Abstract
Military and civilian emergency situations often involve prolonged exposures to warm and very humid environments. We tested the hypothesis that increases in core temperature and body fluid losses during prolonged exposure to warm and very humid environments are dependent on dry bulb temperature. On three occasions, 15 healthy males (23 ± 3 yr) sat in 32.1 ± 0.1°C, 33.1 ± 0.2°C, or 35.0 ± 0.1°C and 95 ± 2% relative humidity normobaric environments for 8 h. Core temperature (telemetry pill) and percent change in body weight, an index of changes in total body water occurring secondary to sweat loss, were measured every hour. Linear regression models were fit to core temperature (over the final 4 h) and percent changes in body weight (over the entire 8 h) for each subject. These equations were used to predict core temperature and percent changes in body weight for up to 24 h. At the end of the 8-h exposure, core temperature was higher in 35°C (38.2 ± 0.4°C, P < 0.01) compared with 32°C (37.2 ± 0.2°C) and 33°C (37.5 ± 0.2°C). At this time, percent changes in body weight were greater in 35°C (−1.9 ± 0.5%) compared with 32°C (−1.4 ± 0.3%, P < 0.01) but not 33°C (−1.6 ± 0.6%, P = 0.17). At 24 h, predicted core temperature was higher in 35°C (39.2 ± 1.4°C, P < 0.01) compared with 32°C (37.6 ± 0.9°C) and 33°C (37.5 ± 0.9°C), and predicted percent changes in body weight were greater in 35°C (−6.1 ± 2.4%) compared with 32°C (−4.6 ± 1.5%, P = 0.04) but not 33°C (−5.3 ± 2.0%, P = 0.43). Prolonged exposure to 35°C, but not 32°C or 33°C, dry bulb temperatures and high humidity is uncompensable heat stress, which exacerbates body fluid losses.
Keywords: heat stress, hyperthermia, hypohydration, modeling, temperature regulation
INTRODUCTION
In the event of disabled submarine, a Pressurized Rescue Module (PRM) may be deployed. The PRM is a small pressurized vehicle capable of holding the rescued submariners and safely delivering them to the surface. Safe deployment of the PRM is dependent on understanding and mitigating the possible challenges in the event of module failure. For example, if the PRM were to become disabled, the environmental conditions could quickly challenge the health and safety of those inside. One such circumstance is a blower failure. The blower helps control the thermal conditions within the PRM. Extrapolating from models predicting how environmental parameters change over time in disabled submarine scenarios (5), it is likely that during a blower failure the air in the PRM will quickly become saturated with water because of the high number of people in a small enclosed space. For similar reasons, dry bulb temperature could quickly rise to 32°C and is predicted to approach 35°C when the PRM is in relatively warm waters. Under such warm conditions, the ambient humidity could rise to levels exceeding 95% relative humidity (RH) due to the additional loss of body water due to sweating.
Recommendations for the management of heat stress in a disabled submarine scenario indicate that dry bulb temperatures between 32°C and 35°C with >95% RH present situations in which submariners will likely be encouraged to consider escaping because of excessive heat stress (20). This risk is exemplified by the potential worst-case scenario (i.e., dry bulb temperature = 35°C, >95% RH). These environmental conditions have been theorized to present a thermally uncompensable condition in which heat production even in resting conditions cannot be fully offset by heat loss promoted by sweating and cutaneous vasodilation (37). Therefore, by increasing core body temperature and stimulating profound sweat loss, the thermal environment in the disabled PRM can, in theory, become dangerous during exposure to these conditions, particularly when the exposures are prolonged. Thus, in the effort to determine emergency resource allocation and/or to aid rescue decision making, there is a need to formally determine the thermoregulatory and body fluid challenges associated with prolonged (i.e., up to 24 h) exposure to warm and extremely humid environments.
To our knowledge there are no models, or empirical data to base them on, that are capable of accurately predicting increases in core temperature and/or body fluid losses over 24 h in a nearly saturated (>95% RH) environment with dry bulb temperatures in excess of 32°C. Current models probably do not accurately predict core temperature or body fluid losses when dry bulb temperature is ≥33°C and humidity is ≥95% RH (5). This is likely because the model predicts that skin temperature will increase linearly with increases in dry bulb temperature above ~33°C (5), which may or may not be physiological. Notably, some of the physiological responses to prolonged exposures to warm and extremely humid environments have been described in occupational, military, and laboratory settings (3, 14, 17, 28). Isolated observations within some of these studies generally support that conditions analogous to a dry bulb temperature of 35°C with >95% RH are thermally uncompensable (3, 17). However, most of these observations were conducted during physical work scenarios, which may not be applicable in resting situations (as would occur in a disabled PRM). With this background, the purpose of our study was to quantify the thermoregulatory responses during 8 h of exposure to normobaric warm and extremely humid environments and to predict increases in core temperature and body fluid losses incurred in these scenarios for up to 24 h. We hypothesized that the measured (through 8 h) and predicted (through 24 h) increases in core temperature and body fluid losses during exposure to warm (dry bulb temperatures: 32–35°C) and 95% RH environments are dependent on dry bulb temperature.
METHODS
Ethical approval.
The study and informed consent were approved by the Institutional Review Board at the University at Buffalo. The study conformed to the standards set by the Declaration of Helsinki, except for registration in a database. Before completing any study-related activities, each subject was fully informed of the experimental procedures and possible risks before giving informed written consent.
Subjects.
Fifteen healthy males participated in this study. The subject characteristics were age: 23 ± 3 yr, height: 179 ± 9 cm, weight: 84.5 ± 18.4 kg, and body surface area: 2.0 ± 0.3 m2. All subjects were nonsmokers, not taking medications, and reported to be free from any known cardiovascular, metabolic, neurological, or renal diseases. Subjects were not heat acclimated and self-reported to regularly engage in physical activity. Females were excluded because females make up a very small percentage of the United States Submarine Force, with the male-to-female ratio being so small that it would be difficult to design a study to represent this ratio, particularly to observe any differences between sexes.
Instrumentation and measurements.
Height and weight were measured with a stadiometer and scale (Satorius, Bohemia, NY). Dry bulb temperature, relative humidity, and barometric pressure were measured within ~1 meter of the subject’s torso every 10 min and are presented every 60 min herein (Kestrel 3000 Weather Meter, Kestrel Instruments, Boothwyn, PA). Nude body weight was measured pre- and postexposure while clothed body weight was measured before and every 60 min during each exposure after towel drying.
Approximately 90 min before experimental testing, each subject swallowed a telemetry pill (HQ Inc., Palmetto, FL) for the measurement of core temperature. Core temperature data were recorded every 10 min and are presented before and every 60 min during exposure. The timing of ingestion was chosen to ensure the temperature pill stayed within the gastrointestinal tract throughout the entire duration of experimental procedures (an ~10-h period), which could not be guaranteed if subjects ingested the pill the recommended 6–8 h before data collection. Moreover, this approach provides a valid measure of core temperature, particularly when drinking is prohibited (31).
Mean skin temperature was measured continually as the weighted average of 12 thermochron iButtons (Maxim Integrated, San Jose, CA) using the following equation: (0.07 × forehead) + (0.14 × forearm) + (0.5 × dorsal hand) + (0.07 × lower leg) + [0.13 × (shin + calf)/2] + [0.19 × (hamstring + anterior thigh)/2] + [0.35 × (chest + abdomen + subscapula + lower back)/4] (18), which has excellent agreement with measures of mean skin temperature incorporating more measurement locations (26). Relative humidity of the skin was measured continually using six hydrochron iButtons (Maxim Integrated) placed within 1–2 cm of a thermochron iButton at the calf, anterior thigh, chest, abdomen, subscapula, and lower back. At each location, the iButton was raised 6 mm off the skin using a custom-made capsule that allowed airflow to pass through. The distance of 6 mm was chosen because it ensured that the humidity sensor of the iButton would not become artificially supersaturated because of the incidental occurrence of a droplet of sweat entering the hygrosensor, which may not be directly related to physiological changes (39, 41, 42). That said, it should be noted that supersaturation (i.e., a reading of 100% RH) was likely to occur in the present study, but this was likely for physiological reasons (e.g., when the microclimate becomes fully saturated with water vapor). Thus, for locations under clothing, a reading of 100% RH was considered to be a physiologically relevant measurement. Skin humidity data were not collected in three subjects. Thus, skin humidity-related measures are presented as n = 12. Skin temperature and skin humidity data are presented as a 5-min average immediately before and every 60 min during exposure.
The rates of oxygen uptake and carbon dioxide production were measured using indirect calorimetry over a 10-min period before and every 60 min during exposure. Because of unforeseen technical issues, these data were collected using the Biopac (Goleta, CA) metabolic system (n = 9) or the ParvoMedics (Provo, UT) metabolic cart (n = 5). Notably, the methods for collecting metabolic data were kept consistent within a subject. Indirect calorimetry measurements were unable to be collected in one subject. Thus, these data are presented as n = 14.
Forearm blood flow was measured before and every 60 min during exposure on the left arm via venous occlusion plethysmography (43) to provide an estimate of skin blood flow (7). This was accomplished by placing a strain gauge (D.E. Hokanson, Bellevue, WA) around the widest circumference of the forearm and pressure cuffs on the wrist and upper arm proximal to the elbow. The strain gauge was kept in the same location throughout all experimental testing. During each measurement period, the wrist cuff was inflated to 250 mmHg while the cuff on the upper arm cycled between 0 and 50 mmHg every 8 s, thereby temporarily occluding venous return. Forearm blood flow during each cycle was determined from the slope of the increase in forearm volume determined from the strain gauge and is presented as the average of six consecutive measurements at each measurement time period (44). Plethysmography data were collected at 50 Hz via a data acquisition system (MP150; Biopac). Systolic and diastolic blood pressure were measured manually in duplicate. Heart rate was recorded continuously from a standard heart rate monitor (Polar Electro, Bethpage, NY) and are reported before and every 60 min during exposure.
Venous blood and urine samples were collected before and 4 and 8 h into each exposure. This sample interval was chosen because it would have been unlikely that subjects could micturate more often during exposure to these conditions without fluid replacement. Moreover, the venous blood samples provided information that was in support of the primary purpose of the study. Thus, more frequent sampling would have unnecessarily increased subject discomfort, study costs, etc. Hemoglobin was measured in duplicate using the Hemopoint H2 (Alere, Orlando, FL), and hematocrit was measured in triplicate using microcentrifugation. Plasma osmolality was measured in duplicate via freezing-point depression (model 3250; Advanced Instruments, Norwood, MA). Urine specific gravity was measured in duplicate using a refractometer (Atago, Bellevue, WA).
Experimental protocol.
Subjects visited the laboratory on four occasions separated by at least 7 days. Visit one was the screening and familiarization visit, while visits 2–4 were the experimental trials. The experimental trials consisted of 8 h of seated exposure to environments with a dry bulb temperature 32°C, 33°C, and 35°C and ~95% relative humidity. The average dry bulb temperature, relative humidity, wet bulb temperature, and water vapor pressure over the 8-h exposures are presented in Table 1. These trials were completed in a random order, and subjects were blinded to the experimental conditions. All experimental testing was completed throughout the calendar year in Buffalo, NY, a climate that has been shown to induce minimal heat acclimatization (1).
Table 1.
Average ambient conditions throughout the 8-h exposures
| Trial | Dry Bulb Temperature, °C | Relative Humidity, % | Wet Bulb Temperature, °C | Water Vapor Pressure, kPa |
|---|---|---|---|---|
| 32°C | 32.1 ± 0.1 | 95 ± 2 | 31.6 ± 0.5 | 4.6 ± 0.1 |
| 33°C | 33.1 ± 0.2* | 94 ± 2 | 32.1 ± 0.4* | 4.8 ± 0.1* |
| 35°C | 35.0 ± 0.1*† | 96 ± 2† | 34.4 ± 0.5*† | 5.4 ± 0.1*† |
Data are means ± SD; n = 15 subjects.
Different from 32°C trial (P ≤ 0.02).
Different from 33°C trial (P ≤ 0.01).
The dry bulb temperatures employed in this study were chosen because a dry bulb temperature of: 1) 32°C is the dry bulb temperature most commonly predicted to occur during a blower failure in a disabled PRM (personal communications with United States Navy Undersea Medical Officers), 2) 33°C is the approximate dry bulb temperature upon which current models for predicting increases in core temperature and/or body fluid losses likely begin to become inadequate during exposure to extremely humid environments (5), and 3) 35°C is the upper limit temperature of what could occur during a blower failure in a disabled PRM (personal communications with U.S. Navy Undersea Medical Officers), which also represents conditions theorized to present a thermally uncompensable condition during rest (37). Notably, in a disabled PRM scenario, the environmental conditions will probably worsen progressively over time. However, it is expected that this would occur quickly because of the high number of people in a small space, although the exact dynamics are unknown. Nevertheless, by design, the environmental conditions studied herein were stable throughout the 8-h exposure. This model likely simulated the worst-case scenarios, with exposure to extreme environments occurring upon entering the environmental chamber and not progressively worsening over time. Despite this potential limitation, we expected that the combination of conditions studied herein would allow for inferences to be made regarding the thermal compensability of the environment at any given time.
Subjects avoided exercise, alcohol, and caffeine for at least 12 h before arrival at the laboratory, and all subjects ate a light meal ~2 h before arrival. Upon arrival, subjects provided a urine sample by completely voiding their bladder in a collection urinal. Euhydration, defined as a urine specific gravity ≤1.020 (34), was confirmed using this urine sample. The actual measured urine specific gravity values upon arrival at the laboratory were 32°C: 1.010 ± 0.006, 33°C: 1.009 ± 0.007, and 35°C: 1.009 ± 0.006, and there were no differences between trials (P = 0.78). After this, subjects drank 250 mL of cool tap water to promote urine production over the subsequent 60 min. Following water consumption, subjects sat and rested quietly for 60 min. During this rest period, a baseline venous blood sample was obtained. After 60 min had elapsed, subjects voided their bladder in a separate urinal to establish a baseline urine flow rate over this 1-h period. After this baseline urine sample, the preexposure data (time = 0 h) were recorded in a moderate thermal environment (dry bulb temperature: 25.5 ± 1.1°C, 39 ± 14% RH), after which the 8-h exposure commenced with data collected as noted above.
Subjects wore a standard uniform of long pants, a short sleeve cotton t-shirt, and athletic shoes at all times. The estimated insulation of the clothing ensemble is 0.47 clo (25). All experimental visits commenced in the morning and took place at the same time of day to control for circadian effects. Subjects remained seated on a mesh chair throughout the duration of each exposure, and subjects were not allowed to drink at any time during the experimental trials.
Calculations.
Ambient water vapor pressure (24) and wet bulb temperature (38) were calculated from dry bulb temperature and relative humidity. Body surface area was calculated from height and weight (13). Mean arterial pressure was calculated as diastolic pressure plus one-third pulse pressure. Forearm vascular conductance was calculated as forearm blood flow divided by mean arterial pressure (7). Percentage changes in plasma volume were estimated from changes in hemoglobin and hematocrit (12). Urine flow rate was calculated as urine volume divided by time. All body weight measurements were corrected for urinary fluid losses. Percentage changes in nude body weight provided an accurate measure of body fluid losses (9) and average hourly sweat rate. Temporal changes in body fluid loss throughout experimental testing were indexed from percentage changes in clothed body weight, as we have done previously (35). This was deemed to be a reasonable estimate because sweat trapped in clothing, determined as pre- to postexposure changes in clothing weight, did not differ between trials (32°C: 0.09 ± 0.19 liters, 33°C: 0.13 ± 0.16 liters, 35°C: 0.14 ± 0.15 liters, P = 0.63). Changes in clothed body weight were not corrected for sweat trapped in clothing given that the temporal dynamics of the clothing becoming saturated with sweat were unknown.
The rate of dry heat loss was estimated as the sum of the rates of heat loss from convection and radiation, which were calculated using standard equations involving mean skin temperature, air velocity (estimated as 0.2 m/s, i.e., still air), and dry bulb temperature (11). Conductive heat exchange between the chair and the subject was considered negligible. The rate of metabolic heat production was calculated from oxygen uptake and the respiratory exchange ratio measured via indirect calorimetry (11).
Relative humidity and skin temperature at each measurement location were used to determine the local partial pressure of water on the skin (Psk) using standard calculations as previously reported (39, 40). The unweighted average of each local Psk measured across the eight locations was used to calculate the rate of evaporative heat loss for still air (11). The rate of evaporative heat loss data during each hour of exposure (in W) was converted to total heat loss (in kJ) and summed over the 8-h exposure, providing an index of total evaporative heat loss. These data were used to estimate sweating efficiency, which was calculated as the ratio of total evaporative heat loss and potential evaporative heat loss, and expressed as a percentage (11). Potential evaporative heat loss over the entire exposure was calculated as the product of the change in nude body weight (i.e., sweat loss over the entire exposure period, in grams) and the latent heat of vaporization of sweat (2,246 J/g). Nude body weight was used in these calculations to provide the most accurate measure of sweat loss, avoiding the potential confounding effect of sweat trapped in clothing that would underestimate sweat loss.
Statistical analyses.
Average ambient conditions and data derived from changes in nude body weight were analyzed using a one-way repeated-measures ANOVA. Thermoregulatory, cardiovascular, and hydration data collected over time were analyzed using two-way repeated-measures ANOVA (trial × time).
Predicted changes in core temperature following 12 and 24 h of exposure to the experimental conditions were estimated using linear regression analyses that were fit over the final 4 h of exposure for each subject in each trial (Fig. 1A). This regression line was only fit to the 4 h to account for any thermoregulatory adjustments occurring over the initial 4-h period. Thus, the slope of the regression line provided an objective measure of the compensability of the environment, with a slope greater than zero indicative of the thermal environment being uncompensable. The obtained regression line slope and y-intercept data were analyzed using both a one-way repeated-measures ANOVA to examine differences between trials. Slope data were also analyzed using a one-sample t test, which permitted an analysis of whether each slope differed from zero. On occasion, a negative slope in the regression line resulted in predicted core temperatures at 12 or 24 h below preexposure levels. In these instances, baseline (time = 0 h) core temperature values were substituted, given that core temperature values below baseline would be nonphysiological in the present paradigm. Measured and predicted changes in core temperature over 8, 12, and 24 h were analyzed using a two-way repeated-measures ANOVA. Predicted changes in percent body weight loss following 12 h and 24 h of exposure to the experimental conditions were also estimated using linear regression analyses that were fit to the entire 8 h of exposure for each subject in each trial with the y-intercept forced through zero (Fig. 1B). It was deemed necessary to force the y-intercept through zero given that there was no physiological rationale that the y-intercept should shift over time in the present experiment (i.e., by design, every subject should start at a 0% change in body weight). Measured and predicted changes in percent body weight loss over 8, 12, and 24 h were analyzed using a two-way repeated-measures ANOVA. The obtained regression line slope data were also analyzed using a one-way repeated-measures ANOVA to examine differences between trials. The use of linear regression models to predicted core temperature and percent changes in body weight loss was determined a priori, and this decision was confirmed upon visual examination of the data.
Fig. 1.
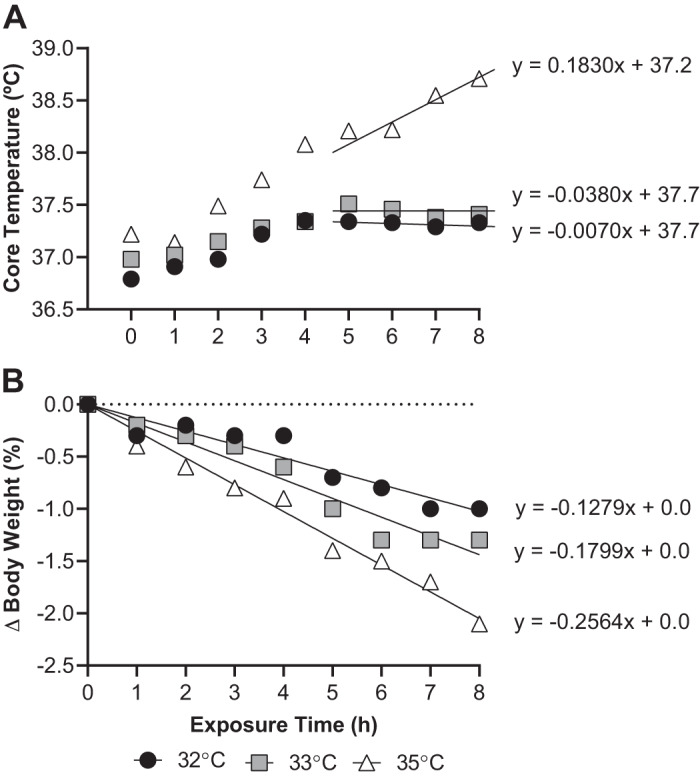
Typical tracing depicting how individual core temperature (A) and percentage changes in body weight (B) were fit using linear regression line. The resulting linear equation was used to predict the core temperatures and percentage changes in body weight occurring subsequent to 12 and 24 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity.
In all instances, when an ANOVA revealed a significant F value, post hoc Tukey’s adjusted pairwise comparisons were made. All data were analyzed using Prism software (version 8; GraphPad Software, La Jolla, CA). A priori statistical significance was set at P ≤ 0.05, and actual P values are reported where possible. Data are reported as means ± SD or as box-and-whisker plots where possible.
RESULTS
Ambient conditions.
By design, average dry bulb temperature, wet bulb temperature, and water vapor pressure were highest in the 35°C trial and lowest in the 32°C trial (P ≤ 0.02, Table 1). Average relative humidity did not statistically differ between the 32°C and 33°C trials (P = 0.56) nor between the 32°C and 35°C trials (P = 0.11, Table 1). However, average relative humidity was incidentally 2 ± 2% higher in the 35°C trial compared with the 33°C trial (P < 0.01, Table 1).
Body temperatures.
Core temperature increased over time in all three trials (P < 0.01, Fig. 2A). The magnitude of increase in core temperature did not statistically differ between the 32°C (0.6 ± 0.3°C) and 33°C (0.7 ± 0.3°C) trials (P = 0.68). However, the increase in core temperature was higher in the 35°C trial (1.4 ± 0.4°C) compared with both the 32°C and 33°C trials (P < 0.01, Fig. 2A). Mean skin temperature was highest in the 35°C trial throughout the 8-h exposure (P < 0.01) while mean skin temperature was statistically lower in the 32°C trial compared with the 33°C trial during the fourth, fifth, and seventh hour of exposure (P ≤ 0.03, Fig. 2B).
Fig. 2.
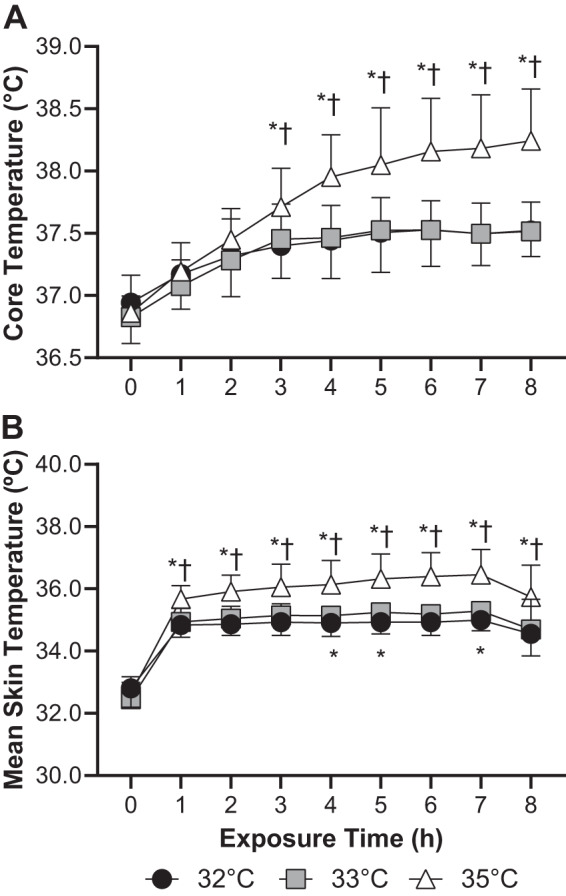
Core temperature (A) and mean skin temperature (B) during 8 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity; n = 15 subjects, means ± SD. *Different from 32°C trial (P ≤ 0.03). †Different from 33°C trial (P < 0.01).
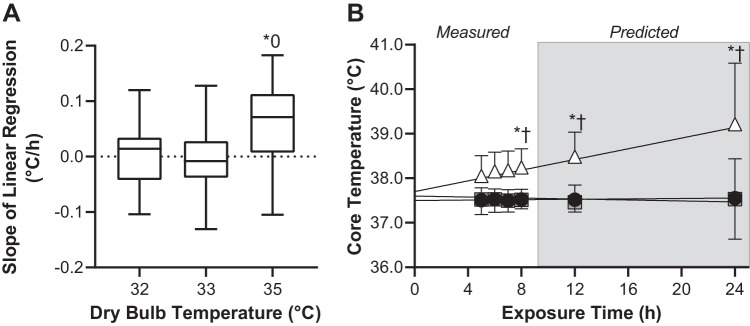
The slope of the linear regression line plotted over the final 4 h of exposure did not statistically differ from zero in the 32°C (P = 0.89) and 33°C (P = 0.73) trials but was greater than zero in the 35°C trial (P = 0.01, Fig. 3A). The slope of the linear regression line did not statistically differ between the 32°C and 33°C trials (P = 0.95), was higher in the 35°C trial compared with the 33°C trial (P = 0.03), but did not statistically differ between the 35°C trial and the 33°C trial (P = 0.06, Fig. 3A). The y-intercept for these linear regression lines did not differ between trials (P = 0.92). The obtained slope and y-intercept for each condition were 32°C: slope = 0.0023 ± 0.0597°C/h, y-intercept = 37.5 ± 0.6°C; 33°C: slope = −0.0055 ± 0.0612°C/h, y-intercept = 37.6 ± 0.5°C; 35°C: slope = 0.0601 ± 0.0783°C/h, y-intercept = 37.8 ± 0.7°C. Measured and predicted core temperatures did not statistically differ at any time during the 32°C and 33°C trials (P ≥ 0.86) but were higher in the 35°C trial compared with both the 32°C and 33°C trials at all time points (P < 0.01, Fig. 3B).
Fig. 3.
The slope of the linear regression line, an index of thermal compensability, during 8 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity (box-and-whisker plot, A) and predicted core temperatures occurring subsequent to 12 and 24 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity (means ± SD, B). For visual purposes, average linear regression lines in B are also presented together with average data from which these regression lines were derived. Please note that the statistical analysis for these data involved only data from 8, 12, and 24 h of exposure; n = 15 subjects. 0Different from zero (P = 0.01). *Different from 32°C trial (P < 0.01). †Different from 33°C trial (P ≤ 0.03).
Hydration.
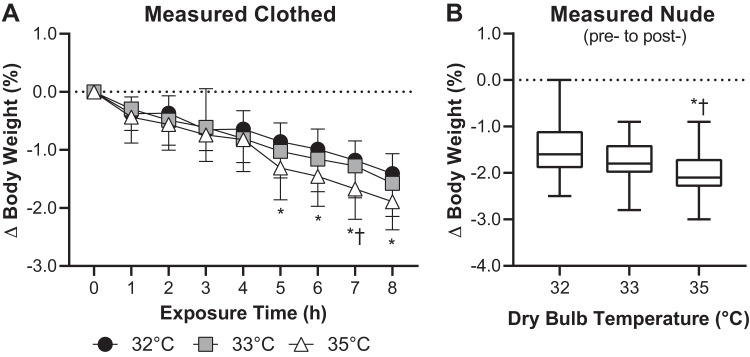
Body weight progressively decreased over time in all three trials, which is indicative of progressive loss of body fluids (P < 0.01, Fig. 4A). Reductions in body weight measured while clothed were greater in the 35°C trial compared with the 32°C trial between the fifth and eighth hour of exposure (P < 0.01) and compared with the 33°C trial in the seventh hour of exposure (P = 0.05, Fig. 4A). Percentage changes in nude body weight measured pre- and postexposure were greater in the 35°C trial compared with both the 33°C trial (P = 0.02) and the 32°C trial (P = 0.03), the latter of which did not statistically differ (P = 0.12, Fig. 4B). Plasma osmolality did not statistically differ between trials at any time point (P ≥ 0.19, Table 2). Reductions in plasma volume were greater in the 35°C trial compared with the 32°C trial at 4 h of exposure (P = 0.05) but did not statistically differ between these trials at 8 h of exposure (P = 0.06, Table 2). Reductions in plasma volume did not differ between the 33°C trial and the 35°C (P ≥ 0.34) or 32°C (P ≥ 0.49) trials at any time point (Table 2). Urine flow rate decreased over time in all three trials (P < 0.01, Table 2). At 4 h of exposure, urine flow rate was lower in the 35°C trial compared with the 33°C trial (P = 0.03) and the 32°C trial (P = 0.23), and by 8 h of exposure urine flow rate in the 35°C trial was lower than the 32°C trial (P < 0.01) but not the 33°C trial (P = 0.39, Table 2).
Fig. 4.
Percentage changes in body weight estimated hourly while clothed over the 8-h exposure (means ± SD, A) and estimated via measurement of nude body weight pre- and postexposure to 32°C, 33°C, and 35°C and ~95% relative humidity (box-and-whisker plot, B); n = 15 subjects. *Different from 32°C trial (P ≤ 0.03). †Different from 33°C trial (P ≤ 0.05).
Table 2.
Indexes of hydration changes over the 8-h exposures
| Exposure Time, h |
|||
|---|---|---|---|
| 0 | 4 | 8 | |
| ΔPlasma volume, % | |||
| 32°C | 0 ± 0 | −3 ± 7 | −4 ± 6 |
| 33°C | 0 ± 0 | −5 ± 7 | −8 ± 10 |
| 35°C | 0 ± 0 | −9 ± 8* | −9 ± 5 |
| Plasma osmolality, mosmol/kgH2O | |||
| 32°C | 285 ± 4 | 283 ± 2 | 284 ± 2 |
| 33°C | 283 ± 3 | 283 ± 2 | 284 ± 3 |
| 35°C | 284 ± 2 | 286 ± 2 | 285 ± 3 |
| Urine flow rate, mL/min | |||
| 32°C | 3.9 ± 3.3 | 1.1 ± 0.4 | 0.6 ± 0.3 |
| 33°C | 3.8 ± 4.2 | 1.3 ± 0.7 | 0.4 ± 0.3 |
| 35°C | 3.7 ± 3.5 | 0.8 ± 0.5*† | 0.3 ± 0.2* |
Data are means ± SD; n = 15 subjects.
Different from 32°C trial (P = 0.05).
Different from 33°C trial (P ≤ 0.01).
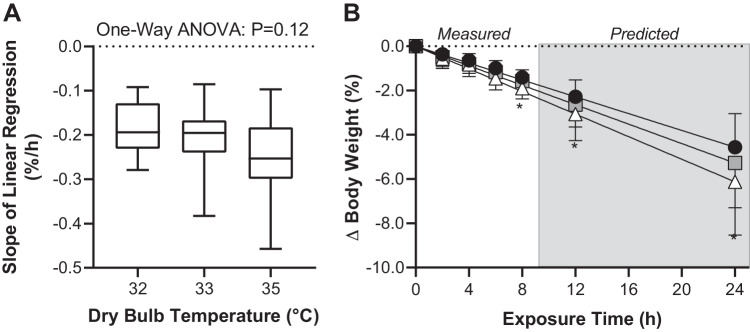
The slope of the linear regression lines for percentage changes in body weight over time did not statistically differ between trials (P = 0.12). The obtained slopes for each condition were 32°C: slope = −0.2 ± 0.1%/h; 33°C: slope = −0.2 ± 0.1%/h; and 35°C: slope = −0.3 ± 0.1%/h. Measured and predicted reductions in body weight were greater in the 35°C trial compared with the 32°C trials at all time points (P ≤ 0.03, Fig. 5). However, measured and predicted reductions in body weight did not statistically differ between the 33°C trial and the 35°C (P ≥ 0.26) or 32°C (P ≥ 0.32) trials at any time point (Fig. 5).
Fig. 5.
The slope of the linear regression line during 8 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity (box-and-whisker plot, A) and predicted percentage changes in body weight occurring subsequent to 12 and 24 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity (means ± SD, B). In B, for visual purposes, average linear regression lines are also presented together with a selection of average data from which these regression lines were derived. Please note that the statistical analysis for these data involved only data from 8, 12, and 24 h of exposure; n = 15 subjects. P value for 1-way ANOVA is reported in A. *Different from 32°C trial (P ≤ 0.03).
Cardiovascular and thermoregulatory responses.
Heart rate increased over time in all three trials (P < 0.01, Table 3). However, although the magnitude of increase in heart rate did not statistically differ between the 32°C (7 ± 9 beats/min) and 33°C (8 ± 11 beats/min) trials (P = 0.97), the increase in heart rate was higher in the 35°C trial (43 ± 18 beats/min) compared with both the 32°C and 33°C trials (P < 0.01, Table 3). Mean arterial pressure did not statistically differ between the three trials until the sixth hour of exposure, after which mean arterial pressure was lower in the 35°C trial compared with the 32°C trial (P ≤ 0.04, Table 3).
Table 3.
Cardiovascular changes over the 8-h exposures
| Exposure Time, h |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Heart rate, beats/min | |||||||||
| 32°C | 70 ± 7 | 75 ± 10 | 73 ± 11 | 74 ± 9 | 74 ± 11 | 76 ± 13 | 77 ± 11 | 79 ± 14 | 77 ± 9 |
| 33°C | 69 ± 10 | 72 ± 10 | 73 ± 9 | 72 ± 9 | 72 ± 8 | 73 ± 12 | 79 ± 7 | 77 ± 14 | 77 ± 9 |
| 35°C | 68 ± 10 | 77 ± 9 | 85 ± 15 † | 90 ± 13*† | 97 ± 21*† | 104 ± 15*† | 102 ± 18*† | 105 ± 17*† | 111 ± 20*† |
| Mean arterial pressure, mmHg | |||||||||
| 32°C | 92 ± 7 | 88 ± 6 | 89 ± 7 | 87 ± 6 | 88 ± 8 | 90 ± 7 | 89 ± 7 | 90 ± 6 | 87 ± 7 |
| 33°C | 90 ± 6 | 90 ± 8 | 89 ± 8 | 87 ± 8 | 88 ± 7 | 88 ± 8 | 89 ± 7 | 88 ± 9 | 86 ± 8 |
| 35°C | 89 ± 5 | 87 ± 8 | 84 ± 10 | 87 ± 8 | 85 ± 9 | 86 ± 7 | 83 ± 7* | 84 ± 10* | 82 ± 9* |
Data are means ± SD; n = 15 subjects.
Different from 32°C trial (P ≤ 0.04).
Different from 33°C trial (P ≤ 0.01).
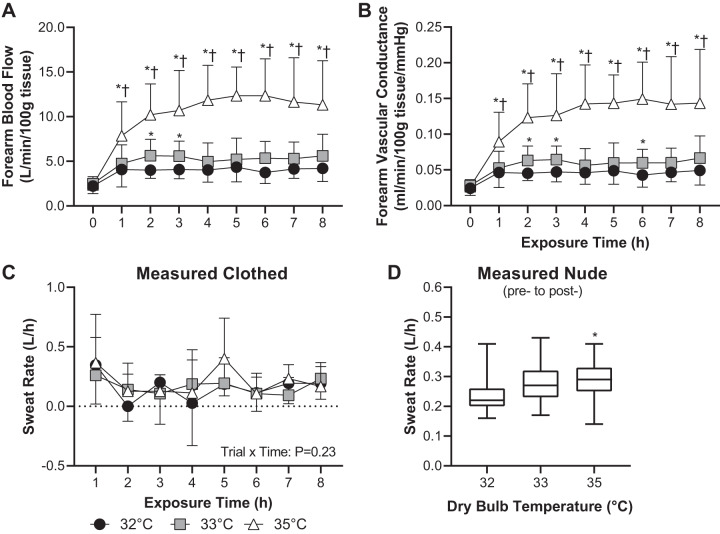
Forearm blood flow and vascular conductance increased over time in all three trials (P < 0.01, Fig. 6, A and B). However, forearm blood flow and vascular conductance were higher throughout the 8-h exposure in the 35°C trial compared with the 32°C (P < 0.01) and 33°C (P < 0.01) trials (Fig. 6, A and B). Compared with the 32°C trial, in the 33°C trial forearm blood flow was higher during the second and third hour (P ≤ 0.02), and forearm vascular conductance was higher during the second, third, and sixth hour (P ≤ 0.02, Fig. 6, A and B). Whole body sweat rate derived from clothed body weight measurements did not statistically differ over time or between trials (trial × time interaction: P = 0.23, Fig. 6C). However, whole body sweat rate derived from pre- to postexposure nude body weight was higher in the 35°C trial compared with the 32°C trial (P = 0.04) but did not statistically differ between the 33°C trial and the 35°C (P = 0.61) or 32°C (P = 0.12) trials (Fig. 6D).
Fig. 6.
Forearm blood flow (means ± SD, A) and vascular conductance (means ± SD, B) during 8 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity and whole body sweat rate estimated hourly while clothed over the 8-h exposure (means ± SD, C) and estimated via measurement of nude body weight pre- and postexposure to 32°C, 33°C, and 35°C and ~95% relative humidity (box-and-whisker plot, D); n = 15 subjects. *Different from 32°C trial (P ≤ 0.04). †Different from 33°C trial (P < 0.01).
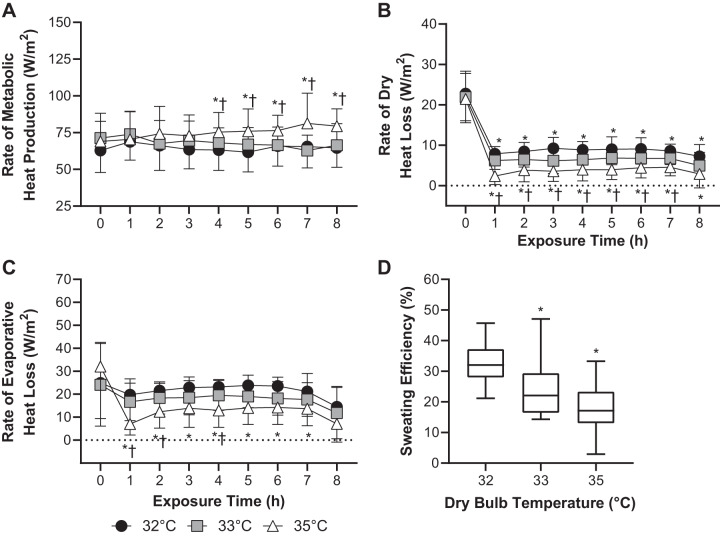
The rate of metabolic heat production was elevated in the 35°C trial compared with the 32°C (P ≤ 0.04) and 33°C (P ≤ 0.05) trials in the fourth through eighth hours of exposure (Fig. 7A). The rate of dry heat loss was lowest in the 35°C trial (P < 0.01) and highest in the 32°C trial (P ≤ 0.04), with the rate of dry heat loss in the 33°C trial being in between (P ≤ 0.04) throughout exposure (Fig. 7B). The rate of evaporative heat loss did not statistically differ between the 32°C and 33°C trials over the 8-h exposures (P ≥ 0.07) but was lower in the 35°C trial compared with the 32°C trial throughout (P ≤ 0.05) and during the first, second, and fourth hour of exposure versus the 33°C trial (P ≤ 0.04, Fig. 7C). Sweating efficiency was lower in the 33°C (P = 0.03) and 35°C (P < 0.01) trials compared with the 32°C trial but did not statistically differ between the 33°C and 35°C trials (P = 0.23, Fig. 7D).
Fig. 7.
The rates of metabolic heat production (n = 14, means ± SD, A), dry heat loss (n = 15 subjects, means ± SD, B), and evaporative heat loss (n = 12, means ± SD, C) and average sweating efficiency (n = 12 subjects, box-and-whisker plot, D) during 8 h exposure to 32°C, 33°C, and 35°C and ~95% relative humidity. Means ± SD. *Different from 32°C trial (P ≤ 0.05). †Different from 33°C trial (P ≤ 0.05).
DISCUSSION
This study tested the hypothesis that the measured (through 8 h) and predicted (through 24 h) increases in core temperature and body fluid losses during exposure to warm and very humid (~95% RH) conditions are dependent on dry bulb temperature and the corresponding changes in water vapor pressure. Three dry bulb temperatures were tested in the current study: 32°C, 33°C, and 35°C (Table 1). These temperatures were chosen for the following three reasons: 1) 32°C is that dry bulb temperature most likely to occur during a blower failure in a disabled PRM; 2) 33°C is the approximate dry bulb temperature upon which models for predicting increases in core temperature and/or body fluid losses likely begin to become inaccurate during exposure to extremely humid environments (5); and 3) 35°C is likely the upper limit temperature of what could occur during a blower failure in a disabled PRM when the PRM is in warm water.
In support of our hypothesis, the increase in measured (Fig. 2A) and predicted (Fig. 3B) core temperature was greater in the 35°C trial compared with both the 32°C and 33°C trials. By contrast, measured (Fig. 2A) and predicted (Fig. 3B) core temperatures did not differ between the 32°C and 33°C trials. The differences in core temperature between the 32°C and 33°C trials and the 35°C trial can likely be explained by a lower rate of dry heat loss (Fig. 7B) that could not be offset by increases in the rate of evaporative heat loss (Fig. 7C), particularly in the presence of a slightly higher rate of metabolic heat production during the latter stages of the exposure (Fig. 7A). The insufficient rate of evaporative heat loss was not likely the result of an inability to increase sweat production given that whole body sweat rate estimated from the measurement of pre- to postexposure nude body weight was higher in the 35°C trial compared with the 32°C trial (Fig. 6D). Rather, evaporative heat loss was likely constrained by the evaporative capacity permitted by the environmental conditions (i.e., the elevated ambient water vapor pressure in the 35°C trial; Table 1), which reduced sweating efficiency (Fig. 7D). Ultimately, the 35°C trial resulted in uncompensable heat stress, as objectively demonstrated by a slope for the change in core temperature over time greater than zero (Fig. 3A).
Our findings support the well-established premise that increases in ambient water vapor pressure impede evaporative heat loss, which can lead to uncompensable heat stress depending on the dry bulb temperature and/or rate of metabolic heat production (e.g., rest versus exercise; see Refs. 8, 17, 23, 29, 33). However, these studies employed exposures that were typically less than ~150 min in duration. Therefore, our study furthers these previous findings by examining the impact of increases in dry bulb temperature and ambient water vapor pressure on thermoregulation over a longer duration of exposure. This is particularly important in the context of the resting exposures used herein, whereby differential thermoregulatory responses [e.g., increases in forearm (skin) blood flow; Fig. 6A] and subsequent differences in dry (Fig. 7B) and evaporative (Fig. 7C) heat exchange (Fig. 7) were observed between trials during the first 1–3 h of exposure. However, this did not result in a sufficient quantity of body heat storage to manifest as a greater increase in core temperature in the 35°C trial until the third hour of exposure (Fig. 2A). The importance of this finding is perhaps best highlighted by the differential conclusions that would have been drawn had data collection been terminated after 3 h of exposure, whereby all dry bulb temperatures studied may have been deemed uncompensable. By contrast, however, the thermoregulatory responses during the initial 1–3 h of exposure were sufficient to achieve heat balance in the 32°C and 33°C trials, but not in the 35°C trial (Fig. 3A).
Also in support of our hypothesis, the magnitude of measured (Fig. 4, A and B) and predicted (Fig. 5) changes in body weight (i.e., body fluid losses) were greater in the 35°C trial compared with the 32°C trial. Interestingly, differences in body fluid losses between the 33°C and 35°C trials were less apparent, with any differences likely within the error of the measurement of when body weight was measured clothed. This is indirectly supported by findings that percentage changes in body weight when estimated via the measurement of pre to post nude body weight demonstrated greater body fluid losses in the 35°C trial compared with both the 32°C and 33°C trials (c.f., Fig. 4, A vs. B). Thus, it is likely that the magnitude of body fluid loss was greatest in the 35°C trial, with no differences between the 32°C and 33°C trials, but that differences were not detected between the 35°C and 33°C trial because of methodological error. As a result, and in the interest of full transparency, we have presented percentage changes in body weight measured both hourly while clothed (Fig. 4A) and pre- to postexposure while nude Fig. 4B).
In the present study, the progressive loss of body water was the result of sweat loss that was unable to be replaced with fluid intake because drinking was prohibited. Given our experimental methodology, we were unable to obtain a complete picture of fluid regulation, which is a function of both fluid output and intake. That said, to our knowledge, the fluid regulatory response to prolonged exposure to warm and very humid environments has never been explored. Thus, it is interesting that we observed a greater reduction in plasma volume in the 35°C trial vs. the 32°C trial (Table 2), which coincided with baroreceptor unloading (i.e., lower blood pressure, Table 3) in the 35°C trial. Interestingly, plasma osmolality did not differ between trials over time (Table 2), and reductions in plasma volume in the 35°C trial between 4 and 8 h did not differ (Table 2), despite continued reductions in body weight (Fig. 4). These data support that the observed body fluid losses resulted in a hypovolemic isoosmotic state, the magnitude of which was greatest in the 35°C trial, and that during the second one-half of the 35°C trial fluid was lost predominantly from the extravascular fluid compartment. Collectively, it is likely that the fluid conservatory hormonal response, often described by increases in vasopressin and aldosterone (10), was augmented in the 35°C trial, particularly compared with the 32°C trial. Although we do not have direct evidence to support this contention, our speculation is supported by greater reductions in urine flow rate during the exposures in the 35°C trial (Table 2).
Perspectives and Significance
To the best of our knowledge, human thermoregulation has never been examined during 8 h of exposure to warm and very humid environments. That said, the true value and novelty of our data likely lies with its applied nature. For instance, our study directly informs emergency resource allocation and/or rescue decision making in disabled PRM or submarine scenarios, and/or any other military or civilian emergency situations (e.g., mine collapse) requiring prolonged exposures to wet bulb temperatures between ~31°C and ~34°C (Table 1). Our study objectively demonstrates that prolonged exposure to a wet bulb temperature of 34°C is uncompensable heat stress (Fig. 3A). Extrapolation of the linear regression equation for this situation estimates that, on average, core temperature will reach 40.5°C, the core temperature associated with the development of life-threatening heat stroke when associated with neuropsychiatric impairment (6) after ~45 h of exposure. Moreover, body fluid loss progressed linearly over time (Fig. 4A), with an average rate of body fluid loss of ~0.2%/h. Thus, the development of 6% body fluid loss, an extreme yet tolerable extent of dehydration, would take ~27 h on average in all conditions. By extension, we estimate that, for an 84.5-kg male, the average body weight for the subjects in the current study, ~3 liters of fluid would be necessary to prevent the development of 6% body fluid loss if the exposure to the warm and very humid environment were to persist up to 48 h. Given these (and other) potential applications of our data, we have reported the slope and y-intercept data to better inform any potential end users.
Moreover, it has been theorized that a wet bulb temperature of 35°C is a thermally uncompensable environment in which autonomically mediated increases in heat loss are unable to fully offset the rate of metabolic heat production even during resting situations (17, 37). Based on this premise, climate change models estimate that many areas across the globe may be unsuitable for sustained human life (37). Our data uniquely demonstrate that this uncompensable wet bulb temperature limit is lower than 35°C and likely between 32°C and 34°C. In addition to the relatively profound thermoregulatory challenge, our data also support that prolonged exposure to a wet bulb temperature of 34°C conditions elicits a profound cardiovascular response that is characterized by elevations in heart rate (Table 3), increases in forearm (skin) blood flow (Fig. 6A), and reductions in blood pressure (Table 3), which could ultimately promote orthostatic intolerance (36) or stimulate a cardiac event in a person with a compromised cardiovascular system (e.g., older adults; see Ref. 21). Thus, the data presented herein could also have important ramifications for refining models predicting the impact of climate change on human health. However, it is important to note that the conditions in the present study do not mimic diurnal changes in dry and wet bulb temperatures and normal human behavior that would occur in free living situations, such as drinking and seeking cooling.
Considerations.
A few methodological considerations warrant mentioning. First, we measured core temperature using a telemetric pill, an index of core temperature that is slower to change than esophageal or pulmonary artery temperature but faster than rectal temperature (30, 31). To date, however, such comparative methodological core temperature studies have only been conducted over a relatively short time duration (≤120 min), with differences between esophageal and intestinal temperature persisting on the time scale of ~30 min (30, 31). Thus, given that changes in core temperature involved a time scale of 1 h in the present study, it is unlikely that our measure of core temperature meaningfully impacted our results. Second, as we have done previously (35), we estimated body fluid loss over time via the measurement of changes in body weight measured while clothed. This was done for logistical reasons, since it was not possible to disrobe every hour while fully instrumented. That said, because of sweat trapped in the clothing, it is likely that our approach increased the error of the measurement, as discussed above. Given that the volume of sweat trapped in the clothing did not statistically differ between the trials, this was likely a systematic error that resulted in an underestimation of the magnitude of body fluid loss in all trials. This is exemplified by a 0.8 ± 0.5% greater body fluid loss when estimated via changes in nude body weight compared with clothed body weight (P < 0.01). Thus, some caution should be exercised in the interpretation of these data. However, we believe that comparisons between conditions likely remain valid. Third, as noted above, the use of linear regression models to predicted core temperature and percent changes in body weight loss was determined a priori, a decision that was later confirmed upon visual inspection of the data (see Figs. 2A and 4). It is possible, however, that a more complex regression model may better fit our data. That said, such an approach would negate the advantage of linear regression in providing a relatively simple method to extrapolate our findings (see Perspectives and Significance). In line with this point, the linear regression models are specific to scenarios in which drinking is not possible. Body fluid loss can independently influence the magnitude of increases in core temperature resulting from impairments in sweat production and cutaneous vasodilation (27). Thus, it is unknown whether the linear regression models predicting increases in core temperature would remain accurate if fluid lost due to sweating is at least partially replaced with drinking. Fourth, for reasons outlined above, our study included healthy young male subjects that self-reported to regularly engage in physical activity. Thus, it remains unknown if our findings would be consistent in females and/or across the menstrual cycle, whether our findings would differ if our subject population was more (or less) aerobically fit, or if our findings would be altered if our subject population was older or less healthy. For instance, maximal evaporative heat loss capacity is lower in females compared with males (15). Moreover, fluid regulation likely differs across the menstrual cycle (16). Therefore, there is reason to believe that thermoregulation and/or changes in hydration status during prolonged exposure to warm and very humid conditions may differ with biological sex. Also, higher levels of aerobic fitness occurring subsequent to regular aerobic exercise can improve heat loss and shift the environmental limits of thermal compensability to more extreme conditions (32), suggesting that our findings may have differed if our subject population was more (or less) aerobically fit. That said, it should be noted that submariners are of mostly average fitness (4). Furthermore, older adults and those with chronic disease (e.g., cardiovascular disease, etc.) often demonstrate attenuated evaporative heat loss and/or cutaneous vascular function during heat exposure (2, 22). Fifth, it is interesting to note that our partitional calorimetry-derived estimates of heat exchange resulted in thermally uncompensable conditions in all experimental trials [i.e., the rate of metabolic heat production (Fig. 7A) exceeded the sum of the rates of dry (Fig. 7B) and evaporative (Fig. 7C) heat loss]. By contrast, our thermometry data demonstrated that only the 35°C trial was uncompensable (Fig. 3A). These conflicting findings highlight the potential error of our partitional calorimetry data. We believe this error was likely due to underestimation of the rate of evaporative heat loss because all skin humidity sensors were placed under clothing. Importantly, we believe that this limitation was a systematic error given that we were still able to discern differences in the rate of evaporative heat loss between trials (Fig. 7C). Sixth, during a disabled PRM scenario, it is likely that a blower failure would occur alongside failures to other systems, including the depressurization system. This is notable, since an increase in ambient pressure, such as would occur at depth during a depressurization system failure, could further reduce sweating efficiency (19), which would, in theory, impact the compensability of the thermal environment during prolonged exposure to a warm and very humid environment. However, this consideration has never been formally explored.
Conclusions.
The findings of the present study indicate that the measured (through 8 h) and predicted (through 24 h) increase in core temperature during exposure to dry bulb temperatures of 32°C, 33°C, and 35°C and very high humidity (~95% RH) is dependent on dry bulb temperature. Specifically, core temperature increased during 8 h resting exposures to each of these conditions. However, only the 35°C trial was thermally uncompensable, as demonstrated by a continued rise in core temperature throughout. The uncompensable nature of the 35°C trial is likely explained by a reduced rate of dry heat loss that could not be offset by increases in the rate of evaporative heat loss, the latter of which was caused by reductions in sweating efficiency that occurred despite a higher sweat rate. Given the increased sweat loss, the magnitude of measured and predicted body fluid loss was greatest in the 35°C trial. These data provide important information regarding emergency resource allocation and/or rescue decision making in disabled pressurized rescue module or submarine scenarios, and/or any other civilian or military emergency situations necessitating prolonged exposures to warm and very humid environments (e.g., mine collapse).
GRANTS
This study was supported by an award from Naval Sea Systems Command (N00024-18-C-4316). BMC was supported by National Institutes of Health (NIH) Award No. K12-HL-138052 to the University at Buffalo and by the National Center for Advancing Translational Sciences of the NIH under award number UL-1TR-001412 to the University at Buffalo.
DISCLOSURES
This article's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Department of Defense. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.J.S., B.D.J., B.M.C., and D.H. conceived and designed research; Z.J.S., B.D.J., R.R.P., J.S., B.M.C., and D.H. performed experiments; Z.J.S. analyzed data; Z.J.S., B.D.J., R.R.P., J.S., B.M.C., and D.H. interpreted results of experiments; Z.J.S. prepared figures; Z.J.S. drafted manuscript; Z.J.S., B.D.J., R.R.P., J.S., B.M.C., and D.H. edited and revised manuscript; Z.J.S., B.D.J., R.R.P., J.S., B.M.C., and D.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for participating in our study.
REFERENCES
- 1.Bain AR, Jay O. Does summer in a humid continental climate elicit an acclimatization of human thermoregulatory responses? Eur J Appl Physiol 111: 1197–1205, 2011. doi: 10.1007/s00421-010-1743-9. [DOI] [PubMed] [Google Scholar]
- 2.Balmain BN, Jay O, Sabapathy S, Royston D, Stewart GM, Jayasinghe R, Morris NR. Altered thermoregulatory responses in heart failure patients exercising in the heat. Physiol Rep 4: e13022, 2016. doi: 10.14814/phy2.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell C, Crowder M, Walters J. The Prediction of Safe Exposure Times for Men Working in Thermally Severe Environments. London, UK: Royal Naval Personnel Research Committee London, 1970. [Google Scholar]
- 4.Bennett BL. Physical fitness in a submarine community as determined by the U.S. Navy Health and Physical Readiness Test. Aviat Space Environ Med 58: 444–451, 1987. [PubMed] [Google Scholar]
- 5.Berglund LG, Yokota M, Potter AW. Thermo-Physiological Responses of Sailors in a Disabled Submarine with Interior Cabin Temperature and Humidity Slowly Rising as Predicted by Computer Simulation Techniques. Natick, MA: U.S. Army Research Institute of Environmental Medicine, 2013. [Google Scholar]
- 6.Casa DJ, DeMartini JK, Bergeron MF, Csillan D, Eichner ER, Lopez RM, Ferrara MS, Miller KC, O’Connor F, Sawka MN, Yeargin SW. National Athletic Trainers’ Association position statement: exertional heat illnesses. J Athl Train 50: 986–1000, 2015. doi: 10.4085/1062-6050-50.9.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaseling GK, Crandall CG, Gagnon D. Skin blood flow measurements during heat stress: technical and analytical considerations. Am J Physiol Regul Integr Comp Physiol 318: R57–R69, 2020. doi: 10.1152/ajpregu.00177.2019. [DOI] [PubMed] [Google Scholar]
- 8.Che Muhamed AM, Atkins K, Stannard SR, Mündel T, Thompson MW. The effects of a systematic increase in relative humidity on thermoregulatory and circulatory responses during prolonged running exercise in the heat. Temperature (Austin) 3: 455–464, 2016. doi: 10.1080/23328940.2016.1182669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheuvront SN, Ely BR, Kenefick RW, Sawka MN. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr 92: 565–573, 2010. doi: 10.3945/ajcn.2010.29490. [DOI] [PubMed] [Google Scholar]
- 10.Cheuvront SN, Kenefick RW. Dehydration: physiology, assessment, and performance effects. Compr Physiol 4: 257–285, 2014. doi: 10.1002/cphy.c130017. [DOI] [PubMed] [Google Scholar]
- 11.Cramer MN, Jay O. Partitional calorimetry. J Appl Physiol (1985) 126: 267–277, 2019. doi: 10.1152/japplphysiol.00191.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) 73: 863–871, 1916. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 14.Ellis FP, Navy R. Thermal comfort in warm, humid atmospheres; observations in a warship in the tropics. J Hyg (Lond) 50: 415–432, 1952. doi: 10.1017/S0022172400019690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagnon D, Kenny GP. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J Physiol 590: 5963–5973, 2012. doi: 10.1113/jphysiol.2012.240739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giersch GE, Charkoudian N, Stearns RL, Casa DJ. Fluid balance and hydration considerations for women: review and future directions. Sports Med 50: 253–261, 2020. doi: 10.1007/s40279-019-01206-6. [DOI] [PubMed] [Google Scholar]
- 17.Haldane JS. The influence of high air temperatures No. I. J Hyg (Lond) 5: 494–513, 1905. doi: 10.1017/S0022172400006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy JD, Dubois EF, Soderstrom GF. The technic of measuring radiation and convection. J Nutr 15: 461–475, 1938. doi: 10.1093/jn/15.5.461. [DOI] [Google Scholar]
- 19.Havenith G, Fiala D. Thermal indices and thermophysiological modeling for heat stress. Compr Physiol 6: 255–302, 2015. doi: 10.1002/cphy.c140051. [DOI] [PubMed] [Google Scholar]
- 20.Horn WG. Summary: Disabled Submarine Heat Stress Conference. Groton, CT: Naval Submarine Medical Research Laboratory, 2009. [Google Scholar]
- 21.Kenney WL, Craighead DH, Alexander LM. Heat waves, aging, and human cardiovascular health. Med Sci Sports Exerc 46: 1891–1899, 2014. doi: 10.1249/MSS.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenney WL, Munce TA. Invited review: Aging and human temperature regulation. J Appl Physiol (1985) 95: 2598–2603, 2003. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kenney WL, Zeman MJ. Psychrometric limits and critical evaporative coefficients for unacclimated men and women. J Appl Physiol (1985) 92: 2256–2263, 2002. doi: 10.1152/japplphysiol.01040.2001. [DOI] [PubMed] [Google Scholar]
- 24.Kerslake DM. The Stress of Hot Environments. London, UK: Cambridge Univ Press, 1972. [PubMed] [Google Scholar]
- 25.McCullough EA, Jones BW, Huck J. A Comprehensive Data Base for Estimating Clothing Insulation. Atlanta, GA: ASHRAE, 1985. [Google Scholar]
- 26.Mitchell D, Wyndham CH. Comparison of weighting formulas for calculating mean skin temperature. J Appl Physiol 26: 616–622, 1969. doi: 10.1152/jappl.1969.26.5.616. [DOI] [PubMed] [Google Scholar]
- 27.Montain SJ, Coyle EF. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol (1985) 73: 1340–1350, 1992. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- 28.Moss KN. Some effects of high air temperatures and muscular exertion upon colliers. Proc R Soc Lond, B 95: 181–200, 1923. doi: 10.1098/rspb.1923.0031. [DOI] [Google Scholar]
- 29.Moyen NE, Mündel T, Du Bois AM, Ciccone AB, Morton RH, Judelson DA. Increasing humidity affects thermoregulation during low-intensity exercise in women. Aviat Space Environ Med 85: 905–911, 2014. doi: 10.3357/ASEM.3993.2014. [DOI] [PubMed] [Google Scholar]
- 30.Mündel T, Carter JM, Wilkinson DM, Jones DA. A comparison of rectal, oesophageal and gastro‐intestinal tract temperatures during moderate‐intensity cycling in temperate and hot conditions. Clin Physiol Funct Imaging 36: 11–16, 2016. doi: 10.1111/cpf.12187. [DOI] [PubMed] [Google Scholar]
- 31.Pearson J, Ganio MS, Seifert T, Overgaard M, Secher NH, Crandall CG. Pulmonary artery and intestinal temperatures during heat stress and cooling. Med Sci Sports Exerc 44: 857–862, 2012. doi: 10.1249/MSS.0b013e31823d7a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravanelli N, Coombs G, Imbeault P, Jay O. Maximum skin wettedness following aerobic training with and without heat acclimation. Med Sci Sports Exerc 50: 299–307, 2018. doi: 10.1249/MSS.0000000000001439. [DOI] [PubMed] [Google Scholar]
- 33.Ravanelli NM, Gagnon D, Hodder SG, Havenith G, Jay O. The biophysical and physiological basis for mitigated elevations in heart rate with electric fan use in extreme heat and humidity. Int J Biometeorol 61: 313–323, 2017. doi: 10.1007/s00484-016-1213-0. [DOI] [PubMed] [Google Scholar]
- 34.Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS; American College of Sports Medicine . American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 35.Schlader ZJ, Gagnon D, Rivas E, Convertino VA, Crandall CG. Fluid restriction during exercise in the heat reduces tolerance to progressive central hypovolaemia. Exp Physiol 100: 926–934, 2015. doi: 10.1113/EP085280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlader ZJ, Wilson TE, Crandall CG. Mechanisms of orthostatic intolerance during heat stress. Auton Neurosci 196: 37–46, 2016. doi: 10.1016/j.autneu.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherwood SC, Huber M. An adaptability limit to climate change due to heat stress. Proc Natl Acad Sci USA 107: 9552–9555, 2010. doi: 10.1073/pnas.0913352107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stull R. Wet-bulb temperature from relative humidity and air temperature. J Appl Meteorol Climatol 50: 2267–2269, 2011. doi: 10.1175/JAMC-D-11-0143.1. [DOI] [Google Scholar]
- 39.Vargas NT, Chapman CL, Johnson BD, Gathercole R, Cramer MN, Schlader ZJ. Thermal behavior alleviates thermal discomfort during steady-state exercise without affecting whole body heat loss. J Appl Physiol (1985) 127: 984–994, 2019. doi: 10.1152/japplphysiol.00379.2019. [DOI] [PubMed] [Google Scholar]
- 40.Vargas NT, Chapman CL, Johnson BD, Gathercole R, Cramer MN, Schlader ZJ. Thermal behavior augments heat loss following low intensity exercise. Int J Environ Res Public Health 17: 20, 2019. doi: 10.3390/ijerph17010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vargas NT, Chapman CL, Johnson BD, Gathercole R, Schlader ZJ. Exercise intensity independently modulates thermal behavior during exercise recovery but not during exercise. J Appl Physiol (1985) 126: 1150–1159, 2019. doi: 10.1152/japplphysiol.00992.2018. [DOI] [PubMed] [Google Scholar]
- 42.Vargas NT, Chapman CL, Johnson BD, Gathercole R, Schlader ZJ. Skin wettedness is an important contributor to thermal behavior during exercise and recovery. Am J Physiol Regul Integr Comp Physiol 315: R925–R933, 2018. doi: 10.1152/ajpregu.00178.2018. [DOI] [PubMed] [Google Scholar]
- 43.Whitney RJ. The measurement of volume changes in human limbs. J Physiol 121: 1–27, 1953. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 52: 631–646, 2001. doi: 10.1046/j.0306-5251.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]