Abstract
Age-related declines in skeletal muscle mass (i.e., sarcopenia) contribute to physical disability in older women. Although a menopause-related increase in fat mass is well documented, whether menopause influences muscle mass and sarcopenia is unclear. We determined the extent to which skeletal muscle mass differs across the stages of the menopause transition in women and whether these differences are associated with estradiol or other sex hormones. This was a cross-sectional study of 144 healthy women (aged 30–70 yr) classified as premenopausal [n = 30, 38 ± 6 yr (means ± SD)], early (n = 31, 50 ± 3 yr) and late (n = 30, 50 ± 4 yr) perimenopausal, and early (n = 26, 55 ± 3 yr) and late (n = 27, 62 ± 4 yr) postmenopausal. Appendicular lean mass (ALM) adjusted by the square of height in meters (ALM index; ALMi) was assessed by dual-energy X-ray absorptiometry. ALMi was lower (P < 0.05) in late perimenopausal and postmenopausal compared with early perimenopausal, with no significant differences between other groups (premenopausal 6.6 ± 0.6, early perimenopausal 6.8 ± 0.8, late perimenopausal 6.1 ± 0.8, early postmenopausal 6.5 ± 1.1, and late postmenopausal 6.2 ± 0.9 kg/m2). The prevalence of sarcopenia (ALMi ≤ 5.67 kg/m2) was 7%, 3%, 30%, 27%, and 32% in premenopausal, early and late perimenopausal, and early and late postmenopausal groups, respectively. ALMi measured across menopause stages was inversely correlated to follicle-stimulating hormone (FSH; r = −0.28, P = 0.003) but not to estradiol (r = 0.088, P = 0.34). The menopause transition appears to be a vulnerable period for the loss of skeletal muscle mass that may begin during the late perimenopausal transition. Future studies are necessary to investigate the potential effect of FSH on skeletal muscle.
NEW & NOTEWORTHY Our data suggest that the late perimenopausal stage may be a vulnerable period for the loss of skeletal muscle, potentially related to elevations in FSH.
Keywords: follicle-stimulating hormone, menopause, muscle mass, sarcopenia
INTRODUCTION
Sarcopenia, the age-related decline in skeletal muscle mass and strength, is one of the main contributors to physical disability in older adults (16). Sarcopenia-associated physical disability results in an annual health care cost of $18.4 billion in the United States (17). Sarcopenia is not a condition that only affects very old individuals but can also be observed in middle-aged ambulatory adults (3). Additionally, increasing evidence suggests that there may be a sexual dimorphism in physical disability and sarcopenia. Older women experience greater physical disability and morbidity compared with older men (44) and have a greater prevalence of sarcopenia beginning at the 4th decade and persisting through old age (16). Moreover, a longitudinal study showed that women with severe sarcopenia were more likely than sarcopenic men to develop a physical disability (15). These findings emphasize the need to mitigate the progression of sarcopenia in aging, particularly in older women.
Menopause may contribute to sarcopenia in women. As opposed to andropause, which occurs in <10% of middle-aged men (10), changes in the sex hormone environment during the menopause transition could be a strong determinant of muscle mass in women. Cross-sectional studies (2, 36, 39) have reported that lean mass is lower in postmenopausal compared with premenopausal women. However, whether muscle mass and the prevalence of sarcopenia differ across the stages of the menopausal transition, specifically during the early to late perimenopausal years, is unknown.
The decline in estradiol is believed to be the most important factor in the menopause-related declines in muscle mass. However, estrogen-based hormone therapy (HT) studies that investigated lean body and skeletal muscle mass in postmenopausal women have provided mixed results. Some studies (7, 31, 34) have reported that estrogen-based HT preserves muscle mass in postmenopausal women, whereas others (1, 9, 18) have shown no effect. A recent rodent study by Liu et al. (20) raised the possibility that increasing levels of circulating follicle-stimulating hormone (FSH) could be another strong factor mediating the decline in skeletal muscle mass during the menopausal transition. Therefore, the purpose of this study was to determine 1) the extent to which appendicular lean mass (ALM) index (ALMi), a surrogate measure of skeletal muscle mass, differs across the stages of the menopause transition, specifically from the early to late perimenopausal years, in healthy women; 2) whether postmenopausal women who previously used HT have a greater ALMi compared with nonusers; and 3) whether the reduction in ALMi across menopausal stages is associated with changes in estradiol or other sex hormones (e.g., FSH).
MATERIALS AND METHODS
Population.
This was a secondary analysis of a cross-sectional study that examined biomarkers of cardiovascular aging across the stages of the menopause transition (25). This secondary analysis includes 144 healthy women aged 30–70 yr. Characteristics of the women have been described previously (25). Menopausal status was assessed by self-reported menstrual cycle, and staging was determined according to the Stages of Reproductive Aging Workshop (STRAW) criteria (35). Briefly, premenopausal women (n = 30) had regular menstrual cycles (21–35 days); early perimenopausal women (n = 31) had >2 cycles with cycle length changes of ≥7 days; late perimenopausal women (n = 30) had ≥2 and <12 mo of amenorrhea; early postmenopausal women (n = 26) had ≥1 yr of amenorrhea but ≤5 yr since menopause; and late postmenopausal women (n = 27) were >5 yr since menopause. Inclusion criteria for this study (25) were 1) no use of oral contraceptives or HT for at least 6 mo (to avoid the effects of estrogen-based HT on vascular function), 2) fasted glucose < 126 mg/dL, 3) blood pressure < 140/90 mmHg, 4) nonsmoker, 5) sedentary/recreationally active (<3 days/wk vigorous aerobic or resistance exercise; to prevent confounding of the influence of physical activity), and 6) healthy as determined by physical examination, medical history, standard blood chemistries (i.e., normal liver, kidney, and thyroid function), and electrocardiography at rest and during a graded exercise treadmill test. Women with a history of or active estrogen-dependent neoplasms, acute liver or gallbladder disease, cardiovascular disease, venous thromboembolism, hypertriglyceridemia, hysterectomy/oophorectomy, and cancer were excluded. Women had not used anti-inflammatory medications or vitamin supplements for at least 4 wk so that they would not influence the testing visit. The protocol was approved by the Colorado Multiple Institutional Review Board, and participants provided written informed consent.
Body composition.
Total body lean mass, ALM (the sum of limb lean mass), ALMi (ALM index, ALM adjusted by the square of height in meters), total body fat mass (FM), FMi (FM index, FM adjusted by the square of height in meters), and trunk FM were measured by dual X-ray absorptiometry (DXA, Hologic Discovery, software version 11.2; Hologic Inc., Waltham, MA) as previously described (6, 12–14, 19, 42). The recommendations of the manufacturer were used to define the regions (e.g., arms, legs, trunk). Lines were initially placed by the computer program and then manually adjusted by a technician. The proximal ends of the lines that separated the arms from the trunk were positioned through the middle of the axilla; they were then angled outward from the body so that they separated the arms from the trunk. A pelvic triangle was positioned so that one horizontal line was just superior to the iliac crests and the two vertical lines angled inferiorly to bisect the femoral neck of both hips to between the legs. The coefficient of variation [95% confidence interval (CI)] for lean and fat are 0.7% (0.5%, 0.8%) and 1.7% (1.3%, 2.1%), respectively. Calibration procedures included spine phantom scans daily, whole body phantom scans three times per week, air scans once a week, and tissue bar scans once a month. Scans were completed by two trained and experienced technicians and reviewed by an investigator to ensure appropriate data acquisition and image analysis.
To confirm the feasibility of DXA (i.e., ALMi) as a surrogate marker of skeletal muscle areas, midthigh muscle area was assessed by computed tomography (CT) in a subgroup of women (n = 37) as previously described (33, 43). Briefly, axial CT images were obtained 20 cm superior to the distal edge of the lateral condyle of the right femur for measurement of thigh muscle and fat areas (120 kVp, 200–300 mAs, and 10-mm slice thickness; General Electric Instrument; Waukesha, WI) by experienced technicians in the University of Colorado Hospital Department of Radiology. Images were analyzed by the technicians at the CT Scan Reading Center. Thigh muscle area was separated from subcutaneous fat area by manually tracing along the deep fascial plane surrounding the muscles. Thigh muscle areas were averaged over the right and left thigh slices. Threshold for inclusion of repeat thigh scans was ±1 cm of baseline scan location. Analysis programs were developed by the University of Colorado CT Reading Center with IDL software (RSI, Inc., Boulder, CO) on a Sparc 20 workstation (Sun Microsystems, Sunnyvale, CA). Scans in the present study were completed by two trained and experienced technicians and reviewed by an investigator to ensure appropriate data acquisition and image analysis. Minimal waist circumferences were measured according to published guidelines (21).
Definition of sarcopenia.
A sex-specific ALMi cut point of ≤5.67 kg/m2 was used to define sarcopenia and determine the prevalence of sarcopenia in each menopause stage, in accordance with the International Working Group on Sarcopenia (IWGS) (3) and Newman et al. (27). Secondarily, we determined the study-specific prevalence of sarcopenia in each menopause stage using the ALMi cut point of ≤ 5.31 kg/m2, corresponding to ≥2 standard deviations (SDs) below the mean ALMi of the premenopausal women in the present study.
Sex hormones.
Serum levels of estradiol, FSH, and progesterone were measured with chemiluminescence, total testosterone with a one-step competitive assay, and estrone with radioimmunoassay by the Colorado Clinical and Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC) core laboratory, as previously described (25). The coefficients of variation (95% CI) for each hormone are as follows: intra-assay CV: estradiol 4.3%, estrone 11.5%, FSH 1.8%, progesterone 4.4%, testosterone, 2.1% and interassay CV: estradiol 8.2%, estrone 19.8%, FSH 3.8%, progesterone 7.9%, and testosterone 5.1% (24).
Physical activity, aerobic exercise capacity, and energy intake.
Physical activity level was determined objectively by pedometer step counts (23) and leisure time physical activity (LTPA) determined subjectively with the Modifiable Activity Questionnaire (28). This questionnaire included the frequency of participation in different activities (work, heavy and light exercise, etc.) and the number of hours spent in sedentary activities (watching TV, sitting, etc.) and finally calculated those in total metabolic equivalent task (MET) per week. Peak oxygen consumption (i.e., the maximal aerobic exercise capacity) was determined with an incremental treadmill running protocol as described previously (40). Energy intake and dietary macronutrient composition (i.e., carbohydrate, fat, and protein in grams) were determined from 3-day food intake records as described previously (37). The CCTSI CTRC Nutrition Core analyzed the dietary food records (41).
Statistical analysis.
All data elements were examined with descriptive statistics and graphical summaries (box plots, profile plots); skewed distributions were improved by transformation. Results are presented as means ± SD for normally distributed variables or median and interquartile range for skewed descriptive variables (i.e., estradiol, estrone, progesterone, testosterone, and LTPA). One-way analysis of variance (ANOVA) was used to determine the main effects of menopause stage on participant characteristics, sex hormones, and body composition. Tukey honestly significant difference (HSD) post hoc tests were used to identify differences among menopause stages. Because prior hormone therapy use could influence the ALM, two-way ANOVA was used to determine the menopause stage main effect (early vs. late postmenopausal), condition main effect (HT users vs. nonusers), and stage × condition interaction for ALMi and FMi. Exploratory analyses were conducted using bivariate Pearson’s correlations to test the association between ALMi and variables of interest (e.g., sex hormones, physical activity markers, CT data). If a significant correlation was found, partial correlations were used to assess whether the significance was maintained after adjusting for other variables (e.g., age, estradiol, estrone). All data were analyzed with IBM SPSS Statistics version 24.0 (IBM/SPSS, Armonk, NY). P < 0.05 was considered statistically significant.
RESULTS
Participants.
There was a main effect of menopause stage on age, total lean mass, ALM, trunk FM, concentrations of estradiol, estrone, FSH, and progesterone, and peak aerobic capacity (Table 1; all P < 0.05). Thirty-one percent and 48% of early and late postmenopausal women, respectively, were prior HT users (average duration: 2.9 ± 2.8 and 4.8 ± 3.6 yr, respectively).
Table 1.
Subject characteristics
| Variable | Pre n = 30 |
EPeri n = 31 |
LPeri n = 30 |
EPost n = 26 |
LPost n = 27 |
P Value |
|---|---|---|---|---|---|---|
| Age, yr | 38 ± 6 | 50 ± 3 | 50 ± 4 | 55 ± 3 | 62 ± 4 | <0.001 |
| Weight, kg | 65.9 ± 9.8 | 71.3 ± 10.9 | 67.3 ± 11.9 | 71.8 ± 12.9 | 66.6 ± 14.0 | 0.19 |
| Height, cm | 165 ± 6 | 165 ± 6 | 166 ± 7 | 165 ± 6 | 161 ± 7 | 0.053 |
| BMI, kg/m2 | 24.3 ± 3.8 | 26.1 ± 3.9 | 24.5 ± 3.9 | 26.6 ± 5.1 | 25.7 ± 5.1 | 0.21 |
| WC, cm | 80.6 ± 8.0 | 84.4 ± 10.4 | 82.1 ± 11.9 | 87.9 ± 13.9 | 83.5 ± 10.7 | 0.23 |
| Total lean mass, kg | 42.2 ± 3.6 | 44.3 ± 5.7 | 40.5 ± 5.1 | 42.3 ± 6.3 | 39.0 ± 5.5 | <0.01 |
| ALM, kg | 17.8 ± 1.7 | 18.7 ± 2.7 | 16.8 ± 2.7 | 17.6 ± 3.1 | 16.0 ± 2.6 | <0.01 |
| Total fat mass, kg | 21.6 ± 7.7 | 24.7 ± 6.9 | 24.8 ± 8.1 | 27.4 ± 8.0 | 25.7 ± 9.6 | 0.10 |
| Trunk fat mass, kg | 9.5 ± 4.0 | 12.1 ± 4.2 | 12.0 ± 4.6 | 13.2 ± 4.8 | 12.4 ± 4.9 | <0.05 |
| Estradiol, pg/mLa,b | 79 [64, 110] | 70 [37, 141] | 34 [10, 94] | 11 [10, 15] | 10 [10, 14] | <0.001 |
| Estrone, ng/dLa,b | 61 [41, 70] | 60 [34, 88] | 43 [30, 69] | 26 [24, 33] | 26 [23, 37] | <0.001 |
| FSH, μIU/mLb | 6.5 ± 3.4 | 22.0 ± 30.0 | 64.1 ± 35.5 | 72.1 ± 26.1 | 84.1 ± 33.3 | <0.001 |
| Progesterone, ng/dLa,b | 0.4 [0.2, 0.6] | 0.5 [0.2, 0.8] | 0.3 [0.2, 0.5] | 0.3 [0.1, 0.4] | 0.2 [0.1, 0.4] | <0.01 |
| Testosterone, ng/dLa,b | 24 [22, 33] | 22 [17, 35] | 20 [17, 25] | 18 [17, 23] | 17 [17, 35] | 0.32 |
| V̇o2peak, mL·kg−1·min−1c | 31.2 ± 6.4 | 28.3 ± 4.8 | 27.5 ± 5.9 | 26.3 ± 3.6 | 24.7 ± 7.2 | <0.001 |
Data are means ± standard deviation or
median [interquartile range] for n subjects.
n = 118,
n = 139.
ALM, appendicular lean mass; BMI, body mass index; EPeri, early perimenopausal; EPost, early postmenopausal; FSH, follicle stimulating hormone; LPeri, late perimenopausal; LPost, late postmenopausal; Pre, premenopausal; V̇o2peak, peak aerobic capacity; WC, waist circumference. Significant P values are in bold.
ALMi and FMi.
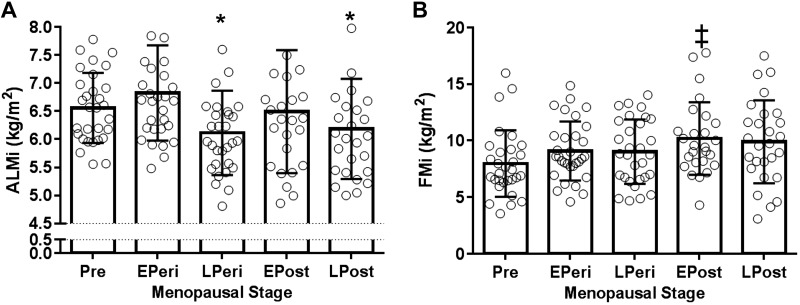
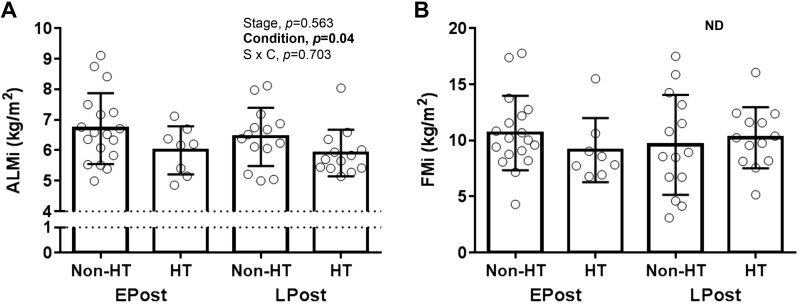
Compared with early perimenopausal women, ALMi was lower in late perimenopausal and late postmenopausal women (P = 0.011 and 0.048, respectively), with no significant differences between other menopause stages (Fig. 1A). Although there was a trend for FMi to be elevated across menopause stages (P = 0.06, premenopausal vs. early postmenopausal), FMi was not significantly different between early and late perimenopausal women (Fig. 1B). ALMi was lower in HT users than nonusers in both early and late postmenopausal stages (main effect of HT use, P = 0.04; Fig. 2A). There were no significant differences in FMi between HT users and nonusers (Fig. 2B).
Fig. 1.
A: appendicular lean mass (ALM) index (ALMi). B: fat mass (FM) index (FMi). Values are means ± SD. ALMi and FMi, ALM and FM adjusted by square of height (m2); EPeri, early perimenopausal; EPost, early postmenopausal; LPeri, late perimenopausal; LPost, late postmenopausal; Pre, premenopausal. *P < 0.05 vs. EPeri; ‡P = 0.06 vs. Pre. n = 144.
Fig. 2.
Comparisons of estrogen-based hormone therapy (HT) users and nonusers on appendicular lean mass (ALM) index (ALMi; A) and fat mass (FM) index (FMi; B). Values are means ± SD. ALMi and FMi, ALM and FM adjusted by square of height (m2); Condition, main condition effect; EPost, early postmenopausal; LPost late postmenopausal; ND, no stage (EPost vs. LPost) and condition (non-HT vs. HT) difference; Stage, main stage effect; S × C, stage × condition interaction. n = 53.
Prevalence of sarcopenia.
When the ALMi cut point of 5.67 kg/m2 was used (3, 27), the prevalence of sarcopenia was 7%, 3%, 30%, 27%, and 32% in premenopausal, early and late perimenopausal, and early and late postmenopausal women, respectively. The study-specific prevalence of sarcopenia (ALMi ≤ 5.31 kg/m2; ≥2 SD below premenopausal) was 0%, 0%, 10%, 11.5%, and 18.5% in premenopausal, early and late perimenopausal, and early and late postmenopausal women, respectively (Table 2).
Table 2.
Prevalence of sarcopenia
| Variable | Pre n = 30 |
EPeri n = 31 |
LPeri n = 30 |
EPost n = 26 |
LPost n = 27 |
|---|---|---|---|---|---|
| Below 5.31 kg/m2 of ALMia | |||||
| Sarcopenic, n (%) | 0 (0) | 0 (0) | 3 (10.0) | 5 (11.5) | 5 (18.5) |
| Below 5.67 kg/m2 of ALMib | |||||
| Sarcopenic, n (%) | 2 (6.7) | 1 (3.2) | 9 (30.0) | 7 (26.9) | 9 (33.3) |
n = no. of subjects. ALMi, appendicular lean mass adjusted by square of height (m2); EPeri, early perimenopausal; EPost, early postmenopausal; LPeri, late perimenopausal; LPost, late postmenopausal; Pre, premenopausal.
≥2 SD below the premenopausal group of the present study.
Dietary intake and physical activity.
We found no significant main effects of menopause stage on energy intake or carbohydrate, fat, or protein consumption (Table 3). The results were unchanged after adjusting for lean mass and total body mass (data not shown). There was no significant main effect of menopause stage on physical activity markers (i.e., pedometer or LTPA; Table 3).
Table 3.
Dietary intake and physical activity
| Variable | Pre | EPeri | LPeri | EPost | LPost | P Value |
|---|---|---|---|---|---|---|
| Dietary intake | ||||||
| Energy, kcal | 1,598 ± 697 | 1,943 ± 565 | 1,791 ± 452 | 1,864 ± 434 | 1,724 ± 478 | 0.445 |
| Carbohydrate, g | 185 ± 84 | 238 ± 89 | 217 ± 78 | 222 ± 76 | 198 ± 75 | 0.416 |
| Fat, g | 66 ± 35 | 72 ± 25 | 70 ± 21 | 73 ± 27 | 74 ± 23 | 0.937 |
| Protein, g | 71 ± 29 | 85 ± 23 | 77 ± 25 | 76 ± 23 | 71 ± 19 | 0.457 |
| Physical activity | ||||||
| Pedometer, steps/day | 5927 ± 2314 | 7364 ± 2491 | 6380 ± 2067 | 7231 ± 3535 | 7648 ± 2761 | 0.267 |
| LTPA, MET h/wka | 8 [4, 18] | 14 [8, 18] | 7 [5, 14] | 12 [6, 15] | 10 [6, 25] | 0.600 |
Data are means ± standard deviation or
median [interquartile range].
n of dietary intake = 85; n of pedometer = 98; n of leisure time physical activity (LTPA) = 117. EPeri, early perimenopausal; EPost, early postmenopausal; LPeri, late perimenopausal; LPost, late postmenopausal; MET, metabolic equivalent; Pre, premenopausal.
Associations.
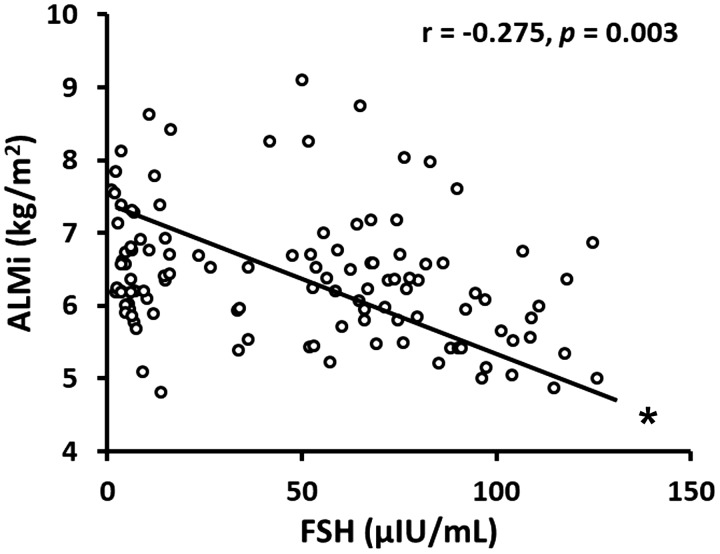
In the pooled population, ALMi was not correlated with estradiol concentrations (r = 0.09, P = 0.50) but was negatively correlated with FSH (r = −0.28, P = 0.003) and positively correlated with protein intake (r = 0.25, P = 0.02) and FMi (r = 0.50, P < 0.001) (Table 4). Within premenopausal women ALMi was negatively correlated with estradiol (r = −0.44, P = 0.04), whereas within perimenopausal women (early and late combined) FSH was negatively correlated with ALMi (r = −0.28, P = 0.046). ALMi was positively correlated with FMi in premenopausal (r = 0.55, P = 0.002), perimenopausal (r = 0.42, P = 0.001), and postmenopausal (r = 0.65, P < 0.001) women.
Table 4.
Correlates of appendicular lean mass index by menopausal categories
| ALMi Pooled |
ALMi Pre |
ALMi Peri |
ALMi Post |
|
|---|---|---|---|---|
| Age | −0.128 | 0.148 | −0.114 | −0.169 |
| Estradiol | 0.088 | −0.441* | 0.094 | 0.077 |
| Estrone | 0.115 | −0.261 | 0.037 | 0.283† |
| FSH | −0.275** | −0.011 | −0.281* | −0.289† |
| Progesterone | −0.025 | 0.164 | −0.054 | −0.180 |
| Testosterone | 0.105 | 0.050 | 0.079 | 0.121 |
| Dietary intake | 0.205† | 0.471 | 0.239 | 0.180 |
| Protein intake | 0.251* | 0.306 | 0.277† | 0.276 |
| LTPA | −0.049 | −0.192 | 0.035 | −0.065 |
| Pedometer | −0.133 | −0.090 | −0.089 | −0.136 |
| FMi | 0.501*** | 0.547** | 0.423** | 0.651*** |
| Partial correlations of ALMi with FSH: control variables | ||||
| Age | −0.215* | −0.037 | −0.272† | −0.270† |
| Estradiol | −0.281** | 0.003 | −0.267† | −0.281† |
| Estrone | −0.251** | −0.076 | −0.280* | −0.242 |
| Progesterone | −0.286** | 0.036 | −0.298* | −0.262† |
| Testosterone | −0.267** | 0.010 | −0.278† | −0.290† |
| Dietary intake | −0.297** | −0.401 | −0.322* | −0.282 |
| Protein intake | −0.282** | −0.452 | −0.313† | −0.261 |
| LTPA | −0.272** | −0.019 | −0.274† | −0.330* |
| Pedometer | −0.254* | 0.209 | −0.300† | −0.280† |
| FMi | −0.366*** | −0.018 | −0.284* | −0.234 |
ALMi, appendicular lean mass normalized to square of height in meters2; FMi, fat mass normalized to square of height in meters2; FSH, follicle-stimulating hormone; LTPA, leisure time physical activity; Peri, early and late perimenopausal; Post, early and late postmenopausal women including both hormone therapy users and nonusers; Pre, premenopausal; Pooled = Pre + Peri + Post. Significant values are in bold.
P ≤ 0.10;
P < 0.05;
P ≤ 0.01;
P ≤ 0.001.
The negative correlations between ALMi and FSH in the pooled population and within the peri- and postmenopausal groups were mostly maintained or tended to be significant after adjusting for age, estradiol, estrone, progesterone, testosterone, dietary intake, protein intake, LTPA, pedometer, and FMi (Table 4, Fig. 3). ALMi was strongly correlated with midthigh muscle area (CT analysis; r = 0.66, P < 0.0001).
Fig. 3.
Association. Follicle-stimulating hormone (FSH) vs. appendicular lean mass (ALM) index (ALMi, ALM adjusted by square of height). n = 144 healthy women (aged 30–70 yr). *Significant correlation.
DISCUSSION
The present study provides novel insights into potential factors that may contribute to sarcopenia in older women. To our knowledge, we are the first to demonstrate that the menopause transition, particularly the late perimenopausal stage, may be a vulnerable period for the loss of skeletal muscle mass, as indicated by a lower ALMi in late compared with early perimenopausal women. We found that the reduction in ALMi across menopausal stages was not associated with lower estradiol but rather with higher FSH levels. Moreover, early and late postmenopausal women who had previously used estrogen-based HT had a lower ALMi than women who never used estrogen-based HT.
Menopause and muscle mass.
It is becoming increasingly evident that menopause may impact muscle mass in women. Cross-sectional studies (2, 36, 39) have reported lower lean mass in post- compared with premenopausal women. However, ALMi across the menopausal transition has not been well characterized. The present study provides new information on ALMi across the stages of the menopause transition in healthy women. Compared with early perimenopausal women, ALMi was significantly lower in late perimenopausal and late postmenopausal women, with no significant differences among the other menopausal stages. It was surprising that ALMi was not significantly different in early postmenopausal compared with early perimenopausal women; however, the greater variability in ALMi in this group suggests diverse responses in muscle mass during the early postmenopausal years. This variability may be partially attributed to the fact that a third of the early postmenopausal women were prior HT users, and thus had not experienced prolonged estrogen deficiency at the time of the study. To our knowledge, no cross-sectional study has demonstrated a significant decline in ALMi from early to late perimenopause. Human experimental studies (30, 45) using gonadotropin-releasing hormone agonist (GnRHAG) treatments partially support our findings in that chronic (4–6 mo) suppression of ovarian hormones caused a decrease in total body lean mass in eugonadal women. Our group has also reported a decrease in total body lean mass in healthy premenopausal women with 5 mo of GnRHAG treatment (22). Because age and menopause could not be uncoupled in the present study, future investigations using an intervention approach (e.g., GnRHAG treatment) are necessary to isolate the effects of the loss of ovarian hormones from the effects of aging on ALM and the underlying mechanism(s) (e.g., skeletal muscle protein metabolism).
Menopause and prevalence of sarcopenia.
Sarcopenia has become one of the most important geriatric conditions and a key risk factor for the development of disability and frailty (16, 26). In a longitudinal comparison of 4,504 older adults, sarcopenic women were more likely than men to develop a physical disability (15), suggesting a critical role of sarcopenia in women’s health and quality of life. In the present study, the prevalence of sarcopenia (ALMi ≤ 5.67 kg/m2) was threefold higher in late perimenopausal and postmenopausal compared with premenopausal and early perimenopausal women. In most previous cross-sectional studies, the prevalence of sarcopenia was studied in women > 65 yr of age. Using an ALMi assessed by DXA in the Health Aging and Body Composition (Health ABC) study, Newman et al. (27) reported that the prevalence of sarcopenia (i.e., ALMi ≤ 5.67 kg/m2) was ~20% in older postmenopausal women (n = 1,549; aged 70–79 yr). Furthermore, Iannuzzi-Sucich et al. (11) found that the prevalence of sarcopenia [i.e., ALMi ≤ 5.45 kg/m2; ≥2 SD below the reference group (4)] was ~23% in women (n = 195; aged 75 ± 5 yr). The prevalence of sarcopenia reported in these studies was lower than that of the postmenopausal women in the present study (i.e., ~33%). When we used the study-specific sarcopenia criterion of ALMi ≤ 5.31 kg/m2 (≥2 SD below premenopausal), the prevalence of sarcopenia was reduced in each stage, and the prevalence of sarcopenia in the late postmenopausal group was comparable to previous studies (11, 27), suggesting that study-specific reference groups are essential to understanding the scope of sarcopenia. An interesting finding in the present study was the increasing prevalence of sarcopenia and decreased ALMi from early to late perimenopause, regardless of the sarcopenia criterion. This suggests that perimenopause is a vulnerable period for skeletal muscle mass.
Estradiol, FSH, and skeletal muscle mass.
Although estrogen deficiency is believed to be the most important factor contributing to loss of lean body mass with menopause, the evidence is mixed. Estrogen-based HT has been shown to preserve skeletal muscle and lean body mass in postmenopausal women (7, 34). In monozygotic postmenopausal twin dyads (31), women treated with estradiol-based HT for 7 yr had greater thigh muscle mass, muscle power, and mobility compared with their non-HT-using twin. Whole body fat-free mass was reduced in premenopausal women treated with 5 mo of GnRHAG (i.e., suppression of overall ovarian hormones) but not in those treated with GnRHAG plus estradiol add-back (22). Contrary to these findings, the present study found lower ALMi in HT users compared with nonusers in both early and late postmenopausal women. Moreover, there was no correlation between estradiol concentrations and ALMi in the pooled population, and ALMi was inversely correlated with estradiol only in the premenopausal women. Taken together, these findings clearly indicate the need for mechanistic studies to test whether estradiol is a catabolic or anticatabolic agent in muscle mass at different stages of the menopause transition.
FSH has recently been implicated as a potential mediator of metabolic actions traditionally attributed to the loss of estradiol. Using an antibody targeting the subunit of FSH, previous studies (38, 46) have suggested that blocking FSH increases bone mass in rodents. A recent paper by Liu et al. (20) expanded these findings to adipose tissues. They demonstrated that rodents treated with the FSH antibody had sharply reduced adiposity, increased brown adipose tissue, beiging of white adipocytes [e.g., enhanced uncoupling protein 1 (UCP1) expression and mitochondrial density], and increased resting energy expenditure. These findings raise the possibility that FSH also plays a critical role in skeletal muscle mass, one of the most metabolically active organs. To our knowledge, no human or clinical studies have reported whether the rise in FSH during the perimenopausal years is associated with the decline in skeletal muscle mass with aging in women. In a study conducted in healthy postmenopausal women (aged 48–65 yr), Garcia-Martin et al. (5) reported an inverse correlation between ALMi and FSH. Similar to the present study, they found no association between ALMi and estradiol concentrations. Our group (22, 33) has previously shown that ovarian suppression of both estradiol and FSH with GnRHAG treatment increased abdominal adiposity and decreased resting energy expenditure and bone mineral density in healthy premenopausal women. Thus, it is possible that beneficial effects of decreased FSH were counteracted, in part, by the unfavorable effects of decreased estradiol. The present study could stimulate paradigm-shifting research investigating the actions of other sex hormones, specifically FSH, on skeletal muscle mass and function in women.
Experimental considerations and potential limitations.
The aim of the study was to examine ALM across stages of the menopause transition and specifically examine the association with estradiol and FSH concentrations. It is recognized that the menopause transition is associated with changes in metabolic, inflammatory, and other factors that could influence ALM. However, it was beyond the scope of this secondary analysis to examine these or other parameters in the present study. The present study also has other important limitations that need to be considered. The first important limitation is the lack of physical function (e.g., muscle strength, mobility) measures to define sarcopenia, as suggested by the International Working Group on Sarcopenia (3). Although sarcopenia is most often associated with advanced age and mobility impairments (3), the present study provides provocative evidence for changes in muscle mass early in the menopause transition in women who are younger, generally healthy, and sedentary or recreationally active. Second, the present study was a secondary analysis of body composition data from an investigation of the biological mechanisms underlying cardiovascular dysfunction with aging and estrogen deficiency in healthy women (25). As such, the present study was not powered to detect differences in ALMi and to make correlations between ALMi and other variables, and the results should be interpreted cautiously. Third, the cross-sectional design precludes discussion of causality. Because menopause stage was determined by self-reported menstrual cycle characteristics, we cannot rule out the possibility that participants have been misclassified. However, circulating sex hormone levels for each of the menopausal stages were comparable to values reported by others (8). Fourth, the interpretation of a single cross-sectional measurement in ALMi is potentially limiting. Future longitudinal studies with a longer history of individual changes in body composition and sex hormones are needed. Fifth, as mentioned above, strength is an important component for defining sarcopenia, and we do not have a measure of strength. Previous studies (29, 32) partially support our finding in that muscle strength also declines around the time of menopause. Whether the late perimenopausal transition would be a vulnerable period for the loss of both skeletal muscle strength and mass should be investigated in future studies. Sixth, this study found that ALMi positively correlated with protein intake. This study focused more on the hormonal factors potentially contributing to muscle mass during the perimenopausal transition; thus we did not discuss in depth dietary protein intake. This is an important topic that should be studied in the future. Additionally, although we requested that all participants attend their study visits well hydrated, we did not measure hydration status per se, and thus it is possible that hydration status influenced the DXA measure. Finally, DXA measures bone-free lean tissue mass in the limbs (ALMi) as a surrogate marker for muscle mass. However, our additional CT analysis in a subgroup of participants showed that ALMi by DXA was strongly correlated with midthigh muscle area (r = 0.66, P < 0.0001).
Conclusions.
The menopausal transition appears to be a vulnerable period for the loss of ALMi that may begin during the late perimenopausal stage. The loss of ALMi was not associated with declines in estradiol but rather with elevated FSH levels. These data suggest that the menopausal transition may trigger mechanisms underlying sarcopenia in women. Thus, the perimenopausal years may be a critical time to introduce strategies that mitigate the changes in muscle mass that contribute to physical disability and frailty later in life. Potential mechanisms underlying menopause-related loss of muscle mass, including the role of FSH, should be explored in the future.
GRANTS
This work was supported by the NIH (R01 AG-027678 and AG-049762 to K.L.M., R01 AG-053489 to C.M.J., R56 HL-114073 to K.L.M., P50 HD-073063 to W.M.K., P30 DK-048520, and UL1 TR-001082) and the Department of Veterans Affairs (Eastern Colorado VA GRECC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.-M.P., C.O., K.L.H., W.M.K., and K.L.M. conceived and designed research; C.O., K.L.H., and K.L.M. performed experiments; Y.-M.P. and K.L.M. analyzed data; Y.-M.P., C.M.J., and K.L.M. interpreted results of experiments; Y.-M.P. prepared figures; Y.-M.P. drafted manuscript; Y.-M.P., C.M.J., C.O., K.L.H., W.M.K., and K.L.M. edited and revised manuscript; Y.-M.P., C.M.J., C.O., K.L.H., W.M.K., and K.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the staff of the University of Colorado Anschutz Medical Campus Energy Balance Core of the Colorado Nutrition and Obesity Research Unit (NORC) and Clinical Translational Research Center (CTRC) for assistance in conducting this study.
REFERENCES
- 1.Brown M, Birge SJ, Kohrt WM. Hormone replacement therapy does not augment gains in muscle strength or fat-free mass in response to weight-bearing exercise. J Gerontol A Biol Sci Med Sci 52A: B166–B170, 1997. doi: 10.1093/gerona/52A.3.B166. [DOI] [PubMed] [Google Scholar]
- 2.Douchi T, Yamamoto S, Nakamura S, Ijuin T, Oki T, Maruta K, Nagata Y. The effect of menopause on regional and total body lean mass. Maturitas 29: 247–252, 1998. doi: 10.1016/S0378-5122(98)00035-8. [DOI] [PubMed] [Google Scholar]
- 3.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M; International Working Group on Sarcopenia . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 83: 229–239, 1997. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Martin A, Reyes-Garcia R, Garcia-Castro JM, Munoz-Garach A, Escobar-Jimenez F, Munoz-Torres M. Gonadotropins are related to lean mass in healthy postmenopausal women. Endocr Res 38: 119–124, 2013. doi: 10.3109/07435800.2012.733987. [DOI] [PubMed] [Google Scholar]
- 6.Gozansky WS, Van Pelt RE, Jankowski CM, Schwartz RS, Kohrt WM. Protection of bone mass by estrogens and raloxifene during exercise-induced weight Loss. J Clin Endocrinol Metab 90: 52–59, 2005. doi: 10.1210/jc.2004-0275. [DOI] [PubMed] [Google Scholar]
- 7.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci 64A: 1071–1081, 2009. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab 92: 3060–3067, 2007. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 9.Hansen RD, Raja C, Baber RJ, Lieberman D, Allen BJ. Effects of 20-mg oestradiol implant therapy on bone mineral density, fat distribution and muscle mass in postmenopausal women. Acta Diabetol 40, Suppl 1: s191–s195, 2003. doi: 10.1007/s00592-003-0063-5. [DOI] [PubMed] [Google Scholar]
- 10.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86: 724–731, 2001. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 11.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 57: M772–M777, 2002. doi: 10.1093/gerona/57.12.M772. [DOI] [PubMed] [Google Scholar]
- 12.Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab 93: 4767–4773, 2008. doi: 10.1210/jc.2007-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankowski CM, Gozansky WS, MacLean PS, Shulman B, Wolfe P, Schwartz RS, Kohrt WM. N-acetyl-4-aminophenol and musculoskeletal adaptations to resistance exercise training. Eur J Appl Physiol 113: 1127–1136, 2013. doi: 10.1007/s00421-012-2529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, Van Pelt RE, Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab 91: 2986–2993, 2006. doi: 10.1210/jc.2005-2484. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc 54: 56–62, 2006. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889–896, 2002. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52: 80–85, 2004. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 18.Kenny AM, Dawson L, Kleppinger A, Iannuzzi-Sucich M, Judge JO. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J Gerontol A Biol Sci Med Sci 58: M436–M440, 2003. doi: 10.1093/gerona/58.5.M436. [DOI] [PubMed] [Google Scholar]
- 19.Kohrt WM, Barry DW, Van Pelt RE, Jankowski CM, Wolfe P, Schwartz RS. Timing of ibuprofen use and bone mineral density adaptations to exercise training. J Bone Miner Res 25: 1415–1422, 2010. doi: 10.1002/jbmr.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, Latif R, Thangeswaran P, Gupta A, Li J, Shnayder V, Robinson ST, Yu YE, Zhang X, Yang F, Lu P, Zhou Y, Zhu LL, Oberlin DJ, Davies TF, Reagan MR, Brown A, Kumar TR, Epstein S, Iqbal J, Avadhani NG, New MI, Molina H, van Klinken JB, Guo EX, Buettner C, Haider S, Bian Z, Sun L, Rosen CJ, Zaidi M. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546: 107–112, 2017. doi: 10.1038/nature22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohman TG, Roche AF, Martorell R (Editors). Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics, 1988. [Google Scholar]
- 22.Melanson EL, Gavin KM, Shea KL, Wolfe P, Wierman ME, Schwartz RS, Kohrt WM. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol (1985) 119: 975–981, 2015. doi: 10.1152/japplphysiol.00473.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau KL, Degarmo R, Langley J, McMahon C, Howley ET, Bassett DR Jr, Thompson DL. Increasing daily walking lowers blood pressure in postmenopausal women. Med Sci Sports Exerc 33: 1825–1831, 2001. doi: 10.1097/00005768-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause 13: 951–958, 2006. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- 25.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley JE, Kim MJ, Haren MT, Kevorkian R, Banks WA. Frailty and the aging male. Aging Male 8: 135–140, 2005. doi: 10.1080/13685530500277232. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB; Health ABC Study Investigators . Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 51: 1602–1609, 2003. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 28.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 29, Suppl: S1–S205, 1997. [PubMed] [Google Scholar]
- 29.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 84: 95–98, 1993. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 30.Revilla R, Revilla M, Villa LF, Cortés J, Arribas I, Rico H. Changes in body composition in women treated with gonadotropin-releasing hormone agonists. Maturitas 31: 63–68, 1998. doi: 10.1016/S0378-5122(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 31.Ronkainen PH, Kovanen V, Alén M, Pöllänen E, Palonen EM, Ankarberg-Lindgren C, Hämäläinen E, Turpeinen U, Kujala UM, Puolakka J, Kaprio J, Sipilä S. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol (1985) 107: 25–33, 2009. doi: 10.1152/japplphysiol.91518.2008. [DOI] [PubMed] [Google Scholar]
- 32.Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing 29: 235–242, 2000. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- 33.Shea KL, Gavin KM, Melanson EL, Gibbons E, Stavros A, Wolfe P, Kittelson JM, Vondracek SF, Schwartz RS, Wierman ME, Kohrt WM. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause 22: 1045–1052, 2015. doi: 10.1097/GME.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sørensen MB, Rosenfalck AM, Højgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res 9: 622–626, 2001. doi: 10.1038/oby.2001.81. [DOI] [PubMed] [Google Scholar]
- 35.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med 10: 843–848, 2001. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 36.Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP Jr. Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc 37: 1195–1202, 2005. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson ET, Davy KP, Seals DR. Hemostatic, metabolic, and androgenic risk factors for coronary heart disease in physically active and less active postmenopausal women. Arterioscler Thromb Vasc Biol 15: 669–677, 1995. doi: 10.1161/01.ATV.15.5.669. [DOI] [PubMed] [Google Scholar]
- 38.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell 125: 247–260, 2006. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 39.Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism 44: 369–373, 1995. doi: 10.1016/0026-0495(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol (1985) 83: 1947–1953, 1997. doi: 10.1152/jappl.1997.83.6.1947. [DOI] [PubMed] [Google Scholar]
- 41.Thompson FE, Byers T. Dietary assessment resource manual. J Nutr 124, Suppl: 2245S–2317S, 1994. doi: 10.1093/jn/124.suppl_11.2245s. [DOI] [PubMed] [Google Scholar]
- 42.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab 282: E1023–E1028, 2002. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]
- 43.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab 90: 4573–4578, 2005. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitson HE, Landerman LR, Newman AB, Fried LP, Pieper CF, Cohen HJ. Chronic medical conditions and the sex-based disparity in disability: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 65A: 1325–1331, 2010. doi: 10.1093/gerona/glq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamasaki H, Douchi T, Yamamoto S, Oki T, Kuwahata R, Nagata Y. Body fat distribution and body composition during GnRH agonist therapy. Obstet Gynecol 97: 338–342, 2001. doi: 10.1016/s0029-7844(00)01181-9. [DOI] [PubMed] [Google Scholar]
- 46.Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF, Sun L, Bian Z, Rosen C, Zallone A, New MI, Zaidi M. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci USA 109: 14574–14579, 2012. doi: 10.1073/pnas.1212806109. [DOI] [PMC free article] [PubMed] [Google Scholar]