Author contributions
- Conceptualization: X.‐N.M.
D.‐F.C., L.S., H.‐K.W., S.‐Y.L. Data curation: D.‐F.C., H.P., R.‐C.C., L.S., H.‐K.W. Formal analysis: X.‐N.M., R.‐C.C., L.S., H.‐K.W. Investigation: H.P., R.‐C.C., L.S., H.‐K.W. Methodology: X.‐N.M., D.‐F.C., R.‐C.C., L.S. Project administration: X.‐N.M., C.‐L.L. Resources: X.‐N.M., C.‐L.L., H.P., S.‐Y.L. Supervision: C.‐L.L., S.‐Y.L. Validation: C.‐L.L., S.‐Y.L. Writing—original draft: X.‐N.M., C.‐L.L., D.‐F.C. Writing—review and editing: X.‐N.M., C.‐L.L., D.‐F.C., S.‐Y.L.
Abbreviations
- AUC
area under the curve
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- OR
odds ratio
- PCT
procalcitonin
- ROC
receiver operating characteristic
- SAA
serum amyloid A
To the Editors:
The coronavirus disease 2019 (COVID‐19) outbreak originated in Wuhan, China, in December 2019. It has now affected 197 countries. By 27 April 2020, it was reported that 2 878 196 individuals were infected, with 6.1% of patients diagnosed with severe illness, and more than 198 000 deaths worldwide. 1 Early identification and prediction of acute exacerbation is of great importance for the management of COVID‐19. However, a serum biomarker for prognosis of COVID‐19 is currently lacking.
Serum amyloid A (SAA) is commonly elevated in the acute phase of inflammatory diseases. 2 We describe levels of serum SAA, C‐reactive protein (CRP) and procalcitonin (PCT) in patients with COVID‐19 during their hospital admission.
We recruited patients with COVID‐19 from 20 January to 10 March 2020, and detected serum markers (SAA, CRP and PCT) within the first day of hospitalization. This study was approved by the Hospital Ethics Review Committee (Ethics No 20201134) and the patients' informed consent was exempted. The outcomes of these patients were recorded as either improved and discharged or acutely exacerbated, according to oxygenation status (oxygen saturation < 93% and arterial partial pressure of oxygen/oxygen concentration ≤ 300 mm Hg) and chest radiograph (>50% lesions progression within 24–48 h in pulmonary imaging). Logistic regression model and receiver operating characteristic (ROC) curve were analysed to investigate the possible roles of SAA, CRP and PCT in prognosis prediction of COVID‐19.
The current observational study enrolled 118 patients with COVID‐19 (64 males) whose average age was (mean ± SD) 49.55 ± 15.95 years and the time of illness onset ranged from 1 to 14 days. On admission, 16 cases were diagnosed as severe COVID‐19 and the remaining 102 patients were identified as ordinary cases, who received further medical surveillance to investigate the prognosis. The levels of SAA, CRP and PCT were markedly elevated in patients with severe illness compared with ordinary cases (SAA (mean ± SD): 40.42 ± 52.62 vs 198.32 ± 55.12 mg/L, P < 0.001; CRP (mean ± SD): 16.55 ± 10.99 vs 46.52 ± 35.21 mg/L, P < 0.001; PCT (mean ± SD): 0.051 ± 0.041 vs 0.125 ± 0.148 ng/mL, P < 0.001) (Fig. 1). Furthermore, all 102 ordinary patients received antiviral therapy (Arbidol, Suzhou, China), of which 71 patients recovered and were discharged, but 31 cases underwent acute exacerbation. Logistic regression showed that SAA, but not CRP or PCT, could serve as an independent predictive factor of disease prognosis (OR: 1.031, 95% CI: 1.004–1.060, P < 0.05). ROC analysis demonstrated that SAA conferred greater diagnostic value than CRP and PCT in predicting disease progression (area under the curve (AUC): 0.9683 vs 0.8089 and 0.7684, both P < 0.05) (Fig. 2).
Figure 1.
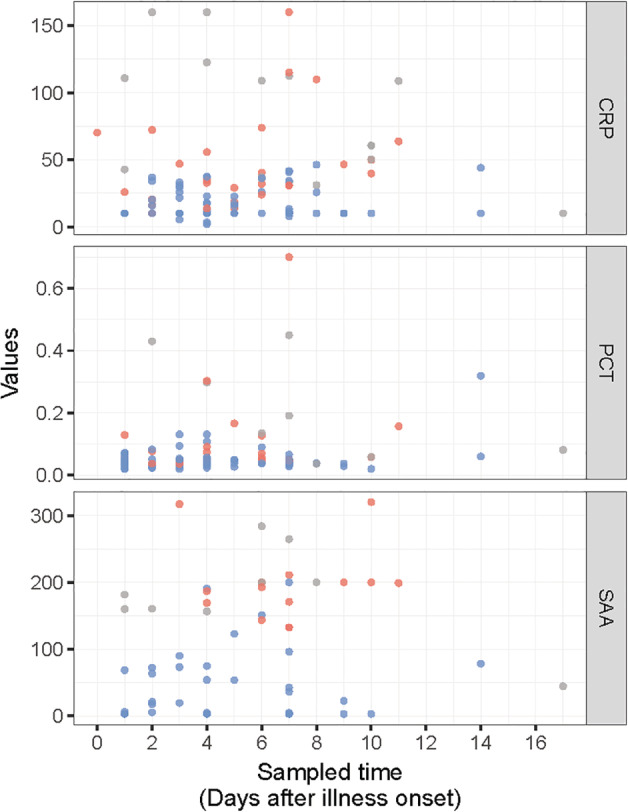
The level of inflammatory indicators in patients with COVID‐19 on admission ( , will worsen;
, will worsen;  , has worsened;
, has worsened;  , remain mild). COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; PCT, procalcitonin; SAA, serum amyloid A.
, remain mild). COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; PCT, procalcitonin; SAA, serum amyloid A.
Figure 2.
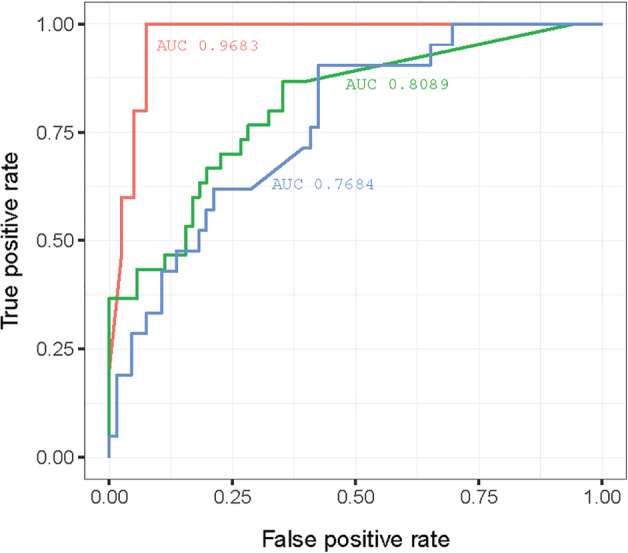
The diagnostic value of inflammatory indicators in predicting acute aggravation of COVID‐19 ( , serum amyloid A, AUC: 0.9683;
, serum amyloid A, AUC: 0.9683;  , C‐reactive protein, AUC: 0.8089;
, C‐reactive protein, AUC: 0.8089;  , procalcitonin, AUC: 0.7684). AUC, area under the curve; COVID‐19, coronavirus disease 2019.
, procalcitonin, AUC: 0.7684). AUC, area under the curve; COVID‐19, coronavirus disease 2019.
Primary inflammation, driven by rapid viral replication and release of potent pro‐inflammatory cytokines, occurs at the early stage of 2019‐novel coronavirus (nCoV) infection. 3 The pulmonary infiltrate and diffuse alveolar damage in COVID‐19, validated by autopsy study, 4 could potentiate further secretion of a variety of inflammatory cytokines. 5 Overall, we found that SAA and CRP were notably elevated in patients with COVID‐19 at the start of their hospital stay, although patients had mild respiratory symptoms with focal pulmonary infiltrates. SAA could be an independent predictive factor of severe COVID‐19, with an accuracy of 89.1% in predicting acute exacerbation (cut‐off value: 122.9), Further evidence needs to be collected to confirm the possible correlation between SAA and the severity of outcome in COVID‐19.
Mo X‐N, Su Z‐Q, Lei C‐L, et al. Serum amyloid A is a predictor for prognosis of COVID‐19. Respirology. 2020;25:764–765. 10.1111/resp.13840
Handled by Co‐Editors in Chief: Phil Bardin and Paul Reynolds
REFERENCES
- 1. World Health Organization . Novel coronavirus (2019‐nCoV) situation report‐98. 2020. [Accessed 27 Apr 2020.] Available from URL: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200427-sitrep-98-covid-19.pdf?sfvrsn=90323472_4
- 2. Zhang Y, Zhang J, Sheng H, Li H, Wang R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv. Clin. Rev. 2019; 90: 25–80. [DOI] [PubMed] [Google Scholar]
- 3. Fu Y, Cheng Y, Wu Y. Understanding SARS‐CoV‐2‐mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020; 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo W, Yu H, Gou J, Li X, Sun Y, Li J, Liu L. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID‐19). Preprints 2020: 2020020407. [Google Scholar]
- 5. Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit. Rev. Immunol. 2012; 32: 335–48. [DOI] [PubMed] [Google Scholar]


