Abstract
Endotracheal intubation is a vital component of many rat in vivo experiments to secure the airway and allow controlled ventilation. Even in the hands of experienced researchers, however, the procedure remains technically challenging. The safest and most reliable way for human intubation is by video laryngoscopy. Previous attempts to apply this technique in rodents have been complicated and expensive. We, hereby, describe a novel, noninvasive method to safely intubate rats orally by video laryngoscopy, thus avoiding the need for a surgical tracheostomy. By repurposing a commercially available ear wax removal device, visualization of the rat larynx can be significantly enhanced. Because of its small diameter, integrated illumination, and a powerful camera with adequate focal length, the device has all of the necessary properties for exploring the upper airway of a rat. After identifying the vocal cords by video laryngoscopy, the insertion of an endotracheal tube (a 14G intravenous catheter) into the trachea under constant visual control is facilitated by using PE50 polyethylene tubing as a stylet (Seldinger technique). The procedure has been performed more than 60 times in our laboratory; all intubations were successful on the first attempt, and no adverse events were observed. We conclude that the described procedure is a simple and effective way to intubate a rat noninvasively, using inexpensive and commercially available equipment.
Keywords: endotracheal intubation, in vivo, mechanical ventilation, respiratory physiology, rodents
INTRODUCTION
Successful intubation of rats provides the basis for many research experiments that can have a significant impact on medical science. Intubation is needed for controlled ventilation, particularly for positive pressure ventilation when neuromuscular blocking agents are used. For survival studies, especially, a less invasive oral intubation technique is preferred over surgical tracheostomy. Therefore, researchers endeavor to improve techniques to make oral intubation safe, reliable, and fast. A popular method is blind oral intubation with improved positioning of the rat (5, 7, 10, 13, 14), but it has the clear disadvantage of often requiring multiple attempts and causing possible harm to the larynx (13). Further development improved the visualization of the larynx by using infant or self-made laryngoscopes (15, 16) or elaborate video laryngoscope (VL) systems (HOPKINS II Telescope; Karl Storz, Tuttlingen, Germany) (11). In the following, we describe a new oral intubation technique for rats, which is very similar to the use of VL for intubation in humans, the most successful technique, especially for difficult airways (3). We use a cost-effective, commercially available device for ear wax removal in humans that has similar features to a VL, and is repurposed for a simple, fast, and safe oral intubation of rats.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University Medical Center, Nashville, Tennessee, and were performed in compliance with the ARRIVE guidelines (8). Prior to the experimentation, all rats were housed in our institute’s animal care facility, with free access to water and food.
Equipment.
An ear wax removal device (5.5 mm in diameter) with an integrated camera, light source (Fig. 1C), and USB connection (Fvgia-Direct, Amazon Seller) was purchased to create a cost-effective and waterproof VL. The ear wax scoop that was also provided was securely attached and used as a blade (Fig. 1C). The VL was connected to a computer (MacBook Pro, Apple, Cupertino, CA) to visualize and record the procedures.
Fig. 1.
Positioning and equipment for video laryngoscopy and intubation of rats. A fast and secure fixation of the rat is performed by loose tape inferior to the upper incisors locked with LEGO bricks on a modified LEGO base plate (A). The anesthetized rat on the board is positioned on the bench at a 45° angle head up (B). The blade is attached to the camera at the upper field of view (C). A stylet (15 cm PE50 polyethylene tubing; Instech Laboratories, Plymouth Meeting, PA) is inserted into a 14 gauge intravenous catheter (Smith Medical ASD, Southington, CT) and advanced 1–2 cm past the catheter tip (D).
Procedure.
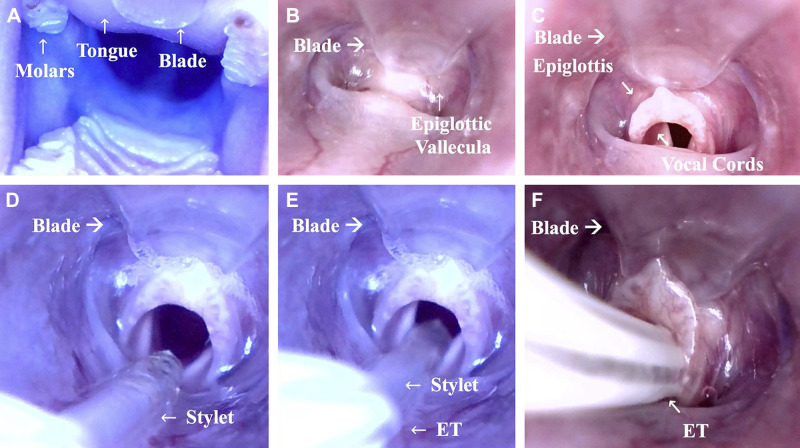
General anesthesia was induced in 60 male rats (Sprague-Dawley; 350–450 g) by intraperitoneal injection of pentobarbital sodium (45 mg/kg). The level of anesthesia (general, surgical plane) was confirmed by the absence of visual movements of the rat and a negative response to a toe pinch stimulus. Each rat was placed on its back on a board and fixed in position with surgical tape (Fig. 1A). The board was then secured in place on the bench at a 45° angle head up (Fig. 1B). A 1¾ inch, 14 gauge catheter (Smith Medical ASD, Southington, CT), used as the endotracheal tube (ET), was prepared with a stylet (15 cm PE50 polyethylene tubing; Instech Laboratories, Plymouth Meeting, PA) that was inserted and advanced 2 cm past the catheter tip (Fig. 1D). The tongue was pulled out slightly, and the tip of the VL, with the blade upwards, was gently introduced into the mouth, on one side of the upper incisors. The VL was gently pushed forward with the blade sliding along the tongue until the tip of the blade was placed at the level of the epiglottic vallecula (Fig. 2, A and B). By pushing the tip of the ear spoon upwards and raising the epiglottis, the glottis was made visible (Fig. 2C). The stylet was inserted into the trachea (Fig. 2, D and E). The ET was pushed over the stylet (Seldinger technique) and, once past the vocal cords, the stylet was pulled back (Fig. 2, E and F) and the VL was carefully removed. The ET was fixed to the upper jaw with 4-0 suture material.
Fig. 2.
Insertion of the video laryngoscope (VL) and field of view during intubation. The VL is introduced into the mouth of the rat with the blade gliding along the tongue (A). The tip of the blade is carefully placed next to the epiglottic valleculae (B). The epiglottis is raised with a slight move of the tip of the blade in ventral direction (C). The stylet is inserted into the glottis (D). Once the stylet has passed the glottis ~1 cm (E), the endotracheal tube (ET) is passed over the stylet into the trachea (F), and finally the stylet is pulled back. The procedure can also be watched in Supplemental Video S1 available at www.doi.org/10.6084/m9.figshare.11891571.v2.
RESULTS
All intubations were successful on the first attempt, being completed in less than 1 min and with proper placement of the ET; the procedure can be watched in Supplemental Video S1 (Supplemental material is available at https://doi.org/10.6084/m9.figshare.11891571.v2). No complications, such as bleeding or bronchospasm, occurred, and all rats were ventilated without problems. The properties of the camera fit exactly to the needs of rat intubation: no sharp edges, waterproof, and the ability to be cleaned with 70% ethanol. A focal length of 1.4–2.0 cm is optimal for this purpose, and the integrated light is sufficient. The device also does not heat up to cause any thermal damage. Importantly, the attached blade improves the field of view by holding the tongue up and away, and by lifting the epiglottis up with its tip. Furthermore, the insertion of the ET under visual inspection indicates if the ET fits the size of the trachea and provides insight about mucus or any foreign objects that might lead to aspiration after extubation.
DISCUSSION
In the past, several different methods with good success rates have been described for rat intubation, but most need a high level of technical effort, training, and/or financial expenditure (7). This VL intubation procedure is a simple, fast, and safe technique. Its costs compared with considerably more expensive VL systems, which in addition to a VL require a separate monitor and a light source (11), are significantly lower. Equally important, this intubation technique is favorable for survival studies by avoiding harm to the larynx and trachea. The device is easy to handle by a single user and is comparable to a human laryngoscope with a straight Miller-Blade. This technique is simple to learn for researchers without previous intubation experience in rodents and enhances the training process of veterinarians, students, and others learning endotracheal intubation.
Because of the small size of the device, its use in smaller animals than ours is entirely possible; proper adjustments of ET tube and stylet size are facilitated by direct visual inspection.
Overall, the properties of this VL technique open up new possibilities beyond intubation. By improving the field of view of the larynx in rats with a high-resolution, wide view on a computer screen, further applications like surgeries and injections related to the oropharynx or vocal cords become possible (1, 4). Under these favorable conditions, intratracheal injections for disease models (e.g., infectious agents) or therapies (pharmacologic, gene, or cell) could be enhanced by safe and rapid execution (2, 6, 9, 12). Because of the short time needed for this procedure, volatile anesthetics like isoflurane could also be used for induction, since rats will not emerge from anesthesia during fast procedures or intubation.
GRANTS
C. Balzer was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Project number 397561247. T. R. Jinka was funded by a Scientist Development Grant (15SDG25830030) from the American Heart Association. Additional support was provided by institutional funds, NIH (National Heart, Lung, and Blood Institute Grant 5R01 HL123227), and a Merit Review Award (I01 BX003482) from the U.S. Department of Veteran Affairs Biomedical Laboratory R&D Service awarded to M. L. Riess.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B., T.R.J., and M.L.R. conceived and designed research; C.B., W.J.C., and M.L.R. performed experiments; C.B. and M.L.R. analyzed data; C.B. and M.L.R. interpreted results of experiments; C.B., W.J.C., T.R.J., and M.L.R. prepared figures; C.B., W.J.C., T.R.J., and M.L.R. drafted manuscript; C.B., W.J.C., T.R.J., and M.L.R. edited and revised manuscript; C.B., W.J.C., T.R.J., and M.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Troy M. Apple of the IACUC at Vanderbilt University Medical Center for consulting on this project.
REFERENCES
- 1.Andreatta RD, Stemple JC, Seward TS, McMullen CA. Subcutaneous neurotrophin 4 infusion using osmotic pumps or direct muscular injection enhances aging rat laryngeal muscles. J Vis Exp (124): 13, 2017. doi: 10.3791/55837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayala P, Torres J, Vivar R, Olmos PR, Meneses M, Borzone GR. Changes in the pattern of fibrosis in the rat lung with repetitive orotracheal instillations of gastric contents: evidence of persistent collagen accumulation. Am J Physiol Lung Cell Mol Physiol 315: L390–L403, 2018. doi: 10.1152/ajplung.00559.2017. [DOI] [PubMed] [Google Scholar]
- 3.Aziz MF, Dillman D, Fu R, Brambrink AM. Comparative effectiveness of the C-MAC video laryngoscope versus direct laryngoscopy in the setting of the predicted difficult airway. Anesthesiology 116: 629–636, 2012. doi: 10.1097/ALN.0b013e318246ea34. [DOI] [PubMed] [Google Scholar]
- 4.Carvajal Monroy PL, Grefte S, Kuijpers-Jagtman AM, Helmich MPAC, Ulrich DJO, Von den Hoff JW, Wagener FADTG. A rat model for muscle regeneration in the soft palate. PLoS One 8: e59193–e59198, 2013. doi: 10.1371/journal.pone.0059193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheong SH, Lee KM, Yang YI, Seo JY, Choi MY, Yoon YC. Blind oral endotracheal intubation of rats using a ventilator to verify correct placement. Lab Anim 44: 278–280, 2010. doi: 10.1258/la.2010.009118. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa-Baba Y, Kubota H, Takata A, Miyagawa M. Intratracheal instillation methods and the distribution of administered material in the lung of the rat. J Toxicol Pathol 27: 197–204, 2014. doi: 10.1293/tox.2014-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastl S, Kotschenreuther U, Hille B, Schmidt J, Gepp H, Hohenberger W. Simplification of rat intubation on inclined metal plate. Adv Physiol Educ 28: 29–32, 2004. doi: 10.1152/advan.00008.2003. [DOI] [PubMed] [Google Scholar]
- 8.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloth C, Gruben N, Ochs M, Knudsen L, Lopez-Rodriguez E. Flow cytometric analysis of the leukocyte landscape during bleomycin-induced lung injury and fibrosis in the rat. Am J Physiol Lung Cell Mol Physiol 317: L109–L126, 2019. doi: 10.1152/ajplung.00176.2018. [DOI] [PubMed] [Google Scholar]
- 10.Lamoureux L, Radhakrishnan J, Gazmuri RJ. A rat model of ventricular fibrillation and resuscitation by conventional closed-chest technique. J Vis Exp (98): 26, 2015. doi: 10.3791/52413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda A, Pêgo JM, Correia-Pinto J. Animal facility videoendoscopic intubation station: tips and tricks from mice to rabbits. Lab Anim 51: 204–207, 2017. doi: 10.1177/0023677216652342. [DOI] [PubMed] [Google Scholar]
- 12.Porzionato A, Zaramella P, Dedja A, Guidolin D, Van Wemmel K, Macchi V, Jurga M, Perilongo G, De Caro R, Baraldi E, Muraca M. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 316: L6–L19, 2019. doi: 10.1152/ajplung.00109.2018. [DOI] [PubMed] [Google Scholar]
- 13.Rivard AL, Simura KJ, Mohammed S, Magembe AJ, Pearson HM, Hallman MR, Barnett SJ, Gatlin DL, Gallegos RP, Bianco RW. Rat intubation and ventilation for surgical research. J Invest Surg 19: 267–274, 2006. doi: 10.1080/08941930600778297. [DOI] [PubMed] [Google Scholar]
- 14.Su CS, Lai HC, Lee WL, Ting CT, Yang YL, Lee HW, Wang LC, Peng CY, Wang KY, Liu TJ. A secure and rapid method for orotracheal intubation of laboratory rats utilising handy instruments. Eur J Anaesthesiol 29: 515–519, 2012. doi: 10.1097/EJA.0b013e328357ce5b. [DOI] [PubMed] [Google Scholar]
- 15.Tomasello G, Damiani F, Cassata G, Palumbo VD, Sinagra E, Damiani P, Bruno A, Cicero L, Cupido F, Carini F, Lo Monte AI. Simple and fast orotracheal intubation procedure in rats. Acta Biomed 87: 13–15, 2016. [PubMed] [Google Scholar]
- 16.Vongerichten A, Aristovich K, dos Santos GS, McEvoy AW, Holder DS. Design for a three-dimensional printed laryngoscope blade for the intubation of rats. Lab Anim (NY) 43: 140–142, 2014. doi: 10.1038/laban.463. [DOI] [PubMed] [Google Scholar]