Abstract
Previous studies indicate that inhibition of food intake by leptin is mediated by an integrated response to activation of hypothalamic and hindbrain receptors. This study tested whether loss of hindbrain leptin receptor signaling changed sensitivity to forebrain leptin. Injections of leptin-conjugated saporin (Lep-Sap) into the medial nucleus of the solitary tract (NTS) were used to destroy hindbrain leptin receptor-expressing neurons of male Sprague–Dawley rats. Controls were injected with saporin conjugated with a nonsense peptide (Blk-Sap). Lep-Sap had no effect on daily food intake or body weight, but expression of phosphorylated signal transducer and activator of transcription 3 (pSTAT3) in the NTS following a peripheral injection of leptin was abolished 26 days after Lep-Sap injections. To test forebrain leptin sensitivity, Lep-Sap and Blk-Sap rats received third-ventricle injections of 0, 0.05, 0.1, 0.25, or 0.5 μg leptin. Food intake was inhibited by 0.25 and 0.5 μg leptin in Blk-Sap rats, but there was no significant effect of leptin on food intake of Lep-Sap rats. There was no difference in hypothalamic pSTAT3 in unstimulated conditions, but pSTAT3 was lower in the dorsomedial region of the ventromedial nucleus of the hypothalamus (VMH) of Lep-Sap rats compared with Blk-Sap rats following a third-ventricle injection of 0.25 μg leptin. These results are consistent with previous data showing that loss of VMH leptin receptor-expressing cells prevents weight loss caused by fourth-ventricle leptin infusion and show that the integrated response between the hindbrain and forebrain is heavily dependent on leptin activity in the VMH.
Keywords: food intake, leptin-conjugated saporin, nucleus of the solitary tract, phosphorylated STAT3, ventromedial nucleus of the hypothalamus
INTRODUCTION
Investigation of the central control of energy balance by leptin has generally focused on the function of leptin receptors located in either the hypothalamus (13) or the hindbrain (23), with the assumption that these populations have independent effects on food intake, energy expenditure, and glucose metabolism. In a previous study, however, we used very low dose infusions of leptin into the third ventricle (3V) and fourth ventricle (4V) to demonstrate a synergy between forebrain and hindbrain leptin receptor activation (9). Doses of leptin that had no effect on food intake or body weight when infused into the 3V or the 4V alone, caused a substantial inhibition of food intake and weight loss when infused simultaneously. Measurement of ΔFosB, an indicator of chronic neuronal activity, was limited to hypothalamic areas that exhibited increased hypothalamic phosphorylation of signal transducer and activator of transcription 3 (pSTAT3) in these rats (10). pSTAT3 is considered a reliable marker of leptin receptor activation (38) and is required for the effects of leptin on energy balance (3). Subsequent studies showed that 4V leptin infusions that caused weight loss were also associated with an increase in hypothalamic pSTAT3 (17). Similar results had previously been reported by Ruiter et al. (36) showing increased pSTAT3 in hypothalamic nuclei following leptin injections into the 4V or nucleus of the solitary tract (NTS). More recent experiments demonstrated that 4V leptin infusions lower the threshold for response to leptin in the forebrain, but that 3V leptin does not affect the response to leptin in the hindbrain (18, 19). Thus we concluded that activation of leptin receptors in the hindbrain facilitated hypothalamic leptin signaling in a site-specific manner.
The hypothalamic area that shows the highest level of STAT3 activation in response to 4V leptin infusions is the dorsomedial region of the ventromedial nucleus of the hypothalamus (VMHdm; 10). We recently reported that deletion of leptin receptor-expressing cells in the VMH using leptin-conjugated saporin (Lep-Sap) attenuates the inhibition of food intake and weight loss caused by 4V leptin infusion (39), which supports the notion that leptin controls energy balance through an integrated circuit. The objective of experiments described here was to obtain further support for this concept by testing whether loss of leptin receptor-expressing cells in the hindbrain of rats attenuated the response to leptin injections in the forebrain.
METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committee of Augusta University. The animals were male Sprague–Dawley rats (Envigo, Prattville, AL), weighing ~300 g at the start of an experiment, housed individually either in wire mesh cages or in calorimetry cages. They had free access to chow and water except when stated otherwise, and each of the rats in wire mesh cages had a Nylabone (Neptune City, NJ) for enrichment. Rooms were maintained at 21–22°C with lights on for 12 h/day from 6 AM. Animals were allowed 5–7 days to adapt to their environment before surgeries were performed. Surgeries were conducted with rats under ketamine (100 mg/kg)-xylazine (10 mg/kg) anesthesia, and the rats received 2 mg/kg Ketofen analgesic (Fort Dodge Animal Health, Fort Dodge, IA) immediately before surgery and again 24 h later. Animals were weighed daily in all studies.
Pilot studies identified the appropriate coordinates for stereotaxic injection into the NTS to be 13.3 mm rostral to the bregma, 0.5 mm lateral to the midline, and 8.1 mm dorsal to the surface of the skull. Injections were performed using a manual stereotaxic injector (Stoelting Co., Wood Dale IL) fitted with a 5-µL Hamilton syringe and 32-gauge needle. All injections were delivered over 1 min, and the needle was left in place for an additional 2 min before being withdrawn. To determine the distribution of leptin-conjugated saporin (Lep-Sap: Leptin-SAP IT-47; Advanced Targeting Systems, San Diego, CA), two rats received bilateral NTS injections of 50 ng Lep-Sap delivered in a volume of 75 nL and were allowed to recover consciousness. Four hours after the injections, rats were anesthetized a second time and perfused pericardially with 300 mL cold heparinized saline followed by 500 mL cold 4% paraformaldehyde solution. The brains were held in paraformaldehyde overnight at 4°C and stored in 25% sucrose-0.1% sodium azide solution at 4°C. Thirty-micrometer sections were made through the hindbrain, and Lep-Sap was visualized by immunohistochemistry using a primary anti-saporin antibody (1:5,000 dilution, AB-15; Advanced Targeting Systems, San Diego, CA) with the secondary antibody Alexa Fluor 488 donkey anti-goat IgG (AffiniPure; Jackson ImmunoResearch Laboratories Inc., West Grove, PA).
To determine how long it took for Lep-Sap to delete leptin receptor-expressing cells, 8 rats received bilateral NTS injections of 50 ng Lep-Sap, and 4 rats received equivalent injections of a control saporin conjugated to an 11-amino acid sequence that has no homology to any known protein (Blk-Sap: Blank-Sap IT-21; Advanced Targeting Systems). Daily food intake, corrected for spillage, was measured from days 10 to 13 and from days 22 to 25 after injection. Fourteen days after the NTS injections, food was removed from the cages of four of the Lep-Sap rats at 7 AM. Starting at 11:30 AM, each rat received an intraperitoneal injection of 1 mg leptin/kg (Recombinant Rat Leptin Protein, CF; R&D Systems, Minneapolis, MN). They were anesthetized with ketamine-xylazine exactly 45 min later and perfused with 4% paraformaldehyde for collection of the brain, as described above. The remaining animals were injected and perfused 26 days after the NTS injections of conjugated saporin. Thirty-micrometer sections were made through the hindbrain, and pSTAT3 was detected using free-floating immunohistochemistry as described by Ellacot et al. (12) with the exception that pSTAT3 immunoreactivity (pSTAT3-IR) was detected using ABC reagents and diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA). pSTAT3-positive nuclei were quantified at bregma −14.08 mm [Plate 76 of the Paxinos and Watson rat brain atlas (31)].
To confirm that Lep-Sap was deleting leptin receptor-expressing cells, expression of leptin receptors in the hindbrain of Lep-Sap- and Blk-Sap-treated rats was evaluated using RNAscope in situ hybridization (Advanced Cell Diagnostics, Middleton, WI). Four rats were treated with Lep-Sap or Blk-Sap, and brains were collected following perfusion with paraformaldehyde 23 days after saporin injections. The fixed brains were frozen in optimal cutting temperature (OCT) compound, and 14-µm sections were collected and mounted onto Superfrost Plus slides. For detection of leptin receptors in hindbrain tissue, RNAscope was performed following the manufacturer’s directions for the 2.5 HD Duplex Assay using RNAscope Probe-Rn-Lepr-3END-1kb-C2 probe (416951-C2; Advanced Cell Diagnostics) with no C1 probe. This approach was used because we were unable to obtain reliable results with the RNAscope 2.5 HD Assay-RED and Probe-Rn-Lepr (415951; Advanced Cell Diagnostics). The pretreatment protocol was modified in that mounted sections were dried at 60°C for 2 h instead of 30 min and exposed to Protease IV for 20 min in place of exposure to Protease Plus for 30 min. Sections were counterstained with 25% hematoxylin and coverslipped with EcoMount mounting medium (Advanced Cell Diagnostics).
To test whether loss of leptin receptor-expressing cells in the hindbrain modified sensitivity to leptin in the forebrain, 14 rats received bilateral NTS injections of Lep-Sap, and 14 rats received bilateral NTS injections of Blk-Sap. Three rats (2 Lep-Sap and 1 Blk-Sap) failed to gain weight after saporin injections and were removed from the experiment. The rats were allowed to recover from the saporin injections for 21 days and were then fitted with a 3V guide cannula as described previously (7). Four days after surgery the correct placement of the guide cannula was confirmed by injecting 10 μg angiotensin II and observing for drinking during the 2 min after injection. Three rats (1 Lep-Sap and 2 Blk-Sap) did not drink and were removed from the experiment. Four days later the rats were housed in a calorimeter (TSE LabMaster, Metabolic Research Platform; TSE Systems International, Chesterfield, MO). Gas was sampled from each cage for 3 min every 39 min. Oxygen and carbon dioxide concentrations of the gas sample from each cage were measured during the last minute of the 3-min sampling period and used to calculate energy expenditure expressed as kcal·h−1·rat−1 and respiratory exchange ratio (RER) as an index of macronutrient oxidation. Food intake data were collected every minute but reported every 39 min. Calorimetry measures were initiated at 8 AM and stopped at 7:20 AM the next day so that only one cycle of measurement was lost each day. Body weight was recorded each morning when food hoppers and water bottles were refilled and cage bedding changed every second day. The experiment was completed with three cohorts of rats and with Blk-Sap and Lep-Sap rats represented within each cohort.
The rats adapted to the calorimeter for 4 days before starting to test for leptin responsiveness. On test days, food was removed from the cages at 7 AM. Starting at 5 PM, the rats received a 2-μL 3V injection of leptin or saline delivered over 1 min from a perfusion pump. The injector was held in place for 1 min at the end of injection. Food was returned to the cages at 6 PM. Each rat was tested with each dose of leptin (0, 0.05, 0.1, 0.25, and 0.5 μg) in random order and with 4 days between tests. Four rats did not complete the experiment either because of loss of the guide cannula (1 rat) or because they stopped eating and rapidly lost weight after the second (2 rats) or fourth (1 rat) injection. Thus, 16 rats completed the experiment: 6 Blk-Sap and 10 Lep-Sap. On the last day of the experiment, food was removed from the cages at 7:30 AM, and starting at 12 PM, rats were anesthetized with ketamine-xylazine and perfused. The brain was collected and sectioned for pSTAT3 immunohistochemistry. pSTAT3 was quantified in the hindbrain at bregma −14.08 mm, in the medial and lateral arcuate nucleus of the hypothalamus (ArcM, ArcL) and VMHdm at bregma −3.14 and −3.30 mm, and in the dorsomedial hypothalamic nucleus, compact part (DMC), at −3.30 mm [Plates 32 and 33 of the Paxinos and Watson brain atlas (31)]. Two Lep-Sap rats were dropped from the study because of control levels of pSTAT3 in the NTS.
The results of the previous experiment indicated that Lep-Sap injections into the NTS reduced sensitivity to 3V leptin, but hypothalamic pSTAT3 was measured at the end of the experiment in nonstimulated conditions. Therefore this study was designed to test whether 3V leptin failed to induce hypothalamic pSTAT3 in Lep-Sap-treated rats. Ten rats received NTS injections of Lep-Sap, and 10 received injections of Blk-Sap. Three weeks later they were fitted with a 3V guide cannula. Five days after cannulation, the rats were housed in the calorimeter, and cannula placement was tested with angiotensin II. Five and nine days after being housed in the calorimeter the rats were tested for leptin responsiveness, acting as their own controls. Food was removed from the cages at 7 AM. Starting at 5 PM, rats received a 3V injection of 0.25 μg leptin or PBS. Food was returned to the cages at 6 PM. Three days after the second leptin test, food was removed from the cages at 7 AM. Starting at 1 PM, each rat received a 3V injection of 0.25 μg leptin, and exactly 60 min after injection it was anesthetized with ketamine-xylazine and perfused for pSTAT3 immunohistochemistry of the hypothalamus and hindbrain. pSTAT3 expression was quantified in the same brain areas as in the previous experiment. Two Blk-Sap rats were removed from the experiment, one because of a misplaced 3V cannula and the other because it lost its cannula partway through the experiment. Three Lep-Sap rats were removed from the experiment, two because of significant levels of pSTAT3 in the NTS and one because of a misplaced 3V cannula.
Because the injection of conjugated saporin into the NTS is close to the surface of the tissue and the 4V, it was determined whether Lep-Sap in the 4V had any potential to diffuse through the subarachnoid space and influence hypothalamic leptin signaling. Eight rats were treated with 50 ng of either Lep-Sap or Blk-Sap injected directly into the 4V. The rats were maintained for 26 days. On day 26, food was removed from the cages at 7 AM. Starting at 12 PM, rats were anesthetized with ketamine-xylazine and received a stereotaxically placed 3V injection of 0.25 μg leptin (R&D Systems, Minneapolis, MN) in 200 nL sterile isotonic saline delivered over 1 min. The injection volume of 200 nL was used to minimize the increase in cerebrospinal fluid volume in the third ventricle and to limit flushing of leptin into the 4V. The needle was held in place for 2 min after injection. Thirty minutes after injection the rats were perfused and the brains collected for pSTAT3 immunohistochemistry. The brains were sectioned, and pSTAT3 was quantified in the Arc, VMHdm, and dorsomedial nucleus of the hypothalamus (DMH) at bregma −3.14 mm, −3.30 mm, and −4.30 mm.
Statistical analysis.
Comparisons between groups were determined using Statistica (StatSoft Inc., Tulsa, OK). Daily measures of food intake, body weight, RER, or energy expenditure were compared by repeated measures analysis of variance with rat number as a covariant. Post hoc comparisons at specific time points were made using Tukey’s honestly significant difference (HSD) test with rat number as a covariant. pSTAT3-IR in the hypothalamus or brain stem of Blk-Sap and Lep-Sap rats was compared by unpaired t test assuming equal variance. Differences were considered significant at P < 0.05.
RESULTS
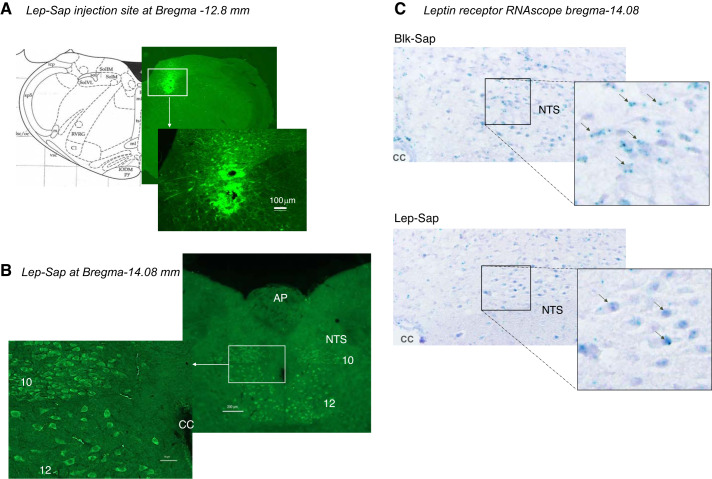
Detection of saporin by immunohistochemistry 4 h after a Lep-Sap injection into the NTS illustrated the distribution of the saporin (Fig. 1A). It was apparent that the Lep-Sap was carried into leptin receptor-expressing cells in the dorsal nucleus of the vagus (10) and the hypoglossal nucleus (12) in addition to the NTS (Fig. 1B). The loss of leptin receptor-expressing cells was confirmed using RNAscope in situ hybridization. Figure 1C shows a substantial loss of leptin receptors in the NTS of a Lep-Sap-injected rat compared with a Blk-Sap-injected rat 23 days after the conjugated saporin injections. It was not possible to test for nonspecific deletion of neurons by the Lep-Sap conjugate because it is not known which neuropeptides are coexpressed with leptin receptors in the hindbrain. Therefore, detection of any peptide known to be expressed in the hindbrain could potentially overlap with detection of leptin receptor-expressing cells, and a change in the level of neuropeptide expression would not be conclusive evidence of lack of specificity of the Lep-Sap.
Fig. 1.
A: immunohistochemistry illustrating the location of a 75-μL leptin-conjugated saporin (Lep-Sap) injection into the nucleus of the solitary tract (NTS). The rat was perfused 4 h after the injection. B: Lep-Sap in the cell bodies of neurons in the NTS, dorsal nucleus of the vagus (10), and hypoglossal nucleus (12) 4 h after Lep-Sap injection. C: RNAscope in situ hybridization detection of leptin receptors in the NTS of representative nonsense peptide-conjugated saporin (Blk-Sap)- and Lep-Sap-injected rats 23 days after injection. Black arrows identify green leptin receptor mRNA. AP, area postrema; CC, central canal.
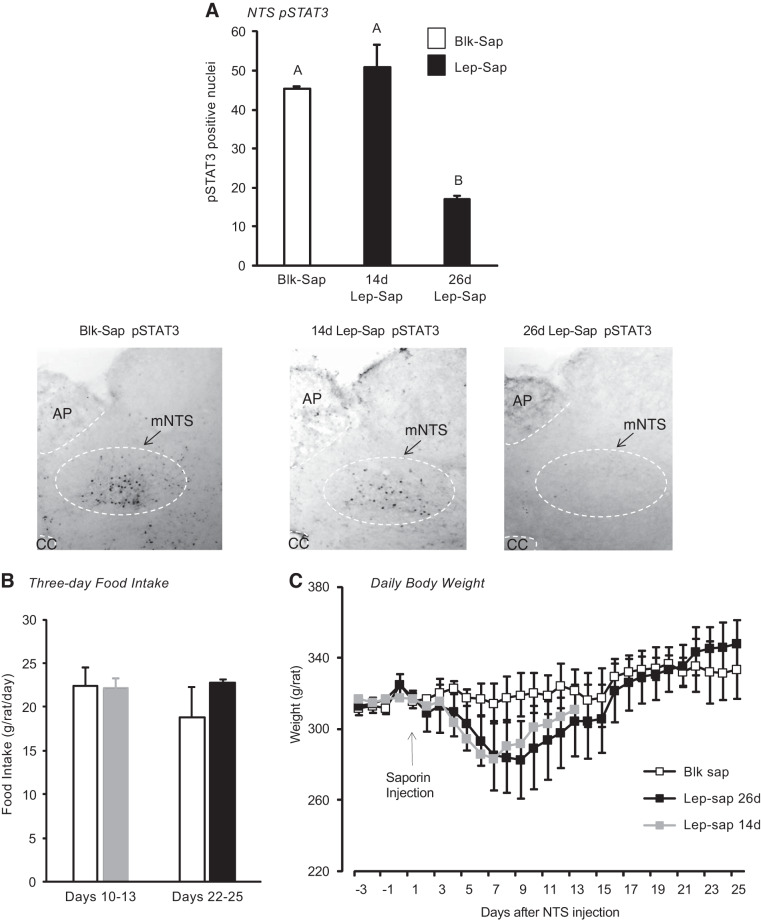
An examination of leptin-induced pSTAT3 in the NTS 14 or 26 days after bilateral injections of Lep-Sap suggested that it took between 2 and 4 wk for the leptin receptor-expressing cells to be deleted (Fig. 2A). Food intake of these rats was measured for 3 days starting either 10 days or 23 days after the saporin injections. There was no effect of Lep-Sap on food intake measured during 10–13 or 22–25 days after injection (Fig. 2B), and there was only a transitory effect of Lep-Sap on body weight of the rats, so that the body weights of Blk-Sap and Lep-Sap rats were the same at 14 days (Lep-Sap 14d) and at 26 days after injection (Lep-Sap 26d; Fig. 2C).
Fig. 2.
A: phosphorylated STAT3 (pSTAT3)-positive nuclei identified by immunohistochemistry in the medial nucleus of the solitary tract (mNTS) of rats at different time intervals after an injection of nonsense peptide-conjugated saporin (Blk-Sap) or leptin-conjugated saporin (Lep-Sap). Rats were perfused 45 min after an ip injection of 1 mg/kg leptin. Values with a different superscript are significantly different. Representative images are also provided for each of the conditions. Images have been adjusted for brightness and contrast. B: daily food intakes of groups of rats injected with Blk-Sap or Lep-Sap measured either 10–13 days or 22–25 days after injection were not different. Data are means ± SE for groups of 4 rats. C: daily body weights of rats injected with Blk-Sap or Lep-Sap on day 0. There were no differences in body weight of Blk-Sap and Lep-Sap rats euthanized 14 days after injection (Lep-Sap 14d) or 26 days after injection (Lep-Sap 26d). Data are means ± SE for groups of 4 rats. AP, area postrema; CC, central canal.
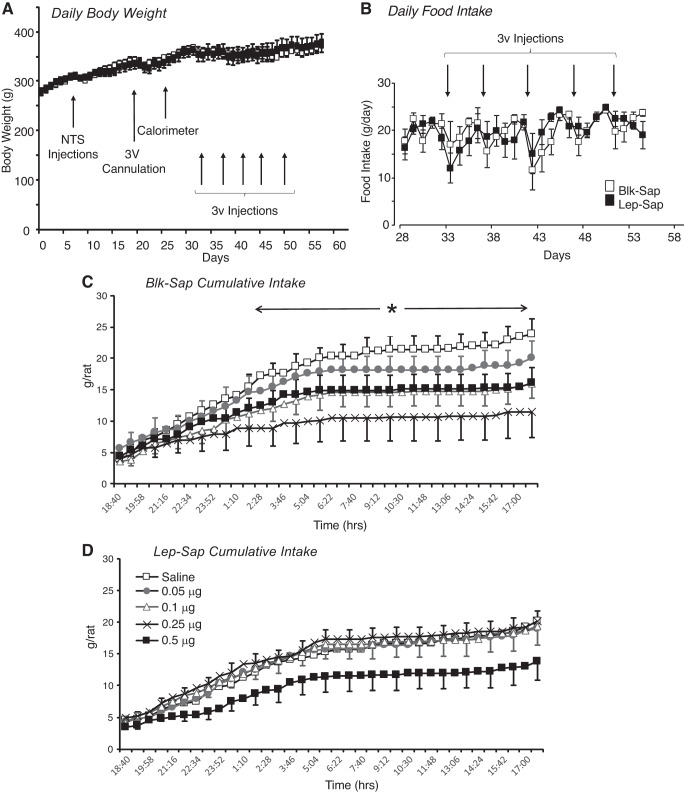
Consistent with results from the pilot studies, there was no effect of Lep-Sap on body weight of the rats used to test for 3V leptin sensitivity (Fig. 3A), nor were there any differences in daily food intakes of the rats on the days that they were housed in the calorimeter (Fig. 3B). Lep-Sap did prevent a significant effect of 3V leptin injection on food intake of the rats (Figs. 3, C and D). Very low doses of leptin were injected into the 3V to test for a threshold response. Comparison of intake during 12-h intervals following the injection found no effect of Lep-Sap or of leptin injection, but there was an interaction between leptin injection and time [Table 1; Lep-Sap, not significant (NS); leptin, NS; time interval, P < 0.0001; leptin × time interval, P < 0.004]. Similarly, comparison of the cumulative intake of all of the rats during the 12 h following injection found no effect of Lep-Sap on intake, but there was a significant interaction between leptin injection and time (Lep-Sap, NS; leptin, NS; time, P < 0.0001; leptin × time, P < 0.0001). Post hoc analysis of data including all rats did not show any time intervals or time points at which there were significant differences between groups; therefore, intake of the rats was compared within treatment group. In Blk-Sap rats, 0.25 μg leptin significantly inhibited food intake during the 24 h following injection (Table 1). Cumulative food intake following a 0.25-μg injection was significantly lower than that following a control, saline injection starting ~6 h after the injection (Fig. 3C; leptin, P < 0.07; time, P < 0.0001; interaction, P < 0.0001). Intake between 12 and 24 h after injection was inhibited by both 0.25 and 0.5 μg leptin. By contrast, there was no significant effect of 3V leptin injections on intervaled (Table 1) or cumulative (Fig. 3D; leptin, P < 0.8; time, P < 0.0001; interaction, P < 1.0) food intake of Lep-Sap-treated animals. There was no effect of any dose of 3V leptin on energy expenditure of either Blk-Sap or Lep-Sap rats during the 24 h following injection (data not shown). Analysis of RER, which is used as an indirect index of the substrate being oxidized, suggested a specific effect of treatment on substrate utilization during the 24 h following leptin injection (saporin, NS; leptin, NS; time, P < 0.0001; saporin × time, P < 0.009; leptin × time, P < 0.0001). Analysis of only the Blk-Sap rats found no significant effect of leptin (leptin, P < 0.4; time, P < 0.001; leptin × time, P < 0.8), whereas there was significant interaction between leptin and time in the Lep-Sap-treated rats (leptin, NS; time, P < 0.001; leptin × time, P < 0.0002). Post hoc analysis identified only one time point at which leptin treatment influenced RER. At ~2:30 PM on the day after leptin injection, RER of rats injected with 0.5 μg leptin was significantly lower than when the rats were injected with saline (data not shown).
Fig. 3.
Daily data from the study testing sensitivity to third-ventricle (3V) leptin injections in nonsense peptide-conjugated saporin (Blk-Sap) and leptin-conjugated saporin (Lep-Sap) rats. A and B: daily body weight (A) and food intake (B) of the rats and outline of the experimental design. There was no effect of Lep-Sap treatment on body weight. C: cumulative food intake of Blk-Sap-treated rats during the 24 h following 3V injection of different doses of leptin. Each rat acted as its own control. Data are means ± SE for 6 or 8 rats. *Time points at which food intake was different following an injection of 0.25 μg leptin compared with the control injection of saline. D: cumulative intake for rats treated with Lep-Sap. There were no significant differences in food intake following the different injections. Data are means ± SE for 7 rats. NTS, nucleus of the solitary tract.
Table 1.
Twelve-hour food intakes of rats receiving 3V injections of leptin
| Leptin Dose |
|||||
|---|---|---|---|---|---|
| 0 μg | 0.05 μg | 0.1 μg | 0.25 μg | 0.5 μg | |
| Blk-Sap rats | |||||
| 0–12 h | 20.4 ± 1.8a | 18.1 ± 2.2ab | 14.6 ± 2.3ab | 10.6 ± 3.8b | 14.8 ± 2.5ab |
| 12–24 h | 2.8 ± 0.5a | 1.9 ± 0.8ab | 1.4 ± 0.5ab | 0.95 ± 0.5b | 1.19 ± 0.3b |
| 24–36 h | 15.5 ± 1.5 | 13.4 ± 1.2 | 13.0 ± 1.7 | 12.5 ± 2.3 | 15.0 ± 1.2 |
| 36–48 h | 6.1 ± 1.1 | 4.9 ± 1.3 | 4.9 ± 0.7 | 5.2 ± 0.7 | 5.6 ± 0.7 |
| Lep-Sap rats | |||||
| 0–12 h | 15.7 ± 2.3 | 15.8 ± 2.5 | 16.5 ± 2.3 | 17.2 ± 1.5 | 11.5 ± 2.4 |
| 12–24 h | 3.9 ± 0.8 | 2.6 ± 0.6 | 2.8 ± 0.7 | 2.6 ± 0.6 | 2.2 ± 0.6 |
| 24–36 h | 16.4 ± 0.9 | 13.7 ± 1.3 | 14.2 ± 1.2 | 14.8 ± 1.3 | 13.9 ± 1.1 |
| 36–48 h | 5.2 ± 0.6 | 6.9 ± 1.2 | 6.1 ± 0.9 | 5.2 ± 0.8 | 6.2 ± 1.1 |
Values are means ± SE for groups of 6 or 8 rats and are given in g·rat−1·12 h−1. Statistical comparisons for a specific time interval within a treatment group were made using one-way analysis of variance using rat number as a covariant. Post hoc analysis was performed using Tukey’s test. Blk-Sap, nonsense peptide-conjugated saporin; Lep-Sap, leptin-conjugated saporin; 3V, 3rd ventricle.
Values that do not share a common superscript are significantly different at P < 0.05.
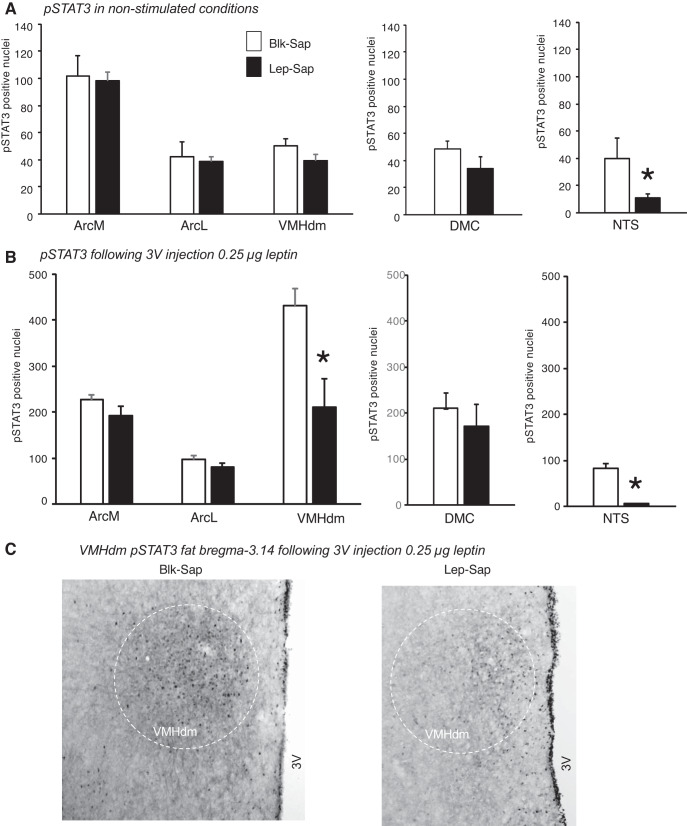
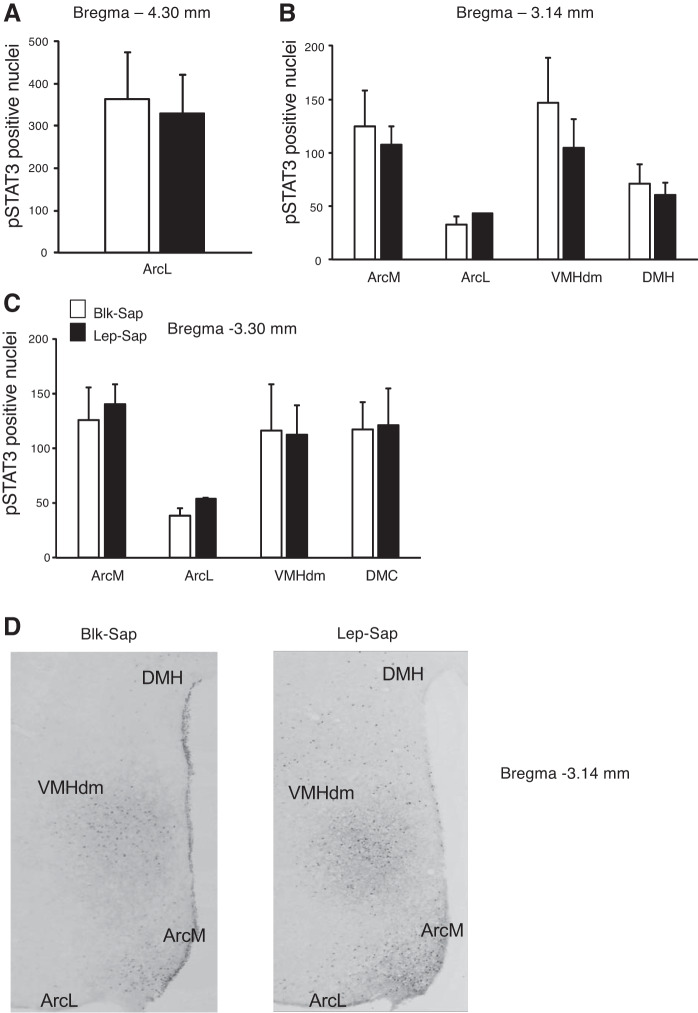
At the end of the experiment, immunohistochemistry for pSTAT3 was performed on the brains of all of the rats in nonstimulated conditions. NTS pSTAT3 levels were used to confirm the effectiveness of the Lep-Sap injections, and as noted above, two Lep-Sap rats were dropped from the study because of significant levels of NTS pSTAT3. The number of pSTAT3-positive nuclei at bregma −3.14 and −3.30 was quantified and summed for each rat. There was no significant effect of Lep-Sap on ArcM, ArcL, or VMHdm pSTAT3 expression (Fig. 4A). DMH pSTAT3 was not quantified at bregma −3.14 mm because it was very low and because the 3V cannula had damaged this tissue in some of the rats. There was no effect of Lep-Sap on pSTAT3 in the DMC at bregma −3.30.
Fig. 4.
Quantification of phosphorylated STAT3 (pSTAT3)-positive nuclei in the hypothalamus of nonsense peptide-conjugated saporin (Blk-Sap) and leptin-conjugated saporin (Lep-Sap) rats in nonstimulated conditions (A) or 1 h after a third-ventricle (3V) injection of 0.25 μg leptin (B). Data are means ± SE for 6–8 rats. *Significant difference between Blk-Sap and Lep-Sap rats. C: representative images of pSTAT3 in the dorsomedial region of the ventromedial nucleus of the hypothalamus (VMHdm) of Blk-Sap and Lep-Sap rats that had been injected with 0.25 μg leptin. Images have been adjusted for brightness and contrast. DMC, dorsomedial hypothalamic nucleus, compact part; NTS, nucleus of the solitary tract. ArcM, medial arcuate nucleus of the hypothalamus; ArcL, lateral arcuate nucleus of the hypothalamus.
When rats were tested only with 3V injections of 0.25 μg leptin, 12-h food intake was nonsignificantly inhibited by leptin in Blk-Sap rats (17.1 ± 1.5 vs. 13.9 ± 1.6 g·rat−1·12 h−1, P < 0.1), but not Lep-Sap rats (15.6 ± 3.0 vs. 14.8 ± 1.9 g·rat−1·12 h−1, P < 0.4). The number of pSTAT3-positive nuclei was substantially higher in all brain areas evaluated, except the NTS of Lep-Sap rats, compared with the previous experiment when the rats were perfused in nonstimulated conditions. For all areas except the VMHdm the leptin injection approximately doubled the amount of pSTAT3 detected. By contrast, pSTAT3 in the VMHdm of Blk-Sap rats increased 10-fold, whereas that in the VMHdm of Lep-Sap rats increased 5-fold (Fig. 4, B and C). Injection of Lep-Sap directly into the 4V had no effect on hypothalamic pSTAT3 activation (Fig. 5, A–C) confirming that changes in the VMHdm leptin response of Lep-Sap rats (Fig. 4B) were due to the loss of receptors in the hindbrain and not because Lep-Sap had diffused and reduced the number of leptin receptor-expressing cells in hypothalamic sites.
Fig. 5.
A–C: phosphorylated STAT3 (pSTAT3)-positive nuclei detected by immunohistochemistry at different levels of the hypothalamus of rats that received fourth-ventricle (4V) injections of nonsense peptide-conjugated saporin (Blk-Sap) or leptin-conjugated saporin (Lep-Sap) 26 days before perfusion. All of the rats received a 3V injection of 0.25 μg leptin 30 min before perfusion. There was no effect of the Lep-Sap on hypothalamic pSTAT3. D: the representative images of pSTAT3 in the hypothalamus of Blk-Sap and Lep-Sap rats have been adjusted for brightness and contrast. VMHdm, dorsomedial region of the ventromedial nucleus of the hypothalamus; DMH, dorsomedial nucleus of the hypothalamus; ArcM, medial arcuate nucleus of the hypothalamus; ArcL, lateral arcuate nucleus of the hypothalamus.
DISCUSSION
We have recently been investigating the importance of simultaneous leptin activation of multiple areas of the brain in mediating leptin-induced inhibition of food intake. The studies were initiated by observations that administration of subthreshold doses of leptin to the 3V and 4V simultaneously caused a dramatic inhibition of food intake and rapid weight loss in rats, whereas administration of each dose independently had no effect (9, 10, 16). These results, combined with data from subsequent experiments, led us to conclude that leptin inhibits food intake only when both hindbrain and hypothalamic leptin receptors are activated and, furthermore, that activation of hindbrain leptin receptors lowers the threshold for initiation of a hypothalamic leptin response (17, 19). Although the Lep-Sap injection was aimed at the NTS, it was clear that Lep-Sap was also taken up by neurons in the dorsal motor nucleus of the vagus and the hypoglossal nucleus. Therefore, in this experiment the change in hypothalamic sensitivity to leptin in Lep-Sap rats cannot be specifically attributed to the NTS. However, leptin-induced pSTAT3 is almost exclusive to the NTS (12, 21), and others have shown that injections of leptin directly into the NTS inhibit food intake (36), suggesting that this hindbrain site is involved in the control of food intake. Previously, leptin signaling in the NTS has been reported to decrease meal size and increase sensitivity to gut-derived satiety signals (24), suppress food intake (47), and increase brown fat thermogenesis (43). If, however, leptin injections into the NTS increase leptin signaling in the hypothalamus (36), then it is possible that these responses are mediated, at least in part, by forebrain leptin signaling.
The objective of the present study was to test whether removing hindbrain leptin receptor-expressing cells influenced the behavioral response to leptin administered in the 3V. Because we were looking for changes in the threshold of response, very low dose 3V leptin injections were tested. The dose-response study indicated that loss of NTS leptin receptor-expressing cells raises the threshold for a feeding response to leptin injected into the 3V. In Blk-Sap-treated rats there appeared to be a dose-response effect of increasing concentrations of leptin on food intake reaching significance with a concentration of 0.25 μg leptin starting 6 h after the injection, whereas in the Lep-Sap rats there was a tendency for the highest-dose injection of 0.5 μg leptin to inhibit food intake during the 12 h following injection, but this was not significant. There was a nonsignificant trend for the food intake of Lep-Sap rats to be lower than that of Blk-Sap rats during the 12 h that immediately followed the 3V injections, including those that received only vehicle. This, however, could not be attributed to a generalized motor or behavioral deficit in the rats because the trend was no longer apparent during the periods 12–24, 24–36, and 36–48 h after injection and must have been secondary to the manipulations involved in giving injections. Daily food intakes of Blk-Sap and Lep-Sap rats were similar during the experimental period, which also argues against a physical deficit caused by the Lep-Sap treatment. The difference in response between Lep-Sap and Blk-Sap rats to 3V leptin implies a requirement for activation of NTS leptin receptors to achieve a full response to 3V leptin, and this was also supported by the finding that a 3V injection of 0.25 μg leptin activated NTS leptin receptors in Blk-Sap rats. The changes in food intake were small, as would be expected for the low concentrations of leptin that were administered. In the Blk-Sap rats, 0.25 μg leptin inhibited food intake during the first 12 h after injection, but 0.5 μg did not inhibit food intake until 12–24 h after injection. We have observed this biphasic response to leptin previously in rats that received low-dose 3V infusions of leptin combined with low-dose 4V leptin injections (18), and it may be due to activation of different leptin signaling pathways. Balogh et al. (1) found a biphasic response in cultured monocytes that was due to activation of phosphatidylinositol 3-kinase (PI3K) and MAPK with low concentrations, but activation of PI3K and PKC at higher concentrations. In this study, only pSTAT3 activation was measured because this is critical for the effects of leptin on energy balance (3), and it is possible that the higher dose of leptin used in this study activated a different signaling cascade that ameliorated the inhibitory effect on food intake. This signaling would likely have to be occurring in the hindbrain because there was no indication of a biphasic effect of leptin in Lep-Sap-treated rats. Others have also reported a biphasic response in proliferation of cultured myometrial muscle cells exposed to increasing doses of leptin. The biphasic response was attributed to activation of NF-κB and IL-6 receptors by the higher concentrations of leptin (2). Again, we did not measure activation of immune pathways in the brains of the rats in this study, but if cytokine receptors were stimulated by leptin, then these receptors would also have to be expressed on leptin receptor-expressing cells in the hindbrain because Lep-Sap rats did not show the biphasic response.
There were no changes in energy expenditure of any the rats, suggesting that stimulation of thermogenesis might only occur with higher, potentially pharmacological doses of leptin. Doses ranging from 3 to 10 μg leptin have been injected into the 3V when testing the effect of leptin on sympathetic outflow (32, 33, 45), and others have injected between 0.05 and 0.2 μg directly into the Arc (25, 48), which was the same range of doses as were injected into the 3V in this study. There was no effect of leptin on RER of any of the rats in this study, which implies that the small changes in food intake that were observed were not sufficient to cause mobilization of lipid stores as energy substrates.
At the end of the dose-response experiment, when rats were perfused in unstimulated conditions, there were no differences in the levels of pSTAT3 in any of the hypothalamic nuclei of Blk-Sap or Lep-Sap rats. By contrast, in the subsequent experiment, loss of leptin receptor-expressing cells in the hindbrain not only prevented activation of the NTS by a 3V injection of 0.25 μg leptin but also substantially attenuated pSTAT3 of the VMHdm of Lep-Sap rats. Previously, we have shown that loss of leptin receptor-expressing cells in the VMH diminishes the response to 4V leptin (39), and the results from the experiments described here demonstrate a reciprocal effect.
It was clear from the experiment described here that leptin caused a much greater increase in pSTAT3 in the VMH than any of the other nuclei. 3V leptin doubled pSTAT3 in the ArcM, ArcL, DMC, and NTS, whereas there was a 10-fold increase in the VMH. These results are similar to those found in previous experiments when pSTAT3 in hypothalamic and hindbrain nuclei of rats receiving simultaneous 3V and 4V leptin infusions was quantified (10). With this level of activation it is surprising how little attention has been paid to the impact of VMHdm leptin receptor activation. Microinjection of leptin into the VMH has been reported to increase sympathetic outflow (42, 44) and to promote glucose uptake in cardiac muscle, spleen, red skeletal muscle, and brown, but not white, fat (27). Others have shown that injection of leptin into the VMH suppresses food intake of rats (22) and also that loss of leptin receptors in the VMH has no effect on the phenotype of mice fed chow, but accelerates onset of diet-induced obesity in high-fat fed mice (5, 11). These observations combined with evidence that unlike the Arc, the VMH does not become leptin resistant with chronic exposure to a high-fat diet (29, 34) lead to the conclusion that VMH leptin receptors are important in preventing weight gain in conditions of positive energy balance, but not the maintenance of body weight in control conditions. By contrast, it is well established that leptin receptors in the Arc are essential for the maintenance of a normal body weight (8, 37). Our observations that loss of leptin receptor-expressing cells in the VMH has no effect on baseline food intake or body weight of chow-fed rats but does prevent a response to central infusions of leptin are consistent with this scenario (39). Similarly, selective replacement of leptin receptors in VMH steroidogenic factor 1 (SF1) neurons of leptin receptor-deficient mice does not prevent excess weight gain in mice fed either chow or high-fat diet (40), thus confirming a critical role for Arc leptin receptors in the maintenance of body weight and for an integrated response of multiple brain areas in mediating leptin’s inhibitory effect on food intake and weight gain.
The integration of leptin signaling between the NTS and VMH appears to be mediated by a neural network rather than diffusion (Fig. 5; 19, 36). The simplest structural network would be a direct projection from hindbrain leptin receptor-expressing neurons to VMHdm leptin receptor-expressing neurons. However, there is no evidence for direct connections from the NTS neurons to the VMHdm (46). Therefore, NTS-to-VMHdm communication must use intermediary brain regions in a multistage network. Existing connectional studies identify two regions as likely intermediate nuclei. These are the lateral parabrachial nucleus (4, 14, 20) and the paraventricular nucleus of the thalamus (28, 41). Both are heavily implicated in the control of energy balance (14, 15, 26, 30, 35), but polysynaptic tract tracing is needed to elucidate the neural communication between leptin receptor-expressing neurons in the NTS and the VMHdm.
It was notable in this study that a significant effect of leptin on intake of Blk-Sap rats was not detected until 6 h after food was returned, although a trend for a reduced intake appeared after 4 h and may have been significant with a larger group size. The rats were food deprived for 10 h during the light period before being tested and were clearly hungry when food was returned to the cage because they rapidly started to eat voraciously. Because the injection was into the 3V, one would anticipate a rapid response, and the immunohistochemistry that was evaluated 1 h after injection indicated that hypothalamic and hindbrain receptors were already activated. Therefore, the delay could be attributed to either an overriding effect of hunger caused by the food deprivation before injection or the need to recruit downstream satiety systems to mediate the inhibition of food intake.
In summary, the results of the studies described here support the notion that activation of leptin receptors in both the hindbrain and hypothalamus is required for a full effect of leptin in the VMHdm. Because Lep-Sap destroys the entire cell, it is possible that this effect is mediated by other receptors expressed on these cells. However, Lep-Sap had no effect on food intake or body weight of the rats in baseline conditions. It has been suggested that leptin receptors in the VMHdm attenuate weight gain in conditions of positive energy balance (5, 11). Injection of leptin would mimic these conditions because a gain in body fat is accompanied by a rise in endogenous leptin (6). It would be of interest to determine whether loss of NTS leptin receptor-expressing cells accelerates weight gain in rats offered a high-fat diet as this would further confirm the requirement for an integrated response between the NTS and VMHdm. Further studies are also needed to identify the neural pathway and the neurotransmitters that mediate this interaction between anatomically distant areas of the brain.
GRANTS
This work was supported by NIH Grant R01DK053903 awarded to R.B.S.H.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.B.S.H. conceived and designed research; performed experiments; analyzed data; interpreted results of experiments; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Balogh Z, Fóris G, Kosztáczky B, Paragh G Jr, Seres I, Zsíros E, Kónya G, Paragh G. The concentration dependent biphasic effect of leptin on endogenous cholesterol synthesis in human monocytes. Peptides 28: 2081–2083, 2007. doi: 10.1016/j.peptides.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Barrichon M, Hadi T, Wendremaire M, Ptasinski C, Seigneuric R, Marcion G, Delignette M, Marchet J, Dumas M, Sagot P, Bardou M, Garrido C, Lirussi F. Dose-dependent biphasic leptin-induced proliferation is caused by non-specific IL-6/NF-κB pathway activation in human myometrial cells. Br J Pharmacol 172: 2974–2990, 2015. doi: 10.1111/bph.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG Jr. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 4.Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 383: 245–281, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 5.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149: 2138–2148, 2008. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348: 159–161, 1996. doi: 10.1016/S0140-6736(96)03173-X. [DOI] [PubMed] [Google Scholar]
- 7.Chotiwat C, Harris RB. Antagonism of specific corticotropin-releasing factor receptor subtypes selectively modifies weight loss in restrained rats. Am J Physiol Regul Integr Comp Physiol 295: R1762–R1773, 2008. doi: 10.1152/ajpregu.00196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson R, Pelleymounter MA, Millard WJ, Liu S, Eppler B. Attenuation of leptin-mediated effects by monosodium glutamate-induced arcuate nucleus damage. Am J Physiol Endocrinol Metab 273: E202–E206, 1997. doi: 10.1152/ajpendo.1997.273.1.E202. [DOI] [PubMed] [Google Scholar]
- 9.Desai BN, Harris RB. Integrated effects of leptin in the forebrain and hindbrain of male rats. Endocrinology 154: 2663–2675, 2013. doi: 10.1210/en.2013-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai BN, Harris RB. Leptin in the hindbrain facilitates phosphorylation of STAT3 in the hypothalamus. Am J Physiol Endocrinol Metab 308: E351–E361, 2015. doi: 10.1152/ajpendo.00501.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147: 3190–3195, 2006. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- 13.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493: 63–71, 2005. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 14.Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JT, Germani M, Jones JC, Rajala M, Satin L, Rhodes CJ, Olson DP, Kennedy RT, Heisler LK, Myers MG Jr. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci 17: 1744–1750, 2014. doi: 10.1038/nn.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG Jr, Heisler LK. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab 20: 1030–1037, 2014. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RB. Evidence that leptin-induced weight loss requires activation of both forebrain and hindbrain receptors. Physiol Behav 120: 83–92, 2013. doi: 10.1016/j.physbeh.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris RB, Desai BN. Fourth-ventricle leptin infusions dose-dependently activate hypothalamic signal transducer and activator of transcription 3. Am J Physiol Endocrinol Metab 311: E939–E948, 2016. doi: 10.1152/ajpendo.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris RB. Low-dose leptin infusion in the fourth ventricle of rats enhances the response to third-ventricle leptin injection. Am J Physiol Endocrinol Metab 313: E134–E147, 2017. doi: 10.1152/ajpendo.00052.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris RB. Low-dose infusions of leptin into the nucleus of the solitary tract increase sensitivity to third ventricle leptin. Am J Physiol Endocrinol Metab 316: E719–E728, 2019. doi: 10.1152/ajpendo.00562.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 21.Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin’s action in the central nervous system. Endocrinology 143: 3498–3504, 2002. doi: 10.1210/en.2002-220077. [DOI] [PubMed] [Google Scholar]
- 22.Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Sherwin RS. The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes 46: 150–152, 1997. doi: 10.2337/diab.46.1.150. [DOI] [PubMed] [Google Scholar]
- 23.Kanoski SE, Alhadeff AL, Fortin SM, Gilbert JR, Grill HJ. Leptin signaling in the medial nucleus tractus solitarius reduces food seeking and willingness to work for food. Neuropsychopharmacology 39: 605–613, 2014. doi: 10.1038/npp.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, Bence KK, Hayes MR, Grill HJ. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab 303: E496–E503, 2012. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zheng H. Leptin-mediated sympathoexcitation in obese rats: role for neuron-astrocyte crosstalk in the arcuate nucleus. Front Neurosci 13: 1217, 2019. doi: 10.3389/fnins.2019.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millan EZ, Ong Z, McNally GP. Paraventricular thalamus: gateway to feeding, appetitive motivation, and drug addiction. Prog Brain Res 235: 113–137, 2017. doi: 10.1016/bs.pbr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48: 287–291, 1999. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 28.Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359: 221–238, 1995. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- 29.Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 30.Ong ZY, Liu JJ, Pang ZP, Grill HJ. Paraventricular thalamic control of food intake and reward: role of glucagon-like peptide-1 receptor signaling. Neuropsychopharmacology 42: 2387–2397, 2017. doi: 10.1038/npp.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain. New York: Academic Press, 1998. [Google Scholar]
- 32.Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol 291: R1613–R1621, 2006. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- 33.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647–652, 2007. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 34.Rizwan MZ, Mehlitz S, Grattan DR, Tups A. Temporal and regional onset of leptin resistance in diet-induced obese mice. J Neuroendocrinol 29: e12481, 2017. doi: 10.1111/jne.12481. [DOI] [PubMed] [Google Scholar]
- 35.Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 7: 11905, 2016. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology 151: 1509–1519, 2010. doi: 10.1210/en.2009-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K, Yoshimasa Y, Nishi S, Hosoda K, Nakao K. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett 224: 149–152, 1997. doi: 10.1016/S0304-3940(97)00163-8. [DOI] [PubMed] [Google Scholar]
- 38.Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology 39: 1872–1879, 2000. doi: 10.1016/S0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 39.Seamon M, Ahn W, Li AJ, Ritter S, Harris RB. Leptin receptor-expressing neurons in ventromedial nucleus of the hypothalamus contribute to weight loss caused by fourth ventricle leptin infusions. Am J Physiol Endocrinol Metab 317: E586–E596, 2019. doi: 10.1152/ajpendo.00205.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senn SS, Le Foll C, Whiting L, Tarasco E, Duffy S, Lutz TA, Boyle CN. Unsilencing of native LepRs in hypothalamic SF1 neurons does not rescue obese phenotype in LepR-deficient mice. Am J Physiol Regul Integr Comp Physiol 317: R451–R460, 2019. doi: 10.1152/ajpregu.00111.2019. [DOI] [PubMed] [Google Scholar]
- 41.Shekhtman E, Geerling JC, Loewy AD. Aldosterone-sensitive neurons of the nucleus of the solitary tract: multisynaptic pathway to the nucleus accumbens. J Comp Neurol 501: 274–289, 2007. doi: 10.1002/cne.21245. [DOI] [PubMed] [Google Scholar]
- 42.Shiuchi T, Toda C, Okamoto S, Coutinho EA, Saito K, Miura S, Ezaki O, Minokoshi Y. Induction of glucose uptake in skeletal muscle by central leptin is mediated by muscle β2-adrenergic receptor but not by AMPK. Sci Rep 7: 15141, 2017. doi: 10.1038/s41598-017-15548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150: 1705–1711, 2009. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sudo M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am J Physiol Endocrinol Metab 261: E298–E303, 1991. doi: 10.1152/ajpendo.1991.261.3.E298. [DOI] [PubMed] [Google Scholar]
- 45.Tanida M, Iwasaki Y, Yamamoto N. Central injection of leptin increases sympathetic nerve outflows to the stomach and spleen in anesthetized rats. In Vivo 33: 1827–1832, 2019. doi: 10.21873/invivo.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience 31: 785–797, 1989. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 47.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes 36: 1522–1528, 2012. doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng H, Liu X, Li Y, Patel KP. A hypothalamic leptin-glutamate interaction in the regulation of sympathetic nerve activity. Neural Plast 2017: 1–11, 2017. doi: 10.1155/2017/2361675. [DOI] [PMC free article] [PubMed] [Google Scholar]