Abstract
In vitro studies have shown that alterations in redox state can cause a range of opposing effects on the properties of the contractile apparatus in skeletal muscle fibers. To test whether and how redox changes occurring in vivo affect the contractile properties, vastus lateralis muscle fibers from seven healthy young adults were examined at rest (PRE) and following (POST) high-intensity intermittent cycling exercise. Individual mechanically skinned muscle fibers were exposed to heavily buffered solutions at progressively higher free [Ca2+] to determine their force-Ca2+ relationship. Following acute exercise, Ca2+ sensitivity was significantly decreased in type I fibers (by 0.06 pCa unit) but not in type II fibers (0.01 pCa unit). Specific force decreased after the exercise in type II fibers (−18%) but was unchanged in type I fibers. Treatment with the reducing agent dithiothreitol (DTT) caused a small decrease in Ca2+-sensitivity in type II fibers at PRE (by ∼0.014 pCa units) and a significantly larger decrease at POST (∼0.035 pCa units), indicating that the exercise had increased S-glutathionylation of fast troponin I. DTT treatment also increased specific force (by ∼4%), but only at POST. In contrast, DTT treatment had no effect on either parameter in type I fibers at either PRE or POST. In type I fibers, the decreased Ca2+ sensitivity was not due to reversible oxidative changes and may have contributed to a decrease in power production during vigorous exercises. In type II fibers, exercise-induced redox changes help counter the decline in Ca2+-sensitivity while causing a small decline in maximum force.
NEW & NOTEWORTHY This study identified important cellular changes occurring in human skeletal muscle fibers following high-intensity intermittent exercise: 1) a decrease in contractile apparatus Ca2+ sensitivity in type I but not type II fibers, 2) a decrease in specific force only in type II muscle fibers, and 3) a redox-dependent increase in Ca2+ sensitivity occurring only in type II fibers, which would help maintain muscle performance by countering the normal metabolite-induced decline in Ca2+ sensitivity.
Keywords: Ca2+ sensitivity, contractile apparatus, fatigue, high-intensity intermittent exercise, reactive oxygen species, troponin I
INTRODUCTION
Repeated or intense activity of skeletal muscle leads acutely to decreased muscle performance, referred to as muscle fatigue, owing to decreases in the Ca2+ sensitivity and maximum force production of the contractile apparatus and/or decreases in Ca2+ release from the sarcoplasmic reticulum (SR) (see Ref. 1 for review). These changes stem primarily from direct deleterious effects of the altered intracellular conditions, in particular increased inorganic phosphate and free Mg2+ concentrations and decreased pH, ATP and glycogen levels (1). In addition, exercise might acutely modify the underlying properties of the contractile apparatus or SR Ca2+ release process, either in a negative or positive way, by altering their redox or phosphorylation state or other aspect. These latter types of changes can be studied by “skinning” muscle fibers and examining the fiber properties under standardized intracellular conditions, thereby removing the strong confounding effects produced by direct actions of the altered intracellular conditions in fatigue.
Many types of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced during muscle contractions (8, 11, 23, 24, 28, 40, 42–44). The range of possible effects of redox alterations on the contractile apparatus is extremely diverse, and the effects can be reversible or irreversible. In vitro studies in rested muscle fibers from rodents and humans have shown that application of particular ROS (e.g., H2O2) can either reversibly increase or decrease the Ca2+ sensitivity of the contractile apparatus in type II [fast-twitch (FT)] fibers, depending on the duration of application, with little effect on maximum Ca2+-activated force, whereas nitric oxide (NO) seemingly only decreases the Ca2+ sensitivity (2–4, 14, 15, 26, 35, 36, 48). The increases and decreases in contractile Ca2+ sensitivity appear to be caused mainly by S-glutathionylation and S-nitrosylation, respectively, of a specific cysteine residue in the FT isoform of troponin I (TnIf) (14, 35). With longer and stronger exposures, ROS and RNS, however, can also cause irreversible decreases in both Ca2+ sensitivity and maximum force in both type I and type II fibers, depending on the particular species of ROS or RNS applied, the amount and duration of the exposure, and the activation state of the fiber (7, 10, 14, 26, 36, 41, 49).
Little is known about the acute effects of short-term exercise on the contractile apparatus properties in human muscle and in particular whether the contractile properties are appreciably modified by any of the many possible redox actions of the ROS and RNS generated during the exercise. Hvid et al. (20) examined the contractile properties of chemically skinned muscle fibers from the vastus lateralis muscle of highly trained athletes obtained before or ∼12 min or 24 h following a 4-h bout of strenuous cycling. It was found that the mean specific force in both type I and type II fibers was decreased by ∼10 to 15% immediately following the exercise, but specific force had recovered to the pre-exercise level following a 24-h rest period. Ca2+ sensitivity was also significantly decreased immediately after the exercise in type II fibers (pCa50, pCa at 50% maximum force, decreased by 0.07 pCa units) but was unchanged in type I fibers. The study did not specifically examine whether the observed effects were due to reversible redox changes. A later study by the same group (18) examined the contractile properties of chemically skinned fibers from biopsies of the triceps brachii muscle of elite cross-country skiers taken before and ∼10 min following four maximal bouts of treadmill skiing, with each bout lasting ∼4 min with 45 min rest in between. There was no significant change in the mean specific force between pre- and postexercise in either the type I or type II fibers, but the mean Ca2+ sensitivity was increased (mean pCa50 increased ∼0.07 pCa units) in both fiber types. A further set of fibers was subjected to strong reducing treatment with DTT before the contractile properties were examined, and in these cases the mean pCa50 was not significantly different between the fibers obtained pre- versus postexercise in either type I or type II fibers. These results appear to indicate that redox effects occurring during the exercise caused an increase in the Ca2+ sensitivity in both the type I and type II fibers of the subjects, which was reversed by the DTT treatment. However, it is possible that the apparent effect of the reducing treatment, particularly in the type I fibers, was actually due to fiber sampling variability, given that the pCa50 values with and without DTT treatment were determined in different pools of fibers, and unusually, the Ca2+ sensitivity (pCa50) in the particular pool of type I fibers sampled pre-exercise was relatively low, showing no significant difference from that in the type II fibers. The findings also differ from a recent study in exercising rats, where exercise was found to cause a reversible increase in Ca2+ sensitivity only in type II fibers (54), which was likely due to S-glutathionylation of TnIf (14, 35, 53), an effect specific to type II fibers.
To further investigate this, the present study examined whether the contractile properties in type I and type II fibers in young heathy and recreationally active humans were modified by repeated brief bouts of intense cycling exercise and, in particular, whether the fiber properties were modified by redox effects induced by the exercise. Biopsies were obtained from vastus lateralis muscle before and immediately after the exercise in each participant, and the contractile properties were examined in fibers freshly skinned by microdissection. To avoid possible problems with fiber sampling variability, the properties of each pre- and postexercise fiber were examined both before and after a strong reducing treatment with DTT. In this way, each fiber acted as its own control, which is a sensitive and accurate way to identify any effects of the reducing treatment. Furthermore, each fiber was subsequently subjected to a standardized S-glutathionylation treatment, as the Ca2+ sensitivity response to such treatment is an indicator of whether TnIf had undergone some irreversible oxidative change during the exercise (36). It was found that the exercise elicited a reversible redox-dependent increase in Ca2+ sensitivity only in type II fibers, an increase that would help counter the decrease in Ca2+ sensitivity occurring due to increased metabolite levels in the contracting fibers. The findings highlight an important compensatory redox action occurring in the fast-twitch muscle fibers in exercising humans and other mammals.
MATERIALS AND METHODS
Participant details and ethical approval.
All protocols and procedures were approved by the Human Research Ethics Committee at Victoria University. Informed consent was obtained in writing from all subjects, and the studies conformed to the standards set by the Declaration of Helsinki. All the experiments on human skinned fibers were performed on fibers obtained from vastus lateralis muscle biopsies from seven participants, comprising four males and three females (age: 27 ± 8 yr; height: 173 ± 11 cm; body mass: 77 ± 15 kg; means ± SD). All participants were healthy and recreationally active but were not involved in regular training.
High-intensity intermittent exercise.
Participants visited the laboratory on two occasions, with the two visits being scheduled within 2 to 7 days for all participants. During their first visit, participants completed a force-velocity test using the isoinertial method (45) on a custom-built bike ergometer equipped with instrumented cranks (Axis; Swift Performance Equipment). The mechanical signals recorded by the cranks were sampled at 100 Hz and processed offline to calculate average crank power (W), crank torque (N.m), and cadence (rpm) from all the pedal cycles completed by the participants during the force-velocity test. Participants performed a total of 79 ± 32 (SD) pedal revolutions during the force-velocity test. For each participant, a power versus cadence relationship was modeled using a third-order polynomial with a fixed y-intercept set at zero (45), using an average of 21 ± 5 data points. During their second visit, participants performed a high-intensity intermittent cycling exercise protocol on the same custom-built bike ergometer that consisted of a series of 15-s maximal efforts produced every 3 min. Cycling exercises were completed against a constant external resistance that was individually selected so that cadence would plateau between 130 and 150 rpm during their first 15-s maximal effort. Between each maximal effort, participants cycled at 80 rpm and 15% of the maximal power predicted at this cadence. Ratings of perceived exertion (RPE) were obtained using the original 6- to 20-point Borg scale (5). Maximal heart rate (HRmax) was estimated for each participant using the age-predicted equation proposed by Tanaka et al. (51) for healthy adults, i.e., 208 – (0.7 × age); HRmax was 189 ± 5 beats/min across participants. We continuously recorded heart rate (HR) during the cycling exercise using a Polar FT1 heart rate monitor system (Polar Electro Oy, Kempele, Finland). Both RPE values were recorded immediately after each sprint. The series of maximal efforts was stopped when participants reported an RPE value of >17, and HR was >150 beats/min (∼80% HRmax) immediately after a maximal effort. We computed average values of cadence (rpm) and crank power at the start (first 3 s) and end (last 3 s) and over the entire duration of the first and last 15-s maximal efforts completed by the participants. Crank power was expressed both in W/kg as well as in percentage of the maximal fatigue-free power calculated for the corresponding cadence, using results from the force-velocity test (17, 45).
Muscle biopsies.
One biopsy was taken at pre-exercise (PRE) and a second post-exercise (POST) from all participants. The protocol to collect the muscle biopsy was similar for both conditions. Briefly, after injection of a local anesthetic (1% lidocaine, Xylocaine; AstraZeneca, Macquarie Park NSW, Australia) into the skin and fascia, a small incision was made in the middle third of the vastus lateralis muscle of each subject and a muscle sample taken using a Bergström biopsy needle (34). The PRE and POST muscle biopsies were taken from the vastus lateralis of the same leg, with separate incisions ∼1 cm apart and from distal to proximal direction. An experienced medical practitioner took all biopsies at approximately constant depth and general location. The PRE and POST biopsies were obtained ∼10 min before and ∼1 min following the exercise, respectively. The excised muscle sample was rapidly blotted on filter paper to remove excess blood and placed in room temperature paraffin oil (Ajax Chemicals, Sydney, Australia), then gradually cooled to ∼10°C for 45 min before individual muscle fibers were dissected.
Fiber mounting and force recording.
The muscle biopsy was pinned at resting length in a petri dish lined with Sylgard 184 (Dow Corning, Midland, MI) and immersed in paraffin oil (Ajax Chemicals, Sydney, Australia) and kept cool (∼10°C) on an icepack. As described previously (12, 27, 29), segments of individual fibers were mechanically skinned using jeweler’s forceps and pinned out unstretched under oil, with the diameter being measured at three places along the fiber. Fiber cross-sectional area was calculated assuming an ellipsoidal profile, with dimensions corresponding to the largest and smallest diameter measurements. The skinned fiber was then mounted at 120% of resting length on a force transducer (AME801; Horten) with a resonance frequency of >2 kHz before being transferred to a 2-ml Perspex bath containing standard K+-based solution that broadly mimicked the intracellular milieu (see below). Force responses were recorded using a Bioamp pod and Powerlab 4/20 series hardware (ADInstruments, Sydney, Australia).
Skinned fiber solutions.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless specified otherwise. As described previously (26, 27, 29), the properties of the contractile apparatus were examined using a mixture of two heavily Ca2+-buffered solutions, namely the relaxing solution and the maximal Ca2+-activating solution. The relaxing solution contained (in mM) 50 EGTA, 90 HEPES, 10.3 total Mg2+ (giving 1 mM free), 126 K+, 36 Na+, 8 total ATP and 10 creatine phosphate, pH 7.10, and pCa ( = −log10[Ca2+]) ∼9. Maximal Ca2+-activating solution contained (in mM) 50 Ca-EGTA, 90 HEPES, 8.1 total Mg2+ (giving 1 mM free), 126 K+, 36 Na+, 8 total ATP and 10 creatine phosphate, pH 7.10, and pCa ∼4.7.
The relaxing solution and maximal Ca2+-activating solutions were mixed in appropriate ratios so as to produce a series of solutions with the free [Ca2+] heavily buffered over an intermediate range (pCa 6.7 to 4.7). In addition, a strontium-based solution (at pSr 5.2, pSr = −log10 [Sr2+]) was made by mixing relaxing solution with a maximal Sr-activating solution containing (mM): 40 SrEGTA, 10 EGTA, 90 HEPES, 8.5 Mg2+ (giving 1 mM free), 126 K+, 36 Na+, 8 ATP, 10 creatine phosphate, pH 7.10, and pSr ∼3.7. Where required, 10 mM dithiodithreitol (DTT) was added to relaxing solution from a 1 M stock prepared in distilled water. A 100 mM stock of reduced glutathione (GSH) was made in relaxing solution, with pH re-adjusted to 7.10 with KOH, and then diluted 20-fold to give 5 mM in the final relaxing solution. A 100 mM stock solution of 2,2′-dithiodipyridine (DTDP) was made in absolute ethanol and diluted 1,000-fold in the final relaxing solution to 100 μM. These stock solutions of DTT, GSH, and DTDP were all freshly prepared just before the experiment.
Force-Ca2+ relationship and analysis.
All measurements on skinned fibers were performed at room temperature (∼23 ± 1°C). The force-Ca2+ relationship in each individual muscle fiber was assessed by exposing the skinned fiber segment to a series of solutions, with the [Ca2+] strongly buffered at progressively higher levels (at pCa 6.7 to 4.7, the latter eliciting maximum force), and then the fiber was fully relaxed again in the relaxing solution. As described previously (30), this sequence was performed twice for each of the four different conditions: 1) control, before any treatment; 2) after 10 min of exposure to 10 mM DTT; 3) after S-glutathionylation treatment, by 2 min of exposure in 100 μM DTDP, followed by 2 min of exposure in 5 mM GSH, and finally, 4) after a further 10 min of exposure to DTT. The fiber was washed for 1 min in relaxing solution between the different conditions. This procedure allows verification of the reproducibility of the responses and also assessment of the small “rundown” occurring with repeated activation of the fiber (14, 30). Finally, each fiber was also tentatively assessed as being type I (slow-twitch) or type II (fast-twitch) according to its response to Sr2+ activation at pSr 5.2, so as to give a preliminary indication of the fiber type, which was subsequently checked by dot blotting of MHC (see below). Fibers containing the slow-twitch isoform of troponin C (TnC) give close to the maximum Ca2+-activated force level at pSr 5.2, whereas fibers containing the fast-twitch isoform of TnC produce <5% of maximum force, and fibers with a mixture of the fast and slow isoforms of TnC produce an intermediate level of force (6, 29, 30, 38).
Isometric force responses produced at each [Ca2+] within a given sequence were expressed as a percentage of the corresponding maximum force generated in that same sequence and analyzed by fitting a Hill curve using GraphPad Prism 6 software to ascertain values of pCa50 (pCa at half-maximum force) and the Hill coefficient (h) for each sequence. The maximum force reached during each sequence (at pCa 4.7) was expressed relative to the control level before any treatment in the given fiber after correcting for the small rundown occurring with each repetition of the sequence.
Fiber typing.
Dot blotting was subsequently performed to determine the fiber type of each muscle fiber segment examined, as described previously (9, 31). Briefly, PVDF membrane was activated with 95% ethanol and equilibrated in transfer buffer, and 1 µL of each sample was applied to the wet membrane and allowed to dry. The dry membrane was then reactivated with 95% ethanol, equilibrated in transfer buffer, washed in TBST for 5 min, and then placed in blocking buffer for 5 min. The presence of myosin heavy chain (MHC) types IIa and I were determined by sequential probing of the membrane with antibodies specific to MHC IIa (mouse monoclonal IgG, clone A4.74, Developmental Studies Hybridoma Bank (DSHB), 1 in 200 in 1% BSA/PBST) and MHC I (mouse monoclonal IgM, clone A4.840, DSHB, 1 in 200 in 1% BSA/PBST). Finally, the membrane was probed for MHC IIx (mouse monoclonal IgM, clone 6H1 DSHB, 1 in 100 in 1% BSA/PBST).
Statistics.
Values are presented as means ± SD (or ± SE where indicated), with n denoting the number of fibers examined and N the number of participants. Statistical significance (P < 0.05) was determined with two-tailed Student’s t-test with repeated measures unless specified otherwise. Pearson’s correlation analyses were performed with GraphPad Prism version 8 (La Jolla, CA).
RESULTS
High-intensity intermittent exercise performance.
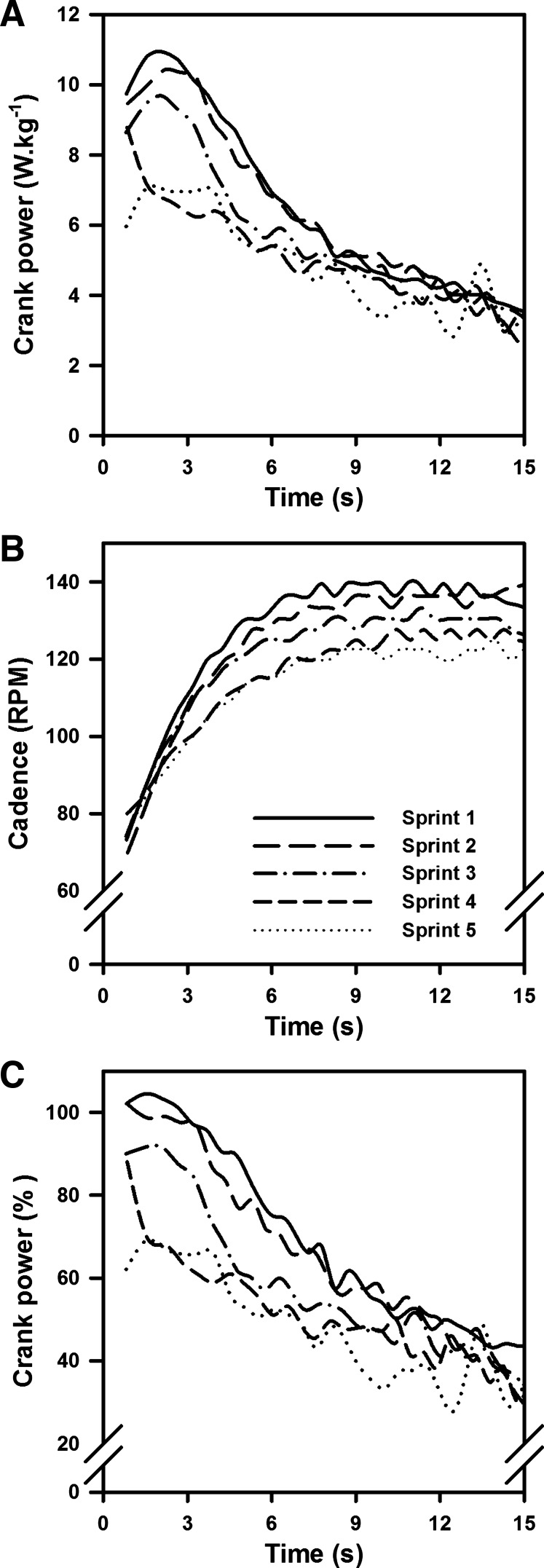
Results from the force-velocity test showed that all individual power versus cadence relationships were well described by third-order polynomial regressions (r2 = 0.940 ± 0.016; standard error of the estimate = 24.8 W), with participants producing maximal levels of crank power of 12.3 ± 3.2 W/kg or 963 ± 363 W at cadences of 116 ± 12 rpm. During the main experimental session, participants completed between three and seven 15-s maximal cycling efforts (see materials and methods). HR and RPE measured immediately after the maximal efforts increased between the first and the last efforts (HR: 150 ± 14 beats/min vs. 164 ± 17 beats/min, respectively; P < 0.05; RPE: 14.6 ± 3.5 vs. 19.1 ± 1.2; P < 0.001). For both the first and last maximal efforts, a significant decrease in cadence-specific relative levels of power were seen between the start and the end of the efforts (89.0 ± 2.8% vs. 49.6 ± 4.8%, P < 0.05; Fig. 1). Additionally, participants reached lower cadences at the end of the 15-s maximal efforts during the last effort compared with the first one (134 ± 6 rpm vs. 144 ± 5 rpm, respectively, P < 0.05), reducing the gap to their optimal cadences (i.e., 116 ± 12 rpm). However, we observed a significant reduction in power production at the end of the last maximal effort compared with the first one (5.0 ± 0.8 W/kg vs. 6.2 ± 0.9 W/kg, P < 0.001). Finally, the average cadence-specific relative levels of power calculated over the entire duration of the 15-s maximal effort were lower during the last maximal effort compared with the first one (23.6 ± 3.3% vs. 37.1 ± 5.0%, respectively, P < 0.05).
Fig. 1.
Representative changes in power production and cadence measured during each of the five 15-s maximal efforts performed by 1 female participant. A: crank power (expressed in W/kg) increased during the first portion of the maximal bouts before decreasing as participants reached the end of their maximal efforts, whereas a lower average level of power was measured during the last maximal effort compared with the first one. B: cadence increased to a plateau between the start and the end of each maximal effort, whereas lower plateau cadences were reached at the end of the last maximal effort, with cadence changes contributing to the variations in power production seen in A. C: crank power (expressed as %maximal power at the same cadence) decreased during each sprint, whereas successive sprints led to power decreases at the start of the next sprint.
Specific force and contractile properties of fibers.
Force responses were measured in a total of 37 skinned muscle fibers before exercise (PRE) and 52 muscle fibers following the exercise (POST). Subsequent dot blotting of MHC (see materials and methods) showed that the sample of PRE fibers consisted of 19 type I, 16 type II, and two “mixed” (type I/II) fibers, and the sample of POST fibers consisted of 26 type I, 22 type II, and four mixed fibers. All type II fibers were IIa or IIax, with no pure IIx. Results for the mixed fibers are not presented here because the proportions of MHC I and MHC II varied greatly between the different fibers; only results for “pure” type I or type II fibers are presented. The force response of contractile apparatus to the Sr2+ solution at pSr 5.2 (see materials and methods) was found to be fully in accord with the MHC typing in each fiber, with the TnC isoform evidently being largely or exclusively the slow isoform in all type I fibers and the largely or exclusively the fast isoform in all type II fibers, similar to our previous studies (12, 30).
The specific force (i.e., maximum Ca2+-activated force per unit cross-sectional area) and Ca2+ sensitivity of the contractile apparatus in each skinned fiber were assessed by activating each fiber in a series of solutions with the free [Ca2+] heavily buffered at progressively higher levels, from <1 nM up to 20 μM (i.e., pCa > 9 to pCa 4.7), as in Fig. 2. Specific force was examined in at least one type I and two type II fibers from each participant both pre- and postexercise (Fig. 3); note that each of these seven subjects showed similar decreases in average power output between their first and last maximal cycling efforts (see above). In the type II fibers the specific force was on average ∼18% lower at POST compared with PRE (P = 0.037, with similar results seen in fibers of all 7 participants), whereas the specific force in type I fibers was not significantly different before and after exercise (P = 0.803) (Fig. 3). On average, the cross-sectional area (CSA) of the POST type II fibers was ∼16% higher than in the PRE type II fibers (see Table 1), although this difference was not statistically significant, owing to the large spread in values between the different individual skinned fibers; in contrast, the average CSA of the type I fibers was very similar POST and PRE.
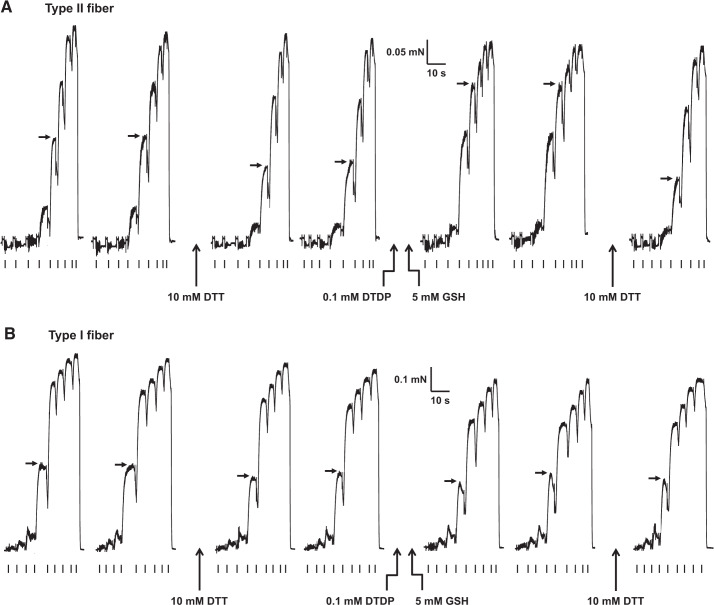
Fig. 2.
Effects of dithiothreitol (DTT) and 2,2′-dithiodipyridine (DTDP)-GSH exposure on maximal activated force and Ca2+ sensitivity of contractile apparatus in human vastus lateralis fibers. Representative force responses in a type II (A) and a type I fiber (B) (both postexercise) elicited by directly activating contractile apparatus with heavily Ca2+-buffered solutions with progressively higher free [Ca2+] (pCa of successive solutions: >9, 6.7, 6.4, 6.22, 6.02, 5.88, 5.75, 5.48, and 4.7, then back to >9, marked by ticks under each force trace). Force-pCa staircases elicited twice successively for each of four different conditions: 1) control; 2) after 10 min of exposure to 10 mM DTT; 3) after 2 min of exposure to 0.1 mM DTDP, followed by 2 min of exposure to 5 mM GSH; and 4) again after 10 min of exposure to DTT (only 1 force-pCa staircase shown). Fiber was washed in relaxing solution for 1 min between different conditions. Horizontal arrows show force levels produced at pCa 5.88 and pCa 6.02 in type II (A) and type I fiber (B), respectively, in the different conditions. Average Ca2+ sensitivities of contractile apparatus (pCa50) values in conditions 1–4 were 5.87, 5.83, 6.02, and 5.81, respectively, in type II fiber and 6.00, 5.99, 5.99, and 5.98, respectively, in type I fiber.
Fig. 3.
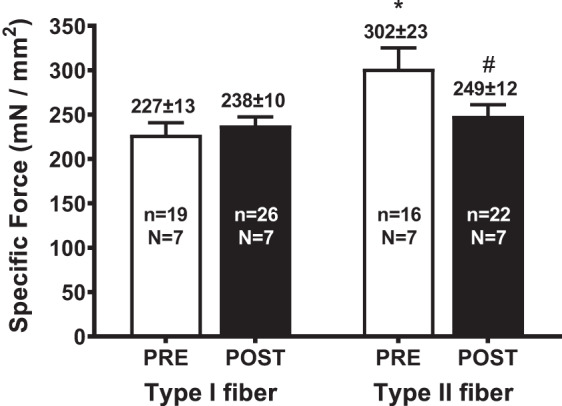
Means +SE of specific force in type I and type II fibers from pre- (POST) and postexercise (POST); specific force assessed by exposing skinned fiber to maximal activation solution; n denotes the no. of fibers and N the no. of participants from which biopsies were taken. *Value significantly different from type I fiber in matching condition; #value significantly different from PRE in same fiber type (Student’s 2-tailed t-test). Mean force and cross-sectional area for each case shown in Table 1.
Table 1.
Maximum force and diameter before and after exercise
| Type I |
Type II |
|||||
|---|---|---|---|---|---|---|
| PRE | POST | %Difference | PRE | POST | %Difference | |
| CSA, µm2 | 3,591 ± 283 | 3,607 ± 281 | +0.5% | 4,026 ± 359 | 4,685 ± 520 | +16.4% |
| Force, mN | 0.79 ± 0.06 | 0.83 ± 0.06 | +6.2% | 1.23 ± 0.15 | 1.16 ± 0.13 | −6.0% |
| n | 19 | 26 | 16 | 22 | ||
Values are means ± SE of maximum Ca2+-activated force and cross-sectional area (CSA) in single skinned fibers sampled pre- (PRE) and postexercise (POST) (n = no. of fibers). No significant difference between PRE and POST values in either fiber type.
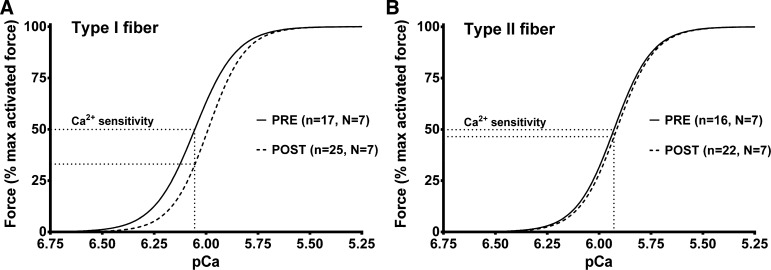
The Ca2+ sensitivity of the type I fibers was found to be lower at POST relative to PRE (pCa50 ∼0.06 pCa units lower, P = 0.008), whereas in type II fibers the Ca2+ sensitivity was not significantly different between POST and PRE (P = 0.440) (Fig. 4 and Table 2). The Hill coefficient (h) in the type I fibers at POST was on average slightly steeper than at PRE, whereas in type II fibers there was no difference (Table 2). As expected from previous work, before the exercise, type II fibers had a lower Ca2+ sensitivity (lower pCa50) and steeper h than type I fibers (Table 2).
Fig. 4.
Type I muscle fibers are less sensitive to Ca2+ following high-intensity intermittent exercise. Average force-Ca2+ relationship in type I (A) and type II fibers (B) from vastus lateralis muscle biopsies pre- (PRE) and postexercise (POST). Mean (± SE) of pCa50 (pCa at half-maximal force) of best-fit Hill curves for each individual fiber was 6.05 ± 0.02 in PRE and 5.98 ± 0.01 in POST (P < 0.05) for the type I fibers and 5.92 ± 0.01 in PRE and 5.91 ± 0.01 in POST for the type II fibers (not significantly different); corresponding h coefficient values, respectively, were 4.3 ± 0.3 and 4.8 ± 0.2 (P < 0.05) and 4.7 ± 0.2 and 4.8 ± 0.2.
Table 2.
Contractile apparatus properties before and after exercise
| Type I Fiber |
Type II Fiber |
|||
|---|---|---|---|---|
| Parameter | PRE | POST | PRE | POST |
| pCa50 | 6.05 ± 0.02 | 5.99 ± 0.01# | 5.92 ± 0.01* | 5.91 ± 0.01* |
| h | 4.3 ± 0.3 | 4.8 ± 0.2# | 4.7 ± 0.2* | 4.8 ± 0.2 |
| (n = 17, N = 7) | (n = 25, N = 7) | (n = 16, N = 7) | (n = 22, N = 7) | |
| ΔpCa50 DTT | −0.002 ± 0.004 | −0.002 ± 0.003 | −0.014 ± 0.002* | −0.035 ± 0.004*# |
| ΔFMax DTT (%) | −0.4 ± 0.2 | −0.6 ± 0.3 | 0.0 ± 0.8 | 4.2 ± 0.9*# |
| (n = 12, N = 6) | (n = 16, N = 7) | (n = 14, N = 6) | (n = 18, N = 7) | |
| ΔpCa50 S-Glut | 0.000 ± 0.002 | 0.005 ± 0.001 | 0.183 ± 0.004* | 0.179 ± 0.004* |
| (n = 2, N = 2) | (n = 7, N = 6) | (n = 15, N = 7) | (n = 15, N = 6) | |
Means ± SE of pCa50, Hill coefficient (h), and change (Δ) in pCa50 and maximum force (FMax) following dithiothreitol (DTT) treatment in type I and type II fibers and change following S-glutathionylation treatment (S-Glut) (as in Fig. 2); n denotes the no. of fibers and N the no. of subjects. PRE, pre-exercise; POST, postexercise. Values corrected for small decline in maximum force and pCa50 occurring upon repeated examination of force-pCa staircase, as gauged by values obtained by repeating controls and with bracketing treatments with DTT.
Value in POST significantly different from matching value in PRE;
value for type II fibers significantly different from that in type I fibers in matching condition (Student’s 2-tailed t-tests).
Effects of DTT and S-glutathionylation.
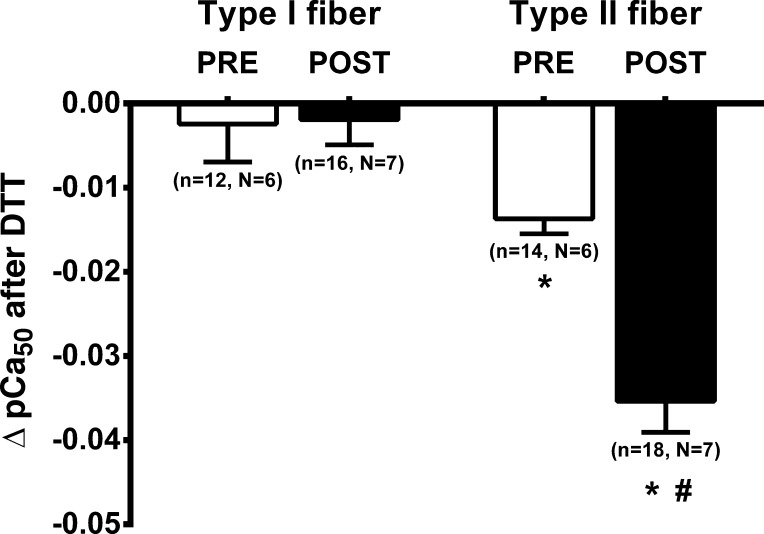
To examine whether the exercise had affected the contractile properties by some reversible oxidative modification, the properties were tested both before and after a 10-min strong reducing treatment in 10 mM DTT (e.g., see Fig. 2). In the PRE fibers, such DTT treatment had no significant effect on maximal force production in either type I or type II fibers (−0.4 ± 0.2% and 0.0 ± 0.8%, respectively). However, in the POST fibers, the reducing treatment increased maximal force production by ∼4% in the type II fibers (P = 0.003) but had no significant effect in the type I fibers (P = 0.723) (Table 2). Importantly, the DTT treatment also caused a significant decrease in the Ca2+ sensitivity in the type II fibers, with the decrease being substantially greater at POST than at PRE (P < 0.001; Fig. 5 and Table 2); similar results were seen in the fibers from all seven subjects. In contrast, the DTT treatment had no effect on the Ca2+ sensitivity in the type I fibers in either condition (P = 0.921).
Fig. 5.
Reducing treatment induces a larger decrease in Ca2+ sensitivity in type II muscle fibers following high-intensity intermittent exercise. Mean (and SE) of change (Δ) in Ca2+ sensitivity (pCa50) value following exposure to dithiothreitol (DTT) (e.g., see Fig. 3); n denotes the no. of fibers and N the no. of subjects from which the biopsies were taken. *Value is significantly different from the type I fiber in the matching condition; #postexercise (POST) value is significantly different from pre-exercise (PRE) value in same fiber type (Student’s 2-tailed t-test).
We have previously shown that treating mammalian type II fibers successively with the sulphydryl-specific oxidant DTDP (100 μM, 5 min) and then reduced glutathione (GSH; 5 mM, 2 min; e.g., Fig. 2), results in S-glutathionylation of the troponin I fast isoform (TnIf) (14), which induces a large increase in myofibrillar Ca2+ sensitivity (14, 30, 35). The increase in Ca2+ sensitivity induced by this treatment is seen only in type II fibers and not in type I fibers. In the present study, this S-glutathionylation treatment (applied after the fibers had been subjected to the first DTT reducing treatment; Fig. 2) was found to cause a very similar large increase in Ca2+ sensitivity in both the PRE (+0.183 pCa units) and POST type II fibers (+0.179 pCa units) (Table 2), which was fully reversed by treating the fibers with DTT again (e.g., Figure 2). In contrast, in the type I fibers, such S-glutathionylation treatment had very little or no effect in either condition (e.g., Figure 2; also see Table 2).
Finally, 1) the size of the decrease in pCa50 to DTT treatment and 2) the size of the increase in pCa50 to subsequent S-glutathionylation treatment in the type II POST fibers showed no apparent dependence of either parameter upon the length of time that the given fiber had been kept in the cool paraffin oil before being skinned and examined. Furthermore, Pearson’s correlation analysis of that data showed no significant relationship of either DTT treatment (r = −0.19, P = 0.44, n = 18) or S-glutathionylation treatment (r = −0.21, P = 0.47, n = 15) with time.
DISCUSSION
High-intensity intermittent exercise.
Irrespective of the exact number of maximal efforts they completed before reaching the exhaustion end point (between 3 and 7), each participant was able to successfully produce repeated high-intensity efforts, as shown by the near-maximal power levels of ∼90% elicited at the start of each 15-s maximal effort (45). During each 15-s maximal effort and in all participants, the levels of power markedly dropped to ∼50% during the last 3 s of the sprints (17). With the resistance kept constant across the maximal efforts, cadence was reduced by ∼10 rpm at the end of the last maximal effort relative to the first one. The decrease in cadence was accompanied by a >1 W/kg decrease in power production between the first and last maximal efforts, although the participants operated over a more favorable portion of their power-cadence relationship in terms of power production (closer to their optimal cadences) during their last maximal effort. Ultimately, the level of cadence-specific power was decreased by ∼15% at the end of the last maximal effort relative to the first one, evidencing an accumulation of fatigue that was expressed by participants who reported RPE values of ∼19 immediately following that last maximal effort. In view of the changes in joint powers reported across the hip, knee, and ankle joints after a similar 15-s maximal cycling effort (33), changes in the contractile properties of the vastii muscles likely made a large contribution to the decreases in power induced by our exercise protocol.
Changes in contractile properties with exercise.
To determine whether the underlying properties of the contractile apparatus were altered by the repetition of maximal cycling efforts, muscle fibers from biopsies obtained just before and immediately after the first and last 15-s maximal efforts, respectively, were skinned by microdissection and examined under set intracellular conditions to remove any direct effects of altered cytoplasmic metabolites on the fiber properties. It was found that the Ca2+ sensitivity in type I fibers postexercise was significantly lower (by ∼0.07 pCa units) than in the type I fibers obtained before exercise (Fig. 4), but the specific force was not significantly different (Fig. 3). The reason for the decrease in Ca2+ sensitivity in the type I fibers is unknown; it was evidently not due to reversible oxidative changes, as it was not reversed by DTT treatment (see Redox effects on contractile apparatus). It may have been the result of some structural change or damage in the fibers, or it might simply have been the result of sampling issues; in contrast, Gejl et al. (18) found an increase in Ca2+ sensitivity in type I fibers in trained athletes following repeated high-intensity exercise. In contrast to the type I fibers, in the type II fibers here, the Ca2+ sensitivity was not significantly different pre- and postexercise, but the specific force was ∼18% lower following the exercise (Figs. 3 and 4). Although the latter change outwardly seems like a profound reduction, it is likely that the functional effect in the subjects was far less pronounced. Here, it needs to be borne in mind that specific force is calculated as the maximum Ca2+-activated force divided by the fiber CSA. In the study here, the CSA of each fiber was measured under paraffin oil, with the fiber still in a similar state as it was in vivo when the biopsy was taken, with any exercise-generated metabolites still trapped within the fiber. During very intense exercise, there is a very large increase in inorganic phosphate levels within each type II fiber, owing to the breakdown of most of the creatine phosphate and ATP present in the cytosol (22), as well as the generation of large amounts of lactate ions (46), which together constitute a large increase in the number of osmotically active particles inside the muscle fiber. This increase causes the osmotically driven influx of extracellular water, leading to substantial fiber swelling, as seen by the ∼10–15% increase in intracellular water content in the quadriceps muscle of humans following exhaustive cycling exercise or maximal dynamic knee extensions (46, 47), which returns to the rested level only 20–30 min after the exercise. Such swelling has also been visualized directly in isolated Xenopus fibers, where single fiber cross-sectional area was increased ∼18% after forty 0.5-s tetani (32). Because the amount of creatine phosphate and ATP broken down (and lactate produced) during intense exercise is substantially higher in type II fibers than in type I fibers (22), it is expected that the extent of fiber swelling is substantially greater in the type II fibers. In the present study, the decrease in specific force seen in the type II fibers (Fig. 3) was largely attributable to such fiber swelling, given that the CSA of the type II fibers examined postexercise was on average ∼16% greater than in the pre-exercise fibers (Table1). (Note that the difference in mean CSA values did not reach significance simply because there was large variability in size of the individual fibers, but the specific force difference was significant because the force in each fiber was normalized to its own CSA.) Two previous studies that examined specific force in human muscle fibers before and after short-term intense exercise [Gejl et al. (18) (see introduction) and Place et al. (39)] did not find any significant change in specific force in either type I or type II fibers. However, in both studies the fibers were chemically skinned and kept for a prolonged period before the CSA of each fiber was measured in a standard solution, and so the values did not reflect the actual CSA of the fibers in vivo pre- and postexercise but did facilitate direct comparison of specific force under standardized conditions. In summary, it seems that although the specific force in type II fibers in vivo declines to a marked extent during very intense exercise, this is largely due to fiber swelling, and the absolute maximum force that each fiber can produce in standard conditions (i.e., in the absence of any effects of raised metabolites levels, etc.) is changed comparatively little. The swelling occurring in vivo does, however, have small direct deleterious effects on both contractile Ca2+ sensitivity and Ca2+ release (see Ref. 52), although it is possible that the swelling nevertheless might aid the speed of contraction by reducing internal drag of the contractile elements (16).
Redox effects on contractile apparatus.
It was evident, nevertheless, that the intense exercise did cause a small oxidation-dependent decrease (∼4%) in maximum force production in the type II fibers, which was reversed by the reducing treatment with DTT (Table 2). No such decrease was seen in the type I fibers. This inhibitory effect on maximum force production could have been due to action of one or more of the many ROS and RNS known to be generated during exercise and previously observed to have a depressing effect in vitro on maximum force production of the contractile apparatus (or myosin MgATPase rate), including superoxide and H2O2 (7, 26, 41), NO (37), and peroxynitrite (13, 49). The lack of effect in the type I fibers was possibly because antioxidant enzyme activity (e.g., superoxide dismutase activity and total glutathione level) is substantially higher in type I fibers than in type II fibers (e.g., ∼5-fold higher glutathione content in type I fibers) (19, 21).
Importantly, the present study further found that the intense exercise caused a reversible redox-dependent increase in the Ca2+ sensitivity of the contractile apparatus, but only in type II fibers (Fig. 5). This effect was evident from the decrease in Ca2+ sensitivity occurring with strong reducing treatment with DTT in every type II fiber. Although the size of the sensitivity shift found in the subjects here was comparatively small, it did produce a substantial (>10–15%) increase in the force elicited at submaximal Ca2+ levels (e.g., see force difference before and after DTT in Fig. 2A). It was also evident that there was a very small level of redox-enhancement of Ca2+-sensitivity (∼0.01 pCa units) in the type II fibers even before the exercise regime (Fig. 5), which has also been seen previously in type II fibers from nonexercised muscle in both young and old human subjects (30) and rats (54). In marked contrast to the type II fibers, the intense exercise regime used here did not elicit any reversible change in Ca2+ sensitivity in type I fibers (Fig. 5), with the DTT reducing treatment having no effect on the sensitivity either before or after exercise. These results in human fibers are all in close accord with recent findings in rat muscle before and after exercise, where DTT reversed an exercise-dependent increase in Ca2+ sensitivity in type II fibers but had no effect at all on Ca2+ sensitivity in type I fibers either before or after exercise (54).
The observed redox-dependent increase in Ca2+-sensitivity in the type II fibers here was most likely due to S-glutathionylation of Cys134 on TnIf, because 1) of all the redox-induced changes examined to date in muscle fibers in vitro, it is one of only two processes seen to induce an increase in Ca2+-sensitivity (26) and the only process to do so exclusively in type II fibers; and 2) such S-glutathionylation of TnIf is seen to occur with exercise in humans (35) and with in vivo stimulation of muscles in rats (53) and mice (25). Interestingly, the increase in Ca2+ sensitivity seen here with exercise was only ∼20% of the maximal increase in sensitivity occurring with S-glutathionylation of TnIf (∼0.035 vs. 0.18 pCa units; Table 2; and see Refs. 14 and 35) and less than half the size of the increase in sensitivity reported in type II fibers in the study by Gejl et al. (18) in trained athletes (∼0.08 pCa units) and in a study on rat muscle stimulated in vivo (53) (∼0.10 pCa units). The reasons for this are not known. It was not the result of some of the level of irreversible oxidation of TnIf (36) that had occurred during the intense exercise, because direct S-gluathionylation treatment (applied after first reversing any existing S-glutathionylation/S-nitrosylation by DTT treatment; Fig. 2) induced a similarly large increase in Ca2+ sensitivity in type II fibers obtained before or after the exercise (both ∼0.18 pCa units; Table 2). It is possible that there was some reversal of the increased sensitivity between the end of the exercise and the time the muscle biopsy was cooled down sufficiently to hinder any such reversal (although note that there was no evidence of any reversal occurring in the later period while the muscle preparation was maintained cool; see results). Certainly, the increase in Ca2+ sensitivity occurring in type II fibers of exercising rats persists to some extent in vivo for ≥1 h after the exercise, but it is fully reversed within 24 h (54). An alternative reason why only a relatively small increase in Ca2+ sensitivity was observed here might be because the experiments are reporting the net change in sensitivity, and it is possible that another reversible oxidative process occurring during the exercise was decreasing the Ca2+ sensitivity. This other oxidative process could have been acting on TnIf or instead on some entirely different target. Here, we note that NO donors produce a reversible decrease in Ca2+ sensitivity of the contractile apparatus (3, 14, 15, 48) due to S-nitrosylation of TnIf (14), with S-nitrosylation and S-glutathionylation acting competitively on the same cysteine residue. Thus, inhibitory effects of the increased NO levels produced in the skeletal muscle fibers during the exercise (40) may have partially countered the potentiating effect on the Ca2+ sensitivity of S-glutathionylation of TnIf in the subjects here. In this regard, it is interesting to note that the intensively exercising subjects in the present study were healthy and recreationally active but were not involved in regular training, whereas in the study by Gejl et al. (18), where a much larger increase in Ca2+ sensitivity was seen, the subjects were highly trained. In view of this, it would be interesting in a future study to use the experimental design employed here to directly compare the extent of the Ca2+ sensitivity increase occurring with different levels of exercise intensity in participants who are sedentary or recreationally active or highly trained, as a greater response in the highly trained participants could be an important part of the training response, possibly reflecting increased S-glutathionylation or decreased S-nitrosylation in the highly trained participants.
The redox-dependent increase in Ca2+-sensitivity in the type II fibers reported in this study is separate from and would act in addition to any Ca2+ sensitivity increase arising from phosphorylation of regulatory myosin light chain (see Ref. 50 for review). It seems likely that there would have been myosin light chain phosphorylation in the fibers of the exercising subjects here, although it is unclear whether this would have been still present at the conclusion of the exercise bouts, given that S-glutathionylation of TnIf, but no myosin light chain phosphorylation, was found at the end of a prolonged bout of intensive in vivo muscle stimulation in rats (53).
Conclusion.
This study examined the contractile properties of mechanically skinned muscle fibers freshly obtained from the vastus lateralis muscle of healthy young adults before and immediately after they performed an exhausting series of high-intensity cycling exercises. The properties of the fibers in each subject were examined on the day of exercise under controlled conditions, with the ATP, phosphate, and other intracellular constituents set close to the normal resting levels, so as to identify any changes in fiber properties occurring independently from those due to the accumulation of exercise-related metabolites. The study established that brief bouts of high-intensity cycling exercise in recreationally active humans elicit a substantial decrease in single-fiber-specific force, attributable largely to fiber swelling, and a reversible redox-dependent increase in Ca2+ sensitivity, but only in type II fibers. This increase in Ca2+ sensitivity would act to help counter the decrease in Ca2+ sensitivity that occurs due to raised metabolite levels in the contracting fibers (principally inorganic phosphate and H+). The findings give a consistent picture of what is likely an important compensatory redox action occurring in the fast-twitch muscle fibers of exercising humans and other mammals that acts to help minimize reduction in muscle performance in the face of unavoidable biochemical and ionic changes occurring in the fibers.
GRANTS
This project was supported by the National Health and Medical Research Council of Australia (grant nos. 1051460 and 1085331).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.L., D.M.R., T.L.D., M.J.M., and G.D.L. conceived and designed research; C.R.L., D.M.R., and T.L.D. performed experiments; C.R.L., D.M.R., T.L.D., and G.D.L. analyzed data; C.R.L., D.M.R., T.L.D., M.J.M., and G.D.L. interpreted results of experiments; C.R.L. and D.M.R. prepared figures; C.R.L., D.M.R., T.L.D., M.J.M., and G.D.L. drafted manuscript; C.R.L., D.M.R., T.L.D., M.J.M., and G.D.L. edited and revised manuscript; C.R.L., D.M.R., T.L.D., M.J.M., and G.D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for their time in completing the study, and we also thank Maria Cellini and Heidy Latchman for technical assistance. The monoclonal antibodies directed against adult human MHC isoforms (A4.840, A4.74, and 6H1) used in the present study were developed by Dr. H. Blau and obtained from the Development Studies Hybridoma Bank under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol 509: 577–586, 1998. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J 15: 309–311, 2001. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- 5.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2: 92–98, 1970. [PubMed] [Google Scholar]
- 6.Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM. MHC isoform composition and Ca2+- or Sr2+-activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol 279: C1564–C1577, 2000. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- 7.Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol (1985) 90: 45–54, 2001. doi: 10.1152/jappl.2001.90.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AJ, Yamada T, Rassier DE, Andersson DC, Westerblad H, Lanner JT. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J Physiol 594: 5149–5160, 2016. doi: 10.1113/JP270650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen D, MacInnis MJ, Zacharewicz E, Xu H, Frankish BP, Murphy RM. A fast, reliable and sample-sparing method to identify fibre types of single muscle fibres. Sci Rep 9: 6473, 2019. doi: 10.1038/s41598-019-42168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darnley GM, Duke AM, Steele DS, MacFarlane NG. Effects of reactive oxygen species on aspects of excitation-contraction coupling in chemically skinned rabbit diaphragm muscle fibres. Exp Physiol 86: 161–168, 2001. doi: 10.1113/eph8602109. [DOI] [PubMed] [Google Scholar]
- 11.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982. doi: 10.1016/S0006-291X(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 12.Dutka TL, Lamboley CR, McKenna MJ, Murphy RM, Lamb GD. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J Appl Physiol (1985) 112: 728–736, 2012. doi: 10.1152/japplphysiol.01331.2011. [DOI] [PubMed] [Google Scholar]
- 13.Dutka TL, Mollica JP, Lamb GD. Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J Appl Physiol (1985) 110: 705–716, 2011. doi: 10.1152/japplphysiol.00739.2010. [DOI] [PubMed] [Google Scholar]
- 14.Dutka TL, Mollica JP, Lamboley CR, Weerakkody VC, Greening DW, Posterino GS, Murphy RM, Lamb GD. S-nitrosylation and S-glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am J Physiol Cell Physiol 312: C316–C327, 2017. doi: 10.1152/ajpcell.00334.2016. [DOI] [PubMed] [Google Scholar]
- 15.Dutka TL, Mollica JP, Posterino GS, Lamb GD. Modulation of contractile apparatus Ca2+ sensitivity and disruption of excitation-contraction coupling by S-nitrosoglutathione in rat muscle fibres. J Physiol 589: 2181–2196, 2011. doi: 10.1113/jphysiol.2010.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204: 3201–3208, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Gardner AS, Martin DT, Jenkins DG, Dyer I, Van Eiden J, Barras M, Martin JC. Velocity-specific fatigue: quantifying fatigue during variable velocity cycling. Med Sci Sports Exerc 41: 904–911, 2009. doi: 10.1249/MSS.0b013e318190c2cc. [DOI] [PubMed] [Google Scholar]
- 18.Gejl KD, Hvid LG, Willis SJ, Andersson E, Holmberg HC, Jensen R, Frandsen U, Hansen J, Plomgaard P, Ørtenblad N. Repeated high-intensity exercise modulates Ca(2+) sensitivity of human skeletal muscle fibers. Scand J Med Sci Sports 26: 488–497, 2016. doi: 10.1111/sms.12483. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi M, Cartier LJ, Chen M, Holloszy JO. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol 40: 281–286, 1985. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- 20.Hvid LG, Gejl K, Bech RD, Nygaard T, Jensen K, Frandsen U, Ørtenblad N. Transient impairments in single muscle fibre contractile function after prolonged cycling in elite endurance athletes. Acta Physiol (Oxf) 208: 265–273, 2013. doi: 10.1111/apha.12095. [DOI] [PubMed] [Google Scholar]
- 21.Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol (1985) 73: 1854–1859, 1992. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- 22.Karatzaferi C, de Haan A, Ferguson RA, van Mechelen W, Sargeant AJ. Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflugers Arch 442: 467–474, 2001. [Erratum in: Pflugers Arch 442: 475, 2001]. doi: 10.1007/s004240100552. [DOI] [PubMed] [Google Scholar]
- 23.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature 372: 546–548, 1994. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 24.Kolbeck RC, She ZW, Callahan LA, Nosek TM. Increased superoxide production during fatigue in the perfused rat diaphragm. Am J Respir Crit Care Med 156: 140–145, 1997. doi: 10.1164/ajrccm.156.1.9610041. [DOI] [PubMed] [Google Scholar]
- 25.Kramer PA, Duan J, Gaffrey MJ, Shukla AK, Wang L, Bammler TK, Qian WJ, Marcinek DJ. Fatiguing contractions increase protein S-glutathionylation occupancy in mouse skeletal muscle. Redox Biol 17: 367–376, 2018. doi: 10.1016/j.redox.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol 546: 149–163, 2003. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb GD, Stephenson DG. Measurement of force and calcium release using mechanically skinned fibers from mammalian skeletal muscle. J Appl Physiol (1985) 125: 1105–1127, 2018. doi: 10.1152/japplphysiol.00445.2018. [DOI] [PubMed] [Google Scholar]
- 28.Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589: 2119–2127, 2011. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamboley CR, Murphy RM, McKenna MJ, Lamb GD. Endogenous and maximal sarcoplasmic reticulum calcium content and calsequestrin expression in type I and type II human skeletal muscle fibres. J Physiol 591: 6053–6068, 2013. doi: 10.1113/jphysiol.2013.265900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD. Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593: 2499–2514, 2015. doi: 10.1113/JP270179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamboley CR, Xu H, Dutka TL, Hanson ED, Hayes A, Violet JA, Murphy RM, Lamb GD. Effect of androgen deprivation therapy on the contractile properties of type I and type II skeletal muscle fibres in men with non-metastatic prostate cancer. Clin Exp Pharmacol Physiol 45: 146–154, 2018. doi: 10.1111/1440-1681.12873. [DOI] [PubMed] [Google Scholar]
- 32.Lannergren J. Volume changes of isolated Xenopus muscle fibres associated with repeated tetanic contractions (Abstract). J Physiol 420: 116P, 1990. [Google Scholar]
- 33.Martin JC, Brown NA. Joint-specific power production and fatigue during maximal cycling. J Biomech 42: 474–479, 2009. doi: 10.1016/j.jbiomech.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 34.McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol 576: 279–288, 2006. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol 590: 1443–1463, 2012. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy RM, Dutka TL, Lamb GD. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol 586: 2203–2216, 2008. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nogueira L, Figueiredo-Freitas C, Casimiro-Lopes G, Magdesian MH, Assreuy J, Sorenson MM. Myosin is reversibly inhibited by S-nitrosylation. Biochem J 424: 221–231, 2009. doi: 10.1042/BJ20091144. [DOI] [PubMed] [Google Scholar]
- 38.O’Connell B, Stephenson DG, Blazev R, Stephenson GM. Troponin C isoform composition determines differences in Sr(2+)-activation characteristics between rat diaphragm fibers. Am J Physiol Cell Physiol 287: C79–C87, 2004. doi: 10.1152/ajpcell.00555.2003. [DOI] [PubMed] [Google Scholar]
- 39.Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevičius G, Paužas H, Mekideche A, Kayser B, Martinez-Redondo V, Ruas JL, Bruton J, Truffert A, Lanner JT, Skurvydas A, Westerblad H. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci USA 112: 15492–15497, 2015. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prochniewicz E, Lowe DA, Spakowicz DJ, Higgins L, O’Conor K, Thompson LV, Ferrington DA, Thomas DD. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol 294: C613–C626, 2008. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pye D, Palomero J, Kabayo T, Jackson MJ. Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol 581: 309–318, 2007. doi: 10.1113/jphysiol.2006.125930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid MB. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic Biol Med 44: 169–179, 2008. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol (1985) 73: 1797–1804, 1992. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 45.Rudsits BL, Hopkins WG, Hautier CA, Rouffet DM. Force-velocity test on a stationary cycle ergometer: methodological recommendations. J Appl Physiol (1985) 124: 831–839, 2018. doi: 10.1152/japplphysiol.00719.2017. [DOI] [PubMed] [Google Scholar]
- 46.Sahlin K, Alvestrand A, Brandt R, Hultman E. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J Appl Physiol 45: 474–480, 1978. doi: 10.1152/jappl.1978.45.3.474. [DOI] [PubMed] [Google Scholar]
- 47.Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol Regul Integr Comp Physiol 248: R190–R196, 1985. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- 48.Spencer T, Posterino GS. Sequential effects of GSNO and H2O2 on the Ca2+ sensitivity of the contractile apparatus of fast- and slow-twitch skeletal muscle fibers from the rat. Am J Physiol Cell Physiol 296: C1015–C1023, 2009. doi: 10.1152/ajpcell.00251.2008. [DOI] [PubMed] [Google Scholar]
- 49.Supinski G, Stofan D, Callahan LA, Nethery D, Nosek TM, DiMarco A. Peroxynitrite induces contractile dysfunction and lipid peroxidation in the diaphragm. J Appl Physiol (1985) 87: 783–791, 1999. doi: 10.1152/jappl.1999.87.2.783. [DOI] [PubMed] [Google Scholar]
- 50.Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol Cell Physiol 264: C1085–C1095, 1993. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37: 153–156, 2001. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe D, Dutka TL, Lamboley CR, Lamb GD. Skeletal muscle fibre swelling contributes to force depression in rats and humans: a mechanically-skinned fibre study. J Muscle Res Cell Motil 40: 343–351, 2019. doi: 10.1007/s10974-019-09521-1. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe D, Kanzaki K, Kuratani M, Matsunaga S, Yanaka N, Wada M. Contribution of impaired myofibril and ryanodine receptor function to prolonged low-frequency force depression after in situ stimulation in rat skeletal muscle. J Muscle Res Cell Motil 36: 275–286, 2015. doi: 10.1007/s10974-015-9409-1. [DOI] [PubMed] [Google Scholar]
- 54.Xu H, Ren X, Lamb GD, Murphy RM. Physiological and biochemical characteristics of skeletal muscles in sedentary and active rats. J Muscle Res Cell Motil 39: 1–16, 2018. doi: 10.1007/s10974-018-9493-0. [DOI] [PubMed] [Google Scholar]