Abstract
Phoenixin is a 20-amino acid peptide (PNX-20) cleaved from the small integral membrane protein 20 (SMIM20), with multiple biological roles in mammals. However, its role in nonmammalian vertebrates is poorly understood. This research aimed to determine whether PNX-20 influences feeding and metabolism in zebrafish. The mRNAs encoding SMIM20 and its putative receptor, super conserved receptor expressed in brain 3 (SREB3), are present in both central and peripheral tissues of zebrafish. Immunohistochemical analysis confirmed the presence of PNX-like immunoreactivity in the gut and in zebrafish liver (ZFL) cell line. We also found that short-term fasting (7 days) significantly decreased smim20 mRNA expression in the brain, gut, liver, gonads, and muscle, which suggests a role for PNX-20 in food intake regulation. Indeed, single intraperitoneal injection of 1,000 ng/g body wt PNX-20 reduced feeding in both male and female zebrafish, likely in part by enhancing hypothalamic cart and reducing hypothalamic/gut preproghrelin mRNAs. Furthermore, the present results demonstrated that PNX-20 modulates the expression of genes involved in glucose transport and metabolism in ZFL cells. In general terms, such PNX-induced modulation of gene expression was characterized by the upregulation of glycolytic genes and the downregulation of gluconeogenic genes. A kinetic study of the ATP production rate from both glycolytic and mitochondrial pathways demonstrated that PNX-20-treated ZFL cells exhibited significantly higher ATP production rate associated with glycolysis than control cells. This confirms a positive role for PNX-20 on glycolysis. Together, these results indicate that PNX-20 is an anorexigen with important metabolic roles in zebrafish.
Keywords: fasting, feeding, glucose metabolism, glycolysis, phoenixin, Seahorse, SMIM20, SREB3, zebrafish
INTRODUCTION
Phoenixin (PNX; used to note the mature peptide), a novel peptide cleaved from the small integral membrane protein 20 (SMIM20; referring to the precursor peptide), was originally identified in silico, and subsequently purified from the rat hypothalamus and bovine heart (26). Several forms of PNX of different amino acid lengths (namely 14, 17, 20, 26, 36, and 42) were identified. Of these, amidated 14- and 20-amino acid chains, referred to as PNX-14 and PNX-20, respectively, are the prominent forms (26). PNX-14 (DVQPPGLKVWSDPF) is a COOH-terminal degraded product of PNX-20 (AGIVQEDVQPPGLKVWSDPF), which lacks the first six amino acids in the NH2-terminal region. PNX is highly conserved across multiple species, including mammals, birds, amphibians, and fish (25, 26). For instance, PNX-14 is identical in human, rat, mouse, porcine, canine, and Xenopus, and phoenixin-20 amide differs in one amino acid between the human, porcine, and canine amino acid sequences (26). These peptides share a common COOH-terminal segment comprised of 14 amino acids, which is essential for its biological activity (26). The main site for PNX synthesis in rats is the hypothalamus (26) although the expression of both mRNA and peptide has been detected in other brain areas and peripheral tissues, including the heart, thymus, gastrointestinal tract, pancreas, and spleen (10, 15, 16, 18, 26). In fish, smim20 (to denote mRNA encoding the precursor) are expressed in the hypothalamus, pituitary, heart, intestine, liver, spleen, kidney, ovary, testis, and muscle (25). In vitro studies using rat pituitary adenoma cells shows that both PNX-20 and PNX-14 potentiate gonadotropin-releasing hormone (GnRH)-mediated luteinizing hormone (LH) release by binding to a cell surface receptor (26). The putative receptor for PNX-20 is the G protein-coupled receptor 173 (GPR173), also named SREB3 (receptor protein; see Ref. 23), a member of the super conserved receptor expressed in brain (SREB) family (12). SREB3 shows a widespread distribution in mammals (12, 20, 22), with the highest levels observed in the brain, ovary, and small intestine.
The first biological action attributed to PNX was a modulatory role in female reproductive processes. In mammals, PNX is reported to have role in GnRH-mediated LH release (20), proliferation of granulosa cells in the developing ovarian follicles, and estradiol production (13). In addition to this, siRNA-mediated gene knockdown of smim20 mRNA resulted in reduced gnrhr expression in the pituitary gland and delayed estrous cycle in female rats (26). Among nonmammals, studies in fish (spotted scat, Scatophagus argus) found that PNX-14 and PNX-20 influence reproduction regulatory genes in the hypothalamus and pituitary (24). Besides its reproductive role, PNX has been reported to have a role in the regulation of several other physiological processes. For instance, it has been shown to modulate cardiac functions (18) and to increase the circulating levels of vasopressin (4) in rats. Exogenously administered PNX-20 suppresses visceral pain (11), and centrally injected PNX-14 generates anxiolytic effects (8) in mice. In addition, PNX-14 induces the proliferation and differentiation of 3T3-L1 cells and rat primary preadipocytes to mature adipocytes (3), which indicates its possible role in the control of body mass regulation and whole energy homeostasis. PNX also enhances glucose-stimulated insulin secretion in INS-1E cells and isolated rat pancreatic islets (2). Finally, a role for PNX in promoting food intake has been reported after intracerebroventricular administration of PNX-14 in rats (19). Overall, PNX is a peptide with multiple biological effects.
Given the pleiotropic roles suggested for PNX in mammals, and the reported observation that smim20 mRNA is responsive to energy status in fish (25), this research aimed to determine whether PNX-20 is a feeding modulatory peptide in zebrafish, a well-characterized model system in comparative endocrinology. In addition, considering the abundance of both PNX and SREB3 in the fish liver (25), we hypothesized that PNX may have a role in the regulation of glucose metabolism in fish. Our specific objectives were to: 1) characterize the expression of smim20 and sreb3 mRNAs in zebrafish tissues and their cellular localization in the gut and zebrafish liver (ZFL) cell line, 2) study whether food deprivation affects the expression of smim20 and sreb3, 3) determine the role of exogenously administered PNX-20 on food intake, 4) test for possible effects of PNX-20 on the tissue-specific expression of mRNAs encoding appetite regulatory peptides, 5) determine the role of PNX-20 on the expression of mRNAs encoding enzymes related to glucose transport and metabolism in ZFL cells, and 6) infer the putative role of PNX-20 in modulating the glycolytic rate by quantifying the ATP production rate in ZFL cells.
MATERIALS AND METHODS
Animals
Zebrafish (Danio rerio; 12 mo old; body wt ∼1 g) were obtained from the Aquatic Toxicology Research Facility at the University of Saskatchewan and housed in 10-liter aquaria with a constant flow of temperature-controlled water (26 ± 1°C), under a simulated 12:12-h light-dark photoperiod (lights on at 0700). Fish were fed daily with commercial slow-sinking pellets (4% body wt; Aqueon, Franklin, WI) at a scheduled feeding time. For all experiments using zebrafish, fish were anesthetized using tricaine methanesulfonate (MS-222; Syndel Laboratories, Nanaimo, BC, Canada) and euthanized by spinal transection. All animal studies complied within the policies of the Canadian Council for Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board (Protocol No. 2012–0082).
ZFL Cell Culture
Zebrafish liver (ZFL) cells were purchased from ATCC (catalog no. CRL-2643; ATCC, Manassas, VA) and cultured at 20°C under a 100% air atmosphere. The media used for cell culture had the following components: 50% Leibovitz's L-15 (Thermo Fisher Scientific, Waltham, MA), 35% Dulbecco’s modified Eagle medium (DMEM) high glucose (Sigma-Aldrich, Oakville, ON, Canada), and 15% Ham's F-12 (ATCC) (all without sodium bicarbonate) supplemented with 0.15 g/L sodium bicarbonate, 15 mM HEPES, 0.01 mg/mL bovine insulin, 50 ng/mL mouse epidermal growth factor (EGF), 5% heat-inactivated fetal bovine serum, and 0.5% trout serum. For studies described in the in vitro experiment section, ZFL cells were seeded at 5 × 105 cells/well in 24-well plates and grown at 80–90% confluency (typically 48–72 h after seeding) before performing the experiment. For the study described in the ATP production experiment, 20,000 cells were seeded in Seahorse XFp cell culture miniplates the day before the experiment.
Experimental Design
In silico analysis.
SMIM20 amino acid sequences of various vertebrates were obtained from GenBank (https://www.ncbi.nlm.nih.gov/protein/). The Clustal Omega multiple-sequence analysis tool (https://www.ebi.ac.uk/Tools/msa/clustalo/) was used to align the sequences. We used the ProtParam (https://web.expasy.org/protparam) bioinformatics data analysis tool for the computation of various physical and chemical parameters of zebrafish SMIM20.
Tissue distribution of SMIM20 and SREB3.
To study the tissue distribution of smim20 and sreb3 mRNAs, the following tissues were collected from six zebrafish: brain (without the hypothalamus), hypothalamus, eye, skin, gills, heart, foregut (intestinal bulb and anteriormost portion of the intestine, equivalent of the J loop region in stomachless fish), hindgut (posteriormost portion of the intestine), liver, spleen, muscle, testis, and ovary. Tissues were immediately frozen in liquid nitrogen and stored at –80°C until quantification of gene expression (described in detail in the qPCR section). To study the cellular localization of PNX-20 and SREB3 using immunocyto/histochemistry, samples of gut fixed in paraformaldehyde and ZFL cells were used.
Fasting-induced changes in the expression of the PNX system.
Zebrafish (adult, 12 mo old) were divided into two groups, one fed daily at the regular feeding time (fed group) and the other kept without feeding (fasted group). On day 7 of the study, six fish (4 male and 2 female) from each tank were euthanized as described above, and samples of brain, gut, liver, gonads, and muscle were collected and stored at –80°C for further analysis.
Effects of exogenous PNX-20 on zebrafish feed intake and mRNA expression.
Two separate experiments testing different doses of PNX-20 were carried out to study the effects of the peptide on food intake. In each experiment, age- and weight-matched male and female zebrafish were grouped (n = 6/group) into three fish per tank and monitored for food intake stabilization. For this, we added prequantified food (>4% body wt ration) to each tank every day at 12:00 PM and removed the leftover food after 1 h of feeding (1:00 PM), which was dried overnight at 30°C for weighing. Feed tracking was continued for 2 wk to ensure that there are no significant differences in food intake levels among the three fish groups. On the experimental day, fish were anesthetized (by immersion in 0.5% TMS-222) and intraperitoneally injected with 0.9% saline alone (control) or containing 1 ng/g or 10 ng/g body wt (experiment 1) or 100 ng/g or 1,000 ng/g body wt (experiment 2) synthetic zebrafish PNX-20 (AGVNQADIQPVGVKVWSDPY, custom synthesized, catalog no. 1711-PAC-21, ≥95% pure; Pacific immunology, Ramona, CA) in a total volume of 4 μL saline. Since the existence and/or abundance of isoforms of PNX endogenously in zebrafish is currently unknown, we decided to use the full-length PNX (20 amino acid long) in this research. After injections, fish were allowed to recover (5 min), and prequantified food was added to each tank. Remaining food was collected after 1 h and quantified as described above. After corroborating that higher doses were more efficient, 100 and 1,000 ng/g body wt PNX-20 were injected intraperitoneally to repeat the study, and tissues [brain, hypothalamus, and whole gut (including foregut and hindgut regions described earlier)] were collected 1 h postinjection and stored at –80°C until further analysis. This experimental design is now shown in Supplemental Fig. S1 (Supplemental data for this article can be found at https://doi.org/10.6084/m9.figshare.11894139.v1.
Effects of PNX-20 on the levels of mRNAs involved in glucose and lipid metabolism in vitro.
ZFL cells were seeded at 5 × 105 cells/well in 24-well plates and grown to confluency as described earlier. Once 80–90% confluency was achieved, media was replaced by 1 mL of fresh media (same recipe, as provided earlier) alone (6 wells) or media containing PNX-20 (1, 10, or 100 nM; 6 wells each). After incubation for 1 or 2 h, medium was removed, and 500 µL of RiboZol RNA isolation reagent (aMReSCO; VWR, Radnor, PA) were added to each well. Cells were then scraped, transferred to tubes, and stored at 80°C until total RNA was extracted (see Total RNA extraction, cDNA synthesis, and real-time quantitative PCR). This experiment was repeated twice. This experimental design is summarized in Supplemental Fig S1 (accessed at https://doi.org/10.6084/m9.figshare.11894139.v1).
Effect of PNX-20 on ATP production rate in ZFL cells.
For this assay, we chose the concentration and time in which PNX-20 exerts the most significant inductions in mRNA expression. ZFL cells were seeded in culture media at 20,000 cells/well in a Seahorse XFp cell culture miniplate (Agilent, Mississauga, ON, Canada) 24 h before the assay. On the day of the assay, culture media was replaced by fresh media alone (3 wells; control) or containing 100 nM PNX-20 (3 wells; treatment), and the plate was incubated for 1.5 h at 20°C. Next, media was replaced by 200 µL of warm (37°C) Seahorse XF media (DMEM, pH 7.4, with 10 mM glucose solution, 1 mM pyruvate solution, and 2 mM glutamine solution; Agilent, ON, Canada), and ATP production rate was measured using the Agilent Seahorse XFp Real-Time ATP Rate Assay Kit (Agilent), following the manufacturer’s protocol. Measurement was performed in a XFp Seahorse Analyzer (Agilent). Data were analyzed using the Wave 2.6.1 software (Agilent Technologies).
Total RNA extraction, cDNA synthesis, and real-time quantitative PCR.
Total RNA extraction, cDNA synthesis, and real-time quantitative PCR (RT-qPCR) were conducted as previously described (17). Total RNA was extracted from zebrafish tissue samples using the RiboZol RNA isolation reagent (aMReSCO). The RNA samples were checked for purity by optical density (OD) absorption ratio (OD 260 nm/OD 280 nm) using a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA). iScript cDNA synthesis kit (Bio-Rad, Mississauga, ON, Canada) was used for the synthesis of cDNA, following the manufacturer’s instructions. Real-time quantitative PCRs were performed using Universal SYBR Green Master Mix (Bio-Rad). The primer sequences and annealing temperature used for each primer set (ordered from IDT, Toronto, ON, Canada) are listed in Table 1. Primer validation and optimization were carried out to find the highest efficiency annealing temperatures before the qPCR analysis of all genes. PCR conditions used were as follows: initial denaturation 95°C (3 min), 35 cycles of denaturation: 95°C (10 s), annealing: specific to each gene (30 s), elongation: 72°C (20 s, when annealing temperature was <55°C). A melting curve analysis was performed from 65 to 95°C (temperature gradient: 0.5°C/5 s), and the absence of any dimer formation or artifacts was confirmed for each primer set. All samples were run in duplicate with negative controls, in which no template DNA (used nuclease-free water instead) was present in the PCR reaction mix. All runs were performed using a CFX Connect Real-Time System (Bio-Rad). The 2−ΔΔCt method (9) was used for the relative data analysis and quantitative expression of different genes, using β-actin as a reference gene.
Table 1.
Primers used for quantifying gene expression by real-time quantitative PCR in zebrafish
| Gene | GenBank Accession No. | Primer Sequence (5′ to 3′) | Annealing Temperature, °C |
|---|---|---|---|
| 18S rRNA | NM_173234.1 | F: GGCGAGGGTTCTGCATAATA R: CATCCTTCGTGTCCTCAACA |
60 |
| β-Actin | NM_131031.2 | F: TTCAAACGAACGACCAACCT R: TTCCGCATCCTGAGTCAATG |
60 |
| cart | GU057836.1 | F: GAGAGACTTGGCTGAGGCAC R: GAAAGTGTTGCAGGCGGTTC |
60 |
| cck | XM_001346104.6 | F: ACGCTGGACTCTGTGTAT R: CTTCATCGTCCTCTGGTTTG |
57 |
| fbp1a | NM_199942.2 | F: TGGCGAGTTCATTCTGGTGG R: TCTGCCACCATTGAGCCTAC |
60 |
| fbp1b | NM_213132.1 | F: GAGTCCCAAGGGCAAGCTAA R: TACAGGAACCCTCTGGTGGA |
60 |
| g6pca1 | BC076446.1 | F: TTGGAGACTGGCTGAACCTC R: TCAGCAAGGATTGAAAGCAACG |
60 |
| g6pcb | XM_002661194.6 | F: CATCTGGACACCACACCCTT R: TGGGTGGTCTGAACGAGTCT |
60 |
| gck | NM_001045385.2 | F: GACACAGGGGACAGAAAGCA R: CCACCCCCACAGTGATCTTT |
60 |
| ghs-r | NM_001146272.1 | F: TTGAGCACGAAAACGGGAC R: GCTTTCGCCCGATGAGACTA |
60 |
| glut2 | DQ098687.1 | F: GGATACAGCTTGGGCGTCAT R: CTCTGTGCCATTTCCCCCTT |
60 |
| glycogen phosphorylase | AY576991.1 | F: TGTAAAGTCCTCGCGCACA R: ACATCCCCGAGTCCTGCTAT |
60 |
| glycogen synthase 2 | NM_001018679.1 | F: GTGCGATGCGACTATCCAGA R: TTCACCCCATTCGTCCACAG |
60 |
| goat | NM_001122944.1 | F: GCACACTGGAAGATGAGCGA R: CGAAGGACGACTGGATGCTT |
60 |
| grl | NM_001083872.1 | F: CAGAAGAGAAGCTGCTGATCCAG R: CTCCAGAAGATTCTGAAGCAC |
58 |
| npy | NM_131074.2 | F: GGCCACCAGATCTCATAAA R: GCGCACATTGACGTATTT |
57 |
| pck1 | NM_214751.1 | F: AGCTCTTCAGGGTCTCGCA R: TAACGTGTGTGTTGCGTGTCTT |
60 |
| pck2 | NM_213192.1 | F: TCCTTCGGCAGTGGTTATGG R: GCTGCTGCAATGTACCGTTT |
60 |
| pfkla | XM_693543.8 | F: AGGTATGAACGCAGCCATCC R: TGCCAATCACTGTTCCTCCC |
60 |
| pfklb | NM_001328389.1 | F: TTTGAGCACAGGATGCCGAA R: TCGATGCTAAGGGTTCGACG |
60 |
| pklr | BC152219.1 | F: CCAGTTTAACACGCGCGGC R: GGGAAGTGTCCTTTGGCTGT |
60 |
| pomc | NM_181438.3 | F: CACTGCTCACACTCTTCA R: GCCCACCTTCGTTTCTAT |
58 |
| pyy | NM_001164371.1 | F: GACATTTGTGGACGCTTATC R: CTTCCGAAGTGGACCTTT |
57 |
| sglt1 | NM_200681.1 | F: TGTCCGTCATGTTGGCTTCA R: TCTGAGCCGTCTGAACGATG |
60 |
| smim20 | NM_001302624.1 | F: TTTGGAGGCTTCGTTGCAG R: GGCTTGTAGGGATCAGACCA |
57 |
| sreb3 | NM_131498.1 | F: CCATTGCCCACCATCGTTTC R: ATGAAGCCTAGCGTGTCGTT |
60 |
F, forward primer; R, reverse primer; cart, cocaine- and amphetamine-regulated transcript; cck, cholecystokinin; fbpase, fructose bisphosphatase; g6pc, glucose 6-phosphatase; gck, glucokinase; ghs-r, growth hormone secretagogue receptor; glut2, glucose transporter 2; goat, ghrelin O-acyltransferase; grl, ghrelin; npy, neuropeptide Y; pck, phosphoenolpyruvate carboxykinase; pfk, phosphofructokinase; pk, pyruvate kinase; pomc, proopiomelanocortin; pyy, peptide YY; smim20, small integral membrane protein 20; sglt1, sodium-glucose cotransporter 1; sreb3, super conserved receptor expressed in brain 3.
Immunohisto(cyto)chemistry.
To identify and localize PNX-20 and SREB3 immunoreactive cells in the zebrafish gut, as well as within ZFL cells, immunohisto(cyto)chemical studies were conducted using methods described earlier (6). We used tissue sections from the foregut (described earlier) of zebrafish for our study. The details of primary antibodies used in our research are as follows: rabbit anti-PNX-14 (1:1,000 dilution; Phoenix Pharmaceuticals, Burlingame, CA) and rabbit anti-SREB3 NH2-terminal (1:500 dilution; Abcam, Cambridge, UK). For immunohistochemical localization of SREB3 in the gut section, an antigen retrieval step (heat-induced epitope retrieval) was included in the tissue sections used as described by the antibody manufacturer (antigen retrieval protocol; Abcam). For immunocytochemical localization of PNX-14 and SREB3 in ZFL cells, ice-cold methanol was used as fixative to avoid the antigen retrieval step (specifically for SREB3 antibody). In both cases, the secondary antibody used was Texas Red Goat anti-rabbit (1:3,000 dilution; Vector Laboratories, Burlingame, CA). Preabsorption (for PNX-like immunoreactivity) and secondary antibody-alone (negative; for PNX-like and SREB3-like immunoreactivity) controls were conducted as previously described (21). Briefly, for the preabsorption control, zebrafish PNX-20 (custom synthesized, 10 µg) was preabsorbed with PNX-14 primary antibody overnight, and this cocktail was used instead of the primary antibody for immunostaining. Slides were mounted using Vectashield medium containing DAPI (Vector Laboratories), imaged using an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan), and analyzed using the DP controller software (Olympus). Despite the use of several controls, we acknowledge that a mammalian primary antibody was used, that it cross-reacts with the precursor, and multiple forms of processed PNX and nonspecific binding might occur. Therefore, we use PNX-like immunoreactivity to refer to the immunostaining obtained. Because of the same considerations on nonspecific binding, SREB3-like immunoreactivity was used to refer to the immunodetection of SREB3.
Statistical Analysis
Student’s t test was used for assessing statistical differences between two experimental groups. One-way ANOVA followed by Tukey’s multiple-comparison test or Tukey Kramer’s (t test) were used for comparisons among multiple groups. Significance was assigned when P < 0.05. PRISM version 5 (GraphPad) and IBM SPSS version 21 (IBM) software were used for statistical analysis. For generating graphs, PRISM version 5 (GraphPad) was used. Data are presented as mean ± SE.
RESULTS
SMIM20/PNX-20 Amino Acid Sequence Is Conserved Among Vertebrates
We found high conservation of amino acids in the PNX-20 region of SMIM20 in vertebrates (Fig. 1A). Zebrafish mature PNX-20 exhibits the highest sequence identity with alligator (70%), followed by mouse, chicken, human (65%), and frog (50%). The zebrafish precursor (SMIM20) amino acid sequence exhibits 65.67% sequence identity with frog, followed by alligator (58.21%), chicken, human (56.72%), and mouse (55.22%).
Fig. 1.
Multiple-sequence alignment of vertebrate preprophoenixin (SMIM20) amino acid sequences and tissue distribution of smim20 and super conserved receptor expressed in brain 3 (sreb3) mRNAs in zebrafish. A: alignment of zebrafish SMIM20 amino acid sequence with sequences from other vertebrates. Active peptide regions are within the arrows. PNX-20, phoenixin-20. *Shared amino acids among sequences. GenBank ID of sequences used for the alignment are as follow: alligator (XP_006260495.1), chicken (NP_001138902.1), frog (XP_002939144.1), human (NP_001138904.1), mouse (NP_001138905.1), and zebrafish (NP_001289553.1). B and C: quantitative analysis of mRNAs encoding smim20 (B) and sreb3 (C) in zebrafish tissues. Data obtained by real-time quantitative PCR (RT-qPCR) were normalized to β-actin and are expressed as means ± SE (n = 6), relative to the tissue with the lowest mRNA expression.
smim20 and sreb3 mRNAs Are Widespread Within Zebrafish Tissues
Expression of smim20 mRNAs was found to be more prominent in the ovary but was detected in several tissues of zebrafish, including the skin, eye, gill, foregut, and liver. The tissue expressing the smallest amount of smim20 mRNAs was the muscle (Fig. 1B). A widespread distribution was also found for mRNAs encoding SREB3. Tissues showing a prominent expression include the whole brain, hypothalamus, and spleen (Fig. 1C). Tabular representation of these data can be seen in Supplemental Fig. S3 (accessed at https://doi.org/10.6084/m9.figshare.11971902.v1).
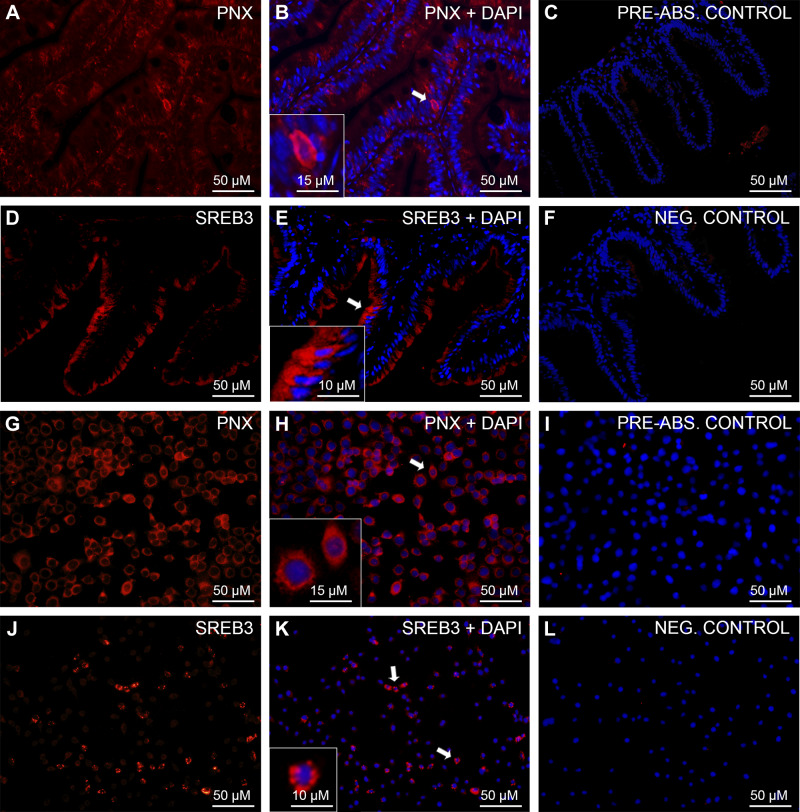
PNX-Like and SREB3-Like Immunoreactivity Was Detected in the Zebrafish Gut and ZFL Cells
Scattered cells positive for PNX-like (red; Fig. 2, A and B) and SREB3-like immunoreactivity (red; Fig. 2, D and E) were found within the epithelium of the zebrafish gut. In ZFL cells, PNX-like immunoreactivity was very prominent and was observed in the cytoplasm of all cells (red; Fig. 2, G and H). Some ZFL cells were also positive for SREB3 (red; Fig. 2, J and K). No immunoreactivity was observed in either preabsorption (Fig. 2, C and I) or secondary antibody-alone negative (Fig. 2, F and L) controls.
Fig. 2.
Immunohistochemical analysis of phoenixin (PNX)-like and super conserved receptor expressed in brain 3 (SREB3)-like in zebrafish gut and zebrafish liver (ZFL) cells. Shown are representative sections of zebrafish gut (A–F) and ZFL (G–L) cells showing PNX-like (red; A–B and G–H) and SREB3-like (red; D–E and J–K) immunoreactivity (ir). No PNX-/SREB-3-like ir was observed in either preabsorption (C and I) or secondary antibody-alone negative control (F and L). Nuclei are shown in blue (DAPI). Scale bars are indicated in each image.
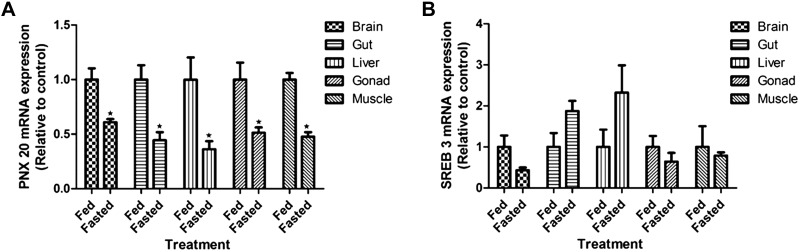
Short-Term Fasting Reduces the Expression of smim20 mRNA in Zebrafish
Food deprivation for 7 days downregulated smim20 mRNA expression in zebrafish brain, gut, liver, gonad, and muscle (Fig. 3A). However, no significant changes in sreb3 mRNA expression were observed in the 7-day fasted fish compared with fed fish (Fig. 3B). Tabular representation of these data is shown in Supplemental Fig. S3 (accessed at https://doi.org/10.6084/m9.figshare.11971902.v1).
Fig. 3.
Effects of short-term (7 days) food deprivation on the mRNA expression of small integral membrane protein 20 (smim20, A) and super conserved receptor expressed in brain 3 (sreb3, B) in zebrafish tissues. Data are presented as means ± SE (*P < 0.05, n = 6 fish/group; 4 males and 2 females). Student’s t test was used for statistical analysis.
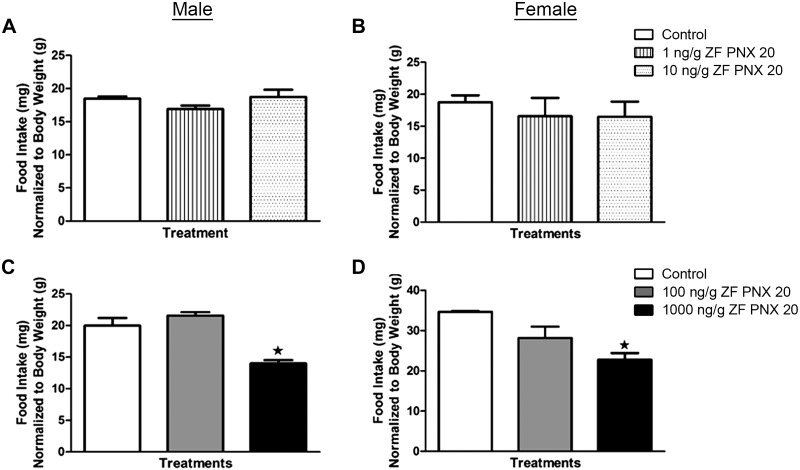
Intraperitoneal Injection of PNX-20 Suppresses Food Intake in Male and Female Zebrafish
A significant decrease in food intake was observed in 1,000 ng/g body wt PNX-20-injected male and female zebrafish compared with saline-injected fish (Fig. 4, C and D). We repeated the 1,000 ng/g dose study two times to confirm the anorexigenic effect of PNX-20 in zebrafish. No significant difference in food intake was observed between controls and fish that were injected with 1, 10, or 100 ng/g body wt PNX-20 in both male and female treatment groups (Fig. 4, A–D). Tabular representation of these data is shown in Supplemental Fig. S3 (https://doi.org/10.6084/m9.figshare.11971902.v1).
Fig. 4.
Effects of exogenously administered phoenixin-20 (PNX-20) on food intake levels of male (left) and female (right) zebrafish (ZF). Shown are food intake levels at 1 h postintraperitoneal administration of saline alone (control) or containing 1 and 10 (A and B) or 100 and 1,000 (C and D) ng/g body wt of PNX-20. Data are presented as means ± SE (n = 6 fish/group). ★P < 0.05, significant differences between control and treated groups. One-way ANOVA followed by Tukey’s multiple-comparison test was used for statistical analysis.
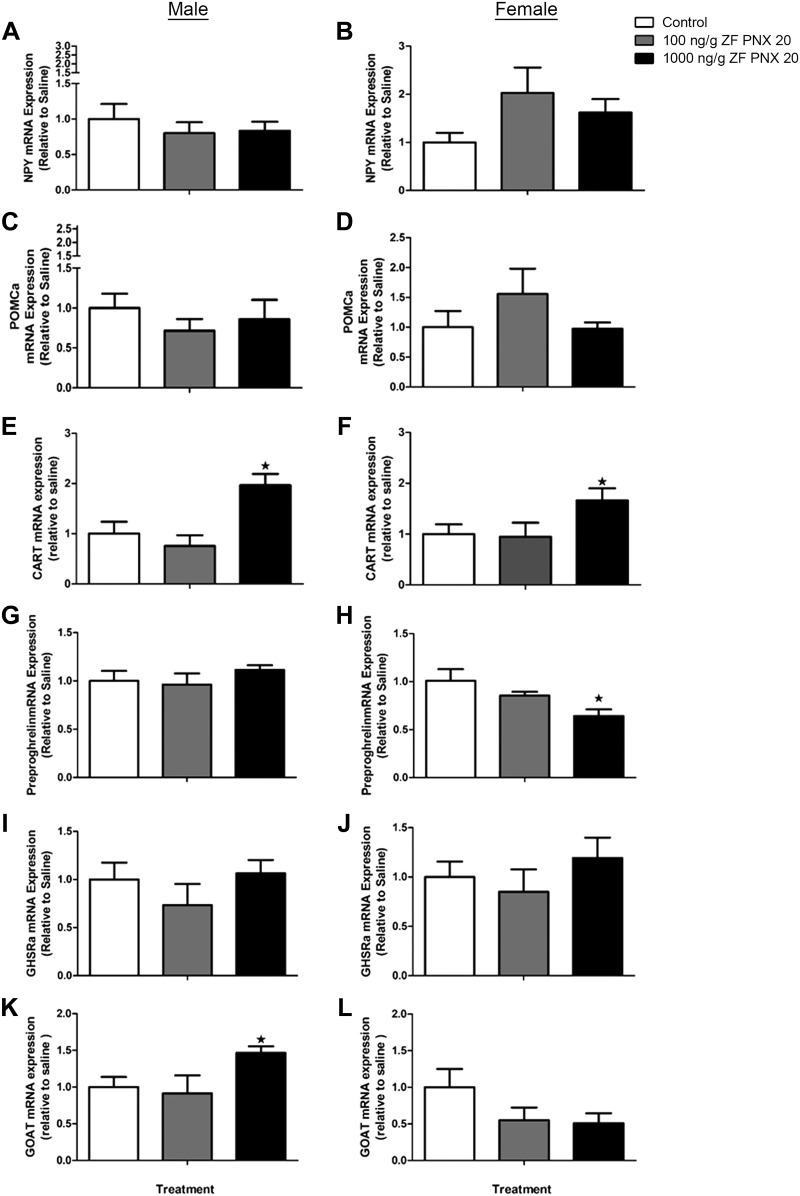
PNX-20 Modulates the Expression of Feeding Regulatory Genes in Zebrafish
Intraperitoneal administration of 1,000 ng/g body wt PNX-20 resulted in a significant increase in cart mRNA expression in the hypothalamus of both male and female zebrafish (Fig. 5, E and F). Preproghrelin mRNA expression was significantly reduced in the hypothalamus of 1,000 ng/g body wt PNX-20-injected female fish (Fig. 5H) but not in male fish (Fig. 5G). However, there was a significant increase in the hypothalamic expression of ghrelin-O-acyl transferase (goat) mRNA expression in male fish treated with 1,000 ng/g body wt PNX-20 (Fig. 5K). There were no significant changes in the growth hormone secretagogue/ghrelin receptor (ghsra) mRNA expression in response to PNX-20 treatment in either male or female fish (Fig. 5, I and J). Similarly, no significant changes in npy or pomca mRNA expression were observed post-PNX-20 administration in the brain of any of the experimental groups (Fig. 5, A–D).
Fig. 5.
Expression levels of mRNAs encoding key appetite-regulating peptides in the brain (A–D) and hypothalamus (E–L) of male (left) and female (right) zebrafish (ZF) 1 h after intraperitoneal administration of 100 and 1,000 ng/g body wt of phoenixin-20 (PNX-20). NPY, neuropeptide Y; POMCa, proopiomelanocortin a; CART, cocaine- and amphetamine-regulated transcript; GHSRa, growth hormone secretagogue receptor-a; GOAT, ghrelin O-acyltransferase. Data obtained by real-time quantitative PCR are presented as means ± SE (n = 6 fish/group). ★P < 0.05, significant differences between control and treated groups. One-way ANOVA followed by Tukey’s multiple-comparison test was used for statistical analysis.
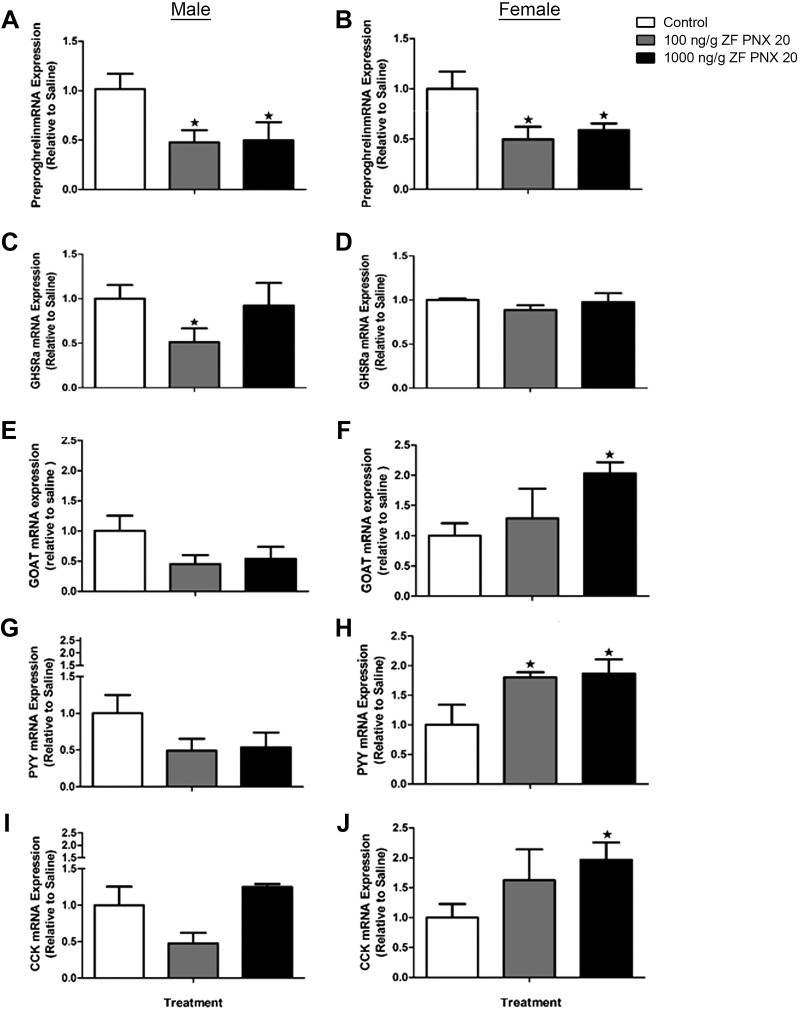
In the gut, both male and female zebrafish showed a significant reduction in preproghrelin mRNA expression in response to 100 and 1,000 ng/g body wt PNX-20 administration (Fig. 6, A and B). Injection of 100 ng/g body wt PNX-20 also caused a significant downregulation of ghsra mRNA levels in the gut of male zebrafish (Fig. 6C), whereas 1,000 ng/g body wt PNX-20 increased goat mRNA levels in the gut of female fish (Fig. 6F). In addition, a significant increase in the expression of peptide YY (pyy) was observed in the gut of female zebrafish injected with 100 and 1,000 ng/g body wt PNX-20 (Fig. 6, H and J). Similarly, 1,000 ng/g body wt PNX-20 significantly increased cholecystokinin (cck) mRNAs in the gut of female zebrafish (Fig. 6J). Tabular representation of these data is shown in Supplemental Fig. S3 (accessed at https://doi.org/10.6084/m9.figshare.11971902.v1).
Fig. 6.
Expression levels of mRNAs encoding key appetite-regulating peptides in the gut of male (left) and female (right) zebrafish (ZF) 1 h after intraperitoneal administration of 100 and 1,000 ng/g body wt of phoenixin-20 (PNX-20). GHSRa, growth hormone secretagogue receptor-a; GOAT, ghrelin O-acyltransferase; PYY, peptide YY; CCK, cholecystokinin. Data obtained by real-time quantitative PCR are presented as means ± SE (n = 6 fish/group). ★P < 0.05, significant differences between control and treated groups. One-way ANOVA followed by Tukey’s multiple-comparison test was used for statistical analysis.
Genes Involved in Glucose Transport and Metabolism Are Modulated by In Vitro Treatment with PNX-20 in ZFL Cells
The effects of in vitro treatment of ZFL cells with PNX-20 during 1 and 2 h on the mRNA expression of genes involved in glucose transport and metabolism are shown in Table 2 [graphical representation of these results is shown in Supplemental Fig. S2 (accessed at https://doi.org/10.6084/m9.figshare.11894232.v2)]. All concentrations of PNX-20 tested (1, 10, and 100 nM) caused a significant increase in the mRNA expression of glucose transporter 2 (glut2) at 2 h and of sodium-glucose cotransporter 1 (sglt1) at 1 h. We also observed increased levels of sglt1 mRNAs at 2 h in response to 1 and 100 nM (but not 10 nM) PNX-20.
Table 2.
In vitro effects of the exposure to PNX on the mRNA expression of genes involved in glucose transport and metabolism in zebrafish liver cells
| Glucose Transport and Metabolism | 1 h |
2 h |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | PNX (1 nM) | PNX (10 nM) | PNX (100 nM) | Control | PNX (1 nM) | PNX (10 nM) | PNX (100 nM) | |
| Transport | ||||||||
| glut2 | 1.00 ± 0.23 | 1.40 ± 0.20 | 1.29 ± 0.13 | 1.14 ± 0.13 | 1.00 ± 0.09 | 1.88 ± 0.13*** | 1.91 ± 0.14*** | 2.96 ± 0.49*** |
| sglt1 | 1.00 ± 0.17 | 1.70 ± 0.26* | 1.64 ± 0.20* | 1.43 ± 0.13* | 1.00 ± 0.15 | 2.06 ± 0.48* | 1.40 ± 0.20 | 1.48 ± 0.08* |
| Glycolysis | ||||||||
| gck | 1.00 ± 0.06 | 1.23 ± 0.07* | 1.24 ± 0.10* | 1.36 ± 0.18* | 1.00 ± 0.12 | 1.60 ± 0.20* | 1.27 ± 0.21 | 0.95 ± 0.11 |
| pfkla | 1.00 ± 0.10 | 1.14 ± 0.09 | 0.92 ± 0.07 | 0.71 ± 0.07* | 1.00 ± 0.06 | 1.18 ± 0.06* | 1.04 ± 0.07 | 0.78 ± 0.07* |
| pfklb | 1.00 ± 0.03 | 1.39 ± 0.19* | 1.40 ± 0.11** | 1.42 ± 0.18* | 1.00 ± 0.13 | 1.49 ± 0.19* | 1.31 ± 0.19 | 0.95 ± 0.22 |
| pklr | 1.00 ± 0.21 | 1.47 ± 0.08* | 1.32 ± 0.11 | 1.97 ± 0.29* | 1.00 ± 0.11 | 1.27 ± 0.15 | 1.14 ± 0.14 | 0.76 ± 0.06 |
| Gluconeogenesis | ||||||||
| pck1 | 1.00 ± 0.10 | 0.85 ± 0.08 | 0.92 ± 0.07 | 0.56 ± 0.06** | 1.00 ± 0.17 | 0.52 ± 0.01** | 0.58 ± 0.13* | 0.61 ± 0.14* |
| pck2 | 1.00 ± 0.14 | 0.81 ± 0.04 | 0.72 ± 0.06* | 0.60 ± 0.07* | 1.00 ± 0.11 | 0.63 ± 0.03** | 0.71 ± 0.06* | 0.73 ± 0.01* |
| fbp1a | 1.00 ± 0.10 | 1.08 ± 0.11 | 0.96 ± 0.13 | 1.00 ± 0.19 | 1.00 ± 0.14 | 0.30 ± 0.05*** | 0.38 ± 0.04*** | 0.28 ± 0.04*** |
| fbp1b | 1.00 ± 0.09 | 1.03 ± 0.02 | 1.14 ± 0.17 | 0.99 ± 0.10 | 1.00 ± 0.04 | 1.06 ± 0.28 | 0.81 ± 0.14 | 0.52 ± 0.10*** |
| g6pca1 | 1.00 ± 0.18 | 0.45 ± 0.06** | 0.45 ± 0.04** | 0.58 ± 0.11* | 1.00 ± 0.17 | 0.79 ± 0.07 | 0.69 ± 0.12 | 0.65 ± 0.10* |
| g6pcb | 1.00 ± 0.05 | 1.31 ± 0.13* | 1.35 ± 0.13* | 1.53 ± 0.08*** | 1.00 ± 0.19 | 1.29 ± 0.15 | 1.30 ± 0.17 | 0.78 ± 0.07 |
| Glycogen metabolism | ||||||||
| glycogen synthase 2 | 1.00 ± 0.07 | 1.17 ± 0.09 | 1.36 ± 0.12* | 1.01 ± 0.03 | 1.00 ± 0.03 | 0.71 ± 0.11* | 1.07 ± 0.19 | 0.98 ± 0.13 |
| glycogen phosphorylase | 1.00 ± 0.06 | 1.01 ± 0.11 | 0.57 ± 0.07*** | 0.71 ± 0.06** | 1.00 ± 0.14 | 1.26 ± 0.24 | 0.77 ± 0.09 | 0.61 ± 0.06* |
Data obtained by real-time quantitative PCR are shown as means ± SE of the results obtained in two different experiments; n = 6 fish in each experiment. Cells were incubated with culture media alone (control) or containing 1, 10, and 100 nM phoenixin-20 (PNX-20) during 1 and 2 h. The graphical representation of the in vitro effects of the exposure to PNX on the mRNA expression of genes involved in glucose transport and metabolism in zebrafish liver cells is shown in Supplemental Fig. S2 (accessed at https://doi.org/10.6084/m9.figshare.11894232.v2)]. fbp1a, fructose 1,6-bisphosphatase 1a; fbp1b, fructose 1,6-bisphosphatase 1b; g6pca1, glucose 6-phosphatase a1; g6pcb, glucose 6-phosphatase b; gck, glucokinase; glut2, glucose transporter 2; pck1, phosphoenolpyruvate carboxykinase 1; pck2, phosphoenolpyruvate carboxykinase 2; pfkla, phosphofructokinase a; pfklb, phosphofructokinase b; pklr, pyruvate kinase; sglt1, sodium-glucose cotransporter 1.
P < 0.05,
P < 0.01, and
P < 0.001, significant differences between control and treated groups within each time point.
Exposure of ZFL to PNX-20 also resulted in a time- and concentration-dependent increase (in general terms) in the mRNA levels of genes encoding glycolytic enzymes, including glucokinase (gck), phosphofructokinase a (pfkla), phosphofructokinase b (pfklb) and pyruvate kinase (pklr). The only exceptions were a decrease in pfkla mRNA at 1 and 2 h and of pklr mRNAs at 2 h, both in response to 100 nM PNX-20. On the contrary, expression of mRNAs encoding gluconeogenic enzymes, including glucose-6-phosphatase a1 (g6pca1), fructose-1,6-bisphosphatase 1a (fbp1a), fructose-1,6-bisphosphatase 1b (fbp1b), phosphoenolpyruvate carboxykinase 1 (pck1), and phosphoenolpyruvate carboxykinase 2 (pck2), were downregulated by PNX-20 exposure. We only observed increased expression of one gluconeogenic gene, glucose 6-phosphatase b (g6pcb), in response to PNX-20 treatment during the 1st h of treatment.
Finally, exposure of ZFL to PNX-20 significantly altered the expression of key genes involved in glycogen metabolism. Specifically, we found a significant increase in glycogen synthase 2 mRNAs with 10 nM PNX-20 at 1 h but a significant decrease with 1 nM PNX-20 at 2 h. In addition, 10 and 100 nM PNX-20 downregulated glycogen phosphorylase mRNA at 1 h, and 100 nM caused a similar effect at 2 h.
PNX-20 Increases glycoATP Production Rate in ZFL Cells
Exposure of ZFL cells to 100 nM PNX-20 during 1.5 h did not result in significant differences in the rate of total ATP production. However, ZFL cells treated with PNX-20 exhibited significantly higher ATP production rate associated with glycolysis compared with control cells (Fig. 7).
Fig. 7.
ATP production rate of zebrafish liver cells exposed to 100 nM phoenixin-20 (PNX-20) during 1.5 h. ATP production rate was measured using the Seahorse XFp Real-Time ATP Rate assay. This assay is based on the kinetic quantification of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). OCR and ECAR rates are first measured in basal conditions. Next, injection of oligomycin results in an inhibition of mitochondrial ATP synthesis that results in a decrease in OCR, allowing mitochondrial ATP production rates to be quantified. Complete inhibition of mitochondrial respiration with rotenone + antimycin A enables calculation of mitochondrial-associated acidification, allowing calculation of glycolytic ATP production. Data are presented as means ± SE (★P < 0.05, n = 3 wells/group). Student’s t test was used for statistical analysis.
DISCUSSION
PNX-20 has emerged as a multifunctional peptide in mammals, with roles in several physiological processes, including reproduction and food intake regulation (27). This study aimed to offer novel information on PNX-20 in zebrafish, an important teleost model. First, we demonstrated that mRNAs encoding PNX-20 are widely expressed within zebrafish tissues, as it was described in other fish (25) and mammals (10, 15, 16, 18, 26). The highest levels of expression were detected in the ovary, in accordance with the well-known modulatory role of PNX on female reproduction (20, 24, 26). Expression of PNX-20 receptor mRNA, sreb3, was also observed in several zebrafish tissues, which were previously reported as sites of this receptor expression in mammals (12, 20, 22). This wide tissue distribution of sreb3 points toward a multifunctional nature of PNX-20 in fish. Specifically, the presence of smim20 and sreb3 mRNAs, as well as the presence of the proteins encoded by both genes (as demonstrated by immunohistochemistry), allowed us to hypothesize for a role of PNX-20 on food intake and/or energy balance regulation. The identity of gut cells expressing PNX and its receptor is currently unknown. It is likely that these cells are enteroendocrine cells or enterocytes, but further studies are required to confirm its identity.
To study the putative role for PNX-20 on food intake regulation, PNX-20 was intraperitoneally administered to both male and female zebrafish, and feed intake levels were quantified at 1 h postadministration. We found that 1,000 ng/g body wt PNX-20 significantly reduced feeding in zebrafish. This anorexigenic action is in accordance with present observations on smim20 mRNA being downregulated by a 7-day fasting period in several zebrafish tissues (brain, gut, liver, gonad, and muscle). A naturally occurring anorexigen is expected to be downregulated in appetite regulatory centers during reduced energy availability to promote an urge to consume food. However, it disagrees with previous studies in mammals reporting that intracerebroventricular injection of PNX-20 induces feeding in rats (19). Previous reports on an orexigenic role for PNX-20 in mammals and the present observation of an anorexigenic action indicate the existence of species-specific effects of PNX-20 on feeding in vertebrates. Another reason for this variability might be the differences in the mode of administration and the type of peptide used. Central administration (icv) of PNX-14 was employed in the rat study (19), whereas intraperitoneal injection of PNX-20 was used in this study.
Given the anorectic nature of PNX-20 in zebrafish, we then explored whether its intraperitoneal injection affects the expression of appetite regulatory peptides. The most notable results include an increase in the mRNA levels of the anorexigen cart (14) in the hypothalamus and a decrease of the orexigen ghrelin (1) in the brain and gut in response to PNX-20, supporting an anorexigenic action of this peptide and its mediators by these two potent appetite regulators. These results also suggest that PNX-20 regulates feeding by influencing both brain and gut hormonal milieu. Interestingly, we observed a differential regulatory pattern in both male and female zebrafish. For instance, in females, but not in males, the gut anorexigens pyy (5) and cck (7) were also upregulated by PNX-20 treatment. This observation not only suggests the involvement of these two hormones in mediating PNX-20 anorectic action, but also supports the hypothesis that PNX-20 has sexually dimorphic regulatory effects in zebrafish. Further studies are needed to elucidate the sex-specific differential action of PNX-20 in zebrafish.
Finally, given the presence of PNX-20 and SREB3 in the zebrafish liver and the prominent downregulation of the hepatic expression of the genes encoding both peptides in response to fasting, we hypothesized that PNX-20 may have a metabolic role in the zebrafish liver. Therefore, we aimed to determine whether PNX-20 affects glucose metabolism by evaluating its effect on the expression of mRNAs involved in glucose homeostasis in vitro using ZFL cells. Major results of our study suggest a role for PNX-20 in stimulating hepatic glucose degradation by glycolysis, as pointed out by the PNX-induced increased expression of mRNAs encoding glycolytic enzymes (gck, pfkla, pfklb, and pklr) and increased glycolysis-derived ATP production. Similarly, the observation that PNX-20 decreases the expression of gluconeogenic enzymes (g6pca1, fbp1a, fbp1b, pck1, and pck2) points toward an inhibitory role of this peptide on gluconeogenesis. These observations are in accordance with the anorectic role for PNX-20 reported here. In addition, we found evidence for a role of PNX-20 in stimulating glycogenesis and inhibiting glycogenolysis, as suggested by the increased glycogen synthase 2 mRNAs and decreased glycogen phosphorylase mRNAs in PNX-20-treated ZFL cells. Together, these results suggest that PNX-20 is likely involved in lowering glucose levels, apparently by both reducing glucose formation (synthesis de novo or derived from glycogen degradation) and stimulating its degradation/accumulation (via glycolysis and glycogenesis) in the liver. To the best of our knowledge, this is the first report of such a role for PNX-20 on glucose homeostasis in animals.
In summary, this research characterized PNX-20 as an anorexigen in zebrafish, with important roles in hepatic glucose regulation. Although the appetite regulatory effects of PNX-20 were found in both males and females, its effects on the expression of other anorexigens and orexigens were sex dependent. This suggests different targets of action for PNX-20 in male and female zebrafish. Originally characterized as a reproductive peptide, PNX-20 is also emerging as a multifunctional peptide in fish, as it is in mammals. The present results add PNX-20 to the complex endocrine network that integrates metabolism and reproduction in fish. Whereas these results are novel and insightful, our research has some limitations. Antibodies developed against fish PNX and its receptor as epitopes are currently unavailable. Although our results show immunoreactive cells for both proteins, it would be more important to have these results confirmed using antibodies against fish proteins. We have not tested the effects of all forms of PNX-20, including PNX-14, in our studies. It will be meaningful to address this in the future. Only the highest dose of the peptide used was found effective in modulating food intake. The endogenous levels of PNX in fish remain unknown, and it is possible that the effective dose found here is pharmacological. In mammals, only central administration was found effective in regulating food intake. It is likely that the higher dose injected peripherally is required to provide a more physiological dose in the brain. In future studies, we will consider the intracerebroventricular route of administration of a lower dose of PNX to study its effect on feeding and metabolism in fish. Additional studies are also essential to determine the mechanism of action of PNX-20 in fish.
Perspectives and Significance
Phoenixin is a recently identified peptide with hormone-like action in mammals. The highly conserved amino acid composition and extensive expression of both PNX and its receptor in central and peripheral tissues suggest multiple regulatory roles of PNX. Our study, similar to what was reported in mammals, indicates that PNX is involved in the regulation of energy homeostasis in fish. We used zebrafish for our study, which is one of the most widely used model organisms in comparative endocrinology. The outcomes of this research added a new peptide to the growing array of metabolic regulatory peptides in fish. Furthermore, it provides a basis for additional research into this molecule. PNX is a potent reproductive regulator in mammals, and the role of PNX on reproduction in fish is currently being explored. Preliminary results from our laboratory and other researchers indicate that PNX indeed has positive effects on reproductive hormones in fish. Now that we found a metabolic role for PNX in fish, it would be meritorious to consider elucidating the potential role of PNX in integrating metabolism and reproduction during nutrient availability and its shortage.
GRANTS
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada, and the University of Saskatchewan Centennial Enhancement Chair in Comparative Endocrinology to S. Unniappan. J. J. Rajeswari is a recipient of a Western College of Veterinary Medicine-College of Graduate and Postdoctoral Studies (WCVM-CGPS) PhD Scholarship. A. M. Blanco is supported by a postdoctoral fellowship from Xunta de Galicia, Spain.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.R., A.M.B., and S.U. conceived and designed research; J.J.R., A.M.B., and S.U. performed experiments; J.J.R. and A.M.B. analyzed data; J.J.R., A.M.B., and S.U. interpreted results of experiments; J.J.R. and A.M.B. prepared figures; J.J.R. and A.M.B. drafted manuscript; J.J.R., A.M.B., and S.U. edited and revised manuscript; J.J.R., A.M.B., and S.U. approved final version of manuscript.
REFERENCES
- 1.Amole N, Unniappan S. Fasting induces preproghrelin mRNA expression in the brain and gut of zebrafish, Danio rerio. Gen Comp Endocrinol 161: 133–137, 2009. doi: 10.1016/j.ygcen.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Billert M, Kołodziejski PA, Strowski MZ, Nowak KW, Skrzypski M. Phoenixin-14 stimulates proliferation and insulin secretion in insulin producing INS-1E cells. Biochim Biophys Acta Mol Cell Res 1866: 118533, 2019. doi: 10.1016/j.bbamcr.2019.118533. [DOI] [PubMed] [Google Scholar]
- 3.Billert M, Wojciechowicz T, Jasaszwili M, Szczepankiewicz D, Waśko J, Kaźmierczak S, Strowski MZ, Nowak KW, Skrzypski M. Phoenixin-14 stimulates differentiation of 3T3-L1 preadipocytes via cAMP/Epac-dependent mechanism. Biochim Biophys Acta Mol Cell Biol Lipids 1863: 1449–1457, 2018. doi: 10.1016/j.bbalip.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Gasparini S, Stein LM, Loewen SP, Haddock CJ, Soo J, Ferguson AV, Kolar GR, Yosten GLC, Samson WK. Novel regulator of vasopressin secretion: phoenixin. Am J Physiol Regul Integr Comp Physiol 314: R623–R628, 2018. doi: 10.1152/ajpregu.00426.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez R, Unniappan S. Molecular characterization, appetite regulatory effects and feeding related changes of peptide YY in goldfish. Gen Comp Endocrinol 166: 273–279, 2010. doi: 10.1016/j.ygcen.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Hatef A, Yufa R, Unniappan S. Ghrelin O-acyl transferase in zebrafish is an evolutionarily conserved peptide upregulated during calorie restriction. Zebrafish 12: 327–338, 2015. doi: 10.1089/zeb.2014.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Himick BA, Peter RE. CCK/gastrin-like immunoreactivity in brain and gut, and CCK suppression of feeding in goldfish. Am J Physiol Regul Integr Comp Physiol 267: R841–R851, 1994. doi: 10.1152/ajpregu.1994.267.3.R841. [DOI] [PubMed] [Google Scholar]
- 8.Jiang JH, He Z, Peng YL, Jin WD, Mu J, Xue HX, Wang Z, Chang M, Wang R. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav Brain Res 286: 39–48, 2015. doi: 10.1016/j.bbr.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Lyu R-M, Cowan A, Zhang Y, Chen Y-H, Dun SL, Chang J-K, Dun NJ, Luo JJ. Phoenixin: a novel brain-gut-skin peptide with multiple bioactivity. Acta Pharmacol Sin 39: 770–773, 2018. doi: 10.1038/aps.2017.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyu R-M, Huang X-F, Zhang Y, Dun SL, Luo JJ, Chang J-K, Dun NJ. Phoenixin: a novel peptide in rodent sensory ganglia. Neuroscience 250: 622–631, 2013. doi: 10.1016/j.neuroscience.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto M, Saito T, Takasaki J, Kamohara M, Sugimoto T, Kobayashi M, Tadokoro M, Matsumoto S, Ohishi T, Furuichi K. An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem Biophys Res Commun 272: 576–582, 2000. doi: 10.1006/bbrc.2000.2829. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen XP, Nakamura T, Osuka S, Bayasula B, Nakanishi N, Kasahara Y, Muraoka A, Hayashi S, Nagai T, Murase T, Goto M, Iwase A, Kikkawa F. Effect of the neuropeptide phoenixin and its receptor GPR173 during folliculogenesis. Reproduction 158: 25–34, 2019. doi: 10.1530/REP-19-0025. [DOI] [PubMed] [Google Scholar]
- 14.Nishio S, Gibert Y, Berekelya L, Bernard L, Brunet F, Guillot E, Le Bail J-C, Sánchez JA, Galzin AM, Triqueneaux G, Laudet V. Fasting induces CART down-regulation in the zebrafish nervous system in a cannabinoid receptor 1-dependent manner. Mol Endocrinol 26: 1316–1326, 2012. doi: 10.1210/me.2011-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pałasz A, Rojczyk E, Bogus K, Worthington JJ, Wiaderkiewicz R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci Lett 592: 17–21, 2015. doi: 10.1016/j.neulet.2015.02.060. [DOI] [PubMed] [Google Scholar]
- 16.Prinz P, Scharner S, Friedrich T, Schalla M, Goebel-Stengel M, Rose M, Stengel A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem Biophys Res Commun 493: 195–201, 2017. doi: 10.1016/j.bbrc.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 17.Rajeswari JJ, Hatef A, Golshan M, Alavi SMH, Unniappan S. Metabolic stress leads to divergent changes in the ghrelinergic system in goldfish (Carassius auratus) gonads. Comp Biochem Physiol A Mol Integr Physiol 235: 112–120, 2019. doi: 10.1016/j.cbpa.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Rocca C, Scavello F, Granieri MC, Pasqua T, Amodio N, Imbrogno S, Gattuso A, Mazza R, Cerra MC, Angelone T. Phoenixin-14: detection and novel physiological implications in cardiac modulation and cardioprotection. Cell Mol Life Sci 75: 743–756, 2018. doi: 10.1007/s00018-017-2661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schalla M, Prinz P, Friedrich T, Scharner S, Kobelt P, Goebel-Stengel M, Rose M, Stengel A. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 96: 53–60, 2017. doi: 10.1016/j.peptides.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, Yosten GLC. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol 311: R489–R496, 2016. doi: 10.1152/ajpregu.00191.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundarrajan L, Yeung C, Hahn L, Weber LP, Unniappan S. Irisin regulates cardiac physiology in zebrafish. PLoS One 12: e0181461, 2017. doi: 10.1371/journal.pone.0181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suszka-Świtek A, Pałasz A, Filipczyk Ł, Menezes IC, Mordecka-Chamera K, Angelone T, Bogus K, Bacopoulou F, Worthington JJ, Wiaderkiewicz R. The GnRH analogues affect novel neuropeptide SMIM20/phoenixin and GPR173 receptor expressions in the female rat hypothalamic-pituitary-gonadal (HPG) axis. Clin Exp Pharmacol Physiol 10.1111/1440-1681.13061. [DOI] [PubMed] [Google Scholar]
- 23.Treen AK, Luo V, Belsham DD. Phoenixin activates immortalized GnRH and kisspeptin neurons through the novel receptor GPR173. Mol Endocrinol 30: 872–888, 2016. doi: 10.1210/me.2016-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Chen H-P, Zhai Y, Jiang D-N, Liu J-Y, Tian C-X, Wu T-L, Zhu C-H, Deng S-P, Li G-L. Phoenixin: expression at different ovarian development stages and effects on genes ralated to reproduction in spotted scat, Scatophagus argus. Comp Biochem Physiol B Biochem Mol Biol 228: 17–25, 2019. doi: 10.1016/j.cbpb.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Deng S-P, Chen H-P, Jiang D-N, Tian C-X, Yang W, Wu T-L, Zhu C-H, Zhang Y, Li G-L. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp Biochem Physiol B Biochem Mol Biol 226: 36–44, 2018. doi: 10.1016/j.cbpb.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Yosten GLC, Lyu R-M, Hsueh AJW, Avsian-Kretchmer O, Chang J-K, Tullock CW, Dun SL, Dun N, Samson WK. A novel reproductive peptide, phoenixin. J Neuroendocrinol 25: 206–215, 2013. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan T, Sun Z, Zhao W, Wang T, Zhang J, Niu D. Phoenixin: a newly discovered peptide with multi-functions. Protein Pept Lett 24: 472–475, 2017. doi: 10.2174/0929866524666170207154417. [DOI] [PubMed] [Google Scholar]