Abstract
Background
The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and coronavirus disease 2019 (COVID‐19), has caused more than 3.9 million cases worldwide. Currently, there is great interest to assess venous thrombosis prevalence, diagnosis, prevention, and management in patients with COVID‐19.
Objectives
To determine the prevalence of venous thromboembolism (VTE) in critically ill patients with COVID‐19, using lower limbs venous ultrasonography screening.
Methods
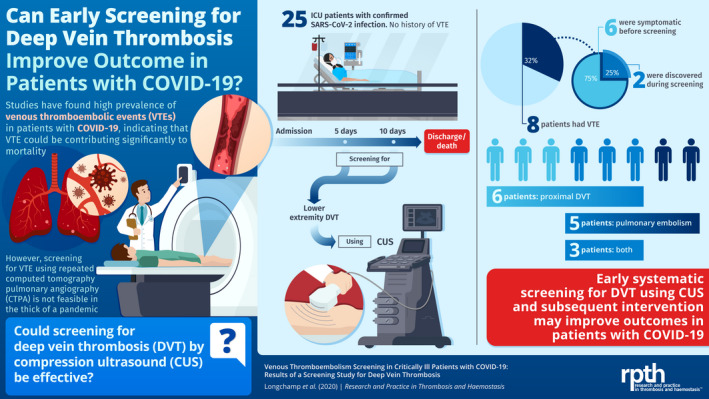
Beginning March 8, we enrolled 25 patients who were admitted to the intensive care unit (ICU) with confirmed SARS‐CoV‐2 infections. The presence of lower extremity deep vein thrombosis (DVT) was systematically assessed by ultrasonography between day 5 and 10 after admission. The data reported here are those available up to May 9, 2020.
Results
The mean (± standard deviation) age of the patients was 68 ± 11 years, and 64% were men. No patients had a history of VTE. During the ICU stay, 8 patients (32%) had a VTE; 6 (24%) a proximal DVT, and 5 (20%) a pulmonary embolism. The rate of symptomatic VTE was 24%, while 8% of patients had screen‐detected DVT. Only those patients with a documented VTE received a therapeutic anticoagulant regimen. As of May 9, 2020, 5 patients had died (20%), 2 remained in the ICU (8%), and 18 were discharged (72%).
Conclusions
In critically ill patients with SARS‐CoV‐2 infections, DVT screening at days 5‐10 of admission yielded a 32% prevalence of VTE. Seventy‐five percent of events occurred before screening. Earlier screening might be effective in optimizing care in ICU patients with COVID‐19.
Keywords: COVID‐19, pulmonary embolism, SARS virus, ultrasonography, venous thrombosis
Essentials.
Initial reports suggest increased coagulation activity in patients with coronavirus disease 2019 (COVID‐19).
In critically ill patients, lower‐extremity deep vein thrombosis (DVT) was systematically assessed by ultrasonography.
The rate of symptomatic venous thromboembolism was 24%, while 8% of patients had screen‐detected DVT.
Early systematic screening for lower‐limb DVT might be warranted in patients with COVID‐19.
1. INTRODUCTION
In December 2019, a novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was discovered, causing more than 3.9 million confirmed cases of the coronavirus disease 2019 (COVID‐19) 1 . Initial reports from China and Italy suggested a high mortality, 2 , 3 especially in critically ill patients requiring invasive mechanical ventilation, of whom more than 80% died. 3 Importantly, most of the patients with COVID‐19 (68%) had increased coagulation activity, and D‐dimer concentrations >1000 μg/L were associated with an increased risk of hospital death 4 . In recent retrospective studies, the prevalence of symptomatic venous thromboembolism (VTE) was 25%‐27%, 5 , 6 which might contribute to the significant mortality rate. Compounding this problem, performance of repeated computed tomography pulmonary angiography (CTPA) is not possible in the context of a pandemic COVID‐19 outbreak and in overwhelmed intensive care units (ICUs). 2 Altogether, this has led many centers to empirically increase the anticoagulant dosage. In the absence of supportive evidence for these practices, we evaluated the role of systematic deep vein thrombosis (DVT) screening with lower‐limb venous compression ultrasound (CUS) and the prevalence of VTE among critically ill patients with COVID‐19.
2. METHODS
2.1. Study population, setting, and data collection
We included patients with laboratory‐confirmed COVID‐19 infection who were admitted to the Sion (Switzerland) hospital ICU for hypoxemic respiratory failure, between March 8 and April 4, 2020. None of the patients were excluded. The hospital was designed as the reference center for the state, and 50 ICU beds were made available. A confirmed case of COVID‐19 was defined as a positive result on a reverse transcriptase–polymerase chain reaction assay of a specimen collected from a nasopharyngeal swab, sputum, or bronchial aspirate. Twenty‐five adults (≥18 years of age) were identified from our institution There were no pregnant women or children admitted to our ICU during the study period. The study protocol was in accordance with the Declaration of Helsinki, and approved by the local ethics committee (2020‐00856). Informed consent was waived. We collected demographic data, information on clinical symptoms or signs at presentation, and laboratory and radiologic results during the ICU stay, from each individual electronic medical record. All laboratory tests and radiologic assessments (including CT pulmonary angiography [CTPA]) were performed at the discretion of the treating physician. As a screening test, lower‐limb venous CUS was systematically performed in all patients by 2 independent vascular specialists, between days 5 and 10 after admission to the ICU.
2.2. Study definitions
Coexisting conditions were ascertained from physician documentation. Acute respiratory distress syndrome was defined according to the Berlin definition. 7 VTE events were defined as a pulmonary embolism (PE) or a proximal lower‐extremity DVT. PEs were diagnosed by CPTA, and DVTs by CUS. Upper‐extremity DVT, catheter‐related thrombosis, and arterial thrombosis were not recorded. Major and nonmajor bleeding were defined according to the ISTH criteria. 8 Disseminated intravascular coagulation scores were calculated using the ISTH scoring system. 9
2.3. Specimen collection and testing
Clinical specimens for COVID‐19 diagnosis were tested with the assay based on the World Health Organization standard, which targets the SARS‐CoV‐2 E gene and RdRp gene. 10 The systematic lower‐limb CUS assessed proximal veins, from the common femoral veins down to the popliteal veins. The criterion for establishing the diagnosis of DVT was the lack of compressibility on B‐mode ultrasound. 11 The criterion for establishing the diagnosis of PE was the presence of an intraluminal filling defect on CTPA in a segmental or more proximal pulmonary artery. 12 D‐dimer levels were quantified with an automated latex‐enhanced quantitative immunoturbidimetric assay, the Innovance D‐Dimer assay (Siemens Medical Solutions, Malvern, PA, USA), on the Behring Coagulation System analyzer.
2.4. Statistical analysis
Descriptive statistics were used to report the data; results are reported as medians and interquartile ranges (IQRs) or means and standard deviations, as appropriate. Categorical variables were summarized as numbers and percentages. No imputation was made for missing data. Analysis was performed with R (RStudio v1.2.5033).
3. RESULTS
3.1. Patients
Of the 25 patients recruited, the mean (± standard deviation) patient age was 68 ± 11 years (range, 49‐82); 64% were men (Table 1). No patients had a history of VTE. Median body mass index was (27.5 ± 4.6). Twenty‐three (92%) patients needed mechanical ventilation, and 19 (76%) patients required hemodynamic support with norepinephrine for >12 hours. Fibrinogen (6.4 g/L; range, 4.5‐7.2) and D‐dimer (2071 μg/L; range, 953‐3606) levels were high. A chest radiograph was obtained in all 25 patients, which showed bilateral infiltrates in all (100%). A CTPA scan was obtained in 7 patients (28%) on admission; all of the computed tomography scans showed bilateral ground glass opacities, and 1 showed multiple bilateral segmental PE (4%). Figure 1 provides representative images.
TABLE 1.
Baseline patient characteristics
| Characteristic and clinical data on admission |
Patients (N = 25) |
|---|---|
| Mean age, y (range) | 68 ± 11 (49‐82) |
| Sex, n (%) | |
| Male | 16 (64) |
| Female | 9 (36) |
| Body mass index, mean (SD) a | 27.5 (4.6) |
| Coexisting disorders, n (%) | |
| Hypertension | 10 (40) |
| Cardiovascular disease | 3 (12) |
| Diabetes mellitus | 1 (4) |
| Chronic obstructive pulmonary disease | 2 (8) |
| Obstructive sleep apnea | 3 (12) |
| Asthma | 1 (4) |
| Current or former tobacco smoker | 6 (24) |
| Active malignancy | 2 (8) |
| History of venous thromboembolism | 0 |
| Mean duration of symptoms before ICU admission, d (SD) | 10 (3) |
| Laboratory data, median (IQR) | |
| Fibrinogen, g/L b | 6.4 (4.5‐7.2) |
| D‐dimer, μg/L c | 2071 (953‐3606) |
| Creatinine, μmol/L | 74 (64‐93) |
| Disseminated intravascular coagulation score, median (IQR) c | 2 (0‐3) |
| Imaging, n (%) | |
| Chest radiography | 25 (100) |
| Chest computed tomography | 7 (28) |
| ICU therapies, n (%) | |
| Invasive mechanical ventilation | 23 (92) |
| Prone position | 14 (56) |
| Vasopressors | 19 (76) |
No patients had cerebrovascular disease, chronic kidney and liver disease, or human immunodeficiency virus infection.
Body mass index is the weight in kilograms divided by the square of the height in meters.
Data were available for 14 of the 25 patients.
Data were available for 12 of the 25 patients.
FIGURE 1.
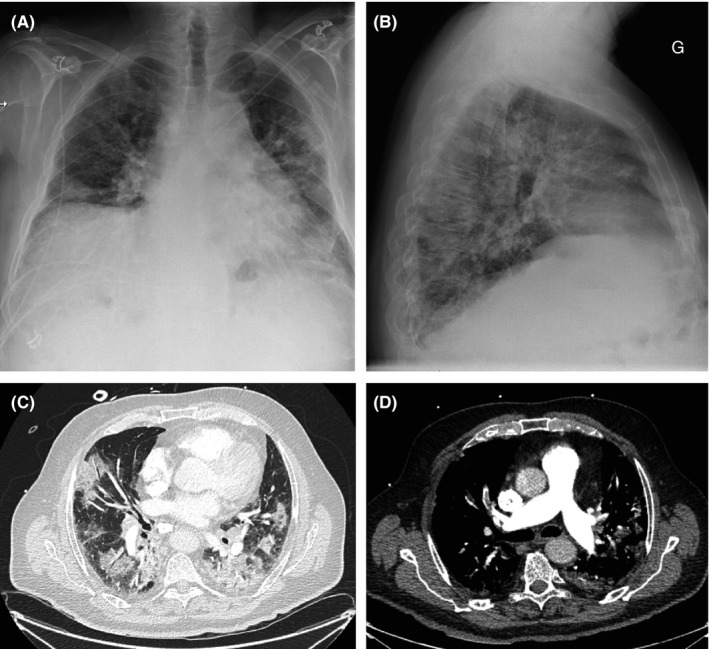
Chest radiograph and computed tomography (CT) images of a patient with coronavirus disease 2019 and pulmonary embolism. (A, B) The chest radiograph (anteroposterior and lateral views) at admission shows bilateral hazy opacities in the lung. (C) Axial CT images demonstrate extensive ground glass opacities and consolidation. (D) Segmental pulmonary embolism
3.2. Venous thromboembolism characteristics
All patients were prescribed a thromboprophylactic regimen, either with continuous intravenous heparin infusion (15 000 IU/24 h, or 20 000 IU/24 h for patients >100 kg), or once‐daily subcutaneous enoxaparin injections (40 mg, or 60 mg for patients >100 kg, Table 2) since the admission. Two patients (8%) were on chronic therapeutic anticoagulation for atrial fibrillation. During the ICU stay, one third (32%) of the patients suffered from a VTE, 6 (24%) a proximal DVT, 5 (20%) PEs, and 3 (12%) had both. All PEs were diagnosed with CTPA in patients with a clinical suspicion. PE locations are described in Table 2. Subsegmental PE was not recorded. Two (8%) DVTs were diagnosed on CUS performed systematically in all patients between days 5 and 10. Three (12%) of the patients with DVT had already been diagnosed with PE, and 1 (4%) had lower‐limb edema. Two patients could not undergo systemic CUS because of early death. Altogether, the rate of symptomatic VTE was 24%, while 8% of patients had screen‐detected DVT.
TABLE 2.
Venous thromboembolism characteristics and outcome
| Laboratory data, thrombotic event, and outcome during ICU stay | Patients (N = 25) |
|---|---|
| Patient with venous thromboembolism event, n (%) | 8 (32) |
| Anticoagulation therapy prior to the event, n/total | |
| Heparin 15 000 IU/24 h | 5/8 |
| Heparin 20 000 IU/24 h | 1/8 |
| Enoxaparin 40 mg/24 h | 1/8 |
| No thromboprophylaxis | 1/8 |
| Median time in ICU until symptomatic venous thromboembolism, d (IQR) | 3 (2‐7) |
| Proximal lower‐extremity DVT, n (%) a | 6 (24) |
| Asymptomatic, CUS screening | 2 (8) |
| Unilateral | 4 (16) |
| Bilateral | 2 (8) |
| Pulmonary embolism, n (%) | 5 (20) |
| Lobar | 3 (12) |
| Multiple segmental | 1 (4) |
| Single segmental | 1 (4) |
| Proximal lower‐extremity DVT and pulmonary embolism, n (%) a | 3 (12) |
| Therapy after the thromboembolic event, n/total | |
| Systemic thrombolysis | 1/8 |
| Therapeutic anticoagulation | 8/8 |
| Bleeding, n (%) | |
| Major | 0 |
| Minor | 2 (8) |
| Outcome, n (%) | |
| Died | 5 (20) |
| Currently in the ICU | 2 (8) |
| Discharged from the ICU | 18 (72) |
CUS, compression ultrasound; DVT, deep vein thrombosis; ICU, intensive care unit; IQR, interquartile range
Twenty‐three of the 25 patients were available for DVT screening.
Of the 6 patients with PE, 1 was administered systemic thrombolysis. All patients with documented VTE received a therapeutic anticoagulant treatment. The median time spent in the ICU before a symptomatic VTE event was 3 days (IQR, 2‐7). During the ICU stay, no patient experienced major bleeding. As of May 9, 2020; 5 patients had died (20%), 2 remained in the ICU (8%), and 18 were discharged (72%).
4. DISCUSSION
In this monocentric case series of 25 critically ill patients admitted to the ICU with COVID‐19–related acute hypoxemic respiratory failure, we found a high rate of VTE events. Indeed, 8 of 25 (32%) patients had an objectively confirmed PE and/or lower‐limb proximal DVT, despite adequate thromboprophylaxis. In contrast to most other studies, 5 , 6 patients were systematically screened for lower‐limb DVT. Thus, this prevalence suggests the maximum DVT rate. Interestingly, the median time spent in the ICU before a symptomatic documented VTE was 3 days, with 75% of events occurring before screening at days 5‐10. This suggests that screening should be performed earlier, which might be effective in optimizing care in ICU patients with COVID‐19.
Initial reports on COVID‐19 reported abnormal coagulation activity, reflected by elevated D‐dimer concentrations. This was associated with an increased risk of in‐hospital death 4 . These studies did not report the occurrence of PE and DVT. 4 , 13 , 14 In 2 recent retrospectives studies, the prevalence of venous thrombotic complications ranged between 25% and 27%, consistent with our findings. In these studies, CUS and CPTA were performed only when thrombotic complications were suspected. Moreover, patients did not receive thromboprophylaxis 6 or it was not standardized. 5 They also failed to describe hemorrhagic complications. It remains unknown whether this high rate of VTE is related to a COVID‐19–specific hypercoagulable state or if it results from the overall condition of these patients, with profound and prolonged sedation, prone positioning, and systemic inflammation. Under adequate thromboprophylaxis, the rate of VTE in acutely ill ICU patients has been reported to be around 10%. 15 However, data on a potentially higher VTE risk in the subgroup of severe acute respiratory syndrome patients is lacking. Postmortem examination of 8 patients infected with SARS‐CoV‐1 identified pulmonary thromboembolism in 4 patients (50%). Three (38%) of these patients also had lower‐extremity DVT. 16 In another retrospective study of patients with influenza A (H1N1) virus pneumonia, 5 (36%) patients had PE on computed tomography scan examination.
The question of a causal effect between VTE and the high mortality rate in these patients was raised, as well as the potential benefit of an enhanced thromboprophylaxis regimen. 17 Together with the low observed bleeding rates in these patients, this has led many centers to empirically increase anticoagulant dosing. However, these practices cannot be supported by available evidence. A prospective randomized open‐label trial comparing 2 different dosing regimens of thromboprophylaxis versus therapeutic doses of anticoagulants in patients admitted to a medical ward or the ICU has just started in Switzerland (NCT04345848).
Another question, which remains still open, is the usefulness of systematic CUS screening for DVT. In our series, the rate of symptomatic VTE was 24%, while 2 (8%) patients were diagnosed by systematic lower‐limb CUS. Although this proportion (25%) of asymptomatic proximal DVT among confirmed VTE events could suggest the usefulness of systematic CUS, 2 points deserve comment. While the current mortality in our series (20%) was low compared to others, 3 , 18 it is impossible to predict if the clinical evolution of these asymptomatic patients would differ if they had not been diagnosed with DVT and were not given anticoagulant treatment. Second, the small sample size of our study precludes any formal conclusion on the benefit of systematic DVT screening. In view of the paucity of data, current screening practices differ widely across ICU wards worldwide.
Our study has several limitations. First, because of our focus on the most severely affected ICU patients, the sample size is small. Second, the dose of prophylactic heparin administered was higher than current guidelines 8 because of the perceived higher risk of VTE as discussed above. 4 , 5 , 19 This was in accordance with current local expert consensus but might have reduced the incidence of VTE.
In conclusion, the prevalence of VTE is high (32%) among critically ill patients with COVID‐19. VTE occurred early in course of the disease, with 75% of events identified before screening at days 5‐10. Thus, early systematic lower‐limb CUS might be more effective in optimizing care in ICU patients with COVID‐19.
RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
AL, JL, SMB, BS, and JD designed the project. SMB, AL, and JL performed the ultrasound. JL, SMB, CH, SJ, MG, JJGM, TB, MC, GM, JMT, RP, VD, AD, GG, and RF collected the data. AL, JL, BS, and JD analyzed the data. AL, JL, SMB, LW, SE, HRE, MR, BS, and JD wrote the manuscript. JD, BS, AL, and JL contributed equally.
Longchamp A, Longchamp J, Manzocchi‐Besson S, et al. Venous thromboembolism in critically Ill patients with COVID‐19: Results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4:842–847. 10.1002/rth2.12376
Alban Longchamp and Justine Longchamp contributed equally.
Funding information
Dr A. Longchamp reports receiving grant support from the Swiss National Science Foundation (SNSF PZ00P3‐185927), the Leenaards, and the Novartis Foundation.
Contributor Information
Alban Longchamp, @AlbanLongchamp.
Bienvenido Sanchez, Email: bienvenido.sanchez@hopitalvs.ch.
Julie Delaloye, Email: Julie.Delaloye@hopitalvs.ch.
REFERENCES
- 1. China WHO . Pneumonia of unknown cause. 2020. [Accessed 2020 January 5] Available from https://www.who.int/csr/don/05‐january‐2020‐pneumonia‐of‐unkown‐cause‐china/en/
- 2. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545. [DOI] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor FB, Toh CH, Hoots WK, Wada H, Levi M, (ISTH) SSoDICDotISoTaH . Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30. [PubMed] [Google Scholar]
- 10. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lensing AWA, Prandoni P, Brandjes D, Huisman PM, Vigo M, Tomasella G, et al. Detection of deep‐vein thrombosis by real‐time B‐mode ultrasonography. N Engl J Med. 1989;320(6):342–5. [DOI] [PubMed] [Google Scholar]
- 12. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. [DOI] [PubMed] [Google Scholar]
- 13. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161(10):1268–79. [DOI] [PubMed] [Google Scholar]
- 16. Chong PY, Chui P, Ling AE, et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128(2):195–204. [DOI] [PubMed] [Google Scholar]
- 17. Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID‐19. Lancet Haematol. 2020;7:e362–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association? Eur Heart J. 2020;41(18):1858. [DOI] [PMC free article] [PubMed] [Google Scholar]


