Abstract
We previously demonstrated that the combined exposure of human pulmonary microvascular endothelial cells (HPMECs) to morphine and viral protein(s) results in the oxidative stress-mediated induction of autophagy, leading to shift in the cells from early apoptotic to apoptosis-resistant proliferative status associated with the angioproliferative remodeling observed in pulmonary arterial hypertension (PAH). In this study, we tried to delineate the major source of HIV-1 protein Tat and morphine induced oxidative burst in HPMECs and its consequences on vascular remodeling and PAH in an in vivo model. We observed switch from the initial increased expression of NADPH oxidase (NOX) 2 in response to acute treatment of morphine and HIV-Tat to later increased expression of NOX4 on chronic treatment in the endoplasmic reticulum of HPMECs without any alterations in the mitochondria. Furthermore, NOX-dependent induction of autophagy was observed to play a pivotal role in regulating the endothelial cell survival. Our in vivo findings showed significant increase in pulmonary vascular remodeling, right ventricular systolic pressure, and Fulton index in HIV-transgenic rats on chronic administration of morphine. This was associated with increased oxidative stress in lung tissues and rat pulmonary microvascular endothelial cells. Additionally, endothelial cells from morphine-treated HIV-transgenic rats demonstrated increased expression of NOX2 and NOX4 proteins, inhibition of which ameliorated their increased survival upon serum starvation. In conclusion, this study describes NADPH oxidases as one of the main players in the oxidative stress-mediated endothelial dysfunction on the dual hit of HIV-viral protein(s) and opioids.
Keywords: apoptosis, autophagy, HIV-Tat, opioids, proliferation
INTRODUCTION
Pulmonary vascular injury is one of the hallmark features linked to the development of pulmonary arterial hypertension (PAH) as well as secondary to chronic airway complications and fibrotic lung diseases (1, 14, 20, 27). The loss of lung parenchyma and increased airway resistance in these diseases is usually linked to endothelial cell apoptosis. Endothelial cell death is considered as an initial trigger for pulmonary vascular injury that subsequently leads to smooth muscle and endothelial hyperproliferation, culminating into the thickening of pulmonary vessels and development of pulmonary hypertension (5, 12).
The oxidative stress plays a pivotal role in altering the endothelial cell function and contributing to the disruption in vascular tone and vascular permeability. The vascular reduced nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase, NOX) enzymes are major producers of reactive oxygen species (ROS) within the lungs (18). The dysregulated activity of NOX enzymes promotes endothelial dysfunction, inflammation, and apoptosis in the pulmonary vessels. Among the four NOX isoforms expressed in vascular cells, human pulmonary endothelial cells mainly express superoxide-generating NOX1 and NOX2 and hydrogen peroxide/superoxide-generating NOX4. Localization of NOX2 is mainly in the plasma membrane, whereas NOX4 is predominantly localized on intracellular membranes, particularly in mitochondria and endoplasmic reticulum (ER) (27). Implication of NOX-derived ROS in the pathogenesis of PAH is well evident (43, 51). Studies have shown that the inhibition of NOX2 or NOX4 is able to reduce pulmonary vascular remodeling and right ventricular hypertrophy in animal models of PAH (4, 23, 45, 52).
Despite the advent of antiretroviral therapy, numerous studies reported increased prevalence of PH in the HIV-infected population, with an estimated range between 2.6 and 15.5% (26, 41, 68). Furthermore, substance abuse is considered a critical factor in the pathogenesis of HIV-associated pulmonary hypertension (22, 33, 48). In support of this notion, we extensively demonstrated that HIV-1 acts in synergism with cocaine and/or opioids in augmenting pulmonary vascular remodeling and development of PAH (8, 15, 64). The role of HIV-1 proteins, such as Nef, Tat, and gp120, in the induction of oxidative stress-mediated apoptosis of pulmonary microvascular endothelial cells has been highlighted in many studies (19, 29, 35, 36, 47, 70). Our in vitro studies demonstrated that the elevated levels of H2O2 and superoxide radicals in primary human pulmonary microvascular endothelial cells (HPMECs) on the chronic exposure of morphine (opioid) and HIV-Tat result in an autophagy-dependent transition of endothelial cells from initial apoptosis to an apoptosis-resistant hyperproliferative state (7). However, the exact major source of oxidative stress leading to this phenomenon remains to be elucidated. In the current study, we now delineate the importance of NOX2 and NOX4 in HIV-1- and morphine-induced oxidative burst and their role in the development of PAH using an in vivo model. To the best of our knowledge, this is a first attempt into exploring the role of NOX in hyperproliferation of apoptosis-resistant endothelial cells in response to HIV protein(s) and drugs of abuse.
MATERIALS AND METHODS
Animal Study
HIV-transgenic (HIV-Tg) and wild-type (WT) Fisher male (n = 8–10/group) or female (n = 5) rats aged 7–8 mo were administered morphine (10 mg/kg body wt ip) or saline once daily for 21 days. The animals were housed at the University of Kansas Medical Center (Kansas City, KS), and the protocol used to perform the study was approved by the University’s Institutional Animal Care and Use Committee (IACUC) guidelines. All experiments were performed in strict accordance with the guidelines and regulations approved in the protocol.
Animals were anesthetized with a ketamine-xylazine mixture (50 and10 mg/kg ip, respectively) and a midline incision was then made to insert the catheters into the left carotid artery and right jugular vein. Mean arterial pressure (MAP) and right ventricle systolic pressure (RVSP) measurements were made as described previously (8) using a PowerLab Data Acquisition System and analyzed with the LabChart System (AD Instruments Inc.). After hemodynamic measurements, the rats were euthanized to harvest heart and lung tissues. Tissues were either fixed in 4% paraformaldehyde or snap-frozen for further experiments as described previously (8). Trichrome staining was carried out on the right ventricle to assess the collagen deposition. Cardiomyocyte diameter size was measured using NIH Image J software (53).
Morphometric Analysis
Quantification of vessel thickness was done by scanning the paraffin-embedded slides into the Aperio system. Subsequently, vessels were divided into three groups: greater than 100 µm, between 50 and 100 µm, and less than 50 µm. Approximately 12–15 vessels per lobe from each size group were measured per rat and then averaged to obtain median wall thickness as described previously (8).
Isolation of Rat Pulmonary Microvascular Endothelial Cells
Rat pulmonary microvascular endothelial cells (RPMECs) were isolated from the left lung lobe. Briefly, the lung tissue was washed in chilled DMEM, chopped, and digested with collagenase. The suspension was then passed through an 18-gauge syringe to make a single-cell suspension and a 100-µm cell strainer (BD Biosciences). The cells were then treated with endothelial cell-specific antibodies [CD31 (BD Biosciences), CD105 (Abcam), and biotin-conjugated Isolectin B4 (Vector Laboratories)] and subsequently with IgG and streptavidin microbeads (Miltenyi Biotec). The magnetic bead-labeled endothelial cells were then pulled using MACS columns. The cells were cultured on a six-well plate in rat endothelial cell media (ECM; Cell Applications) and used until six passages. The purity of cells was checked using endothelial cell-specific markers [von Willebrand factor VIII (vWF) and CD31] and a negative marker [α-smooth muscle actin (SMA)].
Human Pulmonary Microvascular Endothelial Cell Culture and Treatments
The human primary pulmonary microvascular endothelial cells (HPMECs; ScienCell Laboratories, 3000) were grown in endothelial cell basal medium containing 5% fetal bovine serum (FBS), endothelial cell growth supplements, and penicillin-streptomycin (ScienCell Laboratories,1001). At 80% confluency, the medium was replaced with endothelial cell medium containing 0.5% FBS. Cells were then treated with morphine (1 µM, M8777; Sigma-Aldrich) in the presence or absence of recombinant HIV-Tat (25 ng/mL, no. HIV-129; ProSpec) daily for various time intervals. The concentration of morphine and HIV-Tat was based on our previous published findings (6, 61). For oxidative stress inhibitory studies, cells were treated with the NOX inhibitor VAS3947 (10, 25, 50, and µM), the xanthine oxidase inhibitor allopurinol (25, 50, and 100 µM), and mitochondrial inhibitor MitoTempo (25, 50, and 100 µM) for 15 min before morphine and Tat treatment. For transfection experiments, 50% confluent HPMECs were transfected with 5 nM siRNA against NOX2 or NOX4 (ThermoFisher Scientific) or scrambled siRNA using Fugen transfection reagent (Promega) in 0.5% ECM. After 24 h of transfection, media was replaced with fresh 0.5% ECM followed by addition of morphine and HIV-Tat. For autophagic flux experiments, HPMECs were pretreated with 10 nM of bafilomycin (Sigma-Aldrich, 11707) in the presence or absence of NADPH oxidase inhibitor, VAS3947, followed by addition of morphine and Tat for 6 h and subsequently protein extraction for Western blot analysis.
Immunostaining
Immunohistochemical staining was performed on the paraformaldehyde-fixed paraffin-embedded rat lung sections with α-SMA (Abcam) and vWF marker (DakoCytomation) as described previously (8). For immunofluorescence, AlexaFluor 488 anti-rabbit and AlexaFluor 594 anti-mouse secondary antibodies were used. Images were captured using a confocal microscope.
For immunocytochemistry, HPMECs treated with or without morphine and Tat in the presence or absence of 10 nM bafilomycin were fixed in 4% paraformaldehyde and permeabilized with 100% methanol followed by immunostaining using LC3B antibody (Cell Signaling Technology) and AlexaFluor 488 secondary antibody. Images were captured using a Lion Heart imaging system (Biotek Corporation).
Quantification of Oxidative Stress
DNA oxidative damage.
Whole lung tissues from WT/HIV-Tg rats treated with morphine or saline were lysed for DNA isolation using a DNA extraction kit (Qiagen). DNA was then digested using nuclease followed by quantification of oxidatively damaged DNA (8-hydroxydeoxyguanosine residues) using a DNA/RNA Oxidative damage ELISA kit (Cayman Chemicals) per the manufacturer’s instruction.
Lipid hydroperoxide assay.
Whole rat lung tissue lysates from all groups were deproteinized followed by extraction of lipid hydroperoxides using chloroform. The lipid hydroperoxides were then measured using lipid hydroperoxide assay (Cayman Chemicals) per the manufacturer’s instructions.
DCF assay.
To measure total intracellular ROS, pulmonary endothelial cells were plated onto a 96-well plate (6,000 cells/well), and at 80% confluency cells were incubated with specific ROS inhibitors followed by morphine (1 mM) and Tat (25 ng/mL) treatment after 15 min. At 3 h posttreatment, cells were washed with serum-free medium and incubated with 15 mM 5-(and -6)-carboxy-29,79-dichlorodihydroflourescein diacetate (DCFH-DA; Molecular Probes, Inc.) for 30 min. Cells were then lysed, and the amount of ROS generated was quantified by a fluorescent plate reader at an excitation of 485 nm with an emission of 530 nm.
RPMECs isolated from WT or HIV-Tg rats were also plated on 96-well plates (1.25 × 104 cells/well) and cultured in complete ECM. After 48 h of plating, cells were incubated in 0.5% serum containing ECM for 3 and 12 h followed by addition of DCFH-DA dye to measure ROS as mentioned above. RPMECs were also plated in 24-well plates and stained with dihydroethidium (DHE) dye for visualization of oxidative stress visualized using a Lion Heart imaging system (Biotek Corporation).
NADPH Oxidase Activity
NADPH oxidase activity was measured in a cell-free system as described by others (56). In brief, confluent HPMECs in a T-25 flask were treated with morphine and Tat for 3 h. Membrane fraction from total cell extract was isolated using a Mem-PER Plus Membrane Protein Extraction Kit according to the manufacturer’s instructions (ThermoFisher Scientific). For determining NADPH oxidase activity, 50 µg of membrane protein were incubated with 10 mM phosphate buffer, 130 mM NaCl, 1 mM EGTA, 10 µM flavin adenine dinucleotide, 2 mM sodium azide, 40 µM oxidized cytochrome c, and 10 µM guanosine 5′-triphosphate. In parallel, one reaction of each pair contained 200 units of superoxide dismutase. To initiate the reaction, 200 µM of NADPH substrate were added, and, after 1 h, NADPH oxidase activity was measured as SOD-inhabitable increase in the absorbance at 550 nm.
Cell Death Assay
HPMECs (3,000 cells/well) were treated with allopurinol, VAS3947, and MitoTempo (25 and 50 µM) 30 min before morphine and Tat treatment for 3 days followed by cell death/oligonucleosome detection ELISA (1544675001; Roche Applied Science) as described previously (7). Alternatively, cell death assay was also performed on Nox/scrambled siRNA-transfected cells treated with or without morphine and Tat.
Furthermore, RPMECs isolated from WT or HIV-Tg rats treated with morphine and saline controls were plated on 96-well plates (1.25 × 104 cells/well) and grown in complete rat endothelial cell medium (ECM). After 24 h, the media was changed to 0.5% serum containing ECM in the presence or absence of VAS3947 (5 µM) for 24 and 48 h followed by cell death ELISA.
Cell Proliferation Assay
HPMECs were treated with allopurinol, VAS3947, and MitoTempo (25 and 50 µM) 30 min before morphine and Tat treatment for 3 and 6 days followed by cell proliferation assay using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to the manufacturer’s instructions. Alternatively, MTS proliferation assay was performed on Nox siRNA/scrambled transfected cells treated with or without morphine and Tat for 3 and 6 days. For assessing endothelial cell survival in rats, RPMECs (3 × 103 cells/well) were plated, and assay was performed at days 2 and 4 after changing the media to 0.5% serum containing ECM in the presence or absence of VAS3947 (5 µM).
Subcellular Fractionation and Western Blot Analysis
Mitochondrial and microsomal fractions were purified with an Abcam microsome isolation kit (ab206995) per the manufacturer’s instruction. In brief, approximately 6 × 106 cells were pelleted down by centrifugation at 700 g for 5 min at 4°C and sonicated in the homogenization buffer. The homogenate was then centrifuged at 10,000 g for 15 min to separate the mitochondrial fraction as pellet. The supernatant was again centrifuged at 20,000 g for 20 min to pellet down the microsomal fraction.
For Western blots, total cellular protein or protein from mitochondrial and microsome fractions was extracted using radioimmunoprecipitation assay lysis buffer (sc-24948; Santa Cruz Biotechnology). The specificity of fractions was determined by analyzing the expression of mitochondrial specific marker hsp60 using Western blotting (Supplemental Fig. S4; see https://doi.org/10.6084/m9.figshare.11954391). In addition, Western blots were performed to detect the expression of NOX1 (Santa Cruz Biotechnology), NOX2 (Santa Cruz Biotechnology), NOX4 (Abcam), and MAP1LC3B (Cell Signaling Technology) as described previously (15). The blots were stripped and reprobed with β-actin antibody as housekeeping protein for loading control. The NIH ImageJ software was used for densitometric analysis of immunoblots.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) with post hoc Bonferroni correction for multiple comparisons using GraphPad Prism 7 software. Results were judged statistically significant when the Bonferroni-corrected P value was less than 0.05.
RESULTS
NADPH Oxidase is a Major Source of ROS Generation in Morphine and HIV-Tat-treated HPMECs
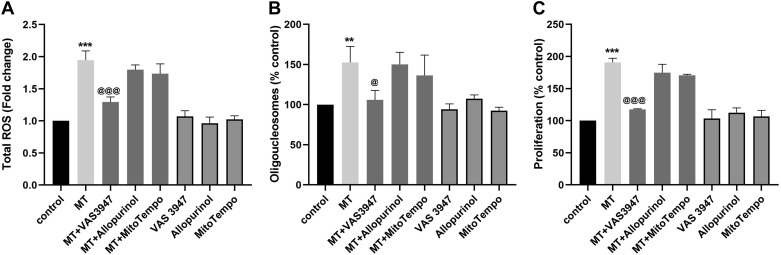
In our previous published findings, we reported that the daily treatment of HPMECs with morphine augments the effect of HIV-Tat-mediated initial apoptosis, with maximum increase in apoptosis at day 3 posttreatment followed by hyperproliferation that peaks at day 6 (7). We also showed that the initial burst of ROS with the maximum increase at 3 h in response to the combined treatment of morphine and HIV-Tat (MT) was partly involved in promoting the early phase of apoptosis. However, continued generation of suboptimal oxidative stress in response to daily MT treatment resulted in autophagy-dependent hyperproliferation of apoptosis-resistant HPMECs (7, 64). To further determine which specific intracellular source is majorly involved in the initial ROS burst in response to MT treatment, we performed DCFH-DA assay in the presence and absence of inhibitors targeting ROS-producing enzyme, namely, VAS3947 to inhibit NOX, allopurinol to inhibit xanthine oxidase-mediated ROS, and MitoTempo to scavenge mitochondrial superoxides. As shown in Fig. 1A, HPMECs pretreated with panNOX inhibitor: VAS3947 at the concentration of 25µM was able to ameliorate MT-mediated increase in ROS levels at 3h post treatment. However, no significant decrease in total ROS levels was observed in HPMECs when pretreated with MitoTempo or allopurinol at the same concentration. However, at higher concentrations of 50 and 100 µM, all three inhibitors were able to prevent MT-mediated ROS/ generation in HPMECs (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.11096168).
Fig. 1.
Inhibition of NADPH oxidase-specific oxidative stress in human pulmonary microvascular endothelial cells (HPMECs) prevents morphine- and Tat-mediated early apoptosis and late hyperproliferation. DCF assay at 3 h (A), cell death assay at day 3 (B), and MTS assay (C) at day 6 posttreatment of HPMECs with morphine (1 µM) and HIV-Tat (25 ng/mL) in the presence or absence of 25 µM of specific inhibitors as follows:VAS3947, allopurinol, or MitoTempo. ROS, reactive oxygen species. Means ± SD of at least 3 independent experiments. **P < 0.01, ***P < 0.001 vs. control. @@@P < 0.001, @P < 0.05 vs. morphine and HIV-Tat (MT).
We next examined the direct effect of specific ROS inhibition on the switching of morphine- and Tat-treated endothelial cells from an early apoptotic state to a later hyperproliferative state. As reported, MT treatment resulted in significant increased endothelial cell death as measured at day 3 posttreatment and significant increased endothelial proliferation as measured at day 6 posttreatment (Fig. 1, B and C). However, the presence of 25 µM NOX inhibitor VAS3947 prevented this morphine- and Tat-mediated increased apoptosis and proliferation of endothelial cells. Similar to the findings from DCFH-DA assay, inhibition of mitochondrial- or xanthine oxidase-specific ROS was not able to prevent MT-mediated increased apoptosis and proliferation of endothelial cells (Fig. 1, B and C).
NOX2 and NOX4 Isoforms Are Involved in Morphine and Tat-Induced Early Apoptosis and Later Proliferation of Endothelial Cells
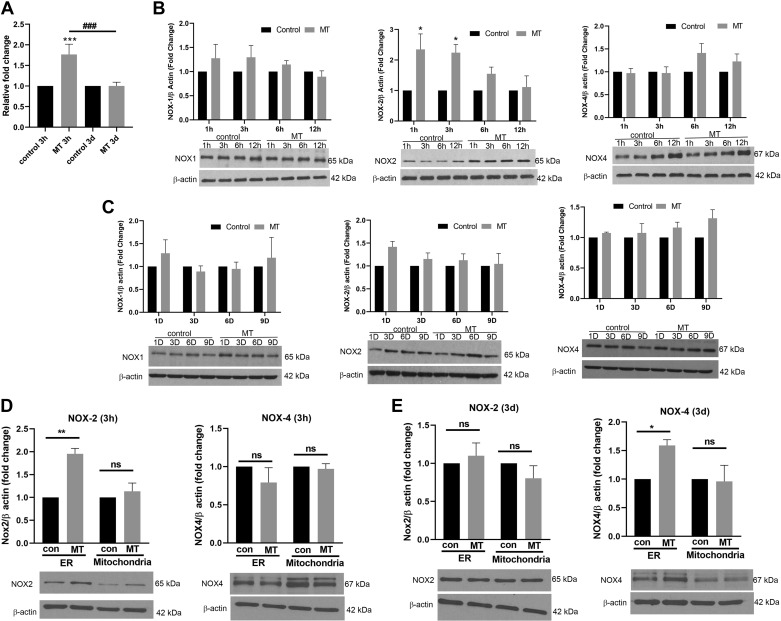
We next determined the changes in total NOX activity and the expression of three major NOX isoforms in HPMECs at early (3 h) and later (3 days) time points of daily MT treatment. As shown in Fig. 2A, we observed a significant increase in NOX activity in the total cellular extract from 3 h MT-treated HPMECs compared with untreated controls. However, no difference in the NOX activity was observed at 3 days posttreatment. In addition, as represented by the densitometry graphs in Fig. 2B, significant increase in NOX2 expression (>2-fold) was observed at 1 and 3 h posttreatment with respect to control. However, no significant change was observed in NOX1 and NOX4 expression at early time points (Fig. 2, B and C). Furthermore, continued daily exposure of HPMECs to MT showed no significant change in any of the NOX isoforms in HPMECs (Fig. 2C) compared with untreated control at later timepoints although a trend toward increased expression of NOX2 at day 1 and NOX4 at days 6 and 9 posttreatment was observed.
Fig. 2.
Elevated NADPH oxidase (NOX) activity and sequential increased expression of endoplasmic reticulum (ER)-specific NOX2 and NOX4 in morphine and HIV-Tat-treated human pulmonary microvascular endothelial cells (HPMECs). Confluent HPMECs were treated with 1 μM morphine and/or 25 ng/mL of HIV-Tat in 0.5% serum-containing medium (A–C). A: NOX activity at 3 h and 3 days after morphine and HIV-Tat (MT) treatment. B and C: HPMECs treated with morphine and/or HIV-Tat were harvested at timepoints 1 h to 12 h (B) and 1 day to 9 days (C) followed by Western blotting for the analysis of NOX1, -2, and -4 expression. D and E: confluent HPMECs were treated with 1 μM morphine and 25 ng/mL of Tat in 0.5% serum-containing medium for 3 h (D) and 3 days (E) followed by isolation of mitochondrial and ER fractions for the analysis of NOX2 and NOX4 expression by Western blotting. All the respective blots were stripped and reprobed for β-actin antibody used as loading control (con). NOX1, -2, and -4 were run with the same samples on different gels due to similar molecular weight. Bar graphs show densitometry analysis of protein expression at various time points from at least 3 independent experiments. Values are means ± SD of at least 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; ns, not significant.
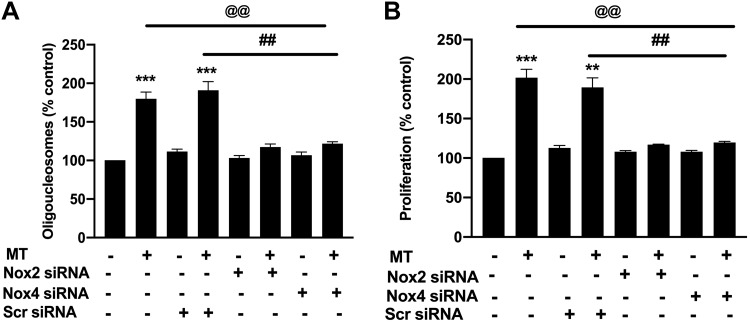
Interestingly, the analysis of mitochondrial and ER fractions found a significant increase in the expression of NOX2 in the ER fraction but not in the mitochondrial fraction of 3 h treated HPMECs and no change in the NOX4 expression in either fraction when compared with the fractions from untreated controls (Fig. 2D). Notably, in HPMECs exposed to the chronic treatment of MT for 3 days, we observed opposite effects where NOX4 was upregulated in the ER fraction and NOX2 was unchanged in both mitochondrial and ER fractions with respect to untreated control (Fig. 2D). To confirm the role of NOX2 and NOX4 in endothelial apoptosis and later survival, we inhibited the expression of NOX2 or NOX4 with specific siRNA before MT treatment. The attenuated expression of NOX2 and NOX4 was confirmed by Western blot (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.11096375). As illustrated in Fig. 3, knockdown of either NOX2 or NOX4 prevented the MT-mediated early apoptosis and later proliferation of HPMECs. However, cells transfected with scrambled siRNA showed significant increase in apoptosis and proliferation in the presence of morphine and Tat (Fig. 3, A and B).
Fig. 3.
Knockdown of NADPH oxidase (NOX) prevents morphine and Tat-mediated human pulmonary microvascular endothelial cell (HPMEC) early apoptosis and later hyperproliferation. HPMECs at 50% confluency were transfected with NOX2 or NOX4-specific siRNA (5 nM) for 24 h and then treated with morphine (1 μM) and HIV-Tat (25 ng/mL) followed by cell death ELISA at 3 days posttreatment (A) or MTS proliferation assay at 6 days posttreatment (B). Values are means ± SD of at least 3 independent experiments. **P < 0.01, ***P < 0.001 vs. control. @@P < 0.01 vs. morphine and Tat (MT) alone. ##P < 0.01 vs. morphine and Tat (MT) transfected with scrambled (Scr) siRNA.
Morphine and Tat-Mediated Induction of Autophagy in HPMECs Is Dependent On NOX
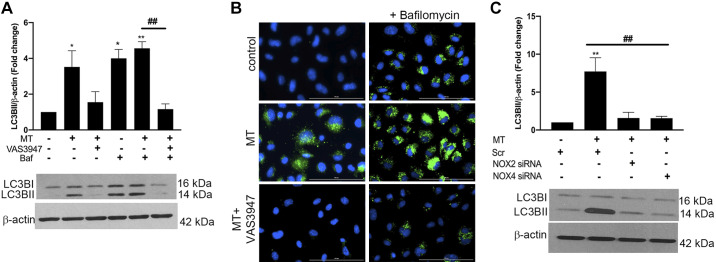
Furthermore, we investigated whether inhibition of NOX regulates MT-mediated augmentation of autophagy in endothelial cells. Consistent with our previous published findings (7), we saw increased expression of LC3BII levels in HPMECs treated with MT at 6 h. However, inhibition of NOX-specific ROS before MT treatment resulted in the reduction of MT-mediated increased expression of LC3B II both in the presence or absence of bafilomycin (Fig. 4A). Concomitantly, immunostaining also revealed a decrease in the number of LC3B puncta in the cells treated with MT in the presence of VAS3947 compared with MT treatment (Fig. 4B). Next, to further confirm the role of specific NOX2 or NOX4 in promoting autophagy, we transfected cells with siRNA against NOX2 or -4 before MT treatment and looked for the expression of LC3BI/II. As shown in Fig. 4C, attenuation of both NOX2 and -4 expression in HPMECs rescued the MT-mediated increase in LC3B II levels. These findings suggest that ROS production due to activation of NOXs in response to MT in HPMECs is directly involved in induction of autophagy, therefore, promoting cell survival.
Fig. 4.
Inhibition or knockdown of NADPH oxidase attenuates morphine- and Tat-mediated autophagy flux. A–C: confluent human pulmonary microvascular endothelial cells (HPMECs) were treated with VAS3947 (25 μM) 30 min before morphine and HIV-Tat addition in the absence or presence of bafilomycin (Baf) for 6 h followed by Western blotting (A and C) and immunocytochemistry (B) for the analysis of LC3B. B: for LC3B staining, cells were fixed with 4% paraformaldehyde post 6 h of treatment and viewed under a Lion Heart fluorescent imaging station at ×40 magnification (scale: 100 µm). C: at 50% confluency, HPMECs were transfected with scrambled (Scr) or NADPH oxidase 2 (NOX2) or NOX4 siRNA (5 nM) for 48 h followed by morphine and HIV-Tat (MT) treatment. All of the respective blots were stripped and reprobed for β-actin antibody used as loading control. Bar graphs show densitometry analyses of protein expression from at least 3 independent experiments. Values are means ± SD of at least 3 independent experiments *P < 0.05, **P < 0.01 vs. control. ##P < 0.001 vs. morphine and HIV-Tat (MT).
Morphine Increases Pulmonary Vascular Remodeling and Right Ventricular Systolic Pressure in HIV-Transgenic Rats
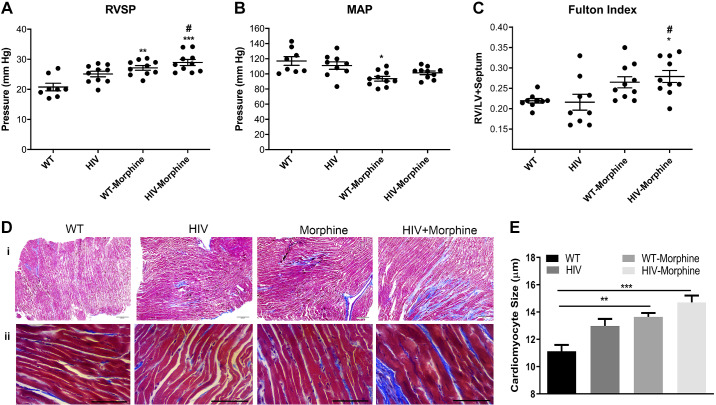
Next, we used a noninfectious HIV-transgenic (Tg) rat model expressing all viral genes except gag and pol (8, 15, 64) for in vivo studies. We did hemodynamic measurements in HIV-Tg rats treated with (HIV-morphine group) or without morphine (HIV group) and in age- and sex-matched wild-type Fisher rats treated with (WT-morphine group) or without morphine (WT group). As shown in Fig. 5A, there was a significant increase in the right ventricular systolic pressure (RVSP) in both HIV-Tg and WT male rats administered with morphine compared with WT male rats. Importantly, increase in RVSP in HIV-morphine rats was also significantly more compared with RVSP of rats from the HIV group. In contrast, there were no differences in mean arterial pressure (MAP) in HIV-morphine rats compared with all other groups (Fig. 5B). Furthermore, male rats from the HIV-morphine group showed significant right ventricle (RV) hypertrophy as depicted by the Fulton Index (RV/LV + septum ratio) compared with male rats from the WT group or HIV group (Fig. 5C). Interestingly, HIV-Tg and WT female rats did not exhibit any RV changes on exposure to morphine (Supplemental Fig. S3; see https://doi.org/10.6084/m9.figshare.11096384). Trichrome staining of the RV tissue demonstrated increased collagen deposition in the HIV-morphine group compared with the other groups (Fig. 5Di), suggesting fibrosis and impaired contractility. In addition, significant increase in cardiomyocyte size due to hypertrophy was observed in both WT and HIV-Tg rats treated with morphine compared with the WT group (Fig. 5, Dii and E).
Fig. 5.
Hemodynamics and right ventricle (RV) hypertrophy in HIV-transgenic (HIV-Tg) rats exposed to morphine. HIV-Tg or wild-type (WT) rats were administered 10 mg/kg body wt of morphine daily for 21 days. HIV-Tg/WT saline controls were used for comparison. Right ventricle systolic pressure (RVSP, A), mean arterial pressure (MAP, B), and Fulton Index (C) from n = 8 or more rats/group. RV, right ventricle; LV, left ventricle. D: Masson’s trichrome staining on formaldehyde-fixed paraffin-embedded heart RV sections. Magnification ×4 (scale: 200 µm, i) and ×40 (scale: 50 µm, ii). E: quantification of cardiomyocyte size in morphine or saline-treated HIV-Tg/WT rats. Values are means ± SE obtained from n = 6 rats/group. ***P < 0.001,**P < 0.01, and *P < 0.05 vs. WT. #P < 0.05 vs. HIV.
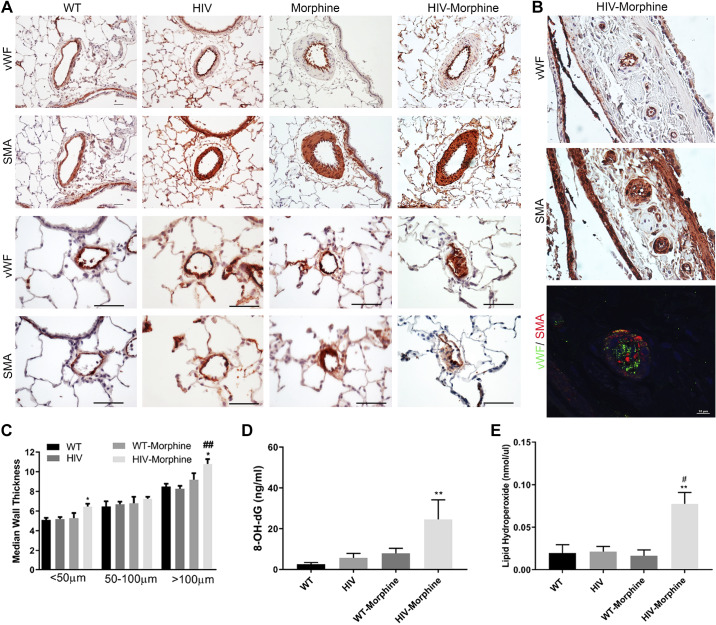
Concomitant to the right heart dysfunction, the pulmonary vessels showed remarkable thickening of the smooth muscle layer in the pulmonary artery vessels of size >100 and <50 µm (Fig. 6, A and C) in HIV-Tg morphine-treated rats compared with the other three groups. We also observed many vessels with disruption of the endothelial monolayer corresponding to endothelial blebbing and endothelial proliferation. The vasa vasorum arteriopathy was also present in rats from the HIV-morphine group, showing significant endothelial proliferation and smooth muscle thickening as shown in Fig. 6B.
Fig. 6.
Immunohistochemical analysis of pulmonary vessels in HIV-transgenic (HIV-Tg) rats exposed to morphine. Right lung was harvested, fixed, paraffin embedded, and sectioned followed by immunostaining for von Willebrand factor (vWF) and α-smooth muscle actin (α-SMA). A and B: representative pictographs showing remodeled pulmonary vessels of size >50 μm (magnification ×20; scale: 50 µm) and of size <50 μm (magnification ×40; scale: 25 µm) (A). Immunofluorescence staining of lung sections from HIV-morphine rat showing vasa vasorum arterioles stained for vWF (green) and α-SMA (red); scale bar = 10 μm (B). C: median smooth muscle wall thickness as assessed by measuring inner and outer diameter of remodeled arteries and arterioles. DNA damage (D) and lipid peroxidation (E) by measuring 8-OH-dG or lipid hydroperoxide levels, respectively, in whole lung tissue homogenate using ELISA in whole lung tissue lysates. Values are means ± SE of n = 6 rats/group. **P < 0.01, *P < 0.05 vs. WT. ##P < 0.01, #P < 0.05 vs. HIV.
Increased Oxidative Stress in the Lungs from HIV-Transgenic Rats Treated with Morphine
The HIV-morphine group showed significant high levels of 8′-hydroxy-2′-deoxyguanosine residues suggestive of oxidative DNA damage and endogenous lipid hydroperoxides due to lipid oxidation in lung tissues compared with WT rats (Fig. 6, D and E). All these parameters suggest the presence of augmented oxidative stress in HIV-Tg rats administered with morphine.
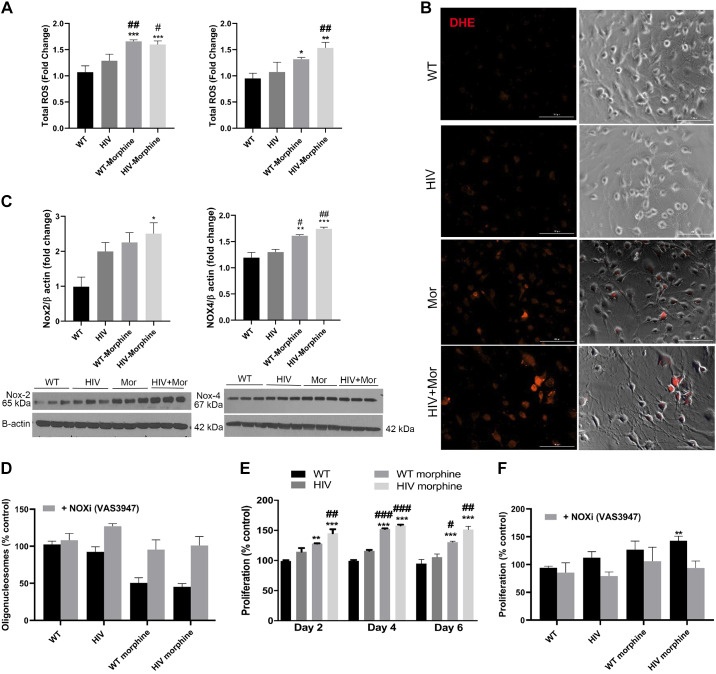
Moreover, RPMECs isolated from these rats also exhibited high levels of ROS generation as determined by DCF assay at 3 h and 12 h of starvation stress due to depletion of serum in the culture media (Fig. 7A). Furthermore, ROS levels were also significantly elevated in RPMECs from the WT-morphine group compared with the placebo WT group (Fig. 7A). These changes were further confirmed by DHE staining, which detects superoxide ions generated due to oxidative stress. The RPMECs isolated from HIV-morphine rats showed increased positivity for DHE staining compared with cells isolated from all other groups (Fig. 7B). Similar to the above-mentioned in vitro studies, we observed enhanced expression of both NOX2 and NOX4 in RPMECs from HIV-morphine group rats and the WT morphine group compared with RPMECs from WT controls (Fig. 7C).
Fig. 7.
Increased oxidative stress in endothelial cells isolated from HIV-transgenic (HIV-Tg) rats administered with morphine. A: RPMECs (3,000 cells/well) were plated on a 96-well plate, and, at 80% confluency, cells were serum starved using 0.5% serum-containing media for 3 (left) and 12 (right) h followed by DCF assay. ROS, reactive oxygen species; WT, wild type. B: for DHE staining, confluent rat endothelial cells were serum starved for 3 h followed by incubation with DHE dye (5 nM) for 30 min. The cells were then fixed with 4% paraformaldehyde, and images were captured using a Lion Heart imaging station (scale: 100 µm). Mor, morphine. C: confluent RPMECs were serum starved for 3 h followed by Western blotting for NADPH oxidase 2 (NOX2) and NOX4 expression. All the respective blots were stripped and reprobed for β-actin antibody used as loading control. NOX2 and -4 were run with the same samples on different gels due to similar molecular weight. D: RPMECs were serum starved 24 h after plating of cells in a 96-well plate followed by cell death ELISA at day 2 posttreatment in the presence or absence of VAS3947. E and F: MTS proliferation assay at days 2, 4, and 6 (E) and at day 4 (F) posttreatment in the presence or absence of VAS3947. Values are means ± SE of n = 3 rats/group. **P < 0.01, *P < 0.05, ***P < 0.001 vs. WT. ##P < 0.01, #P < 0.05, ###P < 0.001 vs. HIV.
Initial Apoptosis and Later Proliferation of RPMECs Isolated from HIV-Morphine Rats
Furthermore, we evaluated the cell apoptosis and survival of RPMECs isolated from morphine-treated HIV-Tg or WT rats in response to serum starvation, in the presence and absence of NOX inhibitor. As shown in Fig. 7D, HIV-morphine RPMECs exhibited a trend toward decreased endothelial cell apoptosis. However, significant increase in the cell survival was observed compared with RPMECs isolated from WT rats and compared with HIV rats (Fig. 7E). The RPMECs from WT-morphine rats also showed significant increase in cell survival compared with cells from WT or HIV rats. Finally, treatment of RPMECs by Nox inhibitor VAS3947 (5 µM) reversed the downward trend of EC apoptosis and prevented the increased proliferation (Fig. 7F) of HIV-morphine RPMECs in serum-starved conditions.
DISCUSSION
Increased survival of people living with HIV with the use of antiretroviral therapy has consequently led to an increase in the prevalence of noninfectious complications, including PAH (1, 5, 6, 13, 37, 55, 71). Among HIV-infected individuals, intravenous drug use (IVDU) is one of the major risk factors in the development of PAH (22, 33, 48, 49). Therefore, it is valuable to understand how drugs of abuse act in concert with HIV infection, leading to enhanced severity in PAH phenotype (21). In this context, we previously reported enhanced pulmonary arteriopathy in the lungs from HIV-infected IVDUs who abused mainly cocaine and/or opioids (15). Furthermore, we used nonhuman primates and HIV-transgenic rats to understand the direct effect of the “double hit” of HIV infection and illicit drug use (8, 64). We observed enhanced vascular remodeling and formation of complex plexiform lesions in SIV-infected macaques exposed to morphine. Similarly, increased RVSP and RV fibrosis along with significant pulmonary vascular remodeling was reported in HIV-Tg rats exposed to cocaine (8, 64). In this study, we now report augmented pulmonary vascular remodeling with significant increase in RVSP and RV hypertrophy in HIV-Tg rats on chronic treatment with morphine. Histological findings showed significant smooth muscle remodeling with the blebbing and proliferation of endothelial cells within the pulmonary vessels, specifically in the small precapillary arterioles in the vasa vasorum that is known to cause vasoconstriction and the development of severe PAH pathology, including plexogenic arteriopathy (65–67).
Interestingly, we found significant changes in hemodynamic measurements or Fulton Index only in the male and not in the female HIV-Tg rats on exposure to morphine. This may be attributed to the fact that female sex hormones like estrogen decrease the vascular oxidative stress by regulating the expression of antioxidant enzymes (3, 42, 46, 54). Importantly, 17β-estradiol has earlier been reported to inhibit NOX2 expression and ROS generation in human umbilical vascular endothelial cells (69). Reduction in the NADPH oxidase activity has also been reported in the cerebral arteries from female rats, and this was found to be associated with estrogen-dependent attenuation in the expression of NOX1 and NOX4 (50). Furthermore, our findings are consistent with a previous report demonstrating lower vulnerability of female rats to monocrotaline-induced PH, and this was associated with the reduced superoxide dismutase and glutathione-S-transferase activity along with increased catalase activity in female rats compared with male rats (3). Lahm et al. also reported the direct protective effect of 17β-estradiol in the hypoxia model of PAH (39).
Endothelial dysfunction is a major cellular event in the pathogenesis of PAH, which is responsible for precapillary vessel occlusion and formation of complex angioobliterative plexiform lesions in the pulmonary vessels (30, 59). HIV proteins like Nef, gp120, and Tat have been extensively studied for their role in apoptosis and proliferation of endothelial cells (34, 47). Similarly, morphine is also known to activate proapoptotic and prosurvival signaling in endothelial cells (44), and we demonstrated that dual hit of HIV/SIV proteins and morphine results in the synergistic increase in initial apoptosis and later proliferation of apoptosis-resistant endothelial cells associated with the development of fully and partially occluded plexiform and other advanced angioproliferative lesions (64). We now further corroborate these findings by reporting initial apoptosis and later increased survival and proliferation of RPMECs isolated from HIV-morphine rats in response to serum starvation.
Oxidative stress has been known to play a pivotal role in endothelial injury-mediated vascular dysfunction (28) and PAH pathogenesis (2, 28, 57, 64). Moreover, elevated oxidative stress is a known phenomenon in patients with HIV infection (32, 61) and substance abuse, including morphine (16, 62). Importantly, we previously reported chronic generation of and H2O2 until day 9 in endothelial cells treated with morphine and HIV-Tat. We observed that the ROS production was at its peak at early timepoints, contributing to endothelial cell death. However, with chronic exposure to Tat and morphine, the ROS amount dropped but still remained higher than the baseline levels in untreated controls. This suboptimal chronic increase in oxidative stress was enough for chronic activation of adaptive autophagy needed to promote endothelial cell survival (7).
Now, in this study, we specifically demonstrate the role of NOX-specific ROS generation in morphine- and Tat-mediated early apoptosis and later hyperproliferation of apoptotic-resistant HPMECs. NADPH oxidase catalyzes the transfer of electrons from cytosolic NADPH to molecular O2, leading to the generation of superoxide radicals and hydrogen peroxide (18, 58). Here we observed that, at 25 µM concentration, only VAS3947 was able to significantly rescue MT-mediated oxidative stress although, at higher concentrations, inhibition of mitochondrial or xanthine oxidase-dependent ROS also significantly abrogated the MT-induced ROS. This could be attributed to the fact that NOX enzymes are predominant ROS producers in vascular endothelial cells (10, 58), and xanthine oxidase-mediated ROS generation is dependent on NOX activity (40). Also, because NOXs are mainly expressed in intracellular membranes, including the mitochondrial membrane, MitoTempo, a mitochondrial ROS scavenger, will eliminate all free radicals generated in the mitochondria, including both electron transport chain- and NOX-mediated superoxide radicals. In this study, we were also able to effectively abolish the ROS-mediated early endothelial apoptosis and later proliferation of both MT-treated HPMECs and serum-starved RPMECs isolated from HIV-morphine rats using the NOX inhibitor VAS3947. All these findings suggest that morphine- and Tat-mediated dysfunction in endothelial cells is mainly dependent on NADPH oxidase-mediated induction of oxidative stress.
Superoxide generating NOX2 is an integral component of the plasma membrane and is known to promote apoptosis in vascular endothelial cells (18, 38). On the contrary, NOX4 is primarily localized in the intracellular membranes of mitochondria and endoplasmic reticulum (ER), and its ER-specific overexpression/activity is known to have a protective function in the vascular wall under chronic stress (60). Here we observed that the initial exposure of morphine and Tat to HPMECs results in the augmented expression of NOX2 in the total cellular extracts with no significant changes in NOX1 or NOX4 expression. Increased NOX2 expression in these cells was in line with our previous findings where we reported a synergistic increase in NOX2 expression in HPMECs on the combined treatment with cocaine and Tat, leading to Ras/Raf/ERK1/2 pathway-dependent disruption of tight junction proteins and hence enhanced permeability of the endothelial monolayer (9).
Alternatively, based on our current findings showing an ER-specific increase in NOX4 expression on chronic exposure of HPMECs to morphine and Tat, we here suggest the involvement of NOX4 in protecting the endothelial cells against chronic stress of HIV protein(s) and illicit drugs, therefore, promoting the cell-survival and switching the cells to an apoptotic-resistant hyperproliferative phase. This is supported by reported observations, including one suggesting the role of NOX4 in maintaining the vessel structure by releasing vessel-healing products, such as nitric oxide via generation of H2O2 (18). Notably, reports demonstrate NOX4 as a major player in the regulation of autophagy via ROS production (60, 63). The elevated NOX4 expression in the ER membrane in glucose-deprived cardiomyocytes and cardiomyocytes from ischemic mice has been reported to be responsible for the regulation of autophagy-dependent cardiomyocyte survival and protection during energy stress (60). Along these lines, we also observed a decrease in the MT-mediated induction of autophagy in HPMECs on the inhibition of NOX-mediated ROS. Therefore, we speculate that the ER-specific NOX4-mediated ROS generation in response to chronic exposure of morphine and Tat may be contributing to an autophagy-dependent regulation of endothelial cell proliferation and survival of HPMECs. However, we observed similar response of NOX2 or NOX4 silencing on endothelial apoptosis and proliferation, and this could be due to the ROS-dependent regulation in the expression of p22phox subunit common to both NOX isoforms (17). Initial inhibition of NOX2-mediated ROS generation may have altered the expression of p22phox and therefore indirectly NOX4 activity, leading to the inhibition of MT-mediated enhanced proliferation as well at later time points.
Interestingly, RPMECs from morphine-exposed HIV-Tg rats with increased RVSP and RV hypertrophy demonstrated significantly increased expression of NOX2 and NOX4 that correlated with the increased endothelial survival in serum-starved conditions. Previous studies in NOX2−/− mice and on arteries from patients with coronary artery disease suggest a role of NOX2-specific ROS in promoting endothelial dysfunction (11, 45). Increased NOX4 expression is reported in the pulmonary vascular media in patients with idiopathic pulmonary arterial hypertension (51). NOX4 was found to be highly expressed in both adventitial and endothelial layers of vessels in an MCT rat model of PAH, where it was responsible for ROS-mediated vascular remodeling and increase in RVSP and hypertrophy (4). Likewise, the role of NOX4 has been reported in the proliferation of adventitial smooth muscle cells and associated vascular remodeling in a hypoxia-induced mice model of PAH (23).
Moreover, morphine alone was able to increase RVSP and RV cardiomyocyte size in WT rats, and this corresponded with increased ROS, NOX4 expression in RPMECs that demonstrated increased survival in serum-starved conditions. However, all these effects were relatively less compared with the HIV-morphine group. Interestingly, morphine treatment tended to decrease systemic pressure, and this effect was significant in WT rats. Morphine is already known to act in a dual manner by enhancing both proapoptotic and prosurvival signals (31, 44) where it can promote both endothelial injury (16) and angiogenesis (25) in endothelial cells. We earlier observed similar effects of morphine on HPMECs where monotreatment of morphine led to increased ROS generation and apoptotic/proliferative behavior. We also observed that morphine is a more potent modulator of ROS compared with HIV-Tat. However, combination of HIV-Tat and morphine showed synergistic effects on ROS generation, apoptosis, and proliferation of pulmonary endothelial cells (7, 64). Furthermore, we also reported that lungs from macaques chronically treated with morphine exhibit higher vasculopathy compared with SIV-infected macaques although relatively far less compared with SIV-infected morphine-treated macaques (64).
In summary, this is the first report to our knowledge that demonstrates the involvement of NADPH oxidase-specific ROS in the endothelial dysfunction associated with the development of HIV-PAH. We speculate that the initial exposure of pulmonary endothelial cells to HIV-Tat in the presence of morphine results in the activation of NOX2, which in turn mediates endothelial apoptotic injury. However, continuous generation of ROS via ER-specific NOX4 in response to the chronic exposure of MT leads to chronic activation of adaptive autophagy and consequently cell survival and hyperproliferation. In light of NOX-derived ROS being pivotal not only in the development of PAH but also in the pathogenesis of various other chronic lung disorders (24), a number of NOX inhibitors are under various stages of development. However, to solely inhibit a specific isoform of NOX is a major challenge to develop efficient therapeutic drugs, and future research aiming at the development and validation of innovative drugs is needed.
GRANTS
This work was supported by National Institutes of Health Grants R01 DA034542, R01 DA042715, and R01 HL129875 awarded to N.K.D.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A., H.S., and N.K.D. conceived and designed research; S.A., H.S., and L.C. performed experiments; S.A., H.S., L.C., and N.K.D. analyzed data; S.A., H.S., and N.K.D. interpreted results of experiments; S.A. and H.S. prepared figures; S.A. and H.S. drafted manuscript; S.A. and N.K.D. edited and revised manuscript; N.K.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Julie Allen, Department of Molecular and Integrative Physiology, KUMC, for helping in animal surgery and Michel Wulser, Department of Internal Medicine, KUMC, for morphometric analysis.
REFERENCES
- 1.Almodovar S. The complexity of HIV persistence and pathogenesis in the lung under antiretroviral therapy: challenges beyond AIDS. Viral Immunol 27: 186–199, 2014. doi: 10.1089/vim.2013.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelova P, Davidoff M. Immunocytochemical demonstration of substance P in hamster Leydig cells during ontogenesis. Z Mikrosk Anat Forsch 103: 560–566, 1989. [PubMed] [Google Scholar]
- 3.Bal E, Ilgin S, Atli O, Ergun B, Sirmagul B. The effects of gender difference on monocrotaline-induced pulmonary hypertension in rats. Hum Exp Toxicol 32: 766–774, 2013. doi: 10.1177/0960327113477874. [DOI] [PubMed] [Google Scholar]
- 4.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 109: 159–165, 2004. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 6.Butrous G. Human immunodeficiency virus-associated pulmonary arterial hypertension: considerations for pulmonary vascular diseases in the developing world. Circulation 131: 1361–1370, 2015. doi: 10.1161/CIRCULATIONAHA.114.006978. [DOI] [PubMed] [Google Scholar]
- 7.Dalvi P, Sharma H, Chinnappan M, Sanderson M, Allen J, Zeng R, Choi A, O’Brien-Ladner A, Dhillon NK. Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: role in HIV-related pulmonary arterial hypertension. Autophagy 12: 2420–2438, 2016. doi: 10.1080/15548627.2016.1238551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalvi P, Spikes L, Allen J, Gupta VG, Sharma H, Gillcrist M, Montes de Oca J, O’Brien-Ladner A, Dhillon NK. Effect of cocaine on pulmonary vascular remodeling and hemodynamics in human immunodeficiency virus-transgenic rats. Am J Respir Cell Mol Biol 55: 201–212, 2016. doi: 10.1165/rcmb.2015-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalvi P, Wang K, Mermis J, Zeng R, Sanderson M, Johnson S, Dai Y, Sharma G, Ladner AO, Dhillon NK. HIV-1/cocaine induced oxidative stress disrupts tight junction protein-1 in human pulmonary microvascular endothelial cells: role of Ras/ERK1/2 pathway. PLoS One 9: e85246, 2014. doi: 10.1371/journal.pone.0085246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damico R, Zulueta JJ, Hassoun PM. Pulmonary endothelial cell NOX. Am J Respir Cell Mol Biol 47: 129–139, 2012. doi: 10.1165/rcmb.2010-0331RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AC. The legislative process of the National Cancer Act, 1970-71. Problems and resolutions. Cancer 78: 2585–2589, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 12.de Raaf MA, Schalij I, Gomez-Arroyo J, Rol N, Happé C, de Man FS, Vonk-Noordegraaf A, Westerhof N, Voelkel NF, Bogaard HJ. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respir J 44: 160–168, 2014. doi: 10.1183/09031936.00204813. [DOI] [PubMed] [Google Scholar]
- 13.Degano B, Guillaume M, Savale L, Montani D, Jaïs X, Yaici A, Le Pavec J, Humbert M, Simonneau G, Sitbon O. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS 24: 67–75, 2010. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 14.Delgado JF, Conde E, Sánchez V, López-Ríos F, Gómez-Sánchez MA, Escribano P, Sotelo T, Gómez de la Cámara A, Cortina J, de la Calzada CS. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail 7: 1011–1016, 2005. doi: 10.1016/j.ejheart.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon NK, Li F, Xue B, Tawfik O, Morgello S, Buch S, Ladner AO. Effect of cocaine on human immunodeficiency virus-mediated pulmonary endothelial and smooth muscle dysfunction. Am J Respir Cell Mol Biol 45: 40–52, 2011. doi: 10.1165/rcmb.2010-0097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding WG, Zhou HC, Cui XG, Li WZ, Guo YP, Zhang B, Liu W. Anti-apoptotic effect of morphine-induced delayed preconditioning on pulmonary artery endothelial cells with anoxia/reoxygenation injury. Chin Med J (Engl) 121: 1313–1318, 2008. doi: 10.1097/00029330-200807020-00013. [DOI] [PubMed] [Google Scholar]
- 17.Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, Görlach A. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med 38: 616–630, 2005. doi: 10.1016/j.freeradbiomed.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab 25: 452–463, 2014. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Duffy P, Wang X, Lin PH, Yao Q, Chen C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res 156: 257–264, 2009. doi: 10.1016/j.jss.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmett CJ, Lawrence JM, Raisman G, Seeley PJ. Cultured epithelioid astrocytes migrate after transplantation into the adult rat brain. J Comp Neurol 311: 330–341, 1991. doi: 10.1002/cne.903110304. [DOI] [PubMed] [Google Scholar]
- 21.George MP, Champion HC, Gladwin MT, Norris KA, Morris A. Injection drug use as a “second hit” in the pathogenesis of HIV-associated pulmonary hypertension. Am J Respir Crit Care Med 185: 1144–1146, 2012. doi: 10.1164/rccm.201204-0609ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghofrani HA, Friese G, Discher T, Olschewski H, Schermuly RT, Weissmann N, Seeger W, Grimminger F, Lohmeyer J. Inhaled iloprost is a potent acute pulmonary vasodilator in HIV-related severe pulmonary hypertension. Eur Respir J 23: 321–326, 2004. doi: 10.1183/09031936.03.00057703. [DOI] [PubMed] [Google Scholar]
- 23.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal 11: 2505–2516, 2009. doi: 10.1089/ars.2009.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 62: 4491–4498, 2002. [PubMed] [Google Scholar]
- 26.Harter ZJ, Agarwal S, Dalvi P, Voelkel NF, Dhillon NK. Drug abuse and HIV-related pulmonary hypertension: double hit injury. AIDS 32: 2651–2667, 2018. doi: 10.1097/QAD.0000000000002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider F, Varga J. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? Arthritis Rheumatol 66: 1967–1978, 2014. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higashi Y, Maruhashi T, Noma K, Kihara Y. Oxidative stress and endothelial dysfunction: clinical evidence and therapeutic implications. Trends Cardiovasc Med 24: 165–169, 2014. doi: 10.1016/j.tcm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Hofman FM, Wright AD, Dohadwala MM, Wong-Staal F, Walker SM. Exogenous tat protein activates human endothelial cells. Blood 82: 2774–2780, 1993. doi: 10.1182/blood.V82.9.2774.2774. [DOI] [PubMed] [Google Scholar]
- 30.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 43: 317–327, 2004. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology 256: 83–91, 2009. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov AV, Valuev-Elliston VT, Ivanova ON, Kochetkov SN, Starodubova ES, Bartosch B, Isaguliants MG. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev 2016: 8910396, 2016. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janda S, Quon BS, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV Med 11: 620–634, 2010. doi: 10.1111/j.1468-1293.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- 34.Kanmogne GD, Kennedy RC, Grammas P. Analysis of human lung endothelial cells for susceptibility to HIV type 1 infection, coreceptor expression, and cytotoxicity of gp120 protein. AIDS Res Hum Retroviruses 17: 45–53, 2001. doi: 10.1089/088922201750056771. [DOI] [PubMed] [Google Scholar]
- 35.Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun 333: 1107–1115, 2005. doi: 10.1016/j.bbrc.2005.05.198. [DOI] [PubMed] [Google Scholar]
- 36.Kline ER, Sutliff RL. The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J Investig Med 56: 752–769, 2008. doi: 10.1097/JIM.0b013e3181788d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klings ES, Farber HW. The pathogenesis of HIV-associated pulmonary hypertension. Adv Cardiol 40: 71–82, 2003. doi: 10.1159/000073176. [DOI] [PubMed] [Google Scholar]
- 38.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal 20: 2794–2814, 2014. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185: 965–980, 2012. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, Mueller M, Drexler H. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol 27: 943–948, 2007. doi: 10.1161/01.ATV.0000258415.32883.bf. [DOI] [PubMed] [Google Scholar]
- 41.Lederman MM, Sereni D, Simonneau G, Voelkel NF. Pulmonary arterial hypertension and its association with HIV infection: an overview. AIDS 22, Suppl 3: S1–S6, 2008. doi: 10.1097/01.aids.0000327509.30385.3b. [DOI] [PubMed] [Google Scholar]
- 42.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest 112: 302–307, 2003. doi: 10.1172/JCI200319429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hänze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 10: 1687–1698, 2008. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 44.Liu HC, Anday JK, House SD, Chang SL. Dual effects of morphine on permeability and apoptosis of vascular endothelial cells: morphine potentiates lipopolysaccharide-induced permeability and apoptosis of vascular endothelial cells. J Neuroimmunol 146: 13–21, 2004. doi: 10.1016/j.jneuroim.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am J Physiol Heart Circ Physiol 295: H466–H474, 2008. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marecki J, Cool C, Voelkel N, Luciw P, Flores S. Evidence for vascular remodeling in the lungs of macaques infected with simian immunodeficiency virus/HIV NEF recombinant virus. Chest 128, Suppl: 621S–622S, 2005. doi: 10.1378/chest.128.6_suppl.621S. [DOI] [PubMed] [Google Scholar]
- 48.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest 118: 1133–1141, 2000. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 49.Mesa RA, Edell ES, Dunn WF, Edwards WD. Human immunodeficiency virus infection and pulmonary hypertension: two new cases and a review of 86 reported cases. Mayo Clin Proc 73: 37–45, 1998. doi: 10.1016/S0025-6196(11)63616-1. [DOI] [PubMed] [Google Scholar]
- 50.Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke 38: 2142–2149, 2007. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 51.Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hänze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 52.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009. doi: 10.1165/2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okumura K, Kato H, Honjo O, Breitling S, Kuebler WM, Sun M, Friedberg MK. Carvedilol improves biventricular fibrosis and function in experimental pulmonary hypertension. J Mol Med (Berl) 93: 663–674, 2015. doi: 10.1007/s00109-015-1251-9. [DOI] [PubMed] [Google Scholar]
- 54.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 55.Pellicelli AM, D’Ambrosio C, Vizza CD, Borgia MC, Tanzi P, Pino P, Zachara E, Soccorsi F. HIV-related pulmonary hypertension. From pathogenesis to clinical aspects. Acta Cardiol 59: 323–330, 2004. doi: 10.2143/AC.59.3.2005190. [DOI] [PubMed] [Google Scholar]
- 56.Pick E. Cell-free NADPH oxidase activation assays: “in vitro veritas”. Methods Mol Biol 1124: 339–403, 2014. doi: 10.1007/978-1-62703-845-4_22. [DOI] [PubMed] [Google Scholar]
- 57.Reis GS, Augusto VS, Silveira AP, Jordão AA Jr, Baddini-Martinez J, Poli Neto O, Rodrigues AJ, Evora PR. Oxidative-stress biomarkers in patients with pulmonary hypertension. Pulm Circ 3: 856–861, 2013. doi: 10.1086/674764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanders KA, Hoidal JR. The NOX on pulmonary hypertension. Circ Res 101: 224–226, 2007. doi: 10.1161/CIRCRESAHA.107.158246. [DOI] [PubMed] [Google Scholar]
- 59.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 8: 443–455, 2011. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ Res 113: 1253–1264, 2013. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res 12: 13–21, 2014. doi: 10.2174/1570162X12666140402100959. [DOI] [PubMed] [Google Scholar]
- 62.Skrabalova J, Drastichova Z, Novotny J. Morphine as a potential oxidative stress-causing agent. Mini Rev Org Chem 10: 367–372, 2013. doi: 10.2174/1570193X113106660031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sobhakumari A, Schickling BM, Love-Homan L, Raeburn A, Fletcher EV, Case AJ, Domann FE, Miller FJ Jr, Simons AL. NOX4 mediates cytoprotective autophagy induced by the EGFR inhibitor erlotinib in head and neck cancer cells. Toxicol Appl Pharmacol 272: 736–745, 2013. doi: 10.1016/j.taap.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spikes L, Dalvi P, Tawfik O, Gu H, Voelkel NF, Cheney P, O’Brien-Ladner A, Dhillon NK. Enhanced pulmonary arteriopathy in simian immunodeficiency virus-infected macaques exposed to morphine. Am J Respir Crit Care Med 185: 1235–1243, 2012. doi: 10.1164/rccm.201110-1909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 21: 134–145, 2006. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 66.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 67.Stenmark KR, Frid MG, Yeager M, Li M, Riddle S, McKinsey T, El Kasmi KC. Targeting the adventitial microenvironment in pulmonary hypertension: a potential approach to therapy that considers epigenetic change. Pulm Circ 2: 3–14, 2012. doi: 10.4103/2045-8932.94817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ten Freyhaus H, Vogel D, Lehmann C, Kümmerle T, Wyen C, Fätkenheuer G, Rosenkranz S. Echocardiographic screening for pulmonary arterial hypertension in HIV-positive patients. Infection 42: 737–741, 2014. doi: 10.1007/s15010-014-0610-8. [DOI] [PubMed] [Google Scholar]
- 69.Wagner AH, Schroeter MR, Hecker M. 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J 15: 2121–2130, 2001. doi: 10.1096/fj.01-0123com. [DOI] [PubMed] [Google Scholar]
- 70.Wang T, Green LA, Gupta SK, Kim C, Wang L, Almodovar S, Flores SC, Prudovsky IA, Jolicoeur P, Liu Z, Clauss M. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS One 9: e91063, 2014. doi: 10.1371/journal.pone.0091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuber JP, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, Nuesch R, Magenta L, Ledergerber B, Jenni R, Speich R, Opravil M; Swiss HIV Cohort Study Group . Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis 38: 1178–1185, 2004. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]