. [Google Scholar]
- Siqueira-Lima P. S., Brito R. G., Araújo-Filho H. G., Santos P. L., Lucchesi A., Araújo A. A. S., et al. (2017). Anti-hyperalgesic effect of Lippia grata leaf essential oil complexed with β-cyclodextrin in a chronic musculoskeletal pain animal model: Complemented with a molecular docking and antioxidant screening. Biomed. Pharmacother. 91, 739–747. 10.1016/j.biopha.2017.05.009 [DOI] [PubMed] [Google Scholar]
- Starkus J., Jansen C., Shimoda L. M. N., Stokes A. J., Small-Howard A. L., Turner H. (2019). Diverse TRPV1 responses to cannabinoids. Channels 13 (1), 172–191. 10.1080/19336950.2019.1619436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiwi S., Subarnas A., Supriyatna S., Abdasah M., Muchtaridi M. (2015). Analysis of chemical composition and its analgesic and anti-inflammatory activity of essential oil of sintoc bark (Cinnamomum sintoc bl.) using in vivo methods. J. Appl. Pharm. Sci. 5, 58–65. 10.7324/JAPS.2015.50209 [DOI] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., et al. (1991). Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 253 (5022), 872–879. 10.1126/science.1678899 [DOI] [PubMed] [Google Scholar]
- Teixeira G. F. D., Vieira-Neto A. E., Da Costa F. N., Silva A. R. A. E., Campos A. R. (2017). Antinociceptive effect of (-)-α-bisabolol in nanocapsules. Biomed. Pharmacother. 91, 946–950. 10.1016/j.biopha.2017.05.024 [DOI] [PubMed] [Google Scholar]
- Thomas A., Baillie G. L., Phillips A. M., Razdan R. K., Ross R. A., Pertwee R. G. (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623. 10.1038/sj.bjp.0707133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen R., Christensen M. H. (2006). MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 49 (11), 3315–3321. 10.1021/jm051197e [DOI] [PubMed] [Google Scholar]
- Viana G. S. B., Matos F. F., Araujo W. L., Matos F. J. A., Craveiro A. A. (1981). Essential oil of lippia grata: Pharmacological effects and main constituents. Pharm. Biol. 19 (1), 1–10. 10.3109/13880208109065203 [DOI] [Google Scholar]
- Vivar A. R., de Cuevas L., Quím C. G.-R. L. (1971). Eupaglabrin a terpene from Eupatorium glabratum. Rev. Latinoamer. Quím. (2), 32–34. [Google Scholar]
- Wagle S., Adhikari A. V., Kumari N. S. (2008). Synthesis of some new 2-(3-methyl-7-substituted-2-oxoquinoxalinyl)-5-(aryl)-1,3,4-oxadiazoles as potential non-steroidal anti-inflammatory and analgesic agents. Indian J. Chem. B. 47 439–448. [Google Scholar]
- Wu Y., OuYang Q., Tao N. (2016). Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 53 (10), 3853–3858. 10.1007/s13197-016-2358-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Peng L., Sang H., Ping Li Q., Cheng S. (2018). Anticancer effects of alpha-Bisabolol in human non-small cell lung carcinoma cells are mediated via apoptosis induction, cell cycle arrest, inhibition of cell migration and invasion and upregulation of P13K/AKT signalling pathway. J. B.U.ON.: Off. J. Balkan Union Oncol. 23 (5), 1407–1412. [PubMed] [Google Scholar]
- Xu H., Blair N. T., Clapham D. E. (2005). Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 25, 8924–8937. 10.1523/JNEUROSCI.2574-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarlaha A., Kourkoumelis N., Stanojkovic T. P., Kovala-Demertzi D. (2014). Cytotoxic activity of essential oil and extracts of ocimum basilicum against human carcinoma cells. Molecular docking study of isoeugenol as a potent cox and lox inhibitor. Dig. J. Nanomater. Biostructures 9 (3), 907–917. [Google Scholar]