Abstract
Studies have demonstrated that comorbidities, especially cardiovascular and endocrine diseases, correlated with poorer clinical outcomes. However, the impact of digestive system diseases has not been issued. The aim of this study is to determine the impact of laryngopharyngeal reflux disease (LPRD) on hospitalized patients with coronavirus disease 2019 (COVID‐19). We extracted clinical data regarding 95 patients in Wuhan Jinyintan Hospital, Wuhan, China, between 26 January and 21 February 2020. The Reflux Symptom Index (RSI) was used to assess the presence and severity of LPRD. An RSI greater than 13 is considered to be abnormal. A total of 95 patients with COVID‐19 were enrolled, with 61.1% (58/95), 32.6% (31/95), and 6.3% (6/95) being moderately ill, severely ill, and critically ill, respectively. In this study, 38.9% (37/95) of the patient had an RSI score over 13, which was indicative of LPRD. In univariable analysis, the age and RSI scores of severely or critically ill patients were statistically significantly higher than patients with moderate disease (P = .026 and P = .005, respectively). After controlling for age difference in a multivariable model, the RSI greater than 13, compared to RSI equal to 0, was associated with significantly higher risk of severe infection (P < .001; odds ratio [OR] = 11.411; 95% confidence interval [CI], 2.95‐42.09) and critical infection (P = .028; OR= 19.61; 95% CI, 1.38‐277.99). Among hospitalized patients with COVID‐19, RSI scores greater than 13, indicative of LPRD, correlated with poorer clinical outcomes. The prevalence of LPRD may be higher than the general population, which indicated that COVID‐19 can impair the upper esophageal sphincter and aggravate reflux.
Keywords: COVID‐19, laryngopharyngeal reflux disease, severe acute respiratory syndrome coronavirus 2
Highlights
The imapct of laryngopharyngeal reflux disease (LPRD) has been analyzed. The prevalence of LPRD in patients with COVID‐19 may be higher than the general population. The RSI score greater than 13, indicative of LPRD, correlated with poorer clinical outcomes.
1. INTRODUCTION
Since the outbreak of coronavirus disease 2019 (COVID‐19) in Wuhan, China, the disease, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, is rapidly sweeping across the world. 1 , 2 , 3 The number of cases and the death toll of COVID‐19 outside China has increased drastically involving 200 countries, states, or territories by 30 March 2020.
Although most of the confirmed cases presented with mild or moderate diseases, 13.8% and 4.7% were classified as severe and critically ill, respectively. 4 Previous studies demonstrated the existence of any comorbidity was associated with poorer clinical outcomes; and a greater number of comorbidities was also correlated with poorer outcomes. 5 , 6 , 7 , 8 A meta‐analysis to assess the prevalence of comorbidities in the patients with COVID‐19 revealed that hypertension and diabetes were the most prevalent comorbidity, followed by cardiovascular diseases and respiratory system diseases. 5 However, the comorbidities in these studies were mostly determined based on self‐report upon admission, which might result in missing data due to the patient's unawareness; and the impact of comorbid digestive system diseases among patients with COVID‐19 have not been fully addressed. To further investigate the impact of gastrointestinal (GI) disorder, this study focused on the GI comorbidity, specifically, laryngopharyngeal reflux disease (LPRD).
According to the recent reports, the severity and clinical manifestations are quite heterogeneous at the time of diagnosis. 2 , 7 , 9 , 10 The most common symptoms were fever and cough, whereas GI presentations were also found in a small number of patients. 2 , 11 Nausea or vomiting was reported in 5% of the cases, and diarrhea was found in 3.8% of the patients. 2 Also, studies have identified the SARS‐CoV‐2 RNA in the stool of infected patients, and angiotensin‐converting enzyme 2 serving as the viral receptor was found to be highly expressed in GI tract, suggesting that the SARS‐CoV‐2 can infect the digestive system. 11 , 12 , 13 LPRD, a subtype of extraesophageal reflux, is a common disorder found in 5% of the people in China and 18.8% in Grace. 14 , 15 The impairment of upper esophageal sphincter (UES) motility may aggravate the reflux of gastric contents beyond the esophagus into pharynx and larynx. On the basis of the fact that SARS‐CoV‐2 can infect the digestive system, we hypothesize that the virus may affect the GI motility and function, leading to repeated reflux of GI content into larynx may further facilitate the attack of the virus on the respiratory system. However, the prevalence and impact of LPRD in patients with COVID‐19 have not been revealed in previous studies.
The Reflux Symptom Index (RSI) is a validated nine‐item questionnaire for the assessment of the presence and severity of commonly reported symptoms, which proved to exhibit excellent construct and criterion‐based validity. An RSI score higher than 13 is considered to be abnormal and indicative of LPRD. 16 , 17 To address the issues mentioned above, the RSI was used in our study to evaluate the prevalence of LPRD in hospitalized patients with COVID‐19 and assess the risk of LPRD in severe patients compared with non‐severe patients.
2. METHODS
A total of 95 patients with confirmed COVID‐19 admitted to Wuhan Jinyintan Hospital, Wuhan, China, were enrolled in this study after receiving approval of the institutional review board between 26 January and 21 February 2020. The diagnosis criteria of COVID‐19 in this study were based on the World Health Organization's interim guidance. 18 The epidemiological, clinical, laboratory, and radiological data were collected to determine the infection of SARS‐CoV‐2. Descriptions of chest X‐ray or computed tomography (CT) were summarized by two separate radiological doctors. Patients whose real‐time reverse‐transcription‐polymerase chain reaction assay for nasal and pharyngeal swab was positive were confirmed with COVID‐19.
All the patients were classified as being mildly, moderately, severely, or critically ill according to the Guidance for Corona Virus Disease 2019 (6th edition) released by the National Health Commission of China. 9 , 19 Briefly, mildly ill denoted minimal symptoms and no findings of pneumonia on chest X‐ray or CT; moderately ill is defined as the presence of symptoms including fever, cough, and so forth, and abnormal radiological findings; severely ill denoted at least one of the following criterion (respiratory rate being 30 times per minute or greater, pulse oxygen saturation being 93% or lower, oxygen being 300 or lower, or rapid progression of pneumonia based on radiological findings in 24 to 48 hours); and critically ill denoted at least one of the criteria (septic shock requiring vasoactive medications, respiratory failure requiring mechanical ventilation, or other organ failure requiring intensive care unit admission).
The RSI was used to assess the presence and intensity of commonly reported LPRD symptoms. 16 The RSI score is from 0 to 5, with 5 being the worst, based on the severity of the following symptoms: hoarseness or a problem with your voice; clearing your throat; excess throat mucus or postnasal drip; difficulty swallowing food, liquids, or pills; coughing after you ate or after lying down; breathing difficulties or choking episodes; troublesome or annoying cough; sensations of something sticking in your throat or a lump in your throat; and heartburn, chest pain, indigestion, or stomach acid coming up. A score greater than 13 was considered to be clinically significant and indicative of LPRD. 16 This study aimed to reveal the correlation of LPRD or RSI with the severity of COVID‐19.
2.1. Statistical analysis
The comparison between patients with different severity of COVID‐19 was performed using univariate analysis, and a χ 2 test or a t test was used depending on the type of variable. Multivariate logistic regression was used to identify independent risk factors for COVID‐19. All statistical analyses were plotted using SPSS 24.0 (IBM) and Origin 2019b (Origin Lab).
3. RESULTS
3.1. Patient characteristics
A total of 95 patients with COVID‐19 were enrolled in this study. The detailed clinical characteristics were displayed in Table 1. The mean age of the patients was 58.8, with a range from 28 to 84. Fifty‐two percent (49/95) of the patients were males, while 48% of the cases were females. About 36.8% (35/95) of the patients had an RSI score of zero; the RSI score for 8.4% (8/95) of the cases was between 1 and 6; 15.8% (15/95) of the patients had an RSI score of between 7 and 13; and about 38.9% (37/95) of the patient had an RSI score over 13, which was indicative LPRD. There are 61.1% (58/95), 32.6% (31/95), and 6.3% (6/95) of the patients evaluated to be moderately ill, severely ill, and critically ill, respectively.
Table 1.
Demographic and clinical characteristics of patients with COVID‐19
| Parameter | |
|---|---|
| Patient characteristics | |
| Mean age, y (range) | 58.8 (28.0‐84) |
| Sex, male:female (number of patients; %) | 49:46 (51.6:48.4) |
| Mean RSI scores (number of patients; %) | |
| 0 | 35 (36.8) |
| 1‐6 | 8 (8.4) |
| 7‐13 | 15 (15.8) |
| >13 | 37 (38.9) |
| Underlying diagnosis styles of COVID‐19 (number of patients; %) | |
| Mildly ill | 58 (61.1) |
| Severely ill | 31 (32.6) |
| Critically ill | 6 (6.3) |
Abbreviations: COVID‐19, coronavirus disease 2019; RSI, Reflux Symptom Index.
3.2. The correlation of RSI scores with the severity of COVID‐19
The characteristics of patients who were moderately ill, severely ill, and critically ill were showed in Table 2. In the univariable analysis, the frequency of males and females among the three groups showed no significant statistical difference (P = .907); however, the median age among the three groups demonstrated significant difference (P = .026). Meanwhile, the median RSI scores for the patient with moderate, severe, and critical diseases were 7, 13, and 14, respectively, which also showed a significant difference (P = .005). The difference of age and RSI scores among three groups was also demonstrated in Figure 1A,B: severely and critically ill patients were significantly older than the moderately ill patients; the RSI scores of severely and critically ill were also significantly higher than that of the moderately ill patients. As a result, the age and RSI score may be risk factors for severely or critically ill patients.
Table 2.
Univariable analysis of risk factors for COVID‐19
| Characteristic | Patients with mild disease n = 58 | Patients with severe disease n = 31 | Patients with critical disease n = 6 | P value |
|---|---|---|---|---|
| Patient age, y; median (range) | 59 (28‐78) | 63 (43‐81) | 69 (54‐84) | .026* |
| Patient sex | ||||
| Male | 29 | 14 | 3 | .907 |
| Female | 29 | 17 | 3 | |
| RSI scores | 7 (0‐34) | 13 (0‐27) | 14 (0‐23) | .005** |
Abbreviations: COVID‐19, coronavirus disease 2019; RSI: Reflux Symptom Index.
P < .05
P < .01.
Figure 1.
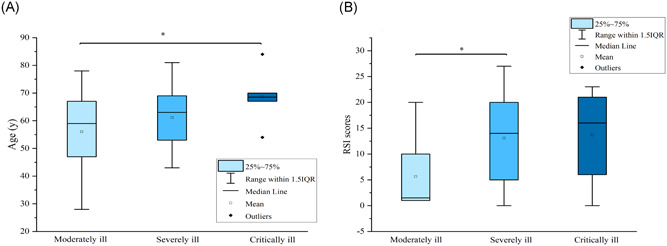
A, Comparison of ages among moderately ill, severely ill, and critically ill patients. B, Comparison of RSI scores among moderately ill, severely ill, and critically ill patients. IQR, interquartile range; RSI, Reflux Symptom Index
In a multivariable model, patients with age ≥ 60 were 3.20 times more likely to have severe COVID‐19 than those with age < 60 (odds ratio [OR] = 3.20; 95% confidence interval [CI], 1.01‐10.15); the risk of severe COVID‐19 for those with RSI greater than 13 was 11.41 times that of the patients with RSI equal to 0 (P < .01; OR = 11.411; 95% CI, 2.95‐42.09). In addition, patients with age ≥ 60 were 14.62 times more likely to have critical COVID‐19 than those with age < 60 (OR = 14.62; 95% CI, 1.17‐182.63); patients with RSI greater than 13 were 19.61 times more likely to be critically ill than patients with RSI equal to 0 (P = .028; OR = 19.61; 95% CI, 1.38‐277.99). As a result, after controlling for the age difference, RSI score greater than 13, indicative of LPRD, was independently associated with a higher risk of severe or critical COVID‐19 (Table 3).
Table 3.
Multivariable analysis of risk factors for severe and critical COVID‐19
| Outcomes | Odds ratio (95% CI) | P value |
|---|---|---|
| Severely ill | ||
| Age ≥ 60 vs age < 60 | 3.20 (1.01‐10.15) | .033* |
| Male vs female | 1.49 (0.53‐4.18) | .452 |
| RSI > 13 vs RSI = 0 | 11.14 (2.95‐42.09) | .000*** |
| 7 < RSI ≤ 13 vs RSI = 0 | 1.91 (0.42‐8.73) | .40 |
| 0 < RSI ≤ 7 vs RSI = 0 | 2.14 (0.31‐14.93) | .45 |
| Critically ill | ||
| Age ≥ 60 vs age < 60 | 14.62 (1.17‐182.63) | .037* |
| Male vs female | 2.28 (0.30‐17.24) | .425 |
| RSI > 13 vs RSI = 0 | 19.61 (1.38‐277.99) | .028* |
| 7 < RSI ≤ 13 vs RSI = 0 | 2.54 (0.128‐50.19) | .541 |
| 0 < RSI ≤ 7 vs RSI = 0 | 6.21 (0.30‐128.94) | .238 |
Abbreviations: COVID‐19, coronavirus disease 2019; RSI: Reflux symptom index.
P < .05
P < .001.
4. DISCUSSION
COVID‐19 has become a global public emergency, resulting in thousands of deaths and affecting more than 1 million people 3 ; therefore, further understanding of the disease helps us to better contain the pandemic. In this study, we retrospectively analyzed the impact of LPRD, a common digestive disorder, on patients with COVID‐19 and found that LPRD was commonly prevalent in hospitalized patients with COVID‐19 and independently associated with risk of severe or critical infection. Our findings have provided further insights to comprehensively assess the prognosis of patients with COVID‐19 in hospital.
Many of the patients with COVID‐19 were found to have at least one comorbidity. A meta‐analysis illustrated that approximately 17% of the cases with COVID‐19 had hypertension; meanwhile, there were 8%, 5%, and 2% of the patients found to have diabetes, cardiovascular diseases, respectively. However, a major limitation of most previous studies was self‐report, which may lead to under‐reporting owing to lack of awareness or diagnostic tests; hence, it might cause missing disorders. Therefore, the impact of digestive system disorder on the COVID‐19 was rarely issued in the previous literature. Our study is the first singe‐institute investigation of the prevalence of LPRD in hospitalized patients with COVID‐19. With RSI, a nine‐item symptom instrument was utilized to assess the presence of coexistence of LPRD, which had more accuracy and validity than self‐recalling. 16 We found that approximately 38.9% (37/95) of the hospitalized patients with COVID‐19 had an RSI score greater than 13, which suggested that a large number of the patients might have comorbid LPRD. As a result, the prevalence of LPRD in patients may be higher than the general population based on the previous data. 14
A large study of 1099 patients from 522 hospitals reported nausea or vomiting in 55 (5.0%) and diarrhea in 42 (4.8%) patients. The frequencies of diarrhea and vomiting varied among previous studies. 1 , 2 , 6 , 9 , 11 In a retrospective that collected data from 140 hospitalized COVID‐19 patients in Wuhan, GI symptoms were found in approximately 39.6% of the patients, including nausea in 24 (17.3%), diarrhea in 18 (12.9%), and vomiting in 7 (5%) patients. 20 In addition, the SARS‐CoV‐2 RNA could be detected in stool specimens of patients with COVID‐19. 13 , 21 , 22 Despite the high variability of clinical presentations, these studies all suggested that the SASR‐CoV‐2 might affect the motility of the GI tract. Therefore, the high prevalence of LPRD in patients with COVID‐19 could have arisen from the impact of the virus on the UES. However, the reason underlying this observation could also be the selection bias due to the small sample size and data extracted from only one institute.
A number of existing studies revealed that patients with comorbidities were more likely to have a severe infection and poorer clinical outcomes. 5 , 8 , 23 In addition to the most common comorbidities among patients with COVID‐19 including circulatory and endocrine diseases, LPRD may be also associated with more severe disease. Our study demonstrated that both age and RSI scores of severely or critically ill patients were significantly higher than moderately ill patients. As with previous studies, older patients were more likely to develop the more severe or critical diseases. 6 , 8 , 9 In our study, patients with age ≥ 60 were 14.62 times more likely to have critical COVID‐19 than those with age < 60 (OR = 14.62; 95% CI, 1.17‐182.63); patients with age ≥ 60 were 3.20 times more likely to have severe COVID‐19 than those with age < 60 (OR = 3.20; 95% CI, 1.01‐10.15). After controlling for age difference in a multivariable analysis, RSI score greater than 13, indicative of LPRD, was independently associated with a higher risk of severe or critical COVID‐19. Our findings, therefore, added to the existing evidence the impact of LPRD, a common GI disorder, in patients with COVID‐19.
This was a retrospective single‐center study, which cannot reflect accurately entire patients with COVID‐19. In addition, the sample size is relatively small in our study, and subsequent studies with larger populations are anticipated to reduce the variability of the data. Besides, we only included hospitalized patients with COVID‐19, who were likely to present more severe disease than nonhospitalized patients, resulting in selection bias. Finally, the pre‐existence of LPRD cannot be determined due to the following reasons. First of all, the past medical history was documented via self‐report, possibly leading to missing GI disorder; hence, only five of the patients had complaints of reflux symptoms in the past, which were not necessarily related to LPRD. Second, the lack of awareness, lack of screening in community settings, and lack of primary care in undeveloped regions could result in under diagnosis of LPRD, and make it more difficult to get accurate laryngopharyngeal medical history. Therefore, we cannot make accurate conclusions about the causes of LPRD. Because of the rapidly evolving pandemic, the ongoing studies with the inclusion of more patients are anticipated to increase the statistical power and support more accurate conclusions about the impact of LRPD.
5. CONCLUSION
Among hospitalized patients with COVID‐19, RSI scores greater than 13, indicative of LPRD, correlated with poorer clinical outcomes. In addition, the prevalence of LPRD may be higher than the general population, which indicated that COVID‐19 can, in turn, impair the UES and aggravate reflux. Assessment of LPRD using the RSI may help us with the risk stratification of patients upon admission.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Literature search: GJ, YC, KX, and XY. Data collection: GJ, YC, YL, JX, YL, and MK. Study design: GJ, YC, and KX. Analysis of data: GJ and YC. Manuscript preparation: GJ and YC. Review of the manuscript: GJ, YC, and KX.
ACKNOWLEDGMENT
The authors would like to acknowledge all the patients and their families for their contributions to this study. No writing assistance was received.
Jiang G, Cai Y, Yi X, et al. The impact of laryngopharyngeal reflux disease on 95 hospitalized patients with COVID‐19 in Wuhan, China: A retrospective study. J Med Virol. 2020;92:2124–2129. 10.1002/jmv.25998
Guiyuan Jiang and Yanping Cai contributed equally to this study.
REFERENCES
- 1. Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020;69:1002‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedford J, Enria D, Giesecke J, et al. COVID‐19: towards controlling of a pandemic. Lancet (London, England). 2020;395:1015‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print February 24, 2020]. JAMA. https://dog.org/10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China [published online ahead of print February 7, 2020]. JAMA. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis [published online ahead of print March 26, 2020]. Eur Respir J. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong SH, Lui RN, Sung JJ. Covid‐19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744‐748. [DOI] [PubMed] [Google Scholar]
- 12. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158:1831‐1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang A, Tong Z, Wang H, et al. Detection of novel coronavirus by RT‐PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen XM, Li Y, Guo WL, Wang WT, Lu M. Prevalence of laryngopharyngeal reflux disease in Fuzhou region of China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51(12):909‐913. [DOI] [PubMed] [Google Scholar]
- 15. Spantideas N, Drosou E, Bougea A, Assimakopoulos D. Laryngopharyngeal reflux disease in the Greek general population, prevalence and risk factors. BMC Ear Nose Throat Disord. 2015;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16(2):274‐277. [DOI] [PubMed] [Google Scholar]
- 17. Kavookjian H, Irwin T, Garnett JD, Kraft S. The Reflux Symptom Index and symptom overlap in dysphonic patients [published online ahead of print February 6, 2020]. Laryngoscope. 10.1002/lary.28506 [DOI] [PubMed] [Google Scholar]
- 18. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/internal‐publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐is‐suspected. Accessed March 10, 2020.
- 19. New coronavirus pneumonia prevention and control program (6th ed.) (in Chinese) . 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf. Accessed May 14, 2020.
- 20. Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China [published online ahead of print February 19, 2020]. Allergy. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 21. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore [published online ahead of print March 3, 2020]. JAMA. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109:531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]


