Abstract
Coronavirus disease 2019 (COVID‐19) is associated with significant hypercoagulability. However, despite prophylactic anticoagulation, critically ill patients with this condition develop thromboses. This forum discusses the lungs as the epicenter for the hemostatic issues, puts forward a proposal for staging COVID‐19 coagulopathy based on available diagnostic markers, and suggest considering current and future treatment options based on these different stages.
Keywords: anticoagulation, COVID‐19, thrombosis
Essentials.
Coronavirus disease 2019 (COVID‐19) is associated with thrombotic complications.
A spectrum of coagulation changes is observed with COVID‐19.
COVID‐19 associated hemostatic changes may be divided into 3 stages.
Current and future treatment possibilities may be stratified based on these stages.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) continues to cause significant morbidity and mortality worldwide. Several recent retrospective studies in the quickly expanding COVID‐19 literature have highlighted a high incidence of hypercoagulability in this condition. 1 , 2 , 3 , 4 In critically ill patients, the range of thromboembolism observed in 3 retrospective studies was 25%‐27% for venous thrombosis and approximately 4% for arterial thrombosis. In one of these studies, 81% of these thrombotic events were reported as pulmonary “embolism” (differentiating embolism from thrombus can be difficult since both appear as filling defects on imaging). 1 It is important to note that all these studies looked at severely ill patients in the intensive care units (ICUs), who have higher risk of thrombosis from their critical illness in addition to COVID‐19 hypercoagulability; it may be that some patients develop thrombi in the lungs, as previously suggested might occur in general populations. 5 Also, recent evidence suggests an increased incidence of arterial thrombosis in this condition, which may not respond normally to anticoagulant therapy. 1
2. PROPHYLAXIS ANTICOAGULATION FAILURE IN COVID‐19
Prophylactic anticoagulation with heparin or low‐molecular‐weight (LMW) heparin has been advised in all hospitalized patients with COVID‐19 with significantly raised D‐dimer in the absence of contraindications. 6 , 7 However, COVID‐19 caregivers are noticing breakthrough thrombosis in some patients treated with prophylactic anticoagulation, leading to rapidly worsening clinical condition requiring ventilatory assistance, with one study reporting a 31% prevalence despite pharmacologic thromboprophylaxis. 1 In another report of 26 consecutive patients with severe COVID‐19 screened for venous thrombosis, 8 were on prophylactic anticoagulation, while 18 patients were on therapeutic anticoagulation. 4 All 8 patients on prophylactic anticoagulation had screen‐detected deep vein thrombosis (DVT), while only 56% of those on therapeutic anticoagulation also were identified to have a DVT. 4 It is difficult to know if these DVTs were present prior to initiation of anticoagulant treatment. The only study so far evaluating hospital ward patients noted a venous thromboembolism (VTE) rate of only 3.2%. 8 Taken together, findings suggest that the thrombotic process has already commenced in the COVID‐19 patient even at admission or while ambulant at home. It worsens with the progression of the underlying disease process manifesting as increasing respiratory distress along with failure of prophylactic anticoagulation. A hypothesis may be made that these patients could benefit from intensive anticoagulation at a stage when the thrombotic process is still limited to the lungs (discussed next).
3. THE LUNGS AS THE EPICENTER OF COVID‐19 COAGULOPATHY
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) gains access predominantly through the respiratory tract. It is now well recognized that the virus causes lung inflammation in all patients progressing to cytokine storm in the most severe cases. Inflammation triggers coagulation activation, with the concept of immunothrombosis well established in the literature. 9 The key feature here is that the inflammatory process involving the alveolar vascular endothelium even in the early stages may trigger the formation of pulmonary clots (untested in the COVID‐19 setting) and stimulation of neutrophil extracellular traps in an attempt to limit viral invasion. 10 These microthrombi may not be detected easily on computed tomography scans, due to their small size and being limited to peripheral microvasculature. Untreated, the intense inflammation or cytokine storm can cause extension of pulmonary microthrombi to become larger thrombi, which would clinically present as worsening respiratory failure and radiologically as perfusion defects. This lung‐specific origin of coagulopathy is supported by 2 recent autopsy reports. 11 , 12 These groups have demonstrated predominant pulmonary pathology in COVID‐19 with thrombi in the lung microvasculature. 10 , 11 Extending hypercoagulability may not be limited to the lungs and may present as lower limb, gastrointestinal tract, cerebrovascular, or coronary ischemia. 5 , 13 , 14
4. CURRENT DIAGNOSTIC MARKERS IN COVID‐19–ASSOCATED HEMOSTATIC ABNORMALITIES
The best currently available laboratory diagnostic marker for COVID‐19–associated hemostatic abnormalities (CAHA) appears to be D‐dimer. Several reports have linked the D‐dimer concentration with poor prognosis. 15 , 16 The platelet count is usually normal in COVID‐19 until the advanced stages, when it decreases to moderately low levels (lowest recorded so far is 49 × 109/L). 15 , 17 Prothrombin time (PT) is also normal in non–critically ill patients, with mild to moderate prolongation observed only in the severe cases. 15 Fibrinogen level is markedly elevated except in terminal patients, where it may decline precipitously. 15 Analyzing published data, we propose a classification of CAHA into 3 stages to aid early recognition and consider possible treatments (see Figure 1). At the outset, we acknowledge that any such classification will require further clinical and laboratory validation.
FIGURE 1.
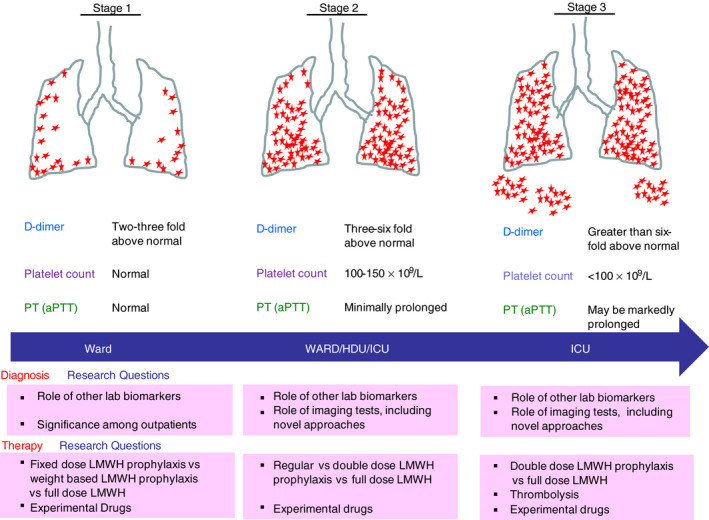
The 3 stages of COVID‐19–associated hemostatic abnormalities (CAHA). In stage 1, regions of microthrombi could be identified throughout the peripheral parenchyma. In stage 2, this extends to most of the lung. In stage 3, further coagulation activation becomes systemic thrombi. aPTT, activated partial thromboplastin time; ICU, intensive care unit; PT, prothrombin time
5. THE STAGES OF CAHA
In stage 1 CAHA, the patient has nonsevere symptoms and may be a hospital inpatient or at home. Currently, we do not have any data for patients with COVID‐19 with symptoms who have not been to a hospital. Future studies examining outcomes in these patients with the help of clinical and laboratory markers would be welcome. The inpatients with nonsevere symptoms usually have elevated D‐dimer (2‐ to 3‐fold above normal) and normal PT, partial thromboplastin time (PTT), and normal or elevated platelet count and fibrinogen. 15 Pulmonary microthrombi may be missed at this stage on computed tomography if performed for respiratory symptoms. Ultrasound‐detected DVT may not be present since the thrombi at this stage may be limited to pulmonary microvasculature. As per the current recommendations, these patients should receive prophylactic LMW heparin in the absence of contraindications. 6 , 7 Given the rapid deterioration reported in many patients after this stage, regular monitoring (once daily for 5‐7 days) of D‐dimer, PT, and platelet count can be advised, along with a high index of suspicion for thrombosis.
In stage 2 CAHA, the patient may develop more severe symptoms and require critical care support. These patients would have markedly elevated D‐dimer (3‐ to 6‐fold above normal), mildly reduced platelet count (between 100‐150 × 109/L) and minor prolongation in PT, while on thromboprophylaxis. 15 These patients may have filling defects noted on CT imaging due to pulmonary thrombi or emboli. They may also have asymptomatic DVT in the lower limbs signifying extensive coagulation activation. Once thrombi are detected, it is standard practice to treat such patients with therapeutic (full‐dose) anticoagulation. Therapeutic anticoagulation may also be provided in patients who have very high clinical suspicion of VTE but radiologic confirmation is difficult due to concerns about exposure of health care workers and staff to the virus. There are several trials currently under way to determine whether full‐dose compared to prophylactic dose anticoagulation may help to prevent worsening of the coagulopathy (see Table 1) and ischemia in the extrapulmonary circulation. 12 , 14 Observations from the study cited above 4 and increasing clinical experience suggest that, although therapeutic anticoagulation is appropriate with definite evidence of thrombi, additional measures could be considered in research settings to block other components of the coagulation pathway including the platelets, complement system, and contact system. For example, contact system activation plays a crucial role in thromboinflammation. 18 Its pharmacologic inhibition prevents both consumptive coagulopathy and pathological systemic inflammatory response in experimental sepsis models. 18 An attractive feature of contact system inhibition is minor impact on hemostasis and host immunity, and thus it may be safe from the perspectives of immunosuppression or bleeding. 18
TABLE 1.
Antithrombotic trials in COVID‐19 listed in ClinicalTrials.gov (https://clinicaltrials.gov/)
| Trial | Design | Primary end point |
|---|---|---|
| Stage 1/2 | ||
|
COVID‐19–Associated Coagulopathy: Safety and Efficacy of Prophylactic Anticoagulation Therapy in Hospitalized Adults With COVID‐19 ClinicalTrials.gov Identifier: NCT04360824 Country: United States |
Prospective, randomized, open‐label, single‐center interventional study comparing the safety and efficacy of 2 LMWH dosing protocols in patients meeting the modified ISTH Overt DIC criteria score ≥ 3. Patients will be randomized to standard prophylactic dose LMWH (standard‐of‐care arm) or intermediate‐dose LMWH (intervention arm). | Mortality at 30 d after intervention and risk of all‐cause mortality |
|
Randomized Trial of Anticoagulation Strategies in COVID‐19 ClinicalTrials.gov Identifier: NCT04359277 Country: United States |
Open‐label randomized trial of higher‐dose anticoagulation with enoxaparin or unfractionated intravenous heparin compared with lower‐dose prophylactic anticoagulation | All‐cause mortality at 1 y and incidence of cardiac arrest, symptomatic deep vein thrombosis, pulmonary embolism, arterial thromboembolism, myocardial infarction, or hemodynamic shock in 21 d |
|
Intermediate or Prophylactic‐Dose Anticoagulation for Venous or Arterial Thromboembolism in Severe COVID‐19 (IMPROVE) ClinicalTrials.gov Identifier: NCT04367831 Country: United States |
Intermediate‐dose anticoagulation with enoxaparin or UFH versus prophylactic dose anticoagulation |
Composite of being alive and without clinically relevant venous or arterial thrombotic events at discharge from ICU (without transfer to another ICU or palliative care unit/hospice) or at 30 d (if ICU duration lasted 30 d or longer). |
|
Preventing COVID‐19 Complications With Low‐ and High‐Dose Anticoagulation (COVID‐HEP) ClinicalTrials.gov Identifier: NCT04345848 Country: Switzerland |
Therapeutic anticoagulation with enoxaparin or intravenous UFH compared with prophylactic enoxaparin or UFH, from admission until the end of hospital stay or clinical recovery. If hospitalized in theICU, an augmented thromboprophylaxis regimen as standard of care. |
Composite outcome of arterial or venous thrombosis, DIC, and all‐cause mortality |
|
Coagulopathy of COVID‐19: A Pragmatic Randomized Controlled Trial of Therapeutic Anticoagulation Versus Standard Care (RAPID COVID COAG) ClinicalTrials.gov Identifier: NCT04362085 Country: United States |
2‐arm, parallel, multicenter, open‐label randomized controlled trial in hospitalized patients with COVID‐19 and an elevated D‐dimer (≥2 times the upper limit of normal). Therapeutic anticoagulation with LMWH or UFH until discharged from hospital, 28 d, or death versus standard care (LMWH, UFH, or fondaparinux at thromboprophylactic doses) |
Composite outcome of ICU admission, noninvasive positive pressure ventilation, invasive mechanical ventilation, or death at 28 d |
|
Trial Evaluating Efficacy and Safety of Anticoagulation in Patients With COVID‐19 Infection, Nested in the Corimmuno‐19 Cohort (CORIMMUNO‐COAG) ClinicalTrials.gov Identifier: NCT04344756 Country: France |
Phase 2 randomized open‐label multicenter clinical trial, where patients will be randomly allocated to anticoagulation versus standard of care |
Survival without ventilation and ventilator‐free survival |
|
Efficacy of Nafamostat in Covid‐19 Patients (RACONA Study) ClinicalTrials.gov Identifier: NCT04352400 Country: Italy |
Adult hospitalized patients with COVID‐19 in randomized, prospective, double‐blind trial to test the clinical efficacy of nafamostat mesylate in addition to best standard of care. | Time to clinical improvement on a 7‐category ordinal scale or live discharge from the hospital, whichever came first. |
| STAGE 3 | ||
|
STARS ("STudy of Alteplase for Respiratory Failure in SARS‐Cov2 (COVID‐19)": A Phase IIa Clinical Trial |
||
|
ClinicalTrials.gov Identifier: NCT04357730 Country: United States |
||
|
Phase IIa, open‐label, modified stepped‐wedge design, testing systemic administration of fibrinolytic therapy with alteplase (t‐PA) versus standard of care for patients with COVID‐19 resulting in severe respiratory failure. |
PaO2/FiO2 improvement from pre‐ to post‐intervention at 48 h after randomization |
|
|
Nebulised rt‐PA for ARDS Due to COVID‐19 (PACA) |
||
|
ClinicalTrials.gov Identifier: NCT04356833 Country: United Kingdom |
||
|
Recombinant tissue‐plasminogen Activator (rt‐PA) given every 6 h for 66 h, in addition to standard of care for COVID‐19 acute respiratory distress syndrome versus standard of care |
Treatment efficacy measured as percentage change in PaO2/FiO2 ratio from baseline and to day 5 and day 7. Safety measured by bleeds and other (non–bleed‐related) adverse events and fibrinogen levels. |
|
|
CORIMUNO19‐ECU: Trial Evaluating Efficacy and Safety of Eculizumab (Soliris) in Patients With COVID‐19 Infection, Nested in the CORIMUNO‐19 Cohort ClinicalTrials.gov Identifier: NCT04346797 Country: France |
Cohort, randomized controlled trial design Randomization between eculizumab plus standard of care versus standard of care . |
Outcomes compared with outcomes of standard‐of‐care–treated patients as well as with outcomes of patients treated with other immune modulators. |
|
Enhanced Platelet Inhibition in Critically Ill Patients With COVID‐19 (PIC‐19) ClinicalTrials.gov Identifier: NCT04368377 Country: Italy |
Compassionate‐use, proof‐of‐concept, phase IIb, prospective, interventional, pilot study of intravenous tirofiban with intravenous acetylsalicylic acid, oral clopidogrel, and subcutaneous fondaparinux 2.5 mg, in patients affected by severe respiratory failure in COVID‐19–associated pneumonia who underwent treatment with continuous positive airway pressure. |
Change in ratio between partial pressure of oxygen in arterial blood and inspired oxygen fraction and change in alveolar‐arterial gradient of oxygen at baseline and after study treatment. |
In stage 3 CAHA, the patient with COVID‐19 is worsening clinically, with need for even higher‐level critical care support (extracorporeal membrane oxygenation), development of overt VTE, multiorgan failure, or ischemia in the gut, limbs, or coronary or cerebral vasculature. The laboratory parameters would have further worsened with much higher D‐dimer (>6‐fold above normal), more significant thrombocytopenia, marked prolongation of PT and PTT, and decreasing fibrinogen. 15 At this stage, there is likely extensive pulmonary thrombi and systemic thrombosis, including disseminated intravascular coagulation in some patients, 15 which may be an unsalvageable scenario. Intensification of antithrombotic therapy as per stage 2 in combination with several other experimental measures may sometimes be effective at stage 3. Barrett and colleagues have provided scientific rationale for the use of fibrinolytic therapy in patients with refractory COVID‐19 with acute respiratory distress syndrome. 19 They propose testing the use of tissue plasminogen activator in patients who have persistent, refractory hypoxemia despite maximal therapy. One of the other potential therapies is designer heparin molecules prepared using a synthetic biology approach. 20 The rationale for this approach is that heparan sulfate binds to SARS‐CoV‐2 spike protein, blocking viral invasion while also neutralizing the activity of proinflammatory proteins like the histones. 20
There are several antithrombotic treatment trials currently being undertaken in patients with COVID‐19, and these are summarized in Table 1, along with illustration of the proposed CAHA stages and relevant research questions for each stage in Figure 1.
6. FUTURE DIAGNOSTIC MARKERS IN CAHA
Despite the rapidly evolving understanding of CAHA, there are no available reliable markers to guide treatment. It is possible that the endothelium holds the clue for recognizing this transition from early‐to‐late CAHA and aiding in appropriate treatment selection. 13 , 21 In this context, it is important to note that SARS‐CoV‐2 can infect endothelial cells, which constitute a third of the lung cellular architecture and carry similar receptors to type 2 alveolar cells. 22 As endothelial cells act as the interface between the immune and hemostatic systems, and severe inflammation triggered by the viral invasion can cause marked endothelial dysfunction. Markers of endothelial activation and dysfunction like von Willebrand factor activity, ADAMTS‐13, the selectins, microparticles, vascular endothelial growth factors, and angiopoietins are possible players in CAHA and should be studied in the context of the proposed stages of CAHA stage and their transition (Figure 1). 23 Readily available assays such as C‐reactive protein, lactate dehydrogenase, ferritin, and interleukin‐6 also warrant study. A recent case report noted von Willebrand factor activity in excess of 500% in a patient with COVID‐19. 24 Tests of global hemostasis (thrombin generation or thromboelastography) may also be useful. Both the Quantra system and thromboelastogram have shown features of hypercoagulability in patients with COVID‐19, and there are hints toward their use in tailoring treatment, but the nonrandomized small studies thus far need confirmation. 25 , 26
7. CONCLUSION
In summary, we propose a hypothesis that the spectrum of CAHA first represents a localized phenomenon of hypercoagulability in the lung, which then becomes extensive and systemic if not treated adequately. The staging proposed here requires validation in relation to well‐defined clinical and laboratory parameters and clinical outcomes in patients with COVID‐19 with CAHA. It might be improved by adding other biomarkers and through a better understanding of the pathophysiological basis. It may also be used as a framework to consider interventions in clinical research settings.
RELATIONSHIP DISCLOSURE
The authors declare nothing to report.
AUTHOR CONTRIBUTIONS
JT conceived the article and wrote the first draft. MC and AS provided critical comments. Final submission was approved by all the authors.
Thachil J, Cushman M, Srivastava A. A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. 2020;4:731–736. 10.1002/rth2.12372
Handling Editor: Pantep Angchaisuksiri
Contributor Information
Jecko Thachil, Email: jecko.thachil@mft.nhs.uk.
Mary Cushman, @MaryCushmanMD.
REFERENCES
- 1. Klok FA, Kruipb MJHA, van der Meerc NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; S0049‐3848(20)30120‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020. 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K, et al. Thrombotic complications of patients admitted to intensive care with COVID‐19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llitjos JF, Leclerc M, Chochois C, Monsallier J‐M, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020. 10.1111/jth.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Langevelde K, Srámek A, Vincken PW, van Rooden JK, Rosendaal FR, Cannegieter SC. Finding the origin of pulmonary emboli with a total‐body magnetic resonance direct thrombus imaging technique. Haematologica. 2013;98(2):309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol. 2020; S0735‐1097(20)35008‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. Preprints epublished. [DOI] [PMC free article] [PubMed]
- 9. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID‐19. J Thromb Haemost. 2020. 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin‐Loeches I, Browne P, et al. COVID‐19 Coagulopathy in Caucasian patients. Br J Haematol. 2020. 10.1111/jth.14844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA, Ferraz da Silva LF, Pierre de Oliveira E, Nascimento Saldiva PH, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020. 10.1111/jth.14844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ. Vander Heide RS. Pulmonary and Cardiac Pathology in Covid‐19: The First Autopsy Series from New Orleans. medRxiv preprint. [DOI] [PMC free article] [PubMed]
- 13. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020; e201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020. 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y,, et al. Thrombocytopenia and its association with mortality in patients with COVID‐19. J Thromb Haemost. 2020. 10.1111/jth.14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shatzel JJ, DeLoughery EP, Lorentz CU, Tucker EI, Aslan JE, Hinds MT, et al. The contact activation system as a potential therapeutic target in patients with COVID‐19. Res Pract Thromb Haemost. 2020. 10.1002/rth2.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrett CD, Moore HB, Moore EE, McIntyre RC, Moore PK, Burke J, et al. Fibrinolytic therapy for refractory COVID‐19 acute respiratory distress syndrome: scientific rationale and review. Res Pract Thromb Haemost. 2020. 10.1002/rth2.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jian Liu J, Li J, Arnold K, Pawlinski R, Key NS. Using heparin molecules to manage COVID‐2019. Res Pract Thromb Haemost. 2020. 10.1002/rth2.12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poor HP, Ventetuolo EC, Tolbert T, et alCOVID‐19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. medRxiv preprint. [DOI] [PMC free article] [PubMed]
- 22. Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G Is COVID‐19 an endothelial disease? Clinical and basic evidence. Preprints. 2020;9(5): E1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Escher R, Breakey N, Lämmle B. Severe COVID‐19 infection associated with endothelial activation. Thromb Res. 2020;15(190):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost. 2020. 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020. 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]


