Abstract
The global prevalence of type 2 diabetes (T2D) has doubled since 1980. Human epidemiological studies support arsenic exposure as a risk factor for T2D, although the precise mechanism is unclear. We hypothesized that chronic arsenic ingestion alters glucose homeostasis by impairing adaptive thermogenesis, i.e., body heat production in cold environments. Arsenic is a pervasive environmental contaminant, with more than 200 million people worldwide currently exposed to arsenic-contaminated drinking water. Male C57BL/6J mice exposed to sodium arsenite in drinking water at 300 μg/L for 9 wk experienced significantly decreased metabolic heat production when acclimated to chronic cold tolerance testing, as evidenced by indirect calorimetry, despite no change in physical activity. Arsenic exposure increased total fat mass and subcutaneous inguinal white adipose tissue (iWAT) mass. RNA sequencing analysis of iWAT indicated that arsenic dysregulated mitochondrial processes, including fatty acid metabolism. Western blotting in WAT confirmed that arsenic significantly decreased TOMM20, a correlate of mitochondrial abundance; PGC1A, a master regulator of mitochondrial biogenesis; and, CPT1B, the rate-limiting step of fatty acid oxidation (FAO). Our findings show that chronic arsenic exposure impacts the mitochondrial proteins of thermogenic tissues involved in energy expenditure and substrate regulation, providing novel mechanistic evidence for arsenic’s role in T2D development.
Keywords: adaptive thermogenesis, arsenic, brown adipose tissue, white adipose tissue
INTRODUCTION
Type 2 diabetes (T2D) is a chronic disease, affecting 8.3% of adults worldwide (41). This metabolic disorder is characterized by hyperglycemia, insulin resistance, and abnormal insulin secretion. Epidemiological studies of people residing in Asia and North and South America show that exposure to arsenic-contaminated drinking water is associated with an increased risk of T2D (13, 37, 50, 55, 56). Arsenic is a surprisingly common contaminant of drinking water and foods, such as rice (59). It is found at levels above 50 μg/L in many drinking water supplies, including private wells in the U.S., despite drinking water standards of 10 or 50 μg/L in many countries (4, 61). Levels in some parts of the world have exceeded 800 μg/L, and more than 200 million people are exposed worldwide (35). The precise mechanism by which arsenic produces T2D is unclear, however, despite considerable research in animal models. Potential mechanisms of arsenic action span multiple tissues, including impaired glucose-stimulated insulin secretion from pancreatic beta cells, unrestrained hepatic gluconeogenesis, and impaired insulin-stimulated glucose uptake in adipose tissue (AT), among others (29, 30, 36). The effects of arsenic on other adipose tissue functions has been less examined. Indeed, an expert panel assembled at a National Toxicology Program (NTP) workshop urged researchers to explore the effects of arsenic on adipose tissue function more thoroughly (31, 53).
Given mitochondrial dysfunction is a common target of arsenic across tissues (22, 40, 55), thermogenesis is an understudied arsenic target. The uptake and oxidative metabolism of both lipids and carbohydrates are critical to the uncoupled mitochondrial respiration that defines AT thermogenesis and as such are commonly measured to approximate AT thermogenic activity (25, 42). A recent study demonstrated that mice chronically exposed to high doses of arsenic at 5 mg/L had a lowered ability to maintain their body temperature in response to acute cold exposure, coupled with impaired expression of RNA and proteins critical to adipogenesis, thermogenesis, and mitochondrial activity in their suprascapular brown adipose tissue (BAT) (68). Adult human supraclavicular BAT is normally activated by cold ambient temperature and insulin and contributes to energy expenditure (6, 34, 38). Another recent study demonstrated that mice exposed to arsenic and acute cold temperature also had reduced BAT expression of UCP1, the uncoupling protein critical to AT thermogenesis (5) (see glossary for definitions of proteins and genes). The impaired activity of adult human supraclavicular BAT has been associated with elevated body mass index, insulin insensitivity, and T2D (8, 39), findings that are supported by experimental BAT transplants and are not unlike reported arsenic associations in humans (13, 19, 36, 49).
BAT may not be the only or primary target tissue responsible for arsenic-impaired thermogenesis given the acute cold exposure used in the experiments of Zuo et al. (68) would elicit shivering thermogenesis from skeletal muscle (11) and could extend to other unexamined thermogenic tissues. For example, experimental studies of cells from the white adipose tissue (WAT) of rodents and humans demonstrate inducible thermogenic capacity of a cell population called beige adipocytes (44, 62, 67). Indeed, it has been suggested that the molecular signature of human supraclavicular AT more closely resembles the molecular signature of mouse beige adipocytes than that of mouse suprascapular brown adipocytes (27, 45, 46, 62). Despite using doses of arsenic beyond what humans would experience in most settings, and the absent survey to identify the target tissue, the effects of arsenic on BAT (5, 68) encourage the exploration of the effects of arsenic on multiple thermogenic tissues using doses of arsenic relevant to human exposure.
The research presented here aims to address this knowledge gap by clarifying the role of different AT depots in arsenic pathogenesis consistent with risk of developing T2D. We hypothesize that impaired adaptive thermogenesis is a key mechanism involved in arsenic-induced metabolic disruption, with both brown and beige adipocytes as targets of arsenic toxicity. We utilized a mouse model of arsenic exposure relevant to public health to evaluate its effects on adaptive thermogenesis. Furthermore, we used a step-wise gradient of cold exposure, rather than acute cold exposure, during indirect calorimetry assessment combined with a survey of thermogenic tissue pathology along with molecular analyses of the tissue response, to better understand the effects of arsenic on thermogenic tissues.
MATERIALS AND METHODS
Animals and treatment.
Male C57BL/6J mice (Mus musculus) were purchased at 5 wk of age from Jackson Laboratory (Bar Harbor, ME). This mouse strain is a well-accepted model for arsenic toxicity (18), as well as diet-induced obesity and insulin resistance (54), and the strain used by the Genome Reference Consortium for the mouse reference genome assembly (20). Mice were group-housed in sterile ventilated cages with Sani-Chip wood bedding (Laboratory Supply, Fort Worth, TX) on a 12-h light-dark cycle at 23°C, after acclimation for 7 days. This study included an initial cohort of 10 mice (n = 5/treatment), and a subsequent cohort of 16 mice (n = 8/treatment), to demonstrate reproducibility. Therefore, this study includes a total of 26 mice (n = 13/treatment). After 1 wk of acclimation, male mice (n = 13/treatment) were administered 300 μg/L inorganic arsenic in the form of sodium (meta)arsenite (NaAsO2, Sigma-Aldrich, purity ≥90%) for 9 wk. NaAsO2 was dissolved at a final concentration of 300 μg/L using autoclaved water. Since mice metabolize arsenic at much faster rates than humans, the administered dose of 300 μg/L is approximately equal to a human equivalent dose (HED) of 58.5 μg/L in water, based on allometric scaling approaches published by the Food and Drug Administration (58). These calculations first require converting the exposure concentration of 300 μg/L to an administered dose, based on the animal’s body weight and daily water intake, i.e., 0.300 mg/L × 2 mL water·mouse−1·day−1 = 0.6 μg·day−1·mouse−1/0.025 kg/mouse = 24 μ·kg−1·day−1. This value is subsequently calculated into a human equivalent of consumption, based on human body weight and daily water intake, i.e., 24 24 μ·kg−1·day−1 × 60 kg = 1,440 μg·day−1·humans−1/2 L·day−1·humans−1 = 720 μg/kg. Lastly, this value is converted into a final HED on the basis of body surface area, using a designated allometric scaling factor, i.e., 720 μg/L/12.3 = 58.5 μg/L (58). The dose of 300 μg/L used in our experiments therefore approximates to the drinking water standard of 50 μg/L used in many countries (64).
Both arsenic-treated and control water were freshly prepared and replaced thrice weekly to minimize oxidation from trivalent to pentavalent arsenical species. The mice were fed ad libitum purified casein-based AIN-76A chow (18.8% protein, 68.8% carbohydrate, and 12.4% fat per kcal) (Teklad Adjusted Vitamins Diet; Harlan Laboratories, Inc.). The AIN-76A diet was selected due to its minimal inorganic arsenic concentrations (<20 μg/L) compared with nonpurified chow diets to minimize biasing effects of dietary arsenic (24).
Body weights and food intake were measured weekly. Caloric intake was calculated by multiplying the weekly total weight in grams consumed by the kilocalories per gram (e.g., 3.8 kcal/g) of the AIN-76A diet consumed, each week. Water intake was measured thrice weekly to coincide with water changes. All mice were euthanized by cervical dislocation following isoflurane overdose. These methods are consistent with the American Veterinary Medical Association Guidelines for the Euthanasia of Animals and approved by both the University of California (UC) Berkeley and UC Davis Animal Care and Use Committees (ACUC), Protocols No. 2015-06-2681 and 20429, respectively. All personnel working with mice were specially trained and followed specific standard operating procedures (SOPs) as required by both ACUC and the National Institute of Health (NIH).
Respirometry via indirect calorimetry.
Respirometry was performed using ventilated, open-circuit indirect calorimetry (CLAMS, Columbus Instruments, Columbus, OH) by the UC Davis Mouse Metabolic Phenotyping Center (MMPC). Indirect calorimetry is the gold standard for assessing in vivo energy expenditure (11, 33). We housed a maximum of 16 mice (8 mice/treatment) individually in metabolic chambers with constant light and dark cycles of 12 h each. We repeated the calorimetry experiment, with a total of 13 mice/treatment. Pulverized diet (AIN-76A) and water were provided ad libitum. Oxygen consumption, carbon dioxide production, food intake, and three plane locomotion were monitored in sequential intervals (35 s). An acclimation period (>24 h) allowed the mice to adjust to their new housing conditions (54). A cold tolerance acclimation test was performed, comprising of a slow, step-wise decrease in temperature to examine impairments in nonshivering thermogenesis by preventing the prolonged muscle shivering associated with acute cold exposure (10–12). The ambient temperature of the metabolic cage environment was set to 30°C, 23°C, 12°C, and 4°C for a 24 h interval at each ambient temperature. Energy expenditure and respiratory exchange ratio (RER), a quotient of V̇co2/V̇o2, was calculated for each treatment, ambient temperature, and photoperiod. Once indirect calorimetry was completed, all mice were transferred back to their long-term vivarium and reacclimated to its ambient temperature (22°C) and standard caging for one week. As is standard practice, reacclimation to vivarium temperatures and standard housing conditions were incorporated to reduce murine stress before additional experiments were performed and henceforth cold tolerance experiments testing adaptive thermogenesis were conducted at week 6, while dissection and molecular analyses were completed at week 9 of exposure. Dissection was performed following a terminal 24 h 4°C cold exposure.
Body composition.
We measured body composition by either EchoMRI (Echo Medical Systems, Houston, TX) or dual-energy X-ray absorptiometry (DEXA) (PixiMus densitometer, GE Medical Systems, LUNAR) of each mouse at week 6 of exposure by the UC Davis MMPC before indirect calorimetry. Body weights and food intake were measured weekly. Tissues were weighed postmortem.
Pyruvate tolerance testing.
A pyruvate tolerance test was performed in cohort 2 (n = 8 mice/treatment). Eight-week-old mice were fasted for 6 h and administered an intraperitoneal injection of 2.0 g sodium pyruvate/kg mouse body wt. Blood glucose was measured from tail vein at 15, 30, 45, 60, 90, and 120 min after bolus injection. Area under the curve for the pyruvate tolerance test was calculated using blood glucose values.
Histopathology.
Upon dissection, liver, BAT, and inguinal WAT (iWAT) were submerged in 10% neutral buffered formalin for at least 24 h at room temperature. Formalin-fixed samples were routinely processed for histology, embedded in paraffin, cut into 5-μM sections, and stained with hematoxylin and eosin (H&E). H&E staining was performed to assess tissue composition and morphology, including the presence of crown-like structures, which are indicative of the browning of WAT and whitening of BAT. We made an a priori decision to not conduct specialized histopathological staining unless the trained veterinary pathologist, who was blinded to treatment status, reported tissue morphology assessments of H&E-stained tissue that significantly differed by treatment. Each of the tissues’ object area fraction (OAF) was calculated at ×200 magnification using a binary threshold of 185 to 238 on red, green, and blue channels using a BX43 Olympus microscope fitted with an Olympus DP74 camera. Hepatic lipidosis was scored based on percentage of hepatocytes that contained lipid: 0 = no lipid; 1 = less than 10% of area within a ×200 field contained lipid; 2 = 11–33% of area within a ×200 field contained lipid; 3 = 34–66% of area within a ×200 field contained lipid; 4 = greater than 66% of area within a ×200 field contained lipid. Fat content of each AT depot was calculated as percent region of interest (ROI) in epididymal, brown, and inguinal fat. The ROI was determined with Cell Sens software gated to distinguish clear spaces (i.e., lipid droplets) in three random OAFs. The average ROI of the three OAFs per mouse averaged across treatments is reported here. All histological sectioning and evaluation were conducted at the UC Davis Comparative Pathology Laboratory.
RNA isolation and sequencing.
Total RNA was isolated and extracted from iWAT (~30 mg) using a lipid-specific RNeasy kit according to the manufacturer’s protocol (Qiagen, Hilden, Germany). Total RNA was quantified using a Qubit Fluorometer (Invitrogen, Burlington, ON, Canada). Library preparation and RNA sequencing were performed at the Beijing Genome Institute (BGI, Hong Kong) using the BGISEQ-500 (BGI, Hong Kong). The sequence was aligned with the Genome Reference Consortium Mouse Build 38 (GRCm38, mm10), generating 20 M mapped reads per sample.
Protein quantification and Western blotting.
iWAT (~250 mg) was placed in RIPA buffer containing a protease and phosphatase inhibitor cocktail (Halt, ThermoFisher Scientific) for tissue disruption (TissueLyser II, Qiagen). A bicinchoninic acid assay (BCA) was performed to quantify protein lysate concentrations (Pierce BCA Protein Assay Kit, ThermoFisher Scientific). Western Blotting was conducted with 4–15% Mini-PROTEAN TGX Precast 15-well protein gels according to the manufacturer’s protocol, using ~50 µg total protein per well (Bio-Rad, Hercules, CA). Western blot analysis was performed with primary antibodies UCP1 (14670; Cell Signaling, Danvers, MA), ELOVL6 (ab69857; Abcam, Cambridge, UK), PGC1A (ab54481; Abcam), CPT1B (ab134988; Abcam), ATGL (ab109251; Abcam), TOMM20 (ab186735; Abcam), and TUBB (T8328; Sigma). The secondary antibodies were goat anti-mouse 680 (925-68020) and goat anti-rabbit 800 (925-32211) (Li-Cor, Lincoln, NE). Proteins were detected by chemiluminescence (Western Lighting Plus-ECL; Perkin Elmer, Waltham, MA), with signal intensity identified with Li-Cor Odyssey Software. Image Studio Lite software (Li-Cor) was used to quantify band intensity, dividing the signal intensity of the protein of interest by the intensity of housekeeping gene TUBB. Protein quantification was performed by dividing the normalized signal intensity of the protein of interest relative to its endogenous control among arsenic-exposed mice by the normalized signal intensity of the protein of interest relative to its endogenous control among the controls.
Statistical analysis.
We performed statistical analyses for treatment differences in body weight, consumption of water and calories, body composition, and tissue weights in R (v. 3.5.2) using linear mixed models based on previously published methods (26). We accounted for cohort as a random effect to capture differences between experimental blocks. To identify the effects of temperature and circadian rhythm on differences in energy expenditure during indirect calorimetry, longitudinal modeling was performed with treatment and percent body fat as fixed effects and both cohort and mouse identifiers as random effects while stratifying by photoperiod and ambient temperature (26). The data from the metabolic cage studies were longitudinally analyzed with mixed models, which have been successfully applied in both rodent (26) and human studies (16); we modeled treatment and body composition as fixed effects and both cohort and mouse identifiers as random effects, while stratifying by photoperiod and ambient temperature. This statistical model was able to control for the potential that the energy expenditure findings obtained were due to increases in total or percent fat or lean mass, an issue that has been repeatedly raised by the metabolic biology community (9, 48, 54).
Since percent body fat differed significantly between arsenic and controls, this was included as a covariate in the indirect calorimetry analyses (9). Downregulated genes that exhibited a log2 fold change less than −1 (i.e., 50% decrease in expression) were considered in Gene Set Enrichment Analysis (GSEA). GSEA was performed in R (v. 3.5.2) using the clusterProfiler package and included the following enrichment analyses: Gene Ontology (GO) for biological processes, Kyoto Encyclopedia of Genes and Genomes (KEGG), and WikiPathways (66). GSEA findings were deemed significant based on Benjamini-Hochberg adjusted P values. Significant directions of changes revealed by GSEA were validated by Western blots, where we expected that the direction of arsenic associated protein expression changes would reflect the direction of arsenic-associated RNA expression changes, and as such this directional change in protein was evaluated by a one-tailed t test (Prism, GraphPad) (21).
RESULTS
Chronic arsenic exposure increased percent body fat but not body weight or food consumption.
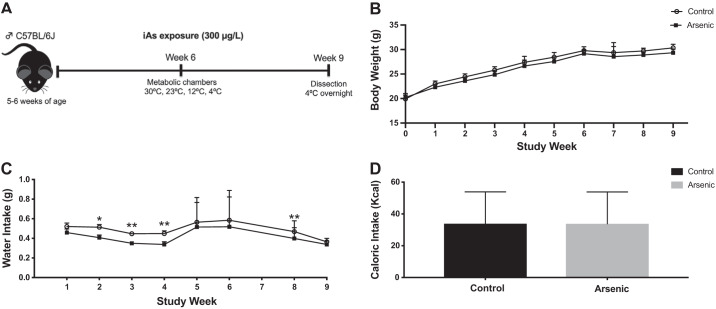
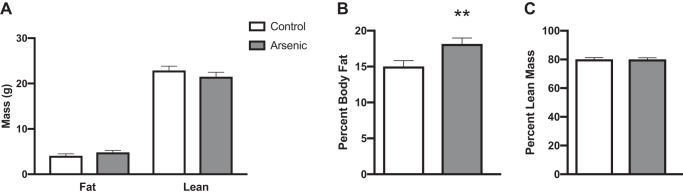
To determine whether chronic arsenic exposure increased body weight and measures of food and water consumption, adult mice were monitored weekly for the status of these parameters (Fig. 1A). A qualitative yet insignificant decrease in body weight was observed for arsenic-exposed mice compared with controls, throughout the duration of the study (Fig. 1B). While caloric intake was not significantly different across treatments throughout the majority of the study duration, significant differences between treatment groups were observed during weeks 1 and 8, when arsenic-exposed mice had increased caloric intake, and week 9, during which controls had increased caloric intake (Fig. 1D). Caloric intake for both arsenic-exposed and control mice decreased during week 5 due to transfer to metabolic cages (Fig. 1D). Water intake was consistently higher among controls, with significant differences noted in weeks 2, 3, 4, and 8 (Fig. 1C). Arsenic-exposed mice had a significantly higher body fat percentage when compared with controls, despite no treatment differences in lean, fat, or total body mass (Fig. 2). An insignificant treatment effect during a pyruvate tolerance test indicated that hepatic gluconeogenesis was not significantly different between treatment groups (Supplemental Fig. S1; all Supplemental Materials are available at https://doi.org/10.6078/D1V10C).
Fig. 1.
Chronic arsenic exposure did not significantly alter body weight. A: study design for male C57BL/6J mice exposed to arsenic (iAs; 300 μg/L) in drinking water for 9 wk; B: body weight; C: body weight adjusted water intake; D: total caloric intake over 9 wk; (n = 26; 13 arsenic vs. 13 controls). Data are represented as least squares means ± SE arsenic vs. controls, with statistical significance determined by linear mixed models. **P < 0.01; *P < 0.05; arsenic vs. controls. Data from week 7 are unavailable in C and D, as mice were in the metabolic chambers.
Fig. 2.
Chronic arsenic exposure significantly increased percent body fat but not lean or fat body mass. A: fat and lean body mass; B: percent body fat; C: percent lean mass, determined via dual-energy X-ray absorptiometry (DEXA) and EchoMRI at study week 6. No significant differences observed comparing arsenic (n = 26; 13 arsenic vs. 13 controls). Data are represented in least squares means, with statistical significance determined by linear mixed models accounting for cohort as a random effect. **P < 0.01.
Chronic arsenic exposure decreased energy expenditure during cold-tolerance acclimation.
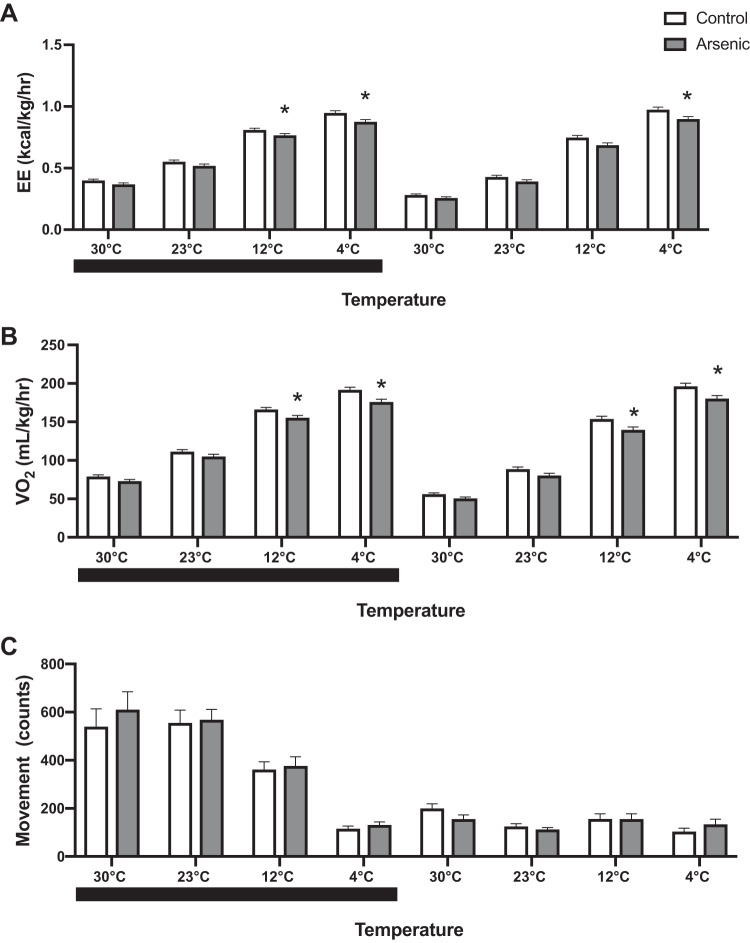
To examine the effect of chronic arsenic exposure on nonshivering thermogenesis, adult mice exposed to water with and without arsenic were subjected to a cold tolerance acclimation test at 6 wk of exposure (11). Since total fat mass, total lean mass, and percent lean mass, were not significantly different between treatment groups (Fig. 2, A and C) and did not modify the treatment effects on calorimetry data, these potential covariates were not included in the final calorimetry models of metabolic cage data. All results from metabolic cages were adjusted by percent body fat (Fig. 2B), which was the only body composition covariate that was significantly different between treatment groups.
Arsenic-treated mice exhibited significantly decreased energy expenditure when challenged with progressively colder ambient temperatures (Fig. 3A). Upon reaching 4°C, arsenic-exposed mice had a 7% reduction in energy expenditure, as compared with controls during both light and dark photoperiods (Fig. 3A). Oxygen consumption reduction by arsenic treatment followed a similar pattern (Fig. 3B). Further the reduction of oxygen consumption decreased by 8% at 4°C, suggestive of impaired nonshivering thermogenesis (12). These significant reductions in energy expenditure and oxygen consumption among arsenic-exposed mice were independent of percent body fat. Despite the reduced energy expenditure and oxygen consumption by arsenic treatment, no significant treatment differences were observed in movement, indicating that decreased energy expenditure by arsenic was independent of physical activity of mice (Fig. 3C). No significant differences were observed in RER (data not shown), suggesting that the effect of arsenic exposure on energy expenditure and oxygen consumption was not mediated by an interference with substrate utilization.
Fig. 3.
Chronic arsenic exposure decreased adaptive thermogenesis during chronic cold tolerance testing. A: energy expenditure (EE); B: oxygen consumption; C: movement at study week 6 (n = 26; 13 arsenic vs. 13 controls). Black bars indicate the dark photoperiod. Data are represented as least squares means ± SE arsenic vs. controls, with statistical significance determined by linear mixed models accounting for treatment and percent body fat and as fixed effects and cohort and mouse ID as random effects, stratified by photoperiod and ambient temperature. *P < 0.05 arsenic vs. controls.
Chronic arsenic exposure increased subcutaneous fat mass.
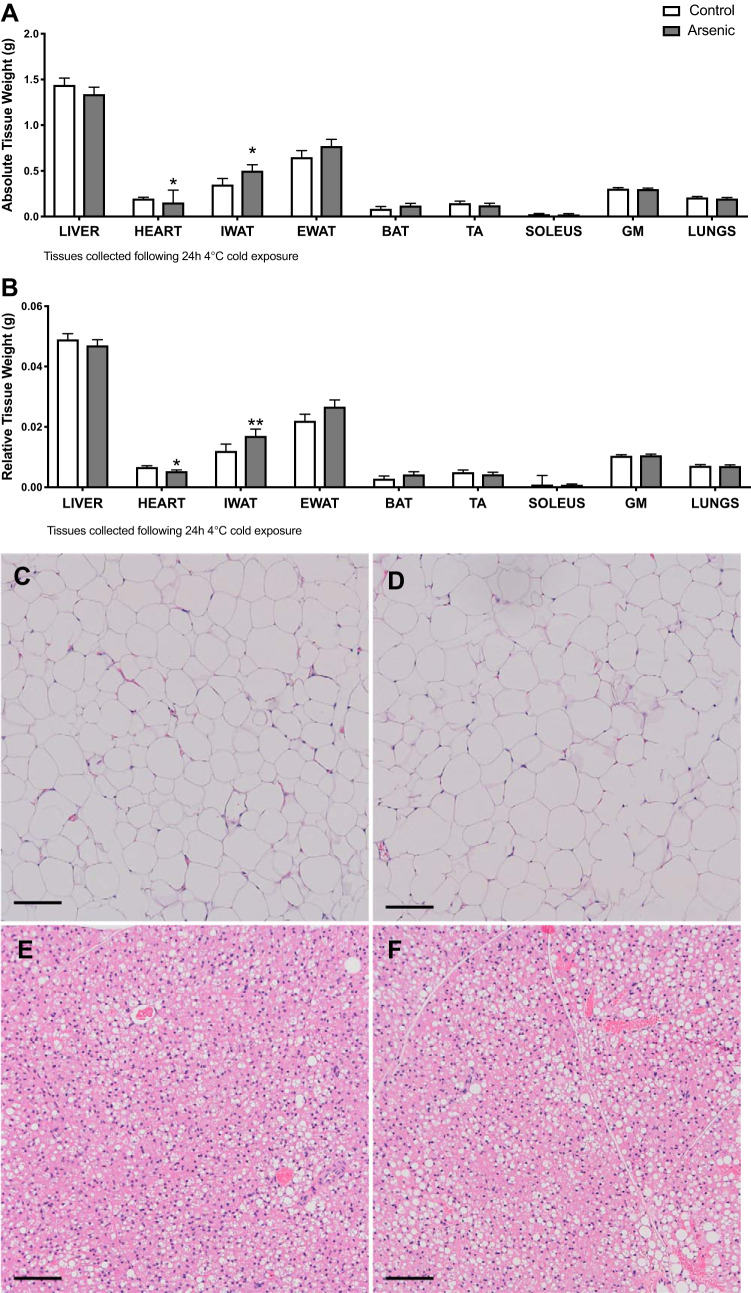
To determine the tissue site responsible for reduced energy expenditure by arsenic-exposed mice challenged by progressively colder ambient temperatures, tissues were weighed and histopathological analysis of thermogenic tissues was conducted (44, 65). Both absolute and relative weights of iWAT were significantly increased among arsenic-exposed mice compared with controls (Fig. 4, A and B). Histopathology of iWAT was suggestive of adipocyte hypertrophy in arsenic-exposed mice compared with controls (Fig. 4, C and D), consistent with unhealthy AT expansion and obesity (3, 51). However, we did not observe an increase in the absolute or relative weight of other thermogenic tissues, e.g., BAT, skeletal muscle, heart, or liver (Fig. 4, A and B). Furthermore, the blinded histopathologist observed an insignificant increase in the vacuolization of BAT from arsenic-exposed mice relative to controls (27.7% vs. 25.8% respective treatment means, P = 0.6; Fig. 4, E and F) and a mean hepatic liver lesion score of 1.4 for arsenic-treated mice and 1 for the control mice (P = 0.14; Supplemental Fig. S2). These data are consistent with iWAT as a target of arsenic toxicity.
Fig. 4.
Chronic arsenic exposure increased inguinal white adipose tissue (iWAT) relative and absolute tissue weight. Absolute (A) and relative tissue weight (B) (n = 13 arsenic vs. n = 13 controls). Representative hematoxylin and eosin (H&E) staining (×10) depicting control iWAT (C); arsenic-treated iWAT (D); control brown adipose tissue (BAT) (E); arsenic-treated BAT (F); n = 10; 5 arsenic vs. 5 controls. Data are represented as least squares means ± SE, with statistical significance determined by linear mixed models accounting for cohort as a random effect. **P < 0.01; *P < 0.05 arsenic vs. controls. Scale bar = 100 μM. eWAT, epididymal WAT; TA, tibilias anterior; GM, gastrocnemius muscle. Tissues were collected following a 24-h 4°C cold exposure. No statistical differences in pancreas absolute or relative weight were observed (data not shown).
Chronic arsenic exposure downregulated the expression of genes and proteins involved thermogenesis and fatty acid oxidation in inguinal WAT.
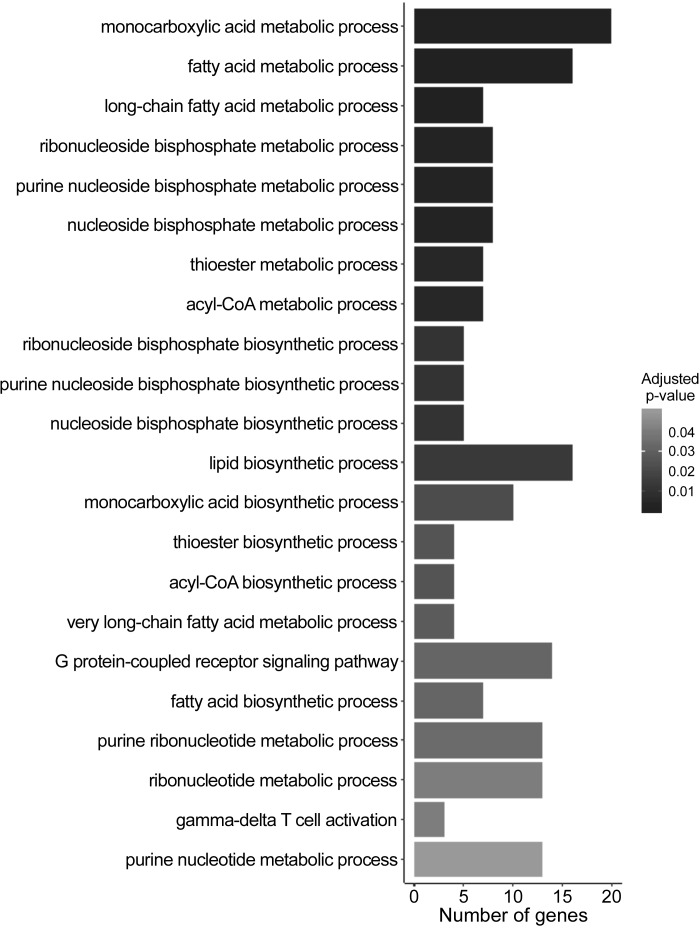
Given arsenic increased iWAT mass (60) and the biomedical evidence for a role of recruitable beige adipocytes in maintaining energy balance (23), we sought to establish whether arsenic exposure interfered with the beiging of iWAT by performing RNA sequencing analysis of iWAT from arsenic-exposed and control mice. Despite increased percent body fat (Fig. 2B) and iWAT mass (Fig. 4, A and B), arsenic significantly decreased the expression of genes involved in regulating adaptive thermogenesis, adipogenesis, lipolysis, inflammation, and fatty acid metabolism (Fig. 5, Table 1, and Supplemental Fig. S3; GEO GSE134982). For example, arsenic exposure decreased the expression of Ucp1 in iWAT, which codes for the primary protein responsible for energy dissipation and nonshivering thermogenesis (Table 1 and Supplemental Fig. S3). The expression of several additional genes critical to beiging, fatty acid oxidation, and energy generation was also significantly reduced with arsenic exposure, including Cpt1b, Cox8b, Acss1, and Acss2 (Table 1).
Fig. 5.
Gene Ontology (GO) Biological Process Enrichment Analysis of Arsenic Downregulated Genes in inguinal white adipose tissue (iWAT) RNA-seq performed on iWAT collected following a 24 h 4°C cold exposure at study week 9. Downregulated biological processes (doubling) shown with Benjamini-Hochberg adjusted P values (n = 6; 3 arsenic vs. 3 controls). Analysis conducted in R using the clusterProfiler package.
Table 1.
KEGG Pathway Analysis of Arsenic Downregulated Genes in iWAT
| Pathway-Specific Genes | Adjusted P Value | Log2 Fold Change (Arsenic/Control) | Decrease in Gene Expression, % |
|---|---|---|---|
| PPAR signaling (8 genes) | 6.47 × 10−6 | ||
| Slc27a2 | −1.67 | 31.4 | |
| Fabp3 | −1.45 | 36.6 | |
| Ucp1 | −1.24 | 42.3 | |
| Acsl5 | −1.13 | 45.7 | |
| Scd2 | −1.12 | 46.0 | |
| Cpt1â | −1.11 | 46.3 | |
| Gyk | −1.04 | 48.6 | |
| Me1 | −1.00 | 50.0 | |
| Metabolism (23 genes) | 1.85 × 10−4 | ||
| Dhrs9 | −1.41 | 37.6 | |
| Lipg | −1.39 | 38.2 | |
| Phospho1 | −1.39 | 38.2 | |
| Cyp2b10 | −1.33 | 40.0 | |
| Acly | −1.32 | 40.0 | |
| Impa2 | −1.30 | 40.1 | |
| Pank1 | −1.22 | 42.9 | |
| St3gal5 | −1.21 | 43.2 | |
| Amd1 | −1.18 | 44.1 | |
| Cyp51 | −1.17 | 44.4 | |
| Cox8b | −1.14 | 45.4 | |
| Acsl5 | −1.13 | 45.7 | |
| Plcd4 | −1.13 | 45.7 | |
| Fbp2 | −1.11 | 46.3 | |
| Idi1 | −1.11 | 46.3 | |
| Dnmt3l | −1.05 | 48.3 | |
| Uqcr10 | −1.05 | 48.3 | |
| Gyk | −1.04 | 48.6 | |
| Acss2 | −1.04 | 48.6 | |
| Odc1 | −1.03 | 49.0 | |
| Plcb2 | −1.03 | 49.0 | |
| Acss1 | −1.01 | 50.0 | |
| Me1 | −1.00 | 50.0 | |
| Chemokine signaling (6 genes) | 3.82 × 10−2 | ||
| Ccl21a | −7.12 | 0.72 | |
| Ccl19 | −1.51 | 35.1 | |
| Cxcr6 | −1.34 | 39.5 | |
| Adcy3 | −1.21 | 43.2 | |
| Ccl8 | −1.09 | 47.0 | |
| Plcb2 | −1.03 | 49.0 |
Pathway-specific Benjamini-Hochberg adjusted P values, with gene-specific log2 fold changes and percent decrease in expression; (n = 6; 3 arsenic vs. 3 controls). Pathway-specific analysis conducted in R using the clusterProfiler package. KEGG, Kyoto Encyclopedia of Genes and Genomes; iWAT, inguinal white adipose tissue. See glossary for definitions of proteins and genes.
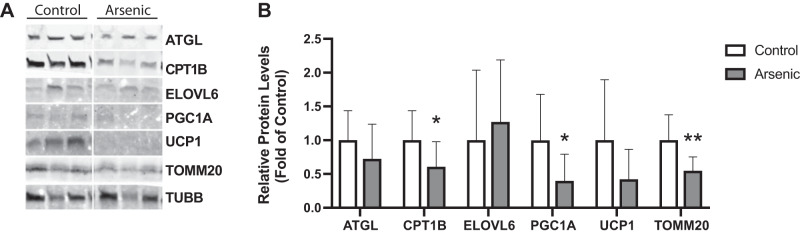
Genes that were expressed differentially with respect to arsenic exposure among the significantly enriched KEGG pathways (Table 1) were validated via protein quantification in iWAT. Arsenic exposure significantly decreased the expression of TOMM20, PGC1A, and CPT1B mitochondrial proteins in iWAT (Fig. 6). Relative to controls, the iWAT of arsenic-exposed mice revealed a 39.3% decrease in CPT1B protein, which corroborated the 46.3% decrease in Cpt1b expression revealed by RNA sequencing (Table 1). Furthermore, the 57.8% decrease in UCP1 protein expression in iWAT from arsenic-exposed mice (P: 0.08) corroborated with the RNA sequencing results, where Ucp1 was decreased 42.3% due to arsenic exposure (Table 1).
Fig. 6.
Chronic arsenic exposure downregulates fatty acid oxidation, adipogenesis, and thermogenesis targets in inguinal white adipose tissue (iWAT). Immunoblot (A) and densitometry (B) analyses of iWAT collected following a 24 h 4°C cold exposure at study week 9 (n = 14; 7 arsenic vs. 7 controls). See glossary for definitions of proteins and genes. Data are represented as relative protein levels (fold of control samples), normalized by TUBB. Statistical significance was determined by an unpaired one-tailed Student’s t test analysis using Prism (GraphPad). **P < 0.01; *P < 0.05 arsenic vs. controls.
DISCUSSION
Our study investigated the hypothesis that chronic arsenic exposure decreases energy expenditure in adult male C57BL/6J mice through effects on thermogenic AT. Our results suggest that chronic arsenic exposure increases WAT mass while reducing adaptive nonshivering thermogenesis and beiging of thermogenic AT. Our molecular findings indicate the latter occurs through arsenic targeting mitochondria, either by altering their numbers or functions or both. Previous experimental research corroborates that arsenic targets mitochondria function in pancreas, liver, and BAT (5, 22, 40, 57, 68).
Based on our findings, we propose that chronic arsenic exposure at doses relevant to humans decreases adaptive thermogenesis and decreases beiging of subcutaneous AT via impaired mitochondrial abundance and fatty acid oxidation (FAO). Since decreased mitochondrial number and function are both implicated in T2D pathogenesis (47), arsenic may increase T2D susceptibility via these pathways. With respect to mitochondrial abundance, chronic cold exposure should have remodeled adipocytes to increase their mitochondria and, consequently, improved their thermogenic capacity (2, 15). Instead, as evidenced by the significantly decreased expression of TOMM20, arsenic exposure appeared to diminish mitochondrial abundance in WAT following cold exposure in alignment with the “anti-thermogenic” phenomenon of increased whitening, also known as beige-to-white adipocyte conversion (1). The decreased nonshivering thermogenesis observed in arsenic-exposed mice may also be associated with impaired mitochondrial FAO, given iWAT of cold challenged arsenic-exposed mice had decreased expression of its rate-limiting enzyme, CTP1B, and fatty acids are a substrate for AT thermogenesis (7, 32). The decreased RNA and protein expression of both CPT1B and UCP1 after arsenic exposure further underscores the biological plausibility of arsenic decreasing mitochondrial lipid oxidation and uncoupled respiration, respectively, to impair adaptive thermogenesis.
There has been some conflict in human observational and experimental studies regarding the effects of arsenic on body mass and composition. However, the obesogenic effects of arsenic observed here are supported by recent reviews of this topic. For example, a recent review of the metabolic toxicities of arsenic conceded that some human observational studies have found a positive association between arsenic exposure and obesity (19). Another review evaluating experimental evidence for and against arsenic as an obesogen concluded arsenic was a potential obesogen (14). We suspect the inconsistencies in investigations of arsenic as an obesogen will later be revealed to relate to the presence of other risk factors.
Although we found no effect of oral arsenic exposure on hepatic histopathology, other experimental studies linking arsenic exposure to fatty liver appear to be restricted to those which fed rodents high-fat or “Western” diets (17, 52, 63), in contrast to our use of a lower fat diet. Human fatty liver is underdiagnosed and its potential relationship with arsenic exposure is uncharacterized but merits examination alongside dietary factors in humans.
A strength of this study lies in the administered exposure concentration, which was designed to be highly relevant to the route and dose of exposure experienced by communities worldwide (31). The only previously published studies on arsenic exposure and thermogenic AT function administered high doses of 5 and 10 mg/L, the lowest of which is roughly equivalent to 1000 μg/L in humans (5, 68). These doses are above most human exposures of arsenic-contaminated drinking water in the United States. Our study also relies on chronic arsenic ingestion via oral exposure, unconfounded by the ingestion of arsenic-rich dietary sources. We also provided a slow cold exposure acclimation period, which avoids shivering due to the lack of acute transfer from vivarium temperatures to 4C (10). This sustained cold acclimation was designed to assess nonshivering thermogenesis specifically (10). This study adds to the experimental animal literature by relying on a study design that is not only applicable to human exposures but also informed by established methodologies in the field of metabolic biology.
A limitation of this study is the lack of measurements of arsenic levels in the target tissues or circulation. This limitation impedes our ability to conduct a direct comparison of internal doses of exposure reported in arsenic-exposed humans in the published literature. However, an allometric scaling approach was used to convert treatment concentrations administered to animals to HEDs (58). According to these calculations, mice exposed to 300 μg/L in water equates to an exposure of 53.6 μg/L in humans, which approximates the current 50 μg/L standard of inorganic arsenic exposure in drinking water established in several nations, including Bahrain, Bangladesh, Bolivia, China, Egypt, India, Indonesia, Oman, Philippines, Saudi Arabia, Sri Lanka, Vietnam, and Zimbabwe (64). An additional limitation of this study is the relatively small sample size (n = 26 per treatment group), which may have limited our ability to detect statistically significant changes in gene expression at this moderate exposure level.
It remains to be determined whether arsenic interferes with adaptive thermogenesis and fatty acid metabolism in iWAT through impaired adipogenesis, through reduced mitochondrial abundance, or by targeting mitochondrial function independent of these pathologies. Future studies should be conducted to examine the dose-dependent effects of arsenic exposure on iWAT remodeling and thermogenesis and its association with metabolic pathologies. Similar studies of adaptive thermogenesis defects in female mice exposed to arsenic should also be performed due to known sex differences in the association of T2D and arsenic exposure in humans (28).
Conclusions.
This is the first study to investigate the effects of inorganic arsenic exposure on adaptive nonshivering thermogenesis. While chronic arsenic exposure is associated with increased susceptibility to T2D in communities worldwide, the exact mechanism by which arsenic exerts its diabetogenic effects remains unclear. Our findings provide additional evidence of potential pathways by which chronic arsenic exposure impairs thermogenesis, FAO, and mitochondrial abundance, increasing the risk of metabolic diseases such as T2D. This study contributes to the emerging relationships between environmental toxicants and thermogenic AT to support a role of environmental chemicals in the growing prevalence of metabolic diseases worldwide.
GRANTS
This work was supported by National Institutes of Health Grants P42 ES-004705 (to UC Berkeley), R01-DK-113019 (to UC Berkeley), R01-ES-024946 (to UC Davis), and U2C DK-092993 (to UC Davis), and the USDA National Institute of Food and Agriculture (Hatch project 1002182 to UC Davis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.C., P.-J.H.Z., M.T.S., J.-C.W., and M.A.L.M. conceived and designed research; F.C., P.-J.H.Z., and S.S.S. performed experiments; F.C., P.-J.H.Z., R.V.P., and M.A.L.M. analyzed data; F.C., P.-J.H.Z., S.S.S., R.V.P., A.H., A.S., M.T.S., J.-C.W., and M.A.L.M. interpreted results of experiments; F.C., P.-J.H.Z., R.V.P., M.T.S., J.-C.W., and M.A.L.M. prepared figures; F.C. and M.T.S. drafted manuscript; F.C., P.-J.H.Z., S.S.S., R.V.P., A.H., A.S., M.T.S., J.-C.W., and M.A.L.M. edited and revised manuscript; F.C., P.-J.H.Z., S.S.S., R.V.P., A.H., A.S., M.T.S., J.-C.W., and M.A.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate the expertise of the University of California (UC) Davis Mouse Metabolic Phenotyping Center for performing the indirect calorimetry and body composition analyses and the UC Davis Comparative Pathology Laboratory for conducting tissue staining and histopathology. The authors acknowledge Kyle Jackson for his involvement in executing the Fragment Analyzer analysis to ascertain RNA sample quality at UC Davis. The authors also thank Jason Tong, Rebecca Lee, and Sarah Elmore for assistance with animal handling and dissections.
GLOSSARY
- Acly
ATP citrate lyase
- Adcy3
Adenylate cyclase 3
- Acot1
Acyl-CoA thioesterase 1
- Acot11
Acyl-CoA thioesterase 11
- Acsl5
Acyl-CoA synthetase long-chain family member 5
- Acss1
Acyl-CoA synthetase short-chain family member 1
- Acss2
Acyl-CoA synthetase short-chain family member 2
- Amd1
S-adenosylmethionine decarboxylase 1
- C/EBPA
CCAAT-enhancer binding protein alpha
- Ccl8
Chemokine (C-C motif) ligand 8
- Ccl19
Chemokine (C-C motif) ligand 19
- Ccl21a
Chemokine (C-C motif) ligand 21a (serine)
- Cidea
Cell death-inducing DNA fragmentation factor, alpha subunit-like effector a
- Cox8b
Cytochrome c oxidase subunit 8b
- Cpt1b
Carnitine palmitoyltransferase 1b
- Cyp2b10
Cytochrome P450, family 2, subfamily b, polypeptide 10
- Cyp51
Cytochrome P450, family 51
- Cxcr6
Chemokine (C-X-C motif) receptor 6
- Dhrs9
Dehydrogenase/reductase (SDR Family) member 9
- Dio2
Deiodinase, iodothyronine, type II
- Dnmt3l
DNA (cytosine-5-)-methyltransferase 3-like
- ELOVL6
ELOVL family member 6, elongation of long chain fatty acids
- Fabp3
Fatty acid binding protein 3
- Fbp2
Fructose bisphosphatase 2
- Gyk
Glycerol kinase
- Idi1
Isopentenyl-diphosphate delta isomerase
- Impa2
Inositol (myo)-1(or 4)-monophosphatase 2
- Lipg
Lipase, endothelial
- Me1
Malic enzyme 1, NADP(+)-dependent, cytosolic
- Odc1
Ornithine decarboxylase, structural 1
- Pank1
Pantothenate kinase 1
- PGC1A
Peroxisome proliferator activated receptor, gamma, coactivator 1 alpha
- Phosopho1
Phosphatase, orphan 1
- Plcb2
Phospholipase c, beta 2
- Plcd4
Phospholipase c, delta 4
- Slc27a2
Solute carrier family 27 (fatty acid transporter), member 2
- Scd2
Stearoyl-coenzyme A desaturase 2
- St3gal5
ST3 Beta-galactoside alpha-2,3-sialyltransferase 5
- TOMM20
Translocase of outer mitochondrial membrane 20
- TUBB
Beta tubulin
- Ucp1
Uncoupling protein 1 (mitochondrial, proton carrier)
- Uqcr10
Ubiquinol-cytochrome c reductase, complex III subunit X
REFERENCES
- 1.Altshuler-Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, Kajimura S. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab 24: 402–419, 2016. doi: 10.1016/j.cmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altshuler-Keylin S, Kajimura S. Mitochondrial homeostasis in adipose tissue remodeling. Sci Signal 10: eaai9248, 2017. doi: 10.1126/scisignal.aai9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arner P, Spalding KL. Fat cell turnover in humans. Biochem Biophys Res Commun 396: 101–104, 2010. doi: 10.1016/j.bbrc.2010.02.165. [DOI] [PubMed] [Google Scholar]
- 4.Ayotte JD, Medalie L, Qi SL, Backer LC, Nolan BT. Estimating the high-arsenic domestic-well population in the conterminous united states. Environ Sci Technol 51: 12443–12454, 2017. doi: 10.1021/acs.est.7b02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae J, Jang Y, Kim H, Mahato K, Schaecher C, Kim IM, Kim E, Ro SH. Arsenite exposure suppresses adipogenesis, mitochondrial biogenesis and thermogenesis via autophagy inhibition in brown adipose tissue. Sci Rep 9: 14464, 2019. doi: 10.1038/s41598-019-50965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakker LE, Boon MR, van der Linden RA, Arias-Bouda LP, van Klinken JB, Smit F, Verberne HJ, Jukema JW, Tamsma JT, Havekes LM, van Marken Lichtenbelt WD, Jazet IM, Rensen PC. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol 2: 210–217, 2014. doi: 10.1016/S2213-8587(13)70156-6. [DOI] [PubMed] [Google Scholar]
- 7.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol 10: 24–36, 2014. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 8.Blondin DP, Labbé SM, Noll C, Kunach M, Phoenix S, Guérin B, Turcotte ÉE, Haman F, Richard D, Carpentier AC. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 64: 2388–2397, 2015. doi: 10.2337/db14-1651. [DOI] [PubMed] [Google Scholar]
- 9.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 11.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes 34, Suppl 1: S7–S16, 2010. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 12.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214: 242–253, 2011. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 13.Castriota F, Acevedo J, Ferreccio C, Smith AH, Liaw J, Smith MT, Steinmaus C. Obesity and increased susceptibility to arsenic-related type 2 diabetes in Northern Chile. Environ Res 167: 248–254, 2018. doi: 10.1016/j.envres.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceja-Galicia ZA, Daniel A, Salazar AM, Pánico P, Ostrosky-Wegman P, Díaz-Villaseñor A. Effects of arsenic on adipocyte metabolism: Is arsenic an obesogen? Mol Cell Endocrinol 452: 25–32, 2017. doi: 10.1016/j.mce.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 7: 30, 2016. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Havel PJ, Keim NL. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr 66: 201–208, 2012. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ditzel EJ, Nguyen T, Parker P, Camenisch TD. Effects of arsenite exposure during fetal development on energy metabolism and susceptibility to diet-induced fatty liver disease in male mice. Environ Health Perspect 124: 201–209, 2016. doi: 10.1289/ehp.1409501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellacott KL, Morton GJ, Woods SC, Tso P, Schwartz MW. Assessment of feeding behavior in laboratory mice. Cell Metab 12: 10–17, 2010. doi: 10.1016/j.cmet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farkhondeh T, Samarghandian S, Azimi-Nezhad M. The role of arsenic in obesity and diabetes. J Cell Physiol 234: 12516–12529, 2019. doi: 10.1002/jcp.28112. [DOI] [PubMed] [Google Scholar]
- 20.Genome Reference Consortium (GRC) Mouse Genome Overview (Online). https://www.ncbi.nlm.nih.gov/grc/mouse
- 21.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31: 337–350, 2016. doi: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseini MJ, Shaki F, Ghazi-Khansari M, Pourahmad J. Toxicity of arsenic (III) on isolated liver mitochondria: a new mechanistic approach. Iran J Pharm Res 12, Suppl: 121–138, 2013. [PMC free article] [PubMed] [Google Scholar]
- 23.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22: 546–559, 2015. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozul CD, Nomikos AP, Hampton TH, Warnke LA, Gosse JA, Davey JC, Thorpe JE, Jackson BP, Ihnat MA, Hamilton JW. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem Biol Interact 173: 129–140, 2008. doi: 10.1016/j.cbi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Labbé SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, Richard D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29: 2046–2058, 2015. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- 26.La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, Buettner C. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PLoS One 9: e103337, 2014. doi: 10.1371/journal.pone.0103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes 38: 170–176, 2014. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, Rudnai P, Leonardi G, Fletcher T, Vahter M. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect 115: 1081–1086, 2007. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, Lin J, Zhao Q, Zhang C, Yuan X, Hu T, Liu L, Huang Y, Zhang L, Wang D, Zhan J, Jong Lee H, Speakman JR, Jin W. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 156: 2461–2469, 2015. doi: 10.1210/en.2014-1598. [DOI] [PubMed] [Google Scholar]
- 30.Martin EM, Stýblo M, Fry RC. Genetic and epigenetic mechanisms underlying arsenic-associated diabetes mellitus: a perspective of the current evidence. Epigenomics 9: 701–710, 2017. doi: 10.2217/epi-2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120: 1658–1670, 2012. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244: 1–14, 1997. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 33.Meyer CW, Reitmeir P, Tschöp MH. Exploration of energy metabolism in the mouse using indirect calorimetry: measurement of daily energy expenditure (DEE) and basal metabolic rate (BMR). Curr Protoc Mouse Biol 5: 205–222, 2015. doi: 10.1002/9780470942390.mo140216. [DOI] [PubMed] [Google Scholar]
- 34.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 54: 523–531, 2013. doi: 10.2967/jnumed.112.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121: 295–302, 2013. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect 114: 641–648, 2006. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 300: 814–822, 2008. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 38.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279, 2011. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96: 192–199, 2011. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 40.Pan X, Jiang L, Zhong L, Geng C, Jia L, Liu S, Guan H, Yang G, Yao X, Piao F, Sun X. Arsenic induces apoptosis by the lysosomal-mitochondrial pathway in INS-1 cells. Environ Toxicol 31: 133–141, 2016. doi: 10.1002/tox.22027. [DOI] [PubMed] [Google Scholar]
- 41.Peng XR, Gennemark P, O’Mahony G, Bartesaghi S. Unlock the thermogenic potential of adipose tissue: pharmacological modulation and implications for treatment of diabetes and obesity. Front Endocrinol (Lausanne) 6: 174, 2015. doi: 10.3389/fendo.2015.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez-Gurmaches J, Tang Y, Jespersen NZ, Wallace M, Martinez Calejman C, Gujja S, Li H, Edwards YJK, Wolfrum C, Metallo CM, Nielsen S, Scheele C, Guertin DA. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab 27: 195–209.e6, 2018. doi: 10.1016/j.cmet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105, 2011. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7: e49452, 2012. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, Nedergaard J, Sidossis LS, Kajimura S. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 21: 389–394, 2015. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 12: 537–577, 2010. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speakman JR. Measuring energy metabolism in the mouse–theoretical, practical, and analytical considerations. Front Physiol 4: 34, 2013. doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinmaus C, Yuan Y, Liaw J, Smith AH. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology 20: 807–815, 2009. doi: 10.1097/EDE.0b013e3181b0fd29. [DOI] [PubMed] [Google Scholar]
- 51.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101, 2011. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan M, Schmidt RH, Beier JI, Watson WH, Zhong H, States JC, Arteel GE. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol 257: 356–364, 2011. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120: 779–789, 2012. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tschöp MH, Speakman JR, Arch JRS, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2011. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tseng CH, Chong CK, Heng LT, Tseng CP, Tai TY. The incidence of type 2 diabetes mellitus in Taiwan. Diabetes Res Clin Pract 50, Suppl 2: S61–S64, 2000. doi: 10.1016/S0168-8227(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 56.Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, Chiou HY, Hsueh YM, Hsu KH, Chen CJ. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect 108: 847–851, 2000b. doi: 10.1289/ehp.00108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol 197: 67–83, 2004. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Food and Drug Administration (FDA) Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. U.S. Food and Drug Administration. Silver Spring, MD: FDA, 2005. https://www.fda.gov/media/72309/download [Google Scholar]
- 59.U.S. Food and Drug Administration (FDA) Arsenic in Rice and Rice Products: Risk Assessment Report. Silver Spring, MD: FDA, 2016. https://www.fda.gov/media/96071/download [Google Scholar]
- 60.Virtue S, Vidal-Puig A. Assessment of brown adipose tissue function. Front Physiol 4: 128, 2013. doi: 10.3389/fphys.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization (WHO) Arsenic in Drinking-Water. Geneva, Switzerland: WHO, 2011. https://www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf [Google Scholar]
- 62.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu J, Liu J, Waalkes MP, Cheng ML, Li L, Li CX, Yang Q. High dietary fat exacerbates arsenic-induced liver fibrosis in mice. Exp Biol Med (Maywood) 233: 377–384, 2008. doi: 10.3181/0710-RM-269. [DOI] [PubMed] [Google Scholar]
- 64.Yamamura S, Bartram J, Csanady M, Gorchev HG, Redekopp A; World Health Organization . Chapter 5: Drinking Water Guidelines and Standards. Geneva, Switzerland: WHO, 2001. https://www.who.int/water_sanitation_health/dwq/arsenicun5.pdf [Google Scholar]
- 65.Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 167: 10–14, 1984. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 66.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16: 284–287, 2012. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci USA 110: 12480–12485, 2013. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zuo Z, Liu Z, Gao T, Yin Y, Wang Z, Hou Y, Fu J, Liu S, Wang H, Xu Y, Pi J. Prolonged inorganic arsenic exposure via drinking water impairs brown adipose tissue function in mice. Sci Total Environ 668: 310–317, 2019. doi: 10.1016/j.scitotenv.2019.03.008. [DOI] [PubMed] [Google Scholar]