Abstract
Excessive alcohol consumption, including binge drinking, is a common cause of fatty liver disease. Binge drinking rapidly induces hepatic steatosis, an early step in the pathogenesis of chronic liver injury. Despite its prevalence, the process by which excessive alcohol consumption promotes hepatic lipid accumulation remains unclear. Alcohol exerts potent effects on the brain, including hypothalamic neurons crucial for metabolic regulation. However, whether or not the brain plays a role in alcohol-induced hepatic steatosis is unknown. In the brain, alcohol increases extracellular levels of adenosine, a potent neuromodulator, and previous work implicates adenosine signaling as being important for the development of alcoholic fatty liver disease. Acute alcohol exposure also increases both the activity of agouti-related protein (AgRP)-expressing neurons and AgRP immunoreactivity. Here, we show that adenosine receptor A2B signaling in the brain modulates the extent of alcohol-induced fatty liver in mice and that both the AgRP neuropeptide and the sympathetic nervous system are indispensable for hepatic steatosis induced by bingelike alcohol consumption. Together, these results indicate that the brain plays an integral role in alcohol-induced hepatic lipid accumulation and that central adenosine signaling, hypothalamic AgRP, and the sympathetic nervous system are crucial mediators of this process.
Keywords: adenosine signaling, AgRP, alcohol, binge drinking, hepatic steatosis
INTRODUCTION
Alcoholic binge drinking is prevalent. In 2015, about 1 in 6 adults in the United States admitted to binge drinking, with an average individual consumption of 7 drinks per episode and 470 binges per year (20). One of the major health consequences of excessive alcohol consumption is the development of acute and chronic liver disease. Hepatic steatosis, seen in more than 90% of heavy alcohol drinkers (1, 11, 45, 46), is an early consequence of excess alcohol consumption, including binge drinking, and can be induced by even a single dose of alcohol in rodents (21, 24, 48). Moreover, alcohol-induced hepatic steatosis predicts the pathogenesis of more chronic and severe diseases, including hepatitis, fibrosis, and ultimately cirrhosis (11, 26, 49).
However, the etiology of alcoholic fatty liver, although likely multifaceted, remains incompletely understood. One potential driver of this process is the direct effect of alcohol on the liver. Ethanol is oxidized by alcohol dehydrogenase (ADH) in the cytosol of hepatocytes to yield acetaldehyde, which is subsequently metabolized by aldehyde dehydrogenase (ALDH) to produce acetate (56). In addition, hepatocytes metabolize ethanol by the microsomal ethanol-oxidizing system, which is catalyzed by cytochrome P450 2E1 (CYP2E1) and catalase in peroxisomes (56). Ethanol also influences the activity of hepatic peroxisome proliferator-activated receptor-α (PPARα), sterol regulatory element binding protein 1c (SREBP-1c), and AMP-dependent protein kinase (AMPK), which in turn inhibit fatty acid oxidation and stimulate de novo lipogenesis, leading to fat accumulation (11, 26, 54). Alcohol may also exert indirect effects on the liver, by disrupting the metabolic function of adipocytes (41), promoting white adipose tissue lipolysis, and enhancing free fatty acid flux to liver (50, 57).
Notably, evidence also suggests that adenosine signaling plays an important role in the etiology of alcoholic liver diseases (13). All cells generate adenosine during the breakdown of ATP. Alcohol increases extracellular adenosine levels by at least two mechanisms (13). First, the liver oxidizes ethanol to yield acetate, which is metabolized to adenosine, thus increasing systemic adenosine levels when alcohol is consumed in excess (6, 40). Second, ethanol inhibits purine reuptake through the type 1 equilibrative nucleoside transporter (ENT1) in tissues including neurons, leading to the buildup of extracellular adenosine (31, 37). There are four adenosine receptors in vertebrates, termed A1, A2A, A2B, and A3 (16). Notably, mice with germline deletion of A1 or A2B, but not A2A, are resistant to alcohol-induced hepatic steatosis (43).
While the roles of the liver and white adipose tissue in alcohol-induced hepatic lipid accumulation have been extensively investigated, the role of the brain, by comparison, has not. Upon consumption, alcohol quickly enters the brain, altering neuronal functions and impairing neurological, cognitive, and social behaviors. Increasing evidence suggests that alcohol may also exert metabolic effects through the brain. For example, binge drinking rapidly induces systemic insulin resistance by impairing hypothalamic insulin signaling (27). Within the hypothalamus, agouti-related protein (AgRP) is a neuropeptide that is expressed by a group of neurons in the mediobasal hypothalamus. AgRP antagonizes the action of α-melanocyte-stimulating hormone (α-MSH), a product of the proopiomelanocortin (POMC)-expressing neurons. Stimulating insulin signaling in AgRP neurons directly inhibits their activity and leads to suppression of hepatic glucose production (23). AgRP neurons regulate peripheral tissues including liver and adipose tissues via modulation of the autonomic nervous system innervating these tissues (2). For example, we recently showed that AgRP inhibits hepatic sympathetic activity and that eliminating AgRP in leptin-deficient mice alters both hepatic lipid accumulation and distribution in an age-dependent manner (29).
Notably, direct alcohol exposure stimulates the electrical activity of AgRP neurons (5), and ethanol dose-dependently increases hypothalamic AgRP immunoreactivity within 2 h of systemic injection (12). Conversely, alcohol-associated overeating is mediated via activation of AgRP neurons (5). Intracerebroventricular infusion of AgRP promotes alcohol drinking, whereas AgRP deficiency reduces ethanol-reinforced lever-pressing and bingelike alcohol drinking (33, 35, 36). Together, these results suggest that AgRP neurons are modulated by alcohol and that some of the metabolic effects of alcohol may depend on AgRP neurons.
Intriguingly, recent evidence also suggests that presynaptic adenosine signaling via the A1 receptor inhibits AgRP neuronal activity (52). Adenosine is a potent neuromodulator, regulating neuronal activity and modulating signaling by other neurotransmitters (17). Alcohol exposure acutely increases central nervous system (CNS) adenosine levels, and elevated adenosine levels mediate the ataxic and sedative effects of ethanol through A1 receptor activation in the cerebellum, striatum, and cerebral cortex (44). In contrast to presynaptic A1 receptors, which are Gi/o-coupled G protein-coupled receptors (GPCRs), A2B is a Gs-coupled postsynaptic GPCR. Of note, Gs signaling potently regulates the function of AgRP neurons (32). This, along with the observations that alcohol increases AgRP neuronal activity (5) and expression (12), and that mice lacking A2B are resistant to alcohol-induced hepatic steatosis (43), led us to investigate if neuronal A2B signaling and AgRP contribute to the development of alcoholic fatty liver disease induced by bingelike drinking.
MATERIALS AND METHODS
Animals
Mice were housed in a barrier facility under a 12-h light cycle (lights on from 7 AM to 7 PM) with ad libitum access to water and standard mouse chow (21.6%, 23.2%, and 55.2% kcal from fat, protein and carbohydrate, respectively; Purina mouse diet no. 5058). Agrp−/− mice were originally provided by Dr. Gregory Barsh at Stanford University. Transgenic mice expressing Cre recombinase under control of the Nestin promoter [B6.Cg-Tg(Nes-cre)1Kln/J] or the AgRP promoter [Agrptm1(cre)Lowl/J] were originally obtained from the Jackson Laboratory. Adora2bflox/flox mice were provided by Dr. Holger Eltzschig at University of Colorado. To generate Agrp−/− mice, Agrp+/− mice were bred with Agrp−/− mice to generate equal ratio of control (Agrp+/−) and mutant (Agrp−/−) mice. Littermates were compared whenever possible to minimize differences in genetic background and litter-specific effects. Adora2bflox/flox females were mated with Nestin-Cre/+; Adora2bflox/+ males to generate mice lacking the A2B receptor in the nervous system (CNS-Adora2b−/−; Nestin-Cre/+, Adora2bflox/flox) and their littermate controls (Nestin-Cre/+; Adora2bflox/+). Since mice carrying Nestin-Cre alone have a body weight phenotype (4), mice expressing Nestin-Cre were used as controls. To generate mice lacking the A2B receptor in AgRP neurons, Adora2bflox/flox females were mated with Adora2bflox/+; Agrp-Cre males. This breeding resulted in control (Adora2bflox/flox or Adora2bflox/+) and AgRP-specific A2B knockout (Agrp-Cre/+; Adora2bflox/flox) mice (AgRP-Adora2b−/−). For the fasting experiment, 23-wk-old mice were fasted at 1000 and euthanized at the onset of the dark phase (1900). All experiments were approved by the University of California San Francisco Institutional Animal Care and Use Committee.
Alcohol Treatment
Preparation, administration, and dosing of alcohol in mice followed the protocol of Bertola et al. (3). Briefly, at 1900 on day 1, mice were given an oral gavage of ethanol (5 g/kg body wt), while control mice were given isocaloric maltose dextrin (control, 9 g/kg body wt). At 800 on day 2, mice received a repeat oral gavage of either ethanol or vehicle and were euthanized at around 1700 The entire experiment was performed under thermoneutral ambient conditions (30°C), as alcohol administration can induce hypothermia in mice housed at room temperature. Blood alcohol content was measured as described previously (49).
RNA-Interference
RNA-interference was used to knockdown Agrp mRNA levels in 8-wk-old C57BL/6J mice (Jackson Laboratories) following a published protocol (47). The experiment was conducted at 30°C thermoneutrality. Briefly, at 1900 on day 1, half of the mice (n = 12, 2 mice per cage) were given an intraperitoneal injection of Agrp-DsiRNA (Integrated DNA Technologies, Inc., MMC.RNAI.N007427.12.1. 5 µg per mouse) while an equal number received a control injection (Integrated DNA Technologies, Inc., Ds NC1, 5 µg per mouse). Subsequently, all mice received an initial ethanol gavage. At 0700 on day 2, all mice received another injection of either Agrp-DsiRNA or control, after which they received a second dose of ethanol. Tissues were then harvested at 1700 on day 2, alternating between control and Agrp-DsiRNA-treated mice.
Chemical Sympathectomy
At 15–16 wk of age, X male C57BL/6J mice underwent chemical sympathectomy by injection with a single dose of 6-hydroxydopamine (6-OHDA, 250 mg/kg in 0.9% NaCl and 10−7 M ascorbic acid, Sigma-Aldrich, St. Louis, MO) versus vehicle (0.9% NaCl and 10−7 M ascorbic acid). Body weights were monitored, and mice were allowed to recover for 10 days. The effectiveness of sympathectomy was validated by confirming the complete ablation of NPY-positive sympathetic fibers in the livers of the 6-OHDA-treated mice.
Body Composition and Metabolic Analysis
Body composition was measured using an EchoMRI-700 machine (Echo Medical Systems, LLC, Houston, TX). Indirect calorimetry, locomotor activity, and food intake were measured over 5 days at room temperature using a comprehensive laboratory animal monitoring system (CLAMS; Columbus Instruments, Inc., Columbus, OH). Data from the first day in the CLAMS were excluded from analysis. Respiratory exchange ratio (RER) was calculated as (V̇co2/V̇o2), and energy expenditure was calculated as (3.815 + 1.232 × RER) × VO2.
Measurement of Plasma and Tissue Lipid Levels
Several different direct and indirect methods were used to measure tissue triacylglycerol (TAG) levels and are specified in each figure.
Enzymatic TAG determination.
Forty to fifty milligrams of liver were homogenized in 500 μl of buffer (250 mM sucrose, 50 mM Tris·HCl pH 7.4), and lipid levels were determined using an enzymatic TAG determination kit (TR0100, Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instructions. This approach measured total tissue glycerol content, which is the sum of both TAG-associated and free glycerol.
Thin layer chromatography.
Forty to fifty milligrams of liver were homogenized in 500 μl of buffer (250 mM sucrose, 50 mM Tris·HCl pH 7.4). Lipids from homogenized samples were extracted in a 2:1 mixture of chloroform and methanol (15), adsorbed onto silica-coated glass slides, and then separated along with serial dilutions of similarly dissolved TAG standards by thin layer chromatography (TLC) using a solvent containing hexane-diethyl ether-acetic acid (80:20:2, vol/vol). This approach allows for direct assessment of tissue TAG levels.
Oil-red-O staining.
Liver sections from mice treated with control and AgRP-DsiRNAs were prepared and stained with Oil-Red-O (Sigma-Aldrich, St. Louis, MO), which stains neutral lipids, including TAG. The protocol for visualizing and quantifying Oil-Red-O staining intensity in liver sections was based on that by Mehlem et al. (30).
Tissue protein content, for normalization, was quantified with Bradford Reagent (B6916, Sigma-Aldrich, St. Louis, MO). Plasma TAG levels were measured using the same determination kit that was used for liver samples. Plasma free fatty acids were measured by the University of California-Davis Mouse Metabolic Phenotyping Center (MMPC).
Lipidomics
Liver samples were weighed and flash frozen. Lipidomic analyses were performed using mass spectrometry as previously described by Louie et al. (28). Specific lipid abundances were normalized to tissue weights and internal standards, i.e., dodecylglycerol for the positive mode and pentadecanoic acid for the negative mode.
Gene Expression Studies
Total RNA from mouse tissues was extracted using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). Gene expression was assessed by quantitative PCR using Taqman gene expression assays (Thermo Fisher Scientific, Inc.). For hypothalamic tissues, relative mRNA levels of Adora2b (mm00839292_m1) and Adora1 (mm01308023_m1) were normalized to that of 18S ribosomal RNA (Rn18S; mm03928990_g1). For liver samples, mRNA levels of the gene encoding alcohol dehydrogenase 1 (Adh1; mm00507711_m1) were normalized to that encoding beta-actin (Actb).
Western Blot Analysis
Approximately thirty milligrams from each liver sample were homogenized in 1 mL of RIPA buffer (sc-364162A), denatured by Laemmli Sample buffer (Bio-Rad no. 1610737) and beta-mercaptoethanol, and then subjected to SDS-PAGE (Bio-Rad no. 4561104) for fractionation. Gels were then electroblotted onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad no. 162-0177) at 120 mA for 100 min. Membranes were then blocked with dried nonfat milk in TBST (20 mM Tris, pH 7.6, 140 mM NaCl, and 0.1% Tween 20) and subsequently incubated with antibodies against either ADH1 (Cell Signaling no. 5295, 1:1,000) or GAPDH (Santa Cruz Biotechnology no. 25778, 1:10,000) in 5% BSA for 12 h at 4°C. After being washed three times, the membranes were incubated with an horseradish peroxidase (HRP)-conjugated anti-rabbit antibody diluted in nonfat milk (1:2,000) for 1 h. Membranes were then washed with TBST five times and developed with the SuperSignal West Pico Chemiluminescent Substrate (Thermoscientific no. 34079) per manufacturer’s protocol. Protein bands were analyzed and quantified using ImageJ.
Immunofluorescence
AgRP-Cre; Adora2bflox/flox mice were anesthetized with ketamine/xylazine and perfused with 4% (wt/vol) paraformaldehyde (PFA). Brains were excised and postfixed in 4% PFA solution for 2 h at 4°C AND then transferred into a 30% sucrose solution for cryoprotection at 4°C overnight. Samples were then embedded in Shandon M-1 embedding matrix (Thermo Fisher Scientific, Inc., Carlsbad, CA) and stored at −80°C. Brain samples were sectioned using a cryostat to obtain 10-µm-thick coronal sections and double stained by simultaneous incubation at 4°C overnight with primary antibodies against c-Fos (1:4,000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and AgRP (1:1,000, Neuromics, Minneapolis, MN). Sections were then washed and incubated at room temperature for 1 h with secondary donkey anti-rabbit Alexa 594 and donkey anti-goat Alexa 488 (1:200, Thermo Fisher Scientific, Inc.) antibodies, respectively. Nuclei were detected by counterstaining for 4′,6-diamidino-2-phenylindole (DAPI, 1:2,000) during incubation with the secondary antibody. Stained sections were prepared using Vectashield (Vector Laboratories, Burlingame, CA) mounting media. For assessing hepatic NPY expression, liver sections were immunostained with a polyclonal antibody against NPY (1:1,000, Peninsula Laboratories, San Carlos, CA) following the same experimental procedures as above.
Images were captured using an Olympus BX51WI microscope equipped with a QImaging Retiga 2000R digital camera. Images were taken with the same exposure, avoiding pixel saturation using QCapture Pro 6 (Qimaging, Surrey, BC, Canada). Only sections processed from the same experiments were compared. Image J (NIH, Bethesda, MD) was used to quantify c-Fos-positive cells. Investigators were blinded during specific cell counting from the images. Sections were matched by anatomical landmarks according to Bregma (anterior, Bregma: −1.84 mm; medial, Bregma −1.94 mm; posterior, Bregma −2.06 mm) using the Mouse Brain Atlas. At least two sections per region per mouse were quantified and then averaged. Only overlapping DAPI and c-Fos signals were used to count c-Fos-positive cell numbers.
Statistical Analysis
Statistical analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA), and specific statistical tests for different experiments are described in the figure legends. Briefly, the two-tailed Student’s t test was used to compare two independent groups of mice, and a two-way ANOVA with multiple comparisons was used when two genotypes and two treatments were compared. Repeated-measures two-way ANOVA was used to analyze data captured from sets of mice monitored repetitively over time.
RESULTS
AgRP-Deficient Mice Have Reduced Hepatic Lipid Accumulation in Response to Bingelike Alcohol Consumption
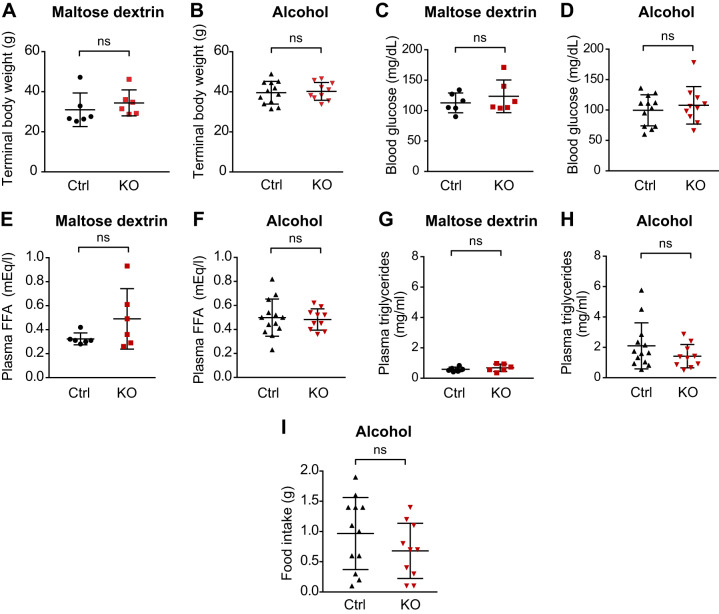
Given that alcohol consumption impacts AgRP neuronal activity and neuropeptide levels (5, 12) and that AgRP neurons are implicated in regulating hepatic lipid metabolism (29), we sought to determine if AgRP plays a role in the development of hepatic steatosis induced by acute bingelike drinking by examining mice that lack the AgRP neuropeptide (Agrp−/− mice). Agrp−/− and wild-type mice had similar body weights and terminal levels of blood glucose, free fatty acids and plasma TAG following receipt of either alcohol or maltose dextrin by oral gavage (Fig. 1, A–H). Food intake was also similar between genotypes (Fig. 1I). These data suggest that under our experimental conditions acute bingelike alcohol consumption or AgRP deficiency does not have significant impact on either energy balance or circulating indicators of metabolic function.
Fig. 1.
Acute bingelike alcohol consumption does not alter energy balance in control or AgRP-deficient mice. Agouti-related protein (AgRP)-deficient mice [Agrp−/−, knockout (KO); n = 10] and control mice (Agrp+/−, Ctrl; n = 12) were given oral gavage of alcohol (5 g ethanol per kg body wt). Another cohort of control and mutant mice was given oral gavage of isocaloric maltose dextrin (n = 6 each). Body weight (A and B), terminal blood glucose (C and D), plasma free fatty acids (E and F), plasma triglycerides (G and H), and food intake (I) were determined. Data are means ± SD. *P < 0.05; ns, nonsignificant. Student’s t test was used to compare 2 independent groups.
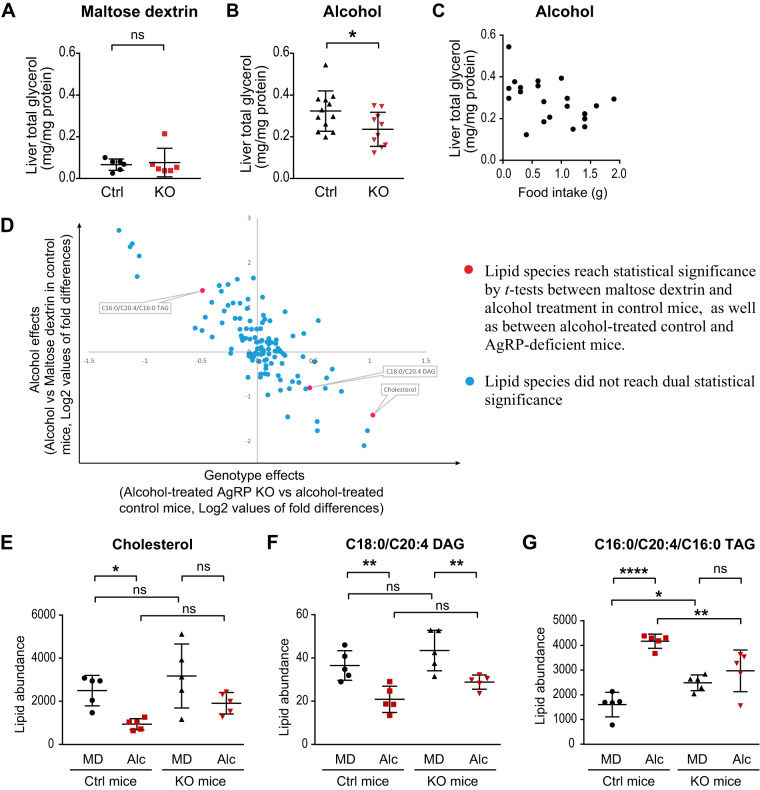
However, this was notably not the case within the liver, as Agrp−/− mice had reduced hepatic total glycerol content (both TAG-associated and free glycerol) following alcohol gavage than did control mice, whereas there were no differences between the two genotypes following gavage with isocaloric maltose dextrin (Fig. 2, A and B). There was no correlation between rates of food intake and hepatic glycerol content (Fig. 2C). Moreover, this protection was not likely due to gross alterations in systemic ethanol metabolism, as both mutant and control mice had similar levels of blood alcohol and hepatic alcohol dehydrogenase (ADH1), assessed by both mRNA and protein expression (Supplemental Fig. S1, A–D; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.11786766).
Fig. 2.
Agouti-related protein (AgRP)-deficient mice have reduced hepatic steatosis in response to bingelike alcohol consumption. A and B: liver total glycerol content [triacylglycerol (TAG)-derived and free glycerol] of control and AgRP-knockout (KO) mice in response to oral gavage of maltose dextrin and alcohol. C: food intake did not show significant correlation with liver total glycerol. D–G: lipidomics analysis of liver tissues from maltose dextrin (MD)- or alcohol (Alc)-treated Agrp+/− control (Ctrl; n = 5) and Agrp−/− (KO; n = 5) mice. Designation of color-coded lipid species are indicated on the graph. With the use of Student’s t test, a 2-tier criterion was applied to identify lipid species that are regulated by alcohol more so in control than in KO mice. E and G: analysis of 3 significant lipid species from D by two-way ANOVA with Sidak’s multiple comparisons test. Lipid abundance represents relative values that were normalized to internal standard dodecylglycerol (positive mode), pentadecanoic acid (negative mode), and tissue weight. Data are means ± SD. *P < 0.05, **P < 0.01, ****P < 0.0001; ns, nonsignificant.
To identify specific hepatic lipid species that are affected by acute alcohol consumption in an AgRP-dependent manner, we performed lipidomic analysis on the livers of Agrp−/− and control mice that were given oral gavage of either maltose dextrin or alcohol (complete data set shown in Supplemental Table S1). Many of the 117 hepatic lipid species we examined were different in abundance following alcohol versus maltose dextrin treatment. To this end, we applied a two-tier criterion to identify lipid species that are regulated by alcohol consumption more so in control than in mutant mice. First, we identified lipid species that were significantly up- or downregulated in alcohol-treated, versus maltose dextrin-treated, control mice. Next, we asked whether the levels of any of these lipid species were significantly different between alcohol-treated control and Agrp−/− mice. Three lipid species, namely C16:0/C20:4/C16:0 TAG (1 of the 6 different TAGs), C18:0/C20:4 diacylglycerol (DAG), and cholesterol (red dots in Fig. 2D) met this criterion. Upon reanalyzing these three lipid species by two-way ANOVA with Sidak’s multiple comparisons test, we found that whereas both hepatic cholesterol and C18:0/C20:4 DAG levels were reduced by alcohol treatment more profoundly in control, versus mutant mice, the absolute hepatic levels of these species were not different between the two genotypes in the context of alcohol treatment (Fig. 2, E and F). By contrast, hepatic C16:0/C20:4/C16:0 TAG content was found to be upregulated by bingelike alcohol consumption in control, but not in Agrp−/− mice and also to have a reduced absolute abundance in alcohol-treated Agrp−/− versus control mice (Fig. 2G). These data together suggest that AgRP mediates the ability of alcohol to alter hepatic levels of C16:0/C20:4/C16:0 TAG, which is a highly abundant hepatic TAG.
Downregulation of AgRP by RNA-Interference Reproduces the Protection against Alcohol-Induced Hepatic Steatosis Seen in Agrp−/− Mice
One caveat to using Agrp−/− mice is the potential for developmental compensation that could confound phenotypic outcomes. Therefore, we sought to knockdown AgRP function in adult animals and examine its effects on alcohol-induced hepatic steatosis. Small interfering RNAs (siRNA) are an effective tool for this purpose (51). Dicer-substrate RNAs (DsiRNAs) are chemically synthesized 27-mer RNA duplexes with a potency up to 100-fold greater than that of traditional siRNAs (22). Notably, peripheral injection of DsiRNAs against AgRP led to specific knockdown of hypothalamic Agrp but not Npy expression (47). This is likely due to the fact that the majority of AgRP neurons are uniquely situated outside the blood-brain barrier, allowing them to readily take up circulating substances (39, 55). To further evaluate the role of AgRP in alcohol-induced hepatic steatosis, we therefore used this DsiRNA approach to acutely knockdown AgRP expression in C57BL/6 mice, an inbred strain sensitive to alcohol.
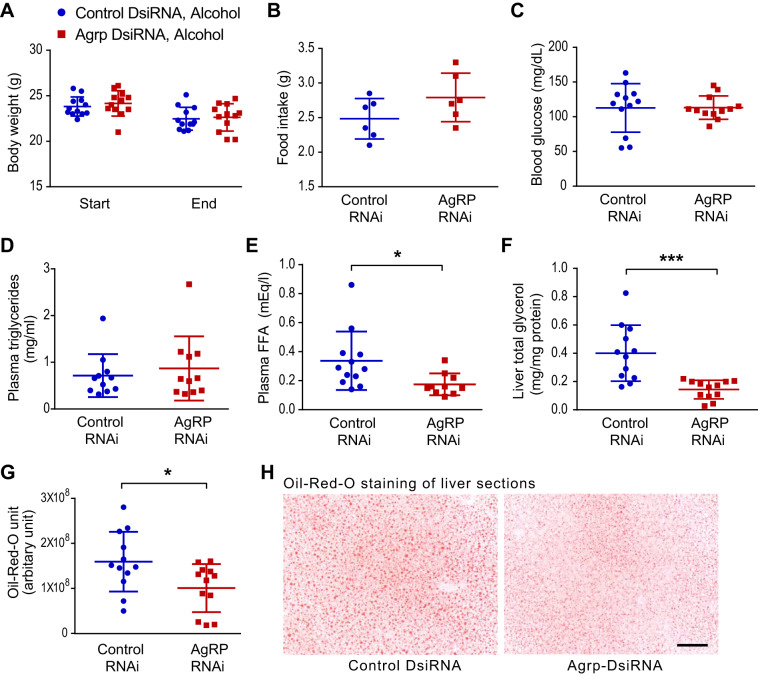
Eight-week-old male C57BL/6J mice were divided into two weight-matched groups and housed under thermoneutral conditions (30°C) to minimize any potential confounding effects of AgRP knockdown on thermogenesis and consequent energy balance. The subsequent treatment protocol took place over two successive days. At both 7 PM on day 1 and 7 AM on day 2, the mice were intraperitoneally injected with either Agrp-DsiRNA or negative control (NC DsiRNA) and then a dose of alcohol by oral gavage. Food intake and blood glucose levels were measured, and tissues were harvested 10 h after the second treatment. Liver lipid content was analyzed using a colorimetric TAG assay from homogenized liver lysates. When compared with NC-DsiRNA, Agrp-DsiRNA treatment did not impact body weight, food intake, blood glucose, or plasma TAG levels (Fig. 3, A–D). However, it reduced both the concentration of plasma free fatty acids and hepatic total glycerol content (TAG-derived and free glycerol) in the setting of alcohol treatment (Fig. 3, E and F). The findings obtained by analyzing total hepatic glycerol content were corroborated by histological data, as the intensity of Oil-Red-O staining, which marks neutral lipids (notably including TAG), was also lower in the liver sections of alcohol-treated Agrp-DsiRNA-treated mice (Fig. 3, G and H). Moreover, direct analysis of hepatic TAG levels resolved by TLC showed a similar trend indicative of reduced acute alcohol-induced hepatic steatosis in the absence of AgRP (Supplemental Fig. S2). Thus acutely knocking down AgRP in adult mice reproduces the protection against alcohol-induced fatty liver seen in Agrp−/− mice.
Fig. 3.
Knockdown of agouti-related protein (AgRP) function by RNA-interference reduces hepatic steatosis induced by bingelike alcohol consumption. Male C57BL6/J mice at 8 wk of age were divided into 2 weight-matched groups (n = 12 in each group) and 2 mice per cage. One group of mice were given intraperitoneal injections of Agrp-DsiRNA (5 μg per mouse) while the other group received negative control (5 μg per mouse). All mice received alcohol via oral gavage (5 g ethanol per kg body wt). Mice were placed at 30°C thermoneutrality during the entire experiment. Two DsiRNA injections and alcohol gavages were given at 7 PM day 1 and 7 AM day 2. Liver tissues were harvested 10 h after the second injection and alcohol gavage. When compared with negative control DsiRNA, treatment with Agrp-DsiRNA did not significantly altered body weight (A), food intake (B), terminal blood glucose (C), or plasma triglyceride levels (D) but resulted in reduced plasma free fatty acid concentrations (E) and liver total glycerol content [triacylglycerol (TAG)-derived and free glycerol] (F). Levels of Oil-Red-O-positive neutral lipids were lower in liver sections from Agrp-DsiRNA-treated mice (G and H). Scale bar = 100 μm. Data are means ± SD. *P < 0.05, ***P < 0.001. Student’s t test was used to compare 2 independent groups.
Adenosine Signaling in the CNS via the A2B Receptor Limits Hepatic Steatosis Induced by Bingelike Drinking
Mice with deletion of the A2B gene (Adora2b) are also protected against the development of alcoholic fatty liver (43). To determine if this protection is mediated within the CNS, we generated mice lacking Adora2b specifically in the CNS by crossing Adora2b flox/flox mice with Tg.nestin-Cre mice (CNS-Adora2b−/−). Since mice expressing the Tg.nestin-Cre transgene are known to have a metabolic phenotype (4), we used control mice that also expressed this transgene (Tg.nestin-Cre, Adora2b flox/+ mice).
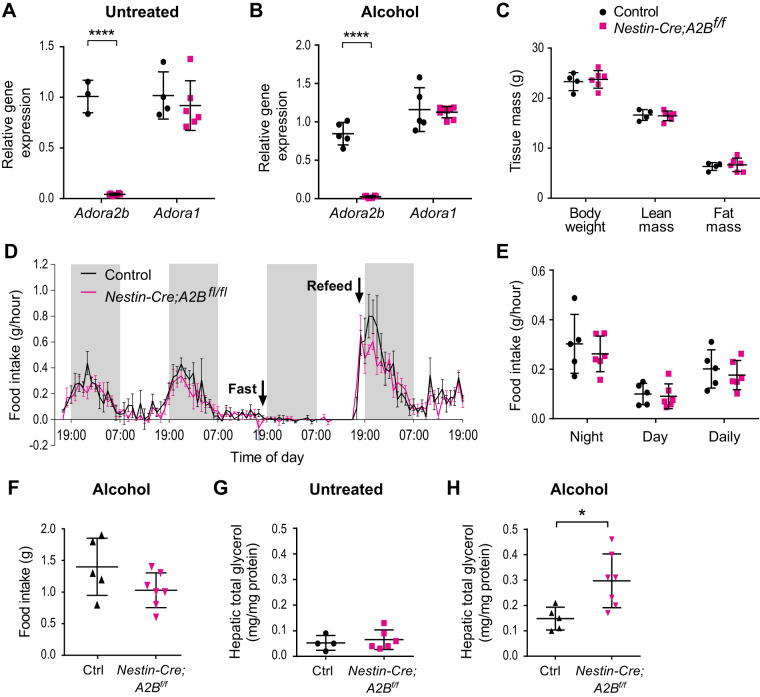
We confirmed the desired specificity of the knockout by observing that hypothalamic Adora2b mRNA expression was abolished in CNS-Adora2b−/− mice, both at baseline and after the alcohol gavage protocol, whereas Adora1 mRNA expression was not affected (Fig. 4, A and B).
Fig. 4.
Adenosine signaling via A2B in the central nervous system (CNS) modulates hepatic steatosis induced by bingelike drinking. A and B: mRNA expression of Adora2b and Adora1 in the hypothalamus of Nestin-Cre; A2Bfl/fl and control mice, either untreated or gavaged with alcohol as indicated. C: body weight and body composition. Mice were weighed at 20–23 wk of age, and body composition was assessed using EchoMRI one day before tissue harvest. D: ad libitum food intake and fasting-refeeding of Nestin-Cre; A2Bfl/fl and control mice during a 4-day measurement in comprehensive laboratory animal monitoring system (CLAMS). Shaded areas represent dark cycle (1900–0700). Animals were fasted toward the dark phase (1800) and food was introduced again after 24 h (n = 5–6, 12–15 wk of age). Arrows indicate time of fasting and refeeding. E: average hourly food intake at nighttime (1900–0700), daytime (0700–1900), and whole day (0700–0700). F: food intake of control and mutant mice during alcohol treatment. G and H: hepatic glycerol [triacylglycerol (TAG)-released and free glycerol] levels in mice that were either untreated or received 2 alcohol binges. All mice were male. *P < 0.05, ****P < 0.0001. Data are means ± SD in A–C and E–H, as determined by unpaired Student’s t test. Data are means ± SE in D; differences were determined by two-way ANOVA repeated measures.
CNS-Adora2b−/− mice showed no alteration in body weight, lean mass, fat mass, respiratory exchange ratio (RER), or energy expenditure (Fig. 4C and Supplemental Fig. 3) nor any changes in ad libitum food intake, as measured by CLAMS (Fig. 4, D and E), when compared with control mice. Although CNS-Adora2b−/− mice did exhibit a nonsignificant trend toward reduced food intake during the initial hours of refeeding (Fig. 4D), their food intake was not different from that of control mice upon exposure to alcohol (Fig. 4F).
By contrast, whereas total hepatic glycerol content (both TAG-associated and free glycerol) was similar between CNS-Adora2b−/− and control mice at baseline (Fig. 4G), CNS-Adora2b−/− mice had significantly higher total hepatic glycerol content after acute alcohol consumption (Fig. 4H). These results suggest that, independent of caloric or macronutrient consumption, A2B signaling in the CNS acts to limit the ability of bingelike alcohol consumption to induce acute hepatic steatosis.
Deletion of Adora2b in AgRP Neurons Is Not Sufficient to Alter Alcohol-Induced Hepatic Steatosis
We next explored the possibility that A2B signaling plays a role in the mechanism by which AgRP neurons impact alcohol-induced hepatic steatosis. To this end, we generated mice that lack Adora2b specifically in AgRP neurons (AgRP-Adora2b−/−). To assess the effects of A2B deficiency on neuronal functions, male control and AgRP-Adora2b−/− mice were euthanized in the late afternoon after a 9-h daytime fast. The mice were then perfused, hypothalamic sections were prepared, and c-Fos expression was examined by immunofluorescence analysis. Fasted AgRP-Adora2b−/− mice had reduced c-Fos expression specifically in the mediobasal arcuate nucleus (ARC) but not in the dorsomedial hypothalamus (DMH) (Supplemental Fig. S4, A–C) Despite this, however, AgRP-Adora2b−/− and control mice had no differences in body weight, body composition (lean and fat mass), food intake, energy expenditure, RER, or locomotor activity (Supplemental Fig. S4D and Supplemental Fig. S5).
To assess the impact of A2B signaling specifically in AgRP neurons on alcohol-induced hepatic steatosis, adiposity-matched AgRP-Adora2b−/− and control mice were given oral gavages of alcohol per the protocol described above. There were no differences in hepatic total glycerol content (TAG-associated and free glycerol) between the two genotypes, either at baseline (untreated) or in response to alcohol gavage (Supplemental Fig. S4, E and F) suggesting that abrogating A2B signaling in AgRP neurons is not sufficient to alter the acute effects of alcohol on hepatic steatosis. Given that we also found that brain-specific removal of Adora2b (CNS-Adora2b−/−) increases the sensitivity to alcohol-induced fatty liver, our data with AgRP-Adora2b−/− mice open up the possibility that A2B signaling may influence alcohol-induced liver fat accumulation either by acting directly on other components of AgRP neuronal circuitry beyond AgRP neurons themselves or on parallel circuits that do not include AgRP neurons.
Chemical Sympathectomy Attenuates the Extent of Hepatic Steatosis Induced by Bingelike Alcohol Consumption
Our results, probing both AgRP and A2B, support the concept that the brain plays an integral role to the development of binge alcohol-induced fatty liver. The brain regulates peripheral lipid metabolism by modulating the function of the autonomic nervous system innervating peripheral metabolic tissues. In particular, the AgRP neuropeptide inhibits sympathetic nervous system (SNS) activity in both the white adipose tissue and liver (29, 38). To this end, we sought to determine if intact SNS function is required for the development of hepatic steatosis induced by bingelike alcohol consumption.
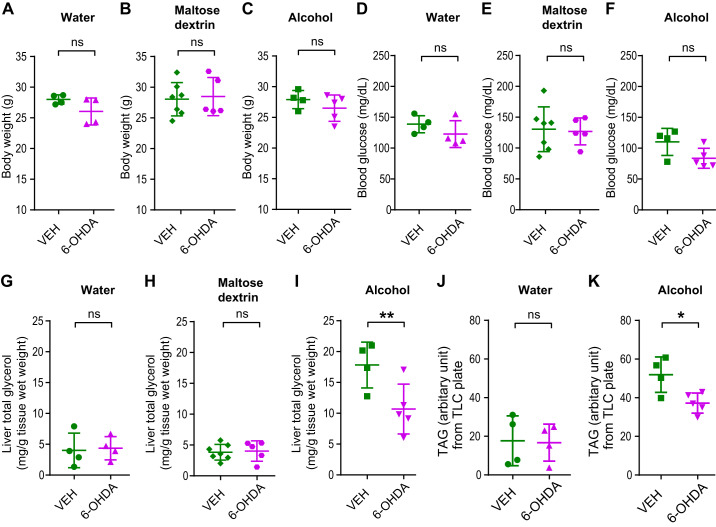
Fifteen- to sixteen-week-old male C57BL/6J mice underwent chemical sympathectomy by injection with a single dose of 6-hydroxydopamine (6-OHDA, 250 mg/kg) or vehicle control (ascorbic acid). The effectiveness of this treatment was validated by confirming the complete ablation of NPY-positive sympathetic fibers in the liver of 6-OHDA-treated mice in pilot experiments (Supplemental Fig. S6). Ten days after 6-OHDA or vehicle injection, weight-matched mice were given a single oral gavage of alcohol. To minimize the likelihood of potential confounds resulting from any effects of SNS activity on carbohydrate metabolism, we opted to use gavage with water in a separate cohort of mice as a second control in addition to isocaloric maltose dextrin in this experiment. After alcohol or control treatment, food was subsequently removed from the cages housing the mice, and their livers were harvested 8 h later. 6-OHDA treatment affected body weight and blood glucose similarly when mice were treated with water, alcohol, or isocaloric maltose dextrin (Fig. 5, A–F). However, whereas chemical sympathectomy did not affect hepatic TAG content in water- or maltose dextrin-treated mice, it reduced hepatic TAG content in alcohol-treated mice, indicating that an intact SNS is needed for the full ability of acute alcohol consumption to induce hepatic steatosis (Fig. 5, G–K, and Supplemental Figs. S7 and S8).
Fig. 5.
Chemical sympathectomy partially blocks the development of hepatic steatosis induced by bingelike alcohol consumption. Fifteen- to sixteen-week-old male C57BL/6J mice underwent chemical sympathectomy by injection with a single dose of 6-hydroxydopamine (6-OHDA; 250 mg/kg in 0.9% NaCl and 10–7 M ascorbic acid, Sigma-Aldrich, St. Louis, MO) or vehicle control (0.9% NaCl and 10–7 M ascorbic acid). After recovery, weight-matched mice were given a single oral gavage of water, maltose dextrin (9 g/kg body wt), or alcohol (5 g/kg body wt). Food was subsequently removed, and liver tissues were harvested 8 h later. 6-OHDA treatment affected body weight and blood glucose equally in water, maltose dextrin, or alcohol-gavaged mice (A–F). Sympathectomy by 6-OHDA did not affect total liver glycerol content [triacylglycerol (TAG) -derived and free glycerol] in water- or maltose dextrin-gavaged mice but caused a reduction in alcohol-gavaged mice (G–I). Similar results were seen with extracted and thin layer chromatography (TLC)-resolved TAG from livers (J and K). Data are means ± SD. *P < 0.05; **P < 0.01; ns, nonsignificant, by two-tailed Student’s t test.
DISCUSSION
Binge drinking is prevalent, is costly, and has serious health consequences (20, 53). Hepatic steatosis develops quickly after acute binge drinking (21, 24, 48), and marks an early stage of liver injury. Alcohol-induced hepatic steatosis is currently understood to be mediated by peripheral mechanisms, such as the direct toxic effects of alcohol in the liver and adipose tissues. In this study, we provide multiple lines of evidence to support the concept that the brain and SNS play important modulatory roles in the full development of hepatic steatosis after bingelike alcohol consumption. Specifically, we show that hypothalamic AgRP, CNS adenosine signaling, and an intact SNS are integral components of a regulatory program impacting hepatic TAG accumulation in the context of acute alcohol consumption.
AgRP neurons have previously been reported to mediate behavioral consequences of alcohol consumption. For example, AgRP infusion into the brain promotes alcohol drinking, whereas AgRP deficiency reduces alcohol-reinforced lever-pressing and bingelike alcohol drinking (33, 35, 36). Here we show that AgRP is required for the full development of acute alcohol-induced hepatic steatosis, an indicator of the role of AgRP in mediating key metabolic consequences of alcohol consumption. Importantly, given that oral alcohol was provided by gavage to both the control and mutant mice, we were able to ensure that any genotype-specific effects we observed were not due to differences in alcohol consumption between groups. To this end, we also found that AgRP deficiency not only diminished the effects of alcohol on hepatic TAG content but also on hepatic cholesterol, although potentially to a lesser extent. In this regard, our findings align with other data suggesting that alcohol drinking impacts both TAG and cholesterol metabolism (8, 14, 18) and that fatty acid and cholesterol synthesis are often coordinately regulated in the liver (19).
It is worth noting that RNAi-mediated knockdown of AgRP expression in adult mice led to a more pronounced reduction of hepatic TAG levels in the context of acute alcohol administration when compared with that seen in mice with germline AgRP-deficiency. This could in part be due to potential compensatory changes induced in Agrp−/− mice due to the absence of AgRP during early development. It could also be attributed to difference in genetic background, as RNAi-treated mice were on a purer C57BL/6J background, which is susceptible to alcohol-induced hepatic steatosis, whereas the Agrp−/− mice were only partially (5–6 generations) backcrossed onto the C57BL/6J background.
One potential caveat of examining the role of AgRP neurons in alcohol-induced liver fat accumulation involves our use of maltose dextrin as an “isocaloric” control agent for comparison against alcohol. It has been recently shown that activation of hypothalamic AgRP neurons increases carbohydrate utilization while decreasing fat utilization (7). As such, maltose dextrin, a carbohydrate, could potentially have exerted independent metabolic effects on hepatic lipid through CNS mechanisms, including those potentially involving AgRP neurons. Although this was mitigated in our studies using 6-OHDA by examining a third, water-treated group of mice alongside those treated with maltose dextrin and alcohol, respectively, future studies may want to utilize isocaloric controls that employ either a noncarbohydrate macronutrient as an additional control or multiple controls employing different carbohydrates. It is technically challenging, however, to develop a variety of controls with similar caloric density to alcohol, to ensure that the volumes of the various gavage agents would be comparable.
The mechanisms by which alcohol regulates AgRP neuronal function remain to be determined. Alcohol exposure rapidly increases hypothalamic AgRP immunoreactivity in C57BL/6J mice (12). Alcohol also increases the activity of AgRP neurons in hypothalamic brain slices (5), suggesting that alcohol may act directly in the brain to regulate AgRP neurons, rather than only doing so indirectly through a peripheral mechanism. Acute alcohol exposure inhibits the activity of equilibrative nucleoside transporter 1 (ENT1) in the brain, leading to an elevation of extracellular adenosine levels (10). As adenosine is a potent neuromodulator (44) and mice lacking the adenosine receptors A1 or A2B are resistant to alcohol-induced hepatic steatosis (43), we evaluated if A2B in the CNS is involved in development of alcohol-induced hepatic steatosis. Deleting Adora2b in the CNS abolished hypothalamic Adora2b mRNA expression, confirming that Adora2b is normally expressed in the hypothalamus. Interestingly, we show that abolishing A2B signaling in the brain potentiates the ability of alcohol consumption to acutely increase liver fat in mice, which could be attributed to overcompensation by other adenosine receptors such as A1 receptor. It should also be acknowledged that Tg.Nestin-Cre:A2B+/+ mice would have been better controls, as the use of Tg.Nestin-Cre, A2B+/− as controls would presumably lead to an underestimate of the phenotypes.
By contrast, our results show that removing A2B only in AgRP neurons does not alter alcohol-induced hepatic steatosis, suggesting that A2B signaling in non-AgRP neurons are responsible for alcohol’s regulatory effects in this context. For example, alcohol also targets POMC neurons (9, 25, 34, 42). Adolescent bingelike alcohol consumption or chronic consumption of alcohol-containing diet reduces hypothalamic α-MSH immunoreactivity (25, 34). The opposing regulatory effects of alcohol on POMC and AgRP neurons support the notion that these neurons are both CNS targets of alcohol, perhaps contributing to alcohol’s metabolic effects in a reciprocal or otherwise integrated manner. Indeed, it is possible that adenosine signaling in POMC neurons, and potentially others, may compensate for the loss of A2B signaling solely in AgRP neurons.
One possible mechanism by which AgRP regulates alcohol-induced hepatic steatosis is via modulating the autonomic nervous system innervating the liver and the adipose tissues. AgRP inhibits sympathetic activity in the white adipose tissue to limit lipolysis (38). We have also shown that intracerebroventricular infusion of AgRP suppresses sympathetic activity in the liver (29). Moreover, AgRP neurons mediate the stimulatory effects of leptin on sympathetic activity in the adipose tissue and liver (2). Thus diminished AgRP function in the brain likely disrupts autonomic function in liver and white adipose tissues, lessening their response to alcohol-induced adipose tissue lipolysis as well as hepatic lipid synthesis. Although our findings suggest that chemical sympathectomy abrogated the ability of alcohol consumption to increase liver fat content by disrupting the mechanism by which AgRP neurons act to stimulate hepatic TAG accumulation, we cannot rule out the possibility that ablating the SNS could also have reduced liver fat content in the context of acute alcohol consumption by other indirect mechanisms.
Taken together, our study indicates that the brain not only underlies the effects of alcohol on cognitive, psychological, and locomotor functions but also mediates the metabolic effects of alcohol, specifically the acute development of hepatic steatosis induced by bingelike drinking. Our results further suggest that alcohol exerts its acute pathological effects, at least in part, by impinging on the central melanocortin system, which in addition to functioning as a key regulator of energy balance, is emerging as an important regulator of peripheral lipid metabolism.
GRANTS
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grant 1R01-AA-022665 (to A.W.X.) and in part by the Joseph and Vera Long Foundation (to A.W.X.). The work was in part supported by National Heart, Lung, and Blood Institute Grants P0I-HL-114457, R01-HL109233, R01-HL119837, and R01-HL133900 and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK-097075, R01-DK109574 (to H.K.E.), as well as American Heart Association Career Development Award 19CDA34660279 and American Thoracic Society Unrestricted Grant to (to X.Y). The work is also partly supported by the Mouse Metabolism Core at the University of California, San Francisco (UCSF) Nutrition Obesity Center (NIDDK Grant 1P30-DK-098722-01A1), the Microscopy Core at the UCSF Diabetes Endocrinology Research Center (NIDDK Grant P30-DK-63720-06A1), and the UCSF Liver Center (NIDDK P30-DK-026743).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.I., M.T.M., E.Y., L.P., R.C., A.V., S.M.L., S.A.W., X.Y., and A.W.X. performed experiments; M.I., M.T.M., E.Y., L.P., R.C., A.V., S.M.L., S.A.W., X.Y., D.K.N., and A.W.X. analyzed data; M.I., M.T.M., E.Y., L.P., R.C., A.V., X.Y., F.W.H., D.K.N., S.K.K., and A.W.X. interpreted results of experiments; M.I., M.T.M., and A.W.X. prepared figures; M.I., M.T.M., and A.W.X. drafted manuscript; M.I., M.T.M., H.K.E., F.W.H., S.K.K., and A.W.X. edited and revised manuscript; M.I., M.T.M., E.Y., L.P., R.C., A.V., S.M.L., S.A.W., X.Y., H.K.E., F.W.H., D.K.N., S.K.K., and A.W.X. approved final version of manuscript; A.W.X. conceived and designed research.
REFERENCES
- 1.Beier JI, Arteel GE, McClain CJ. Advances in alcoholic liver disease. Curr Gastroenterol Rep 13: 56–64, 2011. doi: 10.1007/s11894-010-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell BB, Harlan SM, Morgan DA, Guo DF, Cui H, Rahmouni K. Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin. Mol Metab 8: 1–12, 2018. [Erratum in Mol Metab 14: 158, 2018.] doi: 10.1016/j.molmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8: 627–637, 2013. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briancon N, McNay DE, Maratos-Flier E, Flier JS. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes 59: 3074–3084, 2010. doi: 10.2337/db10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cains S, Blomeley C, Kollo M, Rácz R, Burdakov D. Agrp neuron activity is required for alcohol-induced overeating. Nat Commun 8: 14014, 2017. [Erratum in Nat Commun 8: 15668, 2017.] doi: 10.1038/ncomms14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael FJ, Israel Y, Crawford M, Minhas K, Saldivia V, Sandrin S, Campisi P, Orrego H. Central nervous system effects of acetate: contribution to the central effects of ethanol. J Pharmacol Exp Ther 259: 403–408, 1991. [PubMed] [Google Scholar]
- 7.Cavalcanti-de-Albuquerque JP, Bober J, Zimmer MR, Dietrich MO. Regulation of substrate utilization and adiposity by Agrp neurons. Nat Commun 10: 311, 2019. doi: 10.1038/s41467-018-08239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CC, Lin WY, Li CI, Liu CS, Li TC, Chen YT, Yang CW, Chang MP, Lin CC. The association of alcohol consumption with metabolic syndrome and its individual components: the Taichung community health study. Nutr Res 32: 24–29, 2012. doi: 10.1016/j.nutres.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen CP, Boyadjieva NI, Advis JP, Sarkar DK. Ethanol suppression of the hypothalamic proopiomelanocortin level and the splenic NK cell cytolytic activity is associated with a reduction in the expression of proinflammatory cytokines but not anti-inflammatory cytokines in neuroendocrine and immune cells. Alcohol Clin Exp Res 30: 1925–1932, 2006. doi: 10.1111/j.1530-0277.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci 7: 855–861, 2004. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JI, Nagy LE. Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J Dig Dis 12: 3–9, 2011. doi: 10.1111/j.1751-2980.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cubero I, Navarro M, Carvajal F, Lerma-Cabrera JM, Thiele TE. Ethanol-induced increase of agouti-related protein (AgRP) immunoreactivity in the arcuate nucleus of the hypothalamus of C57BL/6J, but not 129/SvJ, inbred mice. Alcohol Clin Exp Res 34: 693–701, 2010. doi: 10.1111/j.1530-0277.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohrman DP, Diamond I, Gordon AS. The role of the neuromodulator adenosine in alcohol’s actions. Alcohol Health Res World 21: 136–143, 1997. [PMC free article] [PubMed] [Google Scholar]
- 14.Fan AZ, Russell M, Dorn J, Freudenheim JL, Nochajski T, Hovey K, Trevisan M; The Western New York Health Study (WNYHS) . Lifetime alcohol drinking pattern is related to the prevalence of metabolic syndrome. Eur J Epidemiol 21: 129–138, 2006. doi: 10.1007/s10654-005-5457-y. [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 16.Fredholm BB. AP IJ, Jacobson KA, Linden J, and Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev 63: 1–34, 2011. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol 63: 191–270, 2005. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 18.Freiberg MS, Cabral HJ, Heeren TC, Vasan RS, Curtis Ellison R; Third National Health and Nutrition Examination Survey . Alcohol consumption and the prevalence of the Metabolic Syndrome in the US.: a cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care 27: 2954–2959, 2004. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 19.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109: 1125–1131, 2002. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD. Annual total binge drinks consumed by U.S. adults, 2015. Am J Prev Med 54: 486–496, 2018. doi: 10.1016/j.amepre.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khanna JM, Kalant H, Loth J, Seymour F. Effect of 4-methylpyrazole and pyrazole on the induction of fatty liver by a single dose of ethanol. Biochem Pharmacol 23: 3037–3043, 1974. doi: 10.1016/0006-2952(74)90279-2. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol 23: 222–226, 2005. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- 23.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Korkusuz H, Keese D, Raschidi BA, Hübner F, Namgaladze D, Hintereder G, Hammerstingl R, Korkusuz Y, Mönch C, Vogl TJ. Detection of a fatty liver after binge drinking: correlation of MR-spectroscopy, DECT, biochemistry and histology in a rat model. Acad Radiol 18: 1349–1357, 2011. doi: 10.1016/j.acra.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Lerma-Cabrera JM, Carvajal F, Alcaraz-Iborra M, de la Fuente L, Navarro M, Thiele TE, Cubero I. Adolescent bingelike ethanol exposure reduces basal α-MSH expression in the hypothalamus and the amygdala of adult rats. Pharmacol Biochem Behav 110: 66–74, 2013. doi: 10.1016/j.pbb.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34: 9–19, 2004. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Lindtner C, Scherer T, Zielinski E, Filatova N, Fasshauer M, Tonks NK, Puchowicz M, Buettner C. Binge drinking induces whole-body insulin resistance by impairing hypothalamic insulin action. Sci Transl Med 5: 170ra14, 2013. doi: 10.1126/scitranslmed.3005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louie SM, Grossman EA, Crawford LA, Ding L, Camarda R, Huffman TR, Miyamoto DK, Goga A, Weerapana E, Nomura DK. GSTP1 is a driver of triple-negative breast cancer cell metabolism and pathogenicity. Cell Chem Biol 23: 567–578, 2016. doi: 10.1016/j.chembiol.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier MT, Vilhelmsson A, Louie SM, Vagena E, Nomura DK, Koliwad SK, Xu AW. Regulation of hepatic lipid accumulation and distribution by agouti-related protein in male mice. Endocrinology 159: 2408–2420, 2018. doi: 10.1210/en.2018-00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 8: 1149–1154, 2013. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 31.Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem 265: 1946–1951, 1990. [PubMed] [Google Scholar]
- 32.Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S, Lowell BB, Krashes MJ, Wess J. Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun 7: 10268, 2016. [Erratum in Nat Commun 7: 11019, 11019.] doi: 10.1038/ncomms10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE. Effects of melanocortin receptor activation and blockade on ethanol intake: a possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res 29: 949–957, 2005. doi: 10.1097/01.ALC.0000167740.19702.8C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro M, Cubero I, Knapp DJ, Breese GR, Thiele TE. Decreased immunoreactivity of the melanocortin neuropeptide alpha-melanocyte-stimulating hormone (alpha-MSH) after chronic ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res 32: 266–276, 2008. doi: 10.1111/j.1530-0277.2007.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83-132). Neuropeptides 37: 338–344, 2003. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Navarro M, Cubero I, Ko L, Thiele TE. Deletion of agouti-related protein blunts ethanol self-administration and bingelike drinking in mice. Genes Brain Behav 8: 450–458, 2009. doi: 10.1111/j.1601-183X.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther 109: 227–237, 2006. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488, 2007. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci USA 110: E697–E706, 2013. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orrego H, Carmichael FJ, Israel Y. New insights on the mechanism of the alcohol-induced increase in portal blood flow. Can J Physiol Pharmacol 66: 1–9, 1988. doi: 10.1139/y88-001. [DOI] [PubMed] [Google Scholar]
- 41.Parker R, Kim SJ, Gao B. Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nat Rev Gastroenterol Hepatol 15: 50–59, 2018. doi: 10.1038/nrgastro.2017.116. [DOI] [PubMed] [Google Scholar]
- 42.Pastorcic M, Boyadjieva N, Sarkar DK. Comparison of the effects of alcohol and acetaldehyde on proopiomelanocortin mRNA levels and beta-endorphin secretion from hypothalamic neurons in primary cultures. Mol Cell Neurosci 5: 580–586, 1994. doi: 10.1006/mcne.1994.1071. [DOI] [PubMed] [Google Scholar]
- 43.Peng Z, Borea PA, Wilder T, Yee H, Chiriboga L, Blackburn MR, Azzena G, Resta G, Cronstein BN. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest 119: 582–594, 2009. [Erratum in J Clin Invest 119: 1052, 2009.] doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruby CL, Adams CA, Knight EJ, Nam HW, Choi DS. An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev 3: 163–174, 2010. doi: 10.2174/1874473711003030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol 26: 1089–1105, 2011. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- 46.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol 113: 175–194, 2018. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas MA, Tran V, Ryu V, Xue B, Bartness TJ. AgRP knockdown blocks long-term appetitive, but not consummatory, feeding behaviors in Siberian hamsters. Physiol Behav 190: 61–70, 2018. doi: 10.1016/j.physbeh.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wada S, Yamazaki T, Kawano Y, Miura S, Ezaki O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J Hepatol 49: 441–450, 2008. doi: 10.1016/j.jhep.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Wegner SA, Pollard KA, Kharazia V, Darevsky D, Perez L, Roychowdhury S, Xu A, Ron D, Nagy LE, Hopf FW. Limited excessive voluntary alcohol drinking leads to liver dysfunction in mice. Alcohol Clin Exp Res 41: 345–358, 2017. doi: 10.1111/acer.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei X, Shi X, Zhong W, Zhao Y, Tang Y, Sun W, Yin X, Bogdanov B, Kim S, McClain C, Zhou Z, Zhang X. Chronic alcohol exposure disturbs lipid homeostasis at the adipose tissue-liver axis in mice: analysis of triacylglycerols using high-resolution mass spectrometry in combination with in vivo metabolite deuterium labeling. PLoS One 8: e55382, 2013. doi: 10.1371/journal.pone.0055382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 8: 129–138, 2009. [Erratum in Nat Rev Drug Discov 8: 516, 2009 and 9: 412, 2010.] doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang L, Qi Y, Yang Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep 11: 798–807, 2015. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Yoon Y, Chen CM. Liver Cirrhosis Mortality In The United States: National, State, And Regional Trends, 2000–2013. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism Division of Epidemiology and Prevention Research Alcohol Epidemiologic Data System, 2016: p. 1–69. [Google Scholar]
- 54.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol 287: G1–G6, 2004. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 55.Yulyaningsih E, Rudenko IA, Valdearcos M, Dahlén E, Vagena E, Chan A, Alvarez-Buylla A, Vaisse C, Koliwad SK, Xu AW. Acute lesioning and rapid repair of hypothalamic neurons outside the blood-brain barrier. Cell Rep 19: 2257–2271, 2017. doi: 10.1016/j.celrep.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health 29: 245–254, 2006. [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol 180: 998–1007, 2012. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]