To the Editor,
An outbreak of a novel coronavirus, the severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2), has led to over 1 900 000 infected cases and more than 120 000 deaths around the world since December 2019. The World Health Organization has characterized the 2019 novel coronal virus disease (COVID‐19) caused by SARS‐CoV‐2 as pandemic. Certain subgroups of patients are more vulnerable to COVID‐19 and might have poor prognostics, such as patients with acquired immune‐deficiency, malignancies, and tuberculosis. 1 , 2 , 3 Here, we report two particular cases of patients with COVID‐19 who had coexisted human immunodeficiency virus (HIV)‐infection and other comorbidities, and recovered from COVID‐19 after supportive treatment.
CASE 1
On 20 January 2020, a 60‐year‐old male patient presented with generalized myalgia for 2 weeks and intermittent fever around 38.3°C for 5 days and was admitted in our hospital (Figure 1A; patient 1). Before admission, he had accomplished a chest computed tomography (CT) scan on 18 January 2020 that showed bilateral multiple ground‐glass opacities (GGO), prominent on the right lower lobe (Figure 1B; patient 1). The test of influenza virus was negative. Blood cell counts were: leukocyte, 3.41 × 109/L; neutrophil, 2.25 × 109/L; lymphocyte, 0.9 × 109/L; red blood cell, 3.62 × 1012/L; platelet, and 95 × 109/L. The hemoglobin was 125 g/L. Other biochemical test included: procalcitonin (PCT), 0.19 ng/mL; D‐dimer, 204 ng/mL; C‐reactive protein, 191.21 mg/L. On admission, the patient had a fever of 39.3°C. He also complained about fatigue, dyspnea, and productive cough.
Figure 1.
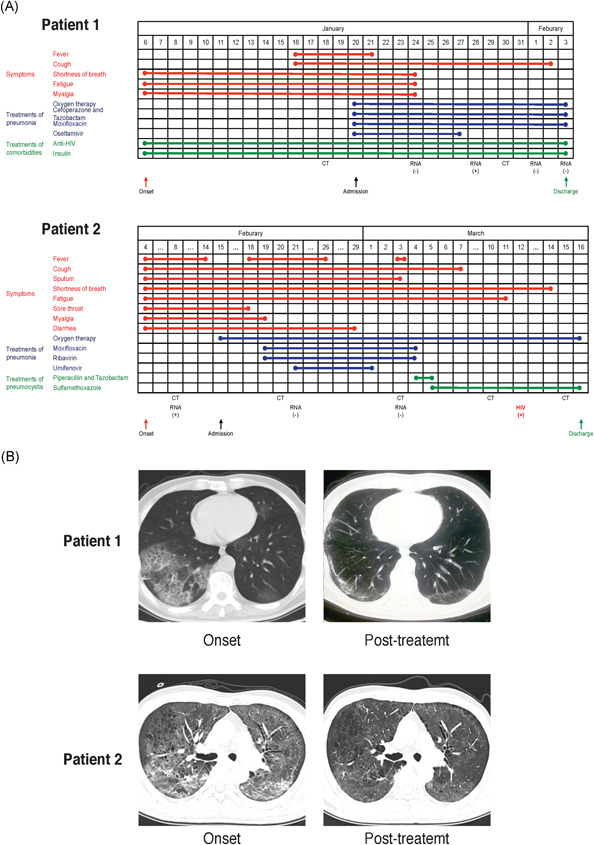
Timeline of disease course and chest CT scans of the two patients. A, Timelines showing durations of symptoms, treatment of pneumonia and treatment of comorbidities were depicted for patients 1 and 2. B, Onset chest CT scans showed typical bilateral multiple ground‐glass opacities in the lungs in both patients. The interstitial pneumonia was significantly resolved following treatment. CT, computed tomography
Regarding medical history, we noted that this patient had confirmed HIV infection in 2014 but had not been treated. He was diagnosed with stage IV diffuse large B‐cell lymphoma and pulmonary tuberculosis in January 2018, for which he received chemotherapy with one cycle of CHOP regimen and seven cycles of EPOCH regimen from 9 April to 10 September 2018. In the meantime, the patient started anti‐HIV treatment with tenofovir disoproxil fumarate, lamivudine, and efavirenz. He then started antituberculosis treatment with Isoniazid, ethambutol, rifabutin, and moxifloxacin for 9 months. The pulmonary tuberculosis was cured and the lymphoma was significantly regressed. Notably, the patient also had a history of type 2 diabetes for 8 years and received insulin to control blood glucose.
SARS‐Cov‐2 nucleic acid detection by quantitative real‐time reverse‐transcriptase polymerase chain reaction assay on January 28 confirmed COVID‐19. Oxygen, antiviral (oseltamivir) and antibiotics treatments (moxifloxacin, ceftriaxone, and tazobactam) were given. During the hospitalization, the patient continued anti‐HIV treatment and glucose control with insulin. Fever disappeared 2 days after admission. Five days later, myalgia, fatigue, and shortness of breath were also significantly mitigated. Two consecutive SARS‐CoV‐2 nucleic acid detections were negative on 1 and 3 February. The patient was considered clinically cured for COVID‐19 and was discharged on 3 February (Figure 1A; patient 1). He was asked to keep isolated at home for another two more weeks before he comes for a review of his disease. A chest CT on April 2 shown a significant improvement of pneumonia (Figure 1B; patient 1).
CASE 2
A 47‐year‐old male patient attended our hospital after 7 days of fever and non‐productive cough on 18 Feburary 2020. He had a highest body temperature of 39.8°C and generalized myalgia, sore throat, cough, intermittent shortness of breath, and diarrhea. He had performed chest CT scan in local hospital which revealed bilateral multiple GGO. The SARS‐CoV‐2 RNA test was positive (Figure 1A; patient 2).
After admission, a chest CT scan showed increased pulmonary exudative lesions on 20 Feburary (Figure 1B; patient 2). Blood cell counts were leukocyte, 10.51 × 109/L; neutrophil, 9.09 × 109/L; lymphocyte, 0.67 × 109/L; red blood cell, 3.99 × 1012/L; and platelet, 302 × 109/L. The hemoglobin was 122 g/L. PCT was 0.05 ng/ml and D‐dimer was 210 ng/mL.
Contrary to case 1 who had known and treated HIV infection, this patient was a newly diagnosed HIV‐infected case that was only confirmed on March 12. In parallel, he was suspected to have pneumocystis pneumonia on chest CT scan and was treat with intravenous Piperacillin and Tazobactam, and then oral Sulfamethoxazole.
For COVID‐19 pneumonia, the patient recieved oxygen, antibiotic (Moxifloxacin), and antiviral (Ribavirin and Umifenovir) treatments. The SARS‐Cov‐2 RNA detections on 21 Februray and 3 March were negative (Figure 1A; patient 2). A chest CT scan on 15 March showed significance resolution of pulmonary exudative lesions (Figure 1B; patient 2). He had no fever, cough, myalgia but still had some dyspnea after labor. He was discharged on 16 March and was asked keep isolated at home for another two more weeks before he comes for a review of his disease. He was recommended to pursue anti‐HIV treatment after recovery from COVID‐19.
DISCUSSION
The clinical manifestations and clinical outcomes of COVID‐19 in HIV‐infected patients remain unclear. In this report, we presented two special COVID‐19 patients with HIV infection and other comorbidities, one with existed and treated HIV‐infection and the other with newly diagnosed HIV‐infection, who were treated with supportive care and recovered from severe pneumonia. The experience of these two cases suggested that COVID‐19 patients with HIV infection could still have satisfactory clinical outcomes following proper medical care. 4
As the number of patients with COVID‐19 augments with time, there are accumulating reports describing the characteristics and management experience of COVID‐19 in patients with HIV‐infected. In line with large‐scaled studies, fever, myalgia, cough, and fatigue were common symptoms of COVID‐19. Noticeably, patient 2 also presented with uncommon diarrhea, which was reported to occur in 3.8% of patients. Consistent with general patients, lymphopenia was detected in both patients. 5 Preliminary results from cases reports indicated that existing HIV‐infection and related lymphopenia might be protective against COVID‐19 deterioration and the use of lopinavir/ritonavir might bring benefit to these patients. 6 , 7 Typical CT signs included multiple patch GGO and consolidation in severe cases. However, mild cases might quick absorption of pulmonary lesions on CT images. 8 Currently, there is no specific treatment to COVID‐19. The best supportive treatments, in particular oxygen support and control of comorbidities, are still the mainstay of treatment. 2 Patients in a state immunocompromised state, such as HIV infection, malignancies are at higher risk of SARS‐CoV‐2 infection. In addition, COVID‐19 patients with comorbidities, such as cancers, diabetes, and tuberculosis were more likely to turn to severe disease and were of poor prognosis. 1 , 2 , 4 Therefore, particular attention should be paid on those patients vulnerable to COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Study design: QW, HZ, and TC. Data collection: QW and HZ. Data analysis: QW, HZ, and TC. Writing of manuscript: QW, HZ, and TC. Critical revision: HZ and TC. Final approval: HZ.
ETHICS STATEMENT
The study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (2020089K).
ACKNOWLEDGMENT
The authors are grateful to both patients who assisted in the implementation of the study. This work was supported by the National Natural Science Foundation of China (81803061).
Funding Information National Natural Science Foundation of China, Grant/Award Number: 81803061
Qiuji Wu and Tielong Chen contributed equally to the work.
REFERENCES
- 1. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335‐337. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu J, Ouyang W, Chua MLK, Xie C. SARS‐CoV‐2 transmission in patients with cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020. 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:529‐530. 10.1002/jmv.25732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan W, Ni Z, Hu, et al. Clinical characteristics of coronavirus disease 2019 in China. New England J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mascolo S, Romanelli A, Carleo MA, Esposito V. Could HIV infection alter the clinical course of SARS‐CoV‐2 infection? When less is better. J Med Virol. 2020. 10.1002/jmv.25881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altuntas Aydin O, Kumbasar Karaosmanoglu H, Kart Yasar K. HIV/SARS‐CoV‐2 co‐infected patients in Istanbul, Turkey. J Med Virol. 2020. 10.1002/jmv.25955 [DOI] [PubMed] [Google Scholar]
- 8. Chen J, Cheng X, Wang R, Zeng X. Computed tomography imaging of an HIV‐infected patient with coronavirus disease 2019 (COVID‐19). J Med Virol. 2020. 10.1002/jmv.25879 [DOI] [PMC free article] [PubMed] [Google Scholar]


