Abstract
Mechanical tension and humoral stimuli can induce transitions in airway smooth muscle phenotype between a synthetic inflammatory state that promotes cytokine secretion and a differentiated state that promotes the expression of smooth muscle phenotype-specific proteins. When tissues are maintained under high tension, Akt activation and eotaxin secretion are suppressed, but expression of the differentiation marker protein, smooth muscle myosin heavy chain (SmMHC), is promoted. When tissues are maintained under low tension, Akt activation and eotaxin secretion are stimulated, and the differentiated phenotype is suppressed. We hypothesized that mechanical stimuli are differentially transduced to Akt-mediated signaling pathways that regulate phenotype expression by α-parvin and β-parvin integrin-linked kinase/PINCH/parvin (IPP) signaling complexes within integrin adhesomes. High tension or ACh triggered paxillin phosphorylation and the binding of phospho-paxillin to β-parvin IPP complexes. This inhibited Akt activation and promoted SmMHC expression. Low tension or IL-4 did not elicit paxillin phosphorylation and triggered the binding of unphosphorylated paxillin to α-parvin IPP complexes, which promoted Akt activation and eotaxin secretion and suppressed SmMHC expression. Expression of a nonphosphorylatable paxillin mutant or β-parvin depletion by siRNA promoted the inflammatory phenotype, whereas the depletion of α-parvin promoted the differentiated phenotype. Results demonstrate that phenotype expression is regulated by the differential interaction of phosphorylated and unphosphorylated paxillin with α-parvin and β-parvin IPP complexes and that these complexes have opposite effects on the activation of Akt. Our results describe a novel molecular mechanism for transduction of mechanical and humoral stimuli within integrin signaling complexes to regulate phenotype expression in airway smooth muscle.
Keywords: airway smooth muscle tissue, Akt/PKB, focal adhesions, integrin signaling, parvin, paxillin
INTRODUCTION
Environmental and mechanical stimuli that are imposed on the airways during breathing stimulate changes in airway caliber that are mediated by the contraction of airway smooth muscle. In addition to its contractile properties, airway smooth muscle possesses an immunomodulary function characterized by the synthesis and secretion of inflammatory mediators (7, 18, 36, 41, 42). The exposure of airway smooth muscle to inflammatory cytokines such as IL-4 or IL-13 induces the activation of STAT6 and the synthetic serine/threonine kinase Akt/PKB, which promotes eotaxin synthesis and suppresses the expression of smooth muscle differentiation marker proteins such as smooth muscle myosin heavy chain (SmMHC) (7, 18, 42). Alterations in the extracellular milieu of airway smooth muscle cells such as changes in cell matrix composition or mechanical tension can also modulate the phenotypic expression and function of airway smooth muscle (2, 4, 5, 7, 18, 30, 33, 37, 42, 44). Subjecting airway muscle tissues to mechanical tension promotes a differentiated phenotype, whereas maintaining them under low mechanical tension promotes a synthetic inflammatory phenotype (7, 42). Mechanical forces experienced by airway smooth muscle tissues may be modulated physiologically by airway edema, extracellular matrix degradation, airway remodeling or the constriction of surrounding airways, all of which can lead to local changes in airway smooth muscle properties and function. Such conditions are particularly likely to be present during airway inflammation and asthma.
Akt is a key regulator of the synthetic functions of airway smooth muscle and is activated by inflammatory cytokines (3, 7, 18, 36). Akt activation is sensitive to the mechanical tension imposed on airway smooth muscle tissues both in vitro and in vivo (7, 42, 44). Mechanical tension suppresses the activation of Akt in airway smooth muscle tissues and inhibits the secretion of eotaxin in response to inflammatory stimuli such as IL-13 or IL-4 (7, 42, 44). In contrast, low levels of mechanical tension potentiate the activation of Akt and the secretion of eotaxin in response to inflammatory stimuli. Akt activation also suppresses the expression of smooth muscle differentiation marker proteins in both airway and vascular smooth muscle by inhibiting the nuclear translocation of serum response factor (SRF) (14, 43), a transcription factor that regulates the expression of genes encoding smooth muscle myosin heavy chain (SmMHC) and other smooth muscle differentiation marker proteins (19, 20). While the effects of mechanical stimuli on airway smooth muscle phenotype expression and Akt activation are well characterized, the mechanism by which mechanical stimuli are transduced to regulate Akt activation and nuclear signaling pathways that modulate phenotype expression are not understood.
Transmembrane integrin proteins act as sensors of the external cellular environment and as transducers for mechanical stimuli (12, 15). The extracellular domains of integrin proteins bind to extracellular matrix proteins and transmit mechanical tension imposed on the tissue to signaling complexes (adhesome complexes) that associate with the cytoplasmic domains of integrin proteins. Adhesome complexes connect to actin cytoskeletal filaments and thereby mediate the transmission of mechanical tension between the contractile apparatus and the extracellular matrix. Adhesome signaling complexes can transduce bidirectional signals between the extracellular matrix and intracellular signaling pathways that modulate both phenotypic expression and functional alterations in the responses of cells and tissues to environmental stimuli (46).
Integrin-linked kinase (ILK) is an integrin-binding protein that plays an important role in regulating phenotype expression in airway smooth muscle (43). ILK binds to Akt and regulates its activation in a PI3-kinase-dependent manner (7, 25, 26, 43). In a previous study, we found that the depletion of ILK protein in airway smooth muscle decreases the activation of Akt and increases the expression of smooth muscle differentiation marker genes and proteins, whereas the overexpression of ILK protein increases Akt activation and suppresses the expression of smooth muscle differentiation marker genes and proteins (43). Thus the regulation of Akt by ILK plays a key role in the modulation of airway smooth muscle phenotype.
ILK is the central component of the widely expressed ILK, PINCH, and parvin (IPP) complex, which is a heterotrimeric signaling module that associates with integrin adhesion complexes in multiple cell types (17, 40, 50). Studies in experimental cell lines, as well as in airway smooth muscle, have demonstrated that the IPP complex assembles in the cytoplasm as a stable inactive complex and is recruited to the membrane in response to extracellular stimuli (49, 50). Parvin is ubiquitously expressed as both α- and β-isoforms, both of which bind directly to the kinase-like domain of ILK (29, 40). The interaction between ILK and each of the parvin isoforms is mutually exclusive; thus an IPP complex can contain either α-parvin or β-parvin but not both (38, 45, 51). Both α-parvin and β-parvin IPP complexes can localize to integrin-associated adhesion complexes by binding directly to the focal adhesion protein paxillin (22, 23, 32). The binding of Akt to the α-parvin IPP complex promotes ILK-mediated Akt activation (8). Although the β-parvin IPP complex also interacts with Akt, molecular imaging studies of parvin-ILK-Akt interactions and studies in cancer cell lines suggest that β-parvin inhibits ILK-mediated signaling to Akt by preventing the interaction of Akt with ILK (16, 21). We therefore hypothesized that α-parvin and β-parvin IPP complexes might serve different functions in the regulation of Akt-mediated signaling pathways that modulate phenotypic expression in airway smooth muscle and that the recruitment and/or activation of α-parvin and β-parvin IPP complexes to integrin adhesomes might be differentially regulated by paxillin.
Focal adhesion kinase (FAK) and its substrate paxillin are key components of adhesome complexes that respond to mechanical stimulation in airway smooth muscle (34, 42). Mechanical tension imposed on airway smooth muscle tissues induces the phosphorylation and activation of FAK and the tyrosine phosphorylation of paxillin (34, 42). We previously demonstrated that FAK regulates the mechanosensitive modulation of airway smooth muscle phenotype; when FAK is activated, Akt activation and eotaxin secretion are suppressed, and the expression of smooth muscle phenotypic marker proteins is promoted (42). Conversely, when FAK activation is inhibited, Akt activation and eotaxin secretion by the muscle tissue are potentiated, and the expression of smooth muscle differentiation marker proteins is suppressed (42). Thus FAK is a critical regulator of signaling pathways that mediate transitions between the differentiated smooth muscle phenotype and the inflammatory synthetic phenotype in airway smooth muscle. However, the molecular link between FAK and the mechanisms that regulate Akt activation is unknown.
FAK is a catalyst for the tyrosine phosphorylation of paxillin; therefore, the effects of FAK on phenotypic expression might be mediated by paxillin. In airway smooth muscle and in other cell types, paxillin can regulate the localization of the IPP complex to adhesome complexes, and ILK can regulate Akt activation and phenotypic expression (22, 23, 32, 43). We therefore hypothesized that paxillin may play a key role in the mechanotransduction process by linking mechanical signals sensed by integrin proteins to the IPP complex, which regulates the activity of Akt.
Our current study provides evidence that mechanical stimuli modulate phenotype expression in airway smooth muscle by regulating the tyrosine phosphorylation of paxillin and that paxillin arbitrates pathways that regulate Akt by differentially recruiting α-parvin versus β-parvin IPP complexes to integrin-associated adhesome complexes. Our results demonstrate a novel molecular mechanism for the transduction of extracellular mechanical stimuli to modulate phenotypic expression and the functional properties of airway smooth muscle.
MATERIALS AND METHODS
All protocols were in accordance with procedures approved by the Institutional Animal Care and Use Committee (IACUC) of Indiana University School of Medicine under the National Research Council's Guide for the Care and Use of Laboratory Animals. Mongrel dogs (20–30 kg, any sex) were procured by the Indiana University Laboratory Animal Resource Center (LARC) at Indiana University School of Medicine from LBL Kennels, Reelsville, IN. Animals were euthanized by LARC personnel by IV injection of Fatal Plus (pentobarbital sodium, 390 mg/mL; propylene glycol, 0.01 mg/mL; ethyl alcohol, 0.29 mg/mL; benzyl alcohol, 0.2 mg/mL) at a dose of ~0.3 mL/kg. The trachea was then immediately removed by laboratory personnel and placed in physiological saline solution (PSS).
Preparation of smooth muscle tissues and measurement of force.
Strips of tracheal smooth muscle tissue (1.0 × 0.2–0.5 × 15 mm) were dissected free of connective and epithelial tissues, attached to force transducers, and maintained in a tissue bath in PSS at 37°C for the measurement of contractile force. Before the beginning of each experimental protocol, muscle tissues were mounted isometrically in organ baths and tissues were stimulated repeatedly with 10−5 M acetylcholine (ACh) to achieve a stable contractile response. Tissues were then used in different protocols as described below.
Protocol for mechanical loading of tissues.
Following the initial equilibration procedure, each tracheal smooth muscle tissue strip was mechanically loaded by suspending a weight of 0.5 g or 1.0 g from one end of the tissue strip. The 1.0 g weight extends the muscle to a tissue length that is approximately the length at which it generates maximal active isometric force, whereas the 0.5 g weight yields a tissue length that is 50–60% of the length of maximal active force and results in a submaximal level of isometric force. The 1.0 g weight results in a tension that is equivalent to the normal physiological tension present on the trachealis muscle in vivo at functional residual capacity (FRC), whereas the 0.5 g weight causes a tension that is significantly lower than that typically experienced by the airway muscle tissues in vivo (9). Mechanically loaded tissue strips were incubated in serum-free DMEM at 37°C for 6 h for each experimental protocol, with the exception of those in which eotaxin secretion was measured, in which tissues were subjected to mechanical loading for 20 h.
Reagents and antibodies.
Antibodies used in these experiments were: rabbit polyclonal anti-human α-parvin (ab154654), rabbit monoclonal anti-human β-parvin (ab154840), mouse monoclonal anti-human β-parvin (ab140384), mouse monoclonal anti-human α-parvin (ab139270), rabbit polyclonal anti-human integrin-linked kinase (ILK) (ab74336) all from Abcam; polyclonal rabbit anti-human fibronectin (F3648), monoclonal mouse anti-human collagen I (C2456), mouse monoclonal anti-human smooth muscle myosin heavy chain (SmMHC) (clone hSM-V, M7786) all from Sigma; mouse anti-human monoclonal Akt (2920S), Cell Signaling; rabbit polyclonal phospho-paxillin tyrosine Y118 (44-722G), rabbit monoclonal phospho-Akt S473 (44-621G) from Invitrogen; mouse monoclonal anti-human paxillin (610052) and mouse monoclonal anti-human PINCH (612711) from BD Biosciences Pharmingen. Polyclonal vinculin Ab (against canine cardiac vinculin) was custom-made by BABCO (Richmond, CA). Secondary antibodies were horseradish peroxidase-conjugated IgG (NA931 and NA934) from GE Healthcare UK Limited, IRDye 680RD [donkey-anti-mouse antibody (926-68070)] and IRDye 800CW [donkey-anti-rabbit antibody (925-32211)] from Li-Cor Biosciences. For immunoblots, all antibodies were diluted by 1:2,000 except for anti-smooth muscle myosin heavy chain and anti-vinculin, which were diluted 1:10,000. All primary antibodies were diluted 1:200 for proximity ligation assays (PLA). All antibodies have been validated in previous studies (24, 35, 43, 49) except for α-parvin and β-parvin, which are both validated in the present study.
Reagents included: Duolink in situ proximity ligation assay kit (PLA) and Duolink anti-mouse Plus, anti-rabbit Minus (Olink Bioscience, Uppsala, Sweden), α-Parvin siRNA(h) and β-parvin siRNA(h) (Santa Cruz), recombinant canine IL-4, and a human eotaxin-1 ELISA kit (R&D Systems). The plasmid vector used was a mutant nonphosphorylatable paxillin construct, pcDNA-paxillin Y31/118F (27). IL-4 was administered to the tissues at a concentration of 50 ng/mL, which consistently stimulated elevated STAT6 activation (7, 42).
Transfection of smooth muscle tissues with siRNA and plasmids.
α-Parvin siRNA, β-parvin siRNA, or plasmids encoding the recombinant paxillin Y31/118F mutant construct were introduced into tracheal smooth muscle strips by the method of reversible permeabilization as previously described (24, 35, 48, 49). Muscle tissues were incubated successively in each of the following solutions: Solution 1, which contained 10 mM EGTA, 5 mM Na2ATP, 120 mM KCl, 2 mM MgCl2, and 20 mM TES (at 4°C, pH 7.1, 100% O2 for 120 min); Solution 2, which contained 0.1 mM EGTA, 5 mM Na2ATP, 120 mM KCl, 2 mM MgCl2, 20 mM TES, and 10 μg of siRNA or plasmids (at 4°C, pH 7.1, 100% O2 overnight); Solution 3, which contained 0.1 mM EGTA, 5 mM Na2ATP, 120 mM KCl, 10 mM MgCl2, and 20 mM TES (at 4°C, pH 7.1,100% O2 for 30 min); and Solution 4, which contained 110 mM NaCl, 3.4 mM KCl, 0.8 mM MgSO4, 25.8 mM NaHCO3, 1.2 mM KH2PO4, and 5.6 mM dextrose (at 22°C, pH 7.4, 95% O2, 5% CO2 for 60 min). After 30 min in Solution 4, CaCl2 was added gradually to reach a final concentration of 2.4 mM. The strips were then incubated in serum-free DMEM containing 5 mM Na2ATP, 100 U/mL penicillin, and 100 μg/mL streptomycin in a CO2 incubator at 37°C for 1–2 days. Sham-treated tissues were subjected to identical procedures except that no siRNA or plasmids were included in Solution 2. The force of contraction in response to ACh was determined before and after treatment with plasmids or siRNA to confirm tissue viability and the effectiveness of the transfection. We have previously demonstrated that ~90% of the cells dissociated from tissues transfected using this method express the recombinant proteins (24, 35, 47, 48).
Immunoblots and immunoprecipitation of proteins.
Frozen muscle tissues were pulverized and proteins were extracted for electrophoresis or immunoprecipitation using methods previously described (10, 11). For immunoprecipitation of paxillin, α-parvin, or β-parvin, muscle extracts were precleared at 4°C with protein A/G UltraLink Resin, incubated with antibodies against α-parvin or β-parvin, and separated by 8% SDS-PAGE. Western blotting of immunoprecipitates or muscle extracts was performed to quantitate proteins. For immunoblots, the nitrocellulose membrane was blocked with 2–5% milk or Li-Cor blocking buffer for 1 h and probed with primary antibodies against proteins of interest overnight followed by secondary antibodies for 1 h. Proteins were visualized by enhanced chemiluminescence (ECL) or by infrared fluorescence using a Li-Cor Odyssey imager.
Dissociation of smooth muscle cells.
Smooth muscle cells were enzymatically dissociated from tracheal muscle strips using a gentle enzymatic procedure as previously described (24, 48). Tracheal muscle strips were minced and transferred to 5 mL of dissociation solution (in mM: 130 NaCl, 5 KCl, 1.0 CaCl2, 1.0 MgCl2, 10 HEPES, 0.25 EDTA, 10 d-glucose, and 10 taurine, pH 7.0) with collagenase (type IV, 400 U/mL), papain (30 U/mL), bovine serum albumin (1 mg/mL), and dithiothreitol (DTT; 1 mM). All enzymes were obtained from Sigma. The strips were then placed in a 37°C shaking water bath at 45 oscillations/min for 15–20 min, followed by three washes with a HEPES-buffered saline solution (in mM: 130 NaCl, 5 KCl, 1.0 CaCl2, 1.0 MgCl2, 20 HEPES, and 10 d-glucose, pH 7.4) and trituration with a pipette to liberate individual smooth muscle cells from the tissue. The solution containing the dissociated cells was poured over glass slides, and the cells were allowed to adhere to the glass for 30–60 min at 37°C. Cells were stimulated with ACh (10−5 M) for 5 min or with IL-4 for 20 min at 37°C or left unstimulated. Stimulated and unstimulated cells were fixed for 10 min in 4% paraformaldehyde (wt/vol) in phosphate-buffered saline (in mM: 137 NaCl, 4.3 Na2HPO4, 1.4 KH2PO4, and 2.7 KCl, pH 7.4) before treatment with primary antibodies. Freshly dissociated primary cells were used for these studies to avoid the changes in cytoskeletal organization and phenotype that occur during the prolonged culture of smooth muscle cells.
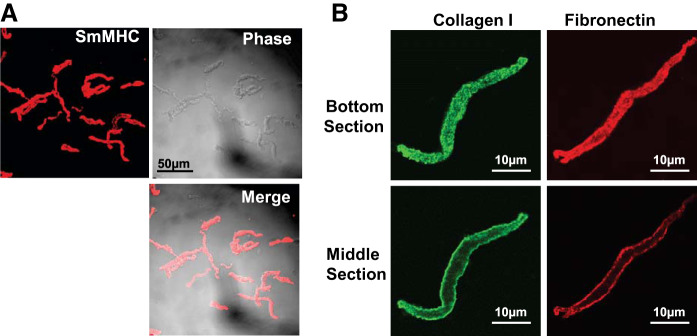
We assessed the proportion of smooth muscle cell in the tissues by staining cells immediately after dissociation from tissue strips for the phenotype-specific marker protein, smooth muscle myosin heavy chain (SmMHC) (Fig. 1A). Virtually all of the cells dissociated from these tissues expressed SmMHC (564 out of 573 cells dissociated from 10 tissue strips, 98.4%), indicating that tracheal smooth muscle tissue strips consist almost entirely of smooth muscle after dissection from other tissue components. We also stained cells for fibronectin and collagen I and visualized them using confocal microscopy to determine whether extracellular matrix (ECM) proteins remained bound to the cells after enzymatic dissociation (Fig. 1B). Both collagen and fibronectin stained intensely on the membrane of the dissociated cells.
Fig. 1.
Immunofluorescence images of airway smooth muscle cells after enzymatic dissociation. A: immunofluorescence and phase-contrast images of freshly dissociated airway smooth muscle cells stained for smooth muscle myosin heavy chain (SmMHC). B: dissociated airway smooth muscle cells stained for the extracellular matrix proteins collagen and fibronectin. Optical sections are at the bottom of the cell where fluorescence is observed over the cell surface, and through the middle of the cell where fluorescence is observed only along the membrane surface.
In situ proximity ligation assay.
In situ proximity ligation assays (Olink Bioscience, Uppsala, Sweden) were performed to detect protein interactions in freshly disassociated airway smooth muscle cells and airway smooth muscle tissue sections (10, 11, 31). PLA yields a fluorescent signal (fluorescent spot) when the target proteins are localized within 40 nm of each other and thus provides a method for assessing the cellular localization of protein complexes (31).
Mechanically weighted tissue strips were immersed in a plastic vessel containing embedding medium (OCT: 20% sucrose, 1:1), and the vessel was quickly dipped in dry ice-chilled isopentane and allowed to freeze for 50 s. Frozen tissues were stored at −80°C and were cryo-sectioned at a thickness of 5 μm at −20°C. Smooth muscle cells were enzymatically dissociated from tracheal muscle strips. Cells were then plated onto glass slides and allowed to adhere for 30–60 min.
Tissue sections or dissociated cells were fixed, permeabilized, and incubated with primary antibodies against target proteins. A pair of oligonucleotide-labeled secondary antibodies (PLA probes) was targeted to primary antibodies. PLA probe hybridization, ligation, amplification and detection media were administered as previously described (10, 11). Randomly selected cells or tissue sections from each treatment group were analyzed for protein interactions by counting PLA fluorescent spots using a Zeiss LSM 510 confocal microscope. All treatment groups imaged during a single experiment were subjected to the same imaging conditions during microscopy. The total number of spots in each individual dissociated cell was quantitated. For tissue sections, spots in each section were quantitated as the average of at least three 50 × 50 µm fields. At least three different sections were analyzed to obtain a value for each tissue. Duolink Images Tool software (Olink Bioscience, Uppsala, Sweden) was used to quantitate PLA signals.
ELISA analysis for eotaxin secretion.
Tissues were incubated for 20 h with or without 50 ng/mL IL-4. Following the incubation, the tissue culture medium from each sample was collected and concentrated to 100 μl using an Amicon Ultra-4 centrifugal filter (Ultracel-3K, Millipore). The culture medium was free of cells. The eotaxin-1 in the concentrate from each sample was quantified by ELISA using an antibody against human eotaxin-1. Absorption was measured using a SpectraMax M2 microplate reader. Eotaxin-1 concentrations were determined by comparison to a standard curve.
Statistical analysis.
Comparisons between groups were performed using paired Student’s t tests or by one-way ANOVA with or without repeated measures using GraphPad statistical software. Analysis of differences between pairs of groups was done using Tukey’s multiple comparison post hoc analysis. All tests used two-tailed analysis, and n represents the number of tissues or cells used to obtain each value. All values represent means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Mechanical tension induces transitions in phenotype of airway smooth muscle between a synthetic inflammatory state and a differentiated state by regulating the tyrosine phosphorylation of paxillin.
We expressed the nonphosphorylatable paxillin mutant paxillin Y31/118F (27) in airway smooth muscle tissues to evaluate the role of paxillin tyrosine phosphorylation in the changes in phenotype expression induced by mechanical tension. Transfected tracheal muscle tissues were maintained under low (0.5 g) or high (1.0 g) mechanical tension for 6 h and then frozen for the analysis of Akt activation and SmMHC expression. A separate set of muscle tissues was also maintained under low or high mechanical tension and incubated with IL-4 for 20 h for the analysis of eotaxin secretion.
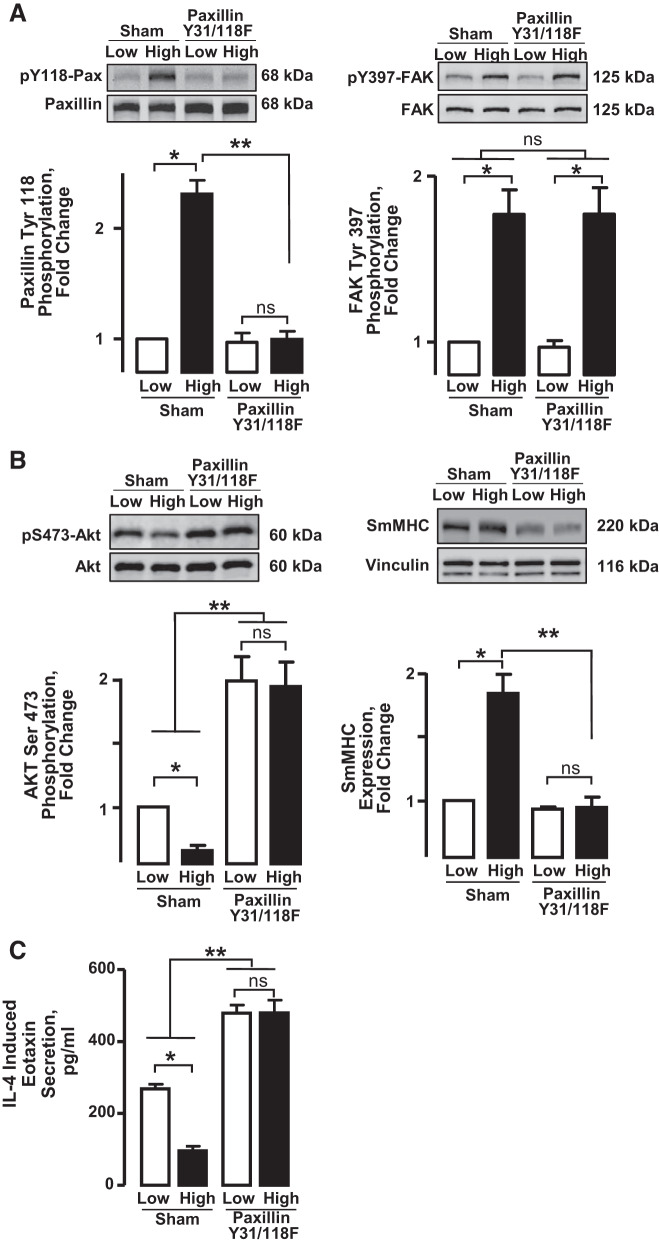
In Sham-treated tissues, incubation under high mechanical tension resulted in significantly higher levels of paxillin tyrosine phosphorylation and FAK activation, as indicated by phosphorylation on Tyr397, than in tissues maintained under low tension (Fig. 2A). The expression of paxillin Y31/118F in the tissues abolished the differences in the mechanosensitivity of paxillin tyrosine phosphorylation, as measured by differences in responses induced by low and high mechanical tension. As expected, the expression of paxillin Y31/118F had no significant effect on the mechanosensitivity of FAK activation, as the activation of FAK is prerequisite to paxillin phosphorylation.
Fig. 2.
Mechanical tension regulates Akt activation, eotaxin secretion, and smooth muscle myosin heavy chain (SmMHC) expression by regulating paxillin phosphorylation. A: smooth muscle tissues were transfected with the paxillin mutant Y31/118F or Sham-treated and maintained for 6 h at low or high mechanical tension. The phosphorylation of paxillin and focal adhesion kinase (FAK) was significantly increased in tissues at high tension relative to low tension. Expression of the paxillin mutant Y31/118F inhibited paxillin phosphorylation (n = 3) but did not affect mechanosensitive FAK activation (n = 5). B: Akt activation was significantly higher in tissues at low tension relative to those at high tension. The inhibition of paxillin phosphorylation significantly potentiated Akt activation in tissues at both high and low tension and abolished the mechanosensitivity of Akt activation (n = 6). SmMHC expression was significantly higher in tissues at high tension relative to those at low tension. The inhibition of paxillin phosphorylation significantly decreased SmMHC expression at high tension and abolished the mechanosensitivity of SmMHC expression (n = 4). C: secretion of eotaxin into the incubation medium was measured in tissues incubated with 50 ng/mL IL-4 at low or high mechanical tension for 20 h. Eotaxin secretion was significantly greater at low mechanical tension than at high tension. The inhibition of paxillin tyrosine phosphorylation potentiated the secretion of eotaxin at both low and high tensions and abolished its mechanosensitivity (n = 6). All values are means ± SE. All data analyzed by one-way ANOVA. *Significant difference between low and high tension. **Significant difference between Sham-treated tissues and tissues expressing the paxillin Y31/118F mutant. P < 0.05.
Incubation of tissues under high mechanical tension suppressed Akt activation and promoted SmMHC expression (Fig. 2B). High mechanical tension also inhibited IL-4-induced eotaxin secretion (Fig. 2C). The inhibition of paxillin tyrosine phosphorylation by the expression of paxillin Y31/118F potentiated Akt activation and suppressed SmMHC expression whether tissues were subjected to low or high mechanical tension (Fig. 2B). When paxillin phosphorylation was inhibited, the mechanosensitivity of Akt activation and SmMHC expression was abolished (Fig. 2B). The inhibition of paxillin tyrosine phosphorylation also potentiated IL-4-induced eotaxin secretion in tissues subjected to either low or high mechanical tension and eliminated the mechanosensitivity of eotaxin secretion (Fig. 2C).
These results demonstrate that changes in paxillin phosphorylation induced by mechanical tension regulate transitions in the expression of the synthetic inflammatory phenotype versus the differentiated phenotype in airway smooth muscle. High tension stimulates the phosphorylation of paxillin, resulting in the expression of SmMHC and inhibition of Akt activity, whereas the inhibition of paxillin phosphorylation promotes an inflammatory phenotype characterized by Akt activation and eotaxin secretion. The inhibition of paxillin tyrosine phosphorylation suppresses the modulation of phenotype expression induced by changes in mechanical tension.
Mechanical tension differentially regulates the association of paxillin with α-parvin and β-parvin IPP complexes.
We next sought to determine the mechanism by which paxillin phosphorylation regulates Akt activation and phenotype expression. Both α-parvin and β-parvin isoforms can bind to paxillin, and the interaction of parvin with paxillin is critical for the localization of the IPP complexes to integrin-associated adhesomes (23, 32, 39).
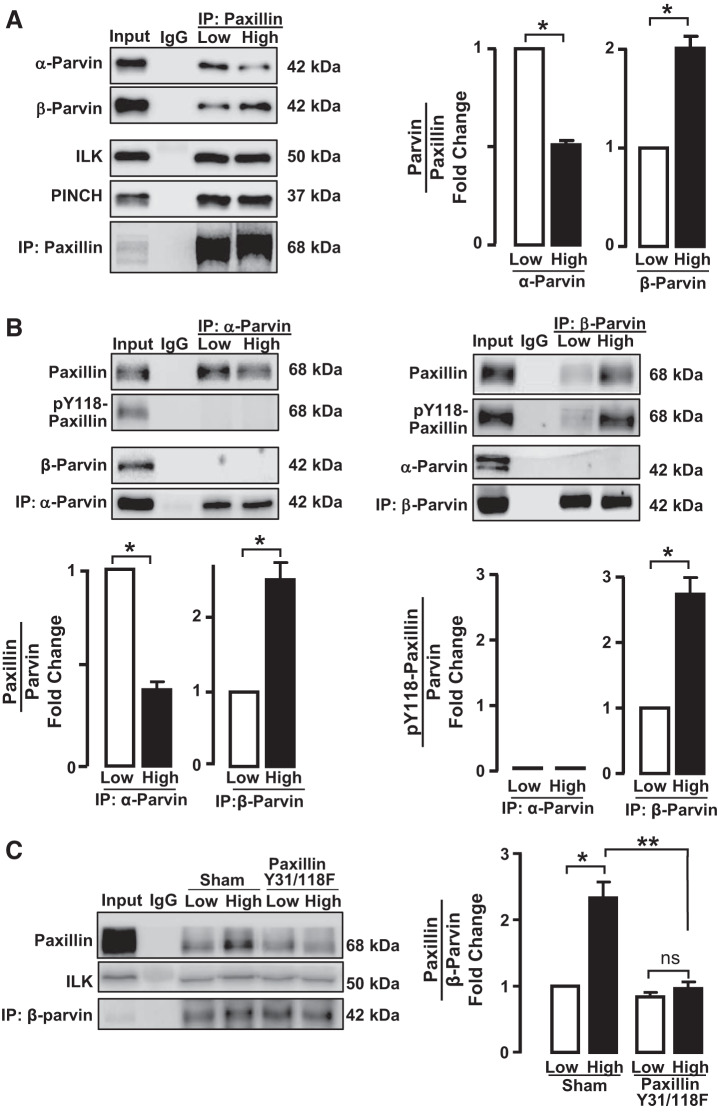
We evaluated the expression of α-parvin and β-parvin IPP complexes in airway smooth muscle tissues maintained under low or high mechanical tension and determined the effect of mechanical tension on their association with paxillin (Fig. 3A). Paxillin was immunoprecipitated, and immunocomplexes were analyzed for ILK, PINCH, α-parvin, and β-parvin. Paxillin immunocomplexes contained similar amounts of ILK and PINCH regardless of mechanical tension; however, significantly more α-parvin was detected in paxillin immunocomplexes from tissues maintained under low tension than from those maintained under high tension. In contrast, more β-parvin was detected in paxillin immunocomplexes from tissues maintained at high tension than from those maintained at low tension (Fig. 3A).
Fig. 3.
The interaction of α-parvin and β-parvin integrin-linked-kinase/PINCH/parvin (IPP) complexes with paxillin is differentially regulated by mechanical tension and is determined by paxillin phosphorylation. The effect of mechanical tension on the interaction of paxillin with α-parvin or β-parvin was determined by immunoprecipitation (IP) analysis of extracts of tracheal smooth muscle tissues incubated at low or high tension. A: paxillin immunocomplexes were analyzed for α-parvin, β-parvin, integrin-linked-kinase (ILK), and PINCH. Significantly more α-parvin precipitated with paxillin from tissues at low tension, and more β-parvin precipitated from tissues at high tension. No tension-dependent differences were found in the amount of ILK or PINCH in paxillin immunocomplexes (n = 4). B: α-parvin and β-parvin were immunoprecipitated from extracts of tissues maintained at low or high tension and immunocomplexes analyzed for the presence of α-parvin, β-parvin, paxillin, and phospho-paxillin. In α-parvin immunocomplexes, significantly more paxillin was detected in tissues at low tension (n = 4), whereas in β-parvin immunocomplexes significantly more paxillin was found at high tension (n = 8). Phospho-paxillin precipitated with β-parvin and was significantly higher in extracts from tissues at high tension (n = 3). Very little phospho-paxillin coprecipitated with α-parvin regardless of mechanical tension (n = 3). C: effects of inhibiting paxillin phosphorylation on the association of β-parvin with paxillin were analyzed in tissues in which paxillin phosphorylation was inhibited by expressing paxillin Y31/118F. The inhibition of paxillin phosphorylation suppressed the interaction between paxillin and β-parvin at high mechanical tension (n = 4). Data analyzed by one-way ANOVA. *Significant difference between low and high tension. **Significant difference between Sham-treated tissues and tissues expressing paxillin Y31/118F mutant. P < 0.05.
We precipitated α-parvin and β-parvin immunocomplexes from tissues maintained at low or high mechanical tension and analyzed them for paxillin and phospho-paxillin (Fig. 3B). α-Parvin and β-parvin precipitated as separate and distinct complexes; no β-parvin was detected in α-parvin immunocomplexes, and no α-parvin was detected in β-parvin immunocomplexes. More paxillin was detected in α-parvin immunocomplexes in tissues maintained at low tension than in tissues maintained at high tension. Conversely, significantly more paxillin was associated with β-parvin in tissues maintained at high tension than in tissues maintained at a low tension. The paxillin in α-parvin immunocomplexes was not phosphorylated, whereas phosphorylated paxillin was detected in β-parvin immunocomplexes (Fig. 3B).
We evaluated the role of paxillin phosphorylation in regulating the association of paxillin with β-parvin by expressing paxillin Y31/118F in the tissues. In Sham-treated muscles, more paxillin was detected in β-parvin immunocomplexes at high mechanical tension than at low mechanical tension. In tissues expressing paxillin Y31/118F, the increase in the association of paxillin with β-parvin at high tension was inhibited (Fig. 3C).
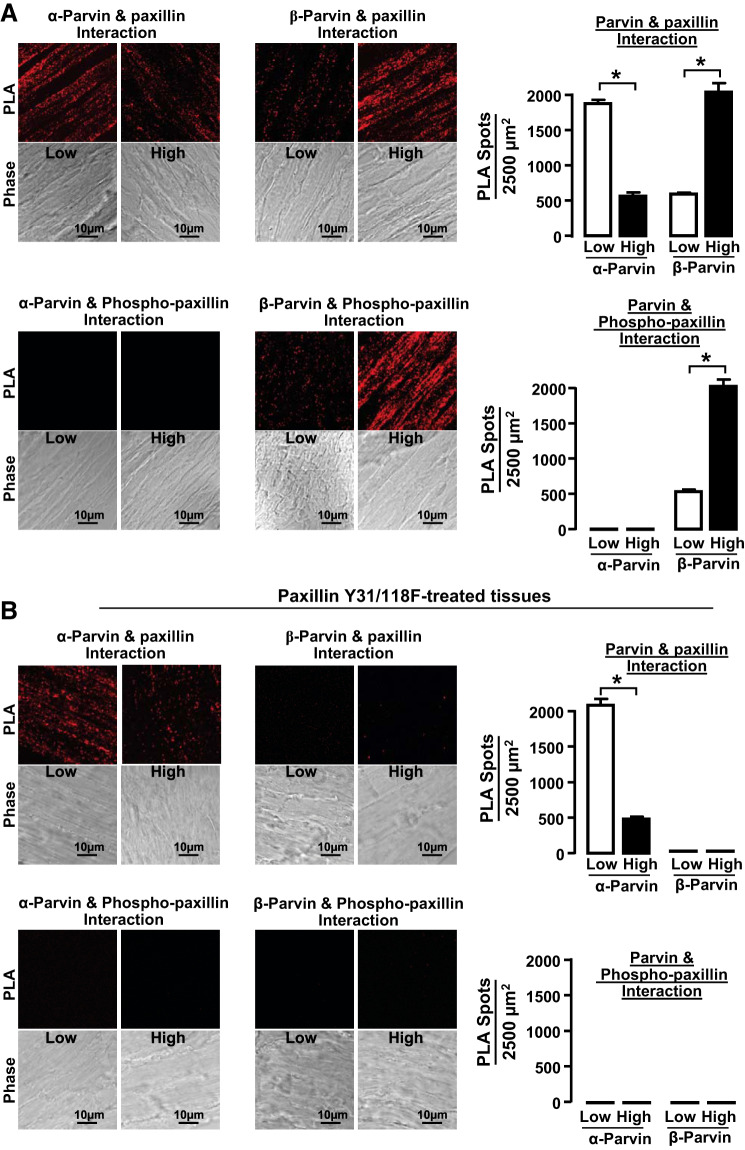
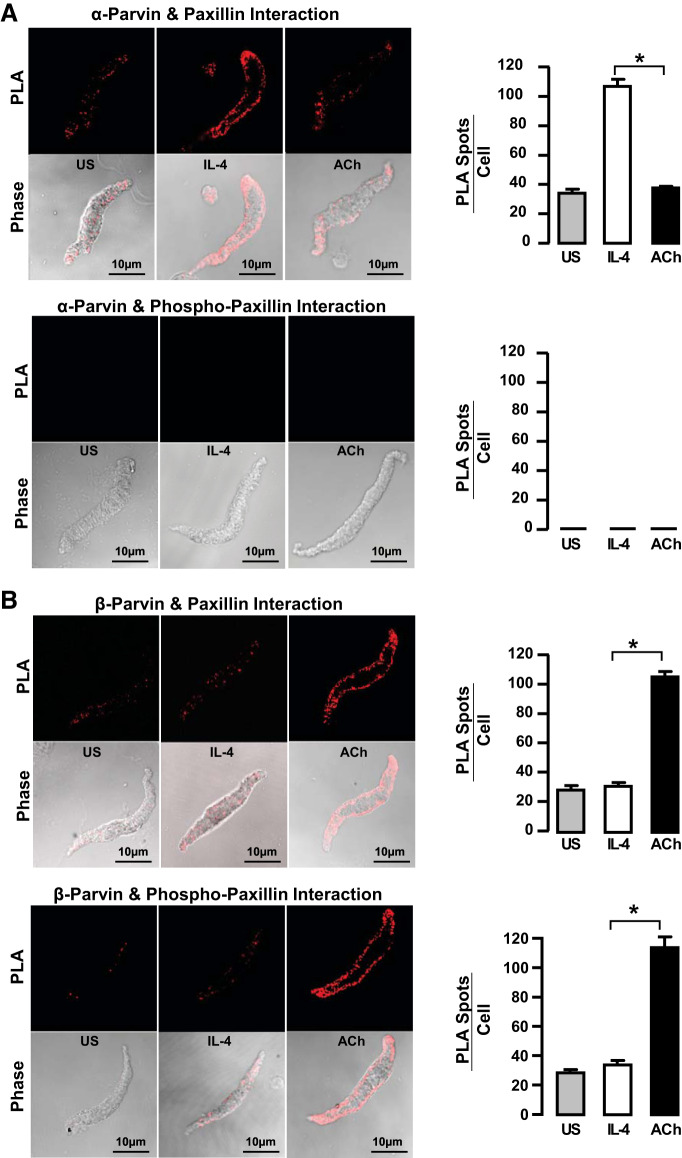
The effect of mechanical tension on the association of paxillin with α-parvin and β-parvin was also evaluated using proximity ligation assays (PLA) on cryosections of smooth muscle tissues that were maintained under low or high mechanical tension for 6 h (Fig. 4A). PLA yields a fluorescent signal (fluorescent spot) when the target proteins are localized within 40 nm of each other (31). In tissues subjected to low tension, significantly more complexes between paxillin and α-parvin were detected compared with tissues subjected to high mechanical tension, whereas, in tissues subjected to high mechanical tension, significantly more complexes between paxillin and β-parvin were detected. The paxillin complexed with α-parvin was not phosphorylated, whereas the paxillin in complexes with β-parvin was phosphorylated.
Fig. 4.
Low mechanical tension promotes interactions between α-parvin and unphosphorylated paxillin, whereas high mechanical tension promotes interactions between β-parvin and phosphorylated paxillin. A: cryo-sections from tissues maintained at low or high mechanical tension for 6 h showing proximity ligation assay (PLA) fluorescence from the interactions between α-parvin or β-parvin with paxillin or pY118-paxillin. More interactions between α-parvin and paxillin were detected in tissues at low mechanical tension, while more interactions between β-parvin and paxillin were detected in tissues at high mechanical tension. pY118-paxillin interacted with β-parvin and not with α-parvin regardless of mechanical tension (n = 9). B: cryo-sections from tissues maintained at low and high mechanical tension after transfection with paxillin Y31/118F to inhibit paxillin phosphorylation. PLA fluorescence for interactions between α-parvin and paxillin was similar to those in untreated tissues, whereas interactions between β-parvin and paxillin were inhibited in tissues treated with paxillin Y31/118F (n = 9). Data analyzed by one-way ANOVA. *Significant difference between low and high tension. P < 0.05.
Sections from tissues expressing paxillin Y31/118F were also analyzed to determine the effect of inhibiting paxillin phosphorylation on the association between paxillin and parvin (Fig. 4B). The interaction between α-parvin and paxillin in tissues expressing the paxillin mutant was similar to that in untreated tissues, but expression of the paxillin mutant inhibited the interaction between β-parvin and paxillin. Very few interactions between phosphorylated paxillin and either parvin could be detected in tissues expressing paxillin Y31/118F.
These results demonstrate that mechanical tension differentially regulates the association of α-parvin and β-parvin IPP complexes with paxillin in tracheal smooth muscle tissues by regulating the phosphorylation state of paxillin. In tissues subjected to high mechanical tension, paxillin phosphorylation is stimulated, which promotes its association with β-parvin IPP complexes. In contrast, low mechanical tension provides less stimulation for paxillin phosphorylation, and unphosphorylated paxillin associates with α-parvin IPP complexes. Thus the mechanosensitivity of paxillin phosphorylation regulates the association of paxillin with α-parvin versus β-parvin IPP complexes.
Mechanical tension regulates the activation of Akt by differentially regulating its association with α-parvin and β-parvin IPP complexes.
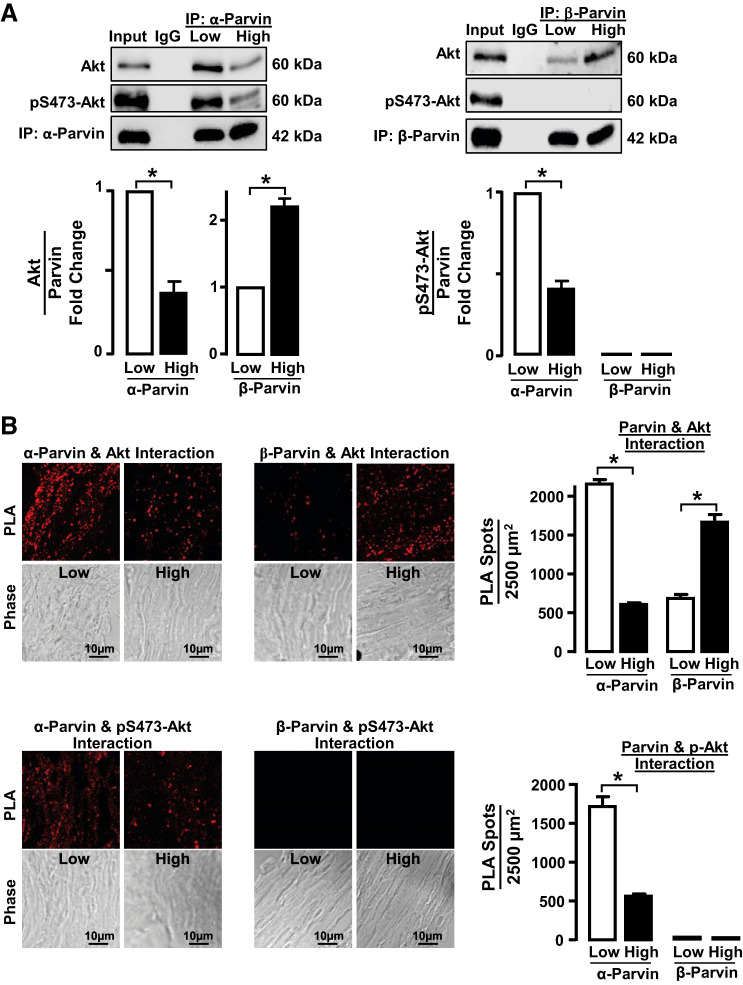
We investigated the hypothesis that mechanical tension might regulate the association of Akt with α-parvin versus β-parvin IPP complexes and that this might determine Akt activation. Coimmunoprecipitation analysis of tissue extracts was performed to analyze the effect of mechanical tension on the association of Akt with α-parvin and β-parvin IPP complexes (Fig. 5A). Significantly more Akt coprecipitated with α-parvin immunocomplexes from tissues maintained under low tension than from tissues maintained under high tension. In contrast, Akt coprecipitated at significantly higher levels with β-parvin immunocomplexes from tissues maintained under high tension than from those maintained under low tension. The Akt that associated with α-parvin was highly phosphorylated on Ser473, indicating Akt kinase activation, whereas the Akt that associated with β-parvin was not phosphorylated, indicating that it was not activated (Fig. 5A).
Fig. 5.
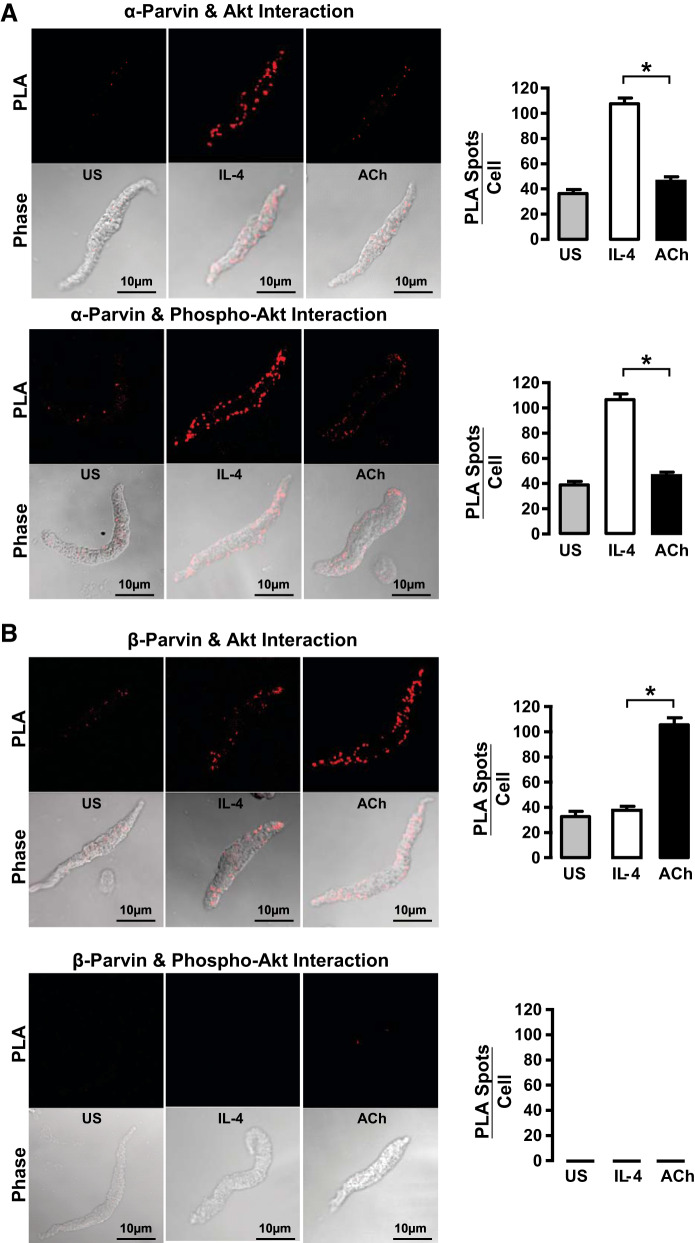
Low mechanical tension promotes interactions between α-parvin and activated Akt, whereas high mechanical tension promotes interactions between β-parvin and inactive Akt. A: α-parvin and β-parvin were immunoprecipitated (IP) from tissues maintained at low or high tension and immunocomplexes analyzed for the presence of Akt and phospho-Akt. Significantly more Akt precipitated with α-parvin in tissues at low tension (n = 4), whereas significantly more Akt precipitated with β-parvin at high tension (n = 9). Akt that precipitated with α-parvin was phosphorylated, whereas the Akt that precipitated with β-parvin was not phosphorylated. Phospho-Akt that precipitated with α-parvin was significantly higher at low tension than at high tension (n = 3). B: cryo-sections of tissues maintained at low or high mechanical tension showing proximity ligation assay (PLA) fluorescence of the interactions between α-parvin or β-parvin with Akt or pS473-Akt. Significantly more interactions between α-parvin and Akt were detected in tissues at low mechanical tension than at high mechanical tension (n = 10). Significantly more interactions between β-parvin and Akt were detected in tissues at high mechanical tension than at low tension (n = 9). pS473-Akt interacted with α-parvin and not with β-parvin regardless of mechanical tension (n = 9). Data analyzed by one-way ANOVA. *Significant difference between low and high tension. P < 0.05.
Similar results were obtained by PLA analysis of sections of tissues subjected to low or high mechanical tension (Fig. 5B). Akt interacted preferentially with α-parvin in tissues subjected to low mechanical tension, whereas Akt interacted preferentially with β-parvin in tissues subjected to high mechanical tension. The Akt that interacted with α-parvin was phosphorylated, indicating that it was activated, whereas the Akt that interacted with β-parvin was not phosphorylated, indicating that it was inactive.
These results demonstrate that high mechanical tension promotes the association of Akt with β-parvin, which prevents Akt activation, whereas low mechanical tension promotes the association of Akt with α-parvin IPP complexes, which promotes Akt activation. Thus the interaction of Akt with α-parvin and β-parvin IPP complexes is differentially regulated by mechanical tension, and this determines the activation of Akt.
The depletion of α-parvin promotes the differentiated phenotype in smooth muscle tissues, whereas the depletion of β-parvin promotes the synthetic inflammatory phenotype.
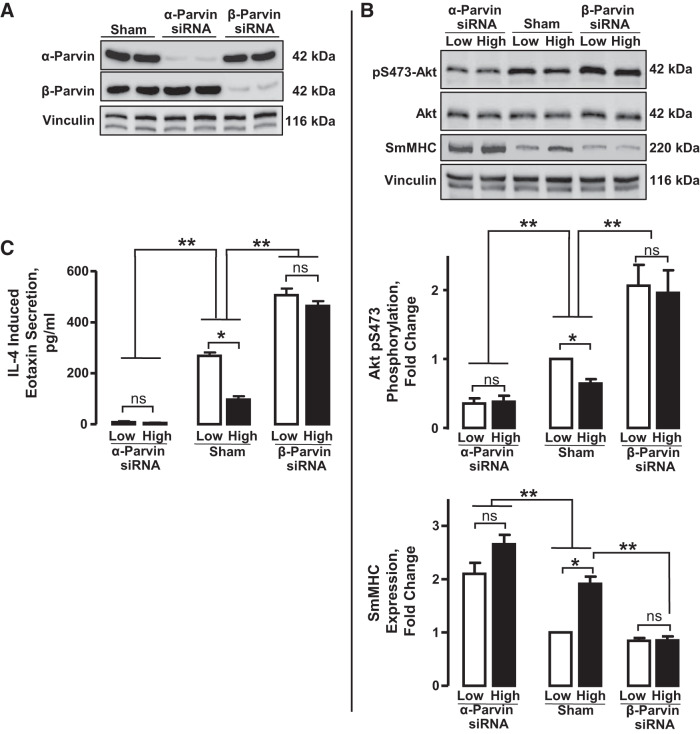
We evaluated the roles of α-parvin and β-parvin in phenotype modulation in airway smooth muscle by selectively depleting either α-parvin or β-parvin from the tracheal muscle tissues using siRNA and then incubating the muscle tissues under low or high mechanical tension. Treatment with α-parvin siRNA depleted α-parvin protein without affecting β-parvin expression, whereas treatment with β-parvin siRNA depleted β-parvin protein without affecting α-parvin expression (Fig. 6A).
Fig. 6.
Depletion of α-parvin from airway smooth muscle tissues promotes the expression of smooth muscle myosin heavy chain (SmMHC) and inhibits Akt activation and eotaxin secretion. Depletion of β-parvin promotes Akt activation and eotaxin secretion and inhibits SmMHC expression. A: tissues were depleted of α-parvin or β-parvin using α-parvin siRNA (α-Prv siRNA) or β-parvin siRNA (β-Prv siRNA) and immunoblotted for α-parvin, β-parvin, and vinculin (loading control). α-parvin protein was significantly depleted in α-Prv siRNA-transfected tissues but not in Sham-treated or β-Prv siRNA-transfected tissues. Conversely, β-parvin expression was significantly depleted in β-Prv siRNA-transfected tissues but not in Sham-treated or α-Prv siRNA-transfected tissues. B: depletion of α-parvin significantly decreased Akt phosphorylation in tissues at both low and high tension and abolished the mechanosensitivity of Akt phosphorylation. In contrast, depletion of β-parvin significantly increased Akt phosphorylation at both low and high tensions and abolished the mechanosensitivity of Akt phosphorylation (n = 4). Depletion of α-parvin significantly increased SmMHC expression at both low and high tensions and reduced the mechanosensitivity of SmMHC expression (n = 6). Depletion of β-parvin significantly inhibited SmMHC expression at high mechanical tension and abolished its mechanosensitivity (n = 6). C: depletion of α-parvin significantly inhibited the eotaxin secretion regardless of mechanical tension and suppressed the mechanosensitivity of eotaxin secretion (n = 6). Depletion of β-parvin significantly enhanced the eotaxin secretion by tissues at low and high tension and inhibited the mechanosensitivity of eotaxin secretion (n = 6). All values are means ± SE. Statistical analysis by one-way ANOVA. *Significant difference between low and high tensions. **Significant difference between Sham-treated tissues and α-parvin- or β-parvin-depleted tissues. ns, not significant. P < 0.05 was considered significant.
The depletion of α-parvin significantly inhibited Akt phosphorylation compared with Sham-treated muscles, whereas β-parvin depletion significantly increased Akt phosphorylation (Fig. 6B). In contrast, α-parvin depletion significantly increased SmMHC protein expression, whereas the depletion of β-parvin significantly decreased SmMHC protein expression. The potentiation or suppression of Akt activation and SmMHC expression caused by the depletion of either parvin occurred at both low and high mechanical tensions. These results suggest that the depletion of β-parvin promotes the interaction of Akt with the α-parvin IPP complex and that the depletion of α-parvin promotes the interaction of Akt with the β-parvin IPP complex. The mechanosensitivity of Akt activation and SmMHC expression was suppressed by the depletion of either parvin protein (Fig. 6B).
We also determined the effect of depleting α-parvin and β-parvin on eotaxin secretion in response to IL-4 stimulation in tissues maintained under a low or high mechanical tension (Fig. 6C). After depleting tissues of α-parvin or β-parvin, tissues were incubated with 50 ng/mL IL-4 for 20 h under low or high mechanical tension, and eotaxin secretion was quantified in the incubation medium by ELISA. High mechanical tension significantly inhibited IL-4-induced eotaxin secretion in Sham-treated tissues compared with low mechanical tension. The depletion of β-parvin significantly potentiated the IL-4-stimulated secretion of eotaxin at both low and high mechanical tension, whereas the depletion of α-parvin almost abolished the IL-4-induced secretion of eotaxin regardless of mechanical tension (Fig. 6C). Thus the mechanosensitivity of eotaxin secretion was suppressed by the depletion of either α- or β-parvin. These results are consistent with the effects of α-parvin and β-parvin depletion on Akt activation.
These results demonstrate that the α-parvin IPP complex promotes the expression of a synthetic inflammatory phenotype in airway smooth muscle, whereas the β-parvin IPP complex promotes the expression of a differentiated phenotype. They further demonstrate that the mechano-modulation of phenotype expression in airway smooth muscle is mediated by the differential activation of α-parvin and β-parvin IPP complexes.
Stimulation of freshly dissociated tracheal smooth muscle cells with ACh or IL-4 induces the recruitment of α-parvin and β-parvin IPP complexes to the smooth muscle cell membrane.
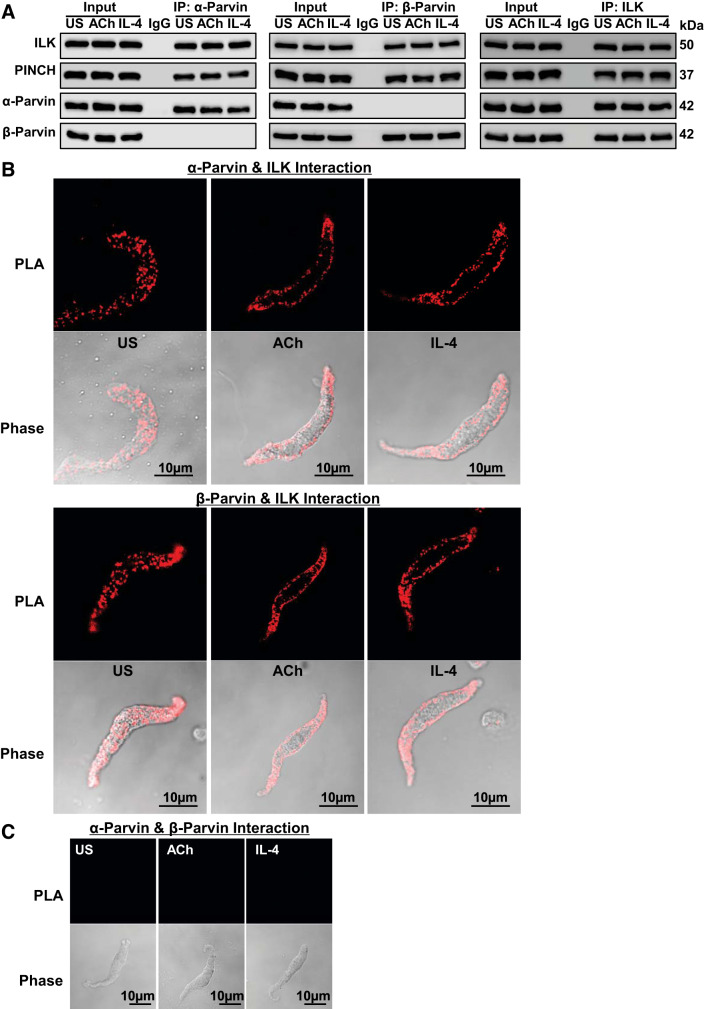
α-Parvin and β-parvin IPP complexes were analyzed in tracheal smooth muscle tissues and dissociated cells to determine the effects of contractile and inflammatory stimuli on their expression and cellular localization. Tissues were stimulated for 5 min with 10−5 M ACh or for 20 min with 50 ng/mL IL-4. α-Parvin and β-parvin were immunoprecipitated from tissue extracts, and immunocomplexes were blotted for ILK, PINCH, α-parvin, and β-parvin (Fig. 7A). ILK and PINCH were expressed in similar amounts in α-parvin and β-parvin immunocomplexes regardless of whether tissues were stimulated with ACh or IL-4 or were unstimulated. No β-parvin was found in α-parvin immunocomplexes, and no α-parvin was found in β-parvin immunocomplexes. Both α-parvin and β-parvin were detected in ILK immunocomplexes. The amount of α-parvin and β-parvin in ILK immunoprecipitates was unaffected by stimulation of the tissues with ACh or IL-4.
Fig. 7.
α-Parvin integrin-linked kinase/PINCH/parvin (IPP) complexes and β-parvin IPP complexes exist in separate pools in airway smooth muscle and are recruited to the membrane in response to stimulation with ACh or IL-4. A: tracheal smooth muscle tissues were stimulated with ACh or with IL-4. α-Parvin, β-parvin, or integrin-linked-kinase (ILK) was immunoprecipitated (IP) from tissue extracts and immunoblotted for ILK, PINCH, α-parvin, and β-parvin. ILK and PINCH were found in both α-parvin and β-parvin immunocomplexes in similar amounts regardless of whether tissues had been stimulated with ACh or IL-4 or were unstimulated (US). ILK, PINCH, α-parvin, and β-parvin were found in ILK immunocomplexes in similar amounts regardless of whether tissues were stimulated or unstimulated. B: proximity ligation assays (PLA) were performed on tracheal smooth muscle cells freshly dissociated from intact tissues and stimulated with ACh or IL-4 or unstimulated (US) to evaluate the cellular localization of α-parvin and β-parvin IPP complexes. PLA probes were targeted to α-parvin and ILK to detect α-parvin IPP complexes or to β-parvin and ILK to detect β-parvin IPP complexes. Both α-parvin and β-parvin IPP complexes were distributed throughout the cytoplasm of unstimulated cells and were localized primarily at the membrane of ACh- or IL-4-stimulated cells. C: PLA probes were targeted to α-parvin and β-parvin. No protein complexes containing both α-parvin and β-parvin were detected in unstimulated cells or in cells stimulated with ACh or with IL-4.
Proximity ligation assays (PLA) were performed on freshly dissociated tracheal smooth muscle cells to evaluate the effect of stimulation with ACh or IL-4 on the cellular localization of α-parvin and β-parvin IPP complexes. PLA probes were targeted to α-parvin and ILK to detect α-parvin IPP complexes or to β-parvin and ILK to detect β-parvin IPP complexes (Fig. 7B). Both α-parvin and β-parvin IPP complexes were distributed throughout the cytoplasm of unstimulated cells but localized preferentially to the membrane of cells stimulated with either ACh or IL-4. No protein complexes containing both α-parvin and β-parvin were detected in either stimulated or unstimulated smooth muscle cells (Fig. 7C).
Our results demonstrate that α-parvin and β-parvin exist in stable complexes with ILK and PINCH (IPP complexes) in tracheal smooth muscle tissues and that they coexist in separate and distinct pools that are unaffected by stimulation with ACh or IL-4. However, stimulation with either ACh or IL-4 induces the recruitment of both α-parvin and β-parvin IPP complexes to membrane sites.
IL-4 stimulates the interaction of paxillin with α-parvin IPP complexes, whereas ACh stimulates the interaction of paxillin with β-parvin IPP complexes.
The stimulation of airway smooth muscle tissues with IL-4 causes the activation of Akt and synthesis and secretion of eotaxin, whereas stimulation with the contractile agonist ACh does not. We compared the effects of IL-4 and ACh on the interactions of α-parvin and β-parvin with paxillin and Akt to determine their roles in the changes in phenotypic expression in airway smooth muscle induced by ACh and IL-4 (7, 42) (Fig. 8A).
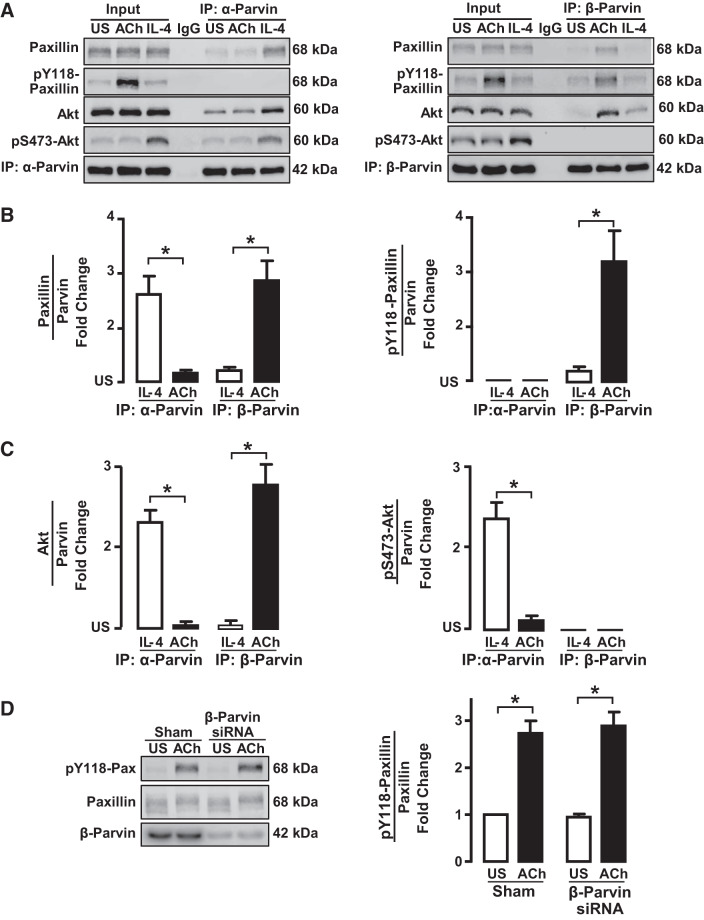
Fig. 8.
IL-4 stimulates the interaction of unphosphorylated paxillin with α-parvin, whereas ACh stimulates the interaction of phosphorylated paxillin with β-parvin. A: tissues were maintained isometrically and stimulated with ACh or IL-4 or were unstimulated (US). α-Parvin or β-parvin was immunoprecipitated (IP) from tissue extracts, and immunoprecipitates were blotted for paxillin and pY118-paxillin, Akt and pS473-Akt, and α-parvin or β-parvin. B: significantly more paxillin coprecipitated with α-parvin immunocomplexes from IL-4-stimulated muscles than from ACh-stimulated muscles or unstimulated US muscles (n = 6). Significantly more paxillin coprecipitated with β-parvin from ACh-stimulated muscles than from IL-4-stimulated or unstimulated muscles (n = 5). Paxillin that coprecipitated with α-parvin was not phosphorylated; phosphorylated paxillin only coprecipitated with β-parvin complexes (n = 5). C: significantly more Akt coprecipitated from α-parvin immunoprecipitates from tissues stimulated with IL-4 than from tissues stimulated with ACh or US tissues. In β-parvin immunoprecipitates, significantly more Akt was found in tissues stimulated with ACh than in tissues stimulated with IL-4 or US tissues. The Akt that precipitated with α-parvin was phosphorylated, but the Akt that coprecipitated with β-parvin was not phosphorylated (n = 5). D: tissues were depleted of β-parvin and stimulated with ACh. Similar amounts of phosphorylated paxillin were found in β-parvin-depleted tissues and Sham-treated tissues (n = 3). Statistical analysis by one-way ANOVA. *P < 0.05.
Muscle tissues were maintained in tissue baths under isometric conditions and stimulated with 10−5 M ACh for 5 min or with 50 ng/mL IL-4 for 20 min before biochemical analysis. Time points for measurements were selected based on our previous experience to obtain maximal activation of signaling pathways by each stimulus (42, 49). ACh stimulates tension development by the tissues and increases the phosphorylation of paxillin on Tyr118, whereas stimulation with IL-4 does not induce tension generation and induces much less paxillin tyrosine phosphorylation. In contrast, the stimulation of muscle tissues with IL-4 induces Akt activation, whereas stimulation with ACh does not (Fig. 8A).
α-Parvin or β-parvin was immunoprecipitated from extracts of smooth muscle tissues, and immunocomplexes were immunoblotted for paxillin, phospho-paxillin, Akt, and phospho-Akt (Fig. 8A). More paxillin was associated with α-parvin than with β-parvin in IL-4-stimulated muscle tissues, whereas more paxillin was associated with β-parvin than α-parvin in ACh-stimulated muscle tissues (Fig. 8B). The paxillin that coimmunoprecipitated with α-parvin was not phosphorylated, whereas the paxillin that coimmunoprecipitated with β-parvin was phosphorylated on Tyr118.
When muscle tissues were stimulated with IL-4, Akt associated preferentially with α-parvin immunocomplexes (Fig. 8C). In tissues stimulated with ACh, Akt associated preferentially with β-parvin immunocomplexes. The Akt that associated with α-parvin in response to IL-4 was highly phosphorylated on Ser473, indicating Akt activation, whereas the Akt associated with β-parvin in response to ACh stimulation was not phosphorylated, indicating that it was inactive (Fig. 8C).
We evaluated the effect of β-parvin depletion on paxillin phosphorylation in response to ACh (Fig. 8D). The depletion of β-parvin had no significant effect on the ACh-stimulated phosphorylation of paxillin. These results confirm an upstream role for paxillin in regulating the activation of β-parvin IPP complexes.
We also used PLA in freshly dissociated airway smooth muscle cells to compare the effects of stimulation with ACh or IL-4 on the interaction of paxillin with α-parvin and β-parvin IPP complexes (Fig. 9, A and B). Stimulation with IL-4 resulted in the interaction of paxillin with α-parvin IPP complexes at the cell membrane but caused very little interaction between paxillin and β-parvin IPP complexes. Conversely, ACh stimulated the interaction of paxillin with β-parvin at the membrane, but it triggered very little interaction between paxillin with α-parvin IPP complexes. In both cases, the paxillin associated with β-parvin IPP complexes was phosphorylated on Tyr118, whereas the paxillin associated with α-parvin IPP complexes was not phosphorylated.
Fig. 9.
IL-4 promotes interactions between α-parvin and unphosphorylated paxillin, whereas ACh promotes interactions between β-parvin and phosphorylated paxillin in freshly dissociated tracheal smooth muscle cells. Proximity ligation assay (PLA) fluorescence images show the interaction of α-parvin or β-parvin with paxillin or pY118-paxillin in dissociated cells stimulated with ACh or IL-4 or unstimulated (US). A: significantly more paxillin interacts with α-parvin in cells stimulated with IL-4 than in cells stimulated with ACh or unstimulated cells. Paxillin that interacts with α-parvin is not phosphorylated. B: significantly more β-parvin interacts with paxillin in cells stimulated with ACh than in cells stimulated with IL-4 or unstimulated cells. Paxillin that interacts with β-parvin is phosphorylated. All values are means ± SE. *Significant difference between IL-4- and ACh-stimulated groups (n = 10–20 cells per group). Statistical analysis by one-way ANOVA. P < 0.05 was considered significant.
These results show that stimulation with ACh, which induced high levels of tyrosine-phosphorylated paxillin, causes paxillin to interact primarily with β-parvin, whereas IL-4, which induces only a small amount of paxillin tyrosine phosphorylation, causes paxillin to interact preferentially with α-parvin.
IL-4 stimulates significantly more interactions between Akt and α-parvin IPP complexes at the cell membrane than with β-parvin IPP complexes. Conversely, ACh stimulates significantly more interactions between Akt and β-parvin IPP complexes than with α-parvin complexes at the smooth muscle cell membrane (Fig. 10). The Akt that interacted with α-parvin IPP complexes was phosphorylated on Ser473, whereas the Akt that was associated with β-parvin IPP complexes was not phosphorylated. These observations are consistent with the results obtained using coimmunoprecipitation and demonstrate that Akt associates preferentially with the α-parvin IPP complex in response to IL-4 and undergoes phosphorylation and activation. In contrast, Akt associates preferentially with the β-parvin IPP complex in response to stimulation with ACh, but the Akt associated with β-parvin IPP complexes does not undergo activation.
Fig. 10.
IL-4 promotes interactions between α-parvin and activated Akt, whereas ACh promotes interactions between β-parvin and inactive Akt in freshly dissociated tracheal smooth muscle cells. Proximity ligation assay (PLA) fluorescence images are of cells stimulated with IL-4 or ACh or unstimulated (US). A: significantly more Akt interacts with α-parvin in cells stimulated with IL-4 than in cells stimulated with ACh or unstimulated cells. Akt that interacts with α-parvin is phosphorylated. B: significantly more β-parvin interacts with Akt in cells stimulated with ACh than in cells stimulated with IL-4 or unstimulated cells. Akt that interacts with β-parvin is not phosphorylated. All values are means ± SE. *Significant difference between IL-4- and ACh-stimulated groups (n = 10–20 cells per group). Statistical analysis by one-way ANOVA. P < 0.05 was considered significant.
Paxillin tyrosine phosphorylation regulates the association of α-parvin and β-parvin IPP complexes with paxillin and Akt in tissues stimulated with ACh or IL-4.
We evaluated the role of paxillin tyrosine phosphorylation in regulating the interaction of paxillin and Akt with α- and β-parvin IPP complexes in response to stimulation with ACh or IL-4 by expressing paxillin Y31/118F in smooth muscle tissues.
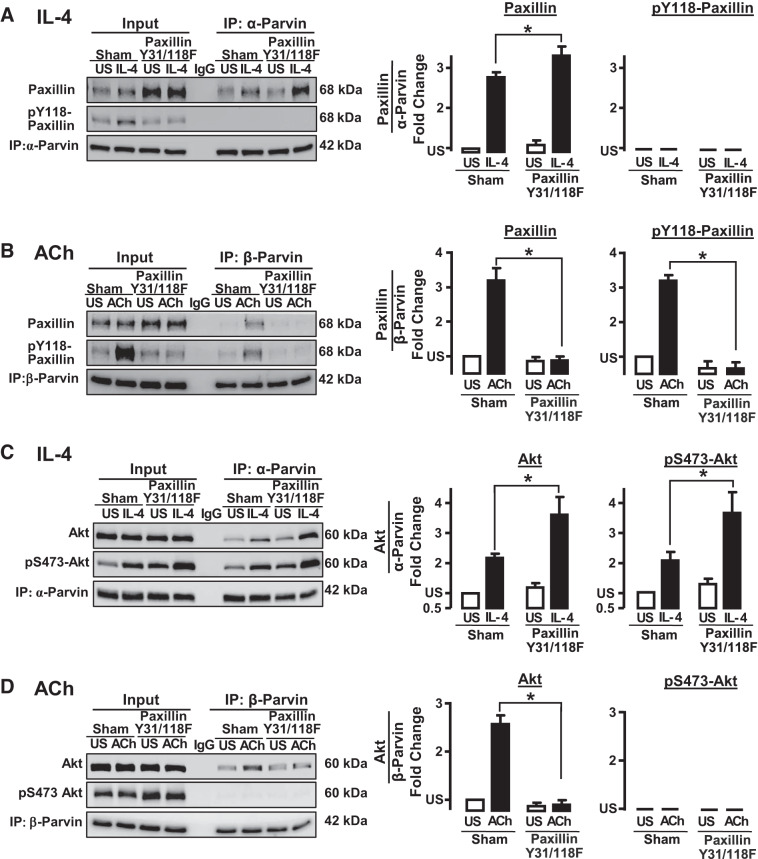
α-Parvin or β-parvin were immunoprecipitated from Sham-treated tissues and from tissues expressing the nonphosphorylatable paxillin mutants. The amount of unphosphorylated paxillin that precipitated with α-parvin IPP complexes in IL-4-stimulated muscles was significantly increased by the inhibition of paxillin phosphorylation (Fig. 11A), whereas the inhibition of paxillin phosphorylation prevented the interaction of β-parvin IPP complexes with paxillin in response to ACh stimulation (Fig. 11B). These results confirm that in tissues stimulated with ACh or IL-4, only phosphorylated paxillin can interact with β-parvin IPP complexes, whereas unphosphorylated paxillin interacts with α-parvin IPP complexes.
Fig. 11.
Paxillin phosphorylation is required for the interaction of β-parvin with paxillin and Akt in response to ACh. Inhibition of paxillin phosphorylation potentiates the interaction of α-parvin with paxillin and Akt in response to IL-4. A: expression of paxillin Y31/118F in smooth muscle tissues inhibited the ACh-induced increase in endogenous paxillin phosphorylation on Tyr118. The expression of paxillin Y31/Y118F significantly increased the IL-4-induced coimmunoprecipitation (IP) of paxillin with α-parvin immunocomplexes. No phosphorylated paxillin precipitated with α-parvin (n = 5). B: inhibition of paxillin phosphorylation suppresses the coimmunoprecipitation paxillin (n = 6) and phospho-paxillin (n = 4) with β-parvin in response to ACh. C: expression of paxillin Y31/Y118F significantly increased IL-4-induced interactions between α-parvin and Akt (n = 5) and phospho-Akt (n = 4). D: expression of paxillin Y31/Y118F inhibits the coimmunoprecipitation of Akt with β-parvin in ACh-stimulated tissues (n = 5). No phosphorylated Akt precipitated with β-parvin. All values are means ± SE. *Significant difference between Sham-treated tissues and tissues expressing paxillin Y31/118F mutant. Statistical analysis by paired t test. P < 0.05 was considered significant. US, unstimulated.
The effect of inhibiting paxillin tyrosine phosphorylation on the interaction of α-parvin and β-parvin IPP complexes with Akt was also analyzed in immunoprecipitates (Fig. 11, C and D). The suppression of paxillin phosphorylation significantly increased the IL-4-stimulated interaction of α-parvin IPP complexes with Akt, and the Akt that associated with α-parvin IPP complexes was phosphorylated (Fig. 11C). In contrast, expression of the paxillin mutant completely inhibited the ACh-stimulated interaction of Akt with β-parvin complexes (Fig. 11D).
These results confirm that paxillin phosphorylation regulates the activation of Akt by determining whether paxillin interacts with α-parvin or β-parvin IPP complexes.
DISCUSSION
Our study demonstrates a novel molecular mechanism by which integrin-associated signaling complexes transduce environmental signals to nuclear signaling pathways that regulate phenotype expression in airway smooth muscle. Akt is a key intermediate in the modulation of airway smooth muscle phenotype: Akt mediates nuclear signaling pathways that initiate the synthesis and secretion of inflammatory cytokines. Akt activation also suppresses the expression of smooth muscle differentiation marker proteins by inhibiting the nuclear localization of the transcription factor, SRF (42, 43). Mechanical tension suppresses Akt activation and inhibits airway smooth muscle inflammation while promoting the expression of a differentiated phenotype (7, 42, 44). Similarly, inflammatory mediators such as IL-4 activate Akt concurrently with STAT6 to initiate cytokine synthesis and suppress the differentiated phenotype. Thus the mechanism by which external stimuli are transduced to modulate the activation of Akt is key to understanding the regulation of phenotypic expression in airway smooth muscle.
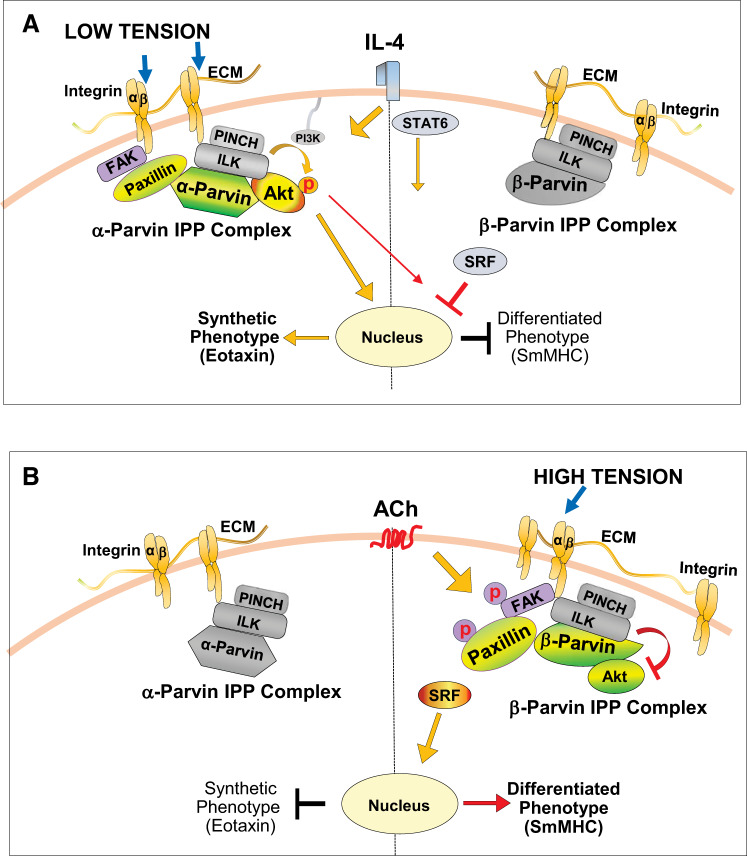
In the present study, we demonstrated that the activation of Akt is regulated by the differential coupling of paxillin to α-parvin and β-parvin IPP complexes, and that α-parvin and β-parvin IPP complexes have opposite effects on the activation of Akt and airway smooth muscle phenotypic expression (Fig. 12). Our results show that airway smooth muscle expresses separate pools of α-parvin and β-parvin IPP complexes, both of which can bind to Akt and to paxillin. We demonstrated that the interaction of α-parvin IPP complexes with Akt results in Akt activation and the expression of an inflammatory phenotype by airway smooth muscle, whereas the interaction of Akt with β-parvin IPP complexes results in the suppression of Akt activation and promotes a differentiated phenotype. We further demonstrate that phosphorylated paxillin promotes the interaction of β-parvin IPP complexes at integrin adhesion sites with Akt, resulting in the inhibition of Akt activation. Conversely, unphosphorylated paxillin mediates the interaction of α-parvin IPP complexes with Akt at integrin adhesion complexes, resulting in Akt activation. As the tyrosine phosphorylation of paxillin is differentially regulated by both mechanical and humoral stimuli, the regulation of paxillin phosphorylation represents a key step in the modulation of phenotype expression by a variety of external stimuli. Mechanical tension and ACh both stimulate the tyrosine phosphorylation of paxillin and the expression of a differentiated phenotype by the muscle tissue, whereas low mechanical tension or the inflammatory stimulus IL-4 activates Akt and results in the expression of an inflammatory phenotype by the muscle tissue (Fig. 12).
Fig. 12.
Proposed model for roles of α-parvin and β-parvin integrin-linked kinase/PINCH/parvin (IPP) complexes in phenotype modulation in airway smooth muscle. A: low mechanical tension or IL-4 stimulates the coupling of the α-parvin IPP complex to unphosphorylated paxillin within integrin adhesome complexes. This facilitates the recruitment of Akt to the α-parvin IPP complex, where it binds to integrin-linked kinase (ILK). The interaction of Akt with ILK promotes Akt activation by PI3-kinase and results in a synthetic inflammatory phenotype in the airway smooth muscle that is characterized by the synthesis of eotaxin. Akt activation suppresses the nuclear localization of serum response factor (SRF), which inhibits expression of the smooth muscle-specific marker protein, smooth muscle myosin heavy chain (SmMHC) (43). The β-parvin IPP complex also localizes to the membrane, but it does not bind to paxillin or couple to Akt. B: high mechanical tension or the stimulation of smooth muscle cells with ACh induces the β-parvin IPP complex to localize to membrane adhesomes, where it binds to tyrosine-phosphorylated paxillin. Akt preferentially binds to β-parvin rather than ILK within the β-parvin IPP complex. β-Parvin prevents the binding of Akt to ILK, which inhibits the activation of Akt. The inhibition of Akt activation promotes the nuclear localization of SRF and expression of the differentiated smooth muscle phenotype. ECM, extracellular matrix; FAK, focal adhesion kinase.
We demonstrated the role of paxillin phosphorylation in regulating phenotype expression by expressing unphosphorylatable paxillin mutants in the airway smooth muscle tissues to inhibit endogenous paxillin tyrosine phosphorylation (Fig. 2). In tissues in which paxillin phosphorylation is inhibited, Akt activation and the secretion of eotaxin is potentiated, and the expression of SmMHC is inhibited (Figs. 2 and 11). We also evaluated the role of paxillin phosphorylation in regulating the association of paxillin with α-parvin versus β-parvin IPP complexes. Expression of the paxillin nonphosphorylatable mutants prevented the interaction of paxillin with β-parvin IPP complexes, but it enhanced the interaction of paxillin with α-parvin IPP complexes (Figs. 3 and 11). The inhibition of paxillin phosphorylation also enhanced the interaction of Akt with α-parvin IPP complexes, but it prevented the interaction of Akt with β-parvin IPP complexes. This suggests that when paxillin phosphorylation is prevented, more unphosphorylated paxillin is available to bind to α-parvin IPP complexes, resulting in the potentiation of Akt activation. A similar effect is observed in tissues in which β-parvin is depleted; this also promotes more binding of Akt to α-parvin IPP complexes and the potentiation of Akt activation (Fig. 6B). The inhibition of paxillin phosphorylation had similar effects on airway smooth muscle tissues whether they were subjected to stimulation with ACh or IL-4 or the modulation of mechanical tension. These results confirm that paxillin tyrosine phosphorylation is a critical regulator of the coupling of Akt to β-parvin IPP complexes, and that unphosphorylated paxillin promotes the association of α-parvin IPP complexes with Akt. The results also confirm that the effect of an extracellular stimulus on the tyrosine phosphorylation of paxillin is critical to determining whether or not it activates Akt and leads to the expression of a synthetic phenotype in airway smooth muscle. Thus paxillin is the regulatory link between the mechanotransduction by integrin complexes and the activation of Akt by ILK and the IPP complex (Fig. 12).
We established the roles of α-parvin and β-parvin proteins in the regulation of phenotype by depleting tissues of either α-parvin or β-parvin proteins and then determined the effects on phenotype expression (Fig. 6). The depletion of α-parvin promoted SmMHC expression and the suppression of Akt activation—the differentiated phenotype—whereas the depletion of β-parvin promoted Akt activation and eotaxin secretion—the synthetic inflammatory phenotype. Thus the different effects of mechanical and humoral stimuli on the coupling of Akt to α-parvin versus β-parvin IPP complexes are key to regulating transitions between a synthetic inflammatory phenotype versus a differentiated smooth muscle phenotype. The activation of α-parvin versus β-parvin IPP complexes is a consequence of whether or not the stimulus induces paxillin phosphorylation.
Our results provide evidence that α-parvin IPP complexes interact selectively with unphosphorylated paxillin, whereas β-parvin IPP complexes interact selectively with tyrosine-phosphorylated paxillin. Paxillin has been shown to bind to both α-parvin and β-parvin (22, 23, 32). The Akt that associates with the α-parvin IPP complex binds to ILK, which promotes its activation by PI3-kinase (16, 51). In contrast, there is evidence that Akt binds preferentially to β-parvin rather than to ILK within the β-parvin IPP complex and that this inhibits Akt activation (16). Thus the differential effects of the coupling of α-parvin versus β-parvin IPP complexes to Akt may be a critical determinant of whether the activation of Akt is enabled by ILK.
ILK was initially characterized as an integrin-linked kinase capable of phosphorylating Akt (6, 25). Although subsequent studies have cast doubt on the idea that ILK is a functional kinase that directly catalyzes Akt phosphorylation (28, 40), the role of the ILK/PINCH/α-parvin signaling module in catalyzing the PI3-kinase-mediated activation of Akt is well established (1, 8, 17, 26). In airway smooth muscle, ILK overexpression promotes Akt activation and suppresses the expression of smooth muscle phenotype-specific proteins, whereas ILK depletion inhibits Akt activation and promotes the expression of smooth muscle phenotype-specific proteins (43). The regulation of smooth muscle phenotype-specific proteins by ILK occurs at the transcriptional level; ILK expression modulates RNA levels and the binding of SRF to the DNA promoters of SmMHC, SM22α, and calponin (43). Thus the interaction of Akt with ILK plays a critical role in regulating airway smooth muscle differentiation.
Molecular imaging studies have provided evidence that β-parvin IPP complexes can inhibit ILK-mediated signaling. Kimura et al. (16) demonstrated that Akt preferentially binds to β-parvin rather than ILK within β-parvin IPP complexes and that β-parvin sterically blocks the interaction of ILK with Akt. The β-parvin IPP complex can displace the α-parvin IPP complex within the integrin-associated adhesome, thereby suppressing the activation of Akt and other downstream pathways activated by the α-parvin IPP complex (13, 16, 21, 51).
The results of our present study are consistent with our previous observations that FAK activity modulates phenotype expression in airway smooth muscle (42). In this study, we found that the inhibition of FAK promotes an inflammatory phenotype, whereas the activation of FAK promotes a differentiated phenotype. As FAK is the catalyst for paxillin tyrosine phosphorylation, the inhibition of FAK prevents paxillin phosphorylation and would thus be expected to promote the interaction of Akt with the α-parvin IPP complex rather than the β-parvin IPP complex.
Our results are also consistent with our previous studies demonstrating that the exposure of airway smooth muscle tissues to neutrophil elastase promotes the activation of Akt and the expression of an inflammatory synthetic phenotype in airway smooth muscle (18). Treatment of the tissues with elastase, which degrades extracellular matrix proteins, caused the disassembly of adhesion junction complexes and suppressed the activation of the adhesome proteins paxillin, focal adhesion kinase, and vinculin. The degradation of the extracellular matrix results in the inactivation of FAK and paxillin and would therefore be expected to promote the activation of the α-parvin IPP complex at integrin adhesion sites. Neutrophil elastase and low mechanical tension both alter the connections between the extracellular matrix and integrin proteins, and they have similar effects on signaling through integrin-associated signaling modules, leading to the potentiation of inflammatory signaling pathways in airway smooth muscle. This suggests that inflammatory conditions in the airways that result in the release of proteases and the disruption of connections between the airway tissue and its surrounding milieu are likely to promote the expression of a synthetic inflammatory phenotype by the muscle tissue.
The effects of environmental conditions on phenotypic expression are well documented for many cell and tissue types and can occur in response to a variety of humoral agents, mechanical forces, and alterations in cell matrix composition and structure that are induced by physiologic or pathophysiologic processes. Integrin proteins play an important role in sensing alterations in the extracellular environment within a tissue. Our results demonstrate that the α-parvin and β-parvin proteins are critical components of a transduction mechanism that translates signals from integrin proteins to nuclear signaling pathways that mediate changes in the functional properties of cells. The key role of the parvins as mediators of phenotype modulation and their regulation by paxillin may be broadly applicable to the processes by which other cell and tissue types modulate their phenotypic properties in response to external conditions that alter the cellular environment.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL029289 and HL109629.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.H. and S.J.G. conceived and designed research; Y.H. performed experiments; Y.H. and S.J.G. analyzed data; Y.H. and S.J.G. interpreted results of experiments; Y.H. and S.J.G. prepared figures; Y.H. and S.J.G. drafted manuscript; Y.H. and S.J.G. edited and revised manuscript; Y.H. and S.J.G. approved final version of manuscript.
REFERENCES
- 1.Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell 14: 4813–4825, 2003. doi: 10.1091/mbc.e03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choe MM, Sporn PHS, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol 35: 306–313, 2006. doi: 10.1165/rcmb.2005-0443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damera G, Tliba O, Panettieri RA Jr. Airway smooth muscle as an immunomodulatory cell. Pulm Pharmacol Ther 22: 353–359, 2009. doi: 10.1016/j.pupt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc 6: 683–692, 2009. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- 5.Dekkers BGJ, Spanjer AIR, van der Schuyt RD, Kuik WJ, Zaagsma J, Meurs H. Focal adhesion kinase regulates collagen I-induced airway smooth muscle phenotype switching. J Pharmacol Exp Ther 346: 86–95, 2013. doi: 10.1124/jpet.113.203042. [DOI] [PubMed] [Google Scholar]
- 6.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 95: 11211–11216, 1998. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai LP, Wu Y, Tepper RS, Gunst SJ. Mechanical stimuli and IL-13 interact at integrin adhesion complexes to regulate expression of smooth muscle myosin heavy chain in airway smooth muscle tissue. Am J Physiol Lung Cell Mol Physiol 301: L275–L284, 2011. doi: 10.1152/ajplung.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda T, Guo L, Shi X, Wu C. CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J Cell Biol 160: 1001–1008, 2003. doi: 10.1083/jcb.200212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunst SJ, Lai-Fook SJ. Effect of inflation on trachealis muscle tone in canine tracheal segments in vitro. J Appl Physiol 54: 906–913, 1983. doi: 10.1152/jappl.1983.54.4.906. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Day RN, Gunst SJ. Vinculin phosphorylation at Tyr1065 regulates vinculin conformation and tension development in airway smooth muscle tissues. J Biol Chem 289: 3677–3688, 2014. doi: 10.1074/jbc.M113.508077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Zhang W, Gunst SJ. Activation of vinculin induced by cholinergic stimulation regulates contraction of tracheal smooth muscle tissue. J Biol Chem 286: 3630–3644, 2011. doi: 10.1074/jbc.M110.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst 1: 53–68, 2004. [PubMed] [Google Scholar]
- 13.Johnstone CN, Mongroo PS, Rich AS, Schupp M, Bowser MJ, Delemos AS, Tobias JW, Liu Y, Hannigan GE, Rustgi AK. Parvin-beta inhibits breast cancer tumorigenicity and promotes CDK9-mediated peroxisome proliferator-activated receptor gamma 1 phosphorylation. Mol Cell Biol 28: 687–704, 2008. doi: 10.1128/MCB.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan-Albuquerque N, Garat C, Desseva C, Jones PL, Nemenoff RA. Platelet-derived growth factor-BB-mediated activation of Akt suppresses smooth muscle-specific gene expression through inhibition of mitogen-activated protein kinase and redistribution of serum response factor. J Biol Chem 278: 39830–39838, 2003. doi: 10.1074/jbc.M305991200. [DOI] [PubMed] [Google Scholar]
- 15.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem 279: 12001–12004, 2004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M, Murakami T, Kizaka-Kondoh S, Itoh M, Yamamoto K, Hojo Y, Takano M, Kario K, Shimada K, Kobayashi E. Functional molecular imaging of ILK-mediated Akt/PKB signaling cascades and the associated role of beta-parvin. J Cell Sci 123: 747–755, 2010. doi: 10.1242/jcs.052498. [DOI] [PubMed] [Google Scholar]
- 17.Legate KR, Montañez E, Kudlacek O, Füssler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7: 20–31, 2006. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 18.Lockett AD, Wu Y, Gunst SJ. Elastase alters contractility and promotes an inflammatory synthetic phenotype in airway smooth muscle tissues. Am J Physiol Lung Cell Mol Physiol 314: L626–L634, 2018. doi: 10.1152/ajplung.00334.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 35: 577–593, 2003. doi: 10.1016/S0022-2828(03)00110-X. [DOI] [PubMed] [Google Scholar]
- 20.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292: C70–C81, 2007. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 21.Mongroo PS, Johnstone CN, Naruszewicz I, Leung-Hagesteijn C, Sung RK, Carnio L, Rustgi AK, Hannigan GE. Beta-parvin inhibits integrin-linked kinase signaling and is downregulated in breast cancer. Oncogene 23: 8959–8970, 2004. doi: 10.1038/sj.onc.1208112. [DOI] [PubMed] [Google Scholar]
- 22.Nikolopoulos SN, Turner CE. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol 151: 1435–1448, 2000. doi: 10.1083/jcb.151.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolopoulos SN, Turner CE. Molecular dissection of actopaxin-integrin-linked kinase-paxillin interactions and their role in subcellular localization. J Biol Chem 277: 1568–1575, 2002. doi: 10.1074/jbc.M108612200. [DOI] [PubMed] [Google Scholar]
- 24.Opazo Saez A, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol 286: C433–C447, 2004. doi: 10.1152/ajpcell.00030.2003. [DOI] [PubMed] [Google Scholar]
- 25.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA 97: 3207–3212, 2000. doi: 10.1073/pnas.97.7.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276: 27462–27469, 2001. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 27.Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Vallés AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol 148: 957–970, 2000. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol 24: 607–613, 2012. doi: 10.1016/j.ceb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sepulveda JL, Wu C. The parvins. Cell Mol Life Sci 63: 25–35, 2006. doi: 10.1007/s00018-005-5355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash YS, Kong H. Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 308: L1125–L1135, 2015. doi: 10.1152/ajplung.00154.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 3: 995–1000, 2006. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 32.Stiegler AL, Draheim KM, Li X, Chayen NE, Calderwood DA, Boggon TJ. Structural basis for paxillin binding and focal adhesion targeting of β-parvin. J Biol Chem 287: 32566–32577, 2012. doi: 10.1074/jbc.M112.367342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci USA 98: 6180–6185, 2001. doi: 10.1073/pnas.111133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang D, Mehta D, Gunst SJ. Mechanosensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Am J Physiol Cell Physiol 276: C250–C258, 1999. doi: 10.1152/ajpcell.1999.276.1.C250. [DOI] [PubMed] [Google Scholar]
- 35.Tang DD, Turner CE, Gunst SJ. Expression of non-phosphorylatable paxillin mutants in canine tracheal smooth muscle inhibits tension development. J Physiol 553: 21–35, 2003. doi: 10.1113/jphysiol.2003.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tliba O, Panettieri RA Jr. Noncontractile functions of airway smooth muscle cells in asthma. Annu Rev Physiol 71: 509–535, 2009. doi: 10.1146/annurev.physiol.010908.163227. [DOI] [PubMed] [Google Scholar]
- 37.Tschumperlin DJ, Drazen JM. Chronic effects of mechanical force on airways. Annu Rev Physiol 68: 563–583, 2006. doi: 10.1146/annurev.physiol.68.072304.113102. [DOI] [PubMed] [Google Scholar]
- 38.Tu Y, Huang Y, Zhang Y, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 153: 585–598, 2001. doi: 10.1083/jcb.153.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Fukuda K, Byeon IJ, Velyvis A, Wu C, Gronenborn A, Qin J. The structure of alpha-parvin CH2-paxillin LD1 complex reveals a novel modular recognition for focal adhesion assembly. J Biol Chem 283: 21113–21119, 2008. doi: 10.1074/jbc.M801270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wickström SA, Lange A, Montanez E, Fässler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 29: 281–291, 2010. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright DB, Trian T, Siddiqui S, Pascoe CD, Johnson JR, Dekkers BG, Dakshinamurti S, Bagchi R, Burgess JK, Kanabar V, Ojo OO. Phenotype modulation of airway smooth muscle in asthma. Pulm Pharmacol Ther 26: 42–49, 2013. doi: 10.1016/j.pupt.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Huang Y, Gunst SJ. Focal adhesion kinase (FAK) and mechanical stimulation negatively regulate the transition of airway smooth muscle tissues to a synthetic phenotype. Am J Physiol Lung Cell Mol Physiol 311: L893–L902, 2016. doi: 10.1152/ajplung.00299.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Huang Y, Herring BP, Gunst SJ. Integrin-linked kinase regulates smooth muscle differentiation marker gene expression in airway tissue. Am J Physiol Lung Cell Mol Physiol 295: L988–L997, 2008. doi: 10.1152/ajplung.90202.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue Z, Zhang W, Desai LP, Gao H, Gunst SJ, Tepper RS. Increased mechanical strain imposed on murine lungs during ventilation in vivo depresses airway responsiveness and activation of protein kinase Akt. J Appl Physiol (1985) 114: 1506–1510, 2013. doi: 10.1152/japplphysiol.01460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H, Mohri H, Ohno S, Ishigatsubo Y. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol 153: 1251–1264, 2001. doi: 10.1083/jcb.153.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci 123: 1385–1388, 2010. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Gunst SJ. Dynamic association between alpha-actinin and beta-integrin regulates contraction of canine tracheal smooth muscle. J Physiol 572: 659–676, 2006. doi: 10.1113/jphysiol.2006.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol 288: C1145–C1160, 2005. doi: 10.1152/ajpcell.00387.2004. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Wu Y, Wu C, Gunst SJ. Integrin-linked kinase regulates N-WASp-mediated actin polymerization and tension development in tracheal smooth muscle. J Biol Chem 282: 34568–34580, 2007. doi: 10.1074/jbc.M704966200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci 115: 4777–4786, 2002. doi: 10.1242/jcs.00166. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Chen K, Tu Y, Wu C. Distinct roles of two structurally closely related focal adhesion proteins, alpha-parvins and beta-parvins, in regulation of cell morphology and survival. J Biol Chem 279: 41695–41705, 2004. doi: 10.1074/jbc.M401563200. [DOI] [PubMed] [Google Scholar]