Abstract
Adult CD34+ hematopoietic stem/progenitor cells (HSPC) in the systemic circulation are bone marrow-derived and have the propensity of maintaining cardiovascular health. Activation of angiotensin-converting enzyme-2 (ACE2)-angiotensin-(1–7)-Mas receptor pathway, the vascular protective axis of the renin-angiotensin system (RAS), stimulates vasculogenic functions of HSPCs. In a previous study, exposure to hypoxia increased the expressions of ACE2 and Mas, and stimulated ACE2 shedding. The current study tested if blood flow restriction exercise (BFR)-induced regional hypoxia recapitulates the in vitro observations in healthy adults. Hypoxia was induced by 80% limb occlusion pressure (LOP) via inflation cuff. Muscle oxygen saturation was determined using near-infrared spectroscopy. Peripheral blood was collected 30 min after quiet sitting (control) or after BFR. Lin−CD45lowCD34+ HSPCs were enumerated by flow cytometry, and ACE and ACE2 activities were determined in plasma and cell lysates and supernatants. Regional hypoxia resulted in muscle oxygen saturation of 17.5% compared with 49.7% in the control condition (P < 0.0001, n = 9). Circulating HSPCs were increased following BFR (834.8 ± 62.1/mL) compared with control (365 ± 59, P < 0.001, n = 7), which was associated with increased stromal-derived factor 1α and vascular endothelial growth factor receptor levels by four- and threefold, respectively (P < 0.001). ACE2 activity was increased in the whole cell lysates of HSPCs, resulting in an ACE2-to-ACE ratio of 11.7 ± 0.5 in BFR vs 9.1 ± 0.9 in control (P < 0.05). Cell supernatants have threefold increase in the ACE2-to-ACE ratio following BFR compared with control (P < 0.001). Collectively, these findings provide strong evidence for the upregulation of ACE2 by acute regional hypoxia in vivo. Hypoxic exercise regimens appear to be promising means of enhancing vascular regenerative capacity.
NEW & NOTEWORTHY Although many studies have explored the mechanisms of skeletal muscle growth and adaptation with hypoxia exercise interventions, less attention has been given to the potential for vascular adaptation and regenerative capacity. This study shows for the first time an acute upregulation of the angiotensin-converting enzyme 2 and increase in CD34+ vasculogenic cells following an acute bout of blood flow restriction with low-intensity exercise. These rapid changes collectively promote skeletal muscle angiogenesis. Therefore, this study supports the potential of hypoxic exercise interventions with low intensity for vascular and muscle health.
Keywords: angiotensin converting enzyme-2, blood flow restriction, CD34+ cells, hypoxia
INTRODUCTION
Vasoregenerative functions of bone marrow-derived CD34+ hematopoietic stem/progenitor cells (HSPCs) have been well documented in experimental and clinical settings, and these cells are quite often termed as endothelial progenitor cells (EPCs; see Ref. 1). CD34+ cells home to the areas of hypoxia or ischemia and stimulate angiogenesis or vasculogenesis by paracrine mechanisms (17, 43). Sensitivity to the hypoxia-regulated factors derived from ischemic injury, such as vascular endothelial growth factor (VEGF) and stromal-derived factor-1α (SDF), stimulate the vasoreparative functions of CD34+ cells, including migration, proliferation, vascular incorporation, and the release of paracrine factors (18). Optimal expression of C-X-C chemokine receptor type 4 (CXCR4), vascular endothelial growth factor receptor (VEGFR) 1, and VEGFR2 enables these cells to respond to the signals of hypoxia (13, 16). Conversely, hypoxic preconditioning increases the surface expression of CXCR4, VEGFR1, and VEGFR2, which may promote the vasoreparative functions of progenitor cells (14). In clinical conditions that are associated with impaired innate vasoreparative potential, hypoxic desensitization of the cells has been observed (3, 16, 18). We have recently shown that exposure of HSPCs to hypoxia stimulates vascular repair-relevant functions, proliferation, and migration and potentiate SDF- or VEGF-mediated responses (21).
Angiotensin-converting enzyme (ACE), the metabolite angiotensin II (ANG II) and angiotensin receptor type 1 (AT1) and type 2 receptors constitute the conventional renin-angiotensin system (RAS). ANG II produces cardiovascular detrimental effects (i.e., hypertension, hypertrophy, oxidative stress, etc.) largely by activating AT1 receptor (AT1R). In contrast, the recently identified ACE2, a monocarboxy peptidase, its product derived from ANG II, angiotensin-(1–7) [Ang-(1–7)], and Mas receptor (MasR) constitute the alternative axis of RAS. Ang-(1–7) produces cardiovascular protective functions largely by activating MasR (36). Previous studies have provided strong evidence for the expression of local RAS in bone marrow and for a regulatory role of angiotensin peptides in the hematopoietic functions in human and murine bone marrow cells (34, 35). We have previously shown that ACE2 and MasR are expressed in human and murine HSPCs, and the activation of this axis stimulates vasoreparative functions of the cells in health or diseases such as diabetes (15, 38). Furthermore, we have recently demonstrated that exposure to hypoxia increases the expression of ACE2 and MasR but not ACE or AT1R in human HSPCs in a hypoxia-inducible factor-1α (HIF-1α)-dependent manner at the transcriptional level, and increased protein expression results in ADAM17-dependent shedding of ACE2 fragment with catalytic activity (21). Therefore, hypoxia-induced vasoprotective functions are at least in part mediated by upregulation of the vasoprotective axis of RAS in HSPCs. Importantly, hypoxia-regulated factors, SDF or VEGF, also induce ACE2 or MasR independent of HIF-1α in this cell population (21). The current study extends these observations to an in vivo situation and tests the hypothesis that regional hypoxia induced by low-intensity exercise with blood flow restriction (6, 13) would stimulate mobilization of HSPCs and increase ACE2 levels in the circulation. Exercise with blood flow restriction has recently been implemented as a new clinical rehabilitative tool to improve musculoskeletal health (14) and warrants further investigation into the mechanistic changes resulting from the unique stimulus.
MATERIALS AND METHODS
Research design and participant characteristics.
Nine males between the ages of 18 and 40 yr were recruited to participate in a randomized crossover research study. Participants were included in the study if they were generally healthy as determined by health questionnaires (41) and were currently participating in both resistance and cardiovascular exercise for a minimum 3 days/wk for the past 6 mo. Exclusion criteria for the study included: body mass index >30 kg/m2; individuals who had smoked cigarettes regularly for the past 6 mo; previous pulmonary embolism; previous dizziness or fainting with blood draws or with the sight of blood; previous injuries to the neck, back, or legs; diagnosed cardiovascular disease; known sickle cell anemia/trait; recent surgery; orthostatic intolerance; and history of exertional rhabdomyolysis. Additional exclusions included those with a prior ligamentous, bony, or other soft tissue reconstruction to the lower extremity, endothelial dysfunction, peripheral vascular disease, diabetes, acute fracture, tumor, or infection, implanted medical devices, or the inability to consent. Females were excluded during this project given deep vein thrombosis has been identified as a hypothetical concern from irresponsible blood flow restriction (BFR) exercise, and oral contraceptive use can increase the risk of deep vein thrombosis (26b, 35a). Male participants also had their deep vein thrombus risk assessed using a risk assessment tool (see Ref. 4), and those classified as moderate or high risk were excluded.
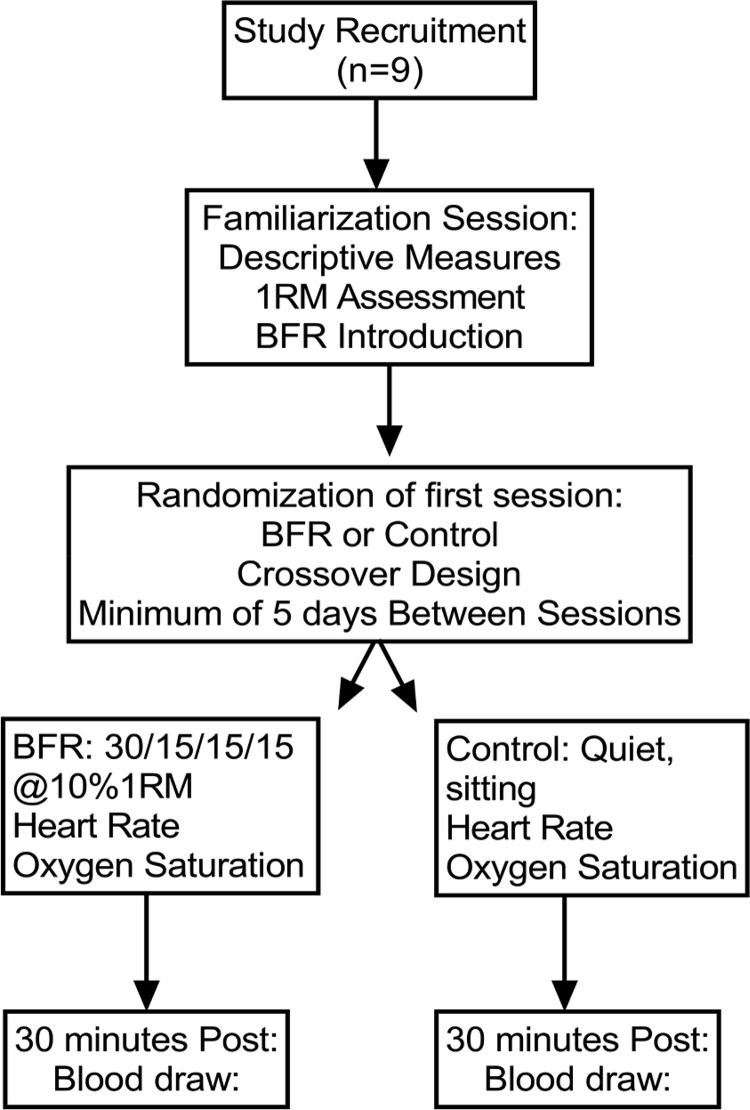
Following screening, all subjects completed a familiarization session where descriptive measurements were obtained (Table 1). Blood pressure was measured using a manual sphygmomanometer cuff and stethoscope. Height and weight were measured using a calibrated stadiometer (Seca 213, Chino, CA) and digital scale (Detecto, Webb City, MO), respectively. In this session, participants were introduced to BFR by a member of the research team, which included the determination of limb occlusion pressure (LOP) using the Delphi Personalized Tourniquet system (Delfi Medical Innovations, Vancouver, BC, Canada). Five to ten brief muscle contractions were completed with the cuff inflated to gain experience with the technique. Participants also completed a one-repetition maximum (1RM) strength assessment for knee extension and flexion after a short warm-up (5 min of low- to moderate-intensity cycling on an ergometer). The 1RM testing consisted of three to five submaximal attempts to get used to the resistance being lifted and the range of motion required. This was followed by increases (5–10 Nm) until the subject can no longer lift the resistance for the full range of motion. After a minimum of 48 h of recovery, subjects were randomized to complete a control session of quiet sitting (control) or BFR exercise session. In the control session, a venous blood sample (50 mL) was obtained under fasted conditions 30 min after 30 min of quiet sitting by a trained nurse at North Dakota State University Student Health Services. In the BFR exercise session, a venous blood sample (50 mL) was obtained under fasted conditions 30 min after BFR exercise at the same location. After completing the first session, participants crossed over and completed the remaining session. There were 6.4 ± 0.8 days between the BFR session and the control session. The protocols for control and BFR are described below. This study was approved by the Institutional Review Board and by the Institutional Biosafety Committee of North Dakota State University. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All subjects provided written consent before testing. The experimental protocol is shown in Fig. 1.
Table 1.
Participant descriptive measurements
| Age, yr | 22.0 ± 2.2 |
| Height, cm | 177 ± 6.3 |
| Body mass, kg | 73.5 ± 8.2 |
| BMI, kg/m2 | 23.1 ± 2.6 |
| Systolic blood pressure, mmHg | 117.1 ± 6.7 |
| Diastolic blood pressure, mmHg | 76.6 ± 6.3 |
| Thigh circumference, cm | 55.0 ± 3.7 |
| Limb occlusion pressure, mmHg | 207.1 ± 29,9 |
| 80% Limb occlusion pressure, mmHg | 165.3 ± 24.1 |
| Right leg knee extension 1RM, N·m | 141.1 ± 27.6 |
| Left leg knee extension 1RM, N·m | 142.2 ± 22.8 |
| Right leg knee flexion 1RM, N·m | 115.0 ± 25.4 |
| Left leg knee flexion 1RM, N·m | 113.3 ± 26.6 |
| Right leg knee extension exercise wt, N·m | 13.9 ± 2.9 |
| Left leg knee extension exercise wt, N·m | 14.1 ± 2.4 |
| Right leg knee flexion exercise wt, N·m | 11.4 ± 2.6 |
| Left leg knee flexion exercise wt, N·m | 11.2 ± 2.6 |
Values are means ± SD; n = 9 experiments. BMI, body mass index; 1RM, one repetition maximum. N·m, newton meter.
Fig. 1.
Experimental protocol in human subjects: Randomized crossover research study. All subjects completed both control and blood flow restriction (BFR) conditions separated by at least 5 days but not more than 7 days. RM, repetition maximum.
Induction of regional hypoxia by blood flow restriction exercise.
The order of control and BFR sessions was randomized for each participant. During both control and BFR sessions, participants were asked to refrain from lower body exercise the day before the study and any exercise the morning of the study. Participants reported to the laboratory in the morning (0700–0900). Upon arrival, participants were fitted with a heart rate monitor and strap (Polar, Bethpage, NY). In addition, A MOXY (Fortiori Design LLC, Hutchinson, MN) device was placed below the applied BFR cuff on the belly of the left vastus lateralis, midway between the greater trochanter and the lateral epicondyle of the femur and secured with adhesive athletic tape (27). In the control session, participants then rested in the seated position for 30 min as heart rate and muscle oxygen saturation were monitored and recorded. Participants were allowed to read books and perform basic activities on their phone or computer during the control session. In the BFR session, participants then proceeded to a cycle ergometer where they completed a 5-min warm-up at a self-paced moderate intensity. They were then fitted into the Biodex dynamometer using the same position and range of motion settings used during 1RM testing. They completed four sets of unilateral blood flow restriction exercise using the 30–15–15–15 repetition protocol. The intensity of the exercise was prescribed at 80% LOP using 10% of maximal knee extension-flexion strength. The cuff was deflated after each set of exercise to allow for increased blood flow and recovery. Heart rate and muscle oxygen saturation were monitored and recorded throughout the exercise session.
Determination of muscle oxygenation and hemodynamics.
Heart rates during control and BFR sessions were recorded using a Polar H7 Bluetooth strap (Polar, Bethpage, NY), saved to the Elite HRV smartphone application (elitehrv.com), and further analyzed for peak heart rate using Kubios software. Muscle oxygen saturation and total oxygenation and deoxygenation hemoglobin were recorded using the MOXY device, which was streamed in real time using Golden Cheetah software. Data were then extracted and averaged over the exercise period in Microsoft Excel.
Enumeration and isolation of HSPCs.
Peripheral blood mononuclear cells (MNCs) were separated by gradient centrifugation (800 g, 30 min) using Ficoll (Ficoll-Paque; GE Healthcare Biosciences). MNCs were collected and freed from plasma by resuspending the cells in PBS with 2% FBS and 1 mM EDTA, and centrifugation. MNCs were either used for analysis flow cytometry analysis of lineage-negative (Lin−) or Lin−CD45lowCD34+ HSPC populations, or for the enrichment of Lin− cell population. A negative selection kit (StemCell Technologies, Vancouver, BC, Canada) was used for the enrichment of Lin− cells by following the supplier's instructions. This kit depletes the following cell types: CD3, CD14, CD16, CD19, CD20, and CD56, all of which will be excluded by using magnetic microparticles. Freshly isolated Lin− cells were used for experiments involving exposure to normoxia or hypoxia. For enzyme activity assays, cell pellets were preserved at −80°C until use.
Flow cytometry.
Flow cytometry was carried out by suspending MNCs in the stain buffer and treated first with FcR blocking reagent (1:100; Miltenyi Biotech) followed by incubation with the fluorescent-conjugated antibodies (Biolegend) allophycocyanin anti-human lineage cocktail (1:500; Biolegend), phycoerythrin anti-human CD45 (1:500), and fluorescein isothiocyanate anti-human CD34 (1 in 250) or isotype control antibodies (1 in 500; Biolegend, San Diego, CA) for 45 min at 4°C. Dead cells were excluded using 7-AAD viability staining solution. Flow cytometry was carried out by using a C6 Accuri cytometer (BD).
ACE and ACE2 activities.
ACE and ACE2 activities were determined in the cell lysates or plasma and cell supernatants by using enzyme-specific fluorogenic substrates (ES005 for ACE and ES007 for ACE2; R&D Systems), as described previously (19). Enzyme-specific inhibitors, captopril and MLN-4760, were used to define ACE- and ACE2-specific enzyme activities, respectively, and enzyme-sensitive fluorescence was expressed as arbitrary fluorescence units per microgram of protein per hour. Chymostatin and Z-prolyl-prolinal are present in the assay buffer for ACE and ACE2 activities, respectively.
Western blotting.
Protein concentrations in plasma samples were determined by using bicinchoninic acid with bovine serum albumin as a standard (Thermo Fisher). Equal amounts of protein (30 µg) were loaded and separated by SDS-PAGE using SurePage 10% precasted gels (Genescript). Proteins were electroblotted on nitrocellulose membranes (Bio-Rad). The blots were blocked using 5% (wt/vol) milk in Tris-buffered saline containing 0.5% (vol/vol) Tween 20. The membranes were then incubated with different antibodies. The anti-ACE2 antibody recognized an epitope within the ectodomain of ACE2 (ab87436; Abcam). To assure that the proteins were transferred on the blot, MemCode staining (ThermoFisher) was used. HRP-conjugated goat anti-mouse (405–306; Biolegend) or donkey anti-rabbit (406–401; Biolegend) secondary antibodies were used at 1:20,000 dilution as recommended by the manufacturer. Primary antibodies were diluted in Tris-buffered saline containing (0.5% vol/vol) Tween 20 (TBS-T) supplemented with 5% milk, whereas secondary antibodies were diluted in TBS-T only. The bands were visualized by incubating the blots with enhanced chemiluminescence reagent (ECL, K15045-D50; Advansta) and developed on X-ray films (Phoenix Research Products). Band intensities were quantified by using Image J software [National Institutes of Health (NIH)].
Ponceau S staining was done by treating the nitrocellulose membranes with electroblotted proteins with Ponceau S stain solution (0.1% wt/vol Ponceau S in 5% acetic acid) for 5 min at room temperature. The membrane was rinsed with deionized water, and images of protein bands were obtained. Protein destaining was carried out by washing the membrane with 0.1 N NaOH solution in 1× TBS-T for 5 min followed by washing with deionized water. Protein bands were quantified by using ImageJ software (NIH). ACE2 protein levels were expressed relative to the corresponding Ponceau S staining of total protein band.
Statistical analysis.
Data are presented as mean values ± SE. Number of experiments (“n”) represents the number of donors used in the experiment. Statistical analyses were performed using GraphPad InStat 3.0 (GraphPad Software, San Diego, CA). Statistical differences in the mean were assessed using paired t-test or repeated-measures analysis of variance with a priori planned comparisons. Pearson correlations were performed using SPSS 26 software (IBM Corp., Armonk, NY). An alpha level of P < 0.05 was used to determine significance for all analyses.
RESULTS
Hemodynamics and muscle oxygenation in individuals experiencing hypoxia.
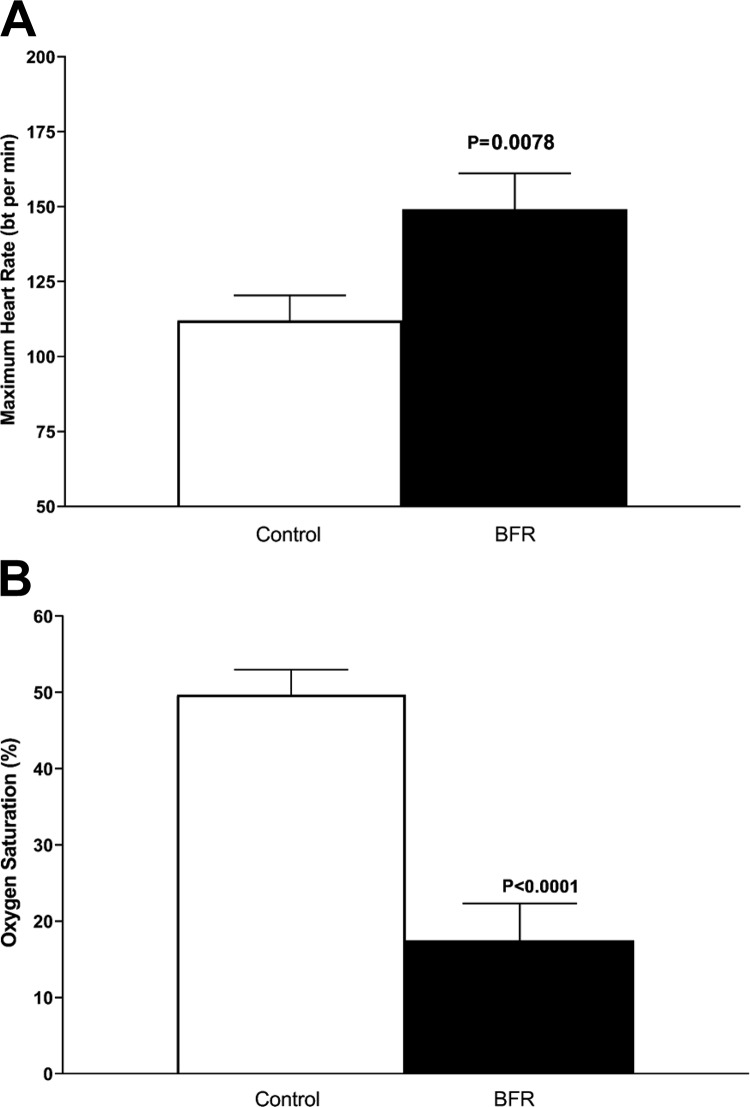
Maximal heart rate was significantly elevated in BFR compared with control (P = 0.008, n = 9, Fig. 2A). Additionally, muscle oxygen saturation decreased significantly in BFR compared with control (P < 0.0001, n = 9, Fig. 2B). Within the BFR session, total hemoglobin was significantly increased [F(1.45,9.79) = 36.93, n = 9, P < 0.0001]. Follow-up a priori established paired t-tests showed the increase was from preexercise to set 1 (P = 0.02), set 1 to set 2 (P = 0.006), and from set 2 to set 3 (P = 0.0004). Oxygenated hemoglobin declined during the BFR session [F(2.36,15.76), P < 0.0001, n = 9]. Follow-up a priori established paired t-tests showed the decline occurred from preexercise to set 1 (P < 0.0001) and from set 1 to set 2 (P < 0.0001). Similarly, deoxygenated hemoglobin increased during the BFR session [F(2.11,14.25) = 82.31, n = 9, P < 0.0001]. Follow-up a priori established paired t-tests showed the increase occurred form preexercise to set 1 (P < 0.0001) and from set 1 to set 2 (P < 0.0001). These data are summarized in Table 2.
Fig. 2.
Hemodynamics and muscle oxygenation. Maximum heart rate (A) and muscle oxygen saturation (B) during control and blood flow restriction (BFR) exercise [P < 0.001 vs. respective control group, n = 9 experiments (repeated-measures ANOVA with a priori planned comparisons)]. bt, Beat.
Table 2.
Changes in hemoglobin oxygenation with BFR across four sets of exercise
| BFR-Pre | Set 1 | Set 2 | Set 3 | Set 4 | |
|---|---|---|---|---|---|
| THb, g/dL | 12.72 ± 0.08 | 12.84 ± 0.07* | 12.97 ± 0.06* | 13.04 ± 0.06* | 13.08 ± 0.06 |
| Oxygenated Hb, g/dL | 6.04 ± 0.10 | 3.79 ± 0.19* | 1.56 ± 0.24* | 2.04 ± 0.32 | 2.28 ± 0.22 |
| Deoxygenated Hb, g/dL | 6.30 ± 0.17 | 9.05 ± 0.17* | 10.95 ± 0.21* | 10.99 ± 0.28 | 10.79 ± 0.24 |
Values are means ± SD; n = 9 experiments. BFR, blood flow restriction; THb, total hemoglobin; Hb, hemoglobin.
Significantly different from previous time point, P < 0.05.
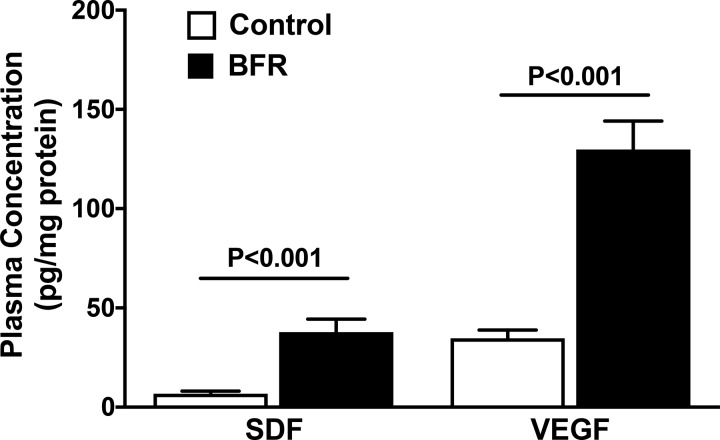
To ensure that the extent of hypoxia is sufficient to activate HIF-1α, the well-known hypoxia-regulated factors, SDF and VEGF, were analyzed in the circulation. Plasma samples derived from individuals following either control or BFR sessions were used for analysis. VEGF levels are higher than SDF in the circulation in control conditions; however, both were robustly increased following hypoxic exercise intervention (Fig. 3). SDF levels were elevated fourfold (P < 0.001) and VEGF levels threefold (P < 0.001) following BFR compared with that observed following control.
Fig. 3.
Circulating stromal-derived factor-1α (SDF) and vascular endothelial growth factor receptor (VEGF) levels following hypoxia. Plasma SDF and VEGF levels, expressed as pg/mg plasma protein, are increased in individuals that have performed blood flow restriction (BFR) exercise compared with the control group (P < 0.001 vs. respective control group, n = 5 experiments, paired t-test).
Regional hypoxia increases the number of circulating HSPCs.
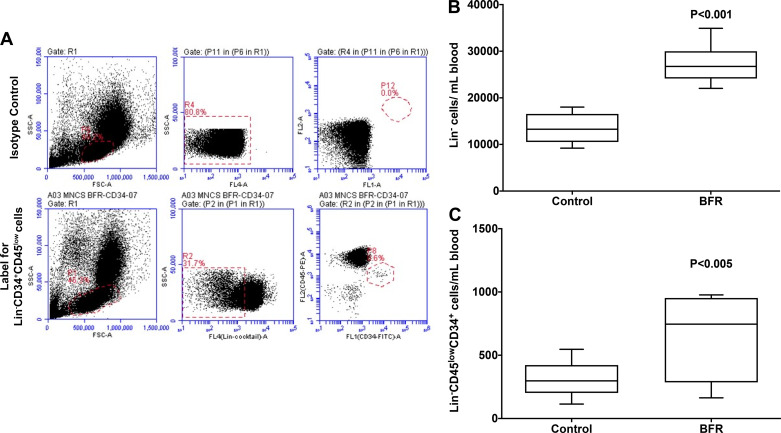
The number of HSPCs, both Lin− and Lin−CD45lowCD34+ populations, was enumerated in the blood samples that were collected half an hour following either control or BFR sessions. Shown in Fig. 4A is a representative flow cytometry dot plot for enumeration. Lin− cells were increased as expressed per milliliter blood in every individual following hypoxia [min-max: (9–18) × 103 cells/mL] compared with that observed following the control session [min-max: (21–36) × 103 cells/mL] (P < 0.001, n = 7, Fig. 4B). Along similar lines, Lin− CD45lowCD34+ were increased following hypoxic intervention (min-max: 114–546 cells/mL) compared with that following the control session (min-max: 163–977 cells/mL) (P < 0.005, n = 7, Fig. 4C.
Fig. 4.
Circulating hematopoietic stem/progenitor cells (HSPCs) following hypoxia: A: representative flow cytometric dot plots for enumeration of HSPC populations as defined by immune-phenotypic markers. Top, using isotype controls for defining gating strategy and for resolving HSPC populations; bottom, depicting resolution of CD45lowCD34+ and CD45high cell populations in the Lin− population. B: Lin− cell population is increased in the circulation in individuals following blood flow restriction (BFR) exercise compared with the control group (P < 0.001 compared with the control). C: Lin− CD45lowCD34+ cells are increased in the circulation in the BFR group compared with the control (P < 0.005 vs. control, n = 9 experiments, paired t-test).
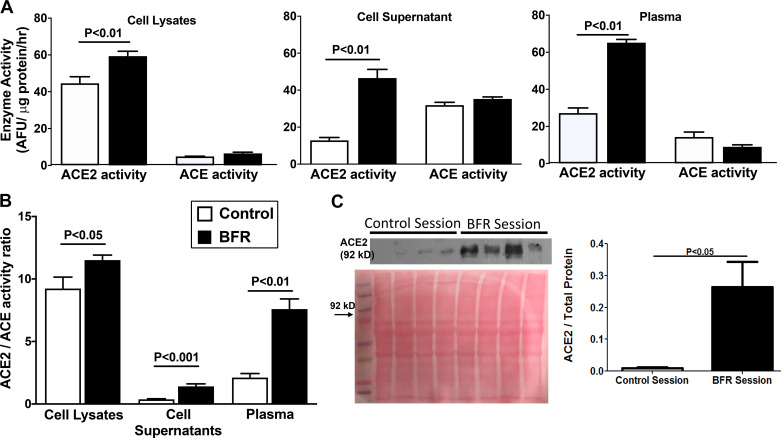
Hypoxia increases ACE2 activity in circulating HSPCs.
Enzyme activities of ACE and ACE2 were determined in Lin− cell lysates, plasma and Lin− cell supernatants that were collected following either control or BFR interventions. ACE2 activity is relatively higher than ACE in cell lysates (P < 0.001) and plasma samples (P < 0.05) but lower than ACE in cell supernatants (P < 0.05, n = 5) in control conditions (Fig. 5A). ACE2 levels in the supernatants indicate shedding of functional protein fragments from the cells as described above. BFR intervention increased ACE2 activity in all three samples compared with the control group; however, no significant changes were observed in ACE activity (Fig. 5A). Accordingly, the ACE2-to-ACE ratio is increased significantly in cell lysates (P < 0.05), cell supernatants (P < 0.001), and in plasma (P < 0.001) (n = 4) following hypoxia (Fig. 5B). Western blotting of ACE2 protein further confirmed the robust increase in the ACE2 activity (Fig. 5C). ACE2 protein levels are higher in the plasma following hypoxic intervention (P < 0.001, n = 4), which could be largely due to shedding of ACE2 from the circulating HSPCs (Fig. 5C).
Fig. 5.
Relative angiotensin-converting enzyme-2 (ACE2) and angiotensin-converting enzyme (ACE) activities in hematopoietic stem/progenitor cells (HSPCs) and plasma. A: enzyme activities of ACE2 and ACE in lysates of HSPCs, cell supernatants, or plasma samples in individuals undergoing control or blood flow restriction (BFR) interventions. ACE2 activity is relatively higher than ACE in cell lysates (P < 0.001) and plasma (P < 0.05) but lower than ACE in cell supernatants (P < 0.05) in control conditions (n = 5 experiments). ACE2 activity was increased in all three samples following BFR compared with the respective control groups (n = 5, paired t-test). B: ACE2-to-ACE ratio is higher in cell lysates, cell supernatants, and plasma in individuals following blood flow restriction (BFR) exercise compared with the control group. Significance level (P) is compared with the respective control group (n = 5, paired t-test). C: ACE2 protein levels in the circulation are increased following BFR exercise compared with control. Top, representative Western blot for ACE2 protein in plasma with Ponceau S red staining for total protein; bottom, summary of protein levels determined by Western blotting (P > 0.05 vs. control group, n = 5, paired t-test). AFU, arbitrary fluorescence units.
Correlations among independent and dependent variables.
All correlations are shown in Table 3. Muscle oxygen saturation was negatively correlated with the circulating Lin−CD45lowCD34+ cells, VEGF, SDF, ACE2, or ACE2/ACE in both plasma and cell supernatants (R = −0.84 to −0.92, P < 0.05). Positive correlation was observed between circulating Lin−CD45lowCD34+ cells and VEGF, SDF, or ACE2/ACE plasma, cell supernatants, or cell lysates (R = 0.71–0.83, P < 0.05). Plasma SDF or VEGF levels were positively correlated with ACE2/ACE in plasma only (R = 0.78, P < 0.01). ACE2/ACE in plasma was significantly and positively associated with ACE2/ACE in cell supernatants (R = 0.89, P < 0.01) further confirming that circulation ACE2 is largely derived from shedding of soluble ectodomain of cell-bound ACE2.
Table 3.
Correlation matrix among independent and dependent measures
| SmO2, % | Lin−CD45lowCD34+ Cells in Blood | Plasma VEGF | Plasma SDF | Plasma ACE2 | Plasma ACE2/ACE |
ACE2/ACE in Cell Supernatants | ACE2/ACE in Cell Lysates | |
|---|---|---|---|---|---|---|---|---|
| SmO2, % | −0.84** | −0.88** | −0.85** | −0.48 | −0.92** | −0.91** | −0.58 | |
| Lin−CD45lowCD34+ cells/mL blood | −0.84** | 0.83** | 0.70* | 0.52 | 0.77** | 0.71* | 0.78** | |
| Plasma VEGF | −0.88** | 0.83** | 0.95** | 0.58 | 0.80** | 0.63 | 0.50 | |
| Plasma SDF | −0.85** | 0.70* | 0.95** | 0.55 | 0.79** | 0.60 | 0.39 | |
| Plasma ACE2 | −0.48 | 0.52 | 0.58 | 0.55 | 0.65 | 0.47 | −0.02 | |
| Plasma ACE2/ACE | −0.92** | 0.76** | 0.80** | 0.79** | 0.65 | 0.89** | 0.52 | |
| ACE2/ACE in cell supernatants | −0.91** | 0.71* | 0.63* | 0.60 | 0.47 | 0.89** | 0.56 | |
| ACE2/ACE in cell lysates | −0.58 | 0.78** | 0.50 | 0.39 | −0.02 | 0.52 | 0.56 |
SDF, stromal-derived factor-1α; VEGF, vascular endothelial growth factor receptor; ACE2, angiotensin-converting enzyme-2; ACE, angiotensin-converting enzyme.
P < 0.05 and
P < 0.01.
DISCUSSION
This study reports several novel findings. Regional hypoxia in the lower limb induced by BFR exercise resulted in elevated circulating SDF and VEGF levels and rapidly induced mobilization of HSPCs in the blood stream from bone marrow. Increased ACE2 protein or activity in the circulation largely due to shedding of ACE2 ectodomain from circulating cells was observed following hypoxic exposure. Results indicate that increased vasoprotective enzyme ACE2 in the vasoreparative cells would rapidly stimulate vascular regeneration, which is likely to be an important underlying mechanism of enhanced myogenesis by hypoxic exercise intervention.
Previous studies that tested the effect of hypoxia on the mobilization of HSPCs in the blood circulation varied in the protocols of hypoxic intervention and the markers that were chosen to identify HSPC population with vasoreparative functions. Circulating CD34+ cells coexpressing VEGFR2, receptor for VEGF, were first reported to have the propensity of inducing vascular regeneration and termed as EPCs (1). Later on, several other markers, including CD133, CD45, VEGFR2, CXCR4, CD14, and CD31, have been used to characterize bone marrow-derived cells with vasculogenic functions in a variety of in vitro and in vivo experimental settings (2, 32, 33). However, clinical studies have clearly indicated a decrease in the number of circulating CD34+ cells is an independent risk factor for cardiovascular events and correlates with outcomes in metabolic syndrome and therefore appears to be the cell population for clinical therapeutics (8). CD34+VEGFR2+ is an equally efficient marker; however, these cells are very few in the circulation in lineage-negative fraction, and VEGFR2 is also present in the circulating endothelial cells. Clinical studies involving administration with the CD34+ cell population have shown evidence for therapeutic feasibility with successful outcomes in patients with critical limb ischemia (7, 9). The current study has specifically enumerated the Lin−CD45lowCD34+ population, which excludes circulating endothelial cells and CD45+ cells with no CD34 expression, and therefore identifies the bone marrow-derived HSPC population as highly relevant for cardiovascular health and for cell-based therapies for cardiovascular disorders.
An acute bout of exercise can be a potent stimulator of CD34+ cells; however, results in human investigations are mixed and are intensity/hypoxia regulated. For instance, Thijssen et al. (39) showed a trend toward an elevation in EPCs after a maximum cycling test. Furthermore, intensive (e.g., 30 min of running at 80% V̇o2max) and moderate (e.g., 30 min of running at 68% of V̇o2max) exercise upregulated EPCs 10 min postexercise while moderate short-term (e.g., 10 min of running at 68% V̇o2max) exercise did not show any change from baseline. “All out” maximal rowing exercise has also been shown to elevate CD34+ cells postexercise (24), which further emphasizes that exercise intensity and hypoxia are the primary factors to mobilize these cells. Unfortunately, not all individuals are able to perform exercise at high intensities/loads due to a variety of circumstances, such as their current cardiovascular health status, previous or current injury, physical environment, lack of access to equipment, or lack of motivation. Low-intensity BFR exercise may be a useful modality in the clinical setting (26); however, little is known about the mechanistic responses to BFR exercise as it pertains to vascular health (6), since the majority of work has been skeletal muscle related (13, 14). The BFR-induced stimulus in the present study resulted in muscle oxygen saturation of 17.5% compared with 49.7% in the control condition, suggesting regional hypoxia. Furthermore, regional hypoxia (muscle oxygen saturation, SmO2%) was negatively correlated with CD34+ HSPCs in circulation. Our results are comparable to a previous study that showed BFR exercise at 60% LOP did not increase HSPCs or CD34+ cells and may blunt the exercise-induced associated response (28). Given our data were compared with resting control and not low intensity exercise without cuff inflation, we cannot rule out this possibility. However, BFR has shown to significantly improve vascular endothelial function in the elderly (37) and tended to improve flow-mediated vasodilation in coronary artery disease patients (22); thus, our CD34+ cell data provide an additional mechanism for these physiological adaptations. Furthermore, this study found profound revelations pertaining to the ACE2-Ang-(1–7)-MasR pathway, which may be critical for future clinical applications of BFR exercise.
Detailed correlation analysis of the data revealed that muscle oxygen levels are negatively correlated with plasma levels of SDF or VEGF, as predicted, and the number of circulating CD34+ cells, ACE2, or ACE2/ACE but not ACE. On the other hand, positive correlation was observed between SDF or VEGF vs. the number of cells, ACE2, or ACE2/ACE. It is important to note that negative and positive correlations of ACE2/ACE with muscle oxygen levels and SDF/VEGF were observed in either plasma or cell supernatants but not in cell lysates, which is in agreement with our previous in vitro observations showing rapid shedding of ACE2 in the extracellular environment, thus leaving relatively lower levels of cellular membrane-bound ACE2 than predicted (21). Collectively, this analysis confirms that changes in the number of bone marrow-derived cells in circulation and ACEs are dependent on the level of hypoxia or hypoxia-regulated factors produced in response to BFR.
It has been well documented that the ACE2-Ang-(1–7)-MasR pathway is counterregulatory to the detrimental ACE-ANG II-AT1R axis and has been shown to be cardiovascular protective in several experimental settings (5). Recently, we have shown evidence for ACE2/ACE imbalance in HSPCs derived from aging or diabetic individuals (20). As shown in another study, in regards to the effect of in vitro hypoxic exposure on ACE2/ACE balance in HSPCs, the current study predicted in vivo hypoxic intervention such as blood flow restriction would enhance ACE2 expression and shedding. While increased ACE2 expression is mainly HIF-1α mediated, shedding of ACE2 is due to ADAM17, which could be upregulated by hypoxia (21, 40, 42). However, shedding of ACE2 from other cells such vascular endothelial cells cannot be ruled out in the present study. Our results confirmed the hypothesis and provided evidence for an important role of the protective axis of RAS in the vasculogenic and myogenic outcomes of hypoxic exercise interventions. While earlier studies in experimental animals showed compelling evidence for activation of the ACE2-Ang-(1–7)-MasR pathway by exercise training and for the associated cardiovascular benefits (10, 11), these were supported by genetic models showing that either ACE2 or MasR deficiency indeed prevents the cardiac benefits of exercise largely due to the overactive ACE-ANG II-AT1R axis (12, 29). However, no human studies have shown evidence for this concept with or without hypoxic intervention (31).
Earlier studies showed rapid increase in both transcription and translation of HIF-1α, VEGF, and VEGFR2, and factors that are involved in myogenesis and muscle repair, such as hepatocyte growth factor (HGF)-, cMet-, MyoD-, and Myf5-regulated factors (23, 25). These studies have used standard pressures (220 mmHg) combined with low-load resistance exercise at 40% maximum strength, and the molecular changes were observed at as early as 4 h postintervention and sustained for 24 h. Hypoxic response and increased VEGF and SDF independently upregulate ACE2 expression and shedding in HSPCs (21), which are also rapidly increased in the blood stream concurrently. Recent studies have provided strong evidence for the existence of local RAS in the skeletal muscle (11). The classical RAS pathway, ACE-ANG II-AT1R promotes muscle wasting via increasing inflammation and oxidative stress, partly via stimulating adiposity. In contrast, the ACE2-Ang-(1–7)-Mas pathway opposes the actions of classical RAS and restores myogenesis (30). Importantly, ACE2 deficiency prevents skeletal adaptations to exercise (29). Although this study does not have direct evidence, based on the available literature, upregulation of circulating ACE2 in concert with an increased number of circulating HSPCs stimulates vasculogenesis, which likely stimulates muscle vascularization and myogenesis. If proven by systematic studies, BFR would be an effective nonpharmacological intervention for individuals requiring muscle growth and cannot adhere to robust exercise regimens.
The small number of subjects involved in this study is a limitation, yet it provides the proof-of-concept that hypoxia regulates ACE2 expression in the in vivo setting and that BFR rapidly stimulates mobilization of vasculogenic progenitor cells. Furthermore, given the participants were only males, we, unfortunately, cannot extend our findings to include female participants. In addition, a more powerful study design could obtain the venous blood sample before and 30 min after BFR exercise instead of the quiet sitting. We chose not to use that design in the current study and instead use quiet sitting as our comparison given 50 mL blood drawn before BFR exercise may increase the chance of a participant getting lightheaded during BFR exercise given the additional shift from plasma into skeletal muscle (26a). Finally, the hypoxia protocol we used involved exercise at 10% of maximum strength at 80% of LOP; therefore, further systematic studies are required to determine the threshold of hypoxia, with or without concurrent muscular activity, necessary to induce mobilization and increase in ACE2 levels.
In conclusion, this study shows that regional hypoxia induced by very low-intensity resistance exercise with BFR rapidly stimulates mobilization of bone marrow HSPCs in the blood stream. This is associated with upregulation of the vasoprotective enzyme of RAS, ACE2. The increase in circulating ACE2 is largely due to the shedding of the ectodomain fragment from HSPCs. An increased number of vasculogenic progenitor cells and the circulating ACE2 would collectively stimulate vascular regeneration in the muscle.
GRANTS
This study was partly supported by National Institutes of Health (NIH) Grant AG-056881 and a North Dakota State University College of Human Sciences and Education Seed Grant. The Core Biology Facility at North Dakota State University was made possible by NIH Grant P30-GM-103332-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J. and Y.P.J. conceived and designed research; S.J., S.M., J.J., L.P., and K.J.H. performed experiments; S.J., S.M., J.J., and L.P. analyzed data; S.J., K.J.H., and Y.P.J. interpreted results of experiments; S.J., K.J.H., and Y.P.J. drafted manuscript; S.J. and Y.P.J. edited and revised manuscript; S.J., S.M., J.J., L.P., K.J.H., and Y.P.J. approved final version of manuscript; S.M. and J.J. prepared figures.
ACKNOWLEDGMENTS
We acknowledge the support from Shane McCullough and Nathan Dicks in data collection and efforts by staff at the Student Health Service, North Dakota State University, Fargo, North Dakota.
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol 26: 758–764, 2006. doi: 10.1161/01.ATV.0000203513.29227.6f. [DOI] [PubMed] [Google Scholar]
- 3.Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 56: 960–967, 2007. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caprini JA, Arcelus JI. State-of-the-art venous thromboembolism prophylaxis. Scope Phlembol Lymphol 1: 228–240, 2001. [Google Scholar]
- 5.Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol 91: 163–198, 2006. doi: 10.1016/j.pbiomolbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Downs ME, Hackney KJ, Martin D, Caine TL, Cunningham D, O’Connor DP, Ploutz-Snyder LL. Acute vascular and cardiovascular responses to blood flow-restricted exercise. Med Sci Sports Exerc 46: 1489–1497, 2014. doi: 10.1249/MSS.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 7.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 209: 10–17, 2010. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Fadini GP, Pagano C, Baesso I, Kotsafti O, Doro D, de Kreutzenberg SV, Avogaro A, Agostini C, Dorigo MT. Reduced endothelial progenitor cells and brachial artery flow-mediated dilation as evidence of endothelial dysfunction in ocular hypertension and primary open-angle glaucoma. Acta Ophthalmol 88: 135–141, 2010. doi: 10.1111/j.1755-3768.2009.01573.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res 116: 1361–1377, 2015. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 10.Frantz EDC, Giori IG, Machado MV, Magliano DC, Freitas FM, Andrade MSB, Vieira AB, Nóbrega ACL, Tibiriçá E. High, but not low, exercise volume shifts the balance of renin-angiotensin system toward ACE2/Mas receptor axis in skeletal muscle in obese rats. Am J Physiol Endocrinol Metab 313: E473–E482, 2017. doi: 10.1152/ajpendo.00078.2017. [DOI] [PubMed] [Google Scholar]
- 11.Frantz EDC, Prodel E, Braz ID, Giori IG, Bargut TCL, Magliano DC, Nobrega ACL. Modulation of the renin-angiotensin system in white adipose tissue and skeletal muscle: focus on exercise training. Clin Sci (Lond) 132: 1487–1507, 2018. doi: 10.1042/CS20180276. [DOI] [PubMed] [Google Scholar]
- 12.Guimarães GG, Santos SH, Oliveira ML, Pimenta-Velloso EP, Motta DF, Martins AS, Alenina N, Bader M, Santos RA, Campagnole-Santos MJ. Exercise induces renin-angiotensin system unbalance and high collagen expression in the heart of Mas-deficient mice. Peptides 38: 54–61, 2012. doi: 10.1016/j.peptides.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Hackney KJ, Downs ME, Ploutz-Snyder L. Blood Flow Restricted Exercise Compared to High Load Resistance Exercise During Unloading. Aerosp Med Hum Perform 87: 688–696, 2016. doi: 10.3357/AMHP.4566.2016. [DOI] [PubMed] [Google Scholar]
- 14.Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med 51: 1003–1011, 2017. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 15.Jarajapu YP, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R, Kent D, Stitt AW, Thut C, Finney EM, Raizada MK, Grant MB. Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes 62: 1258–1269, 2013. doi: 10.2337/db12-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarajapu YP, Caballero S, Verma A, Nakagawa T, Lo MC, Li Q, Grant MB. Blockade of NADPH oxidase restores vasoreparative function in diabetic CD34+ cells. Invest Ophthalmol Vis Sci 52: 5093–5104, 2011. doi: 10.1167/iovs.10-70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res 106: 854–869, 2010. doi: 10.1161/CIRCRESAHA.109.213140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarajapu YP, Hazra S, Segal M, Li Calzi S, Jadhao C, Qian K, Mitter SK, Raizada MK, Boulton ME, Grant MB. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS One 9: e93965, 2014. doi: 10.1371/journal.pone.0093965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi S, Balasubramanian N, Vasam G, Jarajapu YP. Angiotensin converting enzyme versus angiotensin converting enzyme-2 selectivity of MLN-4760 and DX600 in human and murine bone marrow-derived cells. Eur J Pharmacol 774: 25–33, 2016. doi: 10.1016/j.ejphar.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi S, Gomez S, Duran-Mendez M, Quiroz-Overa J, Garcia C, Jarajapu YP. Aging healthy, or with diabetes, is associated with ace2/ace imbalance in the hematopoitic stem progenitor cells (Abstract). FASEB J 33: 514.7, 2019. [Google Scholar]
- 21.Joshi S, Wollenzien H, Leclerc E, Jarajapu YP. Hypoxic regulation of angiotensin-converting enzyme 2 and Mas receptor in human CD34+ cells. J Cell Physiol 234: 20420–20431, 2019. doi: 10.1002/jcp.28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kambič T, Novaković M, Tomažin K, Strojnik V, Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front Physiol 10: 656, 2019. doi: 10.3389/fphys.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc 44: 2077–2083, 2012. doi: 10.1249/MSS.0b013e3182625928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Böhm M, Kindermann W, Nickenig G. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil 12: 407–414, 2005. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 25.Layne AS, Larkin-Kaiser K, MacNeil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Effects of blood-flow restriction on biomarkers of myogenesis in response to resistance exercise. Appl Physiol Nutr Metab 42: 89–92, 2017. doi: 10.1139/apnm-2016-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loenneke JP, Abe T, Wilson JM, Thiebaud RS, Fahs CA, Rossow LM, Bemben MG. Blood flow restriction: an evidence based progressive model (Review). Acta Physiol Hung 99: 235–250, 2012. doi: 10.1556/APhysiol.99.2012.3.1. [DOI] [PubMed] [Google Scholar]
- 26a.Loenneke JP, Fahs CA, Thiebaud RS, Rossow LM, Abe T, Ye X, Kim D, Bemben MG. The acute muscle swelling effects of blood flow restriction. Acta Physiol Hung 99: 400–410, 2012. doi: 10.1556/APhysiol.99.2012.4.4. [DOI] [PubMed] [Google Scholar]
- 26b.Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports 21: 510–518, 2011. doi: 10.1111/j.1600-0838.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 27.McManus CJ, Collison J, Cooper CE. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J Biomed Opt 23: 1–14, 2018. doi: 10.1117/1.JBO.23.1.015007. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery R, Paterson A, Williamson C, Florida-James G, Ross MD. Blood flow restriction exercise attenuates the exercise-induced endothelial progenitor cell response in healthy, young men. Front Physiol 10: 447, 2019. doi: 10.3389/fphys.2019.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motta-Santos D, Dos Santos RA, Oliveira M, Qadri F, Poglitsch M, Mosienko V, Kappes Becker L, Campagnole-Santos MJ, M Penninger J, Alenina N, Bader M. Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res 39: 506–512, 2016. doi: 10.1038/hr.2016.28. [DOI] [PubMed] [Google Scholar]
- 30.Nozato S, Yamamoto K, Takeshita H, Nozato Y, Imaizumi Y, Fujimoto T, Yokoyama S, Nagasawa M, Takeda M, Hongyo K, Akasaka H, Takami Y, Takeya Y, Sugimoto K, Mogi M, Horiuchi M, Rakugi H. Angiotensin 1-7 alleviates aging-associated muscle weakness and bone loss, but is not associated with accelerated aging in ACE2-knockout mice. Clin Sci (Lond) 133: 2005–2018, 2019. doi: 10.1042/CS20190573. [DOI] [PubMed] [Google Scholar]
- 31.Nunes-Silva A, Rocha GC, Magalhaes DM, Vaz LN, Salviano de Faria MH, Simoes E Silva AC. Physical exercise and ACE2-angiotensin-(1-7)-mas receptor axis of the renin angiotensin system. Protein Pept Lett 24: 809–816, 2017. doi: 10.2174/0929866524666170728151401. [DOI] [PubMed] [Google Scholar]
- 32.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95: 952–958, 2000. doi: 10.1182/blood.V95.3.952.003k27_952_958. [DOI] [PubMed] [Google Scholar]
- 33.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol 115: 186–194, 2001. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers K, Xiong S, DiZerega GS. Effect of angiotensin II and angiotensin(1-7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol 51: 97–106, 2003. doi: 10.1007/s00280-002-0509-4. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells 18: 287–294, 2000. doi: 10.1634/stemcells.18-4-287. [DOI] [PubMed] [Google Scholar]
- 35a.Sagar S, Stamatakis JD, Thomas DP, Kakkar VV. Oral contraceptives, antithrombin-III activity, and post-operative deep-vein thrombosis. Lancet 1: 509–511, 1976. doi: 10.1016/s0140-6736(76)90296-8. [DOI] [PubMed] [Google Scholar]
- 36.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu R, Hotta K, Yamamoto S, Matsumoto T, Kamiya K, Kato M, Hamazaki N, Kamekawa D, Akiyama A, Kamada Y, Tanaka S, Masuda T. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol 116: 749–757, 2016. doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- 38.Singh N, Joshi S, Guo L, Baker MB, Li Y, Castellano RK, Raizada MK, Jarajapu YP. ACE2/Ang-(1-7)/Mas axis stimulates vascular repair-relevant functions of CD34+ cells. Am J Physiol Heart Circ Physiol 309: H1697–H1707, 2015. doi: 10.1152/ajpheart.00854.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thijssen DH, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FC, Hopman MT, de Boer HC. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell 5: 495–503, 2006. doi: 10.1111/j.1474-9726.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang XJ, Feng CW, Li M. ADAM17 mediates hypoxia-induced drug resistance in hepatocellular carcinoma cells through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem 380: 57–66, 2013. doi: 10.1007/s11010-013-1657-z. [DOI] [PubMed] [Google Scholar]
- 41.Warburton DER, Jamnik VK, Bredin SSD, Glendhill B. The Physical Activity Readiness Questionanaire for Everyone (PAR-Q+) and Electronic Physical Activity Readiness Medical Examination (ePARmedX+). Health Fit J Can 4: 3–23, 2011. doi: 10.14288/hfjc.v4i2.103. [DOI] [Google Scholar]
- 42.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res 113: 78–87, 2013. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziebart T, Yoon CH, Trepels T, Wietelmann A, Braun T, Kiessling F, Stein S, Grez M, Ihling C, Muhly-Reinholz M, Carmona G, Urbich C, Zeiher AM, Dimmeler S. Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res 103: 1327–1334, 2008. doi: 10.1161/CIRCRESAHA.108.180463. [DOI] [PubMed] [Google Scholar]