Abstract
Air-liquid interface (ALI) cultures are ex vivo models that are used extensively to study the epithelium of patients with chronic respiratory diseases. However, the in vitro conditions impose a milieu different from that encountered in the patient in vivo, and the degree to which this alters gene expression remains unclear. In this study we employed RNA sequencing to compare the transcriptome of fresh brushings of nasal epithelial cells with that of ALI-cultured epithelial cells from the same patients. We observed a strong correlation between cells cultured at the ALI and cells obtained from the brushed nasal epithelia: 96% of expressed genes showed similar expression profiles, although there was greater similarity between the brushed samples. We observed that while the ALI model provides an excellent representation of the in vivo airway epithelial transcriptome for mechanistic studies, several pathways are affected by the change in milieu.
Keywords: air-liquid interface, cell culture, nasal brushing, nasal epithelia, transcriptome
BACKGROUND
Air-liquid interface (ALI) cultures are ex vivo models used extensively to study the epithelium of patients with allergies, asthma, chronic obstructive pulmonary disease (COPD), and chronic rhinosinusitis (1, 3, 9, 11, 12). This model establishes a differentiated epithelium, as seen in vivo, that is useful in performing mechanistic studies of respiratory epithelia, testing drug formulations, and studying the toxicity of inhaled substances, both infectious and noninfectious, none of which can be performed in brushed cells. However, the in vitro conditions impose a milieu different from that encountered in the patient in vivo, and the degree to which this alters gene expression remains unclear. In this study we compared the transcriptome of fresh brushings of nasal epithelial cells with that of ALI-cultured epithelial cells from the same patients. We employed RNA sequencing to determine the correlation between ALI-cultured epithelial cells and epithelial cells obtained from nasal brushing and identify differences that may arise as a result of redifferentiation.
METHODS
Human subjects.
Five former smokers with COPD were enrolled in the study. The research protocol was approved by the Institutional Review Board of Johns Hopkins University, and all the patients gave signed informed consent. The diagnosis of COPD was established using the Global Initiative for Chronic Obstructive Lung Disease (GOLD)-2015 criteria, including ≥10-pack-yr smoking history, ratio of postbronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity <70%, and FEV1 <80% predicted. Participants were former smokers as defined by self-report of no smoking in the last 6 mo and exhaled carbon monoxide <6 ppm. Demographic characteristics of patients with COPD are summarized in Table 1. No known nasal or sinus comorbidities were observed in these patients with COPD.
Table 1.
Demographic characteristics of recruited subjects
| Patient No. |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age, yr | 62 | 68 | 61 | 79 | 75 |
| Sex | Female | Male | Female | Male | Female |
| BMI, kg/m2 | 35.31 | 29.79 | 24.93 | 22.31 | 35.19 |
| Postbronchodilator | |||||
| FVC, %predicted | 84 | 77 | 59 | 99 | 79 |
| FEV1, %predicted | 36 | 65 | 36 | 57 | 58 |
| FEV1/FVC | 0.34 | 0.64 | 0.47 | 0.40 | 0.57 |
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Sinonasal brushing sampling.
Two samples were obtained from the inferior turbinate using a sterile cytology brush (Microvasive, Milford, MA); no topical anesthetic was used. The first brush head was ejected into a vial containing RNA stabilization reagent (RNAlater, Qiagen, MA) and stored at −80°C. The second brush head was suspended in 1× Dulbecco’s phosphate-buffered saline (DPBS; Thermo Fisher Scientific, CA) and immediately processed for cell culture.
Culturing sinonasal epithelia at ALI.
The cell suspension was centrifuged at 1,500 rpm for 10 min to remove 1× DPBS. The pellet was resuspended in PneumaCult-Ex Plus medium (StemCell Technologies, Vancouver, BC, Canada) and amplified on rat tail collagen I (Corning, NY)-coated flasks. Cells were passaged at 80–90% confluency. At subconfluency, cells were plated at 4 × 105 per well onto rat tail collagen I-coated 0.4-μm-pore polyethylene terephthalate clear-membrane 12-mm-diameter Transwell inserts (Corning, NY) with PneumaCult-Ex Plus medium at ALI. At 100% confluency, the inserts were placed in basolateral PneumaCult-ALI medium (StemCell Technologies). Cells were differentiated for 6 wk at ALI to obtain a fully differentiated pseudostratified epithelium. To ensure that the ALI cultures were fully differentiated, we confirmed that the monolayer established a barrier as measured by transepithelial electrical resistance [380–600 Ω·cm2 (average 470 Ω·cm2)] and confirmed the presence of cilia by measuring the percentage of moving pixels [39–53% (average 45.37%)] and ciliary beat frequency [6.8–15.8 Hz (average 8.95 Hz)] based on microscopy, as we reported previously (6, 7). These values are consistent with those reported by others in a well-differentiated nasal epithelial culture (10, 11).
Total RNA preparation and sequencing.
Total RNA was extracted from ALI-cultured nasal epithelia and brushed nasal epithelia using the Invitrogen PureLink RNA Mini Kit (Thermo Fisher Scientific) supplemented with the proteinase K (Qiagen, Germany) and RNase-free DNase set (Qiagen).
RNA quantity was assessed by VICTOR X2 fluorometry (Perkin Elmer, MA) using the Quant-iT RiboGreen RNA assay kit (Thermo Fisher Scientific). The integrity was checked by the Agilent Technologies 4200 TapeStation System with an RNA integrity number ≥7.
cDNA libraries were generated from 500 ng of total RNA using the TruSeq Stranded Total RNA LT Sample Prep Kit (human/mouse/rat) (Illumina, CA). The quality of the cDNA library was assessed by Quant-iT PicoGreen dsDNA assay kit (Thermo Fisher Scientific, MA) and D1000 screen tape analysis.
Quantitative RT-PCR.
cDNA (500 ng/µL) was obtained using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Thermo Fisher Scientific Baltics, Lithuania), and the absence of DNA contamination was verified by exclusion of RT from subsequent PCRs.
cDNA was subjected to PCR using SYBR green PCR master mix (Applied Biosystems, Thermo Fisher Scientific, UK) to amplify the few upregulated genes in brushed nasal epithelia [fatty acid-binding protein 5 (FABP5), 2′-5′-oligoadenylate synthetase 1 (OAS1), and aldehyde dehydrogenase 3 family member A1 (ALDH3A1)] and cultured airway epithelia [ERBB receptor feedback inhibitor 1 (ERRFI1), transglutaminase 2 (TGM2), and caveolin 1 (CAV1)]. Forward primers, reverse primers, and product lengths for the genes are as follows: CTTCCCATCCCACTCCTGATG (forward), CCACAGCTGATGGCAGAAAA (reverse), and 83 bp for FABP1; AGGAAAGGTGCTTCCGAGGTAG (forward), GGACTGAGGAAGACAACCAGGT (reverse), and 127 bp for OAS1; GGGAGAGGCTGTGTCAAAGG (forward), GCTCCGAGTGGATGTAGAGC (reverse), and 334 bp for ALDH3A1; TGAGGAAGACCTACTGGAGCAG (forward), GTATTAGGCGCTCCTGAGCAGA (reverse), and 111 bp for ERRFI1; TAAGAGATGCTGTGGAGGAG (forward), CGAGCCCTGGTAGATAAA (reverse), and 278 bp for TGM2; CCAAGGAGATCGACCTGGTCAA (forward), GCCGTCAAAACTGTGTGTCCCT (reverse), and 113 bp for CAV1; and GTCTCCTCTGACTTCAACAGCG (forward), ACCACCCTGTTGCTGTAGCCAA (reverse), and 131 bp for GAPDH.
Each PCR was carried out as follows: initial denaturation at 94°C for 15 min, 45 cycles of 94°C for 35 s, 60°C for 1 min, and 72°C for 1 min 15 s, followed by a final extension at 72°C for 2 min. Based on the comparative CT method, gene expression levels were calculated; GAPDH was used as the housekeeping gene.
Gene expression analysis.
For analysis of transcriptome data sets, we built an index sequence for STAR using the human reference GENCODE v27 feature that includes protein-coding, as well as noncoding, genes. Before sequence alignment, we applied TrimGalore (version 0.4.3) with the Cutadapt package (version 1.12) (5) to remove unnecessary genomic fragments (e.g., adapter dimers) and low-quality nucleotide sequences from the raw reads. Then we mapped adapter-trimmed sequencing reads to the human reference genome (GRCh38) using STAR aligner (2) and calculated the raw count using featureCounts (gene level) (4).
Data availability.
The data will be made available to qualified investigators on request.
RESULTS
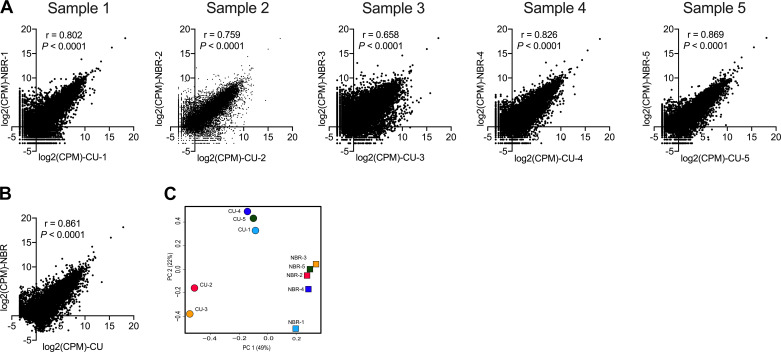
Pair-wise comparison (Fig. 1A, Table 2) and pooled sample analysis (Fig. 1B) showed a strong correlation between cells cultured at ALI and cells from brushed biopsy of nasal epithelia from the same patient. Overall, 96% of expressed genes showed similar expression profiles in cells cultured in vitro and those directly harvested from the patient (Table 2). The reproducibility and the potential outliers were identified by principal component analysis (Fig. 1C), which showed three clusters, one around four of the brushed nasal biopsies, one encompassing three of the cultured cells, and another around the remaining two cultured cells, suggesting close correlations between these groups.
Fig. 1.
Overall gene expression of nasal brushing (NBR) and cultured nasal epithelial (CU) samples. A and B: pair-wise sample (A) and pooled (B) correlation analysis using Pearson’s correlation analysis and log2-transformed count per million reads (CPM) between CU and NBR. C: principal component (PC) analysis of nasal brushing and cultured nasal epithelial cell samples using all genes expressed. Genes that were not expressed in all 5 samples were excluded. P < 0.05 was considered statistically significant.
Table 2.
Sample pair-wise comparison
| Patient No. | CU vs. NBR Correlation, r | Upregulation in CU (log2 FC > 2), TPM | Upregulation in NBR (log2 FC < −2), TPM | Less or No Significant Change | % Correlation |
|---|---|---|---|---|---|
| 1 | 0.802 | 165 | 98 | 19,573 | 98.6 |
| 2 | 0.759 | 637 | 92 | 19,036 | 96.3 |
| 3 | 0.658 | 612 | 160 | 20,006 | 96.2 |
| 4 | 0.826 | 181 | 141 | 19,698 | 98.3 |
| 5 | 0.869 | 180 | 41 | 19,092 | 98.8 |
| Union | 1,149 | 396 |
CU, cultured epithelia; FC, fold change; NBR, nasal brushings; r, Pearson’s correlation coefficient; TPM, transcripts per million (normalized data set).
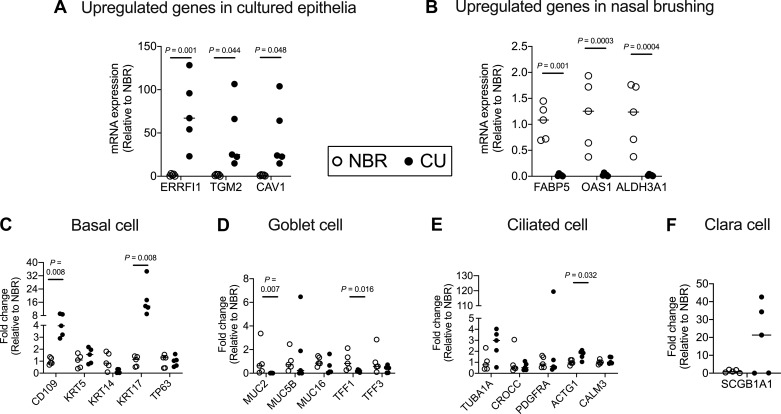
A total of 117 × 106 sequencing reads, with an average of 39 × 106 reads per sample, were used. The genes with >1 count per million reads were considered for analysis. A gene-by-sample matrix of reading counts was analyzed using customized Python script to calculate transcripts per million, and the differentially expressed (>4-fold change) genes (DEGs) were calculated. We validated the DEGs by RT-PCR quantification of 3 upregulated genes randomly selected from the list of top-10 upregulated genes in cultured epithelia and nasal brushing (Fig. 2, A and B). In addition, to determine if cell types in the cultured cells differ from those in the nasal scrapings, we quantified the markers of specific cell types, namely, basal cells, goblet cells, ciliated cells, and Clara cells in the nasal brushings and cultured nasal epithelia (Fig. 2, C–F).
Fig. 2.
Validation of total RNA sequencing data and quantification of cell population makers. A and B: expression of 3 significantly upregulated genes in cultured nasal epithelial (CU) and nasal brushing (NBR) samples was validated using RT-quantitative PCR. C–F: relative expression of basal cell (CD109, KRT5, KRT14, KRT17, TP63), goblet cell (MUC2, MUC5B, MUC16, TFF1, TFF3), ciliated cell (TUB1A, CROCC, PDGFRA, ACTG1, CALM3), and Clara cell (SCGB1A1) genes. Data are represented in scatter plots with median for 5 donors.
We identified specific DEGs that were altered in at least two of the five patients with COPD for subsequent pathway analysis. We performed the functional annotation using Reactome (4, 5) and ingenuity pathways analysis in samples from all five patients with COPD to summarize the functional class and pathway of DEGs (Table 3). Analysis of the functional categories of differentially expressed genes revealed that pathways involved in the cornified epithelium are activated in squamous or flattened epithelium (Table 3, increased expression pathway data). In addition, pathways involved in nuclear receptor and tRNA processing were highly expressed in fresh nasal brushings (Table 3, increased expression pathway data), whereas pathways involved in the extracellular matrix and genes involved in interactions with the extracellular matrix were highly expressed in cultured nasal epithelia (Table 3, decreased expression pathway data). We also performed pathway analysis of DEGs among different clusters observed in principal component analysis of cultured epithelia (Fig. 1C). The cluster of cultured nasal epithelia, i.e., CU2 and CU3, showed an increase in pathways involved in collagen-related extracellular matrix organization (Table 4) and the cluster CU1, CU4, and CU5 showed an increase in pathways involved in innate immunity (Table 4), although the available clinical data do not provide a rationale for these findings.
Table 3.
Pathway analysis of differentially expressed genes in cultured nasal epithelia vs. nasal brushings
| Pathway (Identifiers Found) | Ratio | P Value* |
|---|---|---|
| Pathways with genes with increased expression in NBR vs. CU | ||
| Formation of the cornified envelope (KRT13, SPRR3, SPRR2A, CSTA, SPRR2A) |
0.01 | 2.81E-10 |
| Keratinization (KRT13, SPRR3, SPRR2A, CSTA, SPRR1A) |
0.016 | 1.97E-08 |
| tRNA processing in the mitochondrion (MT-TP, MT-TL1, MT-TT, MT-TW, MT-TS1) |
0.003 | 2.57E-07 |
| Nuclear receptor transcription pathway (NR3C2) |
0.006 | 1.30E-04 |
| tRNA processing (MT-TP, MT-TL1, MT-TT, MT-TW, MT-TS1) |
0.013 | 2.06E-04 |
| rRNA processing in the mitochondrion (MT-TL1, MT-TT, MT-TW) |
0.003 | 2.42E-04 |
| Thyroxine biosynthesis (DU0X2) |
0.002 | 3.00E-03 |
| Developmental biology (KRT13, SPRR3, SPRR2A, CSTA, SPRR1A) |
0.084 | 8.23E-03 |
| MyD88 deficiency (TLR5, MYD88) | 0 | 8.90E-03 |
| Pathways with genes with decreased expression in NBR vs. CU | ||
| ECM organization (FBLN1, LAMC2, LAMA3, COL7A1, SERPINE1, LAMB3, ITGB4, TGFB2, ITGA3, ITGB6, ITGB6, ITGAV, TNC, FN1, LTBP2, COL1A1, CAPN13, COL17A1, SPARC, MMP13, HSPG2, THBS1, ICAM1) |
0.023 | 4.62E-11 |
| Type I hemidesmosome assembly (LAMC2, KRT17, LAMA3, KRT13, LAMB3, COL17A1, ITGB4) |
0.001 | 1.38E-8 |
| Laminin interactions (LAMC2, LAMA3, COL7A1, LAMB3, ITGB4, ITGA3, ITGAV, HSPG2) |
0.002 | 1.66E-8 |
| Non-integrin membrane-ECM interactions (LAMC2, KRT17, LAMA3, KRT13, LAMB3, COL17A1, ITGB4) |
0.004 | 1.88E-8 |
| FOXO-mediated transcription of cell cycle genes (TNC, FN1, LAMA3, SERPINE 1, COL1A1, SPARC, TGFB2, ITGB6, ITGAV, HSPG2) |
0.002 | 1.29E-8 |
| ECM proteoglycans (CDKN1A, CAV1, GADD45A) |
0.005 | 2.01E-7 |
| Integrin-cell surface interactions (TNC, FN1, COL7A1, COL1A1, ITGA3, ITGB6, ITGAV, HSPG2, THBS1, ICAM1) |
0.006 | 4.33E-7 |
| Assembly of collagen fibrils and other multimeric structures (TNC, LAMC2, LAMA3, COL7A1, LAMB3, COL1A1, COL17A1, ITGB4, MMP13) |
0.005 | 5.09E-7 |
| Anchoring fibril formation (LAMC2, LAMA3, COL7A1, LAMB4, COL1A1) |
0.001 | 2.53E-6 |
| MET promotes cell motility (FN1, LAMC2, LAMA3, LAMB3, COL1A1, TNS3, ITGA3) |
0.003 | 3.74E-6 |
| Syndecan interactions (TNC, FN1, COL1A1, ITGB4, ITGAV, THBS1) |
0.002 | 3.78E-6 |
| MET activates PTK2 signaling (FN1, LAMC2, LAMA3, LAMA3, LAMB3, COL1A1, ITGA3) |
0.002 | 6.59E-6 |
| Cell junction organization (FLNA, LAMC2, KRT17, LAMA3, KRT13, LAMB3, COL17A1, ITGB4, CDH3) |
0.007 | 7.8E-6 |
| Degradation of the ECM (TNC, FN1, LAMC2, LAMA3, COL7A1, LAMB3, COL1A1, CAPN13, COL17A1, MMP13, HSPG2) |
0.01 | 8.28E-6 |
| Collagen formation (TNC, LAMC2, LAMA3, COL7A1, LAMB3, COL1A1, COL17A1, ITGB4, MMP13) |
0.007 | 1.72E-5 |
| Molecules associated with elastic fibers (FN1, FBLN1, LTBP2, TGFB2, ITGB6, ITGAV) |
0.003 | 1.73E-5 |
| Cellular senescence (FLNA, CDKN1A, CDKN2A, IGFBP7, JUN, HES4) |
0.014 | 2.52E-5 |
| TP53 regulates transcription of cell cycle genes (PLK2, CDKN1A, GADD45A) |
0.005 | 3.9E-5 |
| IL-4 and IL-13 signaling (VIM, FN1, CDKN1A, SAA1, CCND1, ICAM1) |
0.015 | 4.41E-5 |
| Transcriptional regulation by RUNX3 (CDKN1A, CDKN2A, CTGF, CCND1) |
0.008 | 4.55E-5 |
Functional pathway analysis was carried out on differentially expressed genes from all 5 patients with chronic obstructive pulmonary disease. Ratio refers to percentage of total genes in the pathway. CU, cultured epithelia; ECM, extracellular matrix; MET, mesenchymal-epithelial transition factor; NBR, nasal brushing; PTK2, protein tyrosine kinase 2; RUNX3, runt-related transcription factor 3.
P ≤ 0.01 after Benjamini-Hochberg multiple test correction.
Table 4.
Pathway analysis of differentially expressed genes among cultured epithelial samples: CU2 and CU3 vs. CU1, CU3, and CU5
| Pathway (Identifiers Found) | Ratio | P Value* |
|---|---|---|
| Pathways with genes with increased expression in CU2 and CU3 | ||
| ECM organization (TIMP2, COL6A6, COL6A3, COL6A1, COLA1, COL4A1, COL4A2, ADAM12, DST, TEX10, COL18A1, SPARC. KALRN, COL16A1, LOXL1, LOXL2, HTRA1, LAMC1, PLOD3, LAMC2, PLOD2, PLOD1, P4HA2, SERPINE1, COL12A1, SDC2, PLCD3, FBN1, MFAP2, PMP2, FMNL1, BMP1, CRTAP, SERPINH1, LAMB1, COL5A2, MMP2, ITGA11, COL5A1, LAMB3, ITGA1) |
0.023 | 1.11E-16 |
| Collagen formation (SERPINH1, COL6A6, COL6A3, COL6A1, COL5A2, COL4A1, COL4A2, LAMB3, P3H2, P3H1, DST, PXDN, COL18A1, COL1A1, COL3A1, COL16A1, COL1A2, LOXL1, LOXL2, PLOD3, LAMC2, PLOD2, PLOD1, LAMA3, LOX, P4HA2, COL12A1, PLEC, COL27A1, FMNL1, BMP1, CRTAP) |
0.007 | 5.57E-14 |
| Non-integrin membrane-ECM interactions (LAMC1, SERPINH1, LAMB1, LAMC2, LAMA3, COL5A2, LAMA4, COL4A1, COL4A2, ITGB1, COL5A1, SDC2, LAMB3, DDR2, ITGA2, ITGB5, FN1, PDGFA, PDGFB, COL1A1, ACTN1, COL3A1, COL1A2, THBS1) |
0.004 | 1.06E-12 |
| Assembly of collagen fibrils and other multimeric structures (COL6A6, LAMC2, COL6A3, COL6A1, LAMA3, LOX, COL5A2, COL4A1, COL12A1, COL4A2, COL5A1, LAMB3, PLEC, DST, PXDN, COL18A1, COL1A1, COL27A1, COL3A1, BMP1, COL1A2, LOXL1, LOXL2) |
0.005 | 4.52E-11 |
| Collagen biosynthesis and modifying enzymes (SERPINH1, PLOD3, COL6A6, PLOD2, COL6A1, COL5A2, P4HA2, COL4A1, COL12A1, COL4A2, COL5A1, P3H2, P3H1, COL18A1, COL1A1, COL27A1, COL3A1, COL16A1, FMNL1, BMP1, COL1A2, CRTAP) |
0.005 | 1.04E-10 |
| Degradation of the ECM (TIMP2, LAMB1, COL6A6, COL6A3, COLA1, COL5A2, MMP2, COL4A1, COL4A2, COL5A1, LAMB3, A2M, FN1, MAN1A1, CTSK, TEX10, COL18A1, COL1A1, MMP12, MMP14, KALRN, COL3A1, COL16A1, COL1A2, MYH9, HTRA1, LAMC1, LAMC2, LAMA3, COL12A1, FBN1, ADAMTS1, CLSTN3, BMP1) |
0.01 | 1.64E-10 |
| ECM proteoglycans (LAMC1, LAMB1, COL6A6, COL6A3, COL6A1, LAMA3, COL5A2, LAMA4, COL4A1, SERPINE1, COL4A2, ITGB1, COL5A1, ITGA2, VCAN, ITGB5, ITGB6, SRGAP2, FN1, COL1A1, SPARC, COL3A1, COL1A2) |
0.005 | 5.48E-09 |
| Integrin-cell surface interactions (COL6A6, COL6A3, COL6A1, COL5A2, ITGA11, COL4A1, COL4A2, ITGA11, COL4A1, COL4A2, ITGB1, COL5A1, ITGA1, ITGA2, ITGB5, ITGA4, ITGA5, ITGB6, FBN1, FN1, COL18A1, COL1A1, COL3A1, COL16A1, COL1A2, THBS1) |
0.006 | 5.72E-09 |
| MET activates PTK2 signaling (LAMC1, LAMB1, LAMC2, LAMA3, COL5A2, LAMA4, ITGB1, COL5A1, LAMB3, ITGA2, FN1, COL1A1, COL27A1, COL3A1, COL1A2) |
0.002 | 6.03E-09 |
| Axon guidance (COL6A6, COL6A3, COL6A1, COL4A1, COL4A2, TNS1, TUBB6, CAP1, MYO5A, ABL2, GRB10, MYO5A, ABL2, GRB10, MYO9B, PSMD2, TPRA1, DLG1, KALRN, PPFIBP1, LAMC1, SLIT2, FAP, ABLIM3, SDC2, DPYSL2, SEMA5A, SPTBN1, FGFR1, LIMK1, MAPK11, EFNA1, LAMB1, FLNB, KIF3C, COL5A2, MMP2, COL5A1, ITGA1, ITGA2, ITGA5, SRGAP2, PDLIM7) |
0.04 | 7.77E-09 |
| Elastic fiber formation (LOX, COL4A1, ITGB1, EFEMP2, ITGB5, ITGA5, ITGB6, PLCD3, FBN1, FN1, LTBP2, MFAP2, BMP2, LTBP1, LOXL1, LOXL2) |
0.003 | 1.89E-08 |
| Regulation of IGF transport and uptake by IGFBPs (TIMP2, LAMB1, MMP2, COL4A1, LIMS1, FAM20C, FN1, APOE, CDH2, PAPPA, SPARC, LTBP1, LAMC1, CSF1, LOX, SDC2, VCAN, RCN1, LGALS1, GAS6, FBN1, MELTF, IGFBP6, CLSTN3, IGFBP4, EVA1A, CALU, FSTL3, FSTL1) |
0.009 | 4.59E-08 |
| MET promotes cell motility (LAMC1, LAMB1, LAMC2, LAMA3, COL5A2, LAMA4, ITGB1, COL5A1, LAMB3, ITGA2, TNS4, FN1, COL1A1, COL27A1, COL3A1, COL1A2) |
0.003 | 8.24E-08 |
| Collagen degradation (COL6A6, COL6A3, COL6A1, COL5A2, MMP2, COL4A1, COL12A1, COL4A2, COL5A1, CTSK, COL18A1, COL1A1, MMP12, MMP14, KALRN, COL3A1, COL16A1, COL1A2) |
0.005 | 2.62E-07 |
| Collagen chain trimerization (COL6A6, COL6A3, COLA1, COL5A2, COL4A1, COL12A1, COL4A2 COL5A1, COL18A1, COLA1, COL27A1, COL3A1, COL16A1, COL1A2) |
0.003 | 3.50E-07 |
| Posttranslational protein phosphorylation (TIMP2, LAMB2, COL4A1, LIMS1, FAM20C, FN1, APOE, CDH2, SPARC, LTBP1, LAMC1, CSF1, LOX, SDC2, VCAN, RCN1, LGALS1, GAS6, FBN1, MELTF, IGFBP4, EVA1A, CALU, FSTL3, FSTL1) |
0.008 | 4.20E-07 |
| Syndecan interactions (FN1, COL5A2, ITGB1, COL5A1, SDC2, COL1A1, ACTN1, ITGA2, COL3A1, COL1A2, ITGB5, THBS1) |
0.002 | 7.15E-07 |
| Signaling by receptor tyrosine kinases (SPRY2, COL6A6, COL6A3, COL6A1, COL4A1, COL4A2, TNS4, ADAM12, GRB10, APOE, SPARC, KALRN, LAMC1, ITPR2, LAMC2, PTPRK, ITPR3, AXL, SPTBN1, FGFR1, MAPK11, NEDD4, RALA, VEGFA, CAV1, LAMB1, FLNB, COL5A2, SPHK1, COL5A1, LAMB3, ITGA2, PAG1, RAB11FIP5, FN1, ANOS1, COL1A1, COL3A1, COL1A2, DNM1) |
0.038 | 7.62E-07 |
| Signaling by PDGF (COL6A6, COL6A3, COL6A1, COL5A2, COL4A1, COL4A2, COL5A1, RASA1, NT5DC2, PDGFC, PLAT, PDGFA, PDGFB, COL3A1, THBS3, THBS1) |
0.005 | 1.39E-06 |
| Laminin interactions (LAMC1, LAMB1, LAMC2, LAMA3, LAMA4, COL4A1, COL4A2, ITGB1, LAMB3, COL18A1, ITGA1, ITGA2) |
0.002 | 1.42E-6 |
| Pathways with genes with increased expression in CU1, CU3, and CU5 | ||
| Neutrophil degranulation (ANK3, PRKCD, NAPRT, CYBB, VAMP8, CD55, GSTK1, LRRC6, TUBB4B, S100P, LRG1, CD14, SELL, IDH, SERPINB3, SVIP, RIPOR2, CST3, HPSE, MGST1, CD9, ATP10B, SLCO4C1, FASN, FCGR2A, CSTB, LCN2, DEGS2, TACC2, NFASC, SERPINA1, CRACR2A, IQGAP2, STOM, METTL7A, CTSC, ALDH3B2, PLAC8, CEACAM6, CTSD) |
0.033 | 9.92E-4 |
| Termination of O-glycan biosynthesis (MUC13, MUC15, MUC16, RPIA, MUC1, MUC2, ST6GAL1, ST6GALNAC4, MUC5B, MUC5AC) |
0.002 | 1.37E-3 |
| Defective GALNT12 causes CRCS1 (MUC13, MUC15, MUC16, GLANT12, MUC1, MUC2, MUC5, MUC5AC) |
0.001 | 2.02E-3 |
| Metal sequestration by antimicrobial compounds (S100A7, S100A8, S100A9, TF. LCN2, LTF) |
0.001 | 3.62E-3 |
|
O-linked glycosylation of mucins (MUC13, MUC15, MUC16, RPIA, MUC1, MUC2, CHST4, ST6GALNAC4, GALNT6, GCNT3, B3GNT6, B3GNT3, GALNT12, GALNT14, ST6GAL1, MUC5B, MUC5AC) |
0.005 | 3.96E-3 |
| ERBB2 activates PTK6 signaling (NRG4, ERBB4, ERBB3, BTC) |
0.001 | 4.39E-3 |
| Fatty acids (CYP4B1, CYP2A13, RRAGD, CYP4F3, CYP2J2, CYP2F1, CYP4F12, SORL1, CYP4F11) |
0.002 | 5.74E-3 |
| ERBB2 regulates cell motility (NRG4, ERBB4, ERBB3, BTC) |
0.001 | 5.84E-3 |
| Defective GALNT3 causes familial hyperphosphatemic tumoral calcinosis (MUC13, MUC15M MUC16, MUC1, MUC2, MUC5B, MUC5AC) |
0.001 | 7.62E-3 |
| SHC1 events in ERBB2 signaling (MITF, NRG4, ERBB4, BTC) |
0.001 | 9.77E-3 |
| Defective GALNT3 causes familial C1GALT1C1 causes TNPS (MUC13, MUC15, MUC16, MUC1, MUC2, MUC5B, MUC5AC) |
0.001 | 9.77E-3 |
| GRB2 events in ERBB2 signaling (MITF, NRG4, ERBB4, BTC) |
0.001 | 9.77E-3 |
Ratio refers to percentage of the total genes in the pathway. CRCS1, colorectal cancer 1; CU, cultured epithelia; ECM, extracellular matrix; GALNT12, polypeptide N-acetylgalactosaminyltransferase 12; IGF, insulin-like growth factor; IGFBPs, IGF-binding proteins; MET, mesenchymal-epithelial transition factor; NBR, nasal brushing; PDGF, platelet-derived growth factor; PTK2, protein tyrosine kinase 2; TNPS, Tn polyagglutination syndrome.
P ≤ 0.01 after Benjamini-Hochberg multiple test correction.
DISCUSSION
Although ALI cultures are used routinely for studies, the degree to which these in vitro cultures are representative of cells in patient airways remains in question. This has been an ongoing concern, as these cells are grown in a different environment, in terms of 1) the lack of physical and chemical exposures that occur in the nose from the shear effects of respiration to the various stimuli that are in the air and 2) the difference in the matrix on which cultured cells are grown. However, despite these concerns, ALI cultures are used routinely to test physiologic and pathologic responses of differentiated epithelium to infectious and noninfectious particles and to perform other toxicology studies. To determine the extent to which the ALI cultures are representative of a given individual, we compared the transcriptomics of nasal epithelial cells of a given patient directly extracted from the nose with those grown and differentiated in vitro before RNA extraction. Such an analysis can provide a better assessment of which pathways in cultured cells are highly representative of pathways in the nasal epithelium and which pathways may require additional correlation. It is important to recognize that the samples were collected from diseased (i.e., COPD) patients, and we have evidence that cells derived from patients with COPD behave differently and have significant differences in gene expression compared with cells derived from normal patients (6, 7). However, since the source of the comparators is the same disease condition, it is unlikely that it would affect the interpretation.
There is, in fact, a high degree of correlation in the transcriptome between collected nasal brushings and cells that are cultured and then allowed to differentiate in vitro. Interestingly, pair-wise comparison of specific cell types between the cultured cells and nasal brushings showed similar numbers of ciliated, goblet, Clara, and basal cells, although there were some differences in specific marker expression and a large degree of variability.
Based on principal component analysis, the transcriptomics of nasal brushings are more closely clustered, indicating that, ultimately, these samples are more tightly correlated. The cultured cells form separate clusters, suggesting more variability in their transcriptome. Pathway analysis indicates that one cause of the increased clustering of nasal brushings is contamination from the squamous epithelium, as determined by the increase in pathways involved in cornified epithelium in the nasal vestibule during scraping, as we know that these squamous cells do not grow when cultured in vitro. In addition, once cultured, there may be a downregulation of genes involved in cellular regeneration, which is consistent with the fact that there is a limit to the number of times primary cells can be subcultured. In contrast, ALI-cultured cells had increased expression of extracellular matrix organization and cell-matrix interaction genes. This could be a result of potential downregulation of these pathways that could occur during cellular detachment during scraping of the nares.
We previously reported that primary nasal epithelial cells maintain their innate immune receptor expression profile when grown in prolonged culture in vitro (8). While, based on this comparison, the ALI model of nasal epithelia provides an excellent representation of the in vivo airway epithelial transcriptome that may be utilized for mechanistic studies, it is critical to recognize that certain key pathways, such as cellular regeneration, may not be represented in vitro and that the milieu of the cells plays a significant role.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL-124099 and R01 HL-51107, National Institute of Allergy and Infectious Diseases Grant R01 AI-143731, National Institute on Minority Health and Health Disparities Grant P50 MD-010431, and National Institute of Environmental Health Sciences Grant U01 ES-026721 CA (Clean Air) R01 ES_022607 CURE1 EPA Agreement: 83615001.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R., N.N.H., S.B., and V.K.S. conceived and designed research; B.G., D.B., K.N., M.L., and N.P. performed experiments; B.G. and B.P. analyzed data; B.G. and V.K.S. interpreted results of experiments; B.G. and B.P. prepared figures; B.G. drafted manuscript; B.G., M.R., N.N.H., S.B., and V.K.S. edited and revised manuscript; P.G., M.R., N.N.H., S.B., and V.K.S. approved final version of manuscript.
REFERENCES
- 1.Den Beste KA, Hoddeson EK, Parkos CA, Nusrat A, Wise SK. Epithelial permeability alterations in an in vitro air-liquid interface model of allergic fungal rhinosinusitis. Int Forum Allergy Rhinol 3: 19–25, 2013. doi: 10.1002/alr.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imkamp K, Berg M, Vermeulen CJ, Heijink IH, Guryev V, Kerstjens HAM, Koppelman GH, van den Berge M, Faiz A. Nasal epithelium as a proxy for bronchial epithelium for smoking-induced gene expression and expression quantitative trait loci. J Allergy Clin Immunol 142: 314–317.e15, 2018. doi: 10.1016/j.jaci.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 4.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 5.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17: 10, 2011. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 6.Nishida K, Brune KA, Putcha N, Mandke P, O’Neal WK, Shade D, Srivastava V, Wang M, Lam H, An SS, Drummond MB, Hansel NN, Robinson DN, Sidhaye VK. Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am J Physiol Lung Cell Mol Physiol 313: L581–L591, 2017. doi: 10.1152/ajplung.00074.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishida K, Ghosh B, Chandrala L, Mahmud S, Chen S, Khosla AA, Katz J, Sidhaye VK. Quantifying epithelial plasticity as a platform to reverse epithelial injury (Preprint). bioRxiv: 906008, 2020. doi: 10.1101/2020.01.14.906008. [DOI]
- 8.Ramanathan M Jr, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol 21: 373–377, 2007. doi: 10.2500/ajr.2007.21.3034. [DOI] [PubMed] [Google Scholar]
- 9.Reh DD, Wang Y, Ramanathan M Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy 24: 105–109, 2010. doi: 10.2500/ajra.2010.24.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schagen J, Sly PD, Fantino E. Characterizing well-differentiated culture of primary human nasal epithelial cells for use in wound healing assays. Lab Invest 98: 1478–1486, 2018. doi: 10.1038/s41374-018-0100-1. [DOI] [PubMed] [Google Scholar]
- 11.Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Shields MD, Heaney LG. Nasal epithelial cells can act as a physiological surrogate for paediatric asthma studies. PLoS One 9: e85802, 2014. doi: 10.1371/journal.pone.0085802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Cleemput J, Poelaert KCK, Laval K, Impens F, Van den Broeck W, Gevaert K, Nauwynck HJ. Pollens destroy respiratory epithelial cell anchors and drive α-herpes virus infection. Sci Rep 9: 4787, 2019. doi: 10.1038/s41598-019-41305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be made available to qualified investigators on request.