Abstract
Voluntary force declines during sustained, maximal voluntary contractions (MVC) due to changes in muscle and central nervous system properties. Central fatigue, an exercise-induced reduction in voluntary activation, is influenced by multiple processes. Some may occur independently of descending voluntary drive. To differentiate the effects associated with voluntary drive from other central and peripheral influences, we measured voluntary activation and motoneuron excitability following fatiguing contractions produced voluntarily or by electrical stimulation. On two separate days, participants performed either a 2-min MVC of adductor pollicis muscle or received 2-min continuous supramaximal electrical stimulation of the ulnar nerve. In study 1 (n = 14), the superimposed twitch elicited by ulnar nerve stimulation during brief MVCs was increased, and, hence, voluntary activation was reduced, up to 240 s after the 2-min MVC [−20 ± 12% (SD), P = 0.002] but not the 2-min stimulated contraction (−4 ± 7%), despite large reductions in MVC force (voluntary, −54 ± 18%; stimulated, −46 ± 16%). In study 2 (n = 12), F-waves recorded from the adductor pollicis were reduced in area for 150 s following the 2-min MVC (−21 ± 16%, P = 0.007) but not after the stimulated contraction (5 ± 27%). Therefore, voluntary activation and motoneuron excitability decreased only when descending voluntary drive was present during the fatiguing task. The findings do not exclude a cortical or brain stem contribution to the reduced voluntary activation but suggest that neither sensory feedback from the fatigued muscle nor repetitive activation of motoneurons underlie the changes, whereas they are consistent with motoneuronal inhibition by released factors linked to voluntary drive.
NEW & NOTEWORTHY We demonstrate that reductions in voluntary activation and motoneuron excitability following 2-min isometric maximal contractions in humans occur only when fatigue is produced through voluntary contractions and not through electrically stimulated contractions. This is contrary to studies that suggest that changes in the superimposed twitch and therefore voluntary activation are explained by changes in peripheral factors alone. Thus, the interpolated twitch technique remains a viable tool to assess voluntary activation and central fatigue.
Keywords: central fatigue, interpolated twitch technique, motoneuron excitability, voluntary activation
INTRODUCTION
Muscle fatigue is a progressive decline in maximal voluntary muscle force associated with sustained and/or repeated efforts. Peripheral changes within the muscle, but also failure of the central nervous system to drive the motoneurons adequately, contribute to muscle fatigue (22, 23). Reduced voluntary activation can be measured using the interpolated twitch technique to examine changes in the ability to drive the muscle maximally through voluntary effort during and following fatiguing tasks (6, 25, 47). Supramaximal stimulation of the motor nerve during isometric maximal voluntary contractions (MVCs) produces a twitch-like increment in force, or superimposed twitch, which can be used to measure the efficacy of voluntary drive (voluntary activation, %) by comparing the size of the superimposed twitch to the size of the twitch evoked from the resting muscle with the same stimulus (1, 6, 47). During and shortly after a sustained 2-min MVC, the superimposed twitch evoked by motor nerve stimulation is increased, in the presence of a reduced resting twitch, indicating a reduction in voluntary activation (e.g., 24, 36, 37).
Multiple mechanisms are likely to contribute to reduced voluntary activation during maximal efforts following fatiguing contractions (61, 62). Some may require the fatiguing task to be voluntary and others may not. That is, muscle fatigue produced by electrical stimulation of the muscle (or peripheral nerve) may engage some mechanisms that contribute to central fatigue. For example, small-diameter (groups III and IV) muscle afferents are mechanically and/or chemically sensitive and increase their firing with fatiguing exercise because of conditions in the muscle. These afferents can act supraspinally to reduce voluntary activation (4, 24, 36–38, 59). They can also reduce the excitability of some motoneuron pools (42). Muscle fatigue and accumulation of metabolites may develop at different rates in contractions produced by voluntary drive or by electrical stimulation, particularly for submaximal forces. However, the actions of groups III and IV muscle afferents should remain the same regardless of whether their firing occurs during fatiguing voluntary or stimulated contractions.
Recent findings have suggested that other processes that result in depression of motoneuron excitability during fatiguing exercise and, hence, decrease voluntary activation, may require that the motoneurons are driven through voluntary contraction. Depression of motoneuron excitability during sustained MVCs is shown by decreases in cervicomedullary motor evoked potentials (CMEPs), which are elicited by stimulation of the corticospinal axons at a subcortical level (10, 46, 52). In addition, the amplitude and persistence of F-waves, which represent recurrent activation of the motoneurons through invasion of the soma by antidromic action potentials evoked through supramaximal electrical nerve stimulation, are depressed following MVCs with durations from 5 s to 2 min (39). The degree of motoneuron depression during contractions and the duration of motoneuron depression after contractions are related to both the duration of the fatiguing contraction and the strength of the contraction (40, 45, 46). However, when the neural drive is replaced by supramaximal tetanic stimulation of the motor nerve, no depression in the F-waves occurs (40). These findings suggest that motoneuron excitability is reduced by activity- and time-dependent mechanisms that are not engaged by repetitive antidromic activation of the motoneurons but are associated with voluntary drive to the motoneurons. Interestingly, fatigue produced by voluntary contraction of a nearby muscle also inhibits output from an adjacent motor pool in humans (40). It has been proposed that the spread of neuromodulators (e.g., monoamines) linked to descending drive to the motoneurons may account for this effect on motoneuron excitability (see also 15, 16, 35). One caveat is that the tetanic stimulation applied by Khan et al. (40) lasted only 10 s. During longer-duration (90-s) supramaximal stimulation of the motor axons, F-wave amplitude and persistence are reduced (11).
Previous studies that have examined voluntary activation shortly after muscle fatigue produced by neuromuscular electrical stimulation report mixed findings. Decreases in voluntary activation and no change have both been reported to immediately follow fatiguing electrically evoked muscle contractions (9, 13, 50, 68). However, muscle contractions in those studies were submaximal and intermittent and may not have caused sufficient firing of small-diameter muscle afferents and/or sustained repetitive motoneuron activation to produce effects on voluntary activation. In this study, we aimed to differentiate the effects associated with descending voluntary drive from other factors that depress motoneuron output by examining voluntary activation and F-waves following two fatiguing protocols in the adductor pollicis muscle. The adductor pollicis muscle was chosen because it is feasible to elicit sustained maximal contractions using electrical stimulation (26), and voluntary activation can be measured using supramaximal ulnar nerve stimulation (37). Additionally, F-waves can be reliably recorded from intrinsic muscles of the hand (18, 44). Thus, both changes in voluntary activation and changes in motoneuron excitability can be examined for the same muscle. On separate days, either a 2-min MVC (VOL) or a 2-min electrically evoked maximal contraction (STIM) was used to generate similar levels of muscle fatigue in adductor pollicis. In two separate studies, either changes in voluntary activation or changes in F-waves were measured at similar intervals following each fatiguing contraction to determine the effect of descending drive to the motoneurons on changes in voluntary activation with muscle fatigue.
MATERIALS AND METHODS
Ethical approval.
All experiments were approved by the Human Research Ethics Committee at the University of New South Wales (HC no. 15824) and conformed to the Declaration of Helsinki (2013) apart from registration in a database. All participants gave written, informed consent before participating in the study.
Participants.
In total, 14 healthy individuals who were not taking any medications participated in study 1 [aged 27 ± 9 yr (SD), 7 females]. Fourteen healthy individuals who were not taking any medications were initially enrolled in study 2, but two were excluded because we could not evoke F-waves (aged 25 ± 5 yr, 6 females, n = 12). Five individuals participated in both studies.
Research design.
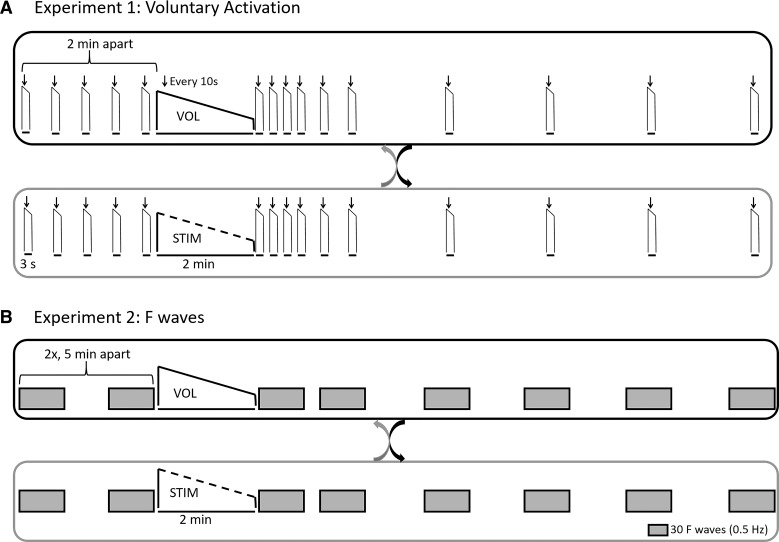
Studies 1 and 2 each comprised two experimental sessions in a crossover design (Fig. 1). In one session, participants performed a 2-min MVC (VOL); in the other, they received electrical stimulation of the ulnar nerve to produce a 2-min stimulated contraction (STIM). Study 1 assessed voluntary activation before and after the fatiguing voluntary and stimulated contractions. Study 2 used F-waves to assess motoneuron excitability before and after the two fatiguing contractions.
Fig. 1.
A: randomized, crossover design of experiment 1 assessing voluntary activation before and after a 2-min maximal voluntary contraction (MVC) (VOL; top) or electrically stimulated maximal contraction (STIM; bottom). Individuals performed 5 brief MVCs with superimposed twitches followed by a resting twitch (not shown) before the fatiguing task. These brief MVCs were then repeated at 5, 20, 40, 60, 90, 120, 240, 360, 480, and 600 s after the fatiguing contraction. B: randomized, crossover design of experiment 2 assessing the effects of 2-min MVC (VOL; top) or electrically stimulated maximal contraction (STIM; bottom) on motoneuron excitability and specifically F-waves. Two baseline sets of 60 F-waves (2 sets of 30 F-waves separated by 1 min) were performed 5 min apart before the fatiguing task. Immediately following the fatiguing tasks, sets of 30 F-waves (0.5 Hz) were recorded at 0–60, 90–150, 210–270, 330–390, 450–510, and 570–630 s after the fatiguing contraction to match the time points in experiment 1.
Setup.
A metal frame was used to position the hand and the thumb to restrict any contribution from any muscles other than the adductor pollicis. Participants' forearms rested on a horizontal surface in a neutral position with 60° elbow flexion (0° = elbow fully extended) and the wrist flexed at ~45°. The wrist was strapped into place using velcro. The back of the hand rested against a densely padded metal support. The thumb was placed in a tightly fitted metal ring that was coupled to a force transducer (linear strain gauge Xtran, Melbourne Australia). Last, the palmar side of the hand was clamped into place using two metal blocks that were slightly padded to prevent movement of the fingers.
Electromyogram.
In both sets of experiments, surface electromyogram (EMG) signals were obtained from the adductor pollicis through adhesive surface EMG electrodes (Ambu White Sensor 7841P ECG electrodes, 16 mm diameter) with one electrode placed over the muscle belly and another on the first metacarpophalangeal joint. A ground electrode was placed on the wrist. EMG signals were amplified (300×) and bandpass filtered (16–1,000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design, Cambridge, UK). All EMG signals were sampled at 5 kHz using a 16-bit A/D converter (CED 1401) and Spike 2 software (version 7, CED).
Peripheral nerve electrical stimulation.
Maximal M-waves, F-waves, superimposed twitches, and maximal resting twitches were elicited through electrical stimulation of the ulnar nerve at the wrist (pulse width 0.2 ms, DS7AH stimulator; Digitimer, Welwyn Garden City, UK). Surface electrodes (Ambu White Sensor 7841P ECG electrodes, 16 mm diameter) were placed ~2 cm apart over the nerve with the cathode positioned distal to the anode and ~2 cm proximal to the wrist. Stimulus intensity was set to 50% above the level required to produce a maximal compound muscle action potential and maximal resting twitch [experiment 1: 68 ± 24 mA (SD); range 30–120 mA; experiment 2: 58 ± 17 mA, range 30–75 mA].
Fatiguing electrically stimulated maximal contraction.
To produce a maximal 2-min contraction through electrical stimulation, surface electrodes were placed over the ulnar nerve at the elbow (Cleartrace; ConMed Corp., Utica, NY) and taped and strapped tightly in place. The cathode was positioned distal to the anode, just below the olecranon, with the anode ~2 cm above. Single stimuli were used to determine the stimulus intensity required to elicit a maximal twitch response from the adductor pollicis (pulse width 0.5 ms; DS7AH stimulator; Digitimer, Welwyn Garden City, UK). The intensity used during the train of stimuli was set to 50% above the level required to produce a maximal resting twitch [experiment 1: 24 ± 9 mA (SD), range 15–42 mA; experiment 2: 24 ± 9 mA, range 14–45 mA). During setup, we compared the force generated through brief stimulus trains of 30 and 40 Hz and selected the stimulation frequency eliciting the higher level of force in each individual. If 40-Hz stimulation produced no more force than 30-Hz stimulation, then the 30-Hz stimulation was used as the default (30 Hz used for 10 of 14 participants in experiment 1, and 8 of 12 participants in experiment 2). This provided the starting point for the 2-min train of stimuli. During the 2-min train, we manipulated the stimulus frequency in an attempt to replicate the changes in motor unit firing rates recorded from the adductor pollicis during a 2-min MVC (7). Thus, stimulus frequency was decreased every 10 s for the first minute and then sustained at ~16 Hz for the last minute of the 2-min contraction. When the resting twitches obtained though electrical stimulation of the ulnar nerve at the wrist were compared with the elbow, twitches were significantly lower following stimulation at the elbow in experiment 2 (wrist: 6.1 ± 2.4 N, elbow: 5.1 ± 2.2 N, P = 0.01) but well matched in experiment 1 (wrist: 6.0 ± 2.7 N, elbow: 5.6 ± 2.6 N, P = 0.07). Adductor pollicis maximal M-waves were smaller when the ulnar nerve was stimulated at the elbow compared with stimulation at the wrist for both experiment 1 (wrist: 14.4 ± 4.4 mV, elbow: 13.4 ± 4.3 mV, P = 0.009) and experiment 2 (wrist: 14.3 ± 2.9 mV, elbow: 12.4 ± 2.4 mV, P = 0.004).
Experiment 1: effect of fatiguing voluntary or stimulated contraction on voluntary activation.
In experiment 1, voluntary activation was measured during brief MVCs before and after both a 2-min isometric MVC, and a 2-min isometric electrically stimulated maximal contraction to examine whether either contraction resulted in decreased voluntary activation.
The protocol for experiment 1 was as follows. Participants visited the laboratory on two separate occasions separated by at least 72 h. During one visit (VOL), they performed a 2-min isometric MVC, and on another day (STIM) stimuli to the ulnar nerve at the elbow produced a maximal 2-min isometric electrically stimulated contraction (Fig. 1A). The order of the visits was randomized. Before the 2-min fatiguing task, participants performed a baseline set of five brief (2–3 s), isometric MVCs, during which supramaximal doublet stimuli (10 ms apart) were delivered to the ulnar nerve at the wrist to produce a superimposed twitch (SIT). With the muscle relaxed, 2–3 s after the maximal contraction, a similar doublet elicited a resting twitch. These brief MVCs were separated by 2 min to ensure that fatigue did not develop in the adductor pollicis muscle. During the 2-min voluntary fatiguing task (VOL), participants received doublets to evoke SITs every 10 s throughout the contraction and a doublet resting twitch immediately upon completion of the 2-min contraction. Following both the VOL and the STIM fatiguing tasks, participants performed brief MVCs with SITs followed by resting twitches at 5, 20, 40, 60, 90, 120, 240, 360, 480, and 600 s (Fig. 1A). During all MVCs, participants received visual feedback of force and were verbally encouraged to produce maximal performance.
Experiment 2: effect of fatiguing voluntary or stimulated contraction on motoneuron excitability (F-waves).
In experiment 2, F-waves were measured before and after a 2-min isometric MVC and a 2-min isometric electrically stimulated contraction to determine whether differences in motoneuron excitability could contribute to the differences in voluntary activation revealed by experiment 1.
The protocol for experiment 2 was as follows. Similarly to experiment 1, participants visited the laboratory on two separate occasions separated by at least 72 h. During one visit they performed a 2-min MVC (VOL), and on another visit they received a 2-min train of supramaximal stimuli of the ulnar nerve to induce a maximal, fatiguing electrically stimulated contraction (STIM) (Fig. 1B). Prior to both 2-min fatiguing tasks, two baseline blocks of F-waves were recorded 5 min apart. F-waves were elicited through supramaximal stimulation of the ulnar nerve at the wrist with stimuli delivered at 2-s intervals (0.5 Hz). Each baseline block consisted of two sets of 30 F-waves (1 min apart). Following the 2-min fatiguing tasks, sets of 30 F-waves were recorded at 0–60 s, 90–150 s, 210–270 s, 330–390 s, 450–510 s, and 570–630 s to encompass similar recovery times to experiment 1 (Fig. 1B). Because F-waves are small and preceded by a maximal compound muscle action potential (M-wave), a second channel of adductor pollicis EMG was recorded with a high-gain (3,000×). High-pass filtering was used (200–1,000 Hz) to remove the slow component of the M-wave and allow better visualization of adductor pollicis F-waves (e.g., Ref. 39).
Data analysis.
All measurements were analyzed offline using Signal (version 4.07, CED) and Spike 2 (version 7, CED) software. For both experiments, the mean forces produced during the first and the last 3 s of the 2-min fatiguing contractions were measured.
For experiment 1, maximal voluntary force was measured as the mean force over the 100 ms before stimulation in each brief MVC. Additionally, the root mean square amplitude of adductor pollicis EMG was measured over the same time window. The amplitudes of the superimposed twitches and doublet resting twitches were measured. Voluntary activation (%) was calculated as follows: (1 − superimposed twitch/doublet resting twitch) × 100. For baseline measurements, the averages of the five MVCs and doublet resting twitches were used for each individual.
For experiment 2, the areas of the compound muscle action potentials (F-waves, M-waves) for all phases of the potentials were calculated using built-in software functions. F-waves were measured from the highly filtered channels (e.g., Ref. 39). Their areas were first normalized to their corresponding maximal M-wave, also from the highly filtered channel, and then averaged over the 30 or 60 sweeps for each time point. F- and M-wave areas were normalized to the average of the two baseline blocks and expressed as a percentage. F-wave persistence (%) was calculated as the percentage of stimuli (of 60 or 30) that evoked an F-wave greater than 20 µV in amplitude (39).
Group averages at each time point are reported in the text and shown in the figures as means ± SD. Differences between days (VOL − STIM) were calculated by subtracting the values obtained after the stimulated contraction from those after the voluntary 2-min contraction at each time point and for each individual. The group averages of these differences are reported as means [95% confidence interval (CI)].
Statistics.
All statistical analyses were performed with SPSS Statistics 22 software. Two-way repeated-measures ANOVAs were used to determine the main effects of contraction type and time, and the interaction effect (contraction type × time) on maximal force, voluntary activation, superimposed twitches, resting twitches, adductor pollicis EMG, normalized F-waves, F-wave persistence, and M waves. In study 1, paired t tests were used to determine whether baseline measurements were significantly different. In study 2, two-way repeated-measures ANOVAs were used to determine whether the two baseline blocks recorded on each day were significantly different within or between days. In addition, paired t tests determined whether there were any significant differences in the forces elicited during the 2-min fatiguing contraction. Mauchly’s test of sphericity was performed, and for data that were not spherical, a Greenhouse-Geisser correction was used. Post hoc Bonferroni-corrected t tests were used to determine the time points when group averages were significantly different between days. Statistical significance was set at P < 0.05.
RESULTS
Experiment 1: effect of fatiguing voluntary or stimulated contraction on voluntary activation.
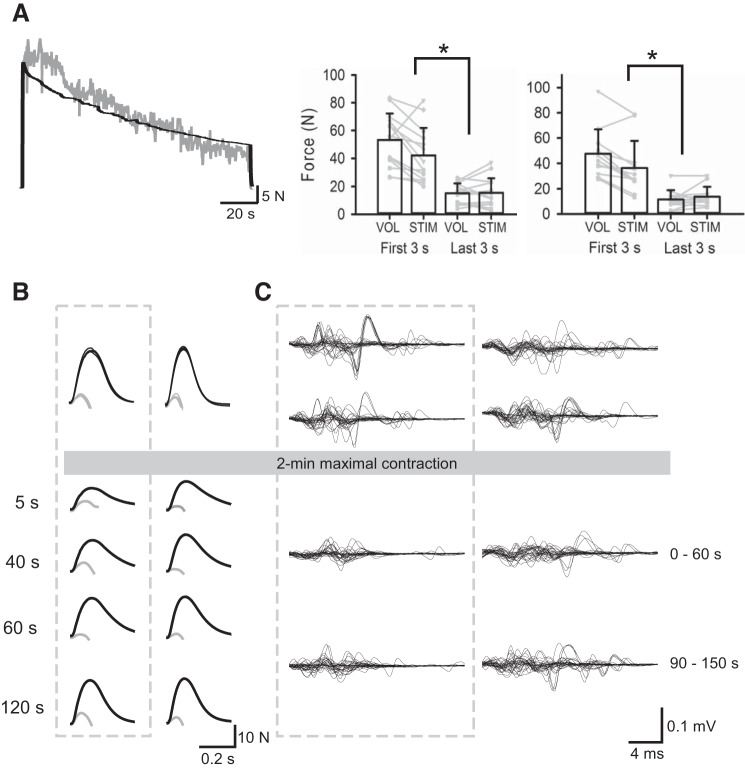
Baseline measurements of voluntary force (means ± SD; VOL: 60.7 ± 19.7 N, STIM: 60.9 ± 22.2 N, P = 0.91), voluntary activation (VOL: 87.5 ± 5.7%, STIM: 88.4 ± 6.0%, P = 0.06), superimposed twitches (VOL: 2.5 ± 1.4 N, STIM: 2.3 ± 1.8 N, P = 0.35), and resting twitches (VOL: 20.1 ± 6.3 N, STIM: 19.3 ± 7.2 N, P = 0.39) were not different before the 2-min fatiguing task on the two days. Additionally, adductor pollicis EMG measured during the brief MVCs was not different between days (VOL: 0.64 ± 0.24 mV, STIM: 0.66 ± 0.26 mV, P = 0.60). Force levels during the initial 3 s of the 2-min fatiguing task were 53.2 ± 19.1 N (87 ± 6% of baseline MVCs) on the VOL day and 42.1 ± 19.7 N (69 ± 18% of baseline MVCs) on the STIM day (P = 0.01; Fig. 2). That is, the initial levels of force able to be elicited through electrical stimulation trains (STIM) were 80 ± 24% of initial VOL force (Fig. 2). During the 2-min fatiguing task, force decreased to 28 ± 8% of its initial value on the voluntary day and to 34 ± 12% of its initial value at the end of the electrically stimulated contraction (P = 0.02). Force levels at the end of each 2-min contraction were similar (VOL: 15.1 ± 7.1 N, STIM: 15.5 ± 10.4 N, P = 0.83; Fig. 2) and for the voluntary contraction voluntary activation was 48.7 ± 25.8%.
Fig. 2.
A, left: raw force traces from 2-min maximal voluntary (gray line) and electrically stimulated (black line) contractions in the same individual. Right: group mean force of the first and last 3 s of the voluntary (VOL) and electrically stimulated (STIM) maximal contractions for experiment 1 (left, n = 14) and experiment 2 (right, n = 12). Individual data are shown by gray lines. B: superimposed (doublet) (gray lines) and resting (doublet) twitches (black lines) for a single participant before and after the 2-min fatiguing maximal contraction on each day. Twitch responses following the 2-min maximal voluntary contraction (MVC) are shown for 5, 40, 60, and 120 s after the maximal contraction and are enclosed within the gray dotted box. Voluntary activation during the VOL visit was 86% at baseline and 54, 73, 91, and 74% at 5, 40, 60, and 120 s postcontraction, respectively. Voluntary activation during the STIM visit was 86% at baseline and 91, 96, 92, and 92%, respectively, at postcontraction times. C: superimposed traces of 30 F-wave responses in an individual participant before and after the 2-min fatiguing maximal contraction on each day. F-waves are shown without the preceding M-waves. Supramaximal ulnar nerve stimuli were delivered 23 ms before the start of each trace. F-waves obtained before and after the 2-min MVC are enclosed within the gray box. Following the 2-min fatiguing MVC, mean F-wave area was decreased by 45 and 34% compared with baseline values at 0–60 s and 90–150 s. Similarly, F-wave persistence decreased by 27 and 17%, respectively. Following the 2-min fatiguing electrically evoked maximal contraction, F-wave area was unchanged at 0–60 s and increased by 9% at 90–150 s. F-wave persistence increased by 7% at 0–60 s and was unchanged at 90–150 s. *P < 0.05.
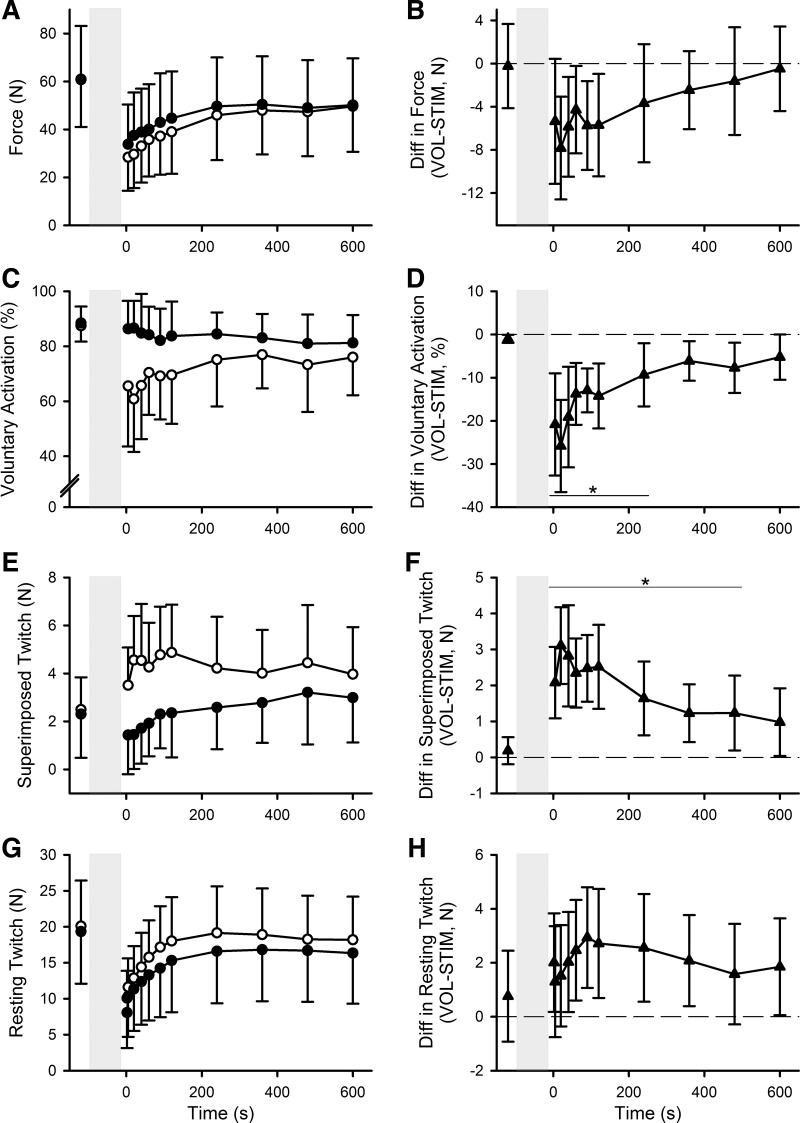
Following the 2-min voluntary and stimulated fatiguing contractions, voluntary force during brief MVCs (Fig. 3, A and B) was reduced by 54 ± 18% and 46 ± 16%, respectively. There was no contraction effect (F1,13 = 4.267, P = 0.06) but a significant time (F2.446,31.80 = 57.83, P < 0.001), and contraction × time interaction effect (F10,130 = 2.953, P = 0.002) with lower maximal force from 20 to 120 s after the voluntary contraction. There was a larger reduction in voluntary activation (Fig. 3, C and D) following the 2-min voluntary contraction (−17 ± 11% across all postcontraction values) compared with the 2-min electrically stimulated contraction (−5 ± 7%) with significant contraction type (F1,13 = 19.25, P = 0.001), time (F2.624,34.107 = 5.47, P = 0.005), and contraction × time interaction effects (F2.776,36.088 = 6.537, P = 0.002). This difference was largest at 5 to 240 s after the fatiguing contractions, with voluntary activation 15 ± 7% lower following the voluntary contraction than the stimulated contraction (Fig. 3D, post hoc Bonferroni-corrected t tests, all P < 0.05). In line with the above findings, superimposed twitches (Fig. 3, E and F) were significantly larger following the 2-min voluntary contraction with significant contraction type (F1,13 = 21.079, P = 0.001), time (F2.637,34.287 = 4.552, P = 0.011), and contraction × time interaction effects (F10,130 = 7.657, P < 0.001). This difference was significant at the 5- to 480-s time points (post hoc testing, all P < 0.05). In contrast, there was a larger reduction in doublet resting twitches (Fig. 3, G and H) following the 2-min electrically stimulated contraction compared with the voluntary contraction with significant contraction type (F1,13 = 5.202, P = 0.04) and time effects (F1.748,22.718 = 68.725, P < 0.001) but no significant contraction × time interaction (F2.429,31.571 = 2.364, P = 0.10). Adductor pollicis EMG amplitude during the brief MVCs was not different between contraction types (F1,13 = 0.10, P = 0.92), and there was no contraction × time interaction (F4.804,62.455 = 1.781, P = 0.13), but there was a significant time effect (F3.298,42.873 = 3.335, P = 0.025).
Fig. 3.
Group means ± SD of force (A), voluntary activation (C), superimposed twitches (E), and resting twitches (G) during brief maximal voluntary contractions (MVCs) before and after 2-min maximal voluntary (VOL; ○) and electrically stimulated (STIM; ●) contractions. Group mean differences ± 95% confidence interval between voluntary fatiguing contraction and electrically stimulated fatiguing contraction for force (B), voluntary activation (D), superimposed twitches (F), and resting twitches (H). Dashed horizontal line indicates no difference between days; n = 14, *P < 0.05.
Experiment 2: effect of fatiguing voluntary or stimulated contraction on motoneuron excitability (F-waves).
The area of adductor pollicis F-waves before the fatiguing voluntary and electrically stimulated 2-min contractions was 4.1 ± 1.7% and 4.4 ± 1.8% Mmax (P = 0.27), and there was high persistence (VOL: 87 ± 9%, STIM: 86 ± 5%, P = 0.68). As in experiment 1, force was lower at the start of the 2-min electrically stimulated contraction compared with the voluntary contraction (36.5 ± 21.2 vs. 47.5 ± 19.3 N, P = 0.002). Electrically evoked force was 74 ± 19% of the force at the start of the 2-min fatiguing MVC. Participants reached similar levels of force at the end of the 2-min contractions (VOL: 11.5 ± 7.4 N, STIM: 13.7 ± 7.8 N, P = 0.26), with a 75 ± 14% reduction in force during the 2-min voluntary contraction and a 62 ± 9% reduction in force during the 2-min electrically stimulated contraction (P < 0.001).
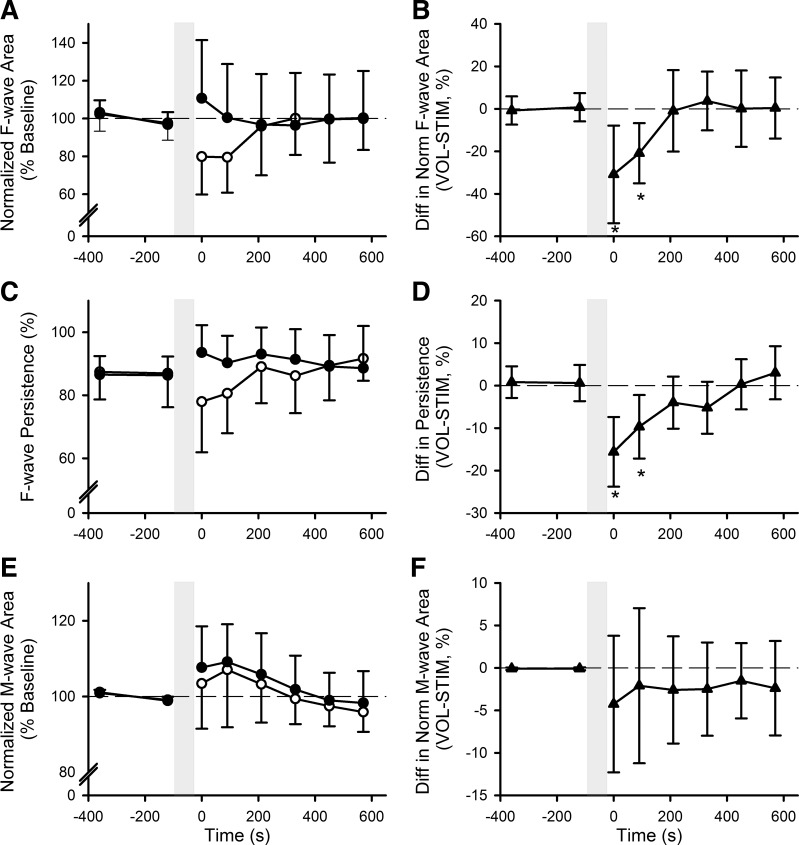
Following the 2-min fatiguing contractions, adductor pollicis F-waves (Fig. 4, A and B) were reduced after the voluntary contraction compared with the electrically stimulated contraction, with a significant difference in area of −26% [−10, −42%] at the 0–60 s and 90–150 s time points (contraction × time interaction: F6,66 = 3.287, P = 0.007). There was no contraction type (F1,11 = 2.245, P = 0.16) or time effect (F3.188,35.068 = 1.329, P = 0.28). F-wave persistence (Fig. 4, C and D) was similarly decreased following the 2-min voluntary contraction and not the 2-min electrically stimulated contraction (contraction × time interaction: F6,66 = 6.201, P < 0.001), with significant differences in persistence of −13% [−6, −20%] at 0–60 s and 90–150 s postcontraction. There was no overall contraction type effect (F1,11 = 4.100, P = 0.07), but there was an effect of time (F6,66 = 2.744, P = 0.02). Maximal M-wave areas (Fig. 4, E and F) were slightly increased following both 2-min fatiguing contractions (time: F2.155,23.709 = 8.901, P = 0.001), but there was no significant difference between contraction types (contraction type: F1,11 = 0.817, P = 0.39; contraction × time interaction: F1.906,20.961 = 0.340, P = 0.71).
Fig. 4.
Group means ± SD of normalized F-wave area (A), F-wave persistence (C), and normalized M-wave area (E) before and after 2-min maximal voluntary (VOL; ○) and electrically stimulated (STIM; ●) contractions. Group mean differences ± 95% confidence interval between voluntary and electrically stimulated fatiguing contraction for normalized F-wave area (B), F-wave persistence (D), and F) normalized M-wave area (F). Dashed horizontal line indicates no difference between days; n = 12, *P < 0.05.
DISCUSSION
Summary of findings.
In the present study, voluntary force declined ~10% more in a 2-min sustained maximal voluntary contraction of adductor pollicis muscle compared with a 2-min sustained contraction produced by electrical stimulation. The mechanisms behind the reductions in force differed. After the maximal voluntary contractions, as expected, both voluntary activation and F-waves decreased (37, 39). However, after the stimulated contraction, no reduction in voluntary activation or F-waves occurred, although the resting twitch declined more than after the voluntary contraction. Hence, a sustained isometric voluntary contraction reduced the excitability of the motoneurons and led to a failure of the nervous system to drive the muscle maximally, whereas the stimulated contraction led only to peripheral fatigue of the muscle.
Factors that differed between the two contractions include the voluntary engagement of corticospinal and other descending systems to drive the motoneurons through synaptic input versus stimulation of motor axons to contract the muscle and antidromically activate the motoneurons. By contrast, both contractions produced fatigue of the muscle fibers and should have activated small-diameter fatigue-sensitive (group III/IV) muscle afferents. Therefore, the lack of a decrease in voluntary activation after the stimulated contraction raises questions about the role of group III/IV muscle afferents in fatigue. In addition, the lack of change in motoneuron excitability after the stimulated contraction confirms that, for hand muscles, depression of motoneuron excitability occurs only when the motoneurons are activated by voluntary drive (5, 40).
Role of group III/IV afferents.
Voluntary activation of exercised muscles, including the adductor pollicis, is reduced when firing of chemically sensitive muscle afferents is maintained after exercise by occlusion of blood flow to trap metabolites in the fatigued muscle (24, 36–38). Group III/IV muscle afferent firing from fatigued muscles can also reduce voluntary activation of nonexercised muscles (36–38, 60). Moreover, blocking group III/IV afferent feedback by using intrathecal fentanyl can ameliorate the decrease in voluntary drive associated with fatigue (3, 4, 59). Taken together, these studies provide strong evidence that sensory feedback from fatigued muscles reduces voluntary activation and, hence, contributes to exercise-related fatigue. This reduction of voluntary activation is likely to occur through supraspinal mechanisms (10, 24, 39, 61), although inhibition of motoneurons may also contribute for some muscles (19, 67, and cf. Ref. 43).
For the adductor pollicis muscle, there is no direct evidence as to whether the motoneurons are excited or inhibited by group III/IV muscle afferent firing. In the current study, voluntary activation and motoneuron excitability were both reduced after the voluntary but not the stimulated 2-min isometric maximal contraction, although the two contractions produced high levels of muscle fatigue as shown by the decreases in maximal voluntary force. Indeed, a larger decrease in resting twitch suggests that fatigue of the muscle itself (peripheral fatigue), and presumably group III/IV afferent firing associated with accumulation of metabolites (e.g., Refs. 34, 57), was greater with the stimulated contraction, yet there was no impairment of voluntary activation. One possible explanation is that group III/IV muscle afferent firing diminishes very quickly on muscle relaxation. In studies that have examined the effects of postexercise blood flow occlusion, resumption of blood flow by release of the cuff leads to recovery of voluntary activation when tested after as little as 30 s in elbow flexors (e.g., Ref. 38). Here, the first brief MVCs were performed 5 s after the fatiguing tasks. A second possibility is that another factor related to voluntary activity makes the nervous system sensitive to group III/IV afferent firing. However, group III/IV afferents from a fatigued muscle can impair voluntary activation of a muscle that has not exercised, so that specific activity in the motor pathway of the tested muscle is not required (37). Finally, it is possible that stimulation somehow facilitates the motor pathway and negates the reduction in motor output resulting from group III/IV afferent firing. Our results on voluntary activation and motoneuron excitability cannot differentiate between possible explanations for the lack of effect of group III/IV afferents.
Consistent with our findings, short-latency stretch reflexes of the first dorsal interosseus muscle were depressed after voluntary contractions but not after electrically evoked contractions (5). As the stretch reflexes were elicited during postexercise occlusion of blood flow, the lack of effect argues that group III/IV afferents do not depress motoneuron excitability (or increase presynaptic inhibition) after stimulated contractions. Moreover, long-latency reflex responses were facilitated by the stimulated contractions in this paradigm, and it was postulated that this might occur through activation of cutaneous afferents (5). Further studies using occlusion to prolong group III/IV afferent firing following electrically stimulated maximal contractions would be useful to elucidate whether these afferents can impair voluntary activation in the adductor pollicis in the absence of preceding voluntary drive.
Role of repetitive motoneuron activation.
Observations in voluntary submaximal fatiguing efforts have shown that repetitive activation affects the excitability of the motoneurons. Motor unit recordings (28, 33) and subcortically evoked motor responses (19, 45) suggest that the intrinsic excitability of active motoneurons is reduced during isometric submaximal, fatiguing voluntary contractions and takes 1–2 min to recover (28). To best match repetitive motoneuron activity in the stimulated and voluntary contractions, the rates of stimulation delivered during the 2-min electrically stimulated maximal contraction mimicked the mean motoneuron firing rates recorded during a 2-min maximal voluntary contraction of the adductor pollicis (7). There were no changes in voluntary activation or F-waves after the 2-min electrically evoked maximal contraction despite presumed antidromic activation of the motoneurons, as well as reflex activation produced synaptically. This confirms previous results in a hand muscle following 10-s trains of tetanic stimuli at similar frequencies to those used in the current study (20 and 40 Hz) (40). It is also consistent with a lack of effect of fatiguing electrically evoked contractions (30 Hz) on H reflexes and short- and medium- latency stretch reflexes (5, 50), although higher-frequency stimulation (60–100 Hz) is reported to produce central effects including decreased H-reflexes (27). The lack of change of F-waves also differs from the reduced F-wave responses that developed during electrical stimulation at 18 Hz to drive thenar muscles over a 90-s period (11). The reduction of F-waves during but not after a stimulated contraction may indicate an increase in short-lasting recurrent or afferent-evoked inhibition elicited by each stimulus (11, 30, 53). Overall, these findings suggest that simple repetitive activation of motoneurons at physiological frequencies does not decrease motoneuron excitability and that any increase in recurrent or afferent-evoked inhibition of the motoneurons has little influence on subsequent voluntary motor output.
Descending drive is essential for changes in voluntary activation and is partly driven by reduced motoneuron excitability.
At the motoneuron level, the presence of descending drive and the associated release of neuromodulators are the major differences between voluntary and stimulated contractions. Several pathways could be involved in the decrease of motoneuronal excitability, but the descending serotonergic system is a likely candidate. Spinal motoneurons receive direct and dense serotonergic innervation from neurons with cell bodies in the medullary raphe nuclei (2, 12, 29, 41, 58). Additionally, increased firing of serotonergic raphe neurons occurs during periods of motor activity, implying increased release of serotonin with increased levels of activity (20, 31, 32, 65). Indeed, it has been suggested that, in humans, serotonin release is graded with the strength of voluntary contraction and that this release is quite diffuse (66). In the adult turtle spinal cord, intense and prolonged serotonergic drive to the motoneurons leads to spillover of synaptically released serotonin, which inhibits motoneuron output via activation of extrasynaptic serotonin 1A subtype (5HT1A) receptors at the axon initial segment of the motoneurons (15). Activation of these specific 5HT1A receptors can depress motoneuron excitability in humans (16), and ingestion of a serotonin reuptake inhibitor to increase available serotonin results in greater reductions in F-wave amplitude and persistence following fatiguing 1-min MVCs (35). Moreover, motoneuron recovery after serotonin spillover has a relatively slow time course of more than 1 but less than 5 min (15, 51), which fits with the depression of F-waves seen in this study. Whatever the mechanism for the decrease in motoneuron excitability after the 2-min voluntary contraction, it likely contributes to reduced voluntary activation. This is consistent with the report that increased available serotonin further depresses voluntary activation and F-waves after fatiguing MVCs (35). However, processes at other sites that were engaged in the voluntary contraction but not the stimulated contraction may also contribute. For example, for the elbow flexor muscles, suboptimal descending drive from the motor cortex after sustained maximal or submaximal efforts is suggested by a reduction in voluntary activation measured using transcranial magnetic stimulation of the motor cortex (63, 64).
Recently, questions have been raised about the validity of the twitch interpolation technique to quantify voluntary activation and study central fatigue (i.e., the contribution of the nervous system to the exercise-related decrease in maximal voluntary force output of a muscle or muscle group) during human exercise (48, 55). Multiple changes within the muscle contribute to loss of force with exercise, and it has been proposed that these peripheral changes may be sufficient to account for exercise-induced fatigue (14, 49, 54, 56). However, fatiguing protocols of human adductor pollicis muscles induced by peripheral electrical stimulation have resulted in reduced, rather than increased, superimposed twitches evoked during the tetanic stimulation (26, 48). Muscle action potential failure may have contributed to the reduced superimposed twitches, as significant reductions in the M-wave occurred, although the initial decline of the superimposed twitch preceded that of the M-wave (8, 21, 26, 48). In the present study, M-waves were slightly increased after both the 2-min MVC and the 2-min stimulated fatiguing contractions. Thus, with the lack of significant differences in M-waves, the absence of a change in superimposed twitches after the stimulated contraction compared with the clear increase in superimposed twitches and consequent decrease in voluntary activation after the 2-min MVC is strong evidence for the occurrence of central fatigue after a voluntary contraction.
Limitations.
The development of muscle fatigue depends on the motor task (17), and the comparisons in this study are between voluntary and electrically evoked isometric maximal contractions of a small hand muscle. Therefore, the findings may not translate to submaximal, intermittent, or dynamic contractions if other neural mechanisms contribute to fatigue in those tasks. Sustained maximal contractions were chosen because they are effective in producing firing of group III/IV muscle afferents given the accumulation of metabolites through the combination of high muscle activity and restricted blood flow to the muscle. They also ensure sustained repetitive firing of most motoneurons supplying the muscle and induce effects dependent on serotonin availability (35). Group III/IV afferent firing influences voluntary motor output and the development of fatigue in dynamic whole body exercise such as cycling (4, 59) as well as isometric contractions, but the contribution of the other processes to fatigue with other tasks is not yet clear.
A second limitation of the study is that muscle activity was not exactly the same in the two contractions. Ulnar nerve stimulation at the elbow caused contraction of all ulnar-innervated muscles of the forearm and hand, with all motor units firing synchronously throughout the stimulation. By contrast, voluntary contraction was aimed at producing maximal thumb adduction. Along with strong activation of the adductor pollicis, there were unknown levels of muscle activity in other hand, forearm, and arm muscles and natural rates of motor unit firing. Effects of interactions among motor units and/or among muscles are unknown, but both types of contraction aimed to activate the adductor pollicis maximally and also engaged various nearby muscles.
Finally, the stimulation was designed to mimic motor unit firing rates during a sustained MVC. Stimulation frequency started at 30–40 Hz and was reduced over the 2-min contraction. Therefore, the study does not give insight into any effects of stimulation at higher frequencies, which are more than physiological for motor units but may not be excessive for large-diameter afferents (e.g., Ref. 27).
Conclusions.
This study demonstrates for the first time that reductions in voluntary activation and motoneuron excitability following 2-min isometric maximal contractions in humans occur only when fatigue is produced through voluntary contraction. Previous work suggests that release of neuromodulators in the spinal cord during voluntary activity is a potential mechanism for these effects. By contrast, the activity of small-diameter muscle afferents seems unlikely to contribute in these conditions. Additionally, our findings demonstrate that changes in the superimposed twitch and, by extension, voluntary activation cannot be explained by changes in peripheral factors alone as suggested by previous modeling and animal studies (14, 55). Thus, we propose that the superimposed twitch technique remains a valid measure of changes in voluntary activation and central fatigue following sustained maximal contractions in humans.
GRANTS
This work was funded by a National Health and Medical Research Council (NHMRC) program grant (no. 1055084). Additionally, S. C. Gandevia was supported by an NHMRC research fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.D., D.M.R., S.C.G., and J.L.T. conceived and designed research; J.M.D. and D.M.R. performed experiments; J.M.D. analyzed data; J.M.D., D.M.R., S.C.G., and J.L.T. interpreted results of experiments; J.M.D. prepared figures; J.M.D. drafted manuscript; J.M.D., D.M.R., S.C.G., and J.L.T. edited and revised manuscript; J.M.D., D.M.R., S.C.G., and J.L.T. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was carried out at Neuroscience Research Australia in Sydney, Australia.
REFERENCES
- 1.Allen GM, Gandevia SC, McKenzie DK. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve 18: 593–600, 1995. doi: 10.1002/mus.880180605. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393: 69–83, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Dempsey JA. Ensemble input of group III/IV muscle afferents to CNS: a limiting factor of central motor drive during endurance exercise from normoxia to moderate hypoxia. Adv Exp Med Biol 903: 325–342, 2016. doi: 10.1007/978-1-4899-7678-9_22. [DOI] [PubMed] [Google Scholar]
- 4.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balestra C, Duchateau J, Hainaut K. Effects of fatigue on the stretch reflex in a human muscle. Electroencephalogr Clin Neurophysiol 85: 46–52, 1992. doi: 10.1016/0168-5597(92)90101-G. [DOI] [PubMed] [Google Scholar]
- 6.Bellemare F, Bigland-Ritchie B. Assessment of human diaphragm strength and activation using phrenic nerve stimulation. Respir Physiol 58: 263–277, 1984. doi: 10.1016/0034-5687(84)90003-3. [DOI] [PubMed] [Google Scholar]
- 7.Bigland-Ritchie B, Johansson R, Lippold OC, Smith S, Woods JJ. Changes in motoneurone firing rates during sustained maximal voluntary contractions. J Physiol 340: 335–346, 1983. doi: 10.1113/jphysiol.1983.sp014765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigland-Ritchie B, Jones DA, Woods JJ. Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contractions. Exp Neurol 64: 414–427, 1979. doi: 10.1016/0014-4886(79)90280-2. [DOI] [PubMed] [Google Scholar]
- 9.Boerio D, Jubeau M, Zory R, Maffiuletti NA. Central and peripheral fatigue after electrostimulation-induced resistance exercise. Med Sci Sports Exerc 37: 973–978, 2005. [PubMed] [Google Scholar]
- 10.Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci 23: 10224–10230, 2003. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler JE, Thomas CK. Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles. J Appl Physiol (1985) 94: 567–575, 2003. doi: 10.1152/japplphysiol.01176.2001. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson A, Magnusson T, Rosengren E. 5-Hydroxytryptamine of the spinal cord normally and after transection. Experientia 19: 359, 1963. doi: 10.1007/BF02152316. [DOI] [PubMed] [Google Scholar]
- 13.Chaubet V, Cormery B, Maitre J, Paillard T. Stimulated contractions delay and prolong central fatigue compared with voluntary contractions in men. J Strength Cond Res 27: 1378–1383, 2013. doi: 10.1519/JSC.0b013e318265a271. [DOI] [PubMed] [Google Scholar]
- 14.Contessa P, Puleo A, De Luca CJ. Is the notion of central fatigue based on a solid foundation? J Neurophysiol 115: 967–977, 2016. doi: 10.1152/jn.00889.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotel F, Exley R, Cragg SJ, Perrier JF. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci USA 110: 4774–4779, 2013. doi: 10.1073/pnas.1216150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amico JM, Butler AA, Héroux ME, Cotel F, Perrier JM, Butler JE, Gandevia SC, Taylor JL. Human motoneurone excitability is depressed by activation of serotonin 1A receptors with buspirone. J Physiol 595: 1763–1773, 2017. doi: 10.1113/JP273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espiritu MG, Lin CS, Burke D. Motoneuron excitability and the F-wave. Muscle Nerve 27: 720–727, 2003. doi: 10.1002/mus.10388. [DOI] [PubMed] [Google Scholar]
- 19.Finn HT, Rouffet DM, Kennedy DS, Green S, Taylor JL. Motoneuron excitability of the quadriceps decreases during a fatiguing submaximal isometric contraction. J Appl Physiol (1985) 124: 970–979, 2018. doi: 10.1152/japplphysiol.00739.2017. [DOI] [PubMed] [Google Scholar]
- 20.Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Exp Neurol 88: 590–608, 1985. doi: 10.1016/0014-4886(85)90074-3. [DOI] [PubMed] [Google Scholar]
- 21.Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460: 549–572, 1993. doi: 10.1113/jphysiol.1993.sp019486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandevia SC. Some central and peripheral factors affecting human motoneuronal output in neuromuscular fatigue. Sports Med 13: 93–98, 1992. doi: 10.2165/00007256-199213020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 24.Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490: 529–536, 1996. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandevia SC, Allen GM, McKenzie DK. Central fatigue. Critical issues, quantification and practical implications. Adv Exp Med Biol 384: 281–294, 1995. doi: 10.1007/978-1-4899-1016-5_22. [DOI] [PubMed] [Google Scholar]
- 26.Gandevia SC, McNeil CJ, Carroll TJ, Taylor JL. Twitch interpolation: superimposed twitches decline progressively during a tetanic contraction of human adductor pollicis. J Physiol 591: 1373–1383, 2013. doi: 10.1113/jphysiol.2012.248989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosprêtre S, Gueugneau N, Martin A, Lepers R. Central contribution to electrically induced fatigue depends on stimulation frequency. Med Sci Sports Exerc 49: 1530–1540, 2017. doi: 10.1249/MSS.0000000000001270. [DOI] [PubMed] [Google Scholar]
- 28.Héroux ME, Butler AA, Gandevia SC, Taylor JL, Butler JE. Time course of human motoneuron recovery after sustained low-level voluntary activity. J Neurophysiol 115: 803–812, 2016. doi: 10.1152/jn.00950.2015. [DOI] [PubMed] [Google Scholar]
- 29.Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26: 331–343, 2003. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Illert M, Kümmel H. Reflex pathways from large muscle spindle afferents and recurrent axon collaterals to motoneurones of wrist and digit muscles: a comparison in cats, monkeys and humans. Exp Brain Res 128: 13–19, 1999. doi: 10.1007/s002210050812. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol 7: 820–825, 1997. doi: 10.1016/S0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40: 45–52, 2002. doi: 10.1016/S0165-0173(02)00187-X. [DOI] [PubMed] [Google Scholar]
- 33.Johnson KV, Edwards SC, Van Tongeren C, Bawa P. Properties of human motor units after prolonged activity at a constant firing rate. Exp Brain Res 154: 479–487, 2004. doi: 10.1007/s00221-003-1678-z. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: I60–I65, 1987. [PubMed] [Google Scholar]
- 35.Kavanagh JJ, McFarland AJ, Taylor JL. Enhanced availability of serotonin increases activation of unfatigued muscle but exacerbates central fatigue during prolonged sustained contractions. J Physiol 597: 319–332, 2019. doi: 10.1113/JP277148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy DS, Fitzpatrick SC, Gandevia SC, Taylor JL. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J Appl Physiol (1985) 118: 408–418, 2015. doi: 10.1152/japplphysiol.00375.2014. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL. Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol (1985) 116: 385–394, 2014. doi: 10.1152/japplphysiol.01166.2013. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL. Firing of antagonist small-diameter muscle afferents reduces voluntary activation and torque of elbow flexors. J Physiol 591: 3591–3604, 2013. doi: 10.1113/jphysiol.2012.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan SI, Giesebrecht S, Gandevia SC, Taylor JL. Activity-dependent depression of the recurrent discharge of human motoneurones after maximal voluntary contractions. J Physiol 590: 4957–4969, 2012. doi: 10.1113/jphysiol.2012.235697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan SI, Taylor JL, Gandevia SC. Unexpected factors affecting the excitability of human motoneurones in voluntary and stimulated contractions. J Physiol 594: 2707–2717, 2016. doi: 10.1113/JP272164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiehn O, Rostrup E, Møller M. Monoaminergic systems in the brainstem and spinal cord of the turtle Pseudemys scripta elegans as revealed by antibodies against serotonin and tyrosine hydroxylase. J Comp Neurol 325: 527–547, 1992. doi: 10.1002/cne.903250406. [DOI] [PubMed] [Google Scholar]
- 42.Martin PG, Gandevia SC, Taylor JL. Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol 95: 3512–3518, 2006. doi: 10.1152/jn.01230.2005. [DOI] [PubMed] [Google Scholar]
- 43.Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26: 4796–4802, 2006. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLeod JG, Wray SH. An experimental study of the F-wave in the baboon. J Neurol Neurosurg Psychiatry 29: 196–200, 1966. doi: 10.1136/jnnp.29.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589: 3533–3544, 2011. doi: 10.1113/jphysiol.2011.207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587: 5601–5612, 2009. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neyroud D, Cheng AJ, Bourdillon N, Kayser B, Place N, Westerblad H. Muscle fatigue affects the interpolated twitch technique when assessed using electrically-induced contractions in human and rat muscles. Front Physiol 7: 252, 2016. doi: 10.3389/fphys.2016.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neyroud D, Maffiuletti NA, Kayser B, Place N. Mechanisms of fatigue and task failure induced by sustained submaximal contractions. Med Sci Sports Exerc 44: 1243–1251, 2012. doi: 10.1249/MSS.0b013e318245cc4d. [DOI] [PubMed] [Google Scholar]
- 50.Papaiordanidou M, Guiraud D, Varray A. Kinetics of neuromuscular changes during low-frequency electrical stimulation. Muscle Nerve 41: 54–62, 2010. doi: 10.1002/mus.21427. [DOI] [PubMed] [Google Scholar]
- 51.Perrier JF, Rasmussen HB, Jørgensen LK, Berg RW. Intense activity of the raphe spinal pathway depresses motor activity via a serotonin dependent mechanism. Front Neural Circuits 11: 111, 2018. doi: 10.3389/fncir.2017.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen NT, Taylor JL, Butler JE, Gandevia SC. Depression of activity in the corticospinal pathway during human motor behavior after strong voluntary contractions. J Neurosci 23: 7974–7980, 2003. doi: 10.1523/JNEUROSCI.23-22-07974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piotrkiewicz M, Młoźniak D. Are human digit muscles devoid of recurrent inhibition? Front Cell Neurosci 9: 507, 2016. doi: 10.3389/fncel.2015.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Place N, Bruton JD, Westerblad H. Mechanisms of fatigue induced by isometric contractions in exercising humans and in mouse isolated single muscle fibres. Clin Exp Pharmacol Physiol 36: 334–339, 2009. doi: 10.1111/j.1440-1681.2008.05021.x. [DOI] [PubMed] [Google Scholar]
- 55.Place N, Yamada T, Bruton JD, Westerblad H. Interpolated twitches in fatiguing single mouse muscle fibres: implications for the assessment of central fatigue. J Physiol 586: 2799–2805, 2008. doi: 10.1113/jphysiol.2008.151910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Place N, Yamada T, Bruton JD, Westerblad H. Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol 110: 1–15, 2010. doi: 10.1007/s00421-010-1480-0. [DOI] [PubMed] [Google Scholar]
- 57.Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99: 368–380, 2014. [Erratum in Exp Physiol 99: 740, 2014.] doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000. doi: 10.1016/S0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 59.Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE, Amann M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128: 44–55, 2017. doi: 10.1016/j.clinph.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M. Spinal μ-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592: 5011–5024, 2014. doi: 10.1113/jphysiol.2014.275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48: 2294–2306, 2016. doi: 10.1249/MSS.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol (1985) 104: 542–550, 2008. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- 63.Taylor JL, Todd G, Gandevia SC. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol 33: 400–405, 2006. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 64.Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551: 661–671, 2003. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei K, Glaser JI, Deng L, Thompson CK, Stevenson IH, Wang Q, Hornby TG, Heckman CJ, Kording KP. Serotonin affects movement gain control in the spinal cord. J Neurosci 34: 12690–12700, 2014. doi: 10.1523/JNEUROSCI.1855-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol 58: 125–137, 1987. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- 68.Zory R, Boërio D, Jubeau M, Maffiuletti NA. Central and peripheral fatigue of the knee extensor muscles induced by electromyostimulation. Int J Sports Med 26: 847–853, 2005. doi: 10.1055/s-2005-837459. [DOI] [PubMed] [Google Scholar]