Abstract
The incidence of asthma has increased from 5.5% to near 8% of the population, which is a major health concern. The hallmarks of asthma include eosinophilic airway inflammation that is associated with chronic airway remodeling. Allergic airway inflammation is characterized by a complex interplay of resident and inflammatory cells. MicroRNAs (miRNAs) are small noncoding RNAs that function as posttranscriptional modulators of gene expression. However, the role of miRNAs, specifically miR-451, in the regulation of allergic airway inflammation is unexplored. Our previous findings showed that oxidant stress regulates miR-451 gene expression in macrophages during an inflammatory process. In this paper, we examined the role of miR-451 in regulating macrophage phenotype using an experimental poly-allergenic murine model of allergic airway inflammation. We found that miR-451 contributes to the allergic induction of CCL17 in the lung and plays a key role in proasthmatic macrophage activation. Remarkably, administration of a Sirtuin 2 (Sirt2) inhibitor diminished alternate macrophage activation and markedly abrogated triple-allergen [dust mite, ragweed, Aspergillus fumigatus (DRA)]-induced lung inflammation. These data demonstrate a role for miR-451 in modulating allergic inflammation by influencing allergen-mediated macrophages phenotype.
Keywords: alternatively activated macrophage, asthma, eosinophilic lung inflammation, microRNA-451
INTRODUCTION
Allergen-induced airway inflammation is characterized by a complex interplay of resident and inflammatory cells (50). Many cells, including Th2 lymphocytes, eosinophils, and macrophages, interact with epithelial and smooth muscle cells resulting in bronchial hyper-reactivity, mucus overproduction, airway narrowing, and deposition of extracellular matrix protein after exposure of inhaled allergens (22). The series of events that lead to chronic airway remodeling are associated with increased morbidity and mortality in severe asthmatics (32). However, the molecular mechanisms leading to these pathogenic allergic responses remain largely unknown.
MicroRNAs (miRNAs) are small noncoding RNAs that function as posttranscriptional modulators of gene expression by either promoting mRNA degradation or blocking protein translation (29). Conserved biogenesis machinery governs the canonical miRNA production in vertebrate cells (20). Canonical miRNAs are generated by sequential cleavage of precursor substrates by the Dicer and Drosha RNase III enzymes with an Argonaute (Ago) protein (51). miRNAs generally decrease protein production by an epigenetic mechanism that involve targeting mRNA for degradation. Recent studies demonstrate that miRNAs are important regulators of gene expression in the immune system and contribute to the pathogenesis of asthma (29, 39, 45). Although various miRNAs have been implicating in allergic asthma, further investigation is required to determine whether they are viable therapeutic targets.
miR-451 is a distinctive microRNA that undergoes Ago2-dependent cytoplasmic biogenesis pathway, independent from Dicer activity (9). Yang et al. reported that the maturation of the highly conserved vertebrate miR-451 bypasses Dicer and instead requires direct cleavage of its precursor hairpin through Ago2 Slicer activity (51). Initially, miR-451 was shown to be involved in a variety of cancer-related pathways (3, 4, 25, 48). To date, miR-451 has been reported to participate in other pathological processes, including arthritis, and chronic obstructive pulmonary disease (COPD) (11, 27, 33). Our previous work highlighted that oxidant stress increases miR-451 gene expression in macrophages with reduction in Ago2 protein (41). Despite increased understanding of the biological functions of miR-451, the impact of miR-451 in allergic disease remains unexplored.
Macrophages are the most prominent immune effector cells that reside on the respiratory epithelial surface and are capable of promoting inflammatory responses in the airways (10). Previously the role of pulmonary macrophages in allergic asthma has been overlooked, but more recently macrophages have emerged as an important cell type in asthma pathogenesis. The macrophage inflammatory phenotype contributes to the inflammatory and repair phases that characterize disease progression (32). We and others have established a pathogenic link between macrophages and the intensity of asthmatic lung inflammation (6, 7, 31, 37, 40).
Here we report that miR-451 is critical for allergen-induced airway inflammation in a poly-allergenic murine model of asthma. By using miR-451-deficient (miR-451 KO) mice, we further demonstrate that miR-451 is involved in allergic induction of CCL17 in the lung. miR-451 KO mice developed accentuated allergic inflammation, which was characterized by increased eosinophil influx and mucus hypersecretion in the lungs of allergen-sensitized and -challenged mice compared with wild-type (WT) control animals. Lung macrophages isolated from miR-451 KO mice expressed increased levels of the CCL17 and Sirtuin 2 (Sirt2). Collectively, our data demonstrate a role for miR-451 in modulating allergic inflammation by influencing allergen-mediated macrophages phenotype.
MATERIALS AND METHODS
Materials.
Unless otherwise stated, all biochemical reagents used in this study were purchased from Sigma (St. Louis, MO). Antibody against Drosha (no. 3364), Dicer (no. 5362), Ago1 (no. 5053), IRF4 (no. 15106), Sirt1 (no. 8469), and β-actin (no. 3700) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against Sirt2 (S8447) and Ago2 (ab186733) were purchased from Sigma and Abcam (Cambridge, MA), respectively. Phycoerythrin (PE)-conjugated anti-SiglecF (no. 552126) was purchased from BD Biosciences (San Diego, CA). APC-conjugated CD11b (no. 101212), PE-Cy7-conjugated CD11c (no. 117318), and CD45 (no. 103132) antibodies were purchased from BioLegend (San Diego, CA).
Mice.
All experiments involving mice were conducted with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Ohio State University. Gene knockout (KO) mice lacking miR-144/451 mice (referred to as miR-451 KO) mice were defective in expressing miR-451 as previously described (41, 52). Wild-type mice with same background (C57BL/6J) were used for the control. The mice were obtained from University of Pennsylvania and housed at the animal facility of the Ohio State University in a standard pathogen free environment.
Induction of murine asthma model.
Triple allergens (DRA) include extracts of dust mite (Dermatophagoides farina), ragweed (Ambrosia artemisiifolia), and Aspergillus fumigatus (Greer Laboratories, Lenoir, NC). Aluminum (Inject Alum; Thermo Scientific) was used for adjuvant. Quantities of allergens for intraperitoneal (100 µl) per mouse were used as follows: D. farina [5 µg, 3–35 EU by means of limulus amebocyte lysate (LAL) assay], ragweed (50 µg, 5 EU), and Aspergillus fumigatus (5 µg, 0.1 EU) (37). Quantities of allergens for intranasal injection (50 µl) were used as follows: D. farina (8.3 µg), ragweed (83.4 µg), and Aspergillus fumigatus (8.3 µg). Briefly, mice (8–12 wk old) were sensitized with the DRA allergen mixture on days 0 and 5 by intraperitoneal injection with alum (Thermo Fisher Scientific). The mice were challenged with the DRA mixture at the same concentration used for sensitization on days 12, 13, and 14 by intranasal delivery. The mice were euthanized on day 15, and bronchoalveolar lavage (BAL) fluid and lung tissues were collected for further analysis. All sensitized animals had elevated levels of total IgE.
Inhibitor administration.
A highly selective Sirt2 inhibitor, AGK2 (10 mg/kg; Selleckchem), was administrated by peritoneal injection with 2% Tween20 and 10% PEG-400 in PBS 30 min before DRA challenge daily for 3 days. Timelines of the DRA models are shown in Fig. 1A.
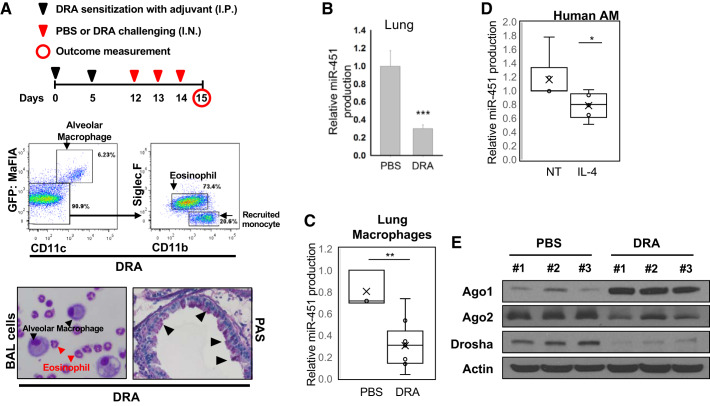
Fig. 1.
miR-451 expression is decreased in asthmatic lungs and alternatively activated macrophages. A: the schematic timeline showed that C57BL/6 mice were sensitized with downregulated in adenoma (DRA) on days 0 and 5 and challenged with DRA on days 12, 13, and 14. All mice were harvested on day 15 for analysis. Eosinophils influx was confirmed by flow cytometry using selective antibodies for cell surface antigen on eosinophils (CD11b, CD11c, and SiglecF). Representative cytospin slides with bronchoalveolar lavage (BAL) fluid showed DRA-induced eosinophil infiltration. Periodic acid-Schiff (PAS) staining was performed for identification of goblet cells in the epithelium. GFP, green fluorescent protein. B: the releative level of miR-451 expression was markedly reduced in DRA-induced asthmatic lungs, compared with nonasthmatic lungs. C: relative expression of miR-451 in collagenase-digested lung macrophages from DRA-challenged mice. D: in vitro studies demonstrated that Th2 cytokines IL-4 significantly reduced miR-451 in healthy human alveolar macrophages (AMs). E: Western blot analysis of the level of protein expression of Ago1, Ago2, and Drosha in DRA-induced asthmatic lungs. Results are shown as means ± SE (n = 5–6). P values were obtained using a t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Lung tissue preparation.
Mouse lung tissue was prepared using pressurized low-melting agarose. Briefly, 1.5% wt/vol low-melting-point agarose was boiled at 60°C and then kept at 42°C in water bath. After tracheostomy was performed, the 1.5% melted agarose was infused through the tracheostomy tube from height of 28 cmH2O to pressurize equally over lung fields. The tracheostomy tube was tied, and lung tissue was put into a formalin container that was refrigerated overnight to facilitate solidification and fixation. Both hematoxylin and eosin (H&E) staining and periodic acid-Schiff (PAS) staining were conducted by the Comparative Pathology and Mouse Phenotyping Shared Resource at the Ohio State University. For quantification of mucus metaplasia, slides were scored using a scale of 0–4 (0: no reactivity; 4: the highest intensity staining) for PAS reactivity. Each slide was scored by two blinded individuals. The intensity was evaluated for each section from each animal and averaged.
BAL differential cell count.
BAL fluid was collected by lavaging the lung with 800 µl of PBS twice via a tracheal catheter and analyzed for total cell counts by countess automated cell counter (Life Technologies). BAL fluid on cytospin slides was stained with HEMA 3 (Thermo Scientific) for differential cell counts. The number of total cells, macrophages, and eosinophils was quantitated and compared for statistical significance.
Flow cytometry.
Cells collected from BAL fluid were incubated with Fc-blocking anti-mouse CD16/32 antibody (no. 553142, BD Bioscience PharMingen) followed by PE-conjugated anti-SiglecF, PE-Cy7-conjugated CD11c, and APC-conjugated anti-CD11b antibodies. For intracellular staining, cells were fixed with BD Bioscience Fixation Buffer for 10 min at room temperature. Cells were washed two times with FACS buffer (2% FBS and 0.5mM EDTA in PBS) and blocked with anti-mouse CD16/CD32 antibody in for 15 min at 4°C. Cell surface were stained with antibodies for SiglecF, CD11c, and CD11b to detect alveolar macrophages population for 30 min at 4°C. After being washed three times with BD Bioscience Permeabilization buffer, cells were stained with Ym1 (Stemcell Technologies, no. 01404) in Permeabilization/Wash buffer for 1 h at 4°C and washed three times in Permeabilization/Wash buffer. Cells were analyzed on a BD LSR II (BD Bioscience) where gating was based on respective unstained cell population and isotype matching control antibodies. The data were analyzed with FlowJo software (TreeStar).
Measurement of cytokines.
Cytokine secretion in culture supernatants was analyzed by ELISA specific for mouse CCL17 and CCL22 (R&D Systems) following the protocols supplied by the manufacturer.
Western blot analysis.
Cells were lysed in RIPA lysis buffer (Millipore, Temecula, CA) with 1× protease inhibitor cocktail (Pierce). Cell lysates containing equal amount of protein were electrophoresed and immunoblotted using appropriate antibodies as described previously (8, 19).
RNA extraction and quantitative real-time RT-PCR.
RNA was extracted from cells or lung tissues homogenates by using a miRNeasy Mini kit (Qiagen) and Direct-zol RNA Kits (Zymo Research) according to the manufacture’s instruction. cDNA synthesis was measured with RevertAid First Strand cDNA Synthesis Kit (Thermo), and gene expression was measured by the change-in-threshold (ΔΔCt) method based on quantitative real-time PCR in a Roche LightCycler 480 (Roche), normalizing to GAPDH expression as an endogenous control.
For microRNA quantitative PCR, total RNA was reverse transcribed using miScript II RT kit (Qiagen) according to instructions. The expression levels of mature miR-451 were analyzed using miScript SYBR Green PCR kit and microRNA-specific RT primers (Qiagen). The primers used were, Hs_miR-451_1 miScript Primer Assay (cat. no. MS00004242) and Mm_miR-451_1 miScript Primer Assay (MS00035658) purchased from Qiagen. Mature miRNA levels were normalized to RNU6B and quantified using the comparative Ct method.
Macrophages cultures.
Murine lung macrophages were isolated from the whole lungs of mice via a collagenase digestion, as previously described (6). Cells were adherence purified for 30 min in serum-free media, nonadherent cells (such as lymphocytes) were washed away, and complete media were replaced for overnight incubation. Lung macrophages were incubated in the presence or absence of recombinant mouse (rm) IL-4 (20 ng/ml).
Mouse alveolar macrophage MH-S cells were cultured in RPMI1640 supplemented with 10% FBS and 1% penicillin/streptomycin (6). In the experiments of transfection, MH-S cells were transfected with miR-451 mimic (Syn-mmu-miR-451a miScript miRNA mimic, cat. no. MSY0001632) and nontargeting control (Qiagen) using Lipofectamine 3000 (Invitrogen).
Measurements of airway hyperresponsiveness.
Mechanical properties of the mouse lung were assessed in diazepam (Valium)-ketamine-anesthetized mice using the forced-oscillation technique as described in previous study (1). Anesthetized mice were mechanically ventilated on a flexiVent computer-controlled piston ventilator (SCIreq, Montreal, Canada) with 8 ml/kg tidal volume at a frequency of 150 breaths/min against 2–3 cmH2O positive end-expiratory pressure. Pressure and flow data (reflective of airway and tissue dynamics) were used to calculate lung resistance, static lung compliance, and dynamic lung compliance at baseline with use of the single-compartment model. Maximal airway responsiveness to bronchoconstrictors was measured following exposure to increasing doses of nebulized methacholine (0.1–50 mg/ml).
Statistical analysis.
Results are expressed as means ± SE. Comparison between two groups were performed with Student’s t-test for unpaired variables. Statistical significance is indicated in figure legends. P < 0.05 was considered statistically significant.
RESULTS
miR-451 expression is decreased in asthmatic lungs and in alternatively activated macrophages.
To determine the role of miR-451 in the pathogenesis of allergic asthma, we examined the expression of miR-451 in mice that were subject to poly-allergenic sensitization and challenge. The DRA model was chosen because it features impressive asthmatic airway changes including peribronchial and alveolar eosinophilia (6, 37). We subjected C57BL/6 mice to sensitization and challenge with DRA, according to the protocol shown in Fig. 1A, top). As expected, DRA challenge induced BAL eosinophilia (in 67.9% eosinophils, SiglecF+CD11c−CD11b+) 24 h after the allergen inhalation (Fig. 1A, middle). We examined lung tissues with periodic acid-Schiff (PAS) staining that detects mucus glycol conjugates in goblet cells indicating marked mucosal metaplasia within the asthmatic airway epithelium (Fig. 1A, bottom). In the DRA model, a significant reduction of miR-451 expression was observed in the lungs of allergen-challenged mice when compared with their control counterparts (Fig. 1B). Similarly, decreased miR-451 expression was seen in macrophages purified from collagenase-digested lung tissue from allergen-challenged mice (Fig. 1C). These murine data are translational to human lung macrophages, which also expressed lower amounts of miR-451 in response to in vitro treatment with IL-4 (Fig. 1D). Since, it is reported that miR-451 undergoes dicer-independent Ago2 dependent alternate miRNA biogenesis pathway (5, 41), we compared Ago1 and Ago2 proteins in the lungs of allergen-challenged mice by Western blots. DRA challenge resulted in a marked decrease in Ago2 protein levels, whereas Ago1 protein production was increased in lung tissue compared PBS control mice (Fig. 1E). This reduction in Ago2 may account for the reduction in miR-451. Although there is a compensatory increase in Ago1, this does not result in production of mature miR-451. Notably, we also observed a remarkable reduction of Drosha protein levels in the lungs of allergen-challenged mice.
Allergic asthmatic inflammation is accentuated in miR-451 KO mice.
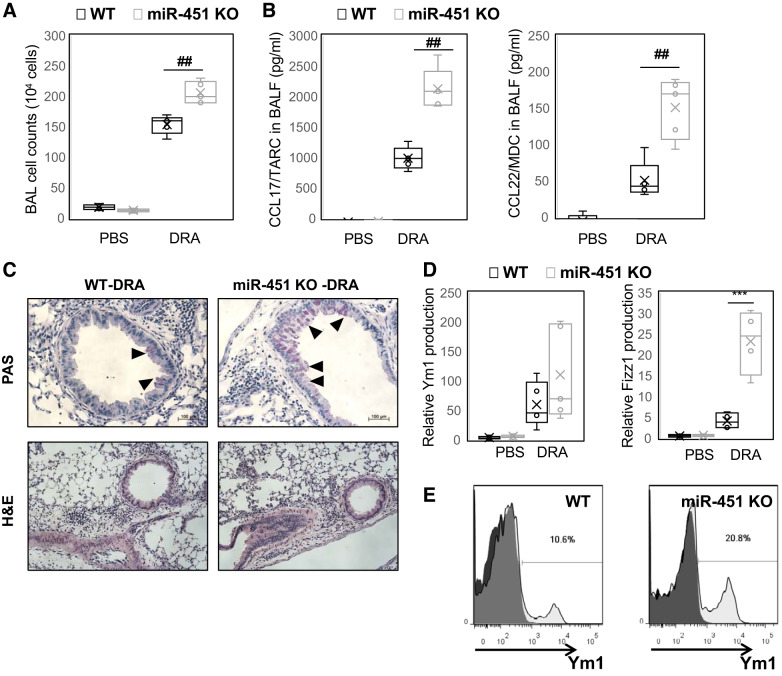
To study the role of miR-451 in regulating asthmatic inflammation, miR-451 KO mice (52) were subjected to sensitization and challenge with DRA. We observed a similar increase in serum IgE in KO compared with WT mice (Supplemental Fig. S2A; see https://doi.org/10.6084/m9.figshare.10257329). As shown in Fig. 2A, the total numbers of BAL cells from DRA-challenged miR-451 KO mice was increased (~33%) compared with those from WT mice. Cytokine array analysis demonstrated a marked increase in TARC/CCL17 and MCP1/CCL2 in miR-451 KO BAL fluid following DRA challenge that was detected by a protein array (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.11871924). These findings are validated by ELISA, which confirmed significant increases in TARC/CCL17 and MDC/CCL22 in miR-451-deficient mice following allergen challenge (Fig. 2B). Lung histology revealed that mucus producing goblet cells was increased in the miR-451 KO mice than WT mice (Fig. 2C). Moreover, there was an increase in the alternative activation markers Ym1 and Fizz1 in whole lung tissue from miR-451 KO mice, compared with WT control (Fig. 2D). Consistent with these findings in lung tissues, Ym1 expression measured by flow cytometry in alveolar macrophages of DRA-challenged miR-451 KO mice was increased compared with WT controls (Fig. 2E, from 10.6% to 20.8%). These data indicate that miR-451 is necessary for development of a proasthmatic macrophage inflammatory phenotype.
Fig. 2.
Allergic asthmatic inflammation is accentuated in miR-451 knockout (KO) mice. Wild-type (WT) and miR-451 KO mice were sensitized and challenged by daily intranasal administration of downregulated in adenoma (DRA). A and B: total number of cells in bronchoalveolar lavage (BAL) fluid were counted based on total amount of BAL cells (A) and CCL17 and CCL22 cytokines were quantified with ELISA in BAL fluid (B). C: histopathology was performed based on hematoxylin and eosin (H&E) staining to determine asthmatic airway inflammation and mucosal metaplasia in PBS- or DRA-treated WT or miR-451 KO mice. Top: periodic acid-Schiff (PAS)-stained lung sections from the mice exposed to PBS or DRA. Black arrowheads indicate PAS-positive goblet cells. Bottom: H&E staining for the lungs. D: expression of mRNA for Ym1 and Fizz1 in DRA-induced murine lung tissues. E: YM1 expression levels on alveolar macrophages (AMs; CD11c+CD11b−SiglecF+) in BAL cells from DRA-challenged mice, analyzed by flow cytometry. Results are shown as means ± SE (n = 4–8). P values were obtained using a t test. ##P < 0.01, ***P < 0.001.
MiR-451 modulates IL-4-induced alternative macrophage polarization by altering Sirtuin2 expression.
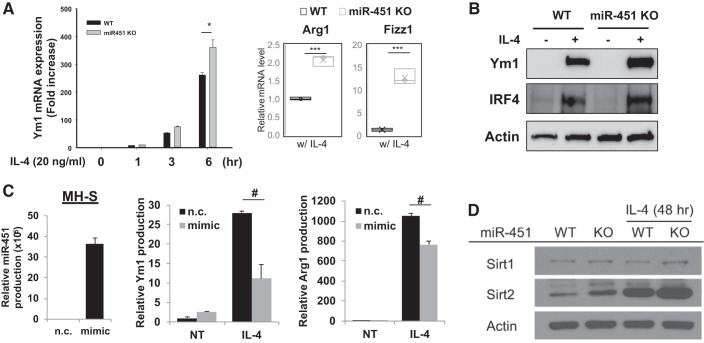
Our previous report defined a role for alternatively activated macrophages in contributing the immune response to allergen (6, 7, 40), the current study seeks to extend these data to determine whether miR-451 has a role in regulating alternative macrophage activation. To do this, we used lung macrophages that were purified from collagenase-digested lung tissue from WT and miR-451 KO mice and incubated with IL-4. There was a time-dependent increase of Ym1 mRNA transcript in the presence of IL-4 stimulation in lung macrophages from WT mice that was most prominent after 6 h. Treatment of lung macrophages from miR-451 KO mice resulted in markedly increase of mRNA expression of Ym1 compared with WT control (Fig. 3A). Next, we examined the expression of Arg1 and Fizz1 after incubation with IL-4 for 48 h in lung macrophages from WT and miR-451 KO mice and found that both Arg1 and Fizz1 mRNA transcripts were significantly increased in lung macrophages from miR-451 KO mice. Consistent with this pattern, we observed M2-related Ym1 and IRF4 protein expression was increased compared with WT lung macrophages (Fig. 3B). Next, we performed a reciprocal “gain of function” experiment where we examined the expression of Ym1 and Arg1 after transfection with miR-451 mimic in MH-S cells. Following transfection with miR-451 mimic (20 nM) for 24 h, MH-S murine alveolar macrophage cells were stimulated with IL-4 (10 ng/ml) for 24 h. We observed that Ym1 and Arg1 mRNA levels were decreased after transfection with miR-451 mimic indicating that miR451 impairs polarization of alternatively activated macrophages (Fig. 3C).
Fig. 3.
miR-451 modulates IL-4-induced alternative macrophage polarization by altering Sirtuin 2 (Sirt2) expression. A: quantitative (q)PCR analysis of Ym1, Arg1, and Fizz1 mRNA expression in IL-4 stimulated lung macropahges from wild-type (WT) and miR-451 knockout (KO) mice. B: M2-related Ym1 and IRF4 protein expressions from IL-4 stimulated WT and miR-451 KO lung macrophages. C: relative miR-451, Ym1, and Arg1 expressions in MH-S cells after transfection with miR-451 mimic. D: Western blot analysis of Sirt1 and Sirt2 proteins in IL-4 stimulated lung macropahges from WT and miR-451 KO mice. NT, not treated; n.c., nontargeting control. Results are shown as means ± SE (n = 4–6). P values were obtained using a t test. *#P < 0.05 and ***P < 0.001.
We recently reported that sirtuin2 (Sirt2), a histone deacetylase, is an important regulator of allergic inflammation via regulating macrophage activation (24). Our previous work demonstrated Sirt2 regulates the production of CCL17 and markers of alternative activated, proasthmatic macrophages in the setting of allergic inflammation. To study the possible association between miR-451 expression and Sirt2 protein levels in alternatively activated macrophages, Sirt2 levels were examined from WT and miR-451 KO lung macrophages. Interestingly miR-451 KO macrophages had increased expression of Sirt2 on in response to IL-4 stimulation (Fig. 3D). As a control we measured Sirt1 expression, which was not different in WT and KO macrophages. Together, these gain- and loss-of-function data indicate that miR-451 is an inhibitor of alternative macrophages activation that is mediated through suppression of Sirt2 protein production.
A highly selective Sirt2 blocker, AGK2, attenuates eosinophilic lung inflammation in miR-451 KO mice.
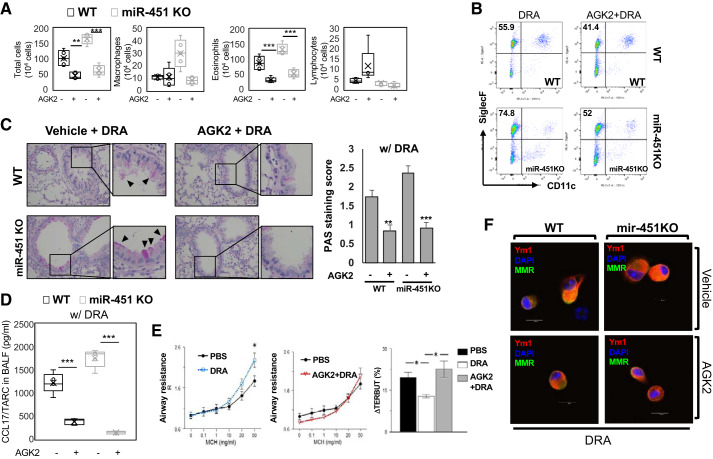
Previously we have shown that AGK2, a highly selective inhibitor of Sirt2 (36), reduced allergic inflammation following DRA challenge in WT mice by regulating lung macrophage phenotype (24). We hypothesized increased Sirt2 expression in miR-451 KO mice drives the development of allergic asthmatic inflammation. DRA-sensitized WT and miR-451 KO mice were administered AGK2 (10 mg/kg ip injection) 1 h before DRA challenge once a day for 3 consecutive days. In response to instillation of DRA, significantly increased eosinophil numbers were also detected in the airways of allergen challenged miR-451 KO compared with WT mice (Fig. 4A). As expected, pretreatment with AGK2 resulted in attenuation of the airway eosinophilia, as evidenced by a 60% reduction in eosinophilic airway inflammation, in BAL fluid (Fig. 4A). This was confirmed by flow cytometric analyses that showed that treatment with AGK2 markedly reduced alveolar eosinophils (SiglecF+CD11c−) in the WT mice challenged with allergens compared with vehicle treated control mice (Fig. 4B and Supplemental Fig. S3B; see https://doi.org/10.6084/m9.figshare.11871924). This is also confirmed by histological examination with marked mucosal metaplasia of PAS-positive goblet cells. As observed in tissue sections (Fig. 4C), compared with DRA-challenged groups, AGK2 reduced the DRA-mediated increases in allergic inflammation and mucosal metaplasia compared with the controls in both WT and miR-451 KO mice.
Fig. 4.
A selective Sirtuin 2 (Sirt2) blocker, acylglycerol kinase 2 (AGK2), attenuates eosinophilic lung inflammation in miR-451 knockout (KO) mice. Wild-type (WT) and miR-451 KO mice were subjected to downregulated in adenoma (DRA) sensitization and challenge as shown in the protocol depicted in Fig. 1A. Before allergen challenge, mice were treated with vehicle or 10 mg/kg of AGK2, a selective Sirt2 inhibitor, on days 12, 13, and 14. A: total cells and macrophages/eosinophils influx in bronchoalveolar lavage (BAL) fluid were counted based on total amount of BAL cells, analyzed by Diff-Quik analysis. B: infiltrated cells in BAL fluid were stained with PE-anti-SiglecF and PE-Cy7-anti-CD11c antibodies. The percentage of eosinophils (Eos, Siglec F+CD11c−) was analyzed by flow cytometry. C: periodic acid-Schiff (PAS)-stained lung sections from the mice exposed to DRA or AGK2 + DRA in allergen-challenged WT and miR-451 KO mice. Black arrowheads indicate PAS-positive goblet cells. D: production of CCL17 was assessed in BAL fluid (BALF) by ELISA. E: airway resistance in increased in the DRA-challenged (blue dashed line) versus vehicle-challenged miR-451 KO mice (black solid line) in response to methacholine (MCH) challenge. Airway resistance in miR-451 KO mice that were treated with AGK2 before challenge with DRA is not different from control mice in response to methacholine challenge testing (MCT). Following the MCT, the mice were treated with terbutaline. All 3 groups had a bronchodilator, response but the response to terbutaline (TERBUT) was significantly less in the DRA challenge group than the control group and response to the AGK2 treated-DRA challenge group was greater than the only DRA challenge group (right). Statistics were done by ANOVA; n for the DRA and DRA/AGK2 group was 12, for the Control group was 6, and the error bars are SE; *P < 0.05. F: representative confocal microscopy image of immunostained YM1 protein from alveolar macrophages (MMR+) in BAL fluid. Results are shown as means ± SE (n = 4–8). P values were obtained using a t test. *P < 0.05, **P < 0.01, and ***P < 0.001.
Data obtained from a cytokine antibody array demonstrated an attenuated Th2/M2 immune response in BAL fluid of AGK2 treated-DRA challenged miR-451 KO mice compared with the only DRA-challenged miR-451 KO mice (Supplemental Fig. S3A; see https://doi.org/10.6084/m9.figshare.11871924). We confirmed this finding by measuring BAL fluid level of TARC/CCL17 and found that both AGK2-treated DRA-challenged WT and miR-451 KO mice had decreased levels of CCL17 (Fig. 4D). The level of Ym1 expression in alveolar macrophages from DRA-challenged miR-451 KO mice was slightly upregulated than that of the WT mice. However, the density of the positively labeled these cells was lower in the AGK2-treated both WT and miR-451 KO mice. Merged images showing immunoreactivity for Ym1 and MMR (an alveolar macrophage marker) in WT and miR-451 KO mice (Fig. 4F). Although there was no difference between IL-5 and IL-13 in BAL fluid of miR-451 KO mice, there was a decrease in IL-5 levels in WT and miR-451 KO mice in response to treatment with AGK2 (Supplemental Fig. S3D; see https://doi.org/10.6084/m9.figshare.11871924). Finally, we measured airway hyperresponsiveness [methacholine challenge testing (MCT)] in DRA-sensitized and -challenged miR-451 KO mice (1). DRA-sensitized and -challenged mice (DRA, blue dashed line) showed a significant increase in airway resistance compared with sensitized miR-451 KO mice that were challenged only with vehicle (PBS) and not DRA (Fig. 4E, left). In contrast, when the miR-451 KO mice were treated with AGK2 before challenging with DRA (AGK2 + DRA), the MCT did not differ from the unchallenged control mice (PBS, black solid line) indicating that the increased airway resistance induced by DRA challenge was ameliorated by treatment with AGK2 (Fig. 4E, middle). At the end of the MCT, the mice were treated with terbutaline (a beta 2 agonist) to test whether the airway resistance was reversible. As shown, airway resistance improved in all three groups but the response to terbutaline was significantly less in the DRA-challenged group (DRA) than the control group (PBS) and the airway resistance was most improved in the AGK2-treated, DRA-challenged group (AGK2 + DRA), which was significantly greater than the only DRA-challenged group (Fig. 4E, right). We also detected a significance difference in the basal airway resistance between the DRA-challenged and the AGK2-treated DRA-challenged groups, but there were no differences in the basal or MCT induced static compliance indicating that the effect of AGK2 is selective on airway resistance (data not shown).
DISCUSSION
Recently, miRNA regulation of allergic inflammation has been reported (13, 16, 17, 35, 42). Nevertheless current knowledge on the role of miRNAs in the pathogenesis of asthma is limited. miRNAs are of considerable importance in immune regulation; therefore, further investigation is required to determine whether they serve as potential therapeutic targets for treating asthma. We have previously shown the functional significance of miR-451 in the regulation of macrophage oxidant stress (41). Our work indicated that there is functional cross talk between miR-451 and reactive oxygen species in the regulation of macrophage oxidant stress through posttranscriptional modulation of Ago2 synthesis. Of note, a previous study also reported that miR-451 expression is downregulated in murine lungs during ovalbumin-induced allergic inflammation (39), but the mechanism was not deciphered. In the current study, we demonstrate for the first time that miR-451-mediated pathways contributing allergic immune response in experimental models of allergen-induced asthmatic airway inflammation.
Over the last two decades, the incidence of asthma has increased from 5.5% to near 8% of the population, which is a major concern for both patients and medical professionals (49). The hallmarks of asthma are increased airway inflammation, mucosal metaplasia, smooth muscle cell hypertrophy, and increased airway reactivity (34). Initially asthma was thought to be exclusively a Th2-cell-mediated disorder. However, only approximately half of asthmatic patients exhibit a typical signs type 2 response (23). The term endotype was proposed in 2008 as a conceptual framework to guide new thinking about the molecular heterogeneity of asthma (12). Asthma has been determined to be divided into at least two subtypes referred to as Th2-low and Th2-high endotypes. Improved understanding of the asthmatic endotypes is expected to provide a rational basis for new or personalized treatments.
We and others have previously shown that the expression of CCL17 and CCL22 was upregulated in the airway upon clinically relevant allergens [e.g., house dust mite (HDM), birch pollen extract, Aspergillus fumigatus, and ragweed] (6, 24, 38, 46, 47). In this study, the three combined allergens (DRA) model was chosen because it features impressive asthmatic airway changes (14) especially peribronchial and alveolar eosinophilia (6, 15), although multiple allergens may create less Th2 phenotypes. In this study, DRA challenge resulted in a marked cellular infiltration of recruited inflammatory cells consisting of mostly eosinophils and is associated with impressive mucosal metaplasia and increased expression of Ym1, a well-known marker of M2 (alternately activated) macrophages (Figs. 2 and 4). Based on these observations, we believe that experiments outlined in this study using the murine DRA-induced allergic asthma model exhibit impressive Th2-mediated airway inflammatory responses. CCR4 and its ligands TARC/CCL17 and MDC/CCL22 are known to have an important role in allergic diseases. Allergen-mediated alveolar eosinophil recruitment is regulated by these two eosinophil chemoattractants. These mediators and Th2/M2 cell-specific proteins are responsible for lung tissue remodeling and fibrosis. In the current study, we demonstrated that miR-451 deficiency accentuates the Th2/M2 allergic immune response. Allergen-challenged miR-451 KO mice have increased peribronchial inflammation and mucosal metaplasia in the airways compared with WT mice. Upon allergen challenge, excessive CCL17 production in miR-451 KO mice leads to alternatively activation of macrophages. There is also increased Ym1, Arg1, and IRF4, which are three markers of proasthmatic lung macrophages in asthma. Although the levels of CCL17 in the airways were dramatically increased in miR-451 KO mice, the levels of IL-5 and IL-13 were not. This discrepancy may indicate that the chemokine CCL17 and CCL22 are predominantly produced by myeloid cells in the immune system (30).
In the present study, we investigated the role of miR-451 in model of allergic asthma using in vivo mouse model. Zhang et al. identified high level of miR-451 in IFNγ + LPS-induced M1 macrophages (53). However, no further studies have examined the role of this miRNA in macrophages. Our findings highlight the impact of miR-451 on the phenotype of alternatively activated macrophages during asthma pathogenesis, including high levels of Ym1 and Fizz1, Arg1, and Irf4 in vitro. A miR-451 mimic decreased markers associated with the phenotype of alternatively activated macrophages, which possibly suggests the dependence of miR-451 in alternatively activated proasthmatic macrophage phenotype. Therefore, validation of potential target genes and further functional studies are required to understand the mechanistic role of miR-451.
Sirtuin-2 (Sirt2) is known for NAD+-dependent protein deacetylase. Previous study using bone marrow-derived macrophages (BMDMs) from Sirt2-deficient mice reported reducing the M2-associated anti-inflammatory pathway (28). The importance of macrophage Sirt2 in allergic inflammation has previously been reported by our group and showed that Sirt2 regulates the production of CCL17 and recruitment of myeloid-derived cells into the lung following sensitization and challenge with allergen in vivo (24). Although Sirt2 is present in other lung cells that could participate in asthma pathogenesis, our published data also showed a marked accentuation of the asthmatic response in WT mice that undergo adoptive transfer of lung macrophages from Sirt2-overexpressing transgenic mice. Together, these findings highlight the novel role of Sirt2 in alternative activation of macrophage.
In this study, we observed substantial upregulation of Sirt2, but not Sirt1, in miR-451 deficient lung macrophages following in vitro IL-4 stimulation. Although the possibility of miR-451 to contribute asthma pathogenesis has already been reported (39), its ability to affect the expression of proasthmatic markers in lung macrophages during allergic inflammation is reported here for the first time. This effect could be due to the modulation of Sirt2 mediated by miR-451. Some of miRNAs have been predicted in silico and/or experimentally determined to target the 3′-untranslated region (UTR) region. In parallel with the direct regulation, miRNAs can suppress protein indirectly through the action of upstream regulators (26, 43). Among all of the genes that were differentially expressed with the miRNA modulation, <20% were predicted miRNA targets (43), suggesting that the underlying coordinated changes in the global patterns of gene expression involve the modulation of core genes (2, 44). It is worth noting that Sirt2 has no 3′-UTR binding regions for miR-451, which further suggests that this might be an indirect effect of miR-451 on allergic airway inflammation. Although we could not detect Sirt2 is a direct target of miR-451, we cannot exclude an indirect effect of miR-451 on CCL17 expression via Sirt2. Therefore, we hypothesized that miR-451 may regulate Sirt2 expression at multiple steps in alternatively activated macrophages and its influence on CCL17 production and inflammatory cell recruit might be the earliest in the allergic airway inflammation. In addition, we found that alternatively spliced Sirt2 isoform 3/5 influences the development of experimental allergic asthmatic inflammation in our previous study (24), suggesting that miR-451 may coordinate an alternative splicing network of Sirt2 during macrophage activation (18, 21).
To confirm the role of Sirt2 in the setting of allergic inflammation in miR-451 KO mice showing aggravated allergic inflammation than WT mice, we treated sensitized miR-451 KO mice with a highly selective Sirt2 inhibitor, AGK2 that does not inhibit Sirt1 (54). Consistent with previous studies (24), treatment with AGK2 reduced alveolar eosinophils in both WT and miR-451 KO mice challenged with DRA allergens as shown by differential cell counting and histology (Fig. 4, A–C). Correspondingly, CCL17 production is reduced in both WT and miR-451 KO mice (Fig. 4D). This trend was also observed in Ym1 expression of lung macrophages from allergen challenged WT and miR-451 KO mice (Fig. 4E). However, Sirt2 inhibition did not impede other Th2-cell derived cytokines such as IL-4 and IL-13 in our study (Supplemental Fig. S3; see https://doi.org/10.6084/m9.figshare.11871924). A possible explanation is that Sirt2 imparts important changes in gene expression by lung macrophages, in mice and humans that are exposed in vivo to an allergen challenge (24). Whether such regulation also occurs in lung macrophages is at present not clear; it will require further experiments.
We have shown that miR-451 is decreased in pulmonary macrophages from sensitized mice that are challenged with DRA allergen. We demonstrate a potential role for miR-451 in the local regulation of allergic immune response in allergen-induced asthmatic inflammation through Sirt2 regulates macrophage activation. Using pharmacologic inhibitor of Sirt2, we demonstrate changes in cellular composition and airway inflammation. In summary, our data indicates that a decrease of miR-451 in lung macrophages of allergen-challenged mice accentuates asthmatic airway inflammation, at least in part, by increasing expression of Sirt2.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-137224 and 5R01-HL-126852. The analytical flow cytometry core is supported by National Cancer Institute Grant P30-CA0-16058) and the Comparative Pathology and Mouse Phenotyping Shared Resource is supported by National Cancer Institute Grant P30-CA-016058.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C. and J.W.C. conceived and designed research; S.C., Y.G.L., and I.C.D. performed experiments; S.C., Y.G.L., and J.W.C. analyzed data; S.C., M.K., J.A.E., M.N.B., I.C.D., G.Y.P., and J.W.C. interpreted results of experiments; S.C. and Y.G.L. prepared figures; S.C. drafted manuscript; S.C., M.K., J.A.E., M.N.B., G.Y.P., and J.W.C. edited and revised manuscript; S.C., Y.G.L., M.K., J.A.E., M.N.B., I.C.D., G.Y.P., and J.W.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mitchell J. Weiss (St. Jude Children's Research Hospital) for the miR-451 knockout mice. We also thank analytical flow cytometry core and Comparative Pathology and Mouse Phenotyping Shared Resource for technical assistance and the Davis Heart and Lung Research Institute.
REFERENCES
- 1.Aeffner F, Woods PS, Davis IC. Ecto-5′-nucleotidase CD73 modulates the innate immune response to influenza infection but is not required for development of influenza-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 309: L1313–L1322, 2015. doi: 10.1152/ajplung.00130.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhtar N, Singh AK, Ahmed S. MicroRNA-17 Suppresses TNF-alpha signaling by interfering with TRAF2 and cIAP2 association in rheumatoid arthritis synovial fibroblasts. J Immunol 197: 2219–2228, 2016. doi: 10.4049/jimmunol.1600360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, Jimenez P, Rodriguez J, Garcia-Foncillas J. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res 15: 2281–2290, 2009. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 4.Bergamaschi S, Morato E, Bazzo M, Neves F, Fialho S, Castro G, Zimmermann A, Pereira I. Tumor markers are elevated in patients with rheumatoid arthritis and do not indicate presence of cancer. Int J Rheum Dis 15: 179–182, 2012. doi: 10.1111/j.1756-185X.2011.01671.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465: 584–589, 2010. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung S, Kim JY, Song MA, Park GY, Lee YG, Karpurapu M, Englert JA, Ballinger MN, Pabla N, Chung HY, Christman JW. FoxO1 is a critical regulator of M2-like macrophage activation in allergic asthma. Allergy 74: 535–548, 2019. doi: 10.1111/all.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung S, Lee TJ, Reader BF, Kim JY, Lee YG, Park GY, Karpurapu M, Ballinger MN, Qian F, Rusu L, Chung HY, Unterman TG, Croce CM, Christman JW. FoxO1 regulates allergic asthmatic inflammation through regulating polarization of the macrophage inflammatory phenotype. Oncotarget 7: 17532–17546, 2016. doi: 10.18632/oncotarget.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S, Ranjan R, Lee YG, Park GY, Karpurapu M, Deng J, Xiao L, Kim JY, Unterman TG, Christman JW. Distinct role of FoxO1 in M-CSF- and GM-CSF-differentiated macrophages contributes LPS-mediated IL-10: implication in hyperglycemia. J Leukoc Biol 97: 327–339, 2015. doi: 10.1189/jlb.3A0514-251R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, Ma E, Mane S, Hannon GJ, Lawson ND, Wolfe SA, Giraldez AJ. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328: 1694–1698, 2010. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draijer C, Peters-Golden M. Alveolar macrophages in allergic asthma: the forgotten cell awakes. Curr Allergy Asthma Rep 17: 12, 2017. doi: 10.1007/s11882-017-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudics S, Venkatesha SH, Moudgil KD. The micro-RNA expression profiles of autoimmune arthritis reveal novel biomarkers of the disease and therapeutic response. Int J Mol Sci 19: 2293, 2018. doi: 10.3390/ijms19082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol 15: 57–65, 2015. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbacki N, Di Valentin E, Huynh-Thu VA, Geurts P, Irrthum A, Crahay C, Arnould T, Deroanne C, Piette J, Cataldo D, Colige A. MicroRNAs profiling in murine models of acute and chronic asthma: a relationship with mRNAs targets. PLoS One 6: e16509, 2011. doi: 10.1371/journal.pone.0016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goplen N, Karim MZ, Liang Q, Gorska MM, Rozario S, Guo L, Alam R. Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma. J Allergy Clin Immunol 123: 925–32.e11, 2009. doi: 10.1016/j.jaci.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noël A, Cataldo DD. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res 58: 845–854, 2009. doi: 10.1007/s00011-009-0054-2. [DOI] [PubMed] [Google Scholar]
- 16.Hu R, Kagele DA, Huffaker TB, Runtsch MC, Alexander M, Liu J, Bake E, Su W, Williams MA, Rao DS, Möller T, Garden GA, Round JL, O’Connell RM. miR-155 promotes T follicular helper cell accumulation during chronic, low-grade inflammation. Immunity 41: 605–619, 2014. doi: 10.1016/j.immuni.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson K, Malmhäll C, Ramos-Ramírez P, Rådinger M. MicroRNA-155 is a critical regulator of type 2 innate lymphoid cells and IL-33 signaling in experimental models of allergic airway inflammation. J Allergy Clin Immunol 139: 1007–1016.e9, 2017. doi: 10.1016/j.jaci.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Kalsotra A, Wang K, Li PF, Cooper TA. MicroRNAs coordinate an alternative splicing network during mouse postnatal heart development. Genes Dev 24: 653–658, 2010. doi: 10.1101/gad.1894310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpurapu M, Wang X, Deng J, Park H, Xiao L, Sadikot RT, Frey RS, Maus UA, Park GY, Scott EW, Christman JW. Functional PU.1 in macrophages has a pivotal role in NF-κB activation and neutrophilic lung inflammation during endotoxemia. Blood 118: 5255–5266, 2011. doi: 10.1182/blood-2011-03-341123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139, 2009. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 21.Kucherenko MM, Shcherbata HR. miRNA targeting and alternative splicing in the stress response - events hosted by membrane-less compartments. J Cell Sci 131: jcs202002, 2018. doi: 10.1242/jcs.202002. [DOI] [PubMed] [Google Scholar]
- 22.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 16: 45–56, 2015. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 23.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 50: 975–991, 2019. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Lee YG, Reader BF, Herman D, Streicher A, Englert JA, Ziegler M, Chung S, Karpurapu M, Park GY, Christman JW, Ballinger MN. Sirtuin 2 enhances allergic asthmatic inflammation. JCI Insight 4: e124710, 2019. doi: 10.1172/jci.insight.124710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Sanda T, Look AT, Novina CD, von Boehmer H. Repression of tumor suppressor miR-451 is essential for NOTCH1-induced oncogenesis in T-ALL. J Exp Med 208: 663–675, 2011. doi: 10.1084/jem.20102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433: 769–773, 2005. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 27.Liu K, Tian H, Zhang Y, Zhao H, Ma K. miR-451 selectively increases sensitivity to cisplatin in ERCC1-high non-small cell lung cancer cells. J Cell Biochem 120: 12074, 2018. doi: 10.1002/jcb.26657. 29315773 [DOI] [Google Scholar]
- 28.Lo Sasso G, Menzies KJ, Mottis A, Piersigilli A, Perino A, Yamamoto H, Schoonjans K, Auwerx J. SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS One 9: e103573, 2014. doi: 10.1371/journal.pone.0103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malmhäll C, Alawieh S, Lu Y, Sjöstrand M, Bossios A, Eldh M, Rådinger M. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol 133: 1429–1438e7, 2014. doi: 10.1016/j.jaci.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Mathie SA, Dixon KL, Walker SA, Tyrrell V, Mondhe M, O’Donnell VB, Gregory LG, Lloyd CM. Alveolar macrophages are sentinels of murine pulmonary homeostasis following inhaled antigen challenge. Allergy 70: 80–89, 2015. doi: 10.1111/all.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira AP, Hogaboam CM. Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J Interferon Cytokine Res 31: 485–491, 2011. doi: 10.1089/jir.2011.0027. [DOI] [PubMed] [Google Scholar]
- 33.Musri MM, Coll-Bonfill N, Maron BA, Peinado VI, Wang RS, Altirriba J, Blanco I, Oldham WM, Tura-Ceide O, García-Lucio J, de la Cruz-Thea B, Meister G, Loscalzo J, Barberà JA. MicroRNA dysregulation in pulmonary arteries from chronic obstructive pulmonary disease. Relationships with vascular remodeling. Am J Respir Cell Mol Biol 59: 490–499, 2018. doi: 10.1165/rcmb.2017-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newcomb DC, Peebles RS Jr. Th17-mediated inflammation in asthma. Curr Opin Immunol 25: 755–760, 2013. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okoye IS, Czieso S, Ktistaki E, Roderick K, Coomes SM, Pelly VS, Kannan Y, Perez-Lloret J, Zhao JL, Baltimore D, Langhorne J, Wilson MS. Transcriptomics identified a critical role for Th2 cell-intrinsic miR-155 in mediating allergy and antihelminth immunity. Proc Natl Acad Sci USA 111: E3081–E3090, 2014. doi: 10.1073/pnas.1406322111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317: 516–519, 2007. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 37.Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y, Chung S, Karpurapu M, Deng J, Ranjan R, Xiao L, Jaffe HA, Corbridge SJ, Kelly EA, Jarjour NN, Chun J, Prestwich GD, Kaffe E, Ninou I, Aidinis V, Morris AJ, Smyth SS, Ackerman SJ, Natarajan V, Christman JW. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med 188: 928–940, 2013. doi: 10.1164/rccm.201306-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J 23: 876–884, 2004. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- 39.Plank MW, Maltby S, Tay HL, Stewart J, Eyers F, Hansbro PM, Foster PS. MicroRNA expression is altered in an ovalbumin-induced asthma model and targeting miR-155 with antagomirs reveals cellular specificity. PLoS One 10: e0144810, 2015. doi: 10.1371/journal.pone.0144810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian F, Deng J, Lee YG, Zhu J, Karpurapu M, Chung S, Zheng JN, Xiao L, Park GY, Christman JW. The transcription factor PU.1 promotes alternative macrophage polarization and asthmatic airway inflammation. J Mol Cell Biol 7: 557–567, 2015. doi: 10.1093/jmcb/mjv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranjan R, Lee YG, Karpurapu M, Syed MA, Chung S, Deng J, Jeong JJ, Zhao G, Xiao L, Sadikot RT, Weiss MJ, Christman JW, Park GY. p47phox and reactive oxygen species production modulate expression of microRNA-451 in macrophages. Free Radic Res 49: 25–34, 2015. doi: 10.3109/10715762.2014.974037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen KD, Simmini S, Abreu-Goodger C, Bartonicek N, Di Giacomo M, Bilbao-Cortes D, Horos R, Von Lindern M, Enright AJ, O’Carroll D. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med 207: 1351–1358, 2010. doi: 10.1084/jem.20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahab SW, Matyunina LV, Hill CG, Wang L, Mezencev R, Walker LD, McDonald JF. The effects of MicroRNA transfections on global patterns of gene expression in ovarian cancer cells are functionally coordinated. BMC Med Genomics 5: 33, 2012. doi: 10.1186/1755-8794-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLOS Comput Biol 3: e131, 2007. doi: 10.1371/journal.pcbi.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C, Barczak AJ, Zlock LT, Blagev DP, Finkbeiner WE, Ansel KM, Arron JR, Erle DJ, Woodruff PG. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med 186: 965–974, 2012. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanović R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. J Allergy Clin Immunol 130: 1404–12.e7, 2012. doi: 10.1016/j.jaci.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Rijt LS, Logiantara A, Canbaz D, van Ree R. Birch pollen-specific subcutaneous immunotherapy reduces ILC2 frequency but does not suppress IL-33 in mice. Clin Exp Allergy 48: 1402–1411, 2018. doi: 10.1111/cea.13254. [DOI] [PubMed] [Google Scholar]
- 48.Wang R, Wang ZX, Yang JS, Pan X, De W, Chen LB. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 30: 2644–2658, 2011. doi: 10.1038/onc.2010.642. [DOI] [PubMed] [Google Scholar]
- 49.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18: 716–725, 2012. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 50.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 15: 271–282, 2015. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 51.Yang JS, Maurin T, Robine N, Rasmussen KD, Jeffrey KL, Chandwani R, Papapetrou EP, Sadelain M, O’Carroll D, Lai EC. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc Natl Acad Sci USA 107: 15163–15168, 2010. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu D, dos Santos CO, Zhao G, Jiang J, Amigo JD, Khandros E, Dore LC, Yao Y, D’Souza J, Zhang Z, Ghaffari S, Choi J, Friend S, Tong W, Orange JS, Paw BH, Weiss MJ. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev 24: 1620–1633, 2010. doi: 10.1101/gad.1942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med 31: 797–802, 2013. doi: 10.3892/ijmm.2013.1260. [DOI] [PubMed] [Google Scholar]
- 54.Zhao T, Alam HB, Liu B, Bronson RT, Nikolian VC, Wu E, Chong W, Li Y. Selective Inhibition of SIRT2 Improves Outcomes in a Lethal Septic Model. Curr Mol Med 15: 634–641, 2015. doi: 10.2174/156652401507150903185852. [DOI] [PMC free article] [PubMed] [Google Scholar]