Abstract
Angiotensin II (ANG II) Agtr1a receptor (AT1A) is expressed in cells of the arcuate nucleus of the hypothalamus that express the leptin receptor (Lepr) and agouti-related peptide (Agrp). Agtr1a expression in these cells is required to stimulate resting energy expenditure in response to leptin and high-fat diets (HFDs), but the mechanism activating AT1A signaling by leptin remains unclear. To probe the role of local paracrine/autocrine ANG II generation and signaling in this mechanism, we bred mice harboring a conditional allele for angiotensinogen (Agt, encoding AGT) with mice expressing Cre-recombinase via the Lepr or Agrp promoters to cause cell-specific deletions of Agt (AgtLepr-KO and AgtAgrp-KO mice, respectively). AgtLepr-KO mice were phenotypically normal, arguing against a paracrine/autocrine AGT signaling mechanism for metabolic control. In contrast, AgtAgrp-KO mice exhibited reduced preweaning survival, and surviving adults exhibited altered renal structure and steroid flux, paralleling previous reports of animals with whole body Agt deficiency or Agt disruption in albumin (Alb)-expressing cells (thought to cause liver-specific disruption). Surprisingly, adult AgtAgrp-KO mice exhibited normal circulating AGT protein and hepatic Agt mRNA expression but reduced Agt mRNA expression in adrenal glands. Reanalysis of RNA-sequencing data sets describing transcriptomes of normal adrenal glands suggests that Agrp and Alb are both expressed in this tissue, and fluorescent reporter gene expression confirms Cre activity in adrenal gland of both Agrp-Cre and Alb-Cre mice. These findings lead to the iconoclastic conclusion that extrahepatic (i.e., adrenal) expression of Agt is critically required for normal renal development and survival.
Keywords: adrenal gland, agouti-related peptide, angiotensinogen, development, leptin
INTRODUCTION
The renin-angiotensin system (RAS) within the brain has been implicated in the control of resting energy expenditure (REE), through tissue-specific actions (8). Angiotensin II (ANG II) in the periphery, acting via its type 2 receptor (AT2, product of the Agtr2 gene), suppresses REE and therefore promotes weight gain. In contrast, ANG II action at its type 1 receptor (AT1A, product of the Agtr1a gene) within the brain promotes REE and weight loss or prevents weight gain (23, 35).
The arcuate nucleus of the hypothalamus (ARC) controls metabolic functions (including feeding and REE) in part by sensing peripheral nutritional status and modifying both behavioral and autonomic functions (57). The adipokine leptin acts through its receptor (LEPR, product of the Lepr gene) at the ARC to control feeding, sympathetic nerve activity (SNA), and REE (31, 57). Leptin’s actions at proopiomelanocortin (Pomc) neurons and agouti-related peptide (Agrp) neurons are known to coordinately control downstream neuronal activity, resulting in increased SNA to several adipose beds, including interscapular brown adipose tissue (BAT; 2, 13, 33, 48).
Recently, we demonstrated that AT1A receptors located on the subset of Lepr-expressing cells that also express Agrp are required for various stimuli, including leptin, to increase thermogenic adipose SNA and thus REE (9). The molecular mechanisms mediating this leptin-AT1A interaction, however, remain unknown. How does leptin, via LEPR, stimulate AT1A signaling in Agrp neurons?
In the circulation, leptin increases levels of angiotensinogen (AGT, product of the Agt gene) and subsequent production of ANG II (26). Furthermore, Agt mRNA is expressed in Agrp neurons of the ARC even though these cells are already bathed in AGT protein produced by glial cells (51). Therefore, we hypothesized that local expression of Agt within Agrp neurons might be required for, and mediate, leptin’s stimulation of SNA and REE via an autocrine or intracrine signaling cascade. To test this hypothesis, animals expressing Cre-recombinase via the Lepr locus (Lepr-ires-Cre; 10) or Agrp locus (Agrp-ires-Cre mice; 58) were bred with animals harboring a conditional endogenous allele for the Agt gene (Agtflox mice; 63). Resulting AgtLepr-KO and AgtAgrp-KO mice exhibited selective deletion of the Agt gene in cells that express either Lepr or Agrp, respectively. No major metabolic phenotype was observed in AgtLepr-KO mice under chow or high-fat diet (HFD)-fed conditions, which provides evidence against our working hypothesis of a neuronal autocrine or intracrine signaling mechanism within the ARC for metabolic control. Surprisingly, AgtAgrp-KO mice exhibited an unexpected reduction in preweaning survival rates. Thus, we utilized this serendipitous observation to further characterize the tissue-specific importance of AGT in development. Data presented herein support the iconoclastic concept that local expression of Agt within the adrenal gland, as opposed to liver-derived circulating AGT protein, is critically required for renal development and survival.
MATERIALS AND METHODS
Animals.
All procedures were approved by the University of Iowa institutional animal care and use committee (IACUC) and Medical College of Wisconsin IACUC and were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (41). Agrp-ires-Cre (stock 012899), Lepr-ires-Cre (stock 008320), Alb-Cre mice (stock 003574), CAG-LSL-hM4Di-pta-mCitrine mice (stock 026219), and CAG-LSL-tdTomato (stock 007909) were obtained from the Jackson Laboratory, and Agtflox (stock 018388) mice were kindly provided by Drs. Lisa A. Cassis and Alan Daugherty (University of Kentucky). Mice were housed at 25°C with a standard 12-h light cycle and provided ad libitum access to water and standard chow (no. 7013; Teklad) or a high-fat diet (HFD; 45% kcal from fat; D12451; OpenSource). Experiments were conducted in mice at 5–25 wk of age. Littermate control mice were used throughout the study, and no phenotypic differences were observed among the various littermate genotypes (including mice with Cre+ Agtflox/wt, Cre− Agtflox/wt, or Cre− Agtflox/flox genotypes).
Recombination was assessed by PCR after DNA extraction from tissues using Qiagen Tissue and Blood DNEasy Kit as per the manufacturer’s instructions. DNA was amplified using One Shot Quick Load Taq-Polymerase 2X Master Mix with Standard Buffer and 0.2 µM each of the following forward and reverse primer set: forward, 5′-ACTCCAAGCCATCCTACTCTAT-3′; reverse, 5′-GGTCTCCATACTTGCTGATGTT-3′. Amplified DNA was immediately run on a 0.5% agarose gel for 60 min at 100 V. Gels were imaged using a BioChemi Systems Dark Room (UVP BioImaging Systems).
Quantitative PCR.
mRNA was isolated from tissues using TRIzol RNA extraction, and cDNA was generated by reverse transcription reaction using SuperScript III First-Strand synthesis system as per the manufacturer’s instructions (Invitrogen). Quantitative PCR was performed on a StepOnePlus real-time PCR system (Applied Biosystems) using either designed primers and SYBR Green DNA polymerase or TaqMan Gene Expression probes and Master Mix (Thermo Fisher Scientific) listed in Table 1. Fold changes were calculated using the method of Livak and Schmittgen (36).
Table 1.
Primers for quantitative PCR
| Gene | Forward Primer | Reverse Primer | TaqMan Probe |
|---|---|---|---|
| Agt | Mm00599662_m1 | ||
| Ren | Mm02342888_gH | ||
| Actb | Mm02619580_g1 | ||
| Slc12a3 (NCC) | 5-AAGTCGGGTGGCACCTATTTCCTT-3 | 5-TTACGGTTTCTGCAAAGCCCACAG-3 | |
| Slc9a3 (NHE3) | 5-TCCTCTCAGCCATTGAGGACATCT-3 | 5-ACTTTGCTGAGGAACTTCCGGTCA-3 | |
| Slc12a1 (NKCC2) | 5-CCATGGTAACCTCTATCACTGGGT-3 | 5-TCAAGCCTATTGACCCACCGAACT-3 | |
| Scnn1a (ENaCα) | 5-ACAATGGTTTGTCCCTGACACTGC-3 | 5-TCACGTTGAAGCCACCATCATCCA-3 | |
| Scnn1b (ENaCβ) | 5-TCTGCCAACCCTGGGACTGAATTT-3 | 5-TGGCATAGATGCCCTCCTCTCTAA-3 | |
| Scnn1g (ENaCγ) | 5-GCCAATCAGTGTGCAAGCAATCCT-3 | 5-TTATTTGCTGGCTTTGGTCCCAGG-3 | |
| Atp1a1 (Na/K ATPase) | 5-TGAAGCTGACACCACGGAGAATCA-3 | 5-TGCCGCTTAAGAATAGGCAGGTT-3 |
Agt, angiotensinogen.
Urine steroids and plasma angiotensinogen.
Urine aldosterone and corticosterone were measured by ELISA (ab136933 and ab108821, respectively; Abcam), per product instructions. For plasma AGT analyses, trunk blood was collected into EDTA-coated tubes, chilled on ice for 20 min, and centrifuged at 2,000 g for 10 min. AGT protein was then measured by ELISA (LS-F22617; LifeSpan BioSciences, Inc.) per product instructions.
Adrenal fluorescent reporter imaging.
Mice were anesthetized with 2% isoflurane, and adrenal glands were collected and flash-frozen using precooled 2-methylbutane. Fresh frozen adrenal glands were embedded in optimal cutting temperature (O.C.T.) compound (Sakura) and stored at −80°C before sectioning via cryostat at 15–20-μm thickness. The sections were direct mounted on Superfrost Plus slides (Thermo Fisher Scientific) and allowed to air dry. The slides were stained with DAPI and coverslipped with ProLong Diamond Antifade Mountant (P36962; Invitrogen). The sections were imaged using either a Zeiss LSM 710 confocal microscope maintained by the Central Microscopy Research Facility at the University of Iowa or a motorized Nikon Eclipse Ni-E fluorescent microscope maintained by the Department of Physiology at the Medical College of Wisconsin.
Brain in situ hybridization/RNAscope.
Mice were euthanized, and brains were collected, immediately flash-frozen in 2-methlybutane and then embedding medium (Tissue-Tek O.C.T. Compound; Sakura Finetek), and stored at −80°C. Coronal sections of 10 µm were cut using a cryostat, and sections underwent RNAscope Multiplex fluorescence in situ hybridization (FISH) for fresh frozen samples (Advanced Cell Diagnostics). Briefly, samples were fixed using 4% paraformaldehyde and underwent protease IV treatment and then hybridization with target probes using the double-Z oligo probe design for genes of interest [Agt 426941, Lepr 402731, and glial fibrillary acidic protein (Gfap) 313211]. Subsequent amplification with RNAscope reagents was then completed. All images were captured using a Zeiss LSM 710 confocal microscope at ×20 or ×40 magnification, maintained by the Central Microscopy Research Facility at the University of Iowa.
Adrenal in situ hybridization/RNAscope.
Adrenal tissue was collected after transcardial perfusion with Dulbecco’s phosphate-buffered saline (DPBS; GIBCO) containing 0.1% heparin followed by 4% paraformaldehyde (PFA, 4% in PBS; Biotium). The adrenal glands were dissected and postfixed in 4% PFA for 24 h, followed by 48 h in 30% sucrose. The tissue was then placed into a cryomold with Tissue-Tek optimal cutting temperature (O.C.T.) compound (Sakura) and snap frozen in dry ice and 4-methylbutane. Tissue was cryosectioned at 10–14 µm and stored at −80°C until use. Endogenous mRNA was detected using the in situ hybridization technique RNAscope 2.5 High Definition-Fluorescent Multiplex Assay (60). Briefly, tissues were placed in 10% neutral buffered formalin (10% NBF; Sigma-Aldrich) for 15 min at 4°C and dehydrated in a serial ethanol dilution up to 100% ethanol before blocking endogenous peroxidase activity with H2O2 and treatment with Protease Plus (ACD Bio). Slides were then incubated for 2 h at 40°C with probes identifying Agt (Mm-Agt-426941; ACD Bio), Agrp (Mm-Agrp-400711-C3; ACD Bio) or positive control probe peptidylprolyl isomerase B (Ppib; Mm-Ppib-313911; ACD Bio), RNA polymerase II subunit A (Mm-Polr2a-C2-312471-C2; ACD Bio), and negative control probe 4-hydroxy-tetrahydrodipicolinate reductase (dapB; 310043; ACD Bio). RNAscope fluorescent multiplex assay protocol was followed for all other amplifications and development steps. Slides were stained with DAPI and coverslipped with ProLong Gold (P10144; Thermo Fisher). Slides were imaged using a fluorescent upright microscope.
Energy balance.
Energy flux was determined using bomb calorimetric methods, as previously described (21). Briefly, mice on chow diet were placed in Nalgene single-mouse metabolic caging systems to permit quantification of food and water intake and quantification and collection of urine and feces at the same time each day for 4 consecutive days, at 8 wk of age. Mice on HFD were singly housed in shoebox-style home cages in which the bedding was replaced by an absorbent pad to allow for feces and food intake measurement, as previously described (9). Mice were acclimated for 72 h, and once-daily measurements were taken at the same time each day for 4 days, at both 9 wk (representing +1 wk on HFD) and 13 wk of age (representing +4 wk on HFD). Energy per gram of feces was assessed using a semimicro bomb calorimeter (Parr), and digestive and energy efficiency were calculated as done previously (21).
In silico reanalysis of public data sets.
The expression for genes of interests was assessed by interrogating publicly available data sets deposited at the National Center for Biotechnology Information (NCBI) through the Gene Expression Omnibus (GEO) data set browser (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser).
DOCA-salt model.
A 50-mg pellet of DOCA (Sigma-Aldrich) was implanted into the subcutaneous space under isoflurane anesthesia. Animals were subsequently singly housed and allowed ad libitum access to standard chow and both tap water and 0.15 M NaCl water for 3 wk (22).
Blood pressure.
Blood pressure was recorded using the Visitech Systems BP-2000 series II noninvasive (tail cuff) blood pressure analysis system for mice. Mice were lightly restrained and artificially heated during recordings. Mice, between the ages of 15 and 20 wk, were trained for a minimum of 2 wk before recording data for analysis.
Renal histology.
Histology was completed in collaboration with the University of Iowa Comparative Pathology Laboratory. Mice were euthanized, and kidneys were collected and immediately placed in formalin until processing. Briefly, tissue underwent a series of dehydrating alcohol washes and embedding in paraffin under vacuum. Ten-micrometer sections were cut using a microtome, placed onto Superfrost microscope slides, and allowed to air dry. Sections were stained using hematoxylin and eosin, and imaging was performed using an Olympus BX53 microscope with an Olympus DP72 camera and scored by a board-certified veterinary pathologist (K. N. Gibson-Corley) at the University of Iowa.
Statistics.
All experiments were performed on both male and female animals as available, although low survival rates of males in the AgtAgrp-KO colony made thorough inclusion impossible for selected end points. Data from each sex were analyzed separately where possible. Quantitative data were analyzed using parametric methods including ANOVA, with repeated measures as appropriate, independent t test, or chi-square analyses as noted. Throughout, differences with P < 0.05 were considered statistically significant, and summary data are presented as means ± SE.
RESULTS
Agt is expressed in Agrp-expressing cells of the ARC.
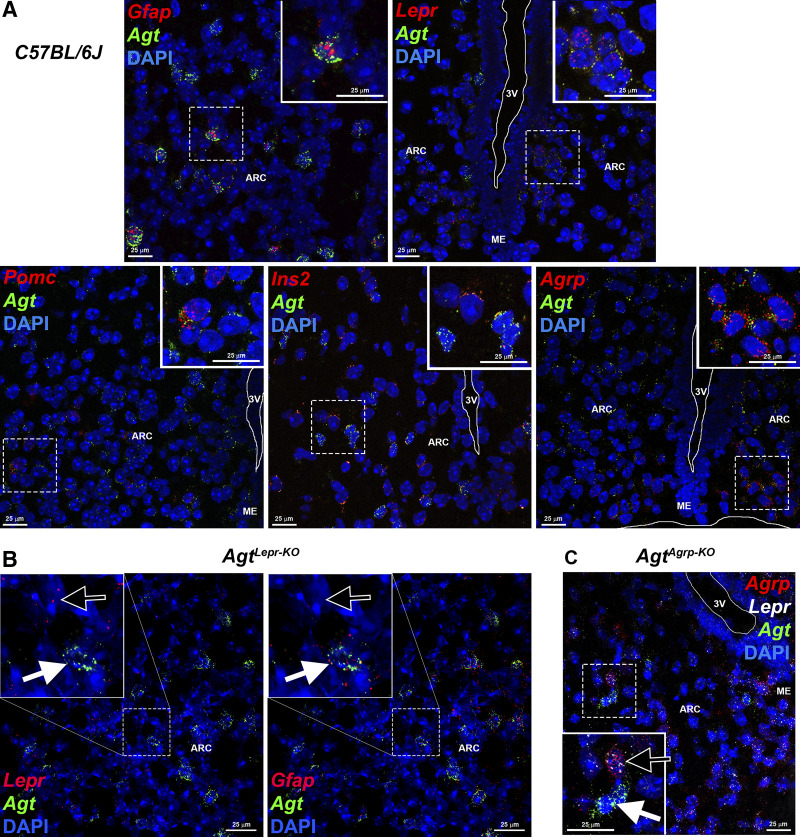
Previously, we utilized in silico reanalysis of a published single-cell RNA-sequencing data set (GSE74672) to examine expression of RAS components within cells of the hypothalamus. We determined that cells expressing Pomc and Agrp (including both “GABA14” and “SST3” subtypes) express Agt (51). Here, we confirmed that various cell types of the ARC express Agt, using in situ hybridization (RNAscope). Agt expression was colocalized with markers of various neuronal populations, including Lepr, Agrp, Pomc, and insulin (Ins1; Fig. 1A). As expected, Agt expression was also localized to cells expressing markers of astrocytes (Gfap). Importantly, colocalization of Agt with Lepr was lost in ARC sections of AgtLepr-KO mice (Fig. 1B), and colocalization of Agt with Agrp was lost in AgtAgrp-KO mice (Fig. 1C); however, expression of Agt by other cell types of the ARC were maintained in both strains. Quantification of single- and dual-fluorescent cells within multiple ARC sections from C57BL/6J, AgtLepr-KO, and AgtAgrp-KO mice confirms knockout of Agt in the targeted cell type (Table 2). Notably, a small number of targeted cells maintained Agt expression in the knockout strains. This was not unexpected, however, as Cre-Lox-mediated recombination in brain tissue is rarely 100% efficient.
Fig. 1.
Localization of angiotensinogen (Agt) within the arcuate nucleus of the hypothalamus (ARC) and confirmation of cell-specific deletion of Agt in AgtLepr-KO and AgtAgrp-KO mice [mice exhibiting selective deletion of the Agt gene in cells that express either leptin receptor (Lepr) or agouti-related peptide (Agrp), respectively]. A: colocalization of Agt mRNA with mRNA for glial fibrillary acidic protein (Gfap), Lepr, proopiomelanocortin (Pomc), insulin II (Ins2), or Agrp in the ARC from a wild-type C57BL/6J mouse using in situ hybridization/RNAscope. DAPI, 4′,6-diamidino-2-phenylindole nuclear stain; ME, median eminence; 3V, third ventricle. B: loss of colocalization of Agt mRNA with Lepr in AgtLepr-KO mice. Black arrows identify a Lepr-positive (panel at left) but Agt-negative cell, whereas white arrows identify an Agt-positive, Gfap-positive neighboring cell. C: loss of colocalization of Agt mRNA with Agrp in AgtAgrp-KO mice. The black arrow identifies an Agrp-/Lepr-positive + Agt-negative cell, whereas the white arrow identifies an Agt-positive + Agrp-/Lepr-negative neighboring cell.
Table 2.
Quantification of in situ hybridization images
| Cell Type | C57BL/6J | AgtLepr-KO | AgtAgrp-KO |
|---|---|---|---|
| Lepr+ | |||
| Sections, n | 5 | 5 | |
| Total Lepr+ | 132 | 52 | |
| Lepr+ and Agt+ | 70 (53%) | 1 (2%); χ2 = 22.4, df = 1, P < 0.0001 | |
| Agrp+ | |||
| Sections, n | 3 | 4 | |
| Total Agrp+ | 130 | 73 | |
| Agrp+ and Agt+ | 79 (61%) | 4 (6%); χ2 = 29.0, df = 1, P < 0.0001 |
Values are counts of individual cells with positive staining for gene of interest, summed across multiple sections of arcuate nucleus of the hypothalamus; percentages are in parentheses. Here, n = no. of sections. AgtLepr-KO and AgtAgrp-KO, mice exhibiting selective deletion of the angiotensinogen (Agt) gene in cells that express either leptin receptor (Lepr) or agouti-related peptide (Agrp), respectively.
Deletion of Agt from Agrp-expressing cells causes reduced preweaning survival.
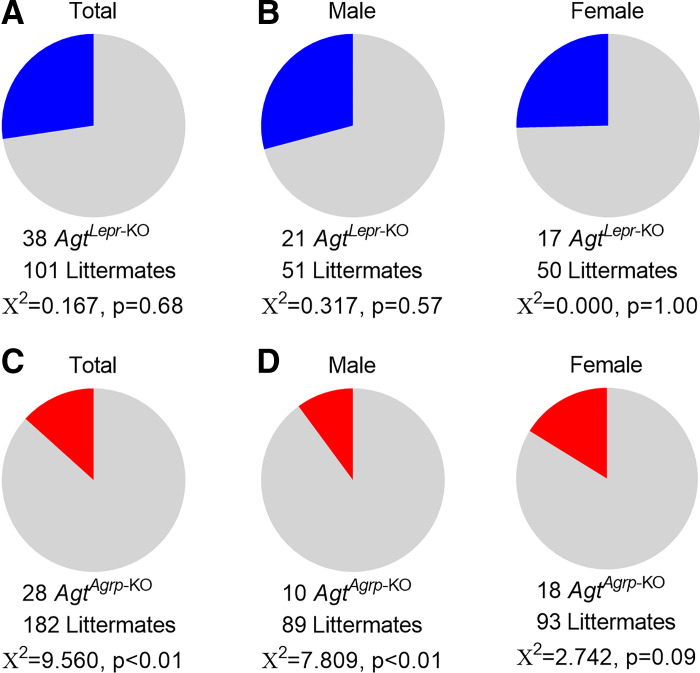
To generate AgtLepr-KO mice, we utilized a breeding scheme that was expected to result in 25% AgtLepr-KO offspring of each sex (sire: Lepr-ires-Cre+ Agtflox/wt x dam: Lepr-ires-Cre− Agtflox/flox). At weaning, 27% of the first 139 offspring from this colony were AgtLepr-KO, and no difference in survival was observed in males or females (Fig. 2, A and B). These findings indicate the deletion of the Agt gene in cells that express Lepr results in viable adult mice, regardless of sex.
Fig. 2.
Reduced preweaning survival of AgtAgrp-KO mice [mice exhibiting selective deletion of the angiotensinogen (Agt) gene in cells that express agouti-related peptide (Agrp)]. A: total live mice at weaning (postnatal day 21, n = 139 mice) from the AgtLepr-KO colony [mice exhibiting selective deletion of the Agt gene in cells that express leptin receptor (Lepr)] illustrates observation of expected 25% survival of AgtLepr-KO mice (i.e., n = 35 mice expected, and 38 mice observed). B: distribution of live AgtLepr-KO and littermate control male (n = 72) vs. female (n = 67) mice from A. C: total live mice at weaning (postnatal day 21, n = 210 mice) from the AgtAgrp-KO colony illustrates significant deviation from expected 25% survival of AgtAgrp-KO mice due to preweaning lethality (i.e., n = 53 mice expected, and only 28 mice observed). D: breakdown of live AgtAgrp-KO and littermate control male (n = 99) vs. female (n = 111) mice from C highlights more penetrant lethal phenotype in male AgtAgrp-KO mice (i.e., n = 25 mice expected, and only n = 10 mice observed) than female AgtAgrp-KO mice (n = 28 mice expected, and 18 mice observed).
A similar breeding scheme was then used to generate AgtAgrp-KO mice (sire: Agrp-ires-Cre+ Agtflox/WT x dam: Agrp-ires-Cre− Agtflox/flox), and therefore we expected to observe 25% AgtAgrp-KO mice at weaning. In contrast to AgtLepr-KO mice, AgtAgrp-KO mice unexpectedly exhibited a major preweaning lethality phenotype, as only 13% of the 210 offspring genotyped at weaning from this colony were AgtAgrp-KO (Fig. 2C). Interestingly, the lethal phenotype was more penetrant in males than females (Fig. 2D). These findings indicate that disruption of the Agt gene in cells that express Agrp results in a major preweaning lethality that limits interpretability of metabolic studies performed in surviving adult AgtAgrp-KO mice and simultaneously highlights an unexpected sex-dependent developmental phenotype worthy of further investigation.
Deletion of Agt from Lepr- or Agrp-expressing cells has no major metabolic consequence.
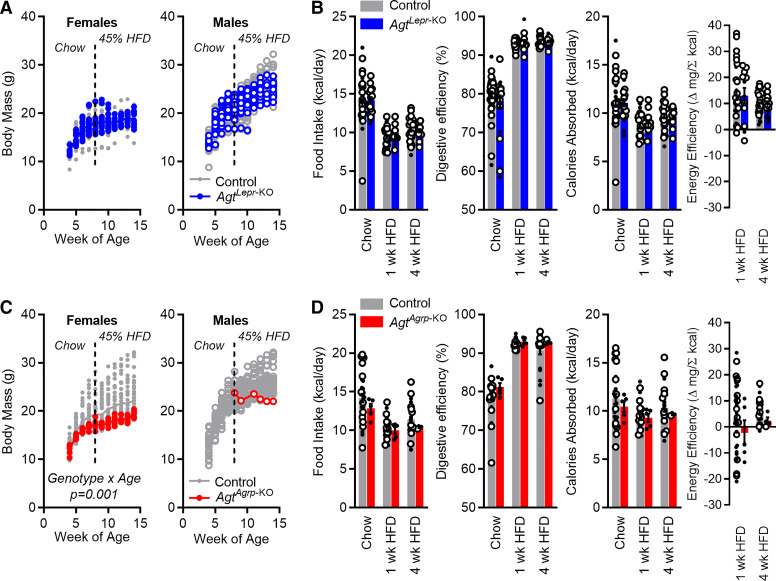
When maintained on a normal chow diet (no. 7013; Teklad) or a 45% HFD (D12451), both male and female AgtLepr-KO mice exhibited similar growth patterns compared with littermate control mice (Fig. 3A). Food intake behavior and digestive efficiency were unaffected by genotype; however, typical effects of HFD to modify behavior and digestive efficiency were detected (Fig. 3B). Energy efficiency, the change in body mass per calorie absorbed, represents an inverse index of total energy expenditure. This index was not affected by genotype, indicating that disruption of Agt in Lepr-expressing cells had no net effect on energy expenditure.
Fig. 3.
Energy homeostasis in AgtLepr-KO and surviving adult AgtAgrp-KO mice [mice exhibiting selective deletion of the angiotensinogen (Agt) gene in cells that express either leptin receptor (Lepr) or agouti-related peptide (Agrp), respectively]. A: body mass vs. age in AgtLepr-KO and littermate control mice. Females: subject P < 0.01, genotype P = 0.20, age P < 0.01, genotype × age P = 0.08 by two-way RM ANOVA. Males: subject P < 0.01, genotype P = 0.81, age P < 0.01, genotype × age P = 0.15 by two-way RM ANOVA. Females: n = 36 control vs. 16 AgtLepr-KO mice. Males: n = 34 control vs. 14 AgtLepr-KO mice. B: food intake per day, digestive efficiency, calories absorbed per day, and energy efficiency in AgtLepr-KO and littermate control mice. Food intake per day: genotype P = 0.68, time/diet P < 0.01, genotype × time P = 0.67. Digestive efficiency: genotype P = 0.38, time/diet P < 0.01, genotype × time P = 0.31. Calories absorbed per day: genotype P = 0.99, time/diet P < 0.01, genotype × time P = 0.94. Energy efficiency: genotype P = 0.82, time/diet P = 0.03, genotype × time P = 0.83. C: body mass vs. age in AgtAgrp-KO and littermate control mice. Females: subject P < 0.01, genotype P = 0.08, age P < 0.01, genotype × age P < 0.01 by two-way RM ANOVA. Females: n = 39 control vs. 5 AgtAgrp-KO mice. Males: n = 31 control mice vs. 1 AgtAgrp-KO mouse. D: food intake per day, digestive efficiency, calories absorbed per day, and energy efficiency in AgtAgrp-KO and littermate control mice. Food intake per day: genotype P = 0.21, time/diet P < 0.01, genotype × time P = 0.76. Digestive efficiency: genotype P = 0.12, time/diet P < 0.01, genotype × time P = 0.66. Calories absorbed per day: genotype P = 0.36, time/diet P = 0.11, genotype × time P = 0.88. Energy efficiency: genotype P = 0.22, time/diet P = 0.31, genotype × time P = 0.79. For all panels, open circles indicate male mice. Summary data are presented as means ± SE. HFD, high-fat diet.
When maintained on a standard chow diet (no. 7013; Teklad), the few surviving adult female and male AgtAgrp-KO mice available for phenotyping exhibited normal total body mass compared with control littermates (Fig. 3C). When switched to 45% HFD at 8 wk of age, however, these few surviving adult AgtAgrp-KO mice were modestly resistant to excess weight gain. No differences in caloric intake behavior (Fig. 3D) or digestive efficiency were observed at 8 wk of age, resulting in normal total daily caloric absorption. No significant change in energy efficiency was observed in AgtAgrp-KO mice, though a nonsignificant trend was observed after 4 wk of HFD feeding. As the number of adult AgtAgrp-KO mice available for metabolic phenotyping was limited by the low survival of these animals, we conclude that the present data set is underpowered to detect this effect; thus, a modest reduction in energy efficiency (implying an increase in total energy expenditure) remains possible in AgtAgrp-KO mice.
We conclude that disruption of local AGT production by leptin-sensitive cells of the ARC, and subsequent disruption of autocrine or intracrine RAS peptide signaling to AT1A specifically between or within Lepr-expressing Agrp neurons within the ARC, is dispensable for integrative control of metabolic functions. Importantly, these studies do not address the role of generalized local production or paracrine RAS signaling within the ARC or compensation of AGT production by those cells in AgtLepr-KO and AgtAgrp-KO mice, however, as glial production of AGT and expression of Agt by other neuronal cell types of the ARC are well documented in wild-type animals (7, 49, 51) and remain intact in both AgtLepr-KO and AgtAgrp-KO animal models (Fig. 1, B and C).
Deletion of Agt from Lepr-expressing cells has no effect on blood pressure.
Previously, we demonstrated that DOCA-salt treatment stimulates REE through activation of AT1A receptors localized to Lepr-expressing cells of the ARC (9, 22). In contrast, stimulation of blood pressure by DOCA-salt is dependent on AT1A signaling within the brain (29, 32, 44), but this is mediated through a distinct population of receptors because genetic disruption of Agtr1a in Lepr-expressing cells had no effect on either baseline blood pressure or blood pressure responses to DOCA-salt (9). Thus, here we performed an experiment to explore whether blood pressure was affected by disruption of Agt in Lepr-expressing cells. As expected, DOCA-salt treatment caused a significant increase in systolic blood pressure, but no effect of genotype was observed [baseline: littermates 119 ± 1 mmHg (n = 8 mice) vs. AgtLepr-KO 121 ± 1 mmHg (n = 7 mice), and after DOCA-salt: littermates 128 ± 3 mmHg (n = 9 mice) vs. AgtLepr-KO 130 ± 3 mmHg (n = 7 mice); genotype P = 0.41, DOCA P < 0.01, interaction P = 0.91]. Thus, we conclude that loss of Agt expression in Lepr-expressing cells is dispensable for both metabolic and blood pressure control. No examinations of blood pressure or responses to DOCA-salt were performed in AgtAgrp-KO mice, because of the low number of animals available for such a study.
Deletion of Agt from Agrp-expressing cells results in pathologies similar to those previously reported with whole body Agt deletion.
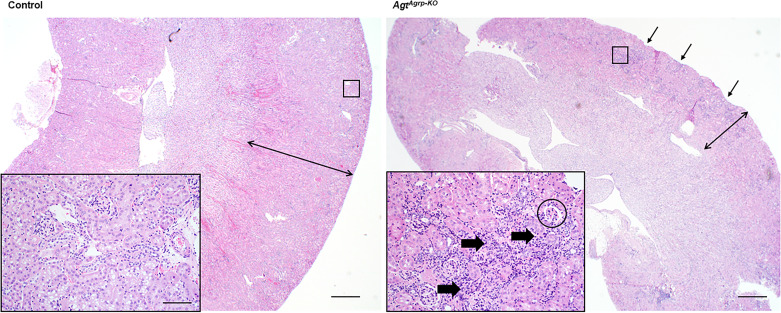
The reduced survival in AgtAgrp-KO offspring was associated with gross morphological changes in kidney structure in surviving adult (18-wk-old) male AgtAgrp-KO mice consistent with “end-stage kidney,” including interstitial fibrosis and inflammation, a thin cortex, glomerulosclerosis, and scattered tubular dilation (Fig. 4), consistent with the concept that altered renal development may mediate the observed reduction in survival of these animals. The renal cortex was diffusely thin, and the capsular surface was pitted. The cortex itself had moderate multifocal to coalescing interstitial fibrosis and mixed inflammation. Many glomeruli were sclerotic, characterized by small, shrunken capillary loops that were thickened and/or obscured by eosinophilic mesangium. Within the cortex and medulla, many tubules were moderately dilated but empty, and scattered tubular epithelial cells had vacuolated cytoplasm. No protein casts were present in tubules. Interestingly, surviving female mice did not exhibit the severe kidney pathologies observed in males (data not shown). No changes in serum creatinine were observed in adult surviving AgtAgrp-KO mice (control n = 9 mice, 0.78 ± 0.06 mg/dL vs. AgtAgrp-KO n = 7 mice, 0.63 ± 0.05 mg/dL, P = not significant). No differences were observed in renal mRNA expression of major solute transporters, although there was a trend toward decreased Nkcc2a and Nhe3 expression in adult surviving AgtAgrp-KO mice compared with littermate controls (Table 3). Although no significant changes in urine electrolytes were observed, these comparisons were underpowered because of the low number of animals available for study and large interindividual variability, and thus such comparisons should be interpreted carefully (Table 3). No major histological changes were observed in surviving AgtAgrp-KO adrenal glands and kidneys from gestational day 15.5 fetuses compared with littermates (data not shown). Meticulous experiments performed throughout various developmental stages in utero and postnatally are therefore required to further clarify the exact timeline and mechanism of preweaning death in AgtAgrp-KO mice.
Fig. 4.
Structural damage in kidneys of AgtAgrp-KO mice [mice exhibiting selective deletion of the angiotensinogen (Agt) gene in cells that express agouti-related peptide (Agrp)]. Representative hematoxylin and eosin (H&E)-stained section of surviving 18-wk-old male kidneys (images from AgtAgrp-KO and age/cage/litter-matched brother). Note the pitted surface of the AgtAgrp-KO kidney (arrows) and thin cortex (double arrow) compared with control. Within the cortex (inset) there was substantial lymphoplasmacytic interstitial nephritis (thick arrows) and glomerulosclerosis (encircled). Bars = 500 µm (large images) and 50 µm (insets).
Table 3.
Renal gene expression and osmolyte handling in surviving adult AgtAgrp-KO mice and littermate controls
| Control | AgtAgrp-KO | P Value | |
|---|---|---|---|
| Renal mRNA (1 male + 7 female control vs. 1 male + 5 female AgtAgrp-KO) | |||
| Ren | 1.000 (0.782–1.279) | 2.672 (1.915–3.726) | 0.03 |
| Slc12a3 (NCC) | 1.000 (0.931–1.075) | 0.904 (0.724–1.130) | 0.69 |
| Slc9a3 (NHE3) | 1.000 (0.870–1.150) | 0.731 (0.644–0.830) | 0.12 |
| Slc12a1 (NKCC2) | 1.000 (0.904–1.106) | 0.732 (0.576–0.930) | 0.23 |
| Scnn1a (ENaCα) | 1.000 (0.935–1.069) | 0.846 (0.753–0.951) | 0.35 |
| Scnn1b (ENaCβ) | 1.000 (0.865–1.156) | 0.958 (0.848–1.082) | 0.82 |
| Scnn1g (ENaCγ) | 1.000 (0.901–1.110) | 0.984 (0.879–1.100) | 0.91 |
| Atp1a1 (Na/K ATPase) | 1.000 (0.953–1.049) | 0.890 (0.780–1.016) | 0.52 |
| Urine osmolytes (1 male + 10 female control vs. 1 male + 8 female AgtAgrp-KO) | |||
| Osmolality, mosmol/kg H2O | 4,818 ± 483 | 5,947 ± 624 | 0.24 |
| UNa, mmol/L | 392 ± 45 | 260 ± 107 | 0.20 |
| UK, mmol/L | 650 ± 54 | 440 ± 171 | 0.17 |
| UCreatinine, mg/dL | 92.8 ± 9.9 | 81.1 ± 15.2 | 0.51 |
Values are means ± SE; comparisons were performed using 2-tailed independent t test. AgtAgrp-KO, mice exhibiting selective deletion of the angiotensinogen (Agt) gene in cells that express agouti-related peptide (Agrp). UNa, UK, and UCreatinine, urinary sodium, potassium, and creatinine, respectively.
Deletion of Agt from Agrp-expressing cells disrupts adrenal Agt expression, but not hepatic Agt expression or circulating AGT, and reduces steroid production.
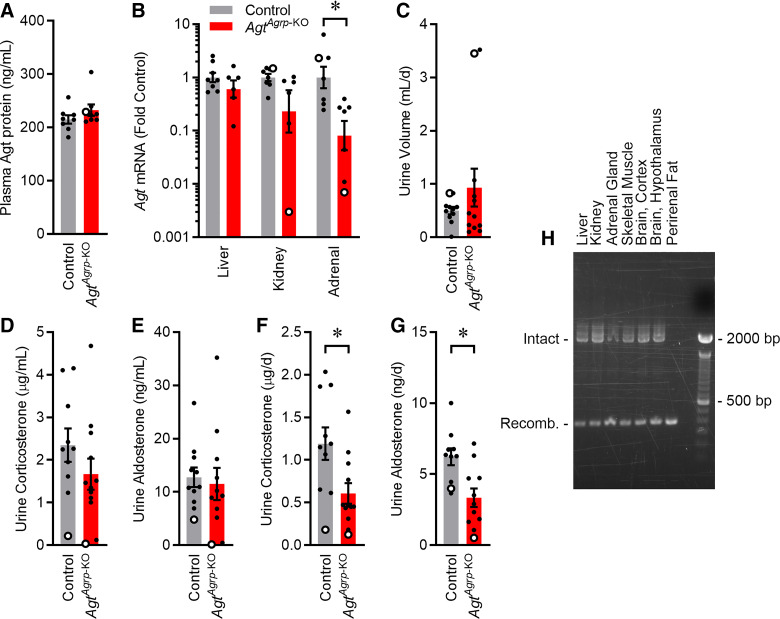
Surviving adult AgtAgrp-KO mice were examined for changes in relevant endocrine functions. First, we examined changes in circulating AGT in surviving adult AgtAgrp-KO mice, as previous studies have implicated liver- and adipose-derived circulating AGT in renal development and survival, whereas Agt expression within the kidney itself is less important (38). Plasma AGT protein concentration was unchanged in surviving adult AgtAgrp-KO mice (Fig. 5A), consistent with no significant change in mRNA Agt levels in liver (Fig. 5B). Similarly, no change in plasma AGT protein concentrations were observed between AgtLepr-KO and their littermate mice of either sex [control male 129 ± 7 ng/mL (n = 11 mice) and female 109 ± 10 ng/mL (n = 10 mice) vs. AgtLepr-KO male 114 ± 8 ng/mL (n = 10 mice) and female 107 ± 9 ng/mL (n = 5 mice); genotype P = 0.36, sex P = 0.16, interaction P = 0.53]. Total daily urine volume was not significantly changed in AgtAgrp-KO mice (Fig. 5C). Urine concentrations of corticosterone and aldosterone were not significantly altered (Fig. 5, D and E); however, total daily elimination rates of corticosterone and aldosterone to urine were reduced in AgtAgrp-KO mice, consistent with reduced rates of steroid production (Fig. 5, F and G). Interestingly, the total daily steroid elimination to urine was suppressed the most in the single male AgtAgrp-KO mouse available for such study, which may help explain the lower survival rates of males. Although Agt expression was not statistically altered in kidneys of AgtAgrp-KO mice, this conclusion is underpowered given the large variability in expression levels among the small number of animals available for study. Renin mRNA expression in kidney was increased by twofold compared with littermate controls (Table 3), similar to what has previously been reported in whole body or “liver” knockout (achieved via Cre-recombinase expression by the albumin promoter) of Agt (38).
Fig. 5.
Altered adrenal expression of angiotensinogen (Agt) in adult AgtAgrp-KO mice [mice exhibiting selective deletion of the Agt gene in cells that express agouti-related peptide (Agrp)]. A: plasma AGT protein; n = 8 mice each. B: Agt mRNA in liver, kidney, and adrenal glands; n = 7–8 mice each. C: urine production rate. D and E: concentration of corticosterone and aldosterone in urine; n = 10–12 mice each. F and G: total daily elimination of corticosterone and aldosterone to urine. For all panels, open circles indicate male mice. Summary data are presented as means ± SE. *P < 0.05 by two-tailed independent t test. H: PCR amplification of the Agt locus from various tissues in AgtAgrp-KO mice. Intact amplicon, 2,009 bp, vs. recombined amplicon, 337 bp.
As the preweaning lethality and renal development phenotypes observed in AgtAgrp-KO mice are similar to previous reports of the consequences of whole body Agt disruption (59), we considered the possibility that Agrp-ires-Cre may be active in peripheral tissues in which Agt expression is known to be critical for development and survival. To test this hypothesis, we examined activity of Cre-recombinase in various peripheral tissues from AgtAgrp-KO mice first by testing for DNA recombination. Recombination of the Agtflox gene (at the DNA level) was detected in all tissues examined including liver, kidney, perirenal adipose, adrenal gland, hypothalamus, cortex, and skeletal muscle (Fig. 5H). Consistent with recombination of the Agtflox gene in adrenal gland, tissue-level expression of Agt mRNA was significantly reduced in the adrenal glands of AgtAgrp-KO mice, although no significant, consistent reduction in Agt mRNA was observed in the other tissues such as liver (Fig. 5B), indicating that DNA recombination in those tissues occurs in cells that are not actively transcribing Agt (though those cells must express Agrp, or have expressed Agrp during development). Again, reduction of Agt expression in adrenal gland was the most severe in the single surviving AgtAgrp-KO male mouse, which may contribute to the reduced steroid levels and survival of these males.
Agrp and Agt genes are expressed in the adrenal gland.
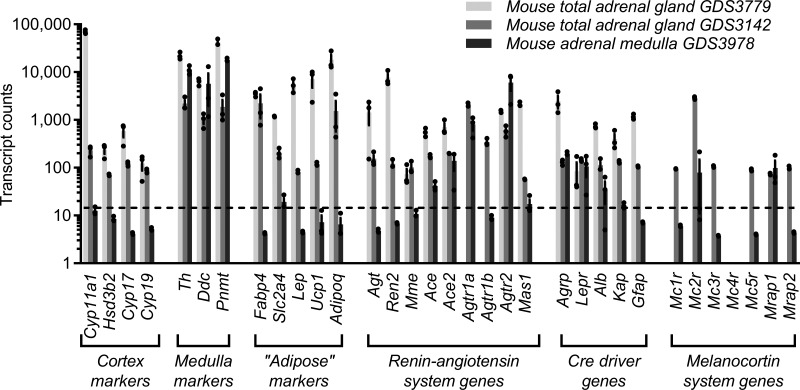
In silico reanalysis was performed using previously published data sets describing the transcriptomes of adult mouse whole adrenal gland (control mice from GDS3779 and mice from GDS3142; 19, 56) and adrenal medulla (control mice from GDS3978; 52). Expression levels of genes involved in steroid synthesis (Cyp11a1, Hsd3b2, Cyp17, and Cyp19) were robust in the whole tissue sample but essentially absent in the medulla tissue sample, whereas genes involved in catecholamine synthesis (Th, Ddc, and Pnmt) were expressed in both, providing a level of confidence in the quality of the medulla isolation and the general adrenal origin of all samples (Fig. 6). Agt expression was strong in whole tissue samples but much lower in adrenal medulla. Agrp expression was evident in both layers of the adrenal gland but expressed more strongly in whole adrenal gland in the GDS3779 study. These findings support the concept that Agt and Agrp are both expressed within the adrenal gland but provide limited information regarding localization at a cellular level. Nonetheless, in concert with reduced Agt mRNA expression in adrenal gland of AgtAgrp-KO mice, Cre activity driven by the Agrp locus in the adrenal gland appears positioned to directly disrupt normal local expression of Agt mRNA in this tissue (Fig. 5B).
Fig. 6.
Expression of angiotensinogen (Agt) and agouti-related peptide (Agrp) in adrenal gland. Expression of endogenous renin-angiotensin system genes, melanocortin system genes, and genes used as tissue-specific promoters in published RNA-sequencing data sets describing whole mouse adrenal gland [GDS3779 (19) and GDS3142 (56)] or mouse adrenal medulla [GDS3978 (52)] Agtr1a, Agtr1b, and Agtr2, ANG II receptor type 1a, 1b, and 2, respectively; Alb, albumin; Gfap, glial fibrillary acidic protein; Kap, kidney androgen response protein; Lepr, leptin receptor.
Notably, genes identifying adipocytes (Fabp4, Slc2a4, Lep, Ucp1, and Adipoq) were expressed in tissue samples from GDS3779 and GDS3142, possibly indicating contamination of these “whole adrenal gland” samples by adipocytes, although this may also indicate that these types of cells also normally express such markers (Fig. 6). Also unexpectedly, given widespread assumptions of the specific expression of the albumin (Alb), kidney androgen response protein (Kap), and glial fibrillary acidic protein (Gfap) genes in liver, kidney, and brain, respectively, we determined that endogenous expression of each is readily detected in the adrenal gland using these data sets.
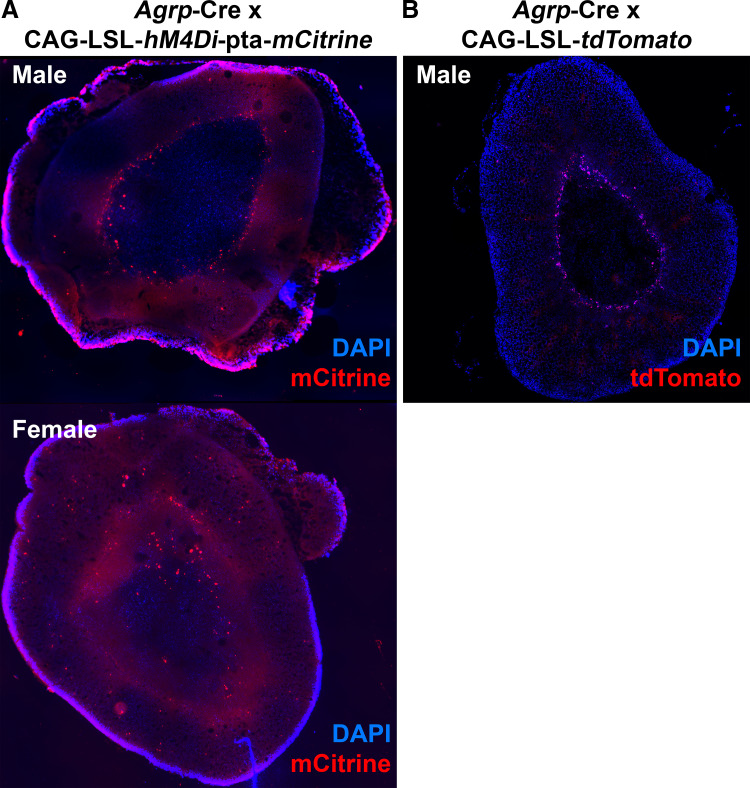
To examine whether Cre is expressed in the adrenal gland of Agrp-ires-Cre mice and to localize Agrp expression within specific cell types of the adrenal gland, we bred these animals with two different reporter strains. First, Agrp-ires-Cre mice were bred with mice harboring a conditionally activated CAG-LSL-hM4Di-pta-mCitrine construct in which Cre activity activates expression of an mCitrine fluorescent protein. Adrenal glands from both male and female offspring carrying both constructs were then examined by fluorescence microscopy. mCitrine-positive cells were observed within the adrenal gland, primarily at the periphery of the medulla (Fig. 7A). Complementing this approach, we also bred Agrp-ires-Cre mice with mice harboring a conditionally activated CAG-LSL-tdTomato construct in which Cre activity activates expression of the tdTomato fluorescent protein. Again, tdTomato-positive cells were observed within the adrenal gland, also primarily at the periphery of the medulla (Fig. 7B). Together, these two reporter strains both support the conclusion that Cre is active within specific cell types of the adrenal gland from Agrp-ires-Cre mice, most prominently at the interface between medulla and cortex.
Fig. 7.
Cre-recombinase activity in adrenal gland of agouti-related peptide (Agrp)-Cre mice. A: fluorescent mCitrine reporter (red pseudocolor) expression in cells of the adrenal gland of Agrp-Cre x CAG-LSL-hM4Di-pta-mCitrine mice. B: fluorescent tdTomato reporter expression within the adrenal gland of a male Agrp-Cre x CAG-LSL-tdTomato mouse.
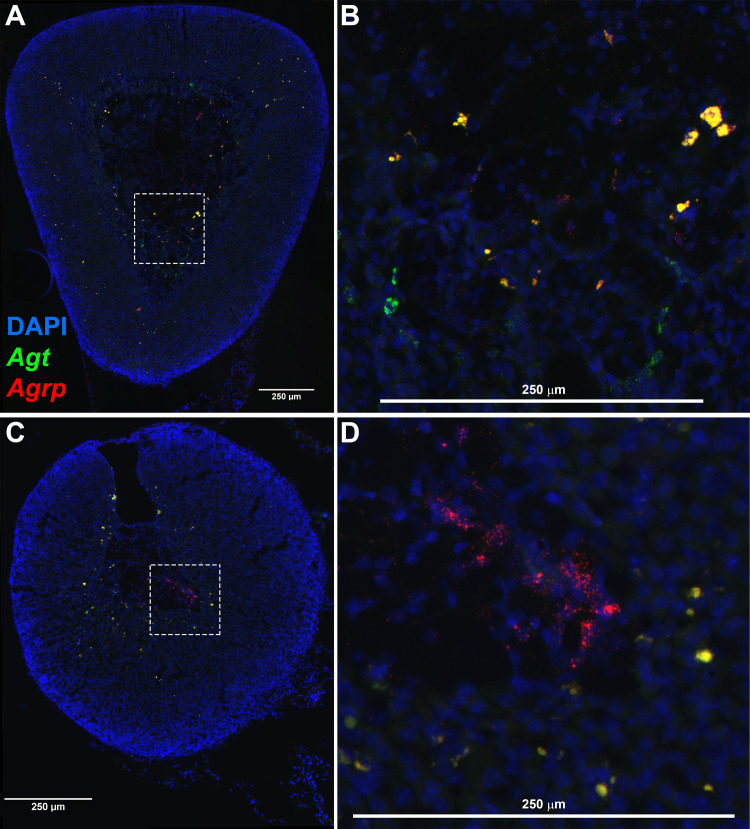
To confirm and extend the findings from activation of fluorescent reporters, we next examined localization and colocalization of endogenous Agrp and Agt expression within the mouse adrenal gland. In both female (Fig. 8, A and B) and male (Fig. 8, C and D) mice, expression of Agrp and Agt was observed in distinct cells in cortex and medulla, and coexpression was observed in cells that constitute the border between cortex and medulla. Notably, this pattern of colocalization is very similar to the pattern of activity of Cre in Agrp-ires-Cre mice as documented using fluorescent reporters (Fig. 7). Thus, we conclude that Agrp is expressed in specific cells of the mouse adrenal gland and Agt expression is frequently colocalized with this Agrp expression.
Fig. 8.
Fluorescent in situ hybridization (RNAscope) detection of endogenous angiotensinogen (Agt) and agouti-related peptide (Agrp) mRNA in adrenal gland of a wild-type mouse. A: in situ hybridization to localize nuclei (DAPI, blue), Agt (green), and Agrp (red) in adrenal gland of a wild-type female mouse. B: image from inset of A, shown at greater magnification. C: similar in situ hybridization in adrenal gland of a wild-type male mouse. D: image from inset of C, shown at greater magnification.
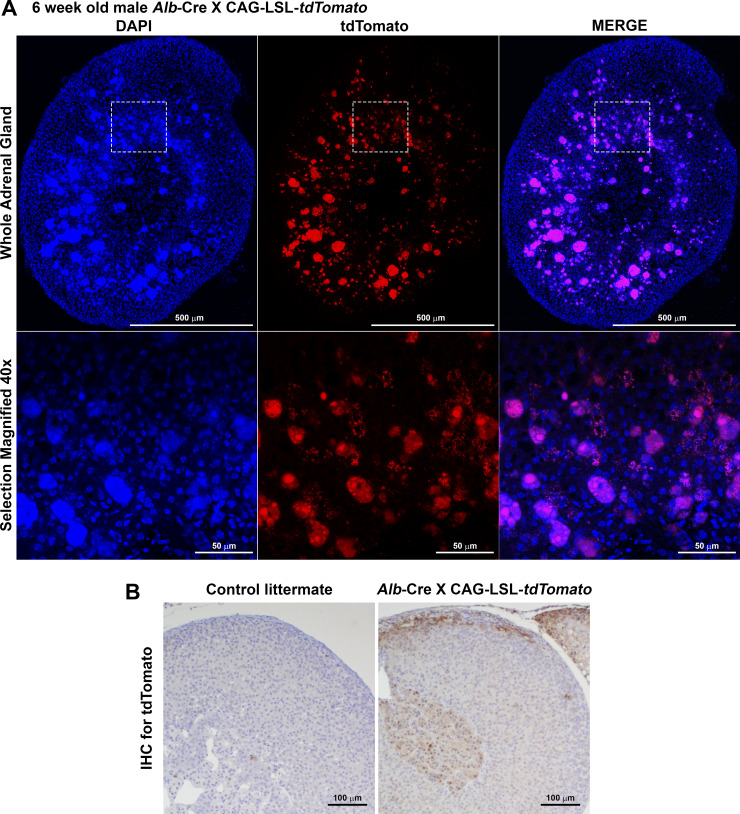
Alb is expressed in the adrenal gland.
Consistent with the detection of endogenous Alb expression in both whole adrenal gland and adrenal medulla (Fig. 6), Cre activity was detected in the adrenal gland of Alb-Cre mice, as determined by the activation of tdTomato reporter gene expression in an Alb-Cre x CAG-LSL-tdTomato mouse (Fig. 9). Interestingly, tdTomato fluorescence in this mouse model was observed in cells on the border between cortex and medulla similar to those identified in Agrp-ires-Cre mice, but oddly, fluorescence was also observed in large structures within the cortex that were unidentified and may represent a background or false fluorescence signal (Fig. 9A). Therefore, to confirm these findings, we performed diaminobenzidine (DAB) immunohistochemical staining for tdTomato protein in neighboring sections of adrenal gland from the same mouse and imaged those slides using light microscopy (Fig. 9B). Importantly, this DAB staining method also supports Cre activity within cells of the medulla-cortex border and possibly within additional cells within the medulla, but Cre activity within the cortex was less obvious. We conclude that the endogenous Alb gene and the Alb-Cre construct are both active in the mouse adrenal gland, possibly in multiple cell types of medulla and cortex.
Fig. 9.
Cre-recombinase activity in adrenal gland of albumin (Alb)-Cre mice. A: fluorescent tdTomato reporter within the adrenal gland of a male Alb-Cre x CAG-LSL-tdTomato mouse. B: immunohistochemical diaminobenzidine (DAB) staining for tdTomato in adrenal sections from the same male Alb-Cre x CAG-LSL-tdTomato mouse. IHC, immunohistochemistry.
Agrp and Agt expression in the kidney.
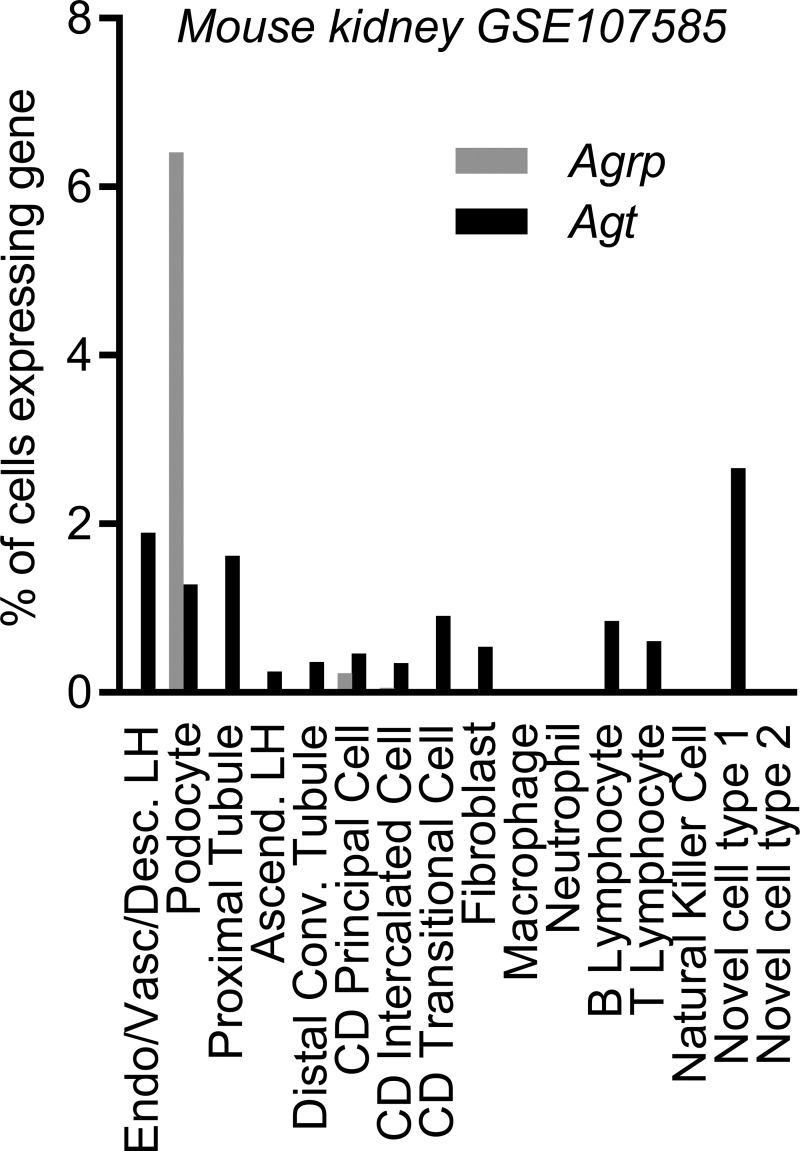
Together, these findings call into question conclusions previously drawn regarding the tissue-specific nature of disrupting Agt expression in “liver” and “kidney” upon kidney development and animal survival through use of the Alb-Cre and Kap-Cre models, respectively (38), and the protective effects of replacing RAS peptide production “in brain” of Agt-deficient mice via Gfap-mediated expression of an ANG II transgene (37). In silico reanalysis of a published single-cell RNA-sequencing data set describing individual cell types of the adult mouse kidney (GSE107585; 45) was next performed to probe for coexpression of Agrp and Agt in layers of this organ. Expression of Agt was observed in a small fraction of multiple cell types of the kidney (Fig. 10). In contrast, Agrp expression was far more restricted, with expression detected in ~6% of podocytes and a very small fraction of collecting duct cells. These findings support the concept that Agrp activity is essentially restricted to a minor fraction of podocytes. As few podocytes express Agt, disruption of Agt in AgtAgrp-KO mice is unlikely to have a major functional consequence in the kidney. By extension, the nonsignificant trends toward reduced Agt mRNA expression in kidney (Fig. 5B) and altered urine electrolyte handling (Table 3) may result from a physiological feedback mechanism, rather than a direct genetic disruption of the Agt gene in this tissue. Because so few animals were available for study, though, one must be careful not to overanalyze these end points; future studies of the expression of Agrp and its colocalization with Agt in the mouse kidney are warranted.
Fig. 10.
Expression of endogenous angiotensinogen (Agt) and agouti-related peptide (Agrp) in the mouse kidney. Expression of endogenous Agrp and Agt in distinct cell types in a previously published single-cell RNA-sequencing data set GSE107585 (45), describing adult mouse kidney. Ascend., ascending limb; CD, collecting duct; Conv., convoluted; Desc., descending limb; Endo, endothelium; LH, loop of Henle; Vasc, vascular.
DISCUSSION
A large body of work has previously demonstrated that selected neuronal populations express components of the RAS, including Agt, despite the fact that glial cells generate and secrete a large amount of AGT protein into the interstitial space and cerebrospinal fluid (1, 51). Here we attempted to further characterize the physiological significance of AGT production by the specific subset of neurons localized within the ARC that express Lepr and Agrp by employing Cre-Lox-mediated disruption of Agt within these cell types. First, we discovered that disruption of Agt in Lepr-expressing cells has no overt consequence on survival, growth, or metabolic control, which argues against the working hypothesis that leptin activates the ARC RAS through stimulation of local Agt expression in these neurons. Second, we discovered that disruption of the Agt gene in Agrp-expressing cells also seems to have no major consequence for metabolic control in adult mice, but surprisingly this manipulation caused a major and unexpected preweaning lethality.
The discovery that Agt deletion from Agrp-expressing cells resulted in a preweaning lethality was surprising because previous studies using cell-specific disruption of Agt have led to the general concept that liver-derived, circulating AGT protein is required for normal kidney development and survival to weaning. Agt-deficient (Agt-KO) mice were first described by Tanimoto et al. in 1994 (55) and Niimura et al. in 1995 (42). These mice exhibited significant decreases in systolic, diastolic, and mean blood pressure compared with heterozygous and wild-type littermate animals. Interestingly, 60% of Agt-KO mice did not survive until weaning, most likely because of severe kidney pathologies described in surviving adults (59). Also similar to the present AgtAgrp-KO mice, Agt-KO mice exhibited reduced aldosterone secretion (59). Similar to observations in Agt-KO mice, disruption of Agt in albumin-expressing cells (via Alb-Cre, which is often assumed to be liver specific) results in a reduction in circulating AGT protein that correlates with altered renal development and reduced preweaning survival, whereas disruption of the Agt gene in cells expressing the kidney androgen promoter (via Kap-Cre, largely restricted to proximal tubule) does not have the same effects (38). These publications precipitated the concept that liver-derived, circulating AGT protein is critical for development and survival. Critically, this interpretation requires the assumption that Agt disruption by Alb-Cre is truly restricted to liver. Furthermore, such studies have not thoroughly assessed the activity of Alb-Cre within the developing adrenal gland, the effect of reduced circulating AGT protein on local adrenal expression of Agt, or the development and function of the adrenal gland when circulating AGT protein is reduced.
Agrp is expressed in a specific subset of neurons of the ARC (51); however, Agrp is also strongly expressed in the adrenal gland of various species, and Agrp is known to function in an autocrine or paracrine manner within the adrenal gland. In addition to data presented above (Fig. 6), Agrp mRNA is detected in adrenal glands by RNA-sequencing/microarray studies of humans (30, 54) and mice (18–20, 43, 56), and Agrp is expressed by H295R human adrenal carcinoma cells (27). Gupta et al. have also recently provided compelling evidence that Agrp is expressed specifically within the adrenal medulla of mice via immunohistochemistry, flow cytometry, and reverse transcription-PCR (24). Similar evidence for medullary localization of Agrp expression in the adrenal gland comes from studies of ducks (39). Complementing these findings, several groups have also suggested that expression of the melanocortin Mc4r receptor and local paracrine action of AGRP within the adrenal gland contribute to the regulation of adrenal steroid production (3, 12, 14–16, 53). The concept that the adrenal gland also expresses components of the renin-angiotensin system, including Agt, has been supported by an array of studies throughout the last few decades. For example, in addition to the sequencing studies reanalyzed herein (Fig. 6), Agt expression in adrenal gland has been documented using in situ hybridization, immunohistochemistry, Northern blot, nuclease S1 analyses of RNA, and RNA-sequencing/microarray studies in humans (11, 47), cows (61), rats (4–6, 20, 40, 46, 50), and mice (17, 20, 43, 52, 56). Similarly, Agt is expressed by human adrenocortical NCI-H295 cells (25), aldosterone-producing adenoma from humans (62), hyperplastic adrenal glands from humans with Cushing’s syndrome (34), bovine chromaffin cells (61), and rat PC12 adrenal gland pheochromocytoma cells (28). Therefore, both Agt and Agrp are expressed in the adrenal gland of many species. By extension, disruption of the Agt gene within the adrenal gland of AgtAgrp-KO mice was not surprising. However, given the previous findings regarding developmental abnormalities resulting from Alb-Cre-mediated disruption of Agt, we were surprised to determine that Agt disruption in Agrp-expressing cells resulted in preweaning lethality, altered renal development, and adrenal steroid production that parallel previous observations from both whole body and Alb-Cre-mediated “liver-specific” Agt-deficient animals.
The observations that the disruption of Agt in Agrp-expressing cells had no effect on hepatic Agt expression or circulating AGT protein levels, along with the documentation of endogenous Alb gene expression in wild-type adrenal gland and evidence for Cre activity within the adrenal gland of Alb-Cre animals, therefore force the reinterpretation of previously published studies concerning the importance of tissue-specific expression of Agt for normal renal development and survival. Whereas previous interpretations conclude that liver-derived, circulating AGT is required for renal development and survival, we propose that it is instead adrenal-derived, paracrine/autocrine AGT that is critical for adrenal function and thereby renal development to ensure preweaning survival (Fig. 11). Such a reinterpretation of the relative importance of tissue-specific manipulations of Agt and the RAS for development and survival may also help explain the confusing observations of Lochard et al. (37), in which transgenic expression of an ANG II transgene via the Gfap promoter rescued development in whole body Agt-deficient mice, as Gfap (and by extension, angiotensin peptides in those transgenic animals) is expressed in the adrenal gland (Fig. 6).
Fig. 11.
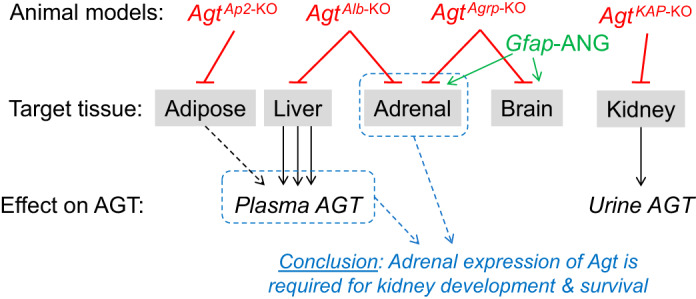
Working model. Tissue-specific disruption of angiotensinogen (Agt) has previously been performed using Cre-recombinase expressing lines that are assumed to be organ specific. Previous work using this approach led to the conclusion that liver (and adipose)-derived AGT is required for renal development and survival (38). The present study demonstrates that disruption of Agt in adrenal and brain tissue causes similar problems in renal development and survival and that Cre-recombinase driven by the albumin (Alb) promoter is unexpectedly active in adrenal gland. Similarly, the endogenous glial fibrillary acidic protein (Gfap) promoter is active in adrenal gland, prompting reconsideration of the mechanism by which expression of an angiotensin II (ANG II) transgene via this promoter rescues the lethality of global Agt knockout (37). Thus, these findings support the iconoclastic hypothesis that adrenal Agt (as opposed to hepatic-derived, circulating AGT protein) is required for renal development and survival, possibly through modulation of steroid synthesis and release. Ap2, adipocyte protein 2; Kap, kidney androgen response protein.
Given the exceptionally limited list of antihypertensives available and recommended or approved for use during hypertensive pregnancy complications (largely limited to methyldopa, labetalol, nifedipine, and hydrochlorothiazide), a more accurate and complete understanding of the mechanisms underlying fetal toxicity is desperately needed. RAS inhibition is contraindicated during pregnancy specifically because of the consequence of failed fetal kidney development. If it is determined that the loss of adrenal function mediates this failed renal development, it is conceivable that simultaneous adrenal modulation or steroid supplementation combined with administration of RAS inhibitors with limited placental transport may represent a novel approach to address maternal hypertensive cardiovascular complications during pregnancy.
Finally, it remains unclear how leptin promotes AT1A signaling within Agrp neurons. Data presented herein argue against the hypothesis that leptin promotes the local autocrine/paracrine RAS through increased Agt expression and thus ANG II ligand production for AT1A. As we previously reviewed (51), however, multiple possible alternative mechanisms exist that could explain leptin-AT1A cross-talk within the ARC. For example, leptin may promote any combination of Agtr1a expression, Agtr1a translation, AT1A posttranslational modifications, AT1A cell surface localization or cycling, or AT1A second-messenger coupling efficiency. Many future studies are required to examine each of these hypothesized mechanisms in detail.
Perspectives and Significance
In conclusion, leptin-mediated induction of Agt expression within the ARC appears dispensable for metabolic control despite our previous demonstration that this effect is dependent on AT1A receptors expressed by Lepr- and Agrp-expressing neurons. Furthermore, these data highlight a previously unappreciated but critical role for adrenal expression of Agt in normal development and survival. Future studies are required to further understand the developmental role of adrenal AGT. Furthermore, careful field-wide reevaluations of the interpretation of “liver”- and “glia”-specific genetic manipulations made via approaches that employ the Alb and Gfap promoters are warranted, as such targeting may result in relevant adrenal dysfunctions.
GRANTS
This work was supported by National Institutes of Health Grants P01 HL084207, R01 HL134850, HL007638, RR025439, and DK106104, American Heart Association Grants 15SFRN23730000, 18EIA33890055, 18PRE33960377, and 19POST34380239, and the Roy J. Carver Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.S., L.L.M., C.D.S., and J.L.G. conceived and designed research; S.A.S., L.L.M., G.D., C.N.P., K.B., V.O., K.E.C., J.G., N.A.P., and K.N.G.-C. performed experiments; S.A.S., L.L.M., G.D., C.N.P., K.B., V.O., K.E.C., J.G., N.A.P., K.N.G.-C., and J.L.G. analyzed data; S.A.S., G.D., K.B., K.E.C., J.G., M.J.P., K.N.G.-C., C.D.S., and J.L.G. interpreted results of experiments; S.A.S., J.G., K.N.G.-C., and J.L.G. prepared figures; S.A.S. drafted manuscript; S.A.S., L.L.M., G.D., C.N.P., K.B., V.O., K.E.C., M.J.P., K.N.G.-C., C.D.S., and J.L.G. edited and revised manuscript; S.A.S., L.L.M., G.D., C.N.P., K.B., V.O., K.E.C., N.A.P., M.J.P., K.N.G.-C., C.D.S., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the input of Drs. Huxing Cui, Kamal Rahmouni, and Julien A. Sebag and the technical assistance of the Comparative Pathology Laboratory in the University of Iowa Department of Pathology.
REFERENCES
- 1.Agassandian K, Grobe JL, Liu X, Agassandian M, Thompson AP, Sigmund CD, Cassell MD. Evidence for intraventricular secretion of angiotensinogen and angiotensin by the subfornical organ using transgenic mice. Am J Physiol Regul Integr Comp Physiol 312: R973–R981, 2017. doi: 10.1152/ajpregu.00511.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baver SB, Hope K, Guyot S, Bjørbaek C, Kaczorowski C, O’Connell KM. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci 34: 5486–5496, 2014. doi: 10.1523/JNEUROSCI.4861-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bicknell AB, Lomthaisong K, Gladwell RT, Lowry PJ. Agouti related protein in the rat adrenal cortex: implications for novel autocrine mechanisms modulating the actions of pro-opiomelanocortin peptides. J Neuroendocrinol 12: 977–982, 2000. doi: 10.1046/j.1365-2826.2000.00543.x. [DOI] [PubMed] [Google Scholar]
- 4.Bruder ED, Lee JJ, Widmaier EP, Raff H. Microarray and real-time PCR analysis of adrenal gland gene expression in the 7-day-old rat: effects of hypoxia from birth. Physiol Genomics 29: 193–200, 2007. doi: 10.1152/physiolgenomics.00245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell DJ, Habener JF. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest 78: 31–39, 1986. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell DJ, Habener JF. Hybridization in situ studies of angiotensinogen gene expression in rat adrenal and lung. Endocrinology 124: 218–222, 1989. doi: 10.1210/endo-124-1-218. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, Rosen ED, Lowell BB, Tsai LT. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 20: 484–496, 2017. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claflin KE, Grobe JL. Control of energy balance by the brain renin-angiotensin system. Curr Hypertens Rep 17: 38, 2015. doi: 10.1007/s11906-015-0549-x. [DOI] [PubMed] [Google Scholar]
- 9.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CML, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest 127: 1414–1424, 2017. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291: 2608–2613, 2001. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 11.Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, Li K, Blake J, Samaha RR, Nikolskaya T. A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol 6: 49, 2008. doi: 10.1186/1741-7007-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhillo WS, Small CJ, Gardiner JV, Bewick GA, Whitworth EJ, Jethwa PH, Seal LJ, Ghatei MA, Hinson JP, Bloom SR. Agouti-related protein has an inhibitory paracrine role in the rat adrenal gland. Biochem Biophys Res Commun 301: 102–107, 2003. doi: 10.1016/S0006-291X(02)02991-1. [DOI] [PubMed] [Google Scholar]
- 13.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Münzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, Tiganis T. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160: 88–104, 2015. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doghman M, Delagrange P, Berthelon MC, Durand P, Naville D, Bégeot M. Sustained inhibitory effect of agouti related protein on the ACTH-induced cortisol production by bovine cultured adrenal cells. Regul Pept 124: 215–219, 2005. doi: 10.1016/j.regpep.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Doghman M, Delagrange P, Blondet A, Berthelon MC, Durand P, Naville D, Bégeot M. Agouti-related protein antagonizes glucocorticoid production induced through melanocortin 4 receptor activation in bovine adrenal cells: a possible autocrine control. Endocrinology 145: 541–547, 2004. doi: 10.1210/en.2003-0605. [DOI] [PubMed] [Google Scholar]
- 16.Doghman M, Soltani Y, Rebuffet V, Naville D, Bégeot M. Role of Agouti-related protein in adrenal steroidogenesis. Mol Cell Endocrinol 265-266: 108–112, 2007. doi: 10.1016/j.mce.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Dzau VJ, Ellison KE, Brody T, Ingelfinger J, Pratt RE. A comparative study of the distributions of renin and angiotensinogen messenger ribonucleic acids in rat and mouse tissues. Endocrinology 120: 2334–2338, 1987. doi: 10.1210/endo-120-6-2334. [DOI] [PubMed] [Google Scholar]
- 18.Fries RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schoenbein GW, O’Connor DT. Neuroendocrine transcriptome in genetic hypertension: multiple changes in diverse adrenal physiological systems. Hypertension 43: 1301–1311, 2004. doi: 10.1161/01.HYP.0000127708.96195.e6. [DOI] [PubMed] [Google Scholar]
- 19.Friese RS, Gayen JR, Mahapatra NR, Schmid-Schönbein GW, O’Connor DT, Mahata SK. Global metabolic consequences of the chromogranin A-null model of hypertension: transcriptomic detection, pathway identification, and experimental verification. Physiol Genomics 40: 195–207, 2010. doi: 10.1152/physiolgenomics.00164.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friese RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schönbein GW, O’Connor DT. Common genetic mechanisms of blood pressure elevation in two independent rodent models of human essential hypertension. Am J Hypertens 18: 633–652, 2005. doi: 10.1016/j.amjhyper.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 21.Grobe JL. Comprehensive assessments of energy balance in mice. Methods Mol Biol 1614: 123–146, 2017. doi: 10.1007/978-1-4939-7030-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grobe JL, Rahmouni K, Liu X, Sigmund CD. Metabolic rate regulation by the renin-angiotensin system: brain vs. body. Pflügers Arch 465: 167–175, 2013. doi: 10.1007/s00424-012-1096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta R, Ma Y, Wang M, Whim MD. AgRP-expressing adrenal chromaffin cells are involved in the sympathetic response to fasting. Endocrinology 158: 2572–2584, 2017. doi: 10.1210/en.2016-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilbers U, Peters J, Bornstein SR, Correa FM, Jöhren O, Saavedra JM, Ehrhart-Bornstein M. Local renin-angiotensin system is involved in K+-induced aldosterone secretion from human adrenocortical NCI-H295 cells. Hypertension 33: 1025–1030, 1999. doi: 10.1161/01.HYP.33.4.1025. [DOI] [PubMed] [Google Scholar]
- 26.Hilzendeger AM, Morais RL, Todiras M, Plehm R, da Costa Goncalves A, Qadri F, Araujo RC, Gross V, Nakaie CR, Casarini DE, Carmona AK, Bader M, Pesquero JB. Leptin regulates ACE activity in mice. J Mol Med (Berl) 88: 899–907, 2010. doi: 10.1007/s00109-010-0649-7. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Dietz JD, Xia C, Knight DR, Loging WT, Smith AH, Yuan H, Perry DA, Keiser J. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology 150: 2211–2219, 2009. doi: 10.1210/en.2008-1512. [DOI] [PubMed] [Google Scholar]
- 28.Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119: 1041–1054, 2004. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 29.Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol Heart Circ Physiol 251: H261–H268, 1986. doi: 10.1152/ajpheart.1986.251.2.H261. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, Shoemaker DD. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science 302: 2141–2144, 2003. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 31.Könner AC, Klöckener T, Brüning JC. Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiol Behav 97: 632–638, 2009. doi: 10.1016/j.physbeh.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. Blockade of angiotensin receptors in the anterior hypothalamic preoptic area lowers blood pressure in DOCA-salt hypertensive rats. Hypertens Res 23: 109–118, 2000. doi: 10.1291/hypres.23.109. [DOI] [PubMed] [Google Scholar]
- 33.Kwon O, Kim KW, Kim MS. Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci 73: 1457–1477, 2016. doi: 10.1007/s00018-016-2133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampron A, Bourdeau I, Hamet P, Tremblay J, Lacroix A. Whole genome expression profiling of glucose-dependent insulinotropic peptide (GIP)- and adrenocorticotropin-dependent adrenal hyperplasias reveals novel targets for the study of GIP-dependent Cushing’s syndrome. J Clin Endocrinol Metab 91: 3611–3618, 2006. doi: 10.1210/jc.2006-0221. [DOI] [PubMed] [Google Scholar]
- 35.Littlejohn NK, Grobe JL. Opposing tissue-specific roles of angiotensin in the pathogenesis of obesity, and implications for obesity-related hypertension. Am J Physiol Regul Integr Comp Physiol 309: R1463–R1473, 2015. doi: 10.1152/ajpregu.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Lochard N, Silversides DW, van Kats JP, Mercure C, Reudelhuber TL. Brain-specific restoration of angiotensin II corrects renal defects seen in angiotensinogen-deficient mice. J Biol Chem 278: 2184–2189, 2003. doi: 10.1074/jbc.M209933200. [DOI] [PubMed] [Google Scholar]
- 38.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol 23: 1181–1189, 2012. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirabella N, Esposito V, Squillacioti C, De Luca A, Paino G. Expression of agouti-related protein (AgRP) in the hypothalamus and adrenal gland of the duck (Anas platyrhynchos). Anat Embryol (Berl) 209: 137–141, 2004. doi: 10.1007/s00429-004-0431-0. [DOI] [PubMed] [Google Scholar]
- 40.Mulrow PJ, Kusano E, Baba K, Shier D, Doi Y, Franco-Saenz R, Stoner G, Rapp J. Adrenal renin: a possible local hormonal regulator of aldosterone production. Cardiovasc Drugs Ther 2: 463–471, 1988. doi: 10.1007/BF00051184. [DOI] [PubMed] [Google Scholar]
- 41.National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 42.Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest 96: 2947–2954, 1995. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4: 163–173, 2006. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Park CG, Leenen FH. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J Korean Med Sci 16: 553–557, 2001. doi: 10.3346/jkms.2001.16.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Suszták K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters J, Kränzlin B, Schaeffer S, Zimmer J, Resch S, Bachmann S, Gretz N, Hackenthal E. Presence of renin within intramitochondrial dense bodies of the rat adrenal cortex. Am J Physiol Endocrinol Metab 271: E439–E450, 1996. doi: 10.1152/ajpendo.1996.271.3.E439. [DOI] [PubMed] [Google Scholar]
- 47.Racz K, Pinet F, Gasc JM, Guyene TT, Corvol P. Coexpression of renin, angiotensinogen, and their messenger ribonucleic acids in adrenal tissues. J Clin Endocrinol Metab 75: 730–737, 1992. doi: 10.1210/jcem.75.3.1381371. [DOI] [PubMed] [Google Scholar]
- 48.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 58: 536–542, 2009. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpár A, Mulder J, Clotman F, Keimpema E, Hsueh B, Crow AK, Martens H, Schwindling C, Calvigioni D, Bains JS, Máté Z, Szabó G, Yanagawa Y, Zhang MD, Rendeiro A, Farlik M, Uhlén M, Wulff P, Bock C, Broberger C, Deisseroth K, Hökfelt T, Linnarsson S, Horvath TL, Harkany T. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci 20: 176–188, 2017. doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sander M, Ganten D, Mellon SH. Role of adrenal renin in the regulation of adrenal steroidogenesis by corticotropin. Proc Natl Acad Sci USA 91: 148–152, 1994. doi: 10.1073/pnas.91.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sapouckey SA, Deng G, Sigmund CD, Grobe JL. Potential mechanisms of hypothalamic renin-angiotensin system activation by leptin and DOCA-salt for the control of resting metabolism. Physiol Genomics 49: 722–732, 2017. doi: 10.1152/physiolgenomics.00087.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider J, Lother A, Hein L, Gilsbach R. Chronic cardiac pressure overload induces adrenal medulla hypertrophy and increased catecholamine synthesis. Basic Res Cardiol 106: 591–602, 2011. doi: 10.1007/s00395-011-0166-z. [DOI] [PubMed] [Google Scholar]
- 53.Soltani Y, Doghman M, Gout J, Rebuffet V, Vigier M, Bekkouche FH, Naville D, Begeot M. Hormonal regulation of the mouse adrenal melanocortinergic system. J Endocrinol Invest 32: 46–51, 2009. doi: 10.1007/BF03345678. [DOI] [PubMed] [Google Scholar]
- 54.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99: 4465–4470, 2002. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K. Angiotensinogen-deficient mice with hypotension. J Biol Chem 269: 31334–31337, 1994. [PubMed] [Google Scholar]
- 56.Thorrez L, Van Deun K, Tranchevent LC, Van Lommel L, Engelen K, Marchal K, Moreau Y, Van Mechelen I, Schuit F. Using ribosomal protein genes as reference: a tale of caution. PLoS One 3: e1854, 2008. doi: 10.1371/journal.pone.0001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10: 679–689, 2017. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11: 998–1000, 2008. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umemura S, Kihara M, Sumida Y, Yabana M, Ishigami T, Tamura K, Nyui N, Hibi K, Murakami K, Fukamizu A, Ishii M. Endocrinological abnormalities in angiotensinogen-gene knockout mice: studies of hormonal responses to dietary salt loading. J Hypertens 16: 285–289, 1998. doi: 10.1097/00004872-199816030-00005. [DOI] [PubMed] [Google Scholar]
- 60.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14: 22–29, 2012. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang JM, Slembrouck D, Tan J, Arckens L, Leenen FH, Courtoy PJ, De Potter WP. Presence of cellular renin-angiotensin system in chromaffin cells of bovine adrenal medulla. Am J Physiol Heart Circ Physiol 283: H1811–H1818, 2002. doi: 10.1152/ajpheart.01092.2001. [DOI] [PubMed] [Google Scholar]
- 62.Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol 195: 39–48, 2007. doi: 10.1677/JOE-07-0037. [DOI] [PubMed] [Google Scholar]
- 63.Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A, Cassis LA. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol 302: R244–R251, 2012. doi: 10.1152/ajpregu.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]